Abstract
The balance between Th17 cells and regulatory T cells (Tregs) has emerged as a prominent factor in regulating autoimmunity and cancer. Th17 cells are vital for host defense against pathogens but have also been implicated in causing autoimmune disorders and cancer, though their role in carcinogenesis is less well understood. Tregs are required for self-tolerance and defense against autoimmunity and often correlate with cancer progression. This review addresses the importance of a functional homeostasis between these two subsets in health and the consequences of its disruption when these forces collide in disease. Importantly, we discuss the ability of Th17 cells to mediate cancer regression in immunotherapy, including adoptive transfer and checkpoint blockade therapy, and the therapeutic possibilities of purposefully offsetting the Th17/Treg balance to treat patients with cancer as well as those with autoimmune diseases.
Keywords: autoimmunity, cancer, immunotherapy, Th17, Treg
Introduction
CD4+ T cells play a critical role in regulating human health and disease by orchestrating the immune system to contend with danger induced by foreign antigens, such as infections or cancer formation. Proper function of the CD4+ T cell compartment relies on an adjustable equilibrium among various T cell subsets, which help trigger the host’s immune system in defense against threat. While the division of CD4 support was previously hypothesized to be dominated solely by Th1 and Th2 helper subsets, mounting evidence over the past decade reveals that Th17 cells and regulatory T cells (Tregs) also play important roles in regulating health and exacerbating autoimmunity and cancer. Herein, we will discuss the delicate balance between Th17 and Treg cells in maintaining a healthy, functioning immune environment, as well as the harmful effects that transpire when homeostasis is disturbed and their therapeutic implications. Finally, we discuss the possibilities of harnessing a Th17 response against cancer in adoptive transfer and checkpoint blockade therapy, thus highlighting an approach whereby therapeutically and purposefully offsetting the Th17/Treg balance could be effective in treating human cancers.
Basics of T helper subsets
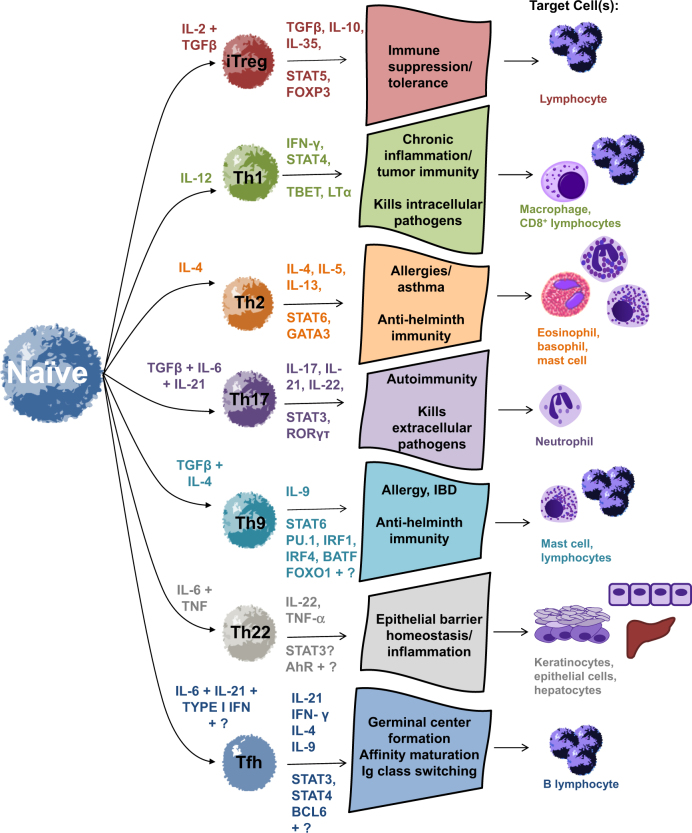
Before the 1980s, helper T cells were believed to be a single subset among T lymphocytes1. Increasing evidence now suggests that there are at least seven distinct T helper subsets differentiated in response to particular combinations of cytokines. These subsets are also controlled by different transcription factors to produce a characteristic milieu of cytokines and exert an effector function against self and foreign antigens. These subsets are described below and summarized visually in Fig. 1.
Fig. 1. CD4+ cell differentiation and effector function.
CD4+ T cells differentiate based on the presence of various cytokines. Under the influence of IL-2 and TGF-β, Treg cells develop and express transcription factors STAT5 and Foxp3 and cytokines TGF-β and IL-10. IL-12 influences Th1 cell development and promotes immunity upon the presence of IFN-γ, STAT4, T-bet, and LTα. Th2 cell differentiation is induced by IL-4, during which cytokine release may manifest in allergies or asthma. Th17 cell development occurs following the influence of TGF-β, IL-6, and IL-21. IL-1β and IL-23 maintain Th17 cell stabilization during clonal expansion. Upon differentiation, Th17 cells are most commonly classified by their expression of RORγt and STAT3. Th9, Th22, and Tfh cells have been most recently described, and transcription factors controlling their differentiation remain under debate
CD4+ T cells differentiate into subsets such as Th1, Th2, Th17, Treg, Th9, Th22, and T follicular helper cells2. Th1 cells rely on the expression of T-bet and eliminate intracellular pathogens through IFN-γ production, which activates macrophages2–4. Th2 cells play a role in the presentation of allergens; promote immunity against parasites through production of IL-4, IL-5, and IL-13, and are regulated by transcription factor GATA35. Interestingly, Th2 differentiation is also mediated by IL-4, creating a positive feedback loop to bolster proliferation2,6. Th17 cells secrete IL-17A, IL-17F, IL-21, IL-22, and CCL20, express master transcription factor RORγt, encoded by Rorc7, and promote inflammation in response to infections8–10. Regulatory T cells (Tregs) suppress effector function through secretion of inhibitory cytokines such as IL-10 and TGF-β or through cell-mediated engagement of inhibitory checkpoint molecules such as TIGIT and CTLA-411. The relevance of Th17 cells has been documented in promoting autoimmunity, carcinogenesis, and antitumor immunity, whereas Treg cells are essential for immune tolerance and have been shown to dampen autoimmunity and antitumor immunity6,12,13.
Most recently, Th9, Th22, and T follicular helper cells (Tfh) have been described as distinct helper populations. As such, knowledge of the programming cytokines and master transcription factors for these subsets is still somewhat under debate. Th9 cells differentiate in response to IL-4 and TGF-β14–16 and produce IL-9 under control of the transcription factors STAT6, PU.117, IRF418, BATF19, and FOXO120. IL-9 recruits lymphocytes and mast cells as effectors21–23 and is enhanced under the influence of IL-1β and transcription factor IRF124. Th22 cells are polarized by IL-6 and TNF-α to secrete IL-2225 and have been shown to exacerbate psoriasis in patients26. Which transcription factor(s) control Th22 differentiation is less clear27,28. However, several groups have identified aryl hydrocarbon receptor (AHR) as having a vital role in Th22 differentiation25,28–30, while the role of T-bet is more controversial25,30. Tfh cells classically secrete IL-21 for B cell development in germinal centers, as well as IFN-γ and IL-4 to aid B-cell immunoglobulin class-switching to IgG and IgE in the lymphoid follicle31. They also secrete IL-9 to promote B cell memory and differentiation of plasma cells31,32. Although Bcl6 has been the widely accepted transcription factor controlling differentiation of Tfh cells, there are also recent reports suggesting STAT4 and T-bet33, c-Maf34, IRF435, and Batf36 are important to differentiation of this lineage.
T helpers in opposition
Our current understanding of T helper function revolves around a theory that subsets are in a state of equilibrium37. Upon activation of one particular subset, other subsets are modulated or inhibited in order to promote the most specific effector response in defense against imminent threat37,38. Historically, this discussion began with the Th1/Th2 hypothesis of distinct opposing T helper subsets, formulated after searching for a T cell responsible for helping antibody production versus one responsible for tissue damage in delayed-type hypersensitivity (DTH)39,40. Early studies claimed that Th1 cells mediated tissue damage in DTH, not the antibodies in serum, and would likely be the cell responsible for mediating tissue damage in various autoimmune diseases40. After several failed attempts to show that tissue damage in murine experimental autoimmune encephalitis (EAE) was mediated by Th1 cells and their effector cytokine, IFN-γ, the characterization of a novel subset called Th17 cells emerged41–45. The Th1/Th17 balance developed, recognizing that IFN-γ and IL-17 have antagonistic properties, as blockade of IFN-γ results in increased IL-17 production by T cells46,47. Finally, an antagonistic relationship between Th17 cells and Tregs has been described, as their differentiation is stimulated by similar cytokines yet they have different functions48–50. Th17 cells serve as an effector lymphocyte population, while Tregs are suppressor cells48–50. Herein, we will focus on the particular Th17/Treg balance of opposing forces in autoimmunity and cancer and the roles of each subset in both promotion of and protection from these pathogenic phenotypes.
The YIN: Th17 cells
Differentiation
The emergence of the distinct Th17 lineage can be attributed to studies of central nervous system autoimmunity. Early studies involving the EAE model revealed that IL-23 knockout (KO) mice were resistant to developing EAE, while IL-12 KO mice remained susceptible51. This surprising series of experiments signified that Th1 polarization was not critical to the autoimmune phenotype, as was previously posited51. IL-23 was subsequently discovered to drive polarization of a pathogenic CD4+ T cell subset characterized by production of IL-17A and IL-17F, which could induce EAE upon adoptive transfer, while IFN-γ producing Th1 cells could not52.
These IL-17 producing cells were considered a distinct lineage, when the milieu of cytokines supporting their differentiation was shown to be independent of the environment of cytokines required for Th1 and Th2 development47,53. In fact, the in vitro differentiation of naive CD4+ T cells to Th17 cells is suppressed by Th1/Th2 cytokines IFN-γ and IL-453 and relies on costimulation by CD28 and ICOS47. The cytokines most important to Th17 differentiation are TGF-β, IL-6, and IL-1β, and the phenotype is maintained long term in the presence of IL-21 and IL-2354,55. The role of each of these individual cytokines is discussed below.
Along with IL-6, TGF-β is well known as a critical cytokine for inducing RORγt in naive CD4+ T cells, which in turn drives their differentiation to a Th17 phenotype. However, new findings have provided insight into exactly how TGF-β regulates RORγt. Interestingly, TGF-β was shown to modulate the SKI–SMAD4 complex56. The SKI–SMAD4 complex suppresses RORγt, as the SKI protein inhibits acetylation of the Rorc locus56. However, in the presence of TGF-β, SKI is degraded, permitting RORγt expression in CD4+ T cells and ultimately driving Th17 differentiation56. Low doses of TGF-β1 also inhibit IL-2-mediated activation of STAT5 and reduce T-bet and GATA3 expression, which inhibits Th1/Th2/Treg differentiation while promoting the Th17 lineage57. Recent findings have also demonstrated that phosphatase and tensin homolog (PTEN) in Th17 cells suppresses IL-2 signaling, reducing STAT5 and the Treg pathway while upregulating STAT3, a transcription factor that supports the Th17 pathway58. It is also important to appreciate that TGF-β and IL-6 induce the IL-23 receptor (IL-23R) in Th17 cells59. IL-23 further activates STAT3, RORα and RORγt in Th17 cells to maintain their long term proinflammatory signature6,7,59,60. Thus, naive CD4+ T cells cultured with TGF-β and IL-6 but without IL-23 still produce IL-17 but also produce anti-inflammatory cytokine IL-1061,62. These non-pathogenic Th17 cells do not induce EAE and have compromised persistence and phenotypic maintenance in vivo 62.
Recent reports have also shed new light on the role of IL-1β and IL-21 in regulating Th17 cells. IL-1β induces alternative splicing of Foxp3, inhibiting Treg differentiation and promoting IL-17A production55. Finally, IL-21 activates STAT3 downstream and can induce Th17 differentiation even in the absence of IL-663. As Th17 cells also produce IL-21, this autocrine signaling amplifies the Th17 response and aids in their maintenance. Globally, transcription factor JunB also supports the Th17 phenotype while repressing alternate CD4+ Th1 and Treg phenotypes64. Collectively, this mounting body of work reveals that various cytokines and key transcription factors are critical for inducing Th17 differentiation and maintaining their function and phenotype long term.
Function
At homeostasis, Th17 cells promote gut barrier defense, granulopoiesis, granulocyte chemotaxis, and immunity against extracellular pathogens. Most Th17 cells reside within the lamina propria of the gut in healthy individuals but are induced at other mucosal sites upon exposure to danger signals, such as infection65. To maintain gut defense, IL-17 upregulates claudins for tight junction formation in the intestinal barrier and IL-22 plays a role in epithelial maintenance66,67. IL-17 induces granulopoeisis indirectly through stimulation of epithelial cells, endothelial cells, and fibroblasts to secrete GM-CSF, IL-6, IL-8, and MIP-268,69. In turn, IL-8, and MIP-2 enhance chemotaxis of neutrophils70. Mice deficient in IL-17R have an impaired ability to repopulate these immune cells after irradiation71. Th17 cells and IL-17 have been implicated in immunity against extracellular pathogens, such as Klebsiella pneumoniae69, Staphylococcus aureus72, Salmonella enterica serovar Enteriditus73, and Shigella flexneri74 among other bacterial species. Th17 cells have also been shown to augment Th1 recruitment in Mycobacterium tuberculosis infection, which is critical to granuloma formation and sequestration of bacteria75. These collective Th17 functions are critical in preserving the health of the host and when compromised can lead to various disease symptoms, as discussed below.
Mutations that result in loss of Th17 cell function manifest in disorders such as Job’s syndrome, also named hyper-IgE syndrome, and chronic mucocutaneous candidiasis (CMC) disease. Job’s syndrome is caused by an autosomal dominant STAT3 inactivating mutation and results in increased susceptibility of patients to S. aureus and Candida albicans76. Phenotypically, this disease presents as a triad of eosiniphilia, eczema, and recurrent skin and pulmonary infections77. CMC disease, manifested by chronic infection of the skin, nails, and mucosa by C. albicans, is related to any of four inheritable gene defects in IL-17RA, IL-17RC, IL-17F, or ACT178. These mutations impair the host Th17 response and increase susceptibility to infection with extracellular pathogens. Thus, Th17 cell function plays a critical role in regulating immune responses in health and in disease within the host. Based on these findings, translational researchers and physician scientists have been actively investigating methods to regulate the Th17 pathway in patients to treat both autoimmunity and infectious disease.
The Yang: Regulatory T cells
Effector functions of the adaptive immune response provide the ability to fight and clear invading pathogens while generating memory against those pathogens for a rapid recall response. Such effector functions, while vital for survival, can be damaging if engaged for too long, driving chronic inflammation, or if directed against self-tissue, causing autoimmunity38. As such, effector T cells must be regulated to prevent immune cell defense from turning to offense.
The discovery and recognition of a distinct T cell subset functioning to suppress immune responses was controversial in the late 20th century, and some debate over lineage still exists today. The first discovery that T cells could dampen immune responses was made in 1970, and thereafter, these cells were termed “suppressor” cells79. These cells were defined by their expression of the “I-J” molecule, which was claimed to be important to suppressive function80. Controversy arose when the I-J coding region could not be identified on murine major histocompatibility complex (MHC) and due to a lack of other concrete identifying markers led to the collapse of the “suppressor” T cell movement81.
Around that time, it was separately noted that immune tolerance could be broken from the beginning of development. Neonatal thymectomy of mice resulted in destruction of the ovaries, which was later correlated with tissue damage in other organs82,83. Identification of two types of “regulatory” T cells followed—one naturally occurring in the thymus and responsible for clonal deletion of T cells specific to self-antigens and one in the periphery, inducible from naive CD4+ T cells.
Differentiation and function
Several cornerstone discoveries about Tregs include the identification of IL-2Rα (CD25) as a functional marker, the importance of IL-2 for tolerogenic function, and designation of Foxp3 as the master transcriptional regulator. In mice, CD25 marks a population of CD4+ T cells that normalize immune function and prevent lethal autoimmunity84,85. Reconstitution of the neonatally thymectomized mice with CD4+ CD25+ T cells, but not with CD25− cells, prevented autoimmune development86. IL-2 is critical for Treg development and function, as IL-2Rα KO, IL-2Rβ KO, and neutralization of IL-2 induce severe autoimmunity87–89. Very recent reports by Dwyer et al. have further revealed that the level of IL-2 signaling is vital to proper Treg function, as chronically reduced IL-2 signaling compromised peripheral tolerance and led to accelerated onset of type 1 diabetes in NOD mice90. Despite its necessity for function, CD25 is not exclusively on Tregs and does not mark the Treg population as effectively in humans as it does in mice. Other Treg surface molecules include glucocorticoid-induced TNF receptor (GITR), PD-1, CTLA-485, TIGIT91, and GARP92, though these markers are also not exclusive to Treg cells. The master transcriptional activator of Tregs is Foxp3, which is a more specific marker for Tregs93 and is important for Treg development and maintenance of function12,94–97. Foxp3 is enhanced by Helios expression98, which correlates with GARP expression and marks a regulatory cell with greater immunosuppressive characteristics99. Bluestone and coworkers found that Foxp3 expression also correlates inversely with CD127 (IL-7Rα) expression, identifying the phenotype of CD4+CD25+CD127lo/− as more representative of human Tregs100. Foxp3 is present in natural and peripheral Tregs, although the exact mechanism of generating peripheral Tregs in the context of antigen specificity is still somewhat under debate101.
Cellular development of Th17 and Treg cells shares a common cytokine, TGF-β, which is needed to induce Foxp3 and RORγt in Treg and Th17 cells, respectively. TGF-β with IL-2 can induce Foxp3+ Treg differentiation peripherally from naive CD4+ T cells, whereas TGF-β plus IL-6 (secreted by the innate immune arm, such as activated dendritic cells) induces the Th17 lineage (Fig. 1)48,49,102. As mentioned previously, IL-6 and IL-21 induce STAT3, which inhibits Foxp3, while IL-2 induces STAT5, which reduces STAT3 binding and inhibits Th17 differentiation59,103–105. Supplemental IL-2 as therapy for autoimmune disease may augment Treg function and boost self-tolerance. The notion is now well appreciated that generating a regulatory response is closely related to differentiating an effector Th17 response against pathogens. Collectively, the immune system tightly regulates Th17/Treg homeostasis via the TGF-β/IL-2 and IL-6 cytokine axis.
Defects in Treg function lead to unregulated immune responses to self-tissue. Treg function depends heavily on migratory activity—during an immune response, Tregs migrate from blood to lymph nodes and tissues, and the ratio of Treg/non-Treg CD4+ cells in those compartments increases106. Recent reports have shown that Treg migration is regulated by bioenergetics. Specifically, glucokinase-dependent glycolysis in Tregs was found to prevent the effector response from generating excess inflammation107. Without the ability to migrate, Tregs cannot relocate to the site of inflammation, as shown by their reduced ability to suppress allograft rejection107.
Similarly, inheritable mutations that render Tregs dysfunctional cause lethal autoimmunity. Mutations in Foxp3 result in a disease known as IPEX, which is characterized by immune dysregulation, polyendocrinopathy, enteropathy, and X-linked syndrome108. This disorder generally presents early after birth with severe dermatitis or psoriaform lesions, watery diarrhea, excessive cytokines, thyroiditis and hypothyroidism and frequently leads to death early in childhood108. This mutation impairs the regulatory host response and permits rampant inflammation from other unopposed immune cell compartments.
Th17/Treg plasticity
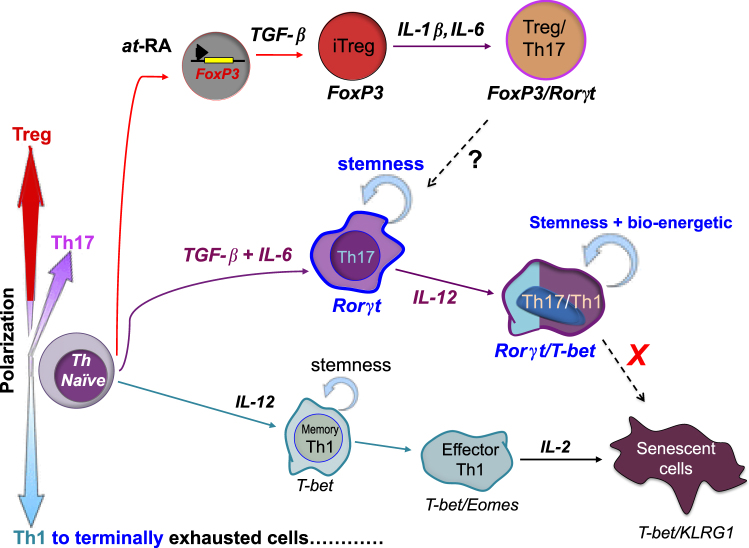
Both Th17 and Treg cells have been described as exhibiting the property of plasticity. Plasticity is defined by the unique ability to adapt to signaling cues in a changing environment. For example, as shown in Fig. 2, Th17 cells can acquire Th1-like characteristics after activation, a property that likely plays a role in enhancing autoimmunity and antitumor immunity. Termed ex-Th17 cells, or non-classical Th1 cells, these cells lose their ability to secrete IL-17 while gaining the capacity to secrete IFN-γ in the presence of proinflammatory signals, such as TCR-triggering or exposure to IL-12 or IL-23109. Although ex-Th17 cells acquire a Th1 profile, they can still be distinguished from classic Th1 cells via unique surface markers, including CD161, CCR6, and IL-17RE110. Moreover, these cells are functionally distinct from classic Th1 cells, secreting more TNF, IL-2, GM-CSF, and IFN-γ111. Interestingly, proliferation of ex-Th17 cells is not suppressed by Treg cells in direct contrast to classical Th1 and Th17 cells, which are inhibited by Treg cells111. These data implicate a possible role for these cells in an unbalanced Th17/Treg autoimmune response. The observed plasticity of Th17 cells occurs only in the direction of Th17 to Th1, as Th1 cells have not been shown to convert to Th17-like T cells112.
Fig. 2. Cytokines induce functional plasticity of Th17s and Tregs.
Both Th17 cells and Tregs exhibit the ability to acquire characteristics of other helper subsets. The presence of Th17-type cytokines IL-1B and IL-6 can generate Treg-producing IL-17. The ability of Th17 to differentiate into a Treg-like phenotype is less well described at this time. Similarly, a Th17 cell reactivated in the presence of IL-12 generates an ex-Th17 or non-classical Th1 cell with self-renewing abilities compared to a classical Th1 cell that progressively differentiates into senescence
As also displayed in Fig. 2, Tregs are able to reacquire characteristics of Th17 cells under a cytokine-driven influence. When Foxp3+ Tregs are exposed to IL-6 with or without IL-1β and IL-23, Foxp3 becomes down-regulated in favor of expressing Th17 genes including IL-17, IL-22, IL-23R, and RORγt113. Such ex-Foxp3 cells have been implicated in the pathogenesis of autoimmune arthritis because they have a reduced ability to suppress cytotoxic CD8+ and effector Th17 cells114. Conversely, the transition to Th17 cells acquiring Treg-like characteristics is less well described but has recently been shown in ovarian and colorectal cancer model. Over time, tumor-infiltrating cells that produce IL-17 and are Foxp3− transdifferentiate into IL-17Aneg-Foxp3+ cells, termed “ex-Th17 Tregs”115. It is hypothesized that this conversion is controlled by TGF-β and prostaglandin E2 (PGE2)115. This dynamic functionality by Th17s and Tregs reveals the complexity of manipulating these subsets therapeutically.
Th17/Treg in Autoimmunity
Th17 cell-mediated immunity is important for maintaining mucosal and hematopoietic homeostasis. However, too strong of a Th17 cell response can induce autoimmunity. Likewise, a lack of Tregs can result in lethal autoimmunity in humans. As detailed in Table 1, the altered homeostasis between Th17 and Treg has been implicated in several autoimmune diseases, including multiple sclerosis116,117, psoriasis118, rheumatoid arthritis119, inflammatory bowel disease120, and systemic lupus erythematosus121. Thus, the relationship between effector Th17 cells and Tregs must remain balanced to preserve functional immunity and health of the host. Some important examples of this Th17/Treg balance in patients are discussed in various autoimmune manifestations below. In addition, recent therapeutics that have been used to reduce the severity of these diseases by modulating the Th17/Treg axis are discussed below.
Table 1.
Th17/Treg role in pathogenesis and therapy for autoimmune diseases
| Th17 role in pathogenesis | Treg role in pathogenesis | Therapeutic intervention | References | ||
|---|---|---|---|---|---|
| Th17-related | Treg-related | ||||
| MS |
 IL-17 in CSF and chronic lesions IL-17 in CSF and chronic lesions |
nL Frequency | Ustekinumab showed no benefit | Baltimore VA looks to induce myelin-specific Tregs | 126–128 |
| IL-23 KO mice resist EAE |
 Function Function |
Secukinumab may show benefit | |||
| Psoriasis |
 IL-17, IL-23, IFN-γ in lesions stimulate keratinocyte proliferation IL-17, IL-23, IFN-γ in lesions stimulate keratinocyte proliferation |
 Function Function |
Secukinumab | None | 130–139 |
| Increased Treg plasticity to Th17-like phenotype | Ustekinumab | ||||
| Guselkumab | |||||
| RA |
 Th17 frequency Th17 frequency |
 Frequency Frequency |
No benefit over standard of care with Secukinumab or Ustekinumab | Tocilizumab restores Th17/Treg imbalance | 119,141–144 |
 Th17 migration to synovium Th17 migration to synovium |
nL Function | Tociluzumab has shown efficacy alone or in combination with methotrexate | |||
| IBD |
 IL-17 in serum and mucosa IL-17 in serum and mucosa |
 Frequency Frequency |
Ustekinumab showed no benefit over placebo; showed efficacy in patients who failed anti-TNF-α therapy | Anti-CD3 shows benefit in UC; may result from accumulation of IL-10+ Tregs in colon | 145–148 |
| IL-23 mediated pathology | nL Function | ||||
| SLE |
 IL-17 compared to healthy controls IL-17 compared to healthy controls |
 Frequency Frequency |
None | MSCT/HSCT induces remission | 121,149–151 |
| IL-23R KO mice show reduced severity of nephritis |
 Function, migration Function, migration |
||||
MS multiple sclerosis, nL normal, RA rheumatoid arthritis, IBD inflammatory bowel disease, UC ulcerative colitis, SLE systemic lupus erythematosus, KO knockout, MSCT mesenchymal stem cell transplant, HSCT hematopoietic stem cell transplant
Multiple sclerosis
Multiple sclerosis (MS) is a chronic inflammatory disease involving destruction of myelin in central nervous system white matter. This disease presents in patients as deficits in sensory or motor function, unilateral vision loss, diplopia, gait disturbance, or loss of bladder control, among other varied symptoms of nervous system malfunction. As discussed previously, the experimental mouse model of MS is EAE, from which came the discovery of Th17 cells and their role in neuroinflammation. In MS patients, myelin-reactive T cells not only secrete IL-17 but also secrete IFN-γ and GM-CSF in contrast to T cells from healthy individuals122. These data suggest, as in the EAE animal model, that Th17 cells play a role in MS in human patients. These data also suggest that these Th17 cells are pathogenic due to their ability to cosecrete multiple cytokines. IL-17 levels have been reported to be increased in the CSF of MS patients during symptomatic relapse123, as well as in chronic lesions116. Interestingly, Th17 cells from patients with active symptoms of MS secrete less IL-10 compared to those who are clinically stable124. These data further suggest that Th17 cells from MS patients with stable disease are non-pathogenic with a regulatory property that might protect the host from aberrant cytotoxic responses against self.
Treg frequency and function have also been studied in MS. Although the frequency of Tregs is generally accepted as unaltered in MS patients117,125, Tregs from MS patients show impaired ability to suppress proliferation and cytokine secretion of other CD4+CD25− T cells compared to healthy individuals117. Both the upregulation of IL-17 and down-regulation of Treg-mediated immunity likely contribute to the pathogenesis of MS in humans.
Due to these Th17/Treg-related causes of MS, therapies that target these pathways are of great interest to the medical community. However, intriguingly, recent trials with IL-12/23 blockade (ustekinumab) did not show efficacy against the disease126. However, blockade of IL-17 with secukinumab showed some efficacy over placebo127. These preliminary findings will need to be evaluated more robustly in future trials. There is also growing interest in inducing suppressive Tregs in patients to reduce the severity of MS. For example, there is a new study led by Jewell at the Baltimore VA Medical Center that seeks to use nanotechnology to program myelin-specific Tregs in lymph nodes through exposure to myelin and toll-like receptor suppressive ligands, but data are not available from this investigation at this time (NIH Award 1I01BX003690-01)128. Perhaps targeting a combination of both Th17 and Treg pathways through IL-17 blockade and a boost of functional Tregs is necessary for disease control in patients with multiple sclerosis.
Psoriasis
MS is not the only disease where pathogenic Th17 cells have been shown to exacerbate autoimmune manifestations. Psoriasis is a chronic inflammatory skin disorder where the over-production of IL-23 by keratinocytes supports pathogenic Th17 cells in the dermis129. Hallmark features of psoriasis include various factors: parakeratosis, elongation and bulbous widening of the rete ridges with thinning of suprapapillary plates, dilated blood vessels and rouleaux formation in the papillary dermis118. Patients with psoriasis have more IL-17 and IL-22-producing cells in their peripheral blood than healthy individuals130. Moreover, psoriatic lesions have been discovered to contain increased IL-17A, IL-17C, and IL-17F compared to non-lesioned skin biopsies131–133 and are populated with more Th17 and Th1 cells134. T cell recruitment and proliferation are induced by CCL20 and IL-23 (secreted by APCs), while IFN-γ and various isoforms of IL-17 stimulate keratinocyte proliferation and APC activation in a cyclic manner134–137.
In addition to the strong link to Th17 hyperactivity, psoriasis has also been associated with altered Treg functionality. Patients have comparable Treg frequency compared to healthy individuals, but suppression of effector T cell function is impaired, similar to what has been shown in MS138. Treg cells from psoriasis patients produce more IL-17 and progressively lose Foxp3 expression more frequently than healthy controls, demonstrating a plasticity in Treg cells that further exacerbates disease pathology139.
Therapeutic options for patients include topical corticosteroids or phototherapy for mild disease, systemic immunosuppression, or TNF-α inhibitors (adalimumab, etanercept). More recently, interventions that target Th17 cells have been FDA approved and include blockade of IL-17 (secukinumab) or IL-12/23 (ustekinumab). A new drug that selectively blocks IL-23 called guselkumab was FDA approved July 2017. This drug was approved based on improved response to selective IL-23 blockade versus adalimumab and ustekinumab140. Therapies that may bolster or correct the faulty Treg function in these patients may also yield improved effects, but such therapies are not currently available. It is possible that modulation of low dose IL-2 or TGF-β could also bolster the generation of suppressive Treg cells in these patients while concomitantly suppressing Th1 or Th17 cells.
Other autoimmune diseases impacted by Th17/Treg axis: RA, IBD, SLE
Similar to MS and psoriasis, it has become clear that the pathogenesis of rheumatoid arthritis (RA), inflammatory bowel disease (IBD), and systemic lupus erythematosus (SLE) are also influenced by an imbalance in Th17/Treg function. These autoimmune diseases are summarized in Table 1. Clinical investigations are underway to understand how drugs that block the IL-17 and IL-23 pathway or potentiate Treg function impact RA, IBD, and SLE. More information on these findings is outlined in these references119,141–151.
A pinch of salt to reduce autoimmunity?
While an obvious way to treat autoimmunity in patients is by blunting the Th17 pathway via IL-17 or IL-23 blockade with FDA-approved drugs, holistic strategies involving simply changing the patient’s diet or modulating the microbiota to dampen Th17-mediated diseases are becoming increasingly more appreciated. A high-salt diet has been shown to induce Th17 cells and exacerbate EAE152. This concept is supported by a recent report by Wilck and team, who found that a high-salt diet triggers an increased number of pathogenic Th17 cells in the peripheral blood of mice, correlating with destruction of the Lactobacillus species in the gut microbiome and hypertension153,154. Repopulating the gut with Lactobacillus species was shown to mitigate the severity of EAE and hypertension152,153. The high-salt diet similarly led to an increase in peripheral circulating Th17 cells in a healthy human cohort153. In a preclinical arthritis model, the gut microbe segmented filamentous bacterium (SFB) was also found to support Th17 cells and exacerbate autoimmunity155. While antibiotics have been shown to reduce SFB and lessen the pathogenesis of Th17 cells, thus reducing these autoimmune diseases, it is less clear how altering the salt intake or administering probiotics in human patients could impact disease outcome. Regardless, it is now clear that modulating the microbiome can also play a major role in shaping the biology of the Th17/Treg axis. As discussed below, we will review the important role of Th17 cells and Tregs in cancer progression as well as ways to manipulate these two subsets to augment cancer immunotherapy.
Th17/Treg in Cancer
The relative contribution of Th17 and Treg cells in carcinogenesis is often related to chronic inflammation. It was first posited in 1863 that tissue injury and resulting cell proliferation could lead to cancer156. Today, it is widely accepted that chronic infection with bacteria, such as Helicobacter pylori157, or viruses, such as human papilloma virus158, can cause cancer. Although present day evidence for the role of Th17 cells in cancer is contradictory, excess inflammation from Th17 cells or too much immunosuppression induced by Treg cells may lead to carcinogenesis.
Th17 cells are found in human tumors159,160. IL-17A promotes the proliferation of malignant cells and induces angiogenic constituents by stimulating fibroblasts to upregulate vascular endothelial growth factor (VEGF), resulting in tumor neovascularization161,162. Whether Th17 cells promote or inhibit cancer development depends on the phenotype of the tumor. Pro-tumorigenic neutrophils, recruited by IL-17, have been reported in breast cancers163. High IL-17A and RORγt expression correlated with reduced disease-free survival rates in patients with colorectal cancer164. In pancreatic cancer, increased IL-22 production by Th17 cells is associated with decreased survival rates in patients165. In non-small cell lung cancer, patients were found to have a greater ratio of Th17/Treg frequencies compared to healthy controls, which correlated with the levels of carcinoembryonic antigen (CEA)166. In breast cancer, though, IL-22 production correlates with decreased tumor formation and a positive prognosis167. In ovarian cancer, increased tumor-associated IL-17 predicted improved patient survival160.
Treg cells, however, are often associated with tumor progression and reduced survival in cancer patients168,169. GARP expression in tumors has been shown to enhance active TGF-β and support Treg induction in the cancer microenvironment, thus hindering immune responses170. Treg presence in the tumor microenvironment has been associated with more advanced stage of malignancy, presence of invasion, and worsened prognosis168,169. Depletion of live Tregs in the murine tumor microenvironment prevented immunosuppression of tumor-infiltrating CD8+ T cells, allowing improved anti-tumor efficacy of endogenous effector T cells171. Apoptotic Tregs in the tumor, however, may also contribute to immune evasion. Recent work by the Zou lab revealed that oxidative stress in the tumor microenvironment induces Treg apoptosis, releasing large amounts of ATP, which is metabolized to immunosuppressive adenosine and signals through the A2a receptor (A2aR)172. This work suggests that selective depletion of Tregs in a patient’s tumor with inhibition of the A2aR pathway could circumvent the immune-evasive tumor microenvironment and thus increase immunity to human malignancies173.
Immunotherapy: a shift in the paradigm?
Despite the evidence suggesting a pro-tumorigenic role of Th17 cells and related cytokines, recent advances in the field of immunotherapy for treating cancer have suggested that Th17 cells may play a powerful role in antitumor immunity. In this case, tipping the Th17/Treg scale towards a dominant Th17 cell influence could be beneficial for patients with aggressive tumors. Herein, we will discuss preclinical and clinical findings that support further investigation of Th17 cells in therapeutically modulating tumor regression.
Adoptive cell transfer
Adoptive T cell transfer therapy (ACT) uses the patient’s own T cells to target and kill tumor cells. In the clinic, T cells can be obtained and logarithmically expanded from the tumor (called tumor-infiltrating lymphocytes or TIL) or can be rendered antigen-specific via genetically engineering peripheral blood T cells using Chimeric Antigen Receptor (CAR) or T cell receptor (TCR) constructs174. Much of ACT has focused on using CD8+ T cells to treat cancer patients; however, CD8+ T cells tend to progressively lose antitumor function as they expand, showing reduced ability to persist and clear tumors in vivo175. Recent clinical trials have now shown that CD4+ tumor-reactive T cells polarized to a Th1 subset are able to regress tumors in humans176, and in recently published work, human CD4+ T cells that express high levels of CD26 were found to cosecrete IL-17 and IFN-γ and be even more effective than CD8+ T cells when infused into mice bearing large human tumors177. However, based on preclinical work, efficacy may ultimately depend on the particular type of T helper subset infused into the cancer patient178–181.
Numerous laboratories have now discovered that Th17 cells can cause tumor regression to a greater extent than Th1 cells when transferred into mice178,179,182,183. While the mechanism by which Th17 cells regress tumors is not fully understood, they may kill tumor cells through synergism with CD8+ T cells or by themselves via direct lysis180,181. The enhanced ability of Th17 to ablate tumors has been attributed to a variety of characteristics. In culture, Th17 cell polarization is known to generate a more differentiated effector memory phenotype (CD62Llo, CCR7lo); However, paradoxically, after transfer, Th17 cells express high CCR7, Lef1, and Tcf7, indicating a durable stem memory phenotype179. In adoptive transfer therapy, it is interesting to note that the ability of Th17 cells to convert to a Th1-like phenotype and generate IFN-γ in vivo is critical for antitumor response178,179. However, compared to their Th1 counterpart, Th17 cells persist far longer and at greater frequencies in the host179. Th17 cells are also resistant to apoptosis, which permits them to oppose activation-induced cell death (AICD)184. Finally, Bowers et al. recently reported that Th17 cells retain their antitumor efficacy and resist senescence compared to Th1 cells even after long term ex vivo expansion for nearly one month185. Given that a large number of T cells are needed to mediate curative responses in mice with large tumors, the fact that Th17 but not CD8+ T cells can expand to ample numbers without losing their therapeutic potency has significant implications for clinical translation.
Despite preclinical success with murine and human Th17 cells, this powerful lymphocyte population has not yet been transferred into patients. One current barrier to successful antitumor response in the clinic includes use of exhausted T cells that do not persist in the blood. Through use of Th17-polarizing conditions in vitro in the clinic, Muranski et al.179 could generate a population that displays improved persistence and long-lived immunity. As many cytokines are needed to generate human Th17 cells, investigators seek to find other methods to effectively enrich and expand these cells without such cumbersome protocols. Most recently, it was demonstrated that CD26, a multifunctional ectoenzyme with costimulatory properties, was associated with enhanced Th17 cell function and activation177. In this work by Bailey and team, high levels of CD26 could be used to enrich human T cells with a type 17 phenotype177,186. T cells with a high expression of CD26 are multifunctional, have enhanced migratory and stem-like properties, resist apoptosis, and have been shown to regress multiple tumors177. Conversely, T cells with low CD26 expression were regulatory T cells, as demonstrated by their high expression of classic hallmark molecules such as high CD25, low CD127 and high expression of Foxp3 and Helios177. While such characteristics of CD26 could be capitalized upon therapeutically by inducing CD26high Th17 cells while concomitantly ablating CD26low Tregs, the possibility of Th17-induced autoimmunity dictates caution for the safe translation of this putative exciting, and perhaps more effective therapy.
Checkpoint blockade
Monoclonal antibodies that inhibit suppressive regulatory receptors (checkpoints) on T cells regress various tumors in patients including melanoma, bladder, breast, renal cell, ovarian, lung, and colorectal cancers187. The role of Th17 cells or other IL-17-producing immune cells has not been fully described in the mechanism for checkpoint blockade therapy, yet immune-related adverse events (irAEs) are frequent toxicities reported with this therapy. Anti-PD-1 therapy has been associated with exacerbated degrees of colitis, pneumonitis, endocrinopathy, lichenoid reactions, eczema, vitiligo, psoriasis, and pemphigoid in patients188–199. Anti-CTLA-4 therapy with ipilumimab has been reported to induce even more toxic side effects than anti-PD-1 therapy, including colitis, arthritis, dermatitis, hepatitis, and endocrinopathies, with less frequent incidence of uveitis, myopathy, and nephritis199–201. Combination therapy with CTLA-4 and PD-1 blockade (ipilimumab and nivolumab, respectively) can result in tumor regression with concurrent autoimmune toxicities related to a secondary immune response and epitope spreading202. PD-1 blockade with nivolumab as a monotherapy in advanced melanoma has been shown to be nearly as effective as combination therapy with ipilumumab; both therapies show significantly increased overall survival compared to ipilumumab alone203,204. Since the combination therapy induces a higher frequency of adverse events, treatment benefits should be weighed against risk of toxicity205.
For many patients, these toxicities are not without benefit. Fortunately, irAEs are often self-limiting or can be managed with high dose corticosteroids or hormone replacement, and especially with ipilumimab, may correlate with antitumor response200,201. Toxicities with ipilumimab have been associated with a greater likelihood of objective response in melanoma. Importantly, it should be noted that use of corticosteroids to treat irAEs has not significantly affected antitumor response206–210. Thus, it is important to understand the underlying reason why irAEs may be associated with better treatment outcome in cancer patients. These adverse events have prompted the search for immune system biomarkers predictive of therapy success. Interestingly, higher baseline IL-17 before treatment with ipilumimab has been associated with more advanced grade III irAEs211. Higher pretreatment serum levels of Th17 lineage related cytokines TGF-β and IL-6 have been associated with lack of melanoma progression and regression free survival with ipilumimab and interferon-α2b treatments, respectively211,212. Thus, autoimmune toxicities have been correlated with IL-17 levels, while antitumor responses have been associated with autoimmune toxicity. Mounting evidence from these various findings warrants future investigations that precisely uncover the role of Th17 cells in modulating these toxic and antitumor events.
Two recent reports suggest that Th17 cells and IL-17 may play an important role in both the efficacy and toxicity of checkpoint blockade therapy in cancer patients. This concept was demonstrated in a 50-year-old male with a history of mild psoriasis and Crohn’s disease who received PD-1 blockade (pembrolizumab) for metastatic colon cancer. The first two rounds of this therapy resulted in a remarkable 50% reduction in CEA213. Interestingly, after the third cycle of pembrolizumab, the patient displayed a severe psoriatic rash that covered 75% of his body along with increased abdominal pain and stool frequency. To resolve the skin manifestations, the patient was treated with secukinumab, an FDA-approved IL-17A blockade indicated for treatment of psoriasis. Although IL-17 blockade improved the symptoms of psoriasis and gastrointestinal pain, the anti-tumor activity was reduced as serum CEA returned to pretreatment levels213. Similarly, in a recent analysis of melanoma patients receiving PD-1 therapy, the frequency of IL-17-producing T helper cells was increased in responders to therapy versus non-responders, though monocytes were most predictive of response to therapy214. Given that several preclinical studies have suggested that Th17 cells and IL-17 may enhance or support tumor immunity, these clinical reports underscore the importance of studying the antitumor qualities of these effector Th17 cells in treating human patients.
The ultimate goal of the immunotherapy field is to uncouple toxicity from durable antitumor immunity. In the future, it is possible that the Th17/Treg axis could be effectively manipulated to augment tumor immunity while suppressing adverse immune side effects to healthy tissue. For example, it is possible that low dose IL-2 therapy following adoptive transfer of Th17 cells could mitigate prolonged autoimmunity once tumors have been cleared by elevating self-antigen-specific Tregs while still supporting antitumor Th17 and CD8+ T cells. Additionally, depletion of Tregs along with inhibition of the adenosine A2aR pathway could empower infiltrating effector immune cells to overcome the immunosuppressive tumor microenvironment. The potential in this field to treat and cure patients could be further enhanced through careful manipulation of both sides of this Th17/Treg balance.
Conclusions
The balance between Th17 and Treg T cells is critical for maintaining homeostasis. A Th17 dominance or dysfunctional Treg surveillance is associated with autoimmune disorders such as MS, psoriasis, RA, IBD, or SLE. Th17 cells and related cytokines can promote either tumorigenesis or tumor suppression, although this role is poorly understood. Future work in the field of immunotherapy in terms of adoptive transfer, vaccines and checkpoint blockade may provide new insights into the power of Th17 cells in regressing tumors, further enhancing our ability to harness the immune system against cancer. Future clinical trials may also use Tregs to quench immune responses to self-tissue without disturbing antitumor Th17 or CD8+ T cell responses in order to maximize efficacy and minimize toxicity in caring for cancer patients. Indeed, these are exciting times in the field of cancer immunotherapy and mounting evidence is converging on the potential to exploit the Th17/Treg axis to profoundly impact the life of patients with cancer and autoimmunity.
Acknowledgements
This work was supported in part by NIH Training grant T32 GM08716 to H.M.K., NIH Fellowship grant F31 CA192787 to S.R.B., NIH Training grant T32 AI132164-01 to C.J.D., NCI Grants R01 CA175061 and R01 CA208514, KL2 South Carolina Clinical & Translational Research grant UL1 TR000062, ACS-IRG grant 016623-004 and MUSC Start-up funds to C.M.P.
Competing interest
The authors declare that they have no competing interest.
Contributor Information
Hannah M. Knochelmann, Email: knochelm@musc.edu
Chrystal M. Paulos, Email: paulos@musc.edu
References
- 1.Tada T, Takemori T, Okumura K, Nonaka M, Tokuhisa T. Two distinct types of helper T cells involved in the secondary antibody response: independent and synergistic effects of Ia- and Ia+ helper T cells. J. Exp. Med. 1978;147:446–458. doi: 10.1084/jem.147.2.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations (*) Annu. Rev. Immunol. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luckheeram RV, Zhou R, Verma AD, Xia B. CD4(+)T cells: differentiation and functions. Clin. Dev. Immunol. 2012;2012:925135. doi: 10.1155/2012/925135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Szabo S, et al. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–669. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 5.Zheng W, Flavell R. The transcription factor GATA3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell. 1997;89:587–596. doi: 10.1016/s0092-8674(00)80240-8. [DOI] [PubMed] [Google Scholar]
- 6.Reiner SL. Development in motion: helper T cells at work. Cell. 2007;129:33–36. doi: 10.1016/j.cell.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 7.Ivanov II, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 8.Liang SC, et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J. Exp. Med. 2006;203:2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang SH, Dong C. A novel heterodimeric cytokine consisting of IL-17 and IL-17F regulates inflammatory responses. Cell. Res. 2007;17:435–440. doi: 10.1038/cr.2007.35. [DOI] [PubMed] [Google Scholar]
- 10.Dong C. TH17 cells in development: an updated view of their molecular identity and genetic programming. Nat. Rev. Immunol. 2008;8:337–348. doi: 10.1038/nri2295. [DOI] [PubMed] [Google Scholar]
- 11.Noack M, Miossec P. Th17 and regulatory T cell balance in autoimmune and inflammatory diseases. Autoimmun. Rev. 2014;13:668–677. doi: 10.1016/j.autrev.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 12.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat. Immunol. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 13.Adeegbe D, Bayer AL, Levy RB, Malek TR. Cutting edge: allogeneic CD4+CD25+Foxp3+T regulatory cells suppress autoimmunity while establishing transplantation tolerance. J. Immunol. 2006;176:7149–7153. doi: 10.4049/jimmunol.176.12.7149. [DOI] [PubMed] [Google Scholar]
- 14.Dardalhon V, et al. IL-4 inhibits TGF-beta-induced Foxp3+T cells and, together with TGF-beta, generates IL-9+IL-10+Foxp3(-) effector T cells. Nat. Immunol. 2008;9:1347–1355. doi: 10.1038/ni.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Veldhoen M, et al. Transforming growth factor-beta ‘reprograms’ the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nat. Immunol. 2008;9:1341–1346. doi: 10.1038/ni.1659. [DOI] [PubMed] [Google Scholar]
- 16.Schmitt E, et al. IL-9 production of naive CD4+T cells depends on IL-2, is synergistically enhanced by a combination of TGF-B and IL-4, and is inhibited by IFN-y. J. Immunol. 1994;153:3989–3996. [PubMed] [Google Scholar]
- 17.Gerlach K, et al. TH9 cells that express the transcription factor PU.1 drive T cell-mediated colitis via IL-9 receptor signaling in intestinal epithelial cells. Nat. Immunol. 2014;15:676–686. doi: 10.1038/ni.2920. [DOI] [PubMed] [Google Scholar]
- 18.Staudt V, et al. Interferon-regulatory factor 4 is essential for the developmental program of T helper 9 cells. Immunity. 2010;33:192–202. doi: 10.1016/j.immuni.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 19.Jabeen R, et al. Th9 cell development requires a BATF-regulated transcriptional network. J. Clin. Invest. 2013;123:4641–4653. doi: 10.1172/JCI69489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malik S, et al. Transcription factor Foxo1 is essential for IL-9 induction in T helper cells. Nat. Commun. 2017;8:815. doi: 10.1038/s41467-017-00674-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nowak EC, et al. IL-9 as a mediator of Th17-driven inflammatory disease. J. Exp. Med. 2009;206:1653–1660. doi: 10.1084/jem.20090246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Townsend M, et al. IL-9 deficient mice establish fundamental roles for IL-9 in pulmonary mastocytosis and goblet cell hyperplasia but not T cell development. Immunity. 2000;13:573–583. doi: 10.1016/s1074-7613(00)00056-x. [DOI] [PubMed] [Google Scholar]
- 23.Elyaman W, et al. IL-9 induces differentiation of TH17 cells and enhances function of FoxP3+natural regulatory T cells. Proc. Natl Acad. Sci. USA. 2009;106:12885–12890. doi: 10.1073/pnas.0812530106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vegran F, et al. The transcription factor IRF1 dictates the IL-21-dependent anticancer functions of TH9 cells. Nat. Immunol. 2014;15:758–766. doi: 10.1038/ni.2925. [DOI] [PubMed] [Google Scholar]
- 25.Duhen T, Geiger R, Jarrossay D, Lanzavecchia A, Sallusto F. Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat. Immunol. 2009;10:857–863. doi: 10.1038/ni.1767. [DOI] [PubMed] [Google Scholar]
- 26.Cai Y, Fleming C, Yan J. New insights of T cells in the pathogenesis of psoriasis. Cell Mol. Immunol. 2012;9:302–309. doi: 10.1038/cmi.2012.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eyerich S, et al. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J. Clin. Invest. 2009;119:3573–3585. doi: 10.1172/JCI40202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trifari S, Kaplan CD, Tran EH, Crellin NK, Spits H. Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from T(H)-17, T(H)1 and T(H)2 cells. Nat. Immunol. 2009;10:864–871. doi: 10.1038/ni.1770. [DOI] [PubMed] [Google Scholar]
- 29.Honda K. IL-22 from T cells: better late than never. Immunity. 2012;37:952–954. doi: 10.1016/j.immuni.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 30.Basu R, et al. Th22 cells are an important source of IL-22 for host protectioni against enteropathogenic bacteria. Immunity. 2012;37:1061–1075. doi: 10.1016/j.immuni.2012.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crotty S. T follicular helper cell differentiation, function, and roles in disease. Immunity. 2014;41:529–542. doi: 10.1016/j.immuni.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Y, et al. Germinal-center development of memory B cells driven by IL-9 from follicular helper T cells. Nat. Immunol. 2017;18:921–930. doi: 10.1038/ni.3788. [DOI] [PubMed] [Google Scholar]
- 33.Weinstein JS, et al. STAT4 and T-bet control follicular helper T cell development in viral infections. J Exp Med. 2017;215:337–355. doi: 10.1084/jem.20170457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andris F, et al. The transcription factor c-Maf promotes the differentiation of follicular helper T cells. Front. Immunol. 2017;8:480. doi: 10.3389/fimmu.2017.00480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bollig N, et al. Transcription factor IRF4 determines germinal center formation through follicular T-helper cell differentiation. Proc. Natl Acad. Sci. USA. 2012;109:8664–8669. doi: 10.1073/pnas.1205834109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ise W, et al. The transcription factor BATF controls the global regulators of class-switch recombination in both B cells and T cells. Nat. Immunol. 2011;12:536–543. doi: 10.1038/ni.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coffman R. Origins of the Th1-Th2 model: a personal perspective. Nat. Immunol. 2006;7:539–541. doi: 10.1038/ni0606-539. [DOI] [PubMed] [Google Scholar]
- 38.Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 2006;24:677–688. doi: 10.1016/j.immuni.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 39.Mossman T, Cherwinski H, Bond M, Giedlin M, Coffman R. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J. Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- 40.Steinman L. A brief history of T(H)17, the first major revision in the T(H)1/T(H)2 hypothesis of T cell-mediated tissue damage. Nat. Med. 2007;13:139–145. doi: 10.1038/nm1551. [DOI] [PubMed] [Google Scholar]
- 41.Billiau A, et al. Enhancement of experimental allergic encephalomyelitis in mice by antibodies against IFN-gamma. J. Immunol. 1988;140:1506–1510. [PubMed] [Google Scholar]
- 42.Voorthuis J, et al. Suppression of experimental allergic encephalomyelitis by intraventricular administration of interferon-gamma in Lewis rats. Clin. Exp. Immunol. 1990;81:183–188. doi: 10.1111/j.1365-2249.1990.tb03315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Willenborg D, Fordhan S, Bernard C, Cowden W, Ramshaw I. IFN-gamma plays a critical down-regulatory role in the induction and effector phase of myelin oligodendrocyte glycoprotein-induced autoimmune encephalomyelitis. J. Immunol. 1996;157:3223–3227. [PubMed] [Google Scholar]
- 44.Krakowski M, Owens T. Interferon-y confers resistance to experimental allergic encephalomyelitis. Eur. J. Immunol. 1996;26:1641–1646. doi: 10.1002/eji.1830260735. [DOI] [PubMed] [Google Scholar]
- 45.Ferber I, et al. Mice with a disrupted IFN-gamma gene are susceptible to the induction of experimental autoimmune encephalitis (EAE) J. Immunol. 1996;156:5–7. [PubMed] [Google Scholar]
- 46.Cruz A, et al. Cutting Edge: IFN- Regulates the Induction and Expansion of IL-17-Producing CD4 T Cells during Mycobacterial Infection. J. Immunol. 2006;177:1416–1420. doi: 10.4049/jimmunol.177.3.1416. [DOI] [PubMed] [Google Scholar]
- 47.Park H, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bettelli E, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 49.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 50.Tato C, O’Shea JJ. What does it mean to be just 17? Nature. 2006;441:166–168. doi: 10.1038/441166a. [DOI] [PubMed] [Google Scholar]
- 51.Cua DJ, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;42:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 52.Langrish CL, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Harrington LE, et al. Interleukin 17-producing CD4+effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 54.Stritesky GL, Yeh N, Kaplan MH. IL-23 promotes maintenance but not commitment to the Th17 lineage. J. Immunol. 2008;181:5948–5955. doi: 10.4049/jimmunol.181.9.5948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mailer RK, et al. IL-1beta promotes Th17 differentiation by inducing alternative splicing of FOXP3. Sci. Rep. 2015;5:14674. doi: 10.1038/srep14674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang S, et al. Reversing SKI-SMAD4-mediated suppression is essential for TH17 cell differentiation. Nature. 2017;551:105–109. doi: 10.1038/nature24283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Qin H, et al. TGF-beta promotes Th17 cell development through inhibition of SOCS3. J. Immunol. 2009;183:97–105. doi: 10.4049/jimmunol.0801986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim HS, et al. PTEN drives Th17 cell differentiation by preventing IL-2 production. J. Exp. Med. 2017;214:3381–3398. doi: 10.1084/jem.20170523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang XO, et al. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J. Biol. Chem. 2007;282:9358–9363. doi: 10.1074/jbc.C600321200. [DOI] [PubMed] [Google Scholar]
- 60.O’Shea JJ, Paul WE. Mechanisms underlying lineage commitment and plasticity of helper CD4+T cells. Science. 2010;327:1098–1102. doi: 10.1126/science.1178334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McGeachy MJ, et al. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat. Immunol. 2007;8:1390–1397. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- 62.McGeachy MJ, et al. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat. Immunol. 2009;10:314–324. doi: 10.1038/ni.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Korn T, et al. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Carr TM, Wheaton JD, Houtz GM, Ciofani M. JunB promotes Th17 cell identity and restrains alternative CD4(+) T-cell programs during inflammation. Nat. Commun. 2017;8:301. doi: 10.1038/s41467-017-00380-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Khader SA, Gaffen SL, Kolls JK. Th17 cells at the crossroads of innate and adaptive immunity against infectious diseases at the mucosa. Mucosal Immunol. 2009;2:403–411. doi: 10.1038/mi.2009.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kinugasa T, Sakaguchi T, Gu X, Reinecker H. Claudins regulate the intestinal barrier in response to immune mediators. Gastroenterology. 2000;118:1001–1011. doi: 10.1016/s0016-5085(00)70351-9. [DOI] [PubMed] [Google Scholar]
- 67.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu. Rev. Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 68.Fossiez F, et al. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J. Ex. Med. 1996;183:2593–2603. doi: 10.1084/jem.183.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ye P, et al. Requirement of Interleukin 17 receptor signalling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J. Exp. Med. 2001;194:519–527. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Laan M, et al. Neutrophil recruitment by human IL-17 via CXC chemokine release in the airways. J. Immunol. 1999;162:2347–2352. [PubMed] [Google Scholar]
- 71.Tan W, Huang W, Zhong Q, Schwarzenberger P. IL-17 receptor knockout mice have enhanced myelotoxicity and impaired hemopoietic recovery following gamma irradiation. J. Immunol. 2006;176:6186–6193. doi: 10.4049/jimmunol.176.10.6186. [DOI] [PubMed] [Google Scholar]
- 72.Cho J, et al. IL-17 is essential for host defense against Staphylococcus aureus infection in mice. J. Clin. Invest. 2010;120:1762–1773. doi: 10.1172/JCI40891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Siegemund S, et al. Production of IL-12, IL-23 and IL-27p28 by bone marrow-derived conventional dendritic cells rather than macrophages after LPS/TLR4-dependent induction by Salmonella Enteritidis. Immunobiology. 2007;212:739–750. doi: 10.1016/j.imbio.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 74.Sellge G, et al. Th17 cells are the dominant T cell subtype primed by Shigella flexneri mediating protective immunity. J. Immunol. 2010;184:2076–2085. doi: 10.4049/jimmunol.0900978. [DOI] [PubMed] [Google Scholar]
- 75.Griffiths KL, et al. Th1/Th17 cell induction and corresponding reduction in ATP consumption following vaccination with the novel Mycobacterium tuberculosis vaccine MVA85A. PLoS ONE. 2011;6:e23463. doi: 10.1371/journal.pone.0023463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ma CS, et al. Deficiency of Th17 cells in hyper IgE syndrome due to mutations in STAT3. J. Exp. Med. 2008;205:1551–1557. doi: 10.1084/jem.20080218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Davis S, Schaller J. Job’s Syndrome: recurrent, “cold”, staphylococcal abcesses. Lancet. 1966;1:1013–1015. doi: 10.1016/s0140-6736(66)90119-x. [DOI] [PubMed] [Google Scholar]
- 78.Okada S, Puel A, Casanova JL, Kobayashi M. Chronic mucocutaneous candidiasis disease associated with inborn errors of IL-17 immunity. Clin. Transl. Immunol. 2016;5:e114. doi: 10.1038/cti.2016.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gershon R, Kondo K. Cell interactions in the induction of tolerance: the role of thymic lymphocytes. Immunology. 1970;19:723–737. [PMC free article] [PubMed] [Google Scholar]
- 80.Green D, Flood P, Gershon R. Immunoregulatory T-cell pathways. Ann. Rev. Immunol. 1983;1:439–463. doi: 10.1146/annurev.iy.01.040183.002255. [DOI] [PubMed] [Google Scholar]
- 81.Kronenberg M, et al. RNA transcripts for I-J polypeptides are apparently not encoded between the I-A and I-E subregions of the murine major histocompatibility complex. Proc. Natl Acad. Sci. USA. 1983;80:5704–5708. doi: 10.1073/pnas.80.18.5704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nishizuka Y, Sakakura T. Thymus and reproduction: sex-linked dysgenesia of the gonad after neonatal thymectomy in mice. Science. 1969;166:753–755. doi: 10.1126/science.166.3906.753. [DOI] [PubMed] [Google Scholar]
- 83.Penhale W, Farmer A, McKenna R, Irvine W. Spontaneous thyroiditis in thymectomized and irradiated wistar rats. Clin. Exp. Immunol. 1973;15:225–236. [PMC free article] [PubMed] [Google Scholar]
- 84.Takahashi H, et al. Immunologic self-tolerance maintained by CD25+ CD4+ naturally anergic and suppressive T cells induction of autoimmune disease by breaking their anergic/suppressive state. Int. Immunol. 1998;10:1969–1980. doi: 10.1093/intimm/10.12.1969. [DOI] [PubMed] [Google Scholar]
- 85.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 86.Asano M, Toda M, Sakaguchi N, Sakaguchi S. Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. J. Exp. Med. 1996;184:387–396. doi: 10.1084/jem.184.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Malek TR, Yu A, Vincek V, Scibelli P, Kong L. CD4 regulatory T cells prevent lethal autoimmunity in IL-2Rbeta-deficient mice. Implications for the nonredundant function of IL-2. Immunity. 2002;17:167–178. doi: 10.1016/s1074-7613(02)00367-9. [DOI] [PubMed] [Google Scholar]
- 88.Willerford D, et al. Interleukin-2 receptor a chain regulates the size and content of the peripheral lymphoid compartment. Immunity. 1995;3:521–530. doi: 10.1016/1074-7613(95)90180-9. [DOI] [PubMed] [Google Scholar]
- 89.Setoguchi R, Hori S, Takahashi T, Sakaguchi S. Homeostatic maintenance of natural Foxp3(+) CD25(+) CD4(+) regulatory T cells by interleukin (IL)-2 and induction of autoimmune disease by IL-2 neutralization. J. Exp. Med. 2005;201:723–735. doi: 10.1084/jem.20041982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dwyer C, et al. Altered homeostasis and development of regulatory T cell subsets represent an IL-2R-dependent risk for diabetes in NOD mice. Sci. Signal. 2017;10:eeam9563. doi: 10.1126/scisignal.aam9563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kurtulus S, et al. TIGIT predominantly regulates the immune response via regulatory T cells. J. Clin. Invest. 2015;125:4053–4062. doi: 10.1172/JCI81187. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 92.Wang R, et al. Expression of GARP selectively identifies activated human FOXP3+regulatory T cells. Proc. Natl Acad. Sci. USA. 2009;106:13439–13444. doi: 10.1073/pnas.0901965106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rudensky AY, Gavin M, Zheng Y. FOXP3 and NFAT: partners in tolerance. Cell. 2006;126:253–256. doi: 10.1016/j.cell.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 94.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat. Immunol. 2005;6:1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 95.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+regulatory T cells. Nat. Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 96.Feng Y, et al. Control of the inheritance of regulatory T cell identity by a cis element in the Foxp3 locus. Cell. 2014;158:749–763. doi: 10.1016/j.cell.2014.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Williams LM, Rudensky AY. Maintenance of the Foxp3-dependent developmental program in mature regulatory T cells requires continued expression of Foxp3. Nat. Immunol. 2007;8:277–284. doi: 10.1038/ni1437. [DOI] [PubMed] [Google Scholar]
- 98.Getnet D, et al. A role for the transcription factor helios in human CD4+CD25+regulatory cells. Mol. Immunol. 2010;47:1595–1600. doi: 10.1016/j.molimm.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Elkord E, Abd Al Samid M, Chaudhary B. Helios, and not FoxP3, is the marker of activated Tregs expressing GARP/LAP. Oncotarget. 2015;6:20026–20036. doi: 10.18632/oncotarget.4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Liu W, et al. CD127 expression inversely correlates with FoxP3 and suppresive function of human CD4+T reg cells. J. Exp. Med. 2006;203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ono M, Tanaka RJ. Controversies concerning thymus-derived regulatory T cells: fundamental issues and a new perspective. Immunol. Cell. Biol. 2016;94:3–10. doi: 10.1038/icb.2015.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhou L, et al. IL-6 programs Th1-7 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat. Immunol. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 103.Laurence A, et al. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26:371–381. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 104.Ichiyama K, et al. Foxp3 inhibits RORgammat-mediated IL-17A mRNA transcription through direct interaction with RORgammat. J. Biol. Chem. 2008;283:17003–17008. doi: 10.1074/jbc.M801286200. [DOI] [PubMed] [Google Scholar]
- 105.Yang XP, et al. Opposing regulation of the locus encoding IL-17 through direct, reciprocal actions of STAT3 and STAT5. Nat. Immunol. 2011;12:247–254. doi: 10.1038/ni.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ding Y, Xu J, Bromberg JS. Regulatory T cell migration during an immune response. Trends Immunol. 2012;33:174–180. doi: 10.1016/j.it.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kishore M, et al. Regulatory T cell migration is dependent on glucokinase-mediated glycolysis. Immunity. 2017;47:875–89 e10. doi: 10.1016/j.immuni.2017.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.van der Vliet HJ, Nieuwenhuis EE. IPEX as a result of mutations in FOXP3. Clin. Dev. Immunol. 2007;2007:89017. doi: 10.1155/2007/89017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lee YK, et al. Late developmental plasticity in the T helper 17 lineage. Immunity. 2009;30:92–107. doi: 10.1016/j.immuni.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Maggi L, et al. Distinctive features of classic and nonclassic (Th17 derived) human Th1 cells. Eur. J. Immunol. 2012;42:3180–3188. doi: 10.1002/eji.201242648. [DOI] [PubMed] [Google Scholar]
- 111.Basdeo SA, et al. Ex-Th17 (Nonclassical Th1) cells are functionally distinct from classical Th1 and Th17 cells and are not constrained by regulatory T cells. J. Immunol. 2017;198:2249–2259. doi: 10.4049/jimmunol.1600737. [DOI] [PubMed] [Google Scholar]
- 112.Muranski P, Restifo NP. Essentials of Th17 cell commitment and plasticity. Blood. 2013;121:2402–2414. doi: 10.1182/blood-2012-09-378653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yang XO, et al. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity. 2008;29:44–56. doi: 10.1016/j.immuni.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Komatsu N, et al. Pathogenic conversion of Foxp3+T cells into TH17 cells in autoimmune arthritis. Nat. Med. 2014;20:62–68. doi: 10.1038/nm.3432. [DOI] [PubMed] [Google Scholar]
- 115.Downs-Canner S, et al. Suppressive IL-17A(+)Foxp3(+) and ex-Th17 IL-17A(neg)Foxp3(+) Treg cells are a source of tumour-associated Treg cells. Nat. Commun. 2017;8:14649. doi: 10.1038/ncomms14649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lock C, et al. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat. Med. 2002;8:500–508. doi: 10.1038/nm0502-500. [DOI] [PubMed] [Google Scholar]
- 117.Viglietta V, Baecher-Allan C, Weiner HL, Hafler DA. Loss of functional suppression by CD4+CD25+regulatory T cells in patients with multiple sclerosis. J. Exp. Med. 2004;199:971–979. doi: 10.1084/jem.20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Martin DA, et al. The emerging role of IL-17 in the pathogenesis of psoriasis: preclinical and clinical findings. J. Invest. Dermatol. 2013;133:17–26. doi: 10.1038/jid.2012.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kaneko S. et al. The RORgammat-CCR6-CCL20 axis augments Th17 cells invasion into the synovia of rheumatoid arthritis patients. Mod. Rheumatol. 2017:1–26. [DOI] [PubMed]
- 120.Skroza N, et al. Correlations between psoriasis and inflammatory bowel diseases. Biomed. Res. Int. 2013;2013:983902. doi: 10.1155/2013/983902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Martin JC, Baeten DL, Josien R. Emerging role of IL-17 and Th17 cells in systemic lupus erythematosus. Clin. Immunol. 2014;154:1–12. doi: 10.1016/j.clim.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 122.Cao Y, et al. Functional inflammatory profiles distinguish myelin-reactive T cells from patients with multiple sclerosis. Sci. Transl. Med. 2015;7:287ra74. doi: 10.1126/scitranslmed.aaa8038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Matusevicius D, et al. Interleukin-17 mRNA expression in blood and CSF mononuclear cells is augmented in multiple sclerosis. Mult. Scler. 1999;5:101–104. doi: 10.1177/135245859900500206. [DOI] [PubMed] [Google Scholar]
- 124.Hu D, et al. Transcriptional signature of human pro-inflammatory TH17 cells identifies reduced IL10 gene expression in multiple sclerosis. Nat. Commun. 2017;8:1600. doi: 10.1038/s41467-017-01571-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Putheti P, Pettersson A, Soderstrom M, Link H, Huang Y. Circulating CD4+CD25+T regulatory cells are not altered in multiple sclerosis and unaffected by disease-modulating drugs. J. Clin. Immunol. 2004;24:155–161. doi: 10.1023/B:JOCI.0000019780.93817.82. [DOI] [PubMed] [Google Scholar]
- 126.Segal BM, et al. Repeated subcutaneous injections of IL12/23 p40 neutralising antibody, ustekinumab, in patients with relapsing-remitting multiple sclerosis: a phase II, double-blind, placebo-controlled, randomised, dose-ranging study. Lancet Neurol. 2008;7:796–804. doi: 10.1016/S1474-4422(08)70173-X. [DOI] [PubMed] [Google Scholar]
- 127.Havrdova E, et al. Activity of secukinumab, an anti-IL-17A antibody, on brain lesions in RRMS: results from a randomized, proof-of-concept study. J. Neurol. 2016;263:1287–1295. doi: 10.1007/s00415-016-8128-x. [DOI] [PubMed] [Google Scholar]
- 128.Jewell C., Tostanoski L., Royal W. Self-assembly of immune signals to induce and control tolerance. J Immunol. 2017;198 (1 Supplement) 81.3.
- 129.Fitch E, Harper E, Skorcheva I, Kurtz S, Blauvelt A. Pathophysiology of psoriasis: recent advances on IL-23 and Th17 cytokines. Curr. Rheumatol. Rep. 2007;9:461–467. doi: 10.1007/s11926-007-0075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kagami S, Rizzo HL, Lee JJ, Koguchi Y, Blauvelt A. Circulating Th17, Th22, and Th1 cells are increased in psoriasis. J. Invest. Dermatol. 2010;130:1373–1383. doi: 10.1038/jid.2009.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wilke CM, Bishop K, Fox D, Zou W. Deciphering the role of Th17 cells in human disease. Trends Immunol. 2011;32:603–611. doi: 10.1016/j.it.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Harper EG, et al. Th17 cytokines stimulate CCL20 expression in keratinocytes in vitro and in vivo: implications for psoriasis pathogenesis. J. Invest. Dermatol. 2009;129:2175–2183. doi: 10.1038/jid.2009.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Johansen C, et al. Characterization of the interleukin-17 isoforms and receptors in lesional psoriatic skin. Br. J. Dermatol. 2009;160:319–324. doi: 10.1111/j.1365-2133.2008.08902.x. [DOI] [PubMed] [Google Scholar]
- 134.Kryczek I, et al. et al. Induction of IL-17+T cell trafficking and development by IFN-gamma: mechanism and pathological relevance in psoriasis. J. Immunol. 2008;181:4733–4741. doi: 10.4049/jimmunol.181.7.4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ramirez-Carrozzi V, et al. IL-17C regulates the innate immune function of epithelial cells in an autocrine manner. Nat. Immunol. 2011;12:1159–1166. doi: 10.1038/ni.2156. [DOI] [PubMed] [Google Scholar]
- 136.Ciric B, El-behi M, Cabrera R, Zhang GX, Rostami A. IL-23 drives pathogenic IL-17-producing CD8+T cells. J. Immunol. 2009;182:5296–5305. doi: 10.4049/jimmunol.0900036. [DOI] [PubMed] [Google Scholar]
- 137.Nograles KE, et al. Th17 cytokines interleukin (IL)-17 and IL-22 modulate distinct inflammatory and keratinocyte-response pathways. Br. J. Dermatol. 2008;159:1092–1102. doi: 10.1111/j.1365-2133.2008.08769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sugiyama H, et al. Dysfunctional blood and target tissue CD4+CD25high regulatory T cells in psoriasis: mechanism underlying unrestrained pathogenic effector T cell proliferation. J. Immunol. 2004;174:164–173. doi: 10.4049/jimmunol.174.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bovenschen H, et al. Foxp3+regulatory T cells of psoriasis patients easily differentiate into IL-17A-producing cells and are found in lesional skin. J. Inv Derm. 2011;131:1853–1860. doi: 10.1038/jid.2011.139. [DOI] [PubMed] [Google Scholar]
- 140.Langley R. G. et al. Efficacy and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: results of the randomized, double-blind, phase III NAVIGATE trial. Br. J. Dermatol. 2017. [DOI] [PubMed]
- 141.Blanco FJ, et al. Secukinumab in active rheumatoid arthritis: a phase III randomized, double-blind, active comparator- and placebo-controlled study. Arthritis Rheumatol. 2017;69:1144–1153. doi: 10.1002/art.40070. [DOI] [PubMed] [Google Scholar]
- 142.Sarkar S, Fox DA. Targeting IL-17 and Th17 cells in rheumatoid arthritis. Rheum. Dis. Clin. North. Am. 2010;36:345–366. doi: 10.1016/j.rdc.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 143.Haque M, Fino K, Lei F, Xiong X, Song J. Utilizing regulatory T cells against rheumatoid arthritis. Front Oncol. 2014;4:209. doi: 10.3389/fonc.2014.00209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Samson M, et al. Brief report: inhibition of interleukin-6 function corrects Th17/Treg cell imbalance in patients with rheumatoid arthritis. Arthritis Rheum. 2012;64:2499–2503. doi: 10.1002/art.34477. [DOI] [PubMed] [Google Scholar]
- 145.Boden EK, Snapper SB. Regulatory T cells in inflammatory bowel disease. Curr. Opin. Gastroenterol. 2008;24:733–741. doi: 10.1097/mog.0b013e328311f26e. [DOI] [PubMed] [Google Scholar]
- 146.Sandborn W, et al. A randomized trial of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with moderate-to-severe Crohn’s Disease. Gastroenterology. 2008;135:1130–1141. doi: 10.1053/j.gastro.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 147.Plevy S, et al. A phase I study of visilizumab, a humanized anti-CD3 monoclonal antibody, in severe steroid-refractory ulcerative colitis. Gastroenterology. 2007;133:1414–1422. doi: 10.1053/j.gastro.2007.08.035. [DOI] [PubMed] [Google Scholar]
- 148.Fujino S, et al. Increased expression of interleukin 17 i inflammatory bowel disease. Gut. 2003;52:65–70. doi: 10.1136/gut.52.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kyttaris VC, Zhang Z, Kuchroo VK, Oukka M, Tsokos GC. Cutting edge: IL-23 receptor deficiency prevents the development of lupus nephritis in C57BL/6-lpr/lpr mice. J. Immunol. 2010;184:4605–4609. doi: 10.4049/jimmunol.0903595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lee HY, et al. Altered frequency and migration capacity of CD4+CD25+regulatory T cells in systemic lupus erythematosus. Rheumatology. 2008;47:789–794. doi: 10.1093/rheumatology/ken108. [DOI] [PubMed] [Google Scholar]
- 151.Wang D, et al. Allogenic mesenchymal stem cell transplantation in severe and refractory systemic lupus erythematosus: 4 years of experience. Cell Transp. 2013;22:2267–2277. doi: 10.3727/096368911X582769c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kleinewietfeld M, et al. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature. 2013;496:518–522. doi: 10.1038/nature11868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wilck N, et al. Salt-responsive gut commensal modulates Th17 axis and disease. Nature. 2017;551:585–589. doi: 10.1038/nature24628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wenzel U, et al. Immune mechanisms in arterial hypertension. J. Am. Soc. Nephrol. 2016;27:677–686. doi: 10.1681/ASN.2015050562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wu HJ, et al. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity. 2010;32:815–827. doi: 10.1016/j.immuni.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Parsonnet J, et al. Helicobacter Pylori infection and the risk of gastric carcinoma. N. Engl. J. Med. 1991;325:1127–1131. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- 158.Munoz N, Bosch F, De Sanjose S, Shah K. The role of HPV in the etiology of cervical cancer. Mutat. Res. 1994;305:293–301. doi: 10.1016/0027-5107(94)90249-6. [DOI] [PubMed] [Google Scholar]
- 159.Sfanos KS, et al. Phenotypic analysis of prostate-infiltrating lymphocytes reveals TH17 and Treg skewing. Clin. Cancer Res. 2008;14:3254–3261. doi: 10.1158/1078-0432.CCR-07-5164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kryczek I, et al. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood. 2009;114:1141–1149. doi: 10.1182/blood-2009-03-208249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Murugaiyan G, Saha B. Protumor vs antitumor functions of IL-17. J. Immunol. 2009;183:4169–4175. doi: 10.4049/jimmunol.0901017. [DOI] [PubMed] [Google Scholar]
- 162.Takahashi H, Numasaki M, Lotze MT, Sasaki H. Interleukin-17 enhances bFGF-, HGF- and VEGF-induced growth of vascular endothelial cells. Immunol. Lett. 2005;98:189–193. doi: 10.1016/j.imlet.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 163.Benevides L, et al. IL17 promotes mammary tumor progression by changing the behavior of tumor cells and eliciting tumorigenic neutrophils recruitment. Cancer Res. 2015;75:3788–3799. doi: 10.1158/0008-5472.CAN-15-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Tosolini M, et al. Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in patients with colorectal cancer. Cancer Res. 2011;71:1263–1271. doi: 10.1158/0008-5472.CAN-10-2907. [DOI] [PubMed] [Google Scholar]
- 165.Martin-Orozco N, Chung Y, Chang SH, Wang YH, Dong C. Th17 cells promote pancreatic inflammation but only induce diabetes efficiently in lymphopenic hosts after conversion into Th1 cells. Eur. J. Immunol. 2009;39:216–224. doi: 10.1002/eji.200838475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Duan M, et al. Disturbed Th17/Treg balance in patients with non-small cell lung cancer. Inflammation. 2015;38:2156–2165. doi: 10.1007/s10753-015-0198-x. [DOI] [PubMed] [Google Scholar]
- 167.Horlock C, et al. The effects of trastuzumab on the CD4+CD25+FoxP3+ and CD4+IL17A+ T-cell axis in patients with breast cancer. Br. J. Cancer. 2009;100:1061–1067. doi: 10.1038/sj.bjc.6604963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Tang Y, et al. An increased abundance of tumor-infiltrating regulatory T cells is correlated with the progression and prognosis of pancreatic ductal adenocarcinoma. PLoS ONE. 2014;9:e91551. doi: 10.1371/journal.pone.0091551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Shou J, Zhang Z, Lai Y, Chen Z, Huang J. Worse outcome in breast cancer with higher tumor-infiltrating FOXP3+Tregs: a systematic review and meta-analysis. BMC Cancer. 2016;16:687. doi: 10.1186/s12885-016-2732-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Metelli Alessandra, et al. Surface Expression of TGFÎ2 Docking Receptor GARP Promotes Oncogenesis and Immune Tolerance in Breast Cancer. Cancer Research. 2016;76:7106–7117. doi: 10.1158/0008-5472.CAN-16-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Budhu B, et al. Budhu B. et al. Blockade of surace-bound TGF-B on regulatory T cells abrogates suppression of effector T cell function in the tumor microenvironment. Sci Signal. 2017;10:eaak9702. doi: 10.1126/scisignal.aak9702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Maj T, et al. Oxidative stress controls regulatory T cell apoptosis and suppressor activity and PD-L1-blockade resistance in tumor. Nat. Immunol. 2017;18:1332–1341. doi: 10.1038/ni.3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Leone RD, Lo YC, Powell JD. A2aR antagonists: next generation checkpoint blockade for cancer immunotherapy. Comput. Struct. Biotechnol. J. 2015;13:265–272. doi: 10.1016/j.csbj.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat. Rev. Immunol. 2012;12:269–281. doi: 10.1038/nri3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Gattinoni L, et al. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+T cells. J. Clin. Invest. 2005;115:1616–1626. doi: 10.1172/JCI24480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Tran EH, et al. Cancer immunotherapy based on mutation-specific CD4+T cells in a patient with epithelial cancer. Science. 2014;344:641–645. doi: 10.1126/science.1251102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Bailey SR, et al. Human CD26 high T cells elicit tumor immunity against multiple malignancies via enhanced migration and persistance. Nat. Commun. 2017;8:1961. doi: 10.1038/s41467-017-01867-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Muranski P, et al. Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood. 2008;112:362–372. doi: 10.1182/blood-2007-11-120998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Muranski P, et al. Th17 cells are long lived and retain a stem cell-like molecular signature. Immunity. 2011;35:972–985. doi: 10.1016/j.immuni.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Martin-Orozco N, et al. T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity. 2009;31:787–798. doi: 10.1016/j.immuni.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Xie Y, et al. Naive tumor-specific CD4(+) T cells differentiated in vivo eradicate established melanoma. J. Exp. Med. 2010;207:651–667. doi: 10.1084/jem.20091921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Paulos CM, et al. The inducible costimulator (ICOS) is critical for the development of human Th17 cells. Sci. Transl. Med. 2018;2:55ra78. doi: 10.1126/scitranslmed.3000448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kryczek I, et al. Human TH17 cells are long-lived effector memory cells. Sci. Transl. Med. 2011;3:104ra0. doi: 10.1126/scitranslmed.3002949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Yu Y, et al. Abundant c-Fas-associated death domain-like interleukin-1-converting enzyme inhibitory protein expression determines resistance of T helper 17 cells to activation-induced cell death. Blood. 2009;114:1026–1028. doi: 10.1182/blood-2009-03-210153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Bowers JS, et al. Th17 cells are refractory to senescence and retain robust antitumor activity after long-term ex vivo expansion. JCI Insight. 2017;2:e90772. doi: 10.1172/jci.insight.90772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Bailey SR, et al. Th17 cells in cancer: the ultimate identity crisis. Front. Immunol. 2014;5:276. doi: 10.3389/fimmu.2014.00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Webb E. S. et al. Immune checkpoint inhibitors in cancer therapy. J. Biomed. Res. 0, 1–10 2017. [DOI] [PMC free article] [PubMed]
- 188.June CH, Warshauer JT, Bluestone JA. Corrigendum: Is autoimmunity the Achilles’ heel of cancer immunotherapy? Nat. Med. 2017;23:1004. doi: 10.1038/nm0817-1004b. [DOI] [PubMed] [Google Scholar]
- 189.Vanderlugt CL, Miller SD. Epitope spreading in immune-mediated diseases: implications for immunotherapy. Nat. Rev. Immunol. 2002;2:85–95. doi: 10.1038/nri724. [DOI] [PubMed] [Google Scholar]
- 190.Linardou H, Gogas H. Toxicity management of immunotherapy for patients with metastatic melanoma. Ann. Transl. Med. 2016;4:272. doi: 10.21037/atm.2016.07.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Muro K, et al. Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): a multicentre, open-label, phase 1b trial. Lancet Oncol. 2016;17:717–726. doi: 10.1016/S1470-2045(16)00175-3. [DOI] [PubMed] [Google Scholar]
- 192.Shi VJ, et al. Clinical and histologic features of lichenoid mucocutaneous eruptions due to anti-programmed cell death 1 and anti-programmed cell death ligand 1 immunotherapy. JAMA Dermatol. 2016;152:1128–1136. doi: 10.1001/jamadermatol.2016.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Hwang SJ, et al. Cutaneous adverse events (AEs) of anti-programmed cell death (PD)-1 therapy in patients with metastatic melanoma: a single-institution cohort. J. Am. Acad. Dermatol. 2016;74:455–61 e1. doi: 10.1016/j.jaad.2015.10.029. [DOI] [PubMed] [Google Scholar]
- 194.Sibaud V, et al. Dermatologic complications of anti-PD-1/PD-L1 immune checkpoint antibodies. Curr. Opin. Oncol. 2016;28:254–263. doi: 10.1097/CCO.0000000000000290. [DOI] [PubMed] [Google Scholar]
- 195.Hua C, et al. Association of vitiligo with tumor response in patients with metastatic melanoma treated with pembrolizumab. JAMA Dermatol. 2016;152:45–51. doi: 10.1001/jamadermatol.2015.2707. [DOI] [PubMed] [Google Scholar]
- 196.Jour G, et al. Autoimmune dermatologic toxicities from immune checkpoint blockade with anti-PD-1 antibody therapy: a report on bullous skin eruptions. J. Cutan. Pathol. 2016;43:688–696. doi: 10.1111/cup.12717. [DOI] [PubMed] [Google Scholar]
- 197.Parakh S, Nguyen R, Opie J, Andrews M. Late presentation of generalised bullous pemphigoid-like reaction in a patient treated with pembrolizumab for metastatic melanoma. Australas. J. Dermatol. 2017;58:109–112. doi: 10.1111/ajd.12488. [DOI] [PubMed] [Google Scholar]
- 198.Bonigen J, et al. Anti-PD1-induced psoriasis: a study of 21 patients. J. Eur. Acad. Dermatol. Venereol. 2017;31:e254–e257. doi: 10.1111/jdv.14011. [DOI] [PubMed] [Google Scholar]
- 199.Maker A, et al. Tumor regression and autoimmunity in patients treated with cytotoxic T lymphocyte-associated antigen 4 blockade and interleukin 2: a phase I/II study. Ann. Surg. Oncol. 2005;12:1005–1016. doi: 10.1245/ASO.2005.03.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Yang J, et al. Ipilumumab (Anti-CTLA4 Antibody) causes regression of metastatic renal cell cancer associated with enteritis and hypophysitis. J. Immunother. 2007;30:825–830. doi: 10.1097/CJI.0b013e318156e47e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Di Giacomo AM, Biagioli M, Maio M. The emerging toxicity profiles of anti-CTLA-4 antibodies across clinical indications. Semin. Oncol. 2010;37:499–507. doi: 10.1053/j.seminoncol.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 202.Sznol M, et al. Pooled analysis safety profile of nivolumab and ipilumumab combination therapy in patients with advanced melanoma. J. Clin. Oncol. 2017;35:3815–3822. doi: 10.1200/JCO.2016.72.1167. [DOI] [PubMed] [Google Scholar]
- 203.Larkin J, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N. Engl. J. Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Wolchok JD, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N. Engl. J. Med. 2017;377:1345–1356. doi: 10.1056/NEJMoa1709684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Hassel JC, et al. Combined immune checkpoint blockade (anti-PD-1/anti-CTLA-4): evaluation and management of adverse drug reactions. Cancer Treat. Rev. 2017;57:36–49. doi: 10.1016/j.ctrv.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 206.Weber JS, et al. Phase I/II study of ipilimumab for patients with metastatic melanoma. J. Clin. Oncol. 2008;26:5950–5956. doi: 10.1200/JCO.2008.16.1927. [DOI] [PubMed] [Google Scholar]
- 207.Attia P, et al. Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anti-cytotoxic T-lymphocyte antigen-4. J. Clin. Oncol. 2005;23:6043–6053. doi: 10.1200/JCO.2005.06.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Phan GQ, et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc. Natl Acad. Sci. USA. 2003;100:8372–8377. doi: 10.1073/pnas.1533209100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Beck KE, et al. Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. J. Clin. Oncol. 2006;24:2283–2289. doi: 10.1200/JCO.2005.04.5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Downey SG, et al. Prognostic factors related to clinical response in patients with metastatic melanoma treated by CTL-associated antigen-4 blockade. Clin. Cancer Res. 2007;13:6681–6688. doi: 10.1158/1078-0432.CCR-07-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Tarhini AA, et al. Baseline circulating IL-17 predicts toxicity while TGF-beta1 and IL-10 are prognostic of relapse in ipilimumab neoadjuvant therapy of melanoma. J. Immunother. Cancer. 2015;3:39. doi: 10.1186/s40425-015-0081-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Yurkovetsky ZR, et al. Multiplex analysis of serum cytokines in melanoma patients treated with interferon-alpha2b. Clin. Cancer Res. 2007;13:2422–2428. doi: 10.1158/1078-0432.CCR-06-1805. [DOI] [PubMed] [Google Scholar]
- 213.Esfahani K, Miller W. Reversal of autoimmune toxicity and loss of tumor response by interleukin-17 blockade. N. Engl. J. Med. 2017;376:1989–1991. doi: 10.1056/NEJMc1703047. [DOI] [PubMed] [Google Scholar]
- 214.Krieg C. High dimensional analysis of the immune landscape during anti-PD-1 immunotherapy of melanoma predicts responsiveness. Nat. Med. 2018;24:144–153. doi: 10.1038/nm.4466. [DOI] [PubMed] [Google Scholar]