Abstract
Neutrophils are heterogeneous with distinct subsets, and can switch phenotypes to exert regulatory functions on immunity. We herein demonstrate that IL-23-treated neutrophils selectively produce IL-17A, IL-17F and IL-22, and display a distinct gene expression profile in contrast to resting and lipopolysaccharide-treated neutrophils. IL-17+ neutrophils are present in the colons of mice with dextran sulfate sodium-induced colitis. Adoptive transfer of IL-23-treated neutrophils significantly promotes pathogenesis in this model. IL-23 induces neutrophil polarization through STAT3-dependent RORγt and BATF pathways. Thus, IL-23-induced neutrophil polarization expresses a unique cytokine-producing profile, which may contribute to IL-23-mediated inflammatory diseases.
Keywords: colitis, IL-17, IL-23, inflammation, neutrophils, polarization
Introduction
Polymorphonuclear neutrophil granulocytes (also called neutrophils) are fully equipped with a variety of granules that are essential for host defense against intruding microorganisms and play a critical role in initiating inflammation and innate immunity.1,2,3,4 However, neutrophils also significantly contribute to various types of acute and chronic pathological damage during infections, autoimmune diseases and graft rejections in either a positive or a negative manner.5,6,7,8 In fact, in addition to their direct cytotoxicity against invading pathogens, neutrophils can switch phenotypes and exert regulatory functions in both innate and adaptive immunity.9,10,11,12 Neutrophils from rheumatoid arthritis patients upregulate MHC-II expression and increase their antigen-presenting ability, leading to T lymphocyte activation.13 New evidence has also shown that in certain situations neutrophils can be activated to play a role as antigen-presenting cells.14 Recent studies have indicated that neutrophils exhibit considerable functional plasticity and polarization. Similar to macrophage activation pathways, neutrophils have distinctive subsets, as presented in infections and tumors.15,16,17 A subpopulation of neutrophils present in the spleen produces CD40L, BAFF, APRIL and interleukin-21 (IL-21) and has the ability to activate marginal-zone B cells, and subsequently promotes immunoglobulin class-switching, somatic hypermutation and antibody production.18 Tumor-associated neutrophils have been proposed to be polarized between antitumorigenic and protumorigenic neutrophil phenotypes.19 However, the extracellular inducing factors, the intracellular molecular regulatory networks, the phenotype characteristics and the biological significance of the polarized neutrophils are unclear and require further determination.
IL-23 is crucial in the pathogenesis of psoriasis, experimental autoimmune encephalomyelitis (EAE), inflammatory bowel disease (IBD) and collagen-induced arthritis (CIA).20,21,22 Genetic polymorphisms in the IL-23 receptor (IL-23R) are important susceptibility factors for Bechet’s disease, ankylosing spondylitis and IBDs such as Crohn’s disease and ulcerative colitis.23,24 It is known that IL-23 is essential for the terminal differentiation of IL-17-producing T effector cells.25,26 However, IL-23R is predominantly found on natural killer cells, innate lymphoid cells (ILCs) and activated memory T cells, and at lower levels on dendritic cells (DCs), monocytes and macrophages in humans, whereas mouse IL-23R is expressed on activated T cells, ILCs, γδ T cells, macrophages and DCs.27,28,29 Importantly, studies have demonstrated that IL-23 induces these innate cells to secrete IL-17 and/or IL-22.30,31 In the present study, we demonstrate that IL-23-treated neutrophils selectively produce IL-17A, IL-17F and IL-22, and display a distinct lineage gene expression profile in sharp contrast to resting and lipopolysaccharide (LPS)-treated neutrophils. IL-17-expressing neutrophils are present in the intestines of mice with dextran sulfate sodium (DSS)-induced colitis. Moreover, adoptive transfer of IL-23-treated neutrophils significantly promotes the severity of the pathogenesis of DSS-induced colitis in WT or IL-17 knockout (KO) recipient animals. IL-23 induces neutrophil polarization through STAT3-dependent BATF and RORγT pathways. Thus, our present studies reveal neutrophil polarization with a unique cytokine-producing gene profile driven by IL-23. This IL-23-induced neutrophil subpopulation may make significant contributions to IL-23-related pathologies.
Materials and methods
Mice
BALB/c and C57BL/6 (B6) mice were purchased from the Beijing University Experimental Animal Center (Beijing, China). IL-17 KO mice were kindly provided by the Key Laboratory of Human Diseases Comparative Medicine, the Ministry of Public Health; Institute of Laboratory Animal Science, CAMS & PUMC (Beijing, China).32 All mice were bred and maintained in specific pathogen-free conditions. Six-to-twelve-week-old sex-matched littermate mice were mainly used for the experiments unless otherwise noted. All experimental manipulations were undertaken in accordance with the Institutional Guidelines for the Care and Use of Laboratory Animals, Institute of Zoology.
Reagents
Anti-mCD11b-PE-Cy5 was purchased from BD Biosciences Pharmingen (San Diego, CA, USA). Anti-mLy6G-PE was purchased from eBioscience (San Diego, CA, USA). Anti-mIL-17A-PE was purchased from Biolegend (San Diego, CA, USA). Anti-mIL-23R-AF488 mAb was purchased from R&D Systems (Minneapolis, MN, USA). Bacterial lipopolysaccharide (E. coli 055:B5) was purchased from Sigma-Aldrich (St Louis, MO, USA). Recombinant mouse cytokines were purchased from PeproTech (Rocky Hill, NJ, USA). A selective STAT3 inhibitor (S3I-201) was purchased from Sigma-Aldrich. Primary antibodies against p-STAT3 (705), BATF were purchased from Cell Signaling Technology (Danvers, MA, USA). RORγT antibody was purchased from Millipore Biotechnology (Billerica, MA, USA). These antibodies were diluted at 1:1000 in 5% bovine serum albumin (BSA). Anti-β-actin mAb was purchased from Sigma-Aldrich and its working concentration (1:50 000) was determined in previous studies.33,34
Recombinant mouse cytokines were used at the following concentrations based on our previous studies:35 recombinant mouse IL-1β (100 ng/ml, PeproTech), IL-2 (10 ng/ml, PeproTech), IL-4 (10 ng/ml, R&D Systems), IL-6 (50 ng/ml, PeproTech), IL-10 (20 ng/ml, PeproTech), IL-12 (5 ng/ml, PeproTech), IL-13 (50 ng/ml, PeproTech), IL-17A (100 ng/ml, PeproTech), IL-21 (50 ng/ml, R&D Systems), IL-23 (10 ng/ml, R&D Systems), IL-33 (100 ng/ml, R&D Systems), LPS (1 μg/ml, Sigma-Aldrich), IFN-γ (100 ng/ml, PeproTech), TNF-α (100 ng/ml, PeproTech), TGF-β1 (5 ng/ml, R&D Systems), GM-CSF (40 ng/ml, R&D Systems) and G-CSF (100 ng/ml, Biovision, Milpitas, CA, USA).
Isolation and differentiation of neutrophils in vitro
Tibiae and femora from BALB/c or B6 mice were removed using sterile techniques, and bone marrow was flushed with PBS. Red blood cells were lysed with ammonium chloride. Primary neutrophils (CD11b+Ly6G+F4/80−) were isolated from bone marrow cells with 52% percoll (GE Healthcare Life Sciences, Piscataway, NJ, USA, 17-0891-01) and sorted by MoFlo XDP (Beckman Coulter) in several cases.36 Primary neutrophils (2 × 106 cells per ml in 48-well plates) were cultured with IL-23 (50 ng/ml) for different time courses as indicated in RPMI 1640 medium supplemented with 2 mM l-glutamine, 10 mM HEPES, 20 μM 2-ME, 150 U/ml streptomycin, 200 U/ml penicillin and 5% heat-inactivated FBS at 37 °C and 5% CO2. The cytokine expressions and phenotypes of neutrophils were characterized by real-time PCR, ELISA assays and flow cytometric analysis.
Microarray hybridization and data acquisition
The neutrophils were stimulated by LPS (1 μg/ml) and IL-23 (50 ng/ml). TRIzol Reagent (Life Technologies, Gaithersburg, MD, USA, 15596-018) was used to extract total RNA following the manufacturer’s instructions. Total RNA was amplified and labeled with a Low Input Quick Amp Labeling Kit, One-Color (Agilent Technologies, 5190-2305), and further purified with an RNeasy mini kit (Cat. No. 74106, Qiagen, Valencia, CA, USA). Each slide was hybridized with 1.65 μg Cy3-labeled cRNA using a Gene Expression Hybridization Kit (Cat. No. 5190-2305, Agilent Technologies, Santa Clara, CA, USA, 5188–5242) in a Hybridization Oven (Agilent Technologies, G2545A), according to the manufacturer’s instructions. After 17 h of hybridization, slides were washed in staining dishes (Cat. No. 121, Thermo Shandon, Watertown, MA, USA) with a Gene Expression Wash Buffer Kit (Agilent Technologies, 5188–5327), following the manufacturer’s instructions. Slides were scanned by an Agilent Microarray Scanner (Agilent Technologies, G2565CA) with default settings: dye channel—green, scan resolution—5 μm, PMT—100%, 10%, 16 bit. Data were extracted with Feature Extraction software 10.7 (Agilent Technologies). Raw data were normalized by Quantile algorithm, Gene Spring Software 11.0 (Agilent Technologies).
Microarray data analysis
Lineage-restricted genes were defined as the genes the expression levels of which were at least two-fold greater in one cell type than in any other cell type. R (version 3.0.2) was used to draw the heatmap of lineage-restricted genes. The fold-changes were calculated as the ratio of the expression level in each cell type versus the second-highest expression level in all cell types. Differentially expressed genes were defined as genes with at least two-fold variance of expression levels in LPS- and IL-23-treated neutrophils compared with untreated neutrophils.
Quantitative PCR analysis
The neutrophils were stimulated by LPS (1 μg/ml) and IL-23 (50 ng/ml) at different time points. Total RNA was isolated with TRIzol (Invitrogen) and reverse transcription was performed with M-MLV superscript reverse transcriptase as described previously. Real-time PCR was performed using multiple kits (SYBR Premix Ex Taq, DRR041A, Takara Bio) on CFX96 (Bio-Rad Laboratories, Hercules, CA, USA). The primers utilized are summarized in Table 1. The mRNA expression level of each gene was normalized to the housekeeping gene hypoxanthinephosphoribosyl transferase (HPRT), as reported previously.33,37 For the inhibitor assay, cells were pretreated with various inhibitors for 30 min, and then treated with IL-23 for 12 h.
Table 1.
Primers used for qRT-PCR analysis
| Genes | Primer sequence (5′–3′) |
|---|---|
| HPRT | |
| Forward primer: | |
| Reverse primer: | |
| TNF-α | |
| Forward primer: | |
| Reverse primer: | |
| CXCL4 | |
| Forward primer: | |
| Reverse primer: | |
| IL-6 | |
| Forward primer: | |
| Reverse primer: | |
| CCL3 | |
| Forward primer: | |
| Reverse primer: | |
| iNOS | |
| Forward primer: | |
| Reverse primer: | |
| IL-17A | |
| Forward primer: | |
| Reverse primer: | |
| IL-17F | |
| Forward primer: | |
| Reverse primer: | |
| IL-22 | |
| Forward primer: | |
| Reverse primer: | |
| CCL4 | |
| Forward primer: | |
| Reverse primer: | |
| CCL7 | |
| Forward primer: | |
| Reverse primer: | |
| CCL9 | |
| Forward primer: | |
| Reverse primer: | |
| CXCL10 | |
| Forward primer: | |
| Reverse primer: | |
| CXCL11 | |
| Forward primer: | |
| Reverse primer: | |
| CXCL12 | |
| Forward primer: | |
| Reverse primer: | |
| CXCL14 | |
| Forward primer: | |
| Reverse primer: | |
| CXCL16 | |
| Forward primer: | |
| Reverse primer: | |
| CCL21a | |
| Forward primer: | |
| Reverse primer: | |
| CCL21c | |
| Forward primer: | |
| Reverse primer: | |
| CCL22 | |
| Forward primer: | |
| Reverse primer: | |
| CCL25 | |
| Forward primer: | |
| Reverse primer: | |
| CSF2 | |
| Forward primer: | |
| Reverse primer: | |
| BATF | |
| Forward primer: | |
| Reverse primer: | |
| RORγT | |
| Forward primer: | |
| Reverse primer: | |
| IRF7 | |
| Forward primer: | |
| Reverse primer: | |
| IRF4 | |
| Forward primer: | |
| Reverse primer: | |
| Sox5 | |
| Forward primer: | |
| Reverse primer: | |
| C-Maf | |
| Forward primer: | |
| Reverse primer: | |
| CCL17 | |
| Forward primer: | |
| Reverse primer: | |
| CXCL3 | |
| Forward primer: | |
| Reverse primer: | |
| CXCL1 | |
| Forward primer: | |
| Reverse primer: | |
| CXCL2 | |
| Forward primer: | |
| Reverse primer: | |
| Ifi204 | |
| Forward primer: | |
| Reverse primer: | |
| Ifit1 | |
| Forward primer: | |
| Reverse primer: | |
| Ifit3 | |
| Forward primer: | |
| Reverse primer: | |
| Ifih1 | |
| Forward primer: | |
| Reverse primer: | |
Flow cytometry
For flow cytometric analysis of surface markers, cells were stained with antibodies in PBS containing 0.1% (w/v) BSA and 0.1% NaN3 as described previously.38 For the detection of intracellular cytokines, neutrophils were treated with GolgiPlug (BD Biosciences, San Jose, CA, USA) for the last 4–6 h of incubation. Cells were fixed and permeabilized according to the manufacturer’s protocol (BD Bioscience) and stained with anti-IL-17 mAb (1:100). Flow cytometry data were acquired on a FACSCalibur (BD Biosciences) or Epics XL (Beckman Coulter, Brea, CA, USA) and analyzed with FCS Express Version 3.0 software (De Novo Software) or FlowJo Version 7.6.5 software (TreeStar Inc., Ashland, OR, USA).
Western blotting
Neutrophils were cultured in RPMI 1640 medium with 5% FCS in 12-well plates. Cells were treated with IL-23 (50 ng/ml) or LPS (1 μg/ml) for the indicated times. After stimulation, cells were washed once in cold PBS, lysed in RIPA buffer (50 mM Tris-HCl pH 7.4, 1% NP-40, 0.25% Na-deoxycholate, 150 mM NaCl, 1 mM EDTA pH 7.4) with protease and phosphatase inhibitor cocktails (Sigma) for 10 min on a rocker at 4 °C as described previously.39 Protein concentration was determined using a BCA assay. Protein samples were analyzed by SDS polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto PVDF membranes (Millipore, Ternicula, CA, USA). Each polyvinylidene fluoride membrane was blocked with TBST (100 mM Tris-HCl pH 7.5, 150 mM NaCl, 0.05% Tween20) with 5% non-fat dried milk for 1 h, then incubated with primary antibodies overnight on a shaker at 4 °C. The appropriate HRP-conjugated secondary antibody was then added and was detected through a Tanon 1600R Gel Image System (Tanon Co., Ltd, Shanghai, China). Actin was used as a protein loading control.
ELISA assays
IL-17A (432508), IL-17F (436107) and IL-22 (436307) ELISA assay kits were purchased from Biolegend. The ELISA assay was performed following the manufacturer’s instructions. Briefly, pre-coated ELISA plates were blocked with 5% BSA/PBS at room temperature for ⩾1 h. After washing three times with PBS/Tween20, standards and samples were added to pre-coated ELISA plates and incubated at room temperature for 2–4 h. After washing, specific cytokine detection antibodies were added for 1 h at 30 °C followed by Avidin-HRP A for 1 h at 30 °C. Plates were developed using Substrate Solution E. The reaction was stopped with the Stop Solution and optical densities were determined at 450 nm using a plate reader.
Immunofluorescent staining
Cells were cultured on coverslips for the indicated times and fixed in 4% paraformaldehyde for 10 min.35 Cells were permeabilized with 0.2% Triton X-100 (v/v) in PBS for 10 min at room temperature and blocked for 1 h in 5% BSA/PBS, and incubated overnight in indicated primary mAbs (1:100 dilution in blocking buffer) at 4 °C. Following PBS washes, secondary Ab (FITC- or PE-labeled goat anti-rabbit Ab, Santa Cruz Biotechnology, Santa Cruz, CA, USA; 1:500 dilution) was applied for 1 h followed by Hoechst 33342 (2 μg/ml) staining for 10 min before the coverslips were washed in PBS and mounted. Photomicrographs were taken using an LSM510META Laser Scanning Microscope (Zeiss, Germany).
IBD model and histological examination
Recipient B6 mice were i.v.-injected with 5 × 106 sorted neutrophils stimulated with IL-23 for 12 h on days 0, 2, 5 and 8. Colitis was induced by the administration of dextran sulfate sodium (2.5%) in drinking water. The disease activity index (DAI)40 and body weight of each animal were monitored daily. DAI was determined by scoring the loss of body weight, characteristics of the stool, and occult blood in stool or hematochezia (over the entire period), according to the classic scoring system by Cooper with modification. The scoring process is given as follows: body weight loss (0, none; 1, 1–5%; 2, 5–20%; 3, >20%), stool consistency (0, normal; 2, loose stool; 3, diarrhea) and stool blood (0, negative; 2, fecal occult blood test positive; 3, gross bleeding). The animals were killed on day 7 of the experiment, and the colonic tissue was removed and cleaned, and then subjected to cell culture, followed by quantitative real-time PCR and histological analyses.
Statistical analysis
All data are presented as the mean±s.d. Two-way ANOVA analysis was used for comparisons among multiple groups with SPSS 16.0 software. A Mann–Whitney U-test was used for comparisons between the two groups. A P-value <0.05 was considered statistically significant.
Results
IL-23 selectively induces Th17 cytokine expression in neutrophils
In addition to participating in primary defense against infections, neutrophils produce and release a large number of cytokines and chemokines either constitutively or upon microenvironmental stimulations that play a critical role in infections and pathologies.41,42,43,44 To explore the roles of different cytokines on the expression of IL-17 family members in neutrophils, we first detected the mRNA expression levels of these genes in freshly isolated neutrophils from mouse bone marrow after different cytokine and LPS stimulation for 24 h by real-time PCR. Among the 17 cytokines and LPS stimulations studied, only IL-23 significantly promoted IL-17A and IL-17F expression, while resting neutrophils expressed nearly undetectable levels of IL-17A and IL-17F (P<0.001, Figure 1a). In addition, IL-23 also specifically induced significant IL-22 expression compared with other cytokines and LPS (P<0.001, Figure 1a). IL-23 induced IL-17A, IL-17F and IL-22 mRNA expression in dose- and time-dependent manners, as determined by real-time PCR (Figures 1b and c). The possible direct effects of IL-23 on neutrophils were supported by the expression of IL-23R on neutrophils as detected by flow cytometry, which showed that approximately 10% of neutrophils are positive for IL-23R (Supplementary Table 1). To exclude the potential contamination of other immune cells such as T cells, B cells and ILCs during the differentiation process in vitro, we sorted the neutrophils of naïve mice to obtain highly purified CD11b+Ly6G+ cells with more than 99% purity and repeated the same induction experiments. Indeed, the sorted CD11b+Ly6G+ cells expressed high levels of IL-17A, IL-17F and IL-22 after IL-23 treatment at both the mRNA and the protein levels (P<0.001, Figure 1d, Supplementary Table 2). The protein levels of IL-17A in the culture medium of IL-23-treated sorted neutrophils were significantly increased with increasing IL-23 doses, as detected by ELISA assays, indicating that IL-23-treated neutrophils have the ability to secrete IL-17A (P<0.01, Figure 1e). The expression of IL-17A in IL-23-treated neutrophils was further confirmed by flow cytometry and confocal microscopy staining (Figures 1f and g). By contrast, the sorted IL-23R-deficient neutrophils failed to express IL-17A, IL-17F and IL-22 after IL-23 treatment (Supplementary Table 3). Thus, IL-23, but not other cytokines or LPS, has the ability to promote the expression of the Th17-type cytokines IL-17A, IL-17F and IL-22 in resting mouse neutrophils.
Figure 1.

The expression of IL-17A, IL-17F and IL-22 in neutrophils is selectively induced by IL-23. (a) Primary neutrophils freshly isolated from the bone marrow of C57BL/6 mice were treated with various cytokines and LPS for 24 h. IL-17A, IL-17F and IL-22 mRNA expression levels were determined by real-time PCR. (b) Quantitative PCR analysis of IL-17A, IL-17F and IL-22 mRNA expression in neutrophils treated with IL-23 (50 ng/ml) for different times. (c) mRNA expression levels of IL-17A, IL-17F and IL-22 in primary neutrophils treated with different concentrations of IL-23 for 12 h were determined by real-time PCR. (d) mRNA expression levels of IL-17A, IL-17F and IL-22 in sorted CD11b+Ly6G+ neutrophils treated with IL-23 (0–50 ng/ml) for 12 h were determined by real-time PCR. (e) The protein levels of IL-17A in the culture medium of sorted CD11b+Ly6G+ neutrophils treated with IL-23 (50 ng/ml) for different times were determined by ELISA assays. The protein expression of IL-17A in neutrophils treated with 50 ng/ml IL-23 for 24 h was determined by flow cytometry (f) and confocal microscopy (g). Data are shown as the mean±s.d. (N=3), representing one of three independent experiments. *P<0.05, **P<0.01 and ***P<0.001 compared with control group. Data were analyzed by Mann–Whitney U-test for two group comparisons or one-way ANOVA analysis for multi-group comparisons using SPSS software. LPS, lipopolysaccharide.
IL-23 induces a distinct gene expression profile in neutrophils
To investigate whether IL-23 induces a unique neutrophil polarization profile, in contrast to the LPS-induced inflammatory neutrophil subpopulation, we determined the gene expression profiles of LPS-polarized neutrophils and IL-23-induced neutrophils by microarray analysis. Indeed, IL-23-induced neutrophils express a unique panel of genes in sharp contrast to resting and LPS-stimulated neutrophils (Figure 2a). Specifically, 1362 genes were upregulated and 1438 genes were downregulated in IL-23-treated neutrophils compared with untreated and LPS-polarized neutrophils (Figures 2a and b). Interestingly, LPS- and IL-23-induced neutrophils express distinctive gene profiles and kinetics of cytokine induction, as indicated by mRNA microarray analysis and confirmed by real-time PCR assays (Figures 2c–e). LPS induces TNF-α and IL-6 expression in neutrophils at the mRNA level (Figure 2d). However, IL-23 fails to induce the expression of TNF-α and IL-6 (Figure 2d). By contrast, IL-23 specifically promotes the expression of IL-17A, IL-17F and IL-22 at both the mRNA and the protein levels in neutrophils (P<0.01, Figures 2d and e). Altogether, these data lead us to speculate that IL-23 may drive the polarization of resting neutrophils into a subpopulation with a distinct panel of cytokines compared with LPS-induced inflammatory neutrophils.
Figure 2.

IL-23-treated neutrophils display a distinct gene expression profile. Sorted primary neutrophils were cultured with LPS or IL-23 in vitro, and microarray analyses of gene expression profiles were performed. (a) A heatmap comparison of gene expression profiles identifies lineage-restricted genes in untreated-Neu, LPS-Neu and IL-23-Neu cells. Genes with predominant expression in one cell type (at least two-fold greater than any other cell type) are organized in the heatmap. (b) Venn diagram showing upregulated genes 1695+916+1362=3973 and downregulated genes 2505+1506+1438=5449 in LPS-Neu and IL-23-Neu compared with untreated neutrophils. Approximately 34% of upregulated genes and 26% of downregulated genes are specifically differentially expressed in IL-23-Neu compared with untreated neutrophils. (c) Expression profiling of related cytokines in untreated-Neu, LPS-Neu and IL-23-Neu cells. Genes with predominant expression in one cell type (at least two-fold change compared with other cell types) are organized in the heatmap. Colors represent genes greater than (red) or less than (blue) the second-highest expression in three cell types. (d) The expression levels of cytokines including iNOS, TNF-α, IL-6, IL-17A, IL-17F and IL-22 were determined by real-time PCR after the isolated neutrophils were induced with LPS and IL-23 for different times. (e) The concentrations of IL-17A, IL-17F and IL-22 in the culture medium of isolated neutrophils induced with LPS and IL-23 for different times were measured by ELISA assays. Data are expressed as the mean±s.d. (N=3–5), with one representative experiment from three independent experiments shown. *P<0.05, **P<0.01 and ***P<0.001 for comparisons between the indicated groups. Data were analyzed by the Mann–Whitney U-test or two-way ANOVA analysis using SPSS software.
IL-23 induces Th17 cytokine expression in neutrophils via STAT3-dependent pathways
It has been reported that RORγT, RORα, IRF-7, IRF4, BATF, Sox5 and C-maf are critical transcription factors for Th17 cell induction.45,46,47 The expression of BATF, RORγT, IRF4 and IRF7 is enhanced in IL-23-treated neutrophils at the mRNA level, and the protein level expression of BATF and RORγT is upregulated in IL-23-treated neutrophils (Figures 3a and b). However, the expression of Sox5 and C-maf in IL-23-treated cells did not appear to be significantly changed (Figure 3a). Thus, the enhanced expression of BATF and RORγT suggested that they are likely involved in IL-23-stimulated neutrophil polarization. It has been reported that IL-23 promotes STAT3 activation in T cells based on the enhanced levels of p-STAT3 (at amino acids Y705 and S727).21,48 The level of p-STAT3 in neutrophils is increased after IL-23 treatment (Figure 3c). Inhibition of STAT3 activation by the STAT3-specific inhibitor S31-201 significantly decreases IL-17A, IL-17F and IL-22 protein expression and IL-17A and IL-17F mRNA expression in IL-23-induced neutrophils in a dose-dependent manner (P<0.001, Figures 3d and e). The STAT3-specific inhibitor S31-201 also decreased STAT3 activation in IL-23-treated neutrophils, as indicated by the levels of p-STAT3 detected by western blot (Figure 3f). Meanwhile, inhibiting STAT3 activity significantly blocks the IL-23-induced expression of BATF, RORγT, IFR4 and, to a lesser degree, IRF7 in neutrophils (Figures 3g and h), indicating that BATF, RORγT, IFR4 and IRF7 are down-stream molecules in an IL-23-activated STAT3 pathway in neutrophils. Thus, IL-23 induces IL-17A, IL-17F and IL-22 expression in neutrophils through a STAT3-dependent pathway.
Figure 3.

The Th17-type cytokine expression of IL-23-treated neutrophils is mediated by STAT3-dependent signaling pathways. (a) mRNA expression levels of BATF, RORγT, IRF7, IRF4, Sox5 and C-maf in neutrophils treated with IL-23 for different times were determined by real-time PCR. (b, c) The BATF, RORγT and p-STAT3 protein levels in neutrophils after IL-23 treatment for the indicated times were determined by western blot. (d) Neutrophils were pretreated with the indicated concentrations of STAT3 inhibitor (S3I-201) for 0.5 h and then stimulated with IL-23 for 12 h. The expression levels of IL-17A, IL-17F and IL-22 were detected by real-time PCR. (e) The concentrations of IL-17A, IL-17F and IL-22 in the supernatants of neutrophils pretreated with STAT3 inhibitor (S3I-201) for 0.5 h and then stimulated with IL-23 for 24 h were determined by ELISA assay. (f, g) p-STAT3 and BATF expression in neutrophils treated with or without IL-23 and/or S3I-201 was determined by western blotting. (h) mRNA expression levels of BATF, RORγT, IRF4 and IRF7 in neutrophils pretreated with STAT3 inhibitor (S3I-201) for 0.5 h and then stimulated with IL-23 for 12 h were determined by real-time PCR. Assays were performed more than three times. Data are shown as the mean±s.d. (n=3). *P<0.05, **P<0.01 and ***P<0.001 for comparisons between the indicated groups. Data were analyzed by one-way ANOVA analysis using SPSS software.
The roles of IL-17-expressing neutrophils in colitis in mice
We observed the presence of IL-17-producing neutrophils in a mouse model of DSS-induced colitis, in which Th17 cells and neutrophils have been reported to play a critical role.14 Mice with DSS-induced colitis showed enhanced IL-17A and IL-22 expression in colon tissues (Figures 4a and b). Importantly, IL-17A+ neutrophils were present in the colons of DSS-induced colitis mice, as detected by flow cytometry (Figure 4c). Using the neutrophil-depleting antibody RB6-8C5, we found that neutrophil depletion significantly reduced the expression of IL-17A, IL-17F and IL-22 in colon tissues (Supplementary Table 4a and b), which supports the contribution of neutrophils to these cytokines in colitis. To identify the role of IL-17-producing neutrophils in the pathogenesis of colitis, we adoptively transferred either induced IL-23-treated or resting neutrophils into recipient mice during the administration of DSS. Mice that received IL-23-treated neutrophils displayed more severe symptoms of acute colitis than mice that either received untreated neutrophils or did not receive neutrophils, as shown by significantly shorter survival times (Figure 4d), rapid body weight loss (Figure 4e), increased macroscopic scores (Figure 4f) and shorter colon lengths (Figure 4g) in the mice that received IL-23-treated neutrophils. The median survival times for mice not receiving neutrophils, and for mice receiving untreated, or IL-23-treated neutrophils were 19, 19 and 16 days, respectively. Histological analysis after 7 days of DSS treatment revealed severe colitis in the mice that received IL-23-treated neutrophils, including massive inflammatory infiltrates and thickened walls (Figure 4h). Meanwhile, the colonic tissues of mice that received IL-23-treated neutrophils expressed significantly more IL-17 and IL-22 than those in mice that received resting neutrophils (Figures 4i and j). Interestingly, the adoptive transfer of IL-23-treated neutrophils with an IL-17A deficiency partially but significantly decreased the enhancement of colitis observed with IL-23-treated neutrophils, as supported by mouse survival time (Figure 4k), body weight loss (Figure 4l), macroscopic score (Figure 4m), colon length (Figure 4n) and histological analysis (Figure 4o) in WT and IL-17A-deficient IL-23-neutrophil-transferred mice. To further demonstrate the endogenous role of IL-17-producing neutrophils in colitis progression, IL17A KO mice were treated with DSS and administered either IL-23-treated or untreated neutrophils. IL-17A KO mice receiving IL-23-treated neutrophils displayed more severe symptoms of colitis compared with mice either receiving control neutrophils or not receiving neutrophils, as shown by survival time (Figure 5a), macroscopic score (Figure 5c), colon length (Figure 5d) and histological analysis (Figure 5e). IL-17 and IL-22 expression levels in colonic tissues were higher in IL-17 KO mice receiving IL-23-treated neutrophils than in mice receiving resting neutrophils (Figures 5f and g). Thus, IL-23-induced neutrophils are present in the colonic tissues of colitis mice and have the capacity to promote colitis pathogenesis in mice.
Figure 4.
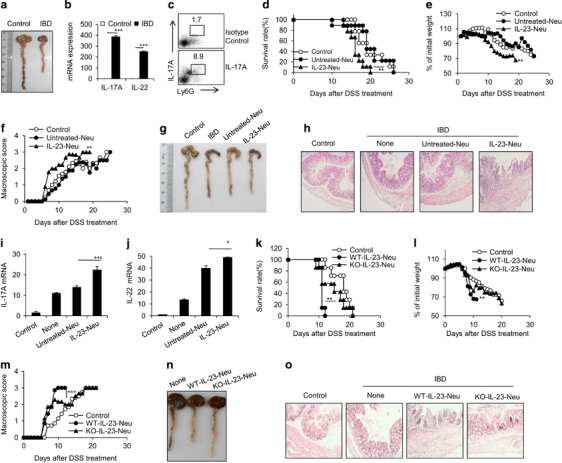
IL-23-treated neutrophils promote dextran sulfate sodium (DSS)-induced colitis. Colitis was induced by adding DSS (2.5%) to drinking water as described in the Materials and Methods. (a) Morphological analysis of colons from wild-type and DSS-induced colitis mice. (b) mRNA expression levels of IL-17A and IL-22 in the sorted CD11b+Ly6G+ neutrophils from control and DSS-treated colonic tissues were detected by real-time PCR. (c) IL-17+ neutrophils in colons of control and DSS-induced colitis mice were detected by flow cytometry. Survival rate (d), body weight (e), macroscopic score (f) and representative pictures (g) of colons from control and DSS-induced colitis mice adoptively transferred with PBS, 5 × 106 untreated-Neu or IL-23-treated-Neu cells (sorted CD11b+Ly6G+ neutrophils) per mouse on day 0, 2 and 5 are summarized. (h) H&E staining of colonic tissues from control or DSS-induced colitis mice treated as described above. mRNA expression levels of IL-17A (i) and IL-22 (j) in colonic tissues from mice treated as described above were detected by real-time PCR. Survival rate (k), body weight (l), macroscopic score (m) and representative pictures (n) of colons of control and DSS-induced colitis mice adoptively transferred with PBS, 5 × 106 untreated neutrophils, IL-23-treated neutrophils or IL-17A-deficient IL-23-treated neutrophils (sorted CD11b+Ly6G+ neutrophils) per mouse on days 0, 2 and 5. (o) H&E staining of colonic tissues from control or DSS-induced colitis mice treated as described above are presented. Data are expressed as the mean±s.d. (n=3–5) and one representative example from three independent experiments with similar results is shown. *P<0.05, **P<0.01 and ***P<0.001 for comparisons between the indicated groups. Data were analyzed by the Mann–Whitney U-test or two-way ANOVA using SPSS software. Survival curves were analyzed by the log-rank test.
Figure 5.

IL-23-treated neutrophils promote dextran sulfate sodium (DSS)-induced colitis in IL-17 knockout (KO) recipients. DSS (2.5%) was administered to IL-17A KO mice for colitis induction. PBS, 5 × 106 untreated or IL-23-treated neutrophils (sorted CD11b+Ly6G+ neutrophils) per mouse were i.v.-injected on days 0, 2 and 5. The survival rate (a), body weight (b), macroscopic score (c) and representative colon pictures (d) of control and DSS-induced colitis mice are shown. (e) H&E staining of colonic tissues from control or DSS-induced colitis mice treated as described above are presented. The mRNA expression levels of IL-17A (f) and IL-22 (g) in colonic tissues from mice treated as described above were detected by real-time PCR. Data are expressed as the mean±s.d. (n=3–5) and one representative example from three independent experiments with similar results is shown. *P<0.05, **P<0.01 and ***P<0.001 for comparisons between the indicated groups. Data were analyzed by the Mann–Whitney U-test or two-way ANOVA using SPSS software. Survival curves were analyzed by the log-rank test.
Discussion
In the present study, we have identified a unique neutrophil subpopulation induced by IL-23 with the following properties: (a) to display distinctive gene expression profiles compared with resting neutrophils and LPS-activated neutrophils; (b) to produce more Th17-type cytokines including IL-17A, IL-17F and IL-22 and less TNF-α and IL-1β; (c) to promote colitis pathogenesis; and (d) to activate the specific transcription factors RORγT and BATF downstream of STAT3. Thus, we provide additional evidence for differentially polarized neutrophils with respect to cytokine production. We believe that relevant studies may provide new insights into the contribution of neutrophils to the pathogenesis of inflammation and immune disorders and may provide new therapeutic approaches to treat neutrophil-related diseases.
Genetic studies have strongly indicated that IL-23 may significantly contribute to inflammatory disease risk in humans.49,50 Deficiency of IL-23 in mice causes resistance to experimental immune-mediated diseases such as IBD.51,52 The promotion of Th17 subsets is highly recognized as a key player for mediating the critical role of IL-23 in these inflammatory diseases.20,53 Our present study showed that IL-17A+ neutrophils are present in colonic tissues with colitis. IL-23 acts directly on neutrophils to induce IL-17A and IL-17F, and the adaptive transfer of IL-23-treated neutrophils significantly increases the severity of colitis in mice, indicating that IL-17A-producing neutrophils may promote the pathogenesis of colitis in mice. The significance of IL-23-induced neutrophil polarization in Th17 cytokine-related inflammatory diseases needs to be studied further. However, it has recently been reported that neutrophils can release IL-17 in areas with amyloid-β deposits and contribute to disease pathogenesis in Alzheimer’s disease-like mouse models.54 It is interesting and suggestive that the serum concentration of IL-23 has been positively correlated with IL-17-producing neutrophils in patients with fungal keratitis.55 Neutrophils in the circulation of arthritic mice spontaneously produce IL-17 in vitro.56 IL-17 produced by neutrophils also participates in inflammation-induced airway neutrophilia.20 Higher levels of IL17+CD177+ neutrophils in the peripheral blood in allergic asthma patients might contribute to disease progression.57 IL-17A-producing RORγt+ neutrophils in the peripheral blood may amplify liver tissue injury in a hepatic ischemia-reperfusion injury mouse model.58 Thus, IL-17A-producing neutrophils contribute to a wide range of inflammatory diseases.
The biological significance and the pro-inflammatory activities of IL-23 in inflammatory and autoimmune diseases include but are not limited to the induction of IL-17 production by Th17 cells and the inhibition of immunosuppressive CD4+CD25+ regulatory T cells.59,60 We observed that IL-23 can significantly induce IL-17A, IL-17F and IL-22 production in neutrophils. Our data are in line with observations showing that human neutrophils also express RORγt and upregulate the expression of IL-17A, IL-17RC and dectin-2 following IL-6 and IL-23 stimulation.44 IL-17 production by neutrophils can be induced in various models including mucosal candidiasis, fusarium corneal infections, kidney ischemia-reperfusion injury and psoriasis.44,61,62,63 Intra-articular administration of zymosan (a TLR2 ligand) results in IL-17 production by neutrophils in an arthritis mouse model.56 Activated peritoneal neutrophils in vitro produce IL-17A and IL-23 in response to myeloperoxidase-specific anti-neutrophil cytoplasmic auto-antibodies via their Fc-regions and the classical complement pathway, which contributes to the occurrence of chronic autoimmune inflammation and ultimately results in the development of local Th17-mediated autoimmunity.64 These data collectively illustrate that neutrophils are one of the major sources of IL-17 under specific inflammatory conditions. It is known that neutrophils can produce many different cytokines such as IL-1β, TNF-α, IL-12, IL-4 and IL-10 under different stimulations.41,42 Therefore, based on the specific cytokine production, neutrophils may have different types of functional polarizations, similar to macrophages.
In addition to producing IL-17, IL-23-induced neutrophils also produce IL-22. The large amount of IL-17 produced by this neutrophil subset significantly contributed to the pathogenesis of DSS-induced colitis in mice. However, it has been reported that IL-22 may have opposing effects on the pathogenesis of colitis by not only enhancing inflammation and antimicrobial immunity but also increasing epithelial cell proliferation and tissue repair.65 Thus, it is clear that the role of IL-22 produced by IL-23-induced neutrophils in colitis needs to be clarified in future studies.
IL-23 signals through IL-23R and IL-12Rβ1 to activate JAK and predominantly leads to phosphorylation and activation of STAT3 in T cells,66 which promotes the transcription of Il23r and Rorc (encoding RORγ) and stabilizes the expression of genes encoding pro-inflammatory effector molecules including IL17A, IL17F, IL22 and CSF2.42,43 RORγT, RORα, IRF-7, IRF4, BATF, Sox5 and C-maf are critical transcription factors for Th17 cell induction.45,46,47 Our results show that IL-23 uses the classical STAT3–RORγT/BATF pathway to induce a pro-Th17 cytokine gene expression profile in neutrophils.
In summary, our present study demonstrates a Th17 type-like polarization of neutrophils induced by IL-23. These polarized neutrophils promote colitis pathogenesis in a mouse model. These findings shed further light on the existence of neutrophil functional plasticity and polarization, which have previously been neglected. The biological significance of polarized neutrophils in other diseases needs to be investigated and may offer novel therapeutic approaches to treat neutrophil-related immune disorders.
Electronic supplementary material
Acknowledgements
We thank Dr Aqeel Javeed for his kind review of the manuscript. This work was supported by grants from the National Basic Research Program of China (2014ZX10002002-001-002, YZ; 2011CB710903, YZ), the National Natural Science Foundation of China for General and Key Programs (81530049, 81130055, YZ), the Knowledge Innovation Program of the Chinese Academy of Sciences (XDA04020202-19, YZ), the Beijing Municipal Hospital Authority ‘Yangfan Program’ (ZYLX201408, XZ), and the CAS/SAFEA International Partnership Program for Creative Research Teams (YZ).
Author contributions
YL, LZ, ZC and TY designed and carried out the experiments, analyzed the data and wrote the manuscript; H-XS analyzed the microarray data; FY, WW and YH performed the animal model experiments and real-time PCR assays; PW performed the ELISA assays; QZ and YT performed the flow cytometry; LZ, XZ and YZ designed the experiments, analyzed the data, wrote the manuscript and provided overall supervision.
Conflict of interest
The authors declare no conflict of interest.
Contributor Information
Lianfeng Zhang, Email: zhanglf@cnilas.org.
Xiaodong Zhang, Email: zhangxiaodong@bjcyh.com.
Yong Zhao, Email: zhaoy@ioz.ac.cn.
Electronic supplementary material
Supplementary Information for this article can be found on the Cellular & Molecular Immunology website 10.1038/cmi.2017.39
References
- 1.Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13:159–175. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 2.Amulic B, Cazalet C, Hayes GL, Metzler KD, Zychlinsky A. Neutrophil function: from mechanisms to disease. Annu Rev Immunol. 2012;30:459–489. doi: 10.1146/annurev-immunol-020711-074942. [DOI] [PubMed] [Google Scholar]
- 3.Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol. 2011;11:519–531. doi: 10.1038/nri3024. [DOI] [PubMed] [Google Scholar]
- 4.Borregaard N. Neutrophils, from marrow to microbes. Immunity. 2010;33:657–670. doi: 10.1016/j.immuni.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 5.Kreisel D, Sugimoto S, Zhu J, Nava R, Li W, Okazaki M, et al. Emergency granulopoiesis promotes neutrophil-dendritic cell encounters that prevent mouse lung allograft acceptance. Blood. 2011;118:6172–6182. doi: 10.1182/blood-2011-04-347823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nemeth T, Mocsai A. The role of neutrophils in autoimmune diseases. Immunol Lett. 2012;143:9–19. doi: 10.1016/j.imlet.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 7.Wu T, Sun C, Chen Z, Zhen Y, Peng J, Qi Z, et al. Smad3-deficient CD11b(+)Gr1(+) myeloid-derived suppressor cells prevent allograft rejection via the nitric oxide pathway. J Immunol. 2012;189:4989–5000. doi: 10.4049/jimmunol.1200068. [DOI] [PubMed] [Google Scholar]
- 8.Colgan SP. Neutrophils and inflammatory resolution in the mucosa. Semin Immunol. 2015;27:177–183. doi: 10.1016/j.smim.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bennouna S, Bliss SK, Curiel TJ, Denkers EY. Cross-talk in the innate immune system: neutrophils instruct recruitment and activation of dendritic cells during microbial infection. J Immunol. 2003;171:6052–6058. doi: 10.4049/jimmunol.171.11.6052. [DOI] [PubMed] [Google Scholar]
- 10.Jaeger BN, Donadieu J, Cognet C, Bernat C, Ordonez-Rueda D, Barlogis V, et al. Neutrophil depletion impairs natural killer cell maturation, function, and homeostasis. J Exp Med. 2012;209:565–580. doi: 10.1084/jem.20111908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiewchengchol D, Midgley A, Sodsai P, Deekajorndech T, Hirankarn N, Beresford MW, et al. The protective effect of GM-CSF on serum-induced neutrophil apoptosis in juvenile systemic lupus erythematosus patients. Clin Rheumatol. 2015;34:85–91. doi: 10.1007/s10067-014-2800-2. [DOI] [PubMed] [Google Scholar]
- 12.Muller I, Munder M, Kropf P, Hansch GM. Polymorphonuclear neutrophils and T lymphocytes: strange bedfellows or brothers in arms? Trends Immunol. 2009;30:522–530. doi: 10.1016/j.it.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 13.Cross A, Bucknall RC, Cassatella MA, Edwards SW, Moots RJ. Synovial fluid neutrophils transcribe and express class II major histocompatibility complex molecules in rheumatoid arthritis. Arthritis Rheum. 2003;48:2796–2806. doi: 10.1002/art.11253. [DOI] [PubMed] [Google Scholar]
- 14.Takashima A, Yao Y. Neutrophil plasticity: acquisition of phenotype and functionality of antigen-presenting cell. J Leukoc Biol. 2015;98:489–496. doi: 10.1189/jlb.1MR1014-502R. [DOI] [PubMed] [Google Scholar]
- 15.Scapini P, Nardelli B, Nadali G, Calzetti F, Pizzolo G, Montecucco C, et al. G-CSF-stimulated neutrophils are a prominent source of functional BLyS. J Exp Med. 2003;197:297–302. doi: 10.1084/jem.20021343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mhawech-Fauceglia P, Kaya G, Sauter G, McKee T, Donze O, Schwaller J, et al. The source of APRIL up-regulation in human solid tumor lesions. J Leukoc Biol. 2006;80:697–704. doi: 10.1189/jlb.1105655. [DOI] [PubMed] [Google Scholar]
- 17.Zindl CL, Lai JF, Lee YK, Maynard CL, Harbour SN, Ouyang W, et al. Il-22-producing neutrophils contribute to antimicrobial defense and restitution of colonic epithelial integrity during colitis. Proc Natl Acad Sci USA. 2013;110:12768–12773. doi: 10.1073/pnas.1300318110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Puga I, Cols M, Barra CM, He B, Cassis L, Gentile M, et al. B cell-helper neutrophils stimulate the diversification and production of immunoglobulin in the marginal zone of the spleen. Nat Immunol. 2012;13:170–180. doi: 10.1038/ni.2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: ‘N1’ versus ‘N2’ TAN. Cancer Cell. 2009;16:183–194. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song X, He X, Li X, Qian Y. The roles and functional mechanisms of interleukin-17 family cytokines in mucosal immunity. Cell Mol Immunol. 2016;13:418–431. doi: 10.1038/cmi.2015.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Teng MW, Bowman EP, McElwee JJ, Smyth MJ, Casanova JL, Cooper AM, et al. IL-12 and IL-23 cytokines: from discovery to targeted therapies for immune-mediated inflammatory diseases. Nat Med. 2015;21:719–729. doi: 10.1038/nm.3895. [DOI] [PubMed] [Google Scholar]
- 22.Tonel G, Conrad C, Laggner U, Di Meglio P, Grys K, McClanahan TK, et al. Cutting edge: a critical functional role for IL-23 in psoriasis. J Immunol. 2010;185:5688–5691. doi: 10.4049/jimmunol.1001538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kadi A, Costantino F, Izac B, Leboime A, Said-Nahal R, Garchon HJ, et al. Brief report: the IL23R nonsynonymous polymorphism rs11209026 is associated with radiographic sacroiliitis in spondyloarthritis. Arthritis Rheum. 2013;65:2655–2660. doi: 10.1002/art.38060. [DOI] [PubMed] [Google Scholar]
- 24.Remmers EF, Cosan F, Kirino Y, Ombrello MJ, Abaci N, Satorius C, et al. Genome-wide association study identifies variants in the MHC class I, IL10, and IL23R-IL12RB2 regions associated with Behcet's disease. Nat Genet. 2010;42:698–702. doi: 10.1038/ng.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McGeachy MJ, Chen Y, Tato CM, Laurence A, Joyce-Shaikh B, Blumenschein WM, et al. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat Immunol. 2009;10:314–324. doi: 10.1038/ni.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Awasthi A, Riol-Blanco L, Jager A, Korn T, Pot C, Galileos G, et al. Cutting edge: IL-23 receptor GFP reporter mice reveal distinct populations of IL-17-producing cells. J Immunol. 2009;182:5904–5908. doi: 10.4049/jimmunol.0900732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aychek T, Mildner A, Yona S, Kim KW, Lampl N, Reich-Zeliger S, et al. IL-23-mediated mononuclear phagocyte crosstalk protects mice from citrobacter rodentium-induced colon immunopathology. Nat Commun. 2015;6:6525. doi: 10.1038/ncomms7525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kastelein RA, Hunter CA, Cua DJ. Discovery and biology of IL-23 and IL-27: related but functionally distinct regulators of inflammation. Annu Rev Immunol. 2007;25:221–242. doi: 10.1146/annurev.immunol.22.012703.104758. [DOI] [PubMed] [Google Scholar]
- 30.Cella M, Fuchs A, Vermi W, Facchetti F, Otero K, Lennerz JK, et al. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 2009;457:722–725. doi: 10.1038/nature07537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paget C, Ivanov S, Fontaine J, Renneson J, Blanc F, Pichavant M, et al. Interleukin-22 is produced by invariant natural killer T lymphocytes during influenza a virus infection: potential role in protection against lung epithelial damages. J Biol Chem. 2012;287:8816–8829. doi: 10.1074/jbc.M111.304758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Du J, Shen X, Zhao Y, Hu X, Sun B, Guan W, et al. Wip1-deficient neutrophils significantly promote intestinal ischemia/reperfusion injury in mice. Curr Mol Med. 2015;15:100–108. doi: 10.2174/1566524015666150114122929. [DOI] [PubMed] [Google Scholar]
- 33.Zhu L, Yang T, Li L, Sun L, Hou Y, Hu X, et al. TSC1 controls macrophage polarization to prevent inflammatory disease. Nat Commun. 2014;5:4696. doi: 10.1038/ncomms5696. [DOI] [PubMed] [Google Scholar]
- 34.Hou Y, Lin H, Zhu L, Liu Z, Hu F, Shi J, et al. Lipopolysaccharide increases the incidence of collagen-induced arthritis in mice through induction of protease HTRA-1 expression. Arthritis Rheum. 2013;65:2835–2846. doi: 10.1002/art.38124. [DOI] [PubMed] [Google Scholar]
- 35.Hou Y, Lin H, Zhu L, Liu Z, Hu F, Shi J, et al. The inhibitory effect of IFN-gamma on protease HTRA1 expression in rheumatoid arthritis. J Immunol. 2014;193:130–138. doi: 10.4049/jimmunol.1302700. [DOI] [PubMed] [Google Scholar]
- 36.Sun B, Hu X, Liu G, Ma B, Xu Y, Yang T, et al. Phosphatase Wip1 negatively regulates neutrophil migration and inflammation. J Immunol. 2014;192:1184–1195. doi: 10.4049/jimmunol.1300656. [DOI] [PubMed] [Google Scholar]
- 37.Yang T, Zhu L, Zhai Y, Zhao Q, Peng J, Zhang H, et al. TSC1 controls IL-1beta expression in macrophages via mTORC1-dependent C/EBPβ pathway. Cell Mol Immunol. 2016;13:640–650. doi: 10.1038/cmi.2015.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen H, Zhang L, Zhang H, Xiao Y, Shao L, Li H, et al. Disruption of TSC1/2 signaling complex reveals a checkpoint governing thymic CD4+ CD25+ Foxp3+ regulatory T-cell development in mice. FASEB J. 2013;27:3979–3990. doi: 10.1096/fj.13-235408. [DOI] [PubMed] [Google Scholar]
- 39.Liu G, Hu X, Sun B, Yang T, Shi J, Zhang L, et al. Phosphatase Wip1 negatively regulates neutrophil development through p38 MAPK-STAT1. Blood. 2013;121:519–529. doi: 10.1182/blood-2012-05-432674. [DOI] [PubMed] [Google Scholar]
- 40.Itoh-Nakadai A, Hikota R, Muto A, Kometani K, Watanabe-Matsui M, Sato Y, et al. The transcription repressors Bach2 and Bach1 promote B cell development by repressing the myeloid program. Nat Immunol. 2014;15:1171–1180. doi: 10.1038/ni.3024. [DOI] [PubMed] [Google Scholar]
- 41.Cassatella MA. Neutrophil-derived proteins: selling cytokines by the pound. Adv Immunol. 1999;73:369–509. doi: 10.1016/S0065-2776(08)60791-9. [DOI] [PubMed] [Google Scholar]
- 42.Tecchio C, Micheletti A, Cassatella MA. Neutrophil-derived cytokines: facts beyond expression. Front Immunol. 2014;5:508. doi: 10.3389/fimmu.2014.00508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yin J, Ferguson TA. Identification of an ifn-gamma-producing neutrophil early in the response to listeria monocytogenes. J Immunol. 2009;182:7069–7073. doi: 10.4049/jimmunol.0802410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taylor PR, Roy S, Leal SM, Jr, Sun Y, Howell SJ, et al. Activation of neutrophils by autocrine IL-17a-IL-17RC interactions during fungal infection is regulated by IL-6, IL-23, RORγt and dectin-2. Nat Immunol. 2014;15:143–151. doi: 10.1038/ni.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ciofani M, Madar A, Galan C, Sellars M, Mace K, Pauli F, et al. A validated regulatory network for Th17 cell specification. Cell. 2012;151:289–303. doi: 10.1016/j.cell.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chung Y, Chang SH, Martinez GJ, Yang XO, Nurieva R, Kang HS, et al. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity. 2009;30:576–587. doi: 10.1016/j.immuni.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huber M, Brustle A, Reinhard K, Guralnik A, Walter G, Mahiny A, et al. Irf4 is essential for IL-21-mediated induction, amplification, and stabilization of the Th17 phenotype. Proc Natl Acad Sci USA. 2008;105:20846–20851. doi: 10.1073/pnas.0809077106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cho ML, Kang JW, Moon YM, Nam HJ, Jhun JY, Heo SB, et al. STAT3 and NF-kappaB signal pathway is required for IL-23-mediated IL-17 production in spontaneous arthritis animal model IL-1 receptor antagonist-deficient mice. J Immunol. 2006;176:5652–5661. doi: 10.4049/jimmunol.176.9.5652. [DOI] [PubMed] [Google Scholar]
- 49.Genetic Analysis of Psoriasis Consortium & the Wellcome Trust Case Control Consortium. Strange A, Capon F, Spencer CC, Knight J, et al. A genome-wide association study identifies new psoriasis susceptibility loci and an interaction between HLA-C and ERAP1. Nat Genet. 2010;42:985–990. doi: 10.1038/ng.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Duerr RH, Taylor KD, Brant SR, Rioux JD, Silverberg MS, Daly MJ, et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science. 2006;314:1461–1463. doi: 10.1126/science.1135245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Murphy CA, Langrish CL, Chen Y, Blumenschein W, McClanahan T, Kastelein RA, et al. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J Exp Med. 2003;198:1951–1957. doi: 10.1084/jem.20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 53.Weaver CT, Elson CO, Fouser LA, Kolls JK. The Th17 pathway and inflammatory diseases of the intestines, lungs, and skin. Annu Rev Pathol. 2013;8:477–512. doi: 10.1146/annurev-pathol-011110-130318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zenaro E, Pietronigro E, Della Bianca V, Piacentino G, Marongiu L, Budui S, et al. Neutrophils promote Alzheimer's disease-like pathology and cognitive decline via LFA-1 integrin. Nat Med. 2015;21:880–886. doi: 10.1038/nm.3913. [DOI] [PubMed] [Google Scholar]
- 55.Karthikeyan RS, Vareechon C, Prajna NV, Dharmalingam K, Pearlman E, Lalitha P. Interleukin 17 expression in peripheral blood neutrophils from fungal keratitis patients and healthy cohorts in Southern India. J Infect Dis. 2015;211:130–134. doi: 10.1093/infdis/jiu381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Milanova V, Ivanovska N, Dimitrova P. TLR2 elicits IL-17-mediated RANKL expression, IL-17, and OPG production in neutrophils from arthritic mice. Mediators Inflamm. 2014;2014:643406. doi: 10.1155/2014/643406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ramirez-Velazquez C, Castillo EC, Guido-Bayardo L, Ortiz-Navarrete V. Il-17-producing peripheral blood CD177+ neutrophils increase in allergic asthmatic subjects. Allergy Asthma Clin Immunol. 2013;9:23. doi: 10.1186/1710-1492-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tan Z, Jiang R, Wang X, Wang Y, Lu L, Liu Q, et al. Rorgammat+il-17+ neutrophils play a critical role in hepatic ischemia-reperfusion injury. J Mol Cell Biol. 2013;5:143–146. doi: 10.1093/jmcb/mjs065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ishida H, Imai T, Suzue K, Hirai M, Taniguchi T, Yoshimura A, et al. Il-23 protection against plasmodium berghei infection in mice is partially dependent on il-17 from macrophages. Eur J Immunol. 2013;43:2696–2706. doi: 10.1002/eji.201343493. [DOI] [PubMed] [Google Scholar]
- 60.Song C, Luo L, Lei Z, Li B, Liang Z, Liu G, et al. Il-17-producing alveolar macrophages mediate allergic lung inflammation related to asthma. J Immunol. 2008;181:6117–6124. doi: 10.4049/jimmunol.181.9.6117. [DOI] [PubMed] [Google Scholar]
- 61.Taylor PR, Leal SM, Jr., Sun Y, Pearlman E. Aspergillus and fusarium corneal infections are regulated by th17 cells and il-17-producing neutrophils. J Immunol. 2014;192:3319–3327. doi: 10.4049/jimmunol.1302235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lin AM, Rubin CJ, Khandpur R, Wang JY, Riblett M, Yalavarthi S, et al. Mast cells and neutrophils release il-17 through extracellular trap formation in psoriasis. J Immunol. 2011;187:490–500. doi: 10.4049/jimmunol.1100123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li L, Huang L, Vergis AL, Ye H, Bajwa A, Narayan V, et al. Il-17 produced by neutrophils regulates ifn-gamma-mediated neutrophil migration in mouse kidney ischemia-reperfusion injury. J Clin Invest. 2010;120:331–342. doi: 10.1172/JCI38702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hoshino A, Nagao T, Nagi-Miura N, Ohno N, Yasuhara M, Yamamoto K, et al. Mpo-anca induces il-17 production by activated neutrophils in vitro via classical complement pathway-dependent manner. J Autoimmun. 2008;31:79–89. doi: 10.1016/j.jaut.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 65.Ratsimandresy RA, Indramohan M, Dorfleutner A, Stehlik C. The aim2 inflammasome is a central regulator of intestinal homeostasis through the il-18/il-22/stat3 pathway. Cell Mol Immunol. 2017;14:127–142. doi: 10.1038/cmi.2016.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Oppmann B, Lesley R, Blom B, Timans JC, Xu Y, Hunte B, et al. Novel p19 protein engages il-12p40 to form a cytokine, il-23, with biological activities similar as well as distinct from il-12. Immunity. 2000;13:715–725. doi: 10.1016/S1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


