Abstract
Inflammatory bowel disease (IBD) is a common chronic intestinal disorder characterised by a loss of epithelial barrier function leading to the unregulated movement of luminal antigenic material into mucosal tissue with resultant inflammation. In IBD, multiple components of the inflammatory response lead to tissue hypoxia. Mucosal hypoxia leads to the inactivation of prolyl hydroxylase domain‐containing (PHD) enzymes, which in turn leads to the stabilisation of the hypoxia‐inducible factor (HIF), which induces the expression of barrier protective genes. Furthermore, pharmacological hydroxylase inhibition has been shown to be protective in colitis, at least in part through enhancing intestinal epithelial barrier function through HIF‐1‐dependent barrier‐protective gene expression. Therefore, targeting hypoxia‐sensitive pathways represents a new and promising therapeutic approach in IBD.
Keywords: hypoxia, inflammation, intestine
Introduction
Inflammatory bowel disease (IBD) is a chronic inflammatory condition affecting the gastrointestinal tract. The core pathology of IBD involves a loss of intestinal barrier function and associated exposure of cells of the mucosal immune system to the antigenic and microbial contents of the gut lumen. This drives inflammation which further compromises intestinal barrier integrity resulting in progressive and cyclical inflammatory disease. The two principal forms of IBD are ulcerative colitis (UC), in which inflammation is restricted to the mucosal tissue of the colon, and Crohn's disease (CD) where inflammation can be transmural and may occur throughout the gastrointestinal tract (Cummins et al. 2013). The prevalence of IBD is highest and rising in Europe and North America. The annual prevalence of IBD in Europe is 505 per 100,000 people for UC and 322 per 100,000 people for CD (Molodecky et al. 2012). The cause(s) of IBD is thought to involve a complex combination of genetic predisposition and exposure to microbial and environmental factors. Symptoms include, but are not limited to, abdominal pain, weight loss, diarrhoea and fatigue (Fakhoury et al. 2014). These symptoms can be debilitating for patients and IBD is associated with a number of comorbidities including increased risk of cardiovascular disease (Wu et al. 2017), inflammation driven tumour development (Axelrad et al. 2016) and associated psychological conditions including depression and anxiety (Bannaga & Selinger, 2015). In a bid to better understand IBD and develop new therapeutic strategies, a number of animal models have been developed. These models vary in their mechanism of action and include chemically induced colitis (e.g. DSS or TNBS colitis) as well as genetically modified mice with increased susceptibility to spontaneous disease (e.g. MDR‐1 knockout mice) (Wirtz & Neurath, 2007). Due to the limitations in the relevance of each of these individual models to human disease, putative new therapeutic approaches to IBD need to be confirmed in multiple model systems.
Of interest, multiple pre‐clinical models of inflammatory bowel disease have demonstrated that the microenvironment of the inflamed mucosa is hypoxic. This is likely a result of a combination of increased oxygen consumption by infiltrating immune cells and resident cells of the inflamed mucosa and decreased perfusion due to microthrombosis in chronically inflamed tissue. Therefore, mucosal hypoxia is a common microenvironmental feature in the inflamed mucosa during IBD (Taylor & Colgan, 2017).
The hypoxia inducible factor
The hypoxia‐inducible factor (HIF) is a transcription factor which governs the cellular transcriptional response to hypoxia. Under conditions of normoxia (where oxygen supply exceeds demand), HIF is generated at a high level but is targeted for immediate, oxygen‐dependent degradation in a manner dependent upon the activity of the HIF PHDs (Fig. 1). There are two HIFα isoforms associated with increased gene expression in hypoxia, termed HIF‐1α and HIF‐2α, which have differential tissue expression patterns and which regulate discreet but overlapping gene cohorts (Kaelin & Ratcliffe, 2008). In hypoxia, PHDs are inhibited and HIF degradation is thereby reduced, allowing HIFα subunits to rapidly accumulate and dimerise with HIF‐1β proteins. These HIFαβ dimers act as transcription factors at hypoxia‐response elements (HRE) and regulate a wide programme of gene transcription to increase cellular oxygen levels but also affecting processes such as metabolism, angiogenesis, proliferation, apoptosis and inflammation (Kaelin & Ratcliffe, 2008). Therefore, HIF has been termed a master regulator of the cellular adaptive response to hypoxia (Semenza, 2012).
Figure 1. The hypoxia‐inducible factor pathway.
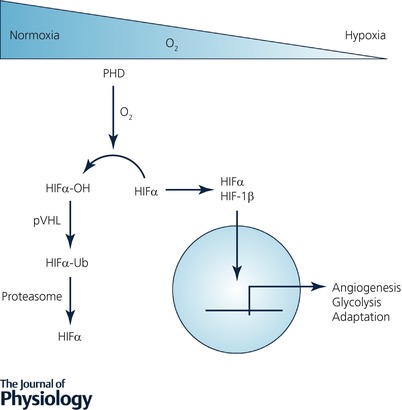
Under conditions where cellular oxygen supply exceeds demand (normoxia), HIF is hydroxylated by PHD enzymes leading to its ubiquitination by the von Hipple Lindau protein and its subsequent degradation. Under conditions where oxygen demand exceeds supply, hydroxylases are inhibited leading to the stabilisation of HIF‐1α subunits which dimerise with HIF‐1β to form a transcriptionally active transcription factor which governs the cellular transcriptional response to hypoxia.
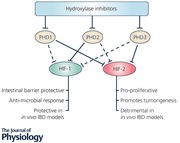
Humans express three prolyl hydroxylase isoforms and siRNA‐based interference experiments have demonstrated that these isoforms (termed PHD1–3) have differential selectivity in relation to the hydroxylation of HIF‐1α and HIF‐2α (Appelhoff et al. 2004). In this study, it was demonstrated that while PHD2 had a greater influence on HIF‐1α than HIF‐2α levels, PHD1 and PHD3 in combination or singly had a greater effect on HIF‐2α levels than HIF‐1α levels. Another study also demonstrated that PHD1 inhibits HIF‐1α accumulation and transcriptional activity (Erez et al. 2003). A caveat in interpreting these data is that different cell types have different expression profiles. Furthermore, PHD2 and PHD3 mRNA levels are inducible by hypoxia while PHD1 is not regulated by such a feedback mechanism (Marxsen et al. 2004). Therefore in designing targeted prolyl hydroxylase inhibitors, consideration must be given to the PHD being targeted as well as the unique PHD expression profile and hypoxic context of the cell or tissue being targeted.
In a murine dextran sulphate sodium (DSS) colitis model it was demonstrated that PHD1 knockout was protective against the disease while PHD3 knockout or PHD2 heterozygous knockout was not (Tambuwala et al. 2010). Furthermore, using a murine DSS model, it was demonstrated that deletion of PHD1 in haematopoietic cells is protective. Similarly to the previously mentioned study, deletion of PHD3 or heterozygous deletion of PHD2 in haematopoietic cells was not protective (Van Welden et al. 2017). Further support for the involvement of PHD1 in IBD is the finding that in human colonic biopsies PHD1 protein levels are elevated in both UC and CD patients (Van Welden et al. 2013). These results imply that PHD1 in particular is associated with colitis in both animal models and the human disease.
In support of the concept that PHD inhibition may be of therapeutic benefit in human colitis, it was demonstrated that the pan‐hydroxylase inhibitor dimethyloxalylglycine (DMOG) is protective against DSS‐induced colitis (Cummins et al. 2008). In a simultaneous report, another pan‐hydroxylase inhibitor (FG‐4497) was shown to be protective in 2,4,6‐trinitrobenzenesulfonic acid (TNBS)‐induced colitis (Robinson et al. 2008). Since then, these results have been reproduced in a variety of colitis models (Cummins et al. 2013). A common factor among these studies has been the finding that compounds which inhibit prolyl hydroxylase enzymes and stabilise hypoxia‐inducible factor (HIF) are therapeutic in pre‐clinical models of colitis (Hindryckx et al. 2010; Hirota et al. 2010; Keely et al. 2014). Therefore, in multiple pre‐clinical models of colitis, genetic PHD1 or pharmacological pan‐hydroxylase inhibition has been shown to be protective. This has led to interest in the use of prolyl hydroxylase inhibitors to manipulate HIF pathway activity as a therapeutic strategy in IBD.
HIF‐1α or HIF‐2α dominant signalling – a determinant of disease progression?
A key question which remains is the relative roles of the HIF‐1 and HIF‐2 isoforms in the protective effects of hydroxylase inhibition in colitis. HIF‐1α and HIF‐2α share 85% sequence identity in their DNA binding basic helix‐loop‐helix domain which allows them to bind identical HRE consensus sequences (Tian et al. 1997). Their N‐terminal transactivation domain is what confers target gene specificity by recruiting different transcriptional cofactors (Hu et al. 2007). As a result of this regulatory system both HIF‐1α and HIF‐2α act as transcription factors for a number of common, as well as many unique, target genes (Keith et al. 2011).
In the context of IBD, HIF‐1α is widely regarded as a protective factor as it regulates the expression of barrier protective proteins such as intestinal trefoil factor and CD73 (Karhausen et al. 2004), as well as anti‐microbial proteins such as β‐defensin‐1 (Kelly et al. 2013). Further evidence for the protective role of HIF‐1α in IBD is the finding that DSS‐induced colitis is more severe in mice with a dendritic cell HIF‐1α knockout compared to wild types (Fluck et al. 2016). Another study using Cre‐loxP‐mediated deletion or constitutive activation of HIF‐1α in the intestinal epithelial cells of mice demonstrated that the absence of HIF‐1α results in more severe TNBS‐induced colitis while constitutive activation of HIF‐1α was protective against TNBS‐induced colitis (Karhausen et al. 2004).
HIF‐2α also plays a critical role in maintaining intestinal barrier homeostasis, and is involved in regulation of pathways involved in proliferation and metabolism required for intestinal injury repair, an essential function for such a highly regenerative tissue (van der Flier & Clevers, 2009). HIF‐2α has been shown to promote the expression of creatine kinases, which are key metabolic enzymes required to maintain epithelial cell adherens junctions and so barrier integrity (Glover et al. 2013). HIF‐2α is also a positive regulator of vascular endothelial growth factor (VEGF) (Rankin et al. 2008), an angiogenic growth factor. However, in some cases, HIF‐2α has been reported to promote deleterious effects in IBD disease models (Xue et al. 2013). In a moderate DSS model of colitis (3% DSS in drinking water) HIF‐2α deletion was reported to be protective, which suggests that HIF‐2α is actively involved in the pathogenesis of the disease. In the same study, the previous finding was further supported by demonstration that overexpression of HIF‐2α in the intestinal epithelial cells exacerbated symptoms of DSS‐induced colitis and chronic overexpression could also induce spontaneous intestinal inflammation. Using immunohistochemical staining of human IBD patient biopsies and mouse colitis model tissue samples, a marked increase in HIF‐2α staining was observed to co‐localise with the epithelial specific marker E‐cadherin. Further evidence for the detrimental influence HIF‐2α has in IBD is that HIF‐2α promotes colorectal cancer progression (Xue et al. 2012) while HIF‐1α does not (Xue et al. 2014). Therefore, while most studies to date concur with HIF‐1 being protective in colitis, the role of HIF‐2α is less clear. Importantly, however, multiple studies to date investigating the effects of pharmacological HIF activation through pharmacological hydroxylase inhibition have shown both isoforms to be protective against mucosal inflammation.
Concluding remarks
Hypoxia is a microenvironmental feature of chronic mucosal inflammation which activates HIF‐dependent adaptive gene expression through the inhibition of PHD enzymes. Furthermore, multiple studies have now demonstrated that pharmacological hydroxylase inhibition is protective in multiple models of colitis. Notably, the PHD1 isoform is emerging as a key target in this protective response, although associated roles for PHD2 and PHD3 cannot be ruled out at this stage. Furthermore, while the HIF‐1 isoform is associated with protection against disease progression, the role of HIF‐2 is more ambiguous. Pharmacological hydroxylase inhibition has been recently introduced to clinical trials for the treatment of chronic kidney disease‐associated anaemia, and initial studies demonstrate that this approach is well tolerated by patients and the inhibitors provide clinical efficacy. Therefore the potential for re‐purposing hydroxylase inhibitors for the treatment of IBD is a realistic possibility. However, systemic administration of hydroxylase inhibitors raises the possibility of unwanted side effects in IBD patients such as erythropoeisis. Therefore, targeted delivery approaches are being developed which allow local administration of drugs to diseased areas of the intestine with minimal systemic exposure (Tambuwala et al. 2015).
In summary, tissue hypoxia is a powerful stimulus for inducing adaptive gene expression through the hypoxia‐inducible factor. Since the elucidation of the role of prolyl hydroxylases in the signalling mechanisms linking tissue hypoxia to HIF activation, the possibility of pharmacological interference with this pathway for therapeutic benefit has arisen. The years ahead will be an exciting time for the field of hypoxia‐related research as the clinical efficacy of this approach is tested in multiple disease models including (but not limited to) IBD.
Additional information
Competing interests
E.B. declares no competing interests. C.T.T. declares he is a member of the Scientific Advisory Board of Akebia Therapeutics.
Author contributions
E.B. and C.T.T. both contributed to the content, the writing, review and editing of this manuscript.
Funding
The laboratory of C.T.T. is funded by the following grants. ERACoSysMed (15/ERA‐CSM/3267) and Science Foundation Ireland (11/PI/1005).
Biographies
Eric Brown is a Postdoctoral Research Fellow at the School of Medicine and Medical Science, University College Dublin, Ireland. Current research being conducted in the laboratory of Cormac Taylor is investigating HIF pathway regulation in ulcerative colitis patients. Previous doctoral research, carried out at University College Dublin, investigated the influence of the immune system on serotonin signalling in the central nervous system.

Cormac Taylor holds an appointment as Professor of Cellular Physiology at the School of Medicine and Medical Science, Systems Biology Ireland and the Conway Institute, University College Dublin, Ireland. Current research in his laboratory is directed towards developing our understanding of the physiological and pathophysiological mechanisms by which changes in microenvironmental oxygen levels regulate gene transcription in eukaryotic cells. A key focus of this work is the identification of new therapeutic targets in inflammatory disease.
Edited by: Harold Schultz & David Grundy
This review was presented at the symposium ‘Advances in cellular and integrative control of oxygen and carbon dioxide homeostasis’, which took place at the XX ISAC meeting, Baltimore, MD, USA, 23–27 July 2017.
References
- Appelhoff RJ, Tian YM, Raval RR, Turley H, Harris AL, Pugh CW, Ratcliffe PJ & Gleadle JM (2004). Differential function of the prolyl hydroxylases PHD1, PHD2, and PHD3 in the regulation of hypoxia‐inducible factor. J Biol Chem 279, 38458–38465. [DOI] [PubMed] [Google Scholar]
- Axelrad JE, Lichtiger S & Yajnik V (2016). Inflammatory bowel disease and cancer: The role of inflammation, immunosuppression, and cancer treatment. World J Gastroenterol 22, 4794–4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannaga AS & Selinger CP (2015). Inflammatory bowel disease and anxiety: links, risks, and challenges faced. Clin Exp Gastroenterol 8, 111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins EP, Doherty GA & Taylor CT (2013). Hydroxylases as therapeutic targets in inflammatory bowel disease. Lab Invest 93, 378–383. [DOI] [PubMed] [Google Scholar]
- Cummins EP, Seeballuck F, Keely SJ, Mangan NE, Callanan JJ, Fallon PG & Taylor CT (2008). The hydroxylase inhibitor dimethyloxalylglycine is protective in a murine model of colitis. Gastroenterology 134, 156–165. [DOI] [PubMed] [Google Scholar]
- Erez N, Milyavsky M, Eilam R, Shats I, Goldfinger N & Rotter V (2003). Expression of prolyl‐hydroxylase‐1 (PHD1/EGLN2) suppresses hypoxia inducible factor‐1alpha activation and inhibits tumor growth. Cancer Res 63, 8777–8783. [PubMed] [Google Scholar]
- Fakhoury M, Negrulj R, Mooranian A & Al‐Salami H (2014). Inflammatory bowel disease: clinical aspects and treatments. J Inflamm Res 7, 113–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluck K, Breves G, Fandrey J & Winning S (2016). Hypoxia‐inducible factor 1 in dendritic cells is crucial for the activation of protective regulatory T cells in murine colitis. Mucosal Immunol 9, 379–390. [DOI] [PubMed] [Google Scholar]
- Glover LE, Bowers BE, Saeedi B, Ehrentraut SF, Campbell EL, Bayless AJ, Dobrinskikh E, Kendrick AA, Kelly CJ, Burgess A, Miller L, Kominsky DJ, Jedlicka P & Colgan SP (2013). Control of creatine metabolism by HIF is an endogenous mechanism of barrier regulation in colitis. Proc Natl Acad Sci USA 110, 19820–19825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindryckx P, De Vos M, Jacques P, Ferdinande L, Peeters H, Olievier K, Bogaert S, Brinkman B, Vandenabeele P, Elewaut D & Laukens D (2010). Hydroxylase inhibition abrogates TNF‐alpha‐induced intestinal epithelial damage by hypoxia‐inducible factor‐1‐dependent repression of FADD. J Immunol 185, 6306–6316. [DOI] [PubMed] [Google Scholar]
- Hirota SA, Fines K, Ng J, Traboulsi D, Lee J, Ihara E, Li Y, Willmore WG, Chung D, Scully MM, Louie T, Medlicott S, Lejeune M, Chadee K, Armstrong G, Colgan SP, Muruve DA, MacDonald JA & Beck PL (2010). Hypoxia‐inducible factor signaling provides protection in Clostridium difficile‐induced intestinal injury. Gastroenterology 139, 259–269.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu CJ, Sataur A, Wang L, Chen H & Simon MC (2007). The N‐terminal transactivation domain confers target gene specificity of hypoxia‐inducible factors HIF‐1α and HIF‐2α. Mol Biol Cell 18, 4528–4542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaelin WG Jr & Ratcliffe PJ (2008). Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell 30, 393–402. [DOI] [PubMed] [Google Scholar]
- Karhausen J, Furuta GT, Tomaszewski JE, Johnson RS, Colgan SP & Haase VH (2004). Epithelial hypoxia‐inducible factor‐1 is protective in murine experimental colitis. J Clin Invest 114, 1098–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keely S, Campbell EL, Baird AW, Hansbro PM, Shalwitz RA, Kotsakis A, McNamee EN, Eltzschig HK, Kominsky DJ & Colgan SP (2014). Contribution of epithelial innate immunity to systemic protection afforded by prolyl hydroxylase inhibition in murine colitis. Mucosal Immunol 7, 114–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith B, Johnson RS & Simon MC (2011). HIF1α and HIF2α: sibling rivalry in hypoxic tumor growth and progression. Nat Rev Cancer 12, 9–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly CJ, Glover LE, Campbell EL, Kominsky DJ, Ehrentraut SF, Bowers BE, Bayless AJ, Saeedi BJ & Colgan SP (2013). Fundamental role for HIF‐1alpha in constitutive expression of human beta defensin‐1. Mucosal Immunol 6, 1110–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marxsen JH, Stengel P, Doege K, Heikkinen P, Jokilehto T, Wagner T, Jelkmann W, Jaakkola P & Metzen E (2004). Hypoxia‐inducible factor‐1 (HIF‐1) promotes its degradation by induction of HIF‐α‐prolyl‐4‐hydroxylases. Biochem J 381, 761–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW & Kaplan GG (2012). Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 142, 46–54.e42; quiz e30. [DOI] [PubMed] [Google Scholar]
- Rankin EB, Rha J, Unger TL, Wu CH, Shutt HP, Johnson RS, Simon MC, Keith B & Haase VH (2008). Hypoxia‐inducible factor (HIF)‐2 regulates vascular tumorigenesis in mice. Oncogene 27, 5354–5358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson A, Keely S, Karhausen J, Gerich ME, Furuta GT & Colgan SP (2008). Mucosal protection by hypoxia‐inducible factor prolyl hydroxylase inhibition. Gastroenterology 134, 145–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza GL (2012). Hypoxia‐inducible factors in physiology and medicine. Cell 148, 399‐408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tambuwala MM, Cummins EP, Lenihan CR, Kiss J, Stauch M, Scholz CC, Fraisl P, Lasitschka F, Mollenhauer M, Saunders SP, Maxwell PH, Carmeliet P, Fallon PG, Schneider M & Taylor CT (2010). Loss of prolyl hydroxylase‐1 protects against colitis through reduced epithelial cell apoptosis and increased barrier function. Gastroenterology 139, 2093–2101. [DOI] [PubMed] [Google Scholar]
- Tambuwala MM, Manresa MC, Cummins EP, Aversa V, Coulter IS & Taylor CT (2015). Targeted delivery of the hydroxylase inhibitor DMOG provides enhanced efficacy with reduced systemic exposure in a murine model of colitis. J Control Release 217, 221–227. [DOI] [PubMed] [Google Scholar]
- Taylor CT & Colgan SP (2017). Regulation of immunity and inflammation by hypoxia in immunological niches. Nat Rev Immunol 17, 774–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian H, McKnight SL & Russell DW (1997). Endothelial PAS domain protein 1 (EPAS1), a transcription factor selectively expressed in endothelial cells. Genes Dev 11, 72–82. [DOI] [PubMed] [Google Scholar]
- van der Flier LG & Clevers H (2009). Stem cells, self‐renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol 71, 241–260. [DOI] [PubMed] [Google Scholar]
- Van Welden S, De Vos M, Wielockx B, Tavernier SJ, Dullaers M, Neyt S, Descamps B, Devisscher L, Devriese S, Van den Bossche L, Holvoet T, Baeyens A, Correale C, D'Alessio S, Vanhove C, De Vos F, Verhasselt B, Breier G, Lambrecht BN, Janssens S, Carmeliet P, Danese S, Elewaut D, Laukens D & Hindryckx P (2017). Haematopoietic prolyl hydroxylase‐1 deficiency promotes M2 macrophage polarization and is both necessary and sufficient to protect against experimental colitis. J Pathol 241, 547–558. [DOI] [PubMed] [Google Scholar]
- Van Welden S, Laukens D, Ferdinande L, De Vos M & Hindryckx P (2013). Differential expression of prolyl hydroxylase 1 in patients with ulcerative colitis versus patients with Crohn's disease/infectious colitis and healthy controls. J Inflamm (Lond) 10, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirtz S & Neurath MF (2007). Mouse models of inflammatory bowel disease. Adv Drug Deliv Rev 59, 1073–1083. [DOI] [PubMed] [Google Scholar]
- Wu P, Jia F, Zhang B & Zhang P (2017). Risk of cardiovascular disease in inflammatory bowel disease. Exp Ther Med 13, 395–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue X, Ramakrishnan S, Anderson E, Taylor M, Zimmermann EM, Spence JR, Huang S, Greenson JK & Shah YM (2013). Endothelial PAS domain protein 1 activates the inflammatory response in the intestinal epithelium to promote colitis in mice. Gastroenterology 145, 831–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue X, Ramakrishnan SK & Shah YM (2014). Activation of HIF‐1alpha does not increase intestinal tumorigenesis. Am J Physiol Gastrointest Liver Physiol 307, G187–G195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue X, Taylor M, Anderson E, Hao C, Qu A, Greenson JK, Zimmermann EM, Gonzalez FJ & Shah YM (2012). Hypoxia‐inducible factor‐2α activation promotes colorectal cancer progression by dysregulating iron homeostasis. Cancer Res 72, 2285–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]


