Abstract
Key points
Expiratory muscles (abdominal and thoracic) can be recruited when respiratory drive increases under conditions of increased respiratory demand such as hypercapnia.
Studying hypercapnia‐induced active expiration in unanaesthetized rats importantly contributes to the understanding of how the control system is integrated in vivo in freely moving animals.
In unanaesthetized rats, hypercapnia‐induced active expiration was not always recruited either in wakefulness or in sleep, suggesting that additional factors influence the recruitment of active expiration.
The pattern of abdominal muscle recruitment varied in a state‐dependent manner with active expiration being more predominant in the sleep state than in quiet wakefulness.
Pulmonary ventilation was enhanced in periods with active expiration compared to periods without it.
Abstract
Expiration is passive at rest but becomes active through recruitment of abdominal muscles under increased respiratory drive. Hypercapnia‐induced active expiration has not been well explored in unanaesthetized rats. We hypothesized that (i) CO2‐evoked active expiration is recruited in a state‐dependent manner, i.e. differently in sleep or wakefulness, and (ii) recruitment of active expiration enhances ventilation, hence having an important functional role in meeting metabolic demand. To test these hypotheses, Wistar rats (280–330 g) were implanted with electrodes for EEG and electromyography EMG of the neck, diaphragm (DIA) and abdominal (ABD) muscles. Active expiratory events were considered as rhythmic ABDEMG activity interposed to DIAEMG. Animals were exposed to room air followed by hypercapnia (7% CO2) with EEG, EMG and ventilation () recorded throughout the experimental protocol. No active expiration was observed during room air exposure. During hypercapnia, CO2‐evoked active expiration was predominantly recruited during non‐rapid eye movement sleep. Its increased occurrence during sleep was evidenced by the decreased DIA‐to‐ADB ratio (1:1 ratio means that each DIA event is followed by an ABD event, indicating a high occurrence of ABD activity). Moreover, was also enhanced (P < 0.05) in periods with active expiration. had a positive correlation (P < 0.05) with the peak amplitude of ABDEMG activity. The data demonstrate strongly that hypercapnia‐induced active expiration increases during sleep and provides an important functional role to support in conditions of increased respiratory demand.
Keywords: breathing control, EEG, EMG, expiratory activity, sleep, wakefulness
Key points
Expiratory muscles (abdominal and thoracic) can be recruited when respiratory drive increases under conditions of increased respiratory demand such as hypercapnia.
Studying hypercapnia‐induced active expiration in unanaesthetized rats importantly contributes to the understanding of how the control system is integrated in vivo in freely moving animals.
In unanaesthetized rats, hypercapnia‐induced active expiration was not always recruited either in wakefulness or in sleep, suggesting that additional factors influence the recruitment of active expiration.
The pattern of abdominal muscle recruitment varied in a state‐dependent manner with active expiration being more predominant in the sleep state than in quiet wakefulness.
Pulmonary ventilation was enhanced in periods with active expiration compared to periods without it.
Abbreviations
- ABDEMG
abdominal EMG
- DIAEMG and ABDEMG
diaphragm and abdominal EMG
- ∫DIAEMG and ∫ABDEMG
integrated DIAEMG and ABDEMG
- NeckEMG
neck muscle EMG
- NREM
non‐rapid eye movement sleep
- OSA
obstructive sleep apnoea
- Peak Amp
peak amplitude
- pFRG
parafacial respiratory group
- REM
rapid eye movement
- RF
respiratory frequency
- RTN
retrotrapezoid nucleus
- TE
expiratory time
ventilation
- TI
inspiratory time
- TTOT
total cycle duration
- VT
tidal volume
- VT/TI
mean inspiratory flow
Introduction
Respiration is a rhythmic process initiated spontaneously in the brainstem respiratory network. Under resting conditions expiration is passive, but expiratory muscles (abdominal and thoracic) can be recruited when respiratory drive increases under conditions such as hypercapnia, hypoxia or exercise (Iscoe, 1998; Iizuka, 2011; Feldman et al. 2013; Jenkin & Milsom, 2014). Recent evidence indicates that the parafacial respiratory group (pFRG), which overlaps to some extent with the retrotrapezoid nucleus (RTN), is a crucial brainstem site for generating active expiration (Janczewski et al. 2002; Mellen et al. 2003; Janczewski & Feldman, 2006; Huckstepp et al. 2015, 2016). Disinhibition of pFRG neurons and stimulation of the RTN/pFRG elicit active expiration in anaesthetized adult rats (Pagliardini et al. 2011), and the expiratory activity induced by hypercapnia is eliminated after chemical inhibition of the RTN/pFRG in in situ preparations (Abdala et al. 2009). This has led to the suggestion that the pre‐Bötzinger complex generates the inspiratory rhythm, while the pFRG generates the expiratory rhythm, and each functions as distinct, but coupled, oscillators (Huckstepp et al. 2016).
The central respiratory control system is strongly influenced by vigilance state (Nattie, 2001; Feldman et al. 2003; Kuwaki et al. 2010; Nattie & Li, 2010; Pagliardini et al. 2012). Burke et al. (2015) recently reported that hypercapnic ventilatory response is highly dependent on vigilance state in rats. Moreover, the activity of most respiratory muscles is reduced in sleep [rapid eye movement (REM) and non‐REM (NREM) sleep] (Sherrey & Megirian, 1977, 1980). The main inspiratory muscle, the diaphragm, is less affected but other accessory muscles exhibiting respiratory activity, such as the genioglossus, are strongly affected (Horner et al. 2002; Horner, 2008; Pagliardini et al. 2012). Interestingly, however, under eupnoeic conditions, where active expiration is absent normally, abdominal activity emerges in the REM‐like sleep state in urethane‐anaesthetized rats as well as in REM sleep, providing stability to breathing (Pagliardini et al. 2012; Andrews & Pagliardini, 2015). Evidence of hypercapnia‐induced expiratory abdominal activity in rats extends from in situ preparations (Abdala et al. 2009; de Britto & Moraes, 2016; Jenkin et al. 2017) to in vivo anaesthetized animals (Iizuka & Fregosi, 2007; Lemes & Zoccal, 2014), but whether active expiration occurs in unanaesthetized animals under hypercapnic conditions in a state‐dependent manner, i.e. differently in sleep and wakefulness, remains to be seen. Finally, active expiration has also been hypothesized to enhance ventilation by producing a more effective breathing pattern. In this context, the impact of hypercapnia‐induced abdominal expiratory activity on the functional aspects of ventilation across vigilance states has been poorly explored. The characterization of CO2‐induced active expiration in unanaesthetized animals may advance our understanding of a rhythmic respiratory‐related behaviour in freely moving animals that is essential to organismic homeostasis by enhancing sustained ventilation in conditions of increased respiratory demand.
Here we characterized active expiration in unanaesthetized, adult Wistar rats during hypercapnia in sleep and wakefulness. Moreover, we evaluated the functional impact of active expiration on the breathing pattern (balance of tidal volume and breathing frequency) in freely moving rats. To do so, combined measurements of EEG, EMG and ventilation (V E) were performed in unanaesthetized, freely moving adult rats. Our data support the view that hypercapnia‐induced active expiration occurs in a state‐dependent manner (in wakefulness and more so in NREM sleep) and improves pulmonary ventilation when recruited.
Methods
Ethical approval and animals
Animal handling and experimental protocols were carried out in compliance with the guidelines of the National Council for Animal Care (CONCEA: Conselho Nacional de Controle de Experimentação Animal) and approved by the local Animal Care and Use Committee (Protocol no. 019289/14) of Sao Paulo State University (UNESP/FCAV). Animals (adult male Wistar rats; 280–330 g) were provided by the animal facility of UNESP (Botucatu campus), and, after transportation to the Jaboticabal campus of UNESP, they were acclimatized to the animal facility for at least 7 days before any procedures were done. Animals were housed under a 12 h dark–light cycle (lights on at 07.00 h) and had free access to water and food. All experiments were performed in the light phase between 09.00 and 16.00 h at room temperature 24–25°C.
Surgical procedures
Electrodes for EMG and EEG recordings were implanted surgically. Bipolar intramuscular EMG electrodes were made of multi‐strand, coated stainless steel wire (A‐M Systems, Sequim, WA, USA) and EEG screw electrodes were commercially acquired (Plastics One Inc., Roanoke, VA, USA). Animals were anaesthetized with ketamine (100 mg kg−1; i.p.) and xylazine (15 mg kg−1; i.p.) to have the EMG and EEG electrodes implanted. For the insertion of EMG electrodes in the diaphragm (DIAEMG) and abdominal (ABDEMG) muscles, animals were placed in the supine position, and after local asepsis, a small horizontal incision was made in the lateral portion of the abdomen. For the ABDEMG, the oblique abdominal muscle was targeted and a pair of electrodes were inserted. On the opposite side, for the DIAEMG, a diagonal incision was made at the superior–lateral portion of the abdomen (from lateral to midline direction), following the ribcage border, from where the diaphragm muscle was accessed and electrodes were inserted. EMG electrode wires were tunnelled under the skin, and attached to an electrical socket secured between the animal's shoulder blades. For implanting the EEG electrodes and EMG electrodes in the neck muscle (NeckEMG), the head and dorsal region of the neck were shaved and animals were placed in a stereotaxic apparatus. Next, the local asepsis was performed (dorsal region of the neck and head) followed by a subcutaneous injection of local anaesthetic (3% lidocaine hydrochloride). A skin midline incision was performed and the skull exposed. Three EEG electrodes were then screwed into the skull (Nattie & Li, 2002; da Silva et al. 2010), as follows: (i) the frontal electrode was placed 2 mm anterior to the bregma and 2 mm lateral to the midline (right side), (ii) the parietal electrode was placed 4 mm anterior to lambda and 2 mm lateral to the midline (right side) and (iii) the ground electrode was placed between the frontal and parietal electrodes (on the left), forming a triangle. A pair of electrodes was inserted into the dorsal cervical neck muscle to record the NeckEMG. A plastic pedestal was used as an electrical socket and covered with a dust cap (Plastics One Inc.). The electrodes were mounted and fixed to the skull using acrylic resin. After surgery, the animals receive injections of antibiotic (enrofloxacin, Baytril; 10 mg kg−1 s.c., Bayer S.A., S.o Paulo, SP, Brazil) and analgesic (Flunixina Meglumina, Banamine; 2.5 mg kg−1 s.c., Schering‐Plough, Kenilworth, NJ, USA)
Physiological recordings
For recordings, an insulated and shielded cable was connected to the plugs/sockets on the animal's head and neck. The cable was attached to an electrical swivel, which permitted free movement of the animal. A custom‐made lid for the experimental chamber contained a hole through which the swivel was inserted and attached. From the swivel in the chamber lid a cable was connected to a four‐channel amplifier (A‐M Systems, model 1700). Signals were amplified (1000×), with 60 Hz notch filters, and band‐pass filtered (low and high cut‐off: 10 and 1000 Hz for EMG signals and 0.1 and 100 Hz for EEG signals, respectively). Signals were acquired (sample rate: 1 kHz) on a PowerLab (ADInstruments, Bella Vista, NSW, Australia) acquisition system and recorded using LabChart8 software. Online digital filtering was applied when necessary.
Ventilation () was obtained by using a well‐established barometric method (whole body plesthymography; for a detailed description see: Nattie & Li, 2002; da Silva et al. 2010, 2011, 2013). Briefly, before connecting the EEG and EMG wires, unanaesthetized rats were placed in the recording chamber and allowed to move freely (∼30 min) while the chamber was flushed with humidified air (1500 ml min−1). The inflow gas was controlled using a gas‐mixing pump. The flow rate through the animal chamber was maintained at 1500 ml min−1. The temperature inside the chamber was continuously monitored. The breathing‐related pressure oscillations were detected by a differential pressure transducer and amplified (MLT141 spirometer, Power Lab, ADInstruments). A volume calibration was performed by injecting a known air volume (1 ml) inside the chamber. Tidal volume (V T) was calculated using the formula described by Drorbaug & Fenn (1955) and Bartlett & Tenney (1970). was calculated as the product of V T and respiratory frequency (RF). From the trace, the inspiratory and expiratory time (T I and T E), total respiratory cycle duration (T TOT) and the mean inspiratory flow (V T/T I) were calculated.
Experimental protocol
Experiments were performed a minimum of 8 days after the surgical procedures. All experiments began at 09.00 h (light phase) and were carried out at room temperature (24–25°C). Animals were placed in a cylindrical plethysmograph chamber (5 litres) and allowed to acclimatize for 30 min before connecting EEG and EMG wires. During this period the chamber was flushed with room air at a flow rate of 1500 ml min−1. Then, small wires for recording EMG and EEG signals were connected to the implanted wires in the animal and connected to an electrical swivel in the internal face of the lid, and from the external face of the lid to the amplifiers (A‐M Systems, model 1700). The use of a swivel was critical to keep the animal undisturbed and the wires untangled throughout the experimental protocol, and this wire arrangement allowed the animal to move freely inside the chamber. With all cables in place, further time (∼30 min) was given before starting to record signals. Room air was continuously flushed during the whole period of adaption. After that, EEG and EMG signals were recorded during room air exposure for a time interval of usually 40–60 min that yielded recording signals in different sleep–wake cycles. The gas was then switched to a hypercarbic gas mixture (7% CO2, 21% O2, N2 balance) provided by a gas mixing system (GSM‐3 Gas Mixer; CWE Inc., Ardmore, PA, USA) at a flow rate of 1500 ml min−1. Animals were exposed to hypercapnia for 60 min, and physiological parameters described above were measured throughout the time of exposure. After the experiments all animals were killed with an overdose of anaesthesia.
Data and statistical analyses
Data were collected electronically for subsequent analyses. The following criteria were applied for data analysis: all periods containing artifacts of animals’ movements, exploratory behaviours and grooming were excluded from analysis, whereas periods of quiet wakefulness and sleep were all analysed. EEG and NeckEMG recordings were used to define sleep or wakefulness states (Nattie & Li, 2002; da Silva et al. 2010). The EMGs from respiratory muscles were integrated (0.05 s), and the muscle activity was calculated as the peak amplitude (Peak Amp) of the integrated DIAEMG (∫DIAEMG) and ABDEMG (∫ABDEMG), respectively. Event detection and Peak Amp analysis were performed on Clampfit software on a peak‐to‐peak basis during different alert states (sleep and wakefulness). The detection and selection of ∫DIAEMG and ∫ABDEMG during the data analysis were all performed within the exact time window for each signal. This allowed the assessment of DIA‐to‐ABD ratio within a given time window of the trace. This ratio expresses the occurrence of ABD events in relation to the DIA. A 1:1 ratio indicates a high occurrence of ABD activity (each DIA event is followed by an ABD event). Hence, the lower the ratio, the higher the occurrence of active expiration. All data were exported and further processed in an Excel spreadsheet where the ∫DIAEMG and ∫ABDEMG data were normalized to their largest integrated (∫) EMG Peak Amp obtained during the wakefulness state for each muscle. The normalized Peak Amp values were used for comparisons. For the purpose of analysis, active expiration was defined by the presence of ABDEMG expiratory activity, i.e. rhythmic burst of ABDEMG activity (between inspiratory DIAEMG bursts) above tonic levels (Pagliardini et al. 2011; Huckstepp et al. 2015). Data were analysed in a state‐dependent manner, separated into two states – quiet wakefulness and non‐REM sleep – both observed consistently throughout the experimental protocol both during room air and hypercapnia exposures. REM sleep periods were very short and in most of the experiments were not present. Hence, all analysis during sleep is reported for NREM sleep. Arousal states were determined based on EEG and neck EMG and categorized as previously described (Nattie & Li, 2001, 2002; Taylor et al. 2006; da Silva et al. 2010). All active expiration events (according to the criteria) in each animal data set were averaged. Distributions (histograms) of the ∫ABDEMG Peak Amp (absolute number of events) were determined individually for each rat in different states during hypercapnia, and these data were also reported in box plots. For ventilatory variables, the analyses during hypercapnia were run in periods with or without active expiration in a state‐dependent manner. Data were expressed as mean ± SEM. Student's t tests and two‐way ANOVA for repeated measures followed by post hoc tests were performed accordingly. A Pearson test was used for correlation analysis. P was set at 0.05 to indicate a significant difference.
Results
Room air
EEG and NeckEMG recordings allowed identification of sleep–wake states. Figure 1 A shows a representative spectrogram, EEG and NeckEMG (top, middle and bottom, respectively) of an animal during room air and hypercapnia exposure. During room air exposure, the percentage of time spent in wakefulness or in NREM sleep in the group data averaged 33.7 and 63.3%, respectively. Figure 1 B and C depict the recorded physiological variables during room air exposure in both wakefulness and sleep. The mean breathing frequency measured by ∫DIAEMG events was 101 ± 5 and 86 ± 4 cycles min−1 in wakefulness and sleep (n = 8), respectively. As for the ABDEMG activity, regardless of the state, no active expiration was observed during room air exposure (n = 8), i.e. no rhythmic expiratory ABDEMG activity was present, either in wakefulness (Fig. 1 B) or in sleep (Fig. 1 C). The absence of active expiration was consistent among animals breathing room air.
Figure 1. State‐dependent activity of abdominal and diaphragm activity during room air and hypercapnia exposure.
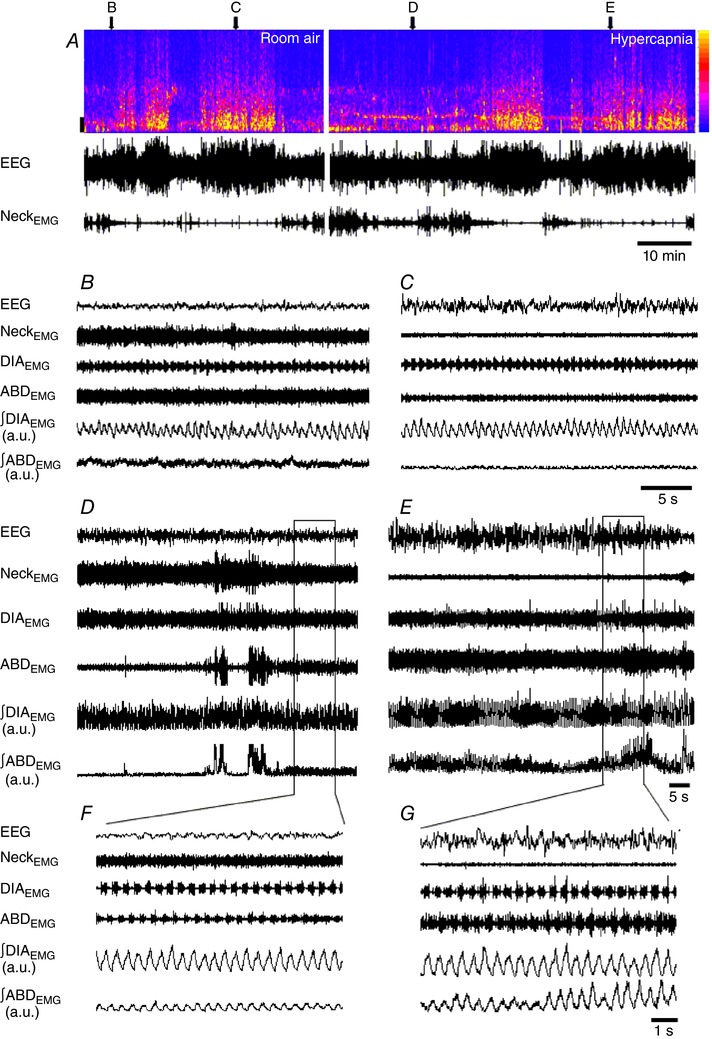
A, EEG spectrogram, EEG and NeckEMG raw recordings (top, middle and bottom, respectively) of a representative animal during a typical experimental protocol in room air (left) and hypercapnia exposure (right). In the EEG spectrogram, the colour intensity, as indicated by the colour calibration bar to the right side, corresponds to the amount of signal power at a given time and frequency. On the left side, frequency calibration bar: 2 Hz. Note that the animal cycles between wake and sleep in both conditions: room air and hypercapnia. Above the spectrogram, the letters and arrows indicate for each of the respective panels where their traces were withdrawn and expanded. The expanded EEG and EMGs [raw and integrated abdominal (∫ABDEMG) and diaphragm (∫DIAEMG) signals] during room air are shown in periods of wakefulness (B) and sleep (C), and during hypercapnia in wakefulness (D) and sleep (E). The horizontal rectangles in D and E depict the expanded trace shown in F and G, respectively. Note the absence of active expiration during room air (B and C) and its recruitment during hypercapnia (D–G). [Color figure can be viewed at http://wileyonlinelibrary.com]
Hypercapnia exposure
During hypercapnia exposure (7% CO2) the percentage of time in wakefulness or in NREM sleep averaged 55.8 and 44.5%, respectively. The hypercapnia‐induced decrease in sleep time was the result of a longer time spent in wakefulness. These effects of hypercapnia in the arousal state have been well documented in the literature (Taylor et al. 2006; Buchanan & Richerson, 2010; Dias et al. 2010; Buchanan et al. 2015; Vicente et al. 2016). The recruitment of abdominal muscles occurred during exposure to CO2 (Fig. 1 D–G). Hypercapnia‐induced active expiration was not present during the entire period of CO2 exposure. Figure 1 D is an example showing that during hypercapnia in a wakefulness period active expiration was not present initially, but that expiratory ABDEMG activity emerged after a brief period of movement. The ∫ABDEMG Peak Amp associated with abdominal expiratory activity during wakefulness was relatively constant (Fig. 1 F). During sleeping periods, however, active expiration was more consistent, and the ∫ABDEMG Peak Amp associated with expiratory ABDEMG activity was often highly variable (Fig. 1 E and G). This general pattern was also observed in other animals (Figs 2 and 3). It is noteworthy that 1 out of 9 rats recorded in the present study did not show any active expiration while awake, but only while sleeping.
Figure 2. Distribution of active expiration (A.E.) events (∫ABDEMG Peak Amp) during hypercapnia in wakefulness and sleep.
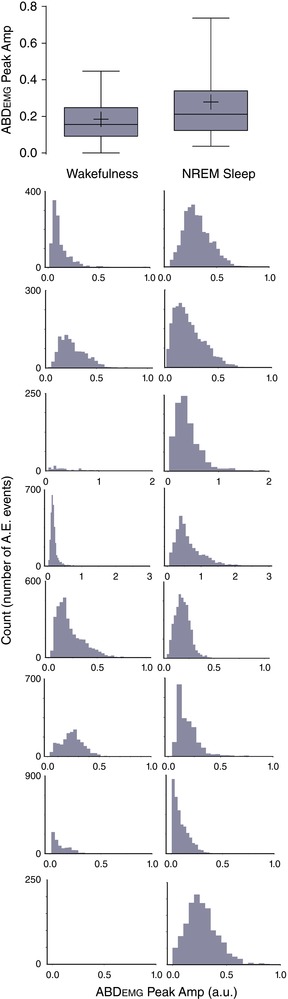
Top panel shows the group data as box plots in which the centre line shows the median; box limits indicate the 25th and 75th percentiles; whiskers extend to 5th and 95th percentiles; and crosses represent sample means. Each histogram (row) represents the distribution for one individual animal in both vigilance states: wakefulness (left column) and sleep (right column). Note that one animal (bottom row) did not present active expiration in wakefulness, but only in sleep. [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 3. Respiratory muscle EMG and breathing frequency during hypercapnia in different vigilance states: wakefulness and sleep.
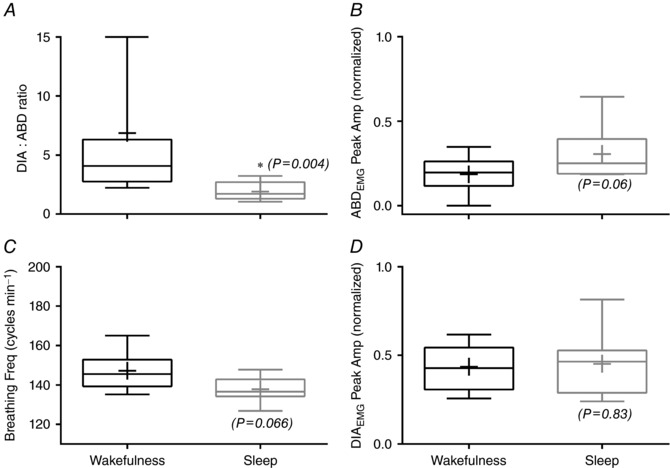
Box plots: centre line shows the median; box limits indicate the 25th and 75th percentiles; whiskers extend to 5th and 95th percentiles; and crosses represent sample means. The panels show the DIA‐to‐ADB ratio (A), ∫ABDEMG Peak Amp (B), breathing frequency (C) and ∫DIAEMG Peak Amp (D) (n = 8). The P values are indicated for the sleep data set compared to wakefulness. *Difference (P < 0.05) of sleep state compared to wakefulness state.
Figure 2 illustrates the group data (box plot) and the distribution (histograms) of ∫ABDEMG Peak Amp (i.e. active expiration events) for each individual animal in different states. The box plot (Fig. 2, top) shows an apparent larger variability of ∫ABDEMG data during sleep. The greater variability during sleep is confirmed by the coefficient of variation (CV) of ∫ABDEMG Peak Amp, which was 96.4 and 65% during sleep and wakefulness, respectively. The ∫DIAEMG had lower variability compared to abdominal activity and was not influenced by vigilance state (the CV of ∫DIAEMG was 35.5 and 28.8% during sleep and quiet wakefulness, respectively). Moreover, the histograms demonstrate that, for most animals, the number of events of active expiration as well as the number of a given ∫ABDEMG Peak Amp tended to be greater during sleep (right column, Fig. 2) when compared to wakefulness (left column, Fig. 2).
The DIA‐to‐ADB ratio, ∫ABDEMG Peak Amp, ∫DIAEMG Peak Amp and breathing frequency during hypercapnia are presented in Fig. 3. The increased occurrence of active expiration during sleep is evidenced by the decreased DIA‐to‐ADB ratio (a 1:1 ratio means that each DIA is followed by an ABD event, indicating a high occurrence of ABD activity). Figure 3 A shows that this ratio decreased during sleep (∼2:1) compared to wakefulness (∼7:1) (mean values: 1.92 ± 0.2 vs. 6.87 ± 3; wakefulness and sleep, respectively; P = 0.02, n = 8). In Fig. 3 B, the amplitude of ∫ABDEMG was 33.2% larger during sleep when compared to wakefulness (0.205 ± 0.02 vs. 0.307 ± 0.05; P = 0.06, n = 8; normalized Peak Amp for wakefulness and sleep, respectively) (Fig. 3 B). Breathing frequency had a ∼6% reduction (147 ± 5 vs. 138 ± 2 cycles min−1, P = 0.066, n = 8) and ∫DIAEMG Peak Amp (0.43 ± 0.4 vs. 0.45 ± 0.06, n = 8) was not different between wakefulness and sleep, respectively (Fig. 3 C and D).
Active expiration and assessment of ventilatory variables
Hypercapnia consistently recruited active expiration, but periods in which active expiration failed to occur were also observed (Fig. 1). To assess the functional role of active expiration during hypercapnia in unanaesthetized freely moving rats, ventilatory measurements were recorded along with EEG and NeckEMG, DIAEMG and ABDEMG. First, ventilatory variables were assessed in the trace periods with active expiration and compared to the same variables in periods without active expiration. Figure 4 shows the relative change of the ventilatory variables analysed separately in periods with or without active expiration. The ventilatory response to hypercapnia was enhanced by active expiration. V T, RF and were higher (P = 0.04, P = 0.026, P = 0.003, n = 7; respectively) when active expiration was present, and this effect was not dependent on sleep–wake cycle. T I was not significantly affected by the presence of active expiration, whereas T E and T TOT decreased (P = 0.02 and P = 0.011, respectively) in periods where active expiration was present. Therefore, in unanaesthetized rats during hypercapnia, whenever present, active expiration enhanced through both increased V T and RF; the reduction in T TOT was mainly achieved by a diminished T E. These effects were independent of vigilance state.
Figure 4. Comparison of ventilatory variables during hypercapnia in periods with or without recruitment of active expiration.
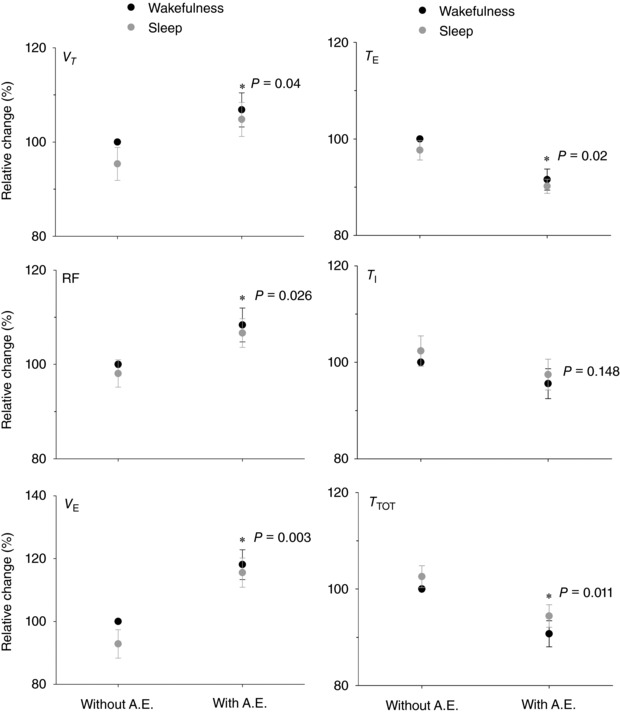
V T and T E top panels, RF and T I middle panels, and VE and T TOT bottom panels. (*) indicates difference (P < 0.05) comparing the ventilatory variables with and without active expiration (A.E.) (n = 7). The P values are indicated.
Figure 5 shows a representative trace of EEG, NeckEMG, DIAEMG and ABDEMG recorded across a sleep–wake cycle during hypercapnia. Note that during the natural transition from sleep to wakefulness there was increasing ABDEMG activity (also seen in Fig. 1 E and G) within the sleeping period, but ABDEMG rhythmic activity ceased completely as soon as the animal woke up. As mentioned earlier, the ∫ABDEMG Peak Amp associated with abdominal expiratory activity during wakefulness was relatively constant (see Fig. 1 F), whereas this type of increasing ∫ABDEMG Peak Amp occurred more during sleep (see Figs 1 E and G and 5).
Figure 5. Functional impact of active expiration with increasing ABDEMG activity on ventilatory variables during hypercapnia.
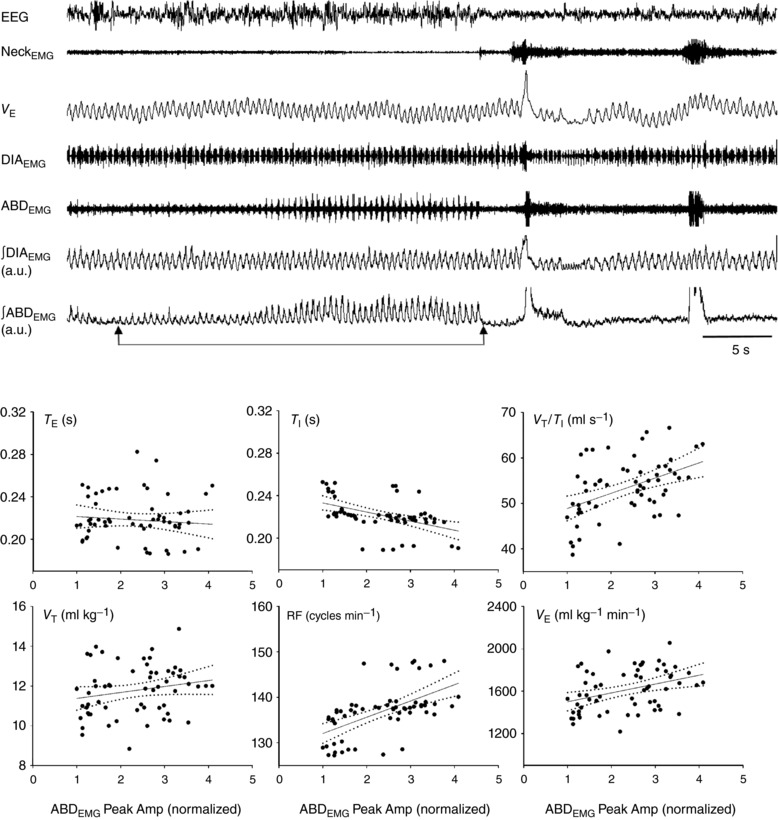
Top traces depict EEG, NeckEMG, barometric V E recording and raw and integrated DIAEMG and ABDEMG. Two arrows below the ∫ABDEMG trace show the interval time with increasing ABDEMG activity from which correlation analyses between active expiration (∫ABDEMG Peak Amp) and ventilation were performed. A breath‐by‐breath analysis was run in 60 events. Scatter plots show the correlation between increasing ∫ABDEMG Peak Amp and T E, T I and V T/T I (top row) and V T, RF and V E (bottom row).
The ventilatory variables were measured breath‐by‐breath over this recording to assess the overall functional impact of the increasing expiratory activity on . A breath‐by‐breath analysis was run by taking each breath and the corresponding ∫ABDEMG Peak Amp (representative trace and scatter plots, Fig. 5). The ∫ABDEMG Peak Amp was positively correlated with RF (r = 0.56, P < 0.001), (r = 0.37, P = 0.003) and V T/T I (r = 0.45, P < 0.001) and negatively correlated with T I (r = −0.47, P < 0.001), while no significant correlation was observed with T E and V T (P = 0.5 and P = 0.11, respectively). Therefore, once active expiration was present, the increasing ABDEMG activity improved through inspiratory actions, mainly due to increases in RF (decrease T I) and V T [augmented mean inspiratory flow (V T/T I)].
Discussion
In the present study, while no active expiration was observed in unanaesthetized animals breathing room air (regardless of sleep–wake state), abdominal muscle expiratory activity was recruited during hypercapnia exposure with an increased occurrence during NREM sleep compared to wakefulness. Moreover, whenever present, active expiration was associated with an increase in compared to periods without it.
Active expiration has gained attention recently arising from data indicating the existence of a brainstem conditional expiratory oscillator, the pFRG, distinct from (but coupled to) the inspiratory oscillator, the pre‐Bötzinger complex (Pagliardini et al. 2011; Huckstepp et al. 2015, 2016). Under eupnoeic conditions (rats breathing room air), urethane‐anaesthetized rats increased abdominal activity switching from tonic to expiratory rhythmic activity (i.e. active expiration) with the transition from NREM to REM‐like sleep (Pagliardini et al. 2012). This has also been reported to occur during natural REM sleep in unanaesthetized rats (Andrews & Pagliardini, 2015). In the present study, we did not observe active expiration (expiratory ABDEMG activity) during sleep in animals breathing room air. The source of this difference is not clear. However, in the urethane‐anaesthetized rats, in ∼46% of recordings (6 out of 13) ABDEMG activity was tonic (no active expiration) and did not change across sleep–wake cycle (Pagliardini et al. 2012). Likewise, in unanaesthetized rats in room air, events of active expiration (that lasted for more than three consecutive breaths as defined by the authors) occurred in only ∼49% of REM epochs (147 out of 300 REM epochs) (Andrews & Pagliardini, 2015). The amount of REM sleep seen in our study was small, suggesting that long‐lasting recording experiments are needed to yield sufficient REM episodes to accurately examine the recurrent activation of ABDEMG activity.
In the present study, abdominal oblique muscles were targeted as representative of hypercapnia‐induced expiratory abdominal activity. Abdominal muscles are innervated by branches from the ventral rami of intercostal nerves of thoracic segments and from lumbar segments, and there is a variable overlap of the abdominal motoneurons in regions of the ventral horn (Iscoe, 1998). The rectus abdominis has little or no respiratory‐related activity in humans and conscious rats (Abe et al. 1996; Iscoe, 1998). In contrast, and in agreement with our data, a consistent response of the abdominal obliques to hypercapnia has been reported in both humans and rats (Jeffries et al. 1984; Takasaki et al. 1989; Abe et al. 1996; Iscoe, 1998; Iizuka, 2011; Pagliardini et al. 2012; Andrews & Pagliardini, 2015).
An increased respiratory drive leads to emergence of expiratory abdominal activity in humans (Badr et al. 1990; Wakai et al. 1992; Abe et al. 1996), dogs (Oliven et al. 1985; Smith et al. 1989, 1990) and rodents (Sherrey et al. 1988; Iizuka & Fregosi, 2007; Lemes & Zoccal, 2014). In rats, evidence of hypercapnia‐induced expiratory ABDEMG activity extends from in situ preparations (Abdala et al. 2009; de Britto & Moraes, 2016; Jenkin et al. 2017) to in vivo anaesthetized animals (Iizuka & Fregosi, 2007; Lemes & Zoccal, 2014). Fewer studies, however, have examined its occurrence in unanaesthetized rats in different vigilance states. In agreement with the literature, we reported that the CO2 drive to breathe (hypercapnia exposure) recruits active expiration in unanaesthetized adult rats. Two observations are noteworthy: (i) in unanaesthetized rats, the expiratory ABDEMG activity was not always recruited during exposure to hypercapnia either in wakefulness or in sleep, suggesting that other factors also influence the recruitment of active expiration; and (ii) the pattern of this recruitment varied in a state‐dependent manner with active expiration being more predominant in NREM sleep state compared to quiet wakefulness.
The observation (i) that active expiration failed to be consistently recruited by hypercapnia in both sleeping and awake rats is consistent with the observation that active expiration was not consistently recruited in REM sleep (Andrews & Pagliardini, 2015). It seems that factors other than levels of chemical drive and state influence abdominal expiratory activity. Some evidence suggests that the posture of the animals may affect the activity of ABDEMG. Although Sherrey et al. (1988) reported that hypercapnia elicited active expiration in rats regardless of posture, when the rat curled its body oppositely from the side of the recording electrodes, the ABDEMG activity was large and decreased to a minimum when the rat was curled toward the electrodes. In humans, body position is also suggested to play a role in the emergence of active expiration, as the abdominal recruitment elicited by increased inspiratory resistance was seen in upright but not supine subjects (Martin & De Troyer, 1982; Badr et al. 1990). Moreover, De Troyer et al. (1989) suggested that, in conscious dogs, proprioceptive inputs from the abdominal wall could promote or modulate ABD activity during expiration. To our knowledge, how the proprioceptive components act to inhibit or facilitate the emergence of active expiration remains uncertain. It is worth mentioning that, in unanaesthetized, freely moving rats, active expiration seems to be a complex respiratory motor behaviour influenced by different components (i.e. chemical drive, state and proprioception); however, how these are computed in the brainstem expiratory generating network to finally express active expiration remains unknown.
The underlying mechanism of the second observation (ii), that active expiration is more predominant in NREM sleep, is also elusive. Recent evidence supports the role of neurons (different from the RTN Phox2b neurons) in the pFRG as the brainstem site that generates active expiration (Huckstepp et al. 2015, 2016). Burke et al. (2015) reported that RTN stimulation caused active expiration only when the rats were awake. In contrast, other reports in the literature showed that active expiration is recruited during sleep in rats and humans (Pagliardini et al. 2012; Andrews & Pagliardini, 2015; and in our present study). The fact that RTN neurons drive active expiration only during wakefulness suggests that different mechanisms to recruit active expiration may be involved during sleep. Alternatively, as suggested by Burke et al. active expiration could yet be triggered in NREM sleep by stimulating a larger fraction of RTN neurons. Serotoninergic (Lemes et al. 2016) and cholinergic (Boutin et al. 2017) neurotransmission in the RTN/pFRG have now been reported to induce active expiration in anaesthetized rats. Disinhibition of these neurons has been shown to underlie the recruitment of active expiration (Pagliardini et al. 2011) and it is quite possible that this disinhibition is influenced by vigilance state. The modulation of pFRG neurons across state as well as the state‐dependent involvement of neurotransmitters is currently not well known. It has also been hypothesized that the activity of different brainstem CO2 chemoreceptor sites varies in a state‐dependent manner (Nattie, 2000; da Silva et al. 2010; Nattie & Li, 2010), and hence it is also possible that the different CO2‐sensitive cell types input into and modulate pFRG neurons to generate active expiration differently in sleep and wakefulness.
The state‐dependent enhancement of CO2‐induced active expiration may also be related to a change in peripheral airway resistance. In humans during NREM sleep, expiratory abdominal muscles are recruited by sustained inspiratory resistive loading (Badr et al. 1990; Henke et al. 1991), obstructive sleep apnoea (Jeffries et al. 1984; Praud et al. 1989) and acute bronchospasm in asthmatics (Issa & Sullivan, 1985), and an interaction of increased airway resistance and chemoreceptor stimuli have been suggested as necessary to recruit abdominal muscles during NREM sleep (Badr et al. 1990; Henke et al. 1992). This suggestion emphasizes the importance of studying active expiration in hypercapnic conditions. In rodents, it has been shown that the activity of airway muscles such as the cricothyroid, nasolabial (Sherrey & Megirian, 1977) and genioglossal muscles (Horner, 2008) changes across state, which implies changes in airway resistance. In the present study, we did not record from other airway respiratory muscles, but it remains possible that the increase in ABDEMG activity during NREM sleep (Fig. 1 E and F) was related to fluctuation of airway resistance during sleep.
Another important aspect of the present study is the description of the functional impact of active expiration on the ventilatory variables. We demonstrate here that, regardless of vigilance state, the presence of active expiration elicited an enhancement of ventilation achieved by increases in both V T and RF (with decrease in T E and T TOT; Fig. 4). This is in agreement with the notion that active expiration can increase V T by recruiting the expiratory reserve volume and increase RF by shortening the expiratory time constant (shorter than the passive time constant of the system) with a decreased T E and T TOT (Jenkin & Milsom, 2014). In humans, the main mechanical consequence of abdominal recruitment, specifically during sustained respiratory loading, was a shortened T E and increased RF (Badr et al. 1990; Henke et al. 1991).
When active expiration was already present and the ABDEMG activity was recruited further in a progressive manner, this was achieved mainly by an increase in RF (i.e. with diminished T I) and increased mean inspiratory flow (V T/T I), although inspiratory DIAEMG activity did not increase to support the increased V T/T I. The lack of further change in V T could reflect maximum recruitment of the expiratory reserve volume. At this point further recruitment of the expiratory muscles can only serve to produce complete exhalation within the same T E. Associated with this may be changes in compliance or resistance (lengthening tau, the passive time constant) reflecting state‐dependent changes in airway muscle activity (and hence resistance). Also, ABDEMG activity could place the diaphragm at a better length for generating tension and improving mechanical output during inspiration. Indeed, these mechanical consequences of expiratory muscle recruitment have been reported during transient events in sleeping humans (Henke et al. 1991, 1992). The observed effect of increasing ABDEMG activity on inspiration (increased V T/T I and decreased T I) could also be associated with a parallel increase in activity of other inspiratory accessory muscles that were not recorded in the present study.
The respiratory control system is dedicated to maintaining the rhythmic activity of respiratory muscles via respiratory motor output in a coordinated fashion. Their rhythmic activities have to ultimately match metabolic demand and maintain organismic homeostasis of blood gases. Using a simple, yet elegant approach, the present study brings fundamental knowledge about control of breathing through the following integrative perspectives on active expiration: the brainstem expiratory generating network, which is influenced by sleep state (although the underlying mechanisms remain to be further explored), and, simultaneously, active expiration enables optimal ventilatory strategies during ventilatory challenge, further supporting important physiological function. By showing the state dependence of the hypercapnia‐induced active expiration, we also have another (patho)physiological aspect. The hypercapnia exposure used in the present study elicits a ∼15 mmHg rise in arterial , reaching values of about 50 mmHg (for detailed values see da Silva et al. 2011). A rise in blood CO2 can be life threatening, and hypercapnia is a significant factor in diseases such as obstructive sleep apnoeas (OSAs) with consequent variable degrees of morbidity (and mortality). For example, an increased occurrence of active expiration during sleep was reported in patients with OSA, compared to subjects without OSA (Jeffries et al. 1984). Hypercapnia was present in patients with OSA and some of them who had significant blood gas abnormalities, i.e. values above 50 mmHg, presented the highest ABD expiratory activity during sleep (Jeffries et al. 1984). Therefore, appropriate respiratory motor outputs as well as ventilatory adjustments to hypercapnia are highly desired.
In conclusion, the present study in unanaesthetized adult rats indicates that while no active expiration is present under normocapnic conditions in any vigilance state, ABDEMG expiratory activity is recruited during hypercapnia predominantly during NREM sleep. We also show that active expiration is associated with improved ventilation achieved by both increased V T and RF, with decreased T E and T TOT.
Additional information
Competing interests
The authors declare no competing interests.
Author contributions
I.P.L. and C.A.S. contributed equally to acquisition, analysis, interpretation of data and manuscript revision; L.H.G. contributed to the design of the work and manuscript revision; G.S.F.S contributed to acquisition, analysis and interpretation of data, conception, study design, drafting and manuscript revision. All authors revised and approved the final version of the manuscript.
Funding
This study was funded by Sao Paulo Research Foundation (FAPESP): Young Investigator Award (Grant nos. 2013/17606‐9 and 2014/12190‐1) and undergraduate scholarships (2016/11061‐9 and 2015/04926‐0).
Acknowledgments
We thank Dr Bill Milsom and Dr Daniel Zoccal for kindly reading the manuscript, for suggesting improvements, and for their insightful discussions and comments. We would also like to thank Dr Lynn Hartzler for her kind help with grammatical corrections in the final version.
I. P. Leirão and C. A. Silva contributed equally to this study.
Linked articles This article is highlighted by a Perspective by O'Halloran. To read this Perspective, visit https://doi.org/10.1113/JP275056.
References
- Abdala AP, Rybak IA, Smith JC & Paton JFR (2009). Abdominal expiratory activity in the rat brainstem–spinal cord in situ: patterns, origins and implications for respiratory rhythm generation. J Physiol 587, 3539–3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe T, Kusuhara N, Yoshimura N, Tomita T & Easton PA (1996). Differential respiratory activity of four abdominal muscles in humans. J Appl Physiol 80, 1379–1389. [DOI] [PubMed] [Google Scholar]
- Andrews CG & Pagliardini S (2015). Expiratory activation of abdominal muscle is associated with improved respiratory stability and an increase in minute ventilation in REM epochs of adult rats. J Appl Physiol 119, 968–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badr MS, Skatrud JB, Dempsey JA & Begle RL (1990). Effect of mechanical loading on expiratory and inspiratory muscle activity during NREM sleep. J Appl Physiol 68, 1195–1202. [DOI] [PubMed] [Google Scholar]
- Bartlett D & Tenney SM (1970). Control of breathing in experimental anemia. Respir Physiol 10, 384–395. [DOI] [PubMed] [Google Scholar]
- Boutin RCT, Alsahafi Z & Pagliardini S (2017). Cholinergic modulation of the parafacial respiratory group. J Physiol 595, 1377–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Britto AA & Moraes DJA (2016). Non‐chemosensitive parafacial neurons simultaneously regulate active expiration and airway patency under hypercapnia in rats. J Physiol 6, 2043–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan GF & Richerson GB (2010). Central serotonin neurons are required for arousal to CO2 . Proc Natl Acad Sci USA 107, 16354–16359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan GF, Smith HR, MacAskill A & Richerson GB (2015). 5‐HT2A receptor activation is necessary for CO2‐induced arousal. J Neurophysiol 114, 233–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke PGR, Kanbar R, Basting TM, Hodges WM, Viar KE, Stornetta RL & Guyenet PG (2015). State‐dependent control of breathing by the retrotrapezoid nucleus. J Physiol 593, 2909–2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias MB, Li A & Nattie E (2010). The orexin receptor 1 (OX1R) in the rostral medullary raphe contributes to the hypercapnic chemoreflex in wakefulness, during the active period of the diurnal cycle. Respir Physiol Neurobiol 170, 96–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drorbaug JE & Fenn WO (1955). A barometric method for measuring ventilation in newborn infants. Pediatrics 16, 81–87. [PubMed] [Google Scholar]
- Feldman JL, Mitchell GS & Nattie EE (2003). Breathing: rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci 26, 239–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman JL, Del Negro CA & Gray PA (2013). Understanding the rhythm of breathing: so near, yet so far. Annu Rev Physiol 75, 423–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henke KG, Badr MS, Skatrud JB & Dempsey JA (1992). Load compensation and respiratory muscle function during sleep. J Appl Physiol 72, 1221–1234. [DOI] [PubMed] [Google Scholar]
- Henke KG, Dempsey JA, Badr MS, Kowitz JM & Skatrud JB (1991). Effect of sleep‐induced increases in upper airway resistance on respiratory muscle activity. J Appl Physiol 70, 158–168. [DOI] [PubMed] [Google Scholar]
- Horner RL (2008). Neuromodulation of hypoglossal motoneurons during sleep. Respir Physiol Neurobiol 164, 179–196. [DOI] [PubMed] [Google Scholar]
- Horner RL, Liu X, Gill H, Nolan P, Liu H & Sood S (2002). Effects of sleep–wake state on the genioglossus vs.diaphragm muscle response to CO2 in rats. J Appl Physiol 92, 878–887. [DOI] [PubMed] [Google Scholar]
- Huckstepp RTR, Cardoza KP, Henderson LE & Feldman JL (2015). Role of parafacial nuclei in control of breathing in adult rats. J Neurosci 35, 1052–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huckstepp RTR, Henderson LE, Cardoza KP & Feldman JL (2016). Interactions between respiratory oscillators in adult rats. Elife 5, 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iizuka M (2011). Respiration‐related control of abdominal motoneurons. Respir Physiol Neurobiol 179, 80–88. [DOI] [PubMed] [Google Scholar]
- Iizuka M & Fregosi RF (2007). Influence of hypercapnic acidosis and hypoxia on abdominal expiratory nerve activity in the rat. Respir Physiol Neurobiol 157, 196–205. [DOI] [PubMed] [Google Scholar]
- Iscoe S (1998). Control of abdominal muscles. Prog Neurobiol 56, 433–506. [DOI] [PubMed] [Google Scholar]
- Issa FG & Sullivan CE (1985). Respiratory muscle activity and thoracoabdominal motion during acute episodes of asthma during sleep. Am Rev Respir Dis 132, 999–1004. [DOI] [PubMed] [Google Scholar]
- Janczewski WA & Feldman JL (2006). Distinct rhythm generators for inspiration and expiration in the juvenile rat. J Physiol 570, 407–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janczewski WA, Onimaru H, Homma I & Feldman JL (2002). Opioid‐resistant respiratory pathway from the preinspiratory neurones to abdominal muscles: in vivo and in vitro study in the newborn rat. J Physiol 545, 1017–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffries B, Brouillette RT & Hunt CE (1984). Electromyographic study of some accessory muscles of respiration in children with obstructive sleep apnea. Am Rev Respir Dis 129, 696–702. [DOI] [PubMed] [Google Scholar]
- Jenkin SEM & Milsom WK (2014). Expiration: Breathing's other face, 1st edn Elsevier, Amsterdam; Available at: 10.1016/B978-0-444-63488-7.00008-2. [DOI] [PubMed] [Google Scholar]
- Jenkin SEM, Milsom WK & Zoccal DB (2017). The Kölliker–Fuse nucleus acts as a timekeeper for late‐expiratory abdominal activity. Neuroscience 348, 63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwaki T, Li A & Nattie E (2010). State‐dependent central chemoreception: a role of orexin. Respir Physiol Neurobiol 173, 223–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemes EV, Colombari E & Zoccal DB (2016). Generation of active expiration by serotoninergic mechanisms of the ventral medulla of rats. J Appl Physiol 121, 1135–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemes EV & Zoccal DB (2014). Vagal afferent control of abdominal expiratory activity in response to hypoxia and hypercapnia in rats. Respir Physiol Neurobiol 203, 90–97. [DOI] [PubMed] [Google Scholar]
- Martin JG & De Troyer A (1982). The behaviour of the abdominal muscles during inspiratory mechanical loading. Respir Physiol 50, 63–73. [DOI] [PubMed] [Google Scholar]
- Mellen NM, Janczewski WA, Bocchiaro CM & Feldman JL (2003). Opioid‐induced quantal slowing reveals dual networks for respiratory rhythm generation. Neuron 37, 821–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nattie E (2000). Multiple sites for central chemoreception: their roles in response sensitivity and in sleep and wakefulness. J Respir Physiol 122, 223–235. [DOI] [PubMed] [Google Scholar]
- Nattie E & Li A (2010). Central chemoreception in wakefulness and sleep: evidence for a distributed network and a role for orexin. J Appl Physiol 108, 1417–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nattie EE (2001). Central chemosensitivity, sleep, and wakefulness. Respir Physiol 129, 257–268. [DOI] [PubMed] [Google Scholar]
- Nattie EE & Li A (2001). CO2 dialysis in the medullary raphe of the rat increases ventilation in sleep. J Appl Physiol 90, 1247–1257. [DOI] [PubMed] [Google Scholar]
- Nattie EE & Li A (2002). CO2 dialysis in nucleus tractus solitarius region of rat increases ventilation in sleep and wakefulness. J Appl Physiol 92, 2119–2130. [DOI] [PubMed] [Google Scholar]
- Oliven A, Jr , Deal EC, Kelsen SG & Cherniack NS (1985). Effects of hypercapnia on inspiratory and expiratory muscle activity during expiration. J Appl Physiol 59, 1560–1565. [DOI] [PubMed] [Google Scholar]
- Pagliardini S, Greer JJ, Funk GD & Dickson CT (2012). State‐dependent modulation of breathing in urethane‐anesthetized rats. J Neurosci 32, 11259–11270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliardini S, Janczewski WA, Tan WB, Dickson CT, Deisseroth K & Feldman JL (2011). Active expiration induced by excitation of ventral medulla in adult anesthetized rats. J Neurosci 31, 2895–2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praud JP, Allest AMD & Nedelcoux H (1989). Sleep‐related abdominal muscle behavior during partial or complete obstructed breathing in prepubertal children. Pediatr Res 26, 347–350. [DOI] [PubMed] [Google Scholar]
- Sherrey JH & Megirian D (1977). State dependence of upper airway respiratory motoneurons: functions of the cricothyroid and nasolabial muscles of the unanesthetized rat. Electroencephalogr Clin Neurophysiol 43, 218–228. [DOI] [PubMed] [Google Scholar]
- Sherrey JH & Megirian D (1980). Respiratory EMG activity of the posterior cricoarythenoid, cricothyroid and diaphragm muscles during sleep. Respir Physiol 39, 355–365. [DOI] [PubMed] [Google Scholar]
- Sherrey JH, Pollard MJ & Megirian D (1988). Proprioceptive, chemoreceptive and sleep state modulation of expiratory muscle activity in the rat. Exp Neurol 101, 50–62. [DOI] [PubMed] [Google Scholar]
- da Silva GSF, Giusti H, Benedetti M, Dias MB, Gargaglioni LH, Branco LGS & Glass ML (2011). Serotonergic neurons in the nucleus raphe obscurus contribute to interaction between central and peripheral ventilatory responses to hypercapnia. Pflugers Arch 462, 407–418. [DOI] [PubMed] [Google Scholar]
- da Silva GSF, Giusti H, Castro OW, Garcia‐Cairasco N, Gargaglioni LH, Branco LGS & Glass ML (2013). Serotonergic neurons in the nucleus raphé obscurus are not involved in the ventilatory and thermoregulatory responses to hypoxia in adult rats. Respir Physiol Neurobiol 187, 139–148. [DOI] [PubMed] [Google Scholar]
- da Silva GSF, Li A & Nattie E (2010). High CO2/H+ dialysis in the caudal ventrolateral medulla (Loeschcke's area) increases ventilation in wakefulness. Respir Physiol Neurobiol 171, 46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CA, Ainsworth DM, Henderson KS & Dempsey JA (1989). Differential responses of expiratory muscles to chemical stimuli in awake dogs. J Appl Physiol 66, 384–391. [DOI] [PubMed] [Google Scholar]
- Smith CA, Ainsworth DM, Henderson KS & Dempsey JA (1990). The influence of carotid body chemoreceptors on expiratory muscle activity. Respir Physiol 82, 123–136. [DOI] [PubMed] [Google Scholar]
- Takasaki Y, Orr D, Popkin J, Xie AL & Bradley TD (1989). Effect of hypercapnia and hypoxia on respiratory muscle activation in humans. J Appl Physiol 67, 1776–1784. [DOI] [PubMed] [Google Scholar]
- Taylor NC, Li A & Nattie EE (2006). Ventilatory effects of muscimol microdialysis into the rostral medullary raphé region of conscious rats. Respir Physiol Neurobiol 153, 203–216. [DOI] [PubMed] [Google Scholar]
- De Troyer A, Gilmartin JJ & Ninane V (1989). Abdominal muscle use during breathing in unanesthetized dogs. J Appl Physiol 66, 20–27. [DOI] [PubMed] [Google Scholar]
- Vicente MC, Dias MB, Fonseca EM, Bícego KC & Gargaglioni LH (2016). Orexinergic system in the locus coeruleus modulates the CO2 ventilatory response. Pflügers Arch 468, 763–774. [DOI] [PubMed] [Google Scholar]
- Wakai Y, Welsh MM, Leevers AM & Road JD (1992). Expiratory muscle activity in the awake and sleeping human during lung inflation and hypercapnia. J Appl Physiol 72, 881–887. [DOI] [PubMed] [Google Scholar]


