Abstract
Among various immunoregulatory molecules, the B7 family of immune-checkpoint receptors consists of highly valuable targets for cancer immunotherapy. Antibodies targeting two B7 family co-inhibitory receptors, CTLA-4 and PD-1, have elicited long-term clinical outcomes in previously refractory cancer types and are considered a breakthrough in cancer therapy. Despite the success, the relatively low response rate (20–30%) warrants efforts to identify and overcome additional immune-suppressive pathways. Among the expanding list of T cell inhibitory regulators, V domain immunoglobulin suppressor of T cell activation (VISTA) is a unique B7 family checkpoint that regulates a broad spectrum of immune responses. Here, we summarize recent advances that highlight the structure, expression, and multi-faceted immunomodulatory mechanisms of VISTA in the context of autoimmunity, inflammation, and anti-tumor immunity.
Keywords: cancer immunotherapy, immune checkpoint, autoimmunity, inflammation, tumor immunity
Introduction
Immunotherapy holds the promise for becoming mainstream therapy for cancer owing to the long-term and significant clinical outcomes in previously refractory malignancies, such as late-stage metastatic melanoma and non-small cell lung cancer.1 During the past five years (2012-2017), there have been over 1,500 registered cancer immunotherapy trials testing the efficacy of immune checkpoint inhibitors specific for CTLA-4, PD-1, and PD-L1 (www.clinicaltrials.gov).
Immune-checkpoint receptors transmit co-inhibitory signaling during T cell activation and directly suppress T cell responses.2,3 Antibodies targeting the immune-checkpoint proteins CTLA-4 (ipilimumab/Yervoy and tremelimumab), PD-1 (pembrolizumab and nivolumab/Opdivo), and PD-L1 (atezolizumab and avelumab) have become breakthrough therapies for cancer.4,5,6,7,8,9 Despite the success, the response rate following CTLA-4, PD-L1, or PD-1-blocking monoclonal antibody (mAb) therapy is generally lower than 30%.6,9,10,11,12,13,14 Efforts are warranted to identify and overcome additional immune-suppressive pathways.
Following the initial identification of the immune checkpoint receptors CTLA-4 (ligands B7.1 and B7.2) and PD-1 (ligands PD-L1 and PD-L2), other Ig superfamily T cell-inhibitory ligands/receptors, such as LAG3 (ligand MHC-II),15 TIM3 (ligands CEACAM-1, HMGB1, and galectin-9),16 TIGIT (ligands CD155 and CD112),17 BTLA (ligand HVEM),18 B7-H3,19 B7-H4,20,21 and VISTA,22 have been indicated as important regulators of anti-tumor immunity.23,24,25,26,27,28 The increasing understanding of regulatory mechanisms involving these ligands and receptors has provided exceptional opportunities to therapeutically target these inhibitory pathways and design synergistic combination anti-cancer therapies. This review will focus on the structure, expression, and multi-faceted immunomodulatory mechanisms of VISTA, a B7 family immune-checkpoint protein.22
The sequence and structure of vista
VISTA (gene Vsir, RIKEN cDNA 4632428N05, aliases Gi24, Dies-1, PD-1H, and DD1α) stands for V domain immunoglobulin suppressor of T cell activation.22,29,30,31 The murine Vsir gene transcript consists of 930 base pairs, which translates into a type I transmembrane protein with 309 amino acids (aa). The extracellular domain of murine VISTA contains a single Ig-V domain of 136 aa, which is linked to a 23-aa stalk region, a 21-residue transmembrane segment, and a 97-aa cytoplasmic domain that does not contain ITAM, ITIM, or ITSM motifs. Putative protein kinase C binding sites and proline-rich regions within the cytoplasmic tail have been identified, but their functional role remains to be characterized.30
VISTA is highly conserved between species, with >80% similarity in protein sequence between murine and human orthologs.22,30,31 Protein sequence analysis has aligned VISTA with the B7 family of ligands and receptors. B7 family ligands, including B7.1, B7.2, PD-L1, PD-L2, and ICOSL, contain Ig-V- and Ig-C-like domains, whereas receptors CD28, PD-1, and ICOS contain one Ig-V-like domain. Despite its ligand function, as shown in previous studies, VISTA contains one Ig-V domain, resembling other B7 family receptors. A genome-wide blast search has identified PD-L1 of the B7 family as the closest evolutionarily related protein.22 This homologous relationship was confirmed by computationally threading the sequence of the VISTA Ig-V domain through the structural model of PD-L1. A structure-based sequence alignment of VISTA with B7 family ligand proteins PD-L1, PD-L2, B7-H3 and B7-H4 or B7 family receptors PD-1, ICOS, and CTLA-4 has identified conserved amino acids that are important for the stability of Ig-V domain-folding in all Ig-V domain-containing proteins, with an expected nine parallel β-strands (ABCC′C″DEFG) with loops connecting the strands.22,30,31 These amino acids include the invariant cysteines (Cys54 and Cys145) and a tyrosine (Tyr143). Irrespective of these similarities, the VISTA Ig-V domain contains several features that distinguish VISTA from the B7 family ligands and receptors. The most notable distinguishing features are the three additional cysteines (Cys44, Cys83, and Cys144) within the Ig-V domain, one cysteine (Cys177) in the stalk region, and the insertion of a loop (IRNFTLQHLQHHGSHLKAN) between the C″ and D strands that are invariant among VISTA orthologs but are absent in all other Ig superfamily members. Collectively, these sequence and structural features identify VISTA as a distant B7 family member.
Vista gene and protein expression at steady state and under inflammatory conditions
At steady state, Vsir gene transcripts were predominantly detected in lymphoid tissues, such as the spleen, thymus, and bone marrow (BM) in adult mice.22,30 Lower levels of VISTA mRNA were detected in non-hematopoietic tissues, such as brain, heart, lung, muscle, testis, kidney, and placenta.
Within the hematopoietic cell lineages, VISTA protein is highly expressed on CD11bHi myeloid cells, including granulocytes, monocytes, macrophages, and myeloid dendritic cells (DCs).22 Intermediate expression levels are seen on CD11bInt myeloid DCs. VISTA is also expressed on NK cells, TCRγδ T cells, naïve CD4+ and CD8+ TCRαβ T cells, and Foxp3+CD4+ regulatory T cells (Tregs). No surface expression of VISTA is detected on splenic B cells at steady state. In T cells, VISTA expression is higher on naïve CD4+ T cells than on CD8+ T cells. Following activation, VISTA expression on T cells was transiently upregulated but downregulated after 24 h in vitro or a few days in vivo.22,30
Similar to the murine homologue, the human VISTA gene is predominantly expressed in hematopoietic cell lineages and in tissues rich in infiltrating leukocytes.32 In human peripheral blood mononuclear cells (PBMC), VISTA is expressed on CD14+ monocytes, neutrophils, myeloid CD11c+ DCs, and CD4+ and CD8+ T cells. VISTA is not expressed on resting CD19+ B cells or CD56Hi NK cells. Following in vitro culture, VISTA expression on human monocytes was downregulated over time.33
Under inflammatory conditions, VISTA expression on immune cell types could be altered. For example, in an imiquimod-induced murine model of psoriasis, VISTA expression on TCRγδ T cells within the inflamed ear tissue was upregulated compared to that on cells in the draining LN.34 Surface expression of VISTA on human PBMC CD14+ monocytes could be upregulated following stimulation of certain Toll-like receptors (TLR), such as TLR3 and TLR5, and cytokines IL-10 and IFN-γ, as well as following HIV-infection.33 At the transcriptional level, VISTA, as well as PD-L1 and PD-1, is directly targeted by tumor suppressor p53.31 Induced transcription occurs following either forced expression of p53 or p53-inducing genotoxic stress. In a murine embryonic stem cell line model, miRNA-125a downregulates VISTA protein expression via suppressing the translational process.35
In murine tumor models, VISTA protein is expressed on tumor-infiltrating leukocytes.36 VISTA expression is upregulated in CD11b+Gr1+ myeloid cells and Foxp3+ Tregs within tumor tissues compared to that in the peripheral lymphoid tissues.36 Several studies have examined VISTA gene or protein expression in human cancers. One study by Oliveira et al. showed that VISTA gene expression is downregulated due to promoter methylation or miRNA-125a overexpression in malignant epithelial cancer cells of breast, colon, and gastric tissue origin compared to that in corresponding normal tissues.37 In contrast, another study of gastric tumor tissues by Boger et al. examined VISTA protein expression by immunohistochemistry and showed that VISTA is detected on tumor-infiltrating lymphocytes in >80% of cases of primary tumor tissues, as well as in minor populations of tumor cells (in <10% of cases).38 Furthermore, the VISTA protein level is correlated with the Lauren classification, tumor localization, Epstein-Barr virus infection, KRAS- and PIK3CA-mutational status, and PD-L1 expression.38 A study of human oral squamous cell carcinoma (OSCC) has shown that VISTA protein level is increased within tumor tissues when compared to normal mucosa.39 The higher protein expression of VISTA together with the low protein level of CD8α within OSCC tumor tissues is significantly correlated with poorer overall survival.39 In a study of human prostate cancer and melanoma following ipilimumab treatment, Gao et al. examined protein levels of VISTA and several other immune-suppressive molecules (i.e., LAG3, TIM3, and PD-L1) within tumor tissues by quantitative immunofluorescence, CyTOF or flow cytometry.40 The percentages of VISTA- and PD-L1-expressing tumor-infiltrating CD4+ and CD8+ T cells as well as CD68+ macrophages are significantly increased following therapy, indicating that both checkpoint proteins may contribute to the immune-suppressive tumor microenvironment (TME) and resistance to CTLA-4 blockade therapy.40
In comparison to VISTA, the regulatory mechanisms that control the gene expression of PD-L1 and PD-1 have been more extensively studied. In contrast to the largely hematopoietic expression of VISTA, PD-L1 is ubiquitously expressed in non-hematopoietic and hematopoietic tissues and cell types. Under inflammatory conditions, PD-L1 gene expression is inducible by common γ chain cytokines IL-2, IL-7 and IL-15, as well as IFN-γ, GM-CSF, and IL-4.41,42,43 PD-L1 is upregulated by HIF-1α within the hypoxic TME.44 Distinct from VISTA, PD-1 is absent on naïve T cells but it is expressed on activated effector T cells and on exhausted T cells.42,45 PD-1 gene expression is upregulated by common γ chain cytokines and VEGF.43,46 At the transcriptional level, the PD-1 promoter is regulated by T-bet, STAT3, STAT4, NFATc1, CTCF, FOXO1, and epigenetic regulator SATB1.47,48,49,50 In cancer, the expression of PD-L1 on tumor cells and PD-1 on TILs has been associated with clinical responses towards antibody-mediated therapy.51 In contrast, VISTA expression is restricted to hematopoietic cell lineages. It is predicted that the clinical response towards VISTA-blocking mAb therapy in solid tumors will be correlated with the VISTA expression level on tumor-infiltrating leukocytes.
Vista is a critical regulator of t cell-mediated immune responses towards self and foreign antigens
The initial evidence for the role of VISTA as a regulator of T cell peripheral tolerance was provided from studies in the murine experimental autoimmune encephalomyelitis (EAE) model, where T cell responses against the self-antigen myelin oligodendrocyte glycoprotein (MOG) drive disease progression.22 Treatment with a VISTA-blocking mAb (clone 13F3) significantly exacerbated disease severity in this model.
Since antibodies could be either antagonistic or agonistic towards the target antigen, the physiological role of VISTA has been assessed by studies in the Vsir gene knockout (KO) mice (Vsir −/−) by several groups.31,34,52,53,54 Since multiple protein aliases (VISTA, PD-1H, and DD1α) have been used to describe the phenotypes of the KO mice that are deficient in the same gene, we will summarize the results from these studies by referring to the gene name.
Studies by Wang et al. showed that although Vsir −/− mice (reported as VISTA KO) were born healthy with apparently normal hematopoietic development, VISTA deficiency resulted in a gradual accumulation of spontaneously activated T cells with hyper-production of inflammatory cytokines such as IFN-γ, IL-17A and TNF-α.52 Accumulation of IFN-γ-inducible chemokines such as Eotaxin, IP-10, MCP-1, and MIG was detected in the serum of KO mice at ~10 months of age.52
The spontaneous T cell activation indicates that VISTA deficiency may have lowered the threshold of TCR-mediated activation towards self-antigens. This hypothesis has been tested by breeding Vsir −/− mice with 2D2 TCR transgenic (Tg) mice, which express a TCR recognizing the self-antigen MOG and are predisposed to developing EAE. VISTA deficiency significantly increased disease incidence and intensity. Disease development was correlated with increased activation of encephalitogenic CD4+ T cells in the periphery and their infiltration into the central nervous system.52,53
In a concanavalin A-induced hepatitis model, Vsir −/− mice (reported as PD-1H KO) developed more severe hepatitis than WT controls.54 Since this disease is largely dependent upon the pathological role of activated CD4+ T cells and NKT cells, this result is consistent with the role of VISTA in negatively regulating T cell-mediated immune responses.
In addition to regulating Th1- and Th17-skewed T cell responses, VISTA also regulates Th2 responses. Studies by Flies et al. have shown that in a murine model of experimental asthma, allergen challenge induced higher levels of Th2 cytokine production and more severe pulmonary inflammation in Vsir −/− mice.55
The ligand-or-receptor paradigm by which vista regulates t cell activation
Due to the lack of knowledge on the cognate receptor for VISTA or associated signaling adaptors, the molecular mechanisms of how VISTA regulates effector T cell activation are not well understood. Despite this limitation, recent studies have demonstrated both cell-extrinsic and intrinsic mechanisms by which VISTA regulates T cell responses. Here, we present the model in which VISTA expressed on both antigen-presenting cells (APCs) and T cells can engage an inhibitory receptor to suppress T cell activation, whereas VISTA on T cells may act as a receptor that directly transmits inhibitory signals (Figure 1). These mechanisms do not contradict each other, as both could contribute to the overall T cell-suppressive function of VISTA.
Figure 1.

The ligand-or-receptor paradigm of VISTA in regulating T cell activation. VISTA is expressed on both APCs and T cells. VISTA suppresses T cell activation via both T cell-extrinsic and intrinsic mechanisms. (a) VISTA acts as a ligand when expressed on APCs, and engages a putative inhibitory receptor on T cells that suppresses T cell proliferation and cytokine production. (b) On the other hand, VISTA expressed on T cells may engage a putative inhibitory receptor on T cells via T cell-T cell interaction or may act as a self-signaling receptor. Both mechanisms will contribute to T cell suppression. The identity of the counter-receptor and detailed signaling mechanisms downstream of VISTA remain to be elucidated and will provide further validation for this paradigm.
The T cell-extrinsic role of VISTA as a ligand
The initial characterization using an immobilized ectodomain of VISTA (ECD) protein has confirmed the ‘ligand’ function of VISTA.22,32 Immobilized VISTA-ECD suppressed T cell proliferation and cytokine production (i.e., IL-2 and IFN-γ) in a dose-dependent manner. This suppression is interpreted as a result of interactions between VISTA-ECD and a co-inhibitory receptor on T cells. The phosphorylation of several key TCR proximal signaling molecules (i.e., LAT, PLC-γ1, and SLP76) and upregulation of early activation markers (i.e., CD69, CD44, and CD25) were inhibited by immobilized VISTA-ECD.22,32,53 Both naïve and memory T cells were sensitive to VISTA-ECD-mediated inhibition. In addition to directly limiting proximal TCR signaling, immobilized VISTA-ECD promoted the conversion of Foxp3− CD4+ T cells into Foxp3+ adaptive Tregs in the presence of exogenous TGF-β. This effect contributes to the overall T cell-inhibitory role of VISTA.22,32
Second, the ‘ligand’ activity of VISTA expressed on APCs is demonstrated by overexpressing VISTA in A20 B lymphoma cells or BM-derived DCs, which were used to stimulate T cells in the presence of cognate peptides.22 VISTA expression on A20 cells or BMDCs reduced the proliferation and cytokine production of co-cultured DO11.10 Tg T cells.
Third, the in vivo T cell-extrinsic inhibitory role of VISTA has been shown in two disease models. In the MCA105 fibrosarcoma model, VISTA expression on tumor cells overcame vaccine-induced anti-tumor immunity and facilitated tumor outgrowth.22 Alternatively, in an adoptive transfer model of EAE, where the disease is induced by adoptively transferred encephalitogenic T cells, VISTA deficiency on host cells significantly accelerated disease progression.52 Importantly, this model also demonstrates a T cell-intrinsic role of VISTA, as adoptive transfer of Vsir −/− T cells accelerated the disease course when with WT T cells. Additive effects were seen when Vsir −/− T cells were transferred into Vsir −/− hosts. Taken together, these results support the T cell-extrinsic role of VISTA when expressed on APCs or tumor cells in controlling T cell activation and indicate a T cell-intrinsic role of VISTA, as discussed below.
The T cell-intrinsic function of VISTA
In addition to myeloid cells, VISTA is expressed on naive CD4+ T cells and CD8+ T cells, and Foxp3+ CD4+ Tregs.22 The T cell-intrinsic role of VISTA has been indicated in vitro, where WT or Vsir −/− CD4+ T cells were stimulated with WT or Vsir −/− APCs.54 VISTA deletion on both T cells and APCs resulted in the extensive proliferation of T cells, whereas VISTA deficiency on either T cells or APCs alone showed an intermediate effect.
Complementary evidence indicating the T cell-intrinsic role of VISTA was presented by the effects of the PD-1H/VISTA-specific mAb MH5A in a murine model of acute GVHD.30,54,56 MH5A suppressed allogeneic T cell response and prevented disease progression. MH5A inhibited the initial expansion and cytotoxic function of WT but not Vsir −/− donor T cells, indicating that VISTA expression on donor T cells is sufficient and is required for the suppressive effect of MH5A.
In VISTA-sufficient WT hosts, MH5A treatment resulted in a transient expansion of Foxp3+ CD4+ Tregs and promoted the conversion of naïve Foxp3− CD4+ T cells to adaptive Foxp3+ Tregs in vivo. Importantly, this result is consistent with the previous study showing that VISTA plays a role in promoting the peripheral differentiation of adaptive Tregs in tumor-bearing hosts.36,56
Despite the strong evidence supporting the T cell-intrinsic role of VISTA, whether VISTA functions as a self-signaling receptor or alternatively engages another transmembrane inhibitory receptor remains to be determined. To date, the counter-receptor for VISTA has not been formally identified. Although it has been reported that VISTA interacts with itself via homotypic interactions of its ECD 31 or interacts with VSIG3 (IGSF11),57,58 whether and how these interactions contribute to T cell suppression have not been determined.
The multi-faceted roles of vista in controlling autoimmune and inflammatory diseases
Studies in multiple murine disease models of cancer, EAE, lupus, GVHD, and psoriasis have demonstrated parallel mechanisms by which VISTA regulates the function of diverse immune cell types (Table 1). In addition to serving as a brake for T cell responses, VISTA plays an important role in regulating the signaling and activation of innate immune cells, including myeloid DCs, macrophages, and TCRγδ T cells. Here, we will summarize recent studies demonstrating these new mechanisms by which VISTA controls autoimmune and inflammatory diseases.
Table 1.
VISTA regulates the responses of diverse immune cell types in cancer, autoimmune and inflammatory diseases
| Immune cell types | Function of VISTA in cancer, autoimmune, and inflammatory diseases |
|---|---|
| CD4+ and CD8+ effector T cells | Suppress T cell proliferation, effector cytokine production (IFN-g, IL-2 etc), and cytotoxicity (Cancer, GVHD)Suppress the differentiation of Th17 CD4+ T cells (EAE) |
| Foxp3+ CD4+ Tregs | Promote the induction of tumor-specific adaptive CD4+ Foxp3+ Tregs (Cancer, GVHD) |
| TCRgd T cells | Inhibit the peripheral homeostasis and cytokine production of IL-17-producing CD27− TCRgd T subsets (Psoriasis) |
| Myeloid dendritic cells and macrophages | Inhibit the production of inflammatory cytokines and chemokines (Cancer, lupus, and psoriasis) |
An initial examination of Vsir −/− mice (VISTA KO) at 8–9 months of age has revealed spontaneous immune-cell infiltration in lung, liver, and pancreas, as well as accumulation of myeloid chemokines (Eotaxin, IP-10, MCP-1, MIG) in the serum.52 These inflammatory phenotypes have been attributed to the spontaneous T cell activation due to loss of T cell peripheral tolerance. Splenomegaly was accompanied by increased cell number in the CD11b+ myeloid lineages, including myeloid DCs, macrophage, and neutrophils. Despite the presence of immune infiltrates in multiple organs, overt lupus-like systemic autoimmunity was not fully developed, and similar levels of anti-dsDNA and anti-nuclear antibodies were detected in the serum of Vsir −/− and WT mice.52
However, when bred onto a disease-prone transgenic background, VISTA deficiency exacerbated disease in the EAE model of multiple sclerosis, as well as the sle1.sle3 model of lupus.52,59 Consistently, treatment with VISTA-blocking mAb led to more severe disease in the EAE model and the BWF1 model of lupus.22,60 In both disease models, VISTA blockade significantly enhanced CD4+ T cell activation. Furthermore, VISTA deficiency in the sle1.sle3 background promoted the activation of myeloid cells by upregulating the expression of costimulatory molecules CD80, CD40 and MHCII and the production of cytokines (IFN-γ and IL-10) following LPS treatment.59 In contrast, a high level of ectopic VISTA expression in PBMC monocytes from chronically HIV-infected individuals induced production of cytokines such as IL-8, IL-1β, IL-6, IL-10, and TNF-α.33 The reasons for this contradictory result are not clear. We speculate that the regulatory role of VISTA in myeloid cells may depend upon the exogenous stimuli and the activation state of the myeloid cells in different studies.
Supporting the anti-inflammatory role of VISTA, our recent study has established the roles of VISTA as a negative regulator of the IL-23/IL17-mediated inflammatory axis.34 In an imiquimod (IMQ)-induced murine model of skin inflammation, VISTA deficiency exacerbated IMQ-induced psoriasiform inflammation. Mechanistically, enhanced IMQ/TLR7 signaling in Vsir −/− myeloid DCs resulted in the hyper-activation of Erk1/2 and Jnk1/2 and augmented the production of IL-23. IL-23, in turn, promoted the expression of IL-17A in both TCRγδ T cells and CD4+ Th17 cells. Although the molecular mechanisms by which VISTA controls TLR signaling in myeloid cells remain to be elucidated, these results suggest that the ectodomain of VISTA is sufficient to downregulate DC activation following TLR7 stimulation, thus pointing to the role of another surface receptor in mediating the inhibitory signaling of VISTA. In addition to regulating the activation of myeloid cells, our study further demonstrates that VISTA restricts IL-7-mediated peripheral homeostasis of CD27− TCRγδ T cells, which is pre-committed to producing IL-17. Thus, VISTA deficiency or mAb-mediated blockade promotes the expansion of CD27− TCRγδ T cells in the periphery and increase the production of IL-17 that amplify the inflammatory responses.34
In addition to regulating the activation and cytokine production of myeloid cells in response to TLR stimulation, VISTA also regulates other aspects of myeloid cell-mediated innate immunity. One study noted the role of VISTA in regulating the expression of the C5a receptor on neutrophils and bone marrow-derived macrophages and reported that VISTA deficiency or treatment with an immuno-suppressive VISTA mAb (clone 8G8) resulted in attenuated disease in the collagen-II antibody-induced arthritis (CAIA) model.61 The molecular mechanisms and the relevance of this finding to human arthritis remain to be investigated. Another study by Yoon et al. has revealed a role of DD-1α/VISTA in facilitating the clearance of apoptotic cells by phagocytes via homotypic interactions between VISTA expressed on apoptotic cells and phagocytes.31 Aged Vsir −/− (reported as DD1α KO) mice have shown an accumulation of anti-dsDNA and anti-nuclear antibodies in the serum, spontaneous glomerulonephritis, and severe inflammatory phenotypes in the skin, eyes, and ears.31,34,52 Although the severity of the inflammatory phenotypes differs between different reports, these studies collectively support the roles of VISTA as a negative regulator of both innate and adaptive immunity during the development of autoimmune and inflammatory diseases.
The role of vista in controlling anti-tumor immunity
Multiple studies, both by us and others, support the role of VISTA as a key regulator of anti-tumor immunity. An initial study showed that overexpressing VISTA in MCA105 tumor cells subverted the vaccine-induced T cell-mediated adaptive immunity against tumors.22 Analyses of Vsir −/− (VISTA KO) mice bearing B16 melanoma demonstrated that, although tumors grew similarly in unmanipulated WT and Vsir −/− hosts, VISTA deficiency endowed greater anti-tumor immunity following vaccination with tumor peptides and TLR agonists as adjuvants, and it potently inhibited tumor growth.52 In another study of the murine glioma model, it was shown that VISTA deficiency significantly enhanced CD4+ T cell-mediated anti-tumor responses following low-dose radiation therapy, resulting in tumor rejection in Vsir −/− hosts.54
Consistent with results from the KO mice, treatment with a VISTA-specific blocking mAb (13F3) significantly enhanced both CD4+ and CD8+ T cell-mediated anti-tumor immunity in multiple tumor models (i.e., MB49 bladder tumor, B16 melanoma, and an inducible melanoma model Tyr CreERT2 Braf LSL-V600E/+ Pten +/+).36,62 Mechanistically, VISTA-blocking mAb treatment improves anti-tumor immunity by targeting multiple immune cell types.36 First, VISTA-blocking mAb significantly enhanced the tumor infiltration, proliferation, cytokine production, and cytotoxic effector function of both CD4+ and CD8+ T cells. Second, VISTA-blocking mAb impaired the suppressive function of Foxp3+CD4+ Tregs and reduced the induction of adaptive Tregs from tumor-specific naïve CD4+ T cells in tumor-bearing hosts. Third, VISTA-blocking mAb induced the activation of tumor-associated myeloid DCs by upregulating the expression of CD80 and MHC-II and elevating the production of IL-12 and TNF-α. VISTA mAb also decreased the amount of tumor-infiltrating myeloid-derived suppressor cells (MDSCs). Whether VISTA regulates the suppressive function of tumor-associated MDSCs remains to be investigated. In this regard, a recent study by Green et al. has described the role of VISTA in contributing to MDSC-mediated suppression of B cells in a murine HIV infection model.63
These studies indicate that VISTA is a critical immune-checkpoint protein that hampers the generation of effective anti-tumor immunity. VISTA blockade not only directly enhances tumor-specific effector T cell activation but also reduces the induction of adaptive Tregs, impairs the suppressive function of Tregs, and potentiates the inflammatory responses mediated by myeloid APCs and TCRγδ T cells. Thus, the in vivo therapeutic outcome of VISTA-targeted therapies may depend upon the complex interplay between multiple innate and adaptive immune cell types (Figure 2). The precise contribution of VISTA-regulated inflammatory responses to the development of an effective anti-tumor immunity remains to be further defined.
Figure 2.
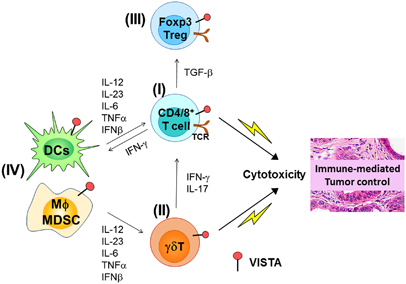
VISTA regulates anti-tumor immunity via controlling both innate and adaptive immune responses. VISTA is expressed in multiple immune cell types, including CD4+/CD8+ T cells, TCRγδ T cells, and myeloid cells, such as dendritic cells and macrophages. VISTA regulates both innate and adaptive immunity against cancer. The therapeutic outcomes of VISTA-targeted therapies will depend upon the complex interplay between these various immune cells. (I) Tumor-specific effector CD4+ and CD8+ T cells are negatively regulated by VISTA expressed on both myeloid APCs and CD4+/CD8+ T cells. As a result, the production of T cell-derived effector cytokines such as IFN-γ and T cell-mediated cytotoxic killing of tumor cells are impaired by VISTA. (II) VISTA expressed on TCRγδ T cells controls the peripheral homeostasis of the CD27− subset and inhibits their production of IL-17. Blocking VISTA may promote the expansion of IL-17-producing TCRγδ T cells and amplify the IL-23/IL-17-mediated inflammatory axis. (III) VISTA is expressed on Foxp3+CD4+ Tregs and contributes to the induction and suppressive function of peripheral Foxp3+CD4+ Tregs. (IV) VISTA expressed on myeloid cells suppresses their activation following TLR signaling, reducing the production of inflammatory cytokines (i.e., IL-12, IL-23, IL-6, TNF-α, IFN-β, etc.). Blocking VISTA may alter the inflammatory milieu within the TME, which skews the effector function of tumor-specific T cells. The precise role of each cytokine in mediating the therapeutic outcome of VISTA-targeted therapies remains to be defined. For example, both tumor-promoting and tumor-inhibitory roles of IL-23 and IL-17 have been reported in previous studies. The contribution of the IL-23/IL-17 inflammatory axis to the development of an effective anti-tumor immunity following VISTA-targeted therapies remains to be clarified.
In the field of cancer immunotherapy, there is significant interest in improving the anti-tumor efficacy of immune checkpoint inhibitors. One hypothesis is that the synergistic activation of T cells could be achieved by targeting distinct immune checkpoint proteins simultaneously. Supporting this, recent clinical studies have shown that combined treatment with CTLA-4 and PD-1 mAb improved the response rate from ~10–20% using single agents to ~40–60%.14,65
To understand the potential redundancy between VISTA and PD-L1/PD-1-mediated T cell regulation, the immunoregulatory roles of VISTA and PD-1 in mice with single or combined gene deletions have been examined and compared.53 Mice deficient in either VISTA or PD-1 showed comparable loss of peripheral tolerance and T cell activation, but the double-KO mice developed the most severe inflammatory phenotypes, manifested as the highest level of spontaneous activation of peripheral CD4+ and CD8+ T cells, multi-organ immune cell infiltration and tissue damage. The double-KO mice developed EAE disease with over 90% penetrance when bred onto the 2D2 T-cell receptor transgenic mice, which is significantly higher than either the PD-1 or VISTA signal KO mice. At the level of TCR signaling, both VISTA and PD-L1 were found to inhibit the phosphorylation of proximal signaling molecules (LAT, SLP76, PLC-γ1) and the activation of Akt and Erk1/2. Following peptide immunization, antigen-specific CD4+ and CD8+ T cell responses were synergistically enhanced in VISTA/PD-1 double KO mice. Combined treatment of VISTA and PD-1-specific mAbs led to synergistic anti-tumor T cell responses and optimal tumor-clearing therapeutic efficacy in B16 melanoma and CT26 colon cancer models. Together, these results indicate that VISTA and PD-1 non-redundantly regulate T cell-mediated adaptive immunity. Since the VISTA/PD-1 and VISTA/PD-L1 double-KO mice did not develop lethal autoimmunity, in contrast to the lethality seen in the PD-1/LAG3 double-KO mice and the CTLA-4-deficient mice,15,66,67 we predict that a combinatorial blockade of VISTA with PD-L1 or PD-1 may achieve synergistic efficacy with less severe immune-related adverse effects. In another study of murine squamous carcinoma, Kondo et al. compared the effects of blocking VISTA, PD-1, and CTLA4 and concluded that VISTA blockade is better combined with CTLA4-blocking mAb than with PD-1-blocking mAb.64 The lack of efficacy of VISTA and PD-1 combination blockade may be due to the difference in antibodies, treatment regimes, or tumor models.
Conclusions and future perspectives
VISTA is an immune-checkpoint protein that has distinct expression patterns and controls a broad spectrum of innate and adaptive immune responses. Studies in murine disease models, including cancer, EAE, lupus, and psoriasiform inflammation, have demonstrated the multi-faceted roles of VISTA in regulating the signaling and activation of an array of immune cell types, including myeloid cells, TCRγδ T cells, CD4+ and CD8+ T cells, and Foxp3+CD4+ Tregs.
The molecular mechanisms by which VISTA regulates diverse immune cell types are not fully understood. In the context of T cell activation, sufficient evidence supports both T cell-extrinsic and intrinsic roles of VISTA. How VISTA acts as both a ligand and a receptor to regulate T cell activation requires further investigation. The emerging role of VISTA in regulating the diverse functions of myeloid cells has just begun to be explored. Multiple studies support the role of VISTA in dampening the production of inflammatory cytokines and controlling myeloid cell-mediated inflammation. Identifying the VISTA-interacting receptor(s) and understanding the mechanisms of VISTA-mediated signaling will help to clarify the role of VISTA in the context of autoimmune and inflammatory diseases.
In the context of cancer immunotherapy, preclinical studies have demonstrated an unambiguous role of VISTA in controlling anti-tumor immunity. MAbs and small molecules targeting human VISTA have been developed and are currently in early-stage clinical trials for cancer treatment (Janssen Inc, NCT02671955; Curis Inc, NCT02812875). While the clinical efficacy data have not been forthcoming, we predict that blocking VISTA will elicit a range of inflammatory responses that may promote or hamper T cell-mediated anti-tumor immunity, depending upon tumor types or inflammatory stimuli present in the host. To better define biomarkers or design combination approaches that improve VISTA-targeted therapies, future studies need to focus on understanding the inflammatory cell types and the induced soluble inflammatory mediators following VISTA-targeted therapies, either alone or in combination with other types of treatment such as radiation, chemotherapy, cancer vaccines, or other immune checkpoint inhibitors.
In addition to cancer, VISTA regulates the development of autoimmune and inflammatory diseases. The multi-faceted roles of VISTA in regulating both innate and adaptive immune responses have placed VISTA as a key regulator of several types of autoimmune inflammatory diseases. It is conceivable that antagonists or agonistic agents modulating VISTA and its interacting partners will greatly benefit the treatment of autoimmune and inflammatory diseases.
Acknowledgements
This work is supported by research funding from NCI R01 CA164225 (LW), Advancing A Healthier Wisconsin Research and Education Program (AHW REP) fund (LW), the Office of the Assistant Secretary of Defense for Health Affairs through the Peer Reviewed Cancer Research Program under Award No. W81XWH-14-1-0587 (LW), Cancer Research Institute CLIP Grant (LW), pilot grant 504057 from the Melanoma Research Alliance (LW), Worldwide Cancer Research grant 16-1161 (LW and SM), NIH R01 AI102893 (SM), NCI R01 CA179363 (SM), the Nicholas Family Foundation (SM), and the Gardetto Family endowment (SM).
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Topalian SL, Weiner GJ, Pardoll DM. Cancer immunotherapy comes of age. J Clin Oncol. 2011;29:4828–4836. doi: 10.1200/JCO.2011.38.0899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharpe AH, Freeman GJ. The B7-CD28 superfamily. Nat Rev Immunol. 2002;2:116–126. doi: 10.1038/nri727. [DOI] [PubMed] [Google Scholar]
- 3.Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. 2013;13:227–242. doi: 10.1038/nri3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol. 2008;8:467–477. doi: 10.1038/nri2326. [DOI] [PubMed] [Google Scholar]
- 5.Pardoll D, Drake C. Immunotherapy earns its spot in the ranks of cancer therapy. J Exp Med. 2012;209:201–209. doi: 10.1084/jem.20112275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Callahan MK, Postow MA, Wolchok JD. CTLA-4 and PD-1 Pathway Blockade: Combinations in the Clinic. Front Oncol. 2015;4:385. doi: 10.3389/fonc.2014.00385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348:56–61. doi: 10.1126/science.aaa8172. [DOI] [PubMed] [Google Scholar]
- 8.Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015;161:205–214. doi: 10.1016/j.cell.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Postow MA, Callahan MK, Wolchok JD. Immune Checkpoint Blockade in Cancer Therapy. J Clin Oncol. 2015;33:1974–1982. doi: 10.1200/JCO.2014.59.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167–3175. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372:2006–2017. doi: 10.1056/NEJMoa1414428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woo SR, Turnis ME, Goldberg MV, Bankoti J, Selby M, Nirschl CJ, et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 2011;72:917–927. doi: 10.1158/0008-5472.CAN-11-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med. 2010;207:2187–2194. doi: 10.1084/jem.20100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnston RJ, Comps-Agrar L, Hackney J, Yu X, Huseni M, Yang Y, et al. The immunoreceptor TIGIT regulates antitumor and antiviral CD8(+) T cell effector function. Cancer Cell. 2014;26:923–937. doi: 10.1016/j.ccell.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 18.Derre L, Rivals JP, Jandus C, Pastor S, Rimoldi D, Romero P, et al. BTLA mediates inhibition of human tumor-specific CD8+ T cells that can be partially reversed by vaccination. J Clin Invest. 2010;120:157–167. doi: 10.1172/JCI40070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zang X, Thompson RH, Al-Ahmadie HA, Serio AM, Reuter VE, Eastham JA, et al. B7-H3 and B7x are highly expressed in human prostate cancer and associated with disease spread and poor outcome. Proc Natl Acad Sci U S A. 2007;104:19458–19463. doi: 10.1073/pnas.0709802104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sica GL, Choi IH, Zhu G, Tamada K, Wang SD, Tamura H, et al. B7-H4, a molecule of the B7 family, negatively regulates T cell immunity. Immunity. 2003;18:849–861. doi: 10.1016/S1074-7613(03)00152-3. [DOI] [PubMed] [Google Scholar]
- 21.Kryczek I, Zou L, Rodriguez P, Zhu G, Wei S, Mottram P, et al. B7-H4 expression identifies a novel suppressive macrophage population in human ovarian carcinoma. J Exp Med. 2006;203:871–881. doi: 10.1084/jem.20050930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang L, Rubinstein R, Lines JL, Wasiuk A, Ahonen C, Guo Y, et al. VISTA, a novel mouse Ig superfamily ligand that negatively regulates T cell responses. J Exp Med. 2011;208:577–592. doi: 10.1084/jem.20100619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gavrieli M, Sedy J, Nelson CA, Murphy KM. BTLA and HVEM cross talk regulates inhibition and costimulation. Adv Immunol. 2006;92:157–185. doi: 10.1016/S0065-2776(06)92004-5. [DOI] [PubMed] [Google Scholar]
- 24.Yi KH, Chen L. Fine tuning the immune response through B7-H3 and B7-H4. Immunol Rev. 2009;229:145–151. doi: 10.1111/j.1600-065X.2009.00768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anderson AC, Joller N, Kuchroo VK. Lag-3, Tim-3, and TIGIT: Co-inhibitory Receptors with Specialized Functions in Immune Regulation. Immunity. 2016;44:989–1004. doi: 10.1016/j.immuni.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andrews LP, Marciscano AE, Drake CG, Vignali DA. LAG3 (CD223) as a cancer immunotherapy target. Immunol Rev. 2017;276:80–96. doi: 10.1111/imr.12519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lines JL, Sempere LF, Broughton T, Wang L, Noelle R. VISTA is a novel broad-spectrum negative checkpoint regulator for cancer immunotherapy. Cancer Immunol Res. 2014;2:510–517. doi: 10.1158/2326-6066.CIR-14-0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Janakiram M, Shah UA, Liu W, Zhao A, Schoenberg MP, Zang X. The third group of the B7-CD28 immune checkpoint family: HHLA2, TMIGD2, B7x, and B7-H3. Immunol Rev. 2017;276:26–39. doi: 10.1111/imr.12521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aloia L, Parisi S, Fusco L, Pastore L, Russo T. Differentiation of embryonic stem cells 1 (Dies1) is a component of bone morphogenetic protein 4 (BMP4) signaling pathway required for proper differentiation of mouse embryonic stem cells. J Biol Chem. 2010;285:7776–7783. doi: 10.1074/jbc.M109.077156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Flies DB, Wang S, Xu H, Chen L. Cutting edge: A monoclonal antibody specific for the programmed death-1 homolog prevents graft-versus-host disease in mouse models. J Immunol. 2011;187:1537–1541. doi: 10.4049/jimmunol.1100660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoon KW, Byun S, Kwon E, Hwang SY, Chu K, Hiraki M, et al. Control of signaling-mediated clearance of apoptotic cells by the tumor suppressor p53. Science. 2015;349:1261669. doi: 10.1126/science.1261669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lines JL, Pantazi E, Mak J, Sempere LF, Wang L, O'Connell S, et al. VISTA Is an Immune Checkpoint Molecule for Human T Cells. Cancer Res. 2014;74:1924–1932. doi: 10.1158/0008-5472.CAN-13-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bharaj P, Chahar HS, Alozie OK, Rodarte L, Bansal A, Goepfert PA, et al. Characterization of programmed death-1 homologue-1 (PD-1H) expression and function in normal and HIV infected individuals. PLoS One. 2014;9:e109103. doi: 10.1371/journal.pone.0109103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li N, Xu W, Yuan Y, Ayithan N, Imai Y, Wu X, et al. Immune-checkpoint protein VISTA critically regulates the IL-23/IL-17 inflammatory axis. Scientific Reports. 2017;7:1485. doi: 10.1038/s41598-017-01411-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parisi S, Battista M, Musto A, Navarra A, Tarantino C, Russo T. A regulatory loop involving Dies1 and miR-125a controls BMP4 signaling in mouse embryonic stem cells. FASEB J. 2012;26:3957–3968. doi: 10.1096/fj.12-211607. [DOI] [PubMed] [Google Scholar]
- 36.Le Mercier I, Chen W, Lines JL, Day M, Li J, Sergent P, et al. VISTA Regulates the Development of Protective Antitumor Immunity. Cancer Res. 2014;74:1933–1944. doi: 10.1158/0008-5472.CAN-13-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oliveira P, Carvalho J, Rocha S, Azevedo M, Reis I, Camilo V, et al. Dies1/VISTA expression loss is a recurrent event in gastric cancer due to epigenetic regulation. Sci Rep. 2016;6:34860. doi: 10.1038/srep34860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boger C, Behrens HM, Kruger S, Rocken C. The novel negative checkpoint regulator VISTA is expressed in gastric carcinoma and associated with PD-L1/PD-1: A future perspective for a combined gastric cancer therapy? Oncoimmunology. 2017;6:e1293215. doi: 10.1080/2162402X.2017.1293215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu L, Deng WW, Huang CF, Bu LL, Yu GT, Mao L, et al. Expression of VISTA correlated with immunosuppression and synergized with CD8 to predict survival in human oral squamous cell carcinoma. Cancer Immunol Immunother. 2017;66:627–636. doi: 10.1007/s00262-017-1968-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gao J, Ward JF, Pettaway CA, Shi LZ, Subudhi SK, Vence LM, et al. VISTA is an inhibitory immune checkpoint that is increased after ipilimumab therapy in patients with prostate cancer. Nat Med. 2017;23:551–555. doi: 10.1038/nm.4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamazaki T, Akiba H, Iwai H, Matsuda H, Aoki M, Tanno Y, et al. Expression of programmed death 1 ligands by murine T cells and APC. J Immunol. 2002;169:5538–5545. doi: 10.4049/jimmunol.169.10.5538. [DOI] [PubMed] [Google Scholar]
- 42.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kinter AL, Godbout EJ, McNally JP, Sereti I, Roby GA, O'Shea MA, et al. The common gamma-chain cytokines IL-2, IL-7, IL-15, and IL-21 induce the expression of programmed death-1 and its ligands. J Immunol. 2008;181:6738–6746. doi: 10.4049/jimmunol.181.10.6738. [DOI] [PubMed] [Google Scholar]
- 44.Noman MZ, Desantis G, Janji B, Hasmim M, Karray S, Dessen P, et al. PD-L1 is a novel direct target of HIF-1alpha, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med. 2014;211:781–790. doi: 10.1084/jem.20131916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pauken KE, Wherry EJ. Overcoming T cell exhaustion in infection and cancer. Trends Immunol. 2015;36:265–276. doi: 10.1016/j.it.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Voron T, Colussi O, Marcheteau E, Pernot S, Nizard M, Pointet AL, et al. VEGF-A modulates expression of inhibitory checkpoints on CD8+ T cells in tumors. J Exp Med. 2015;212:139–148. doi: 10.1084/jem.20140559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kao C, Oestreich KJ, Paley MA, Crawford A, Angelosanto JM, Ali MA, et al. Transcription factor T-bet represses expression of the inhibitory receptor PD-1 and sustains virus-specific CD8+ T cell responses during chronic infection. Nat Immunol. 2011;12:663–671. doi: 10.1038/ni.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Staron MM, Gray SM, Marshall HD, Parish IA, Chen JH, Perry CJ, et al. The transcription factor FoxO1 sustains expression of the inhibitory receptor PD-1 and survival of antiviral CD8(+) T cells during chronic infection. Immunity. 2014;41:802–814. doi: 10.1016/j.immuni.2014.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Austin JW, Lu P, Majumder P, Ahmed R, Boss JM. STAT3, STAT4, NFATc1, and CTCF regulate PD-1 through multiple novel regulatory regions in murine T cells. J Immunol. 2014;192:4876–4886. doi: 10.4049/jimmunol.1302750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stephen TL, Payne KK, Chaurio RA, Allegrezza MJ, Zhu H, Perez-Sanz J, et al. SATB1 Expression Governs Epigenetic Repression of PD-1 in Tumor-Reactive T Cells. Immunity. 2017;46:51–64. doi: 10.1016/j.immuni.2016.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nishino M, Ramaiya NH, Hatabu H, Hodi FS. Monitoring immune-checkpoint blockade: response evaluation and biomarker development. Nat Rev Clin Oncol. 2017;14:655–668. doi: 10.1038/nrclinonc.2017.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang L, Le Mercier I, Putra J, Chen W, Liu J, Schenk AD, et al. Disruption of the immune-checkpoint VISTA gene imparts a proinflammatory phenotype with predisposition to the development of autoimmunity. Proc Natl Acad Sci USA. 2014;111:14846–14851. doi: 10.1073/pnas.1407447111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu J, Yuan Y, Chen W, Putra J, Suriawinata AA, Schenk AD, et al. Immune-checkpoint proteins VISTA and PD-1 nonredundantly regulate murine T-cell responses. Proc Natl Acad Sci USA. 2015;112:6682–6687. doi: 10.1073/pnas.1420370112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Flies DB, Han X, Higuchi T, Zheng L, Sun J, Ye JJ, et al. Coinhibitory receptor PD-1H preferentially suppresses CD4+ T cell-mediated immunity. J Clin Invest. 2014;124:1966–1975. doi: 10.1172/JCI74589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu H, Li X, Hu L, Zhu M, He B, Luo L, et al. A crucial role of the PD-1H coinhibitory receptor in suppressing experimental asthma. Cell Mol Immunol. 2017 doi: 10.1038/cmi.2017.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Flies DB, Higuchi T, Chen L. Mechanistic Assessment of PD-1H Coinhibitory Receptor-Induced T Cell Tolerance to Allogeneic Antigens. J Immunol. 2015;194:5294–5304. doi: 10.4049/jimmunol.1402648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang W, Padkjaer SB, Wang J, Sun Z, Shan B, Yang L, et al. Construction of a versatile expression library for all human single-pass transmembrane proteins for receptor pairings by high throughput screening. J Biotechnol. 2017;260:18–30. doi: 10.1016/j.jbiotec.2017.08.023. [DOI] [PubMed] [Google Scholar]
- 58.Prodeus A, Abdul-Wahid A, Sparkes A, Fischer NW, Cydzik M, Chiang N, et al. VISTA.COMP—an engineered checkpoint receptor agonist that potently suppresses T cell-mediated immune responses. JCI Insight. 2017;2:94308. doi: 10.1172/jci.insight.94308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ceeraz S, Sergent PA, Plummer SF, Schned AR, Pechenick D, Burns CM, et al. VISTA Deficiency Accelerates the Development of Fatal Murine Lupus Nephritis. Arthritis Rheumatol. 2017;69:814–825. doi: 10.1002/art.40020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sergent PA, Plummer SF, Pettus J, Mabaera R, DeLong JK, Pechenick DA et al. Blocking the VISTA pathway enhances disease progression in (NZB x NZW) F1 female mice. Lupus 2017; 961203317716322. [DOI] [PMC free article] [PubMed]
- 61.Ceeraz S, Eszterhas SK, Sergent PA, Armstrong DA, Ashare A, Broughton T, et al. VISTA deficiency attenuates antibody-induced arthritis and alters macrophage gene expression in response to simulated immune complexes. Arthritis Res Ther. 2017;19:270. doi: 10.1186/s13075-017-1474-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dankort D, Curley DP, Cartlidge RA, Nelson B, Karnezis AN, Damsky WE, Jr., et al. Braf(V600E) cooperates with Pten loss to induce metastatic melanoma. Nat Genet. 2009;41:544–552. doi: 10.1038/ng.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Green KA, Wang L, Noelle RJ, Green WR. Selective Involvement of the Checkpoint Regulator VISTA in Suppression of B-Cell, but Not T-Cell, Responsiveness by Monocytic Myeloid-Derived Suppressor Cells from Mice Infected with an Immunodeficiency-Causing Retrovirus. J Virol. 2015;89:9693–9698. doi: 10.1128/JVI.00888-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kondo Y, Ohno T, Nishii N, Harada K, Yagita H, Azuma M. Differential contribution of three immune checkpoint (VISTA, CTLA-4, PD-1) pathways to antitumor responses against squamous cell carcinoma. Oral Oncol. 2016;57:54–60. doi: 10.1016/j.oraloncology.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 65.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 67.Waterhouse P, Penninger JM, Timms E, Wakeham A, Shahinian A, Lee KP, et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270:985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]


