Tissue-resident immune cells are known to be derived from their circulating precursors and migrate to tissues for host defense. Recent studies have revealed heterogeneity in the origin and proliferation properties of macrophages, which are a prototype tissue-resident immune cell. The tissue-resident macrophages can originate from embryonic and fetal sources and are self-maintained by continuous local self-renewal.1,2 As accessory cells, tissue-resident macrophages support parenchymal cells (their ‘client cells’), and their functions range from tissue development to tissue homeostasis (for example, osteoclasts, lung macrophages, microglia, splenic red-pulp macrophages, and Kupffer cells). Although macrophages are known as the major immune cell with tissue-specific functions, new frameworks integrating the functions of other immune cells with the maintenance of tissue homeostasis will be likely established in the near future.
The transcriptional master regulators (for example, PU.1 and C/EBP-β) determine the macrophage lineage before the cells enter tissues.3,4 These master regulators control a universal transcription module that determines the phenotype shared by all macrophage subsets. In adults, tissue-resident macrophages are differentiated from monocytes that migrate from the bone marrow via blood vessels. The cells obtain their identity by cooperation between macrophage-lineage-differentiation factors (for example, M-CSF and GM-CSF) and tissue-specific factors that include environmental signals such as tissue trophic factors or functional demands. For example, heme and retinoic acid drive the differentiation of splenic red-pulp macrophages and peritoneal macrophages via the transcription factors Spi-C and GATA-6, respectively.5,6,7 In addition, the alveolar macrophage is differentiated from fetal monocytes by the GM-CSF-PPAR-γ axis.8 However, beside these few specific cases the tissue-specific signals required for tissue macrophage differentiation have not been elucidated.
Tissue-resident macrophages continuously monitor endogenous and exogenous changes to maintain tissue homeostasis. Thus, these cells play a crucial role in balancing immune protection and immune tolerance. Immune protection is especially important in host defense against invading pathogens and tumor formation. Tolerance is indispensable for inhibition of immunopathology and autoimmunity. Interestingly, homeostatic programs of tissue resident-macrophages seem to be exploited by cancer cells.3 One well-known example is that tumor-associated macrophages create a tumor microenvironment favoring tumor growth. Recent studies by Anandasabapathy and colleagues reported a factor critical in maintaining tissue-immune homeostasis and demonstrated how immune homeostasis links to tumor growth.9 Previous studies demonstrated that skin migratory dendritic cells (DCs) have the ability to maintain tolerance to self-antigen and suppress ongoing immunity to foreign antigens.10,11 This ability depends upon the NF-κB-centered transcription of the 227 gene signature, including many tolerance-related genes, such as PD-L1, IDO and CD200. The most surprising observation in the study9 is that in both human macrophages and mouse skin migratory DCs, the 227 gene signature overlaps with IFN-γ-induced transcripts. These findings indicate that IFN-γ is a sufficient instructive cue for homeostatic programming in mononuclear phagocytes and skin migratory DCs. These results reflect the homeostatic role of type I IFN.12 Although it is not known whether tissues constitutively produce low levels of IFN-γ, tonic activity of the IFN-γ signaling pathway may be used to maintain tissue homeostasis. The essential part of IFN-γ homeostatic programs is balancing immunity and immune tolerance in healthy tissues. It is known that tumors co-opt tissue homeostatic programs controlled by mononuclear phagocytes and are IFN-γ dependent. The 227 gene homeostatic signature is enriched in multiple human primary cancers, and there is higher expression of the signature genes than is found in normal skin or nevi. The IFN-γ and 227 gene signatures are positively associated with melanoma survival. Thus, the homeostatic program appears to be tipped toward immune activation (for example, DC activation and cross-priming) in melanoma cancer patient pools. SOCS2 is a negative regulator of the IFN-γ signaling pathway and is one of the most highly differentially expressed genes in primary cutaneous melanoma. Furthermore, over activation of the immune system is well controlled in melanoma. DC-specific SOCS2 gene deletion promotes tumor rejection and adaptive T cell priming by DCs. Cumulatively, tumors are considered abnormal tissues that co-opt normal tissue physiology and alter immune surveillance and escape.
IFN-γ creates an inflamed tumor microenvironment, which is a prognostic biomarker for immunotherapy.13 Thus, harnessing this mechanism for immunotherapy has a positive impact on antitumor responses.14 However, chronic stimulation of IFN-γ (also type I IFN) results in potent immune suppression and is exemplified in tumor and chronic viral infection. In this case, STAT1 regulates an IFN-γ-mediated multigenic resistance program that includes T cell inhibitor receptors leading to T cell exhaustion and immune checkpoint blockade.15 It is currently unclear how IFN-γ signaling has dual and opposite effects. The strength and duration of IFN-γ signaling are presumably the most important factors that govern the outcome of the pathway. Figure 1 provides a summary of the findings reported by Anadasabapathy’s group and other researchers.
Figure 1.
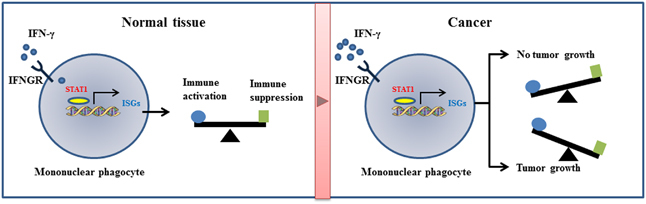
IFN-γ signatures in healthy tissue and cancer. Low levels of IFN-γ are thought to be constitutively expressed in normal tissue. The IFN-γ receptor (IFNGR) may have a tonic activity in stimulating STAT1, which results in expression of low levels of interferon-stimulated genes (ISGs). This homeostatic program balances immune activation and immune suppression. In cancer, high levels of IFN-γ stimulate ISGs involved in immune activation, thereby inhibiting tumor growth. When chronic IFN-γ stimulation occurs, the IFN-γ signatures are shifted toward dominant expression of T cell inhibitory receptors and provide a tumor environment favoring tumor growth.
Acknowledgements
BK laboratory is supported by a grant (2015-0367) from University of Ulsan.
Conflict of interest
The author declares no conflict of interest.
References
- 1.Epelman S, Lavine KJ, Randolph GJ. Origin and functions of tissue macrophages. Immunity. 2014;41:21–35. doi: 10.1016/j.immuni.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ginhoux F, Jung S. Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat Rev Immunol. 2014;14:392–404. doi: 10.1038/nri3671. [DOI] [PubMed] [Google Scholar]
- 3.Okabe Y, Medzhitov R. Tissue biology perspective on macrophages. Nat Immunol. 2016;17:9–17. doi: 10.1038/ni.3320. [DOI] [PubMed] [Google Scholar]
- 4.Amit I, Winter DR, Jung S. The role of the local environment and epigenetics in shaping macrophage identity and their effect on tissue homeostasis. Nat Immunol. 2016;17:18–25. doi: 10.1038/ni.3325. [DOI] [PubMed] [Google Scholar]
- 5.Haldar M, Kohyama M, So AYL, KC W, Brisseno CG, Satpathy AT, et al. Heme-mediated SPI-C induction promotes monocyte differentiation into iron-recycling macrophages. Cell. 2014;156:1223–1234. doi: 10.1016/j.cell.2014.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Okabe Y, Medzhitov R. Tissue-specific signals control reversible program of localization and functional polarization of macrophages. Cell. 2014;47:832–844. doi: 10.1016/j.cell.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosas M, Davies LC, Giles PJ, Liao CT, Kharfan B, Stone TC, et al. The transcription factor Gata6 links tissue macrophage phenotype and proliferative renewal. Science. 2014;344:645–648. doi: 10.1126/science.1251414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schneider C, Nobs SP, Kurrer M, Rehrauer H, Thiele C, Kopf M. Induction of the nuclear receptor PPAR-γ by the cytokine GM-CSF is critical for the differentiation of fetal monocytes into alveolar macrophages. Nat Immunol. 2014;15:1026–1034. doi: 10.1038/ni.3005. [DOI] [PubMed] [Google Scholar]
- 9.Nirschl CJ, Suarez-Farinas M, Izar B, Prakadan S, Dannenfelser R, Tirosh I, et al. IFN- γ-dependent tissue-immune homeostasis is co-opted in the tumor microenvironment. Cell. 2017;170:127–141. doi: 10.1016/j.cell.2017.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baratin M, Foray C, Demaria O, Habbeddine M, Pollet E, Maurizio J, et al. Homeostatic NF-κB signaling in steady-state migratory dendritic cells regulates immune homeostasis and tolerance. Immunity. 2015;42:627–639. doi: 10.1016/j.immuni.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 11.Anaddasabapathy N, Feder R, Mollah S, Tse SW, Longhi MP, Mehandru S, et al. Classical Flt3L-dependent dendritic cells control immunity to protein vaccine. J Exp Med. 2014;211:1875–1891. doi: 10.1084/jem.20131397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gough DJ, Messina NL, Clarke CJP, Johnstone RW, Levy DE. Constitutive type I interferon modulates homeostatic balance through tonic signaling. Immunity. 2012;36:166–174. doi: 10.1016/j.immuni.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harlin H, Meng Y, Peterson AC, Zha Y, Tretiakova M, Slingluff C, et al. Chemokine expression in melanoma metastases associated with CD8+ T-cell recruitment. Cancer Res. 2009;69:3077–3085. doi: 10.1158/0008-5472.CAN-08-2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kang SW, Lee SC, Park SH, Kim J, Kim HH, Lee HW, et al. Anti-CD137 cancer immunotherapy suppresses tumor growth by blockig the CD137 ligand reverse signaling pathway leading to tumor immune tolerance. Cancer Res. 2017 doi: 10.1158/0008-5472.CAN-17-0610. [DOI] [PubMed] [Google Scholar]
- 15.Benci JL, Xu B, Qui Y, Wu TJ, Dada H, Twyman-Saint Victor C, et al. Tumor interferon signaling regulates a multigenic resistance program to immune checkpoint blockdade. Cell. 2017;167:1540–1554. doi: 10.1016/j.cell.2016.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]


