Abstract
The carotid bodies (CBs) are multi‐modal sensory organs located bilaterally at the bifurcation of the carotid artery and innervated by the carotid sinus nerve (Hering's nerve), a branch of the IX cranial nerve. While the CBs (or embryologically analogous structures) are well known as the dominant oxygen‐sensing organ in vertebrates, in mammals there is evidence that the CBs may also sense glucose and temperature, and respond to circulating hormones and other factors. Additionally, the CBs likely participate in regulating baseline levels of sympathetic tone. In this brief review, we focus on the evolution of our efforts to understand ‘what else’ beyond oxygen sensing the CBs do in humans.
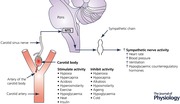
Introduction
The carotid bodies (CBs), or embryologically analogous structures, are well known as the dominant oxygen‐sensing organ in vertebrates (Hempleman & Warburton, 2013). Our interest in what else the CBs do started by chance when one of us (M.J.J.) heard an impressive Physiology departmental seminar by Professor Jose Lopez‐Barneo given at the Mayo Clinic some time during the later 1990s or early 2000s (Pardal & Lopez‐Barneo, 2002). The work presented by Lopez‐Barneo showed that low glucose could stimulate release of neurotransmitters from isolated CB Type 1 cells (aka: glomus cells), and that the magnitude of the release varied depending on the partial pressure of oxygen of the physiological saline solution bathing the cells. At about the same time or shortly before, studies by Koyama et al. working with David Wasserman emerged showing that carotid denervation markedly attenuated the counterregulatory responses (i.e. release of hormones such as glucagon, adrenaline, cortisol, etc. to mobilize or synthesize glucose) to hypoglycaemia in conscious dogs and also altered their responses to prolonged exercise (Koyama et al. 2000, 2001). Over the next 5 or 10 years, evidence from a variety of animal models demonstrated that the CBs either directly or indirectly sensed blood glucose, or a related phenomenon, and potentially played at least some role in the physiological responses to hypoglycaemia (Holmes et al. 2012). This was also true in humans (Ward et al. 2007).
As these sorts of observations progressed, two possible experimental models that might be useful in studying related questions in humans came to mind. First, rare patients exist who have undergone bilateral carotid body resection to treat glomus tumours. Baroreflex function has been studied in such patients (Smit et al. 2002). Additionally, because the Mayo Clinic is a major surgical referral centre in the USA, a search of our medical records system showed that a number of potential patients might be available for study. Second, there was evidence from a number of sources that acute hyperoxia can ‘turn off’ the CBs, or at least markedly suppress baseline afferent nerve activity from the CBs (Fitzgerald & Lahiri, 1986).
What follows is a brief description of our interests on this topic and some of the studies we have done to address them. Where relevant, we cite the work of other labs to highlight our evolving thinking on related topics.
Hyperoxia, CB resection and counterregulation during hypoglycaemia
In our first study on this topic we collaborated with our colleagues Bob Rizza, Rita Basu, and Andy Basu to perform hypoglycaemic, hyperinsulinaemic clamps in healthy adults (Wehrwein et al. 2010). Each subject underwent two clamps on separate days, one during normoxia and the other during hyperoxia. Glucose was clamped at ∼3 mmol L−1 (60 mg dL−1) for 2 h, and during both trials the subjects wore a close‐fitting face mask. During the hyperoxia trial, they were exposed to 100% , which generated a of ∼400 mmHg. As expected, clamping blood glucose in a hypoglycaemic range during normoxia stimulated an increase in the plasma level of counterregulatory hormones to mobilize or synthesize glucose, including glucagon, adrenaline, cortisol, growth hormone, dopamine and noradrenaline. During hypoglycaemia paired with hyperoxia, we observed a ∼50% attenuation of the rise in plasma levels of these counterregulatory hormones compared to normoxic conditions. Additionally, the glucose infusion rate was significantly higher during the hyperoxia trial, consistent with the interpretation that endogenous glucose production was blunted when counterregulatory hormones were suppressed during the hyperoxia trial. These results are broadly consistent with the idea that the CBs can sense blood glucose in humans and participate in the physiological responses to hypoglycaemia.
Our next study was fundamentally similar to our hyperoxia study except it included five generally healthy patients who had undergone bilateral carotid body resection many years prior to the clamps (Wehrwein et al. 2015). In this study, hyperoxia had no impact on either the counterregulatory responses to hypoglycaemia or the glucose infusion rate. The absence of an effect of hyperoxia is consistent with the idea that the CBs have a role in sensing hypoglycaemia and counterregulation. Of note during normoxia, the glucose infusion rates in the resected patients were inversely related to the residual hypoxic ventilatory response (HVR) such that patients with the highest HVR had the lowest glucose infusion rates and those with essentially no HVR needed the highest glucose infusion rates. This latter point is consistent with a role for the CBs in the counterregulatory response to hypoglycaemia.
Intermittent hypoxia and blood glucose
There is ample evidence in the literature that hypoxia acts at the level of the CBs to increase sympathetic nervous system activity. Hypoxia also enhances the sympathetic response to subsequent hypoxic episodes and causes prolonged elevation of sympathetic activity after removal of the hypoxic stimulus (Xie et al. 2001; Lusina et al. 2006). With this, long‐term intermittent hypoxia increases sympathetic activity and CB chemosensitivity during acute hypoxia in humans. However, the effect of intermittent hypoxia on glucose regulation has been less clear. Obstructive sleep apnoea is frequently associated with Type II diabetes and this association is independent of other comorbidities (e.g. obesity, age) (Meslier et al. 2003). In 2014, a group of investigators from Johns Hopkins showed that mice exposed to intermittent hypoxia exhibited fasting hyperglycaemia. Furthermore, this hyperglycaemia was attenuated with CB denervation (Shin et al. 2014). Together these data supported the idea that increases in CB activity may play a causal role that links hypoxic exposures common in sleep apnoea and impairments in glucose regulation. Our group sought to translate these ideas to humans. Eight young, healthy individuals visited the lab on two occasions. After a controlled dinner and overnight fast, subjects were randomized to 3 h of intermittent hypoxia (mild, 90% , 25 events per hour) or continuous normoxia. We found, quite surprisingly, immediate (30 min) and prolonged (up to 180 min) increases in circulating glucose (∼0.3 mmol L−1) during acute hypoxic exposures sufficient to acutely increase carotid body chemosensitivity (Newhouse et al. 2017). Furthermore, these increases in glucose occurred independently of changes in insulin sensitivity – lending support to the idea that activation of the CBs played a role in the increased glucose concentrations (Newhouse et al. 2017).
Exercise and glucose homeostasis
Our interest in the contribution of the CBs to glucoregulation during exercise branched out from our work using hyperinsulinaemic clamps to induce hypoglycaemia and the emerging evidence that the CBs are activated by circulating insulin (Ribeiro et al. 2013; Limberg et al. 2014). Evidence from CB‐resected dogs demonstrated that during prolonged exercise, blood glucose was ∼6% lower and the release of some glucoregulatory hormones was attenuated following CB resection (Koyama et al. 2001). Furthermore, ‘turning off’ the CBs by breathing hyperoxia during cycle ergometer exercise in humans resulted in a substantial reduction in the rate of glucose appearance versus a hypoxic condition (Cooper et al. 1986). Therefore, we thought that blunting CB activation during prolonged exercise would be an excellent experimental model to induce hypoglycaemia without the possible confounding influence of hyperinsulinaemia. After 3 days of dietary (50% carbohydrate, 30% fat, 20% protein) and exercise control, we had exercise‐trained subjects (n = 12) participate in two cycle ergometer exercise sessions at 65% of peak oxygen consumption for up to 2 h (Johnson et al. 2018). One session was completed while a low dose of dopamine was infused to blunt CB activation (Limberg et al. 2016), and saline was infused during the other session. During the dopamine infusion, the cumulative plasma glucose response during exercise was ∼13% lower and a trend for an attenuated noradrenaline response was also observed. These data indicate that the CBs contribute to the complex glucoregulatory response to prolonged aerobic exercise.
Temperature and ventilation
Respiratory‐related mortality is increased during heat waves (Anderson & Bell, 2009) and hyperthermia is typically associated with a rise in ventilation in humans (Gaudio & Abramson, 1968; Cabanac & White, 1995). There is also some evidence that hyperventilation during hyperthermia modulates total body heat loss (Cain et al. 1990). Carotid sinus nerve activity increases when isolated CBs are heat stressed (McQueen & Eyzaguirre, 1974; Gallego et al. 1979), which provides evidence that the CBs contribute to the ventilatory response to increases in body temperature. In humans, the ventilatory response to hypoxia is augmented in the heat (Natalino et al. 1977), and hyperthermia‐induced hyperventilation is reduced when breathing hyperoxia (Fujii et al. 2008), which suggests that the CBs contribute to hyperthermia‐induced hyperventilation. In collaboration with Dr Craig Crandall's laboratory, we examined ventilation and CB chemosensitivity during hyperthermia. Similar to our approach to examine the contribution of the CBs to glucoregulation, we infused low dose dopamine in an attempt to blunt CB activation during hyperthermia (∼1.4°C increase in intestinal temperature) in healthy subjects (n = 20). However, we found that a subset of subjects (n = 13) did not have an attenuated ventilatory response to hypoxia (i.e. decreased CB chemosensitivity) during normothermic dopamine infusion along with the expected increase in ventilation (>2 L min−1) during the saline hyperthermia trial – highlighting the limits of using experimental low‐dose dopamine to attenuate CB activation (Limberg et al. 2016). Therefore, we separately analysed data from seven subjects that had a lower ventilatory response to hypoxia under normothermia during dopamine infusion and a >2 L min−1 increase in ventilation during hyperthermia with saline infusion. We found that ventilation did not increase during hyperthermia in the dopamine trial whereas ventilation significantly increased during hyperthermia in the saline trial (Johnson et al. 2015). The partial pressure of end‐tidal carbon dioxide also indicated that dopamine infusion prevented hyperventilation during hyperthermia whereas the during hyperthermia in the saline trial was reduced. Collectively, we interpreted these currently unpublished results as confirming that the CBs contribute to the ventilatory response to hyperthermia.
Insulin, the CBs and sympathoexcitation
As noted above, early in our quest to explore the potential role of the CBs in glucose regulation using the hyperinsulinaemic hypoglycaemic clamp technique, Silvia Conde's lab in Portugal found insulin receptors on the CBs and reported data including an increase in neurosecretion from the CBs in the presence of insulin (Ribeiro et al. 2013). Therefore, in addition to our studies using prolonged exercise (Johnson et al. 2018), we also conducted hyperinsulinaemic–euglycaemic clamps with the goal of exploring a role for the CBs in insulin‐mediated sympathoexcitation (Anderson et al. 1991). We measured cardiorespiratory and neuroendocrine function, including muscle sympathetic nerve activity (MSNA), during hyperinsulinaemic–euglycaemic clamps in healthy humans. These studies were done under a variety of conditions known to increase (hypoxia) or decrease (hyperoxia, dopamine) CB afferent activity both independently and in the presence of high circulating insulin. As to be expected, we observed a robust increase in sympathetic nervous system activity in the presence of systemic insulin (Anderson et al. 1991; Limberg et al., 2017). However, there was a negligible effect of hyperoxia and/or low‐dose dopamine on acute insulin‐mediated sympathoexcitation in the lean, healthy adults studied (Limberg et al., 2017). These data are in agreement with our exercise data (mentioned above) and suggest that it is unlikely that any acute effect of high insulin on the CBs confounded previous findings in healthy humans (Wehrwein et al. 2010). However, there are data to suggest the CBs may contribute to sympathoexcitation observed with more chronic insulin exposures, such as that seen with insulin resistance and/or diabetes (Ribeiro et al. 2013; Limberg et al. 2014).
Pathophysiology
In addition to our own work in humans, there is evidence in clinically relevant animal models that tonic activation of the CBs contributes to the sympathoexcitation seen in some patients with hypertension and also in congestive heart failure (Abdala et al. 2012; Del Rio et al. 2013). Importantly, bilateral or unilateral CB denervation in these models reverses hypertension and also many of the sympathetically mediated pathophysiological events associated with congestive heart failure. A critical and sometimes overlooked feature of congestive heart failure is that some patients show excessive ventilation during exercise. This response is associated with poor long term survival and is likely part of a suite of events associated with excessive input from the CBs in some patients with congestive heart failure (Tumminello et al. 2007). When these responses are viewed in the context of the linkage between intermittent hypoxia (e.g. sleep apnoea) and hyperglycaemia, it is clear that all are associated with sympathoexcitation. Whether this sympathoexcitation is generalized or specific to organ systems affected in a given pathophysiological conditions remains to be determined.
There is also some retrospective data from a large series of patients who have undergone either unilateral or bilateral CB tumour resection that is supportive of the idea that the CBs play a role in hypertension and might be a therapeutic target in humans (Fudim et al. 2015). This concept is supported by some early phase interventional studies in humans, but a series of rigorous developmental and subsequent randomized clinical trials will be required before exciting observations at the proof‐of‐concept stage are ready for routine use in patients with complex conditions such as resistant hypertension or congestive heart failure (Narkiewicz et al. 2016; Niewinski et al. 2017).
Adding to this body of knowledge, our group recently studied a single patient with unilateral CB resection (Larson et al. 2017). Quite surprisingly, we found although she exhibited an intact blood pressure response to typical sympathoexcitatory stimuli (e.g. cold pressor test, isometric handgrip exercise), she achieved this rise in blood pressure despite complete silence of MSNA. We speculate this blood pressure response was achieved via an increase in cardiac output perhaps with an adaptive inhibitory effect of any remaining baroreflex input. It is important to highlight that the individual studied underwent unilateral carotid body resection. Other work by our group in bilaterally resected patients has shown that although resting baroreflex function is relatively normal, significant impairments in baroreflex sensitivity are uncovered during stress (hypoglycaemia) (Limberg et al. 2015). Together these data suggest there may be unforeseen autonomic consequences of CB resection, even in relatively healthy adults.
Summary
Our efforts to study the CBs as sensors involved in physiological regulatory responses beyond the maintenance of arterial oxygen homeostasis had a long period of germination. This germination was based on an extremely well‐presented seminar detailing activation of the CBs to low glucose (Pardal & Lopez‐Barneo, 2002) and what might be described, in the era of knock‐out mice, as an old‐school, large animal denervation model (i.e. CB‐denervated dogs) (Koyama et al. 2000). The data obtained in this denervated dog model was also a clear cut demonstration of the role of CBs in glucoregulation. It also led us to pursue these ideas in humans via patient ‘experiments in nature’ (bilateral CB tumour resection) and also using interventions like hyperoxia and dopamine infusions that can acutely suppress sensory activity in the CBs. In our series of human studies using models to suppress CB activity, we started exploring hypoglycaemic counterregulation with striking results that led us to further explore a much more complex role of the CBs in integrative human physiology. We showed (1) the CBs play an important role in hypoglycaemic counterregulation, likely through modulation of sympathetic activity and glucose mobilization, (2) intermittent hypoxia results in an increase in plasma glucose and an increased CB sensitivity independently of changes in insulin sensitivity, (3) blunted CB activation reduces glucose mobilization during aerobic exercise, and (4) the CBs modulate the ventilatory response to hyperthermia. Taken together, our data support a key role for the CBs in numerous human physiological functions. The CBs may serve as direct or indirect sensors for glucose and insulin. They may also serve to directly modulate important central afferent inputs from the carotid sinus nerve to key brain regions involved in autonomic control, baroreflex, endocrine, feeding, temperature control, and more. With our evidence that the CBs are important in a variety of health and disease states, this opens the door for potential novel therapeutic approaches to modulating CB activity in common diseases such as hypertension, heart failure, sleep apnoea and diabetes (Paton et al. 2013). In the coming years, it will be interesting to see if therapies that target or exploit these ‘other functions’ of the CBs emerge as safe and effective treatments and if the current trials using CB denervation in resistant hypertension patients reveal a deeper understanding of the role of the CBs.
Additional information
Competing interests
M.J.J. has consulted for GSK and Cibiem on issues related to the CBs as therapeutic targets for disease management in diabetes and hypertension.
Author contributions
All authors have approved the final version of the manuscript and agree to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
Our studies on these topics were funded by NIH DK90541 (M.J.J.), DK84624 (E.A.W.), DK07352, HL120570 (J.K.L.), HL130339 (J.K.L.) and American Heart Association Midwest Affiliate Grant 13POST‐14380027 (B.D.J.).
Acknowledgements
The authors thank the many volunteer subjects and patients who have participated in their studies on related topics. We also thank the technical support staff of the Joyner Lab and Mayo Clinical Research Unit.
Biographies
The carotid bodies (CBs) do more than just sense the partial pressure of oxygen in the arterial circulation. The concepts reviewed in this paper are about what else the CBs do. The data presented are in large part the result of collaborations that emerged in the Human and Integrative Physiology (Joyner) lab at the Mayo Clinic in the late 2000s. Michael Joyner was pleased to facilitate the work of his collaborators and help them pull many interesting ideas together and study them in humans.

Jackie Limberg, now an Assistant Professor at the University of Missouri has focused on the role of the CBs in evoking sympathetic activation.
Erica Wehrwein, (left in photo) now an Assistant Professor at Michigan State University, was instrumental in asking questions about the role of the CBs in responding to hypoglycemia.
Blair Johnson, now an Assistant Professor at the University at Buffalo, extended the CB glucose story to exercise and was also interested in the CBs and thermoregulation. The authors note that in an era where vast resources are being devoted to finding therapeutic targets based on the genome, the CBs have emerged as a potential therapeutic target for a number of clinical conditions.
Edited by: Kim Barrett & Harold Schultz
This review was presented at the symposium ‘Advances in cellular and integrative control of oxygen and carbon dioxide homeostasis’ which took place at XX ISAC meeting, Baltimore, MD, USA, 23–27 July 2017.
References
- Abdala AP, McBryde FD, Marina N, Hendy EB, Engelman ZJ, Fudim M, Sobotka PA, Gourine AV & Paton JF (2012). Hypertension is critically dependent on the carotid body input in the spontaneously hypertensive rat. J Physiol 590, 4269–4277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BG & Bell ML (2009). Weather‐related mortality: how heat, cold, and heat waves affect mortality in the United States. Epidemiology 20, 205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson EA, Hoffman RP, Balon TW, Sinkey CA & Mark AL (1991). Hyperinsulinemia produces both sympathetic neural activation and vasodilation in normal humans. J Clin Invest 87, 2246–2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabanac M & White MD (1995). Core temperature thresholds for hyperpnea during passive hyperthermia in humans. Eur J Appl Physiol Occup Physiol 71, 71–76. [DOI] [PubMed] [Google Scholar]
- Cain JB, Livingstone SD, Nolan RW & Keefe AA (1990). Respiratory heat loss during work at various ambient temperatures. Respir Physiol 79, 145–150. [DOI] [PubMed] [Google Scholar]
- Cooper DM, Wasserman DH, Vranic M & Wasserman K (1986). Glucose turnover in response to exercise during high‐ and low‐FIO2 breathing in man. Am J Physiol 251, E209–E214. [DOI] [PubMed] [Google Scholar]
- Del Rio R, Marcus NJ & Schultz HD (2013). Carotid chemoreceptor ablation improves survival in heart failure: rescuing autonomic control of cardiorespiratory function. J Am Coll Cardiol 62, 2422–2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald R & Lahiri, S (1986). Reflex responses to chemoreceptor stimulation In Handbook of Physiology, Section 3, The Respiratory System, Vol. II, Control of Breathing, ed. Fishman AP, Cherniack NS, Widdicombe JG, Geiger SR, pp. 313–362. American Physiological Society, Bethesda, MD, USA. [Google Scholar]
- Fudim M, Groom KL, Laffer CL, Netterville JL, Robertson D & Elijovich F (2015). Effects of carotid body tumor resection on the blood pressure of essential hypertensive patients. J Am Soc Hypertens 9, 435–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii N, Honda Y, Hayashi K, Kondo N, Koga S & Nishiyasu T (2008). Effects of chemoreflexes on hyperthermic hyperventilation and cerebral blood velocity in resting heated humans. Exp Physiol 93, 994–1001. [DOI] [PubMed] [Google Scholar]
- Gallego R, Eyzaguirre C & Monti‐Bloch L (1979). Thermal and osmotic responses of arterial receptors. J Neurophysiol 42, 665–680. [DOI] [PubMed] [Google Scholar]
- Gaudio R Jr & Abramson N ( 1968). Heat‐induced hyperventilation. J Appl Physiol 25, 742–746. [DOI] [PubMed] [Google Scholar]
- Hempleman SC & Warburton SJ (2013). Comparative embryology of the carotid body. Respir Physiol Neurobiol 185, 3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes AP, Hauton D & Kumar P (2012). The interaction between low glucose and hypoxia in the in vitro, rat carotid body. Adv Exp Med Biol 758, 123–127. [DOI] [PubMed] [Google Scholar]
- Johnson BD, Peinado AB, Ranadive SM, Curry TB & Joyner MJ (2018). The effects of intravenous low dose dopamine infusion on glucose regulation during prolonged aerobic exercise. Am J Physiol Regul Integr Comp Physiol 314, R49–R57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BD, Schlader ZJ, Crandall CG & Joyner MJ (2015). Attenuating ventilation by inhibiting the carotid body chemoreceptors during hyperthermia modulates thermal sensation. Med Sci Sports Exerc 47, S492. [Google Scholar]
- Koyama Y, Coker RH, Denny JC, Lacy DB, Jabbour K, Williams PE & Wasserman DH (2001). Role of carotid bodies in control of the neuroendocrine response to exercise. Am J Physiol Endocrinol Metab 281, E742–E748. [DOI] [PubMed] [Google Scholar]
- Koyama Y, Coker RH, Stone EE, Lacy DB, Jabbour K, Williams PE & Wasserman DH (2000). Evidence that carotid bodies play an important role in glucoregulation in vivo. Diabetes 49, 1434–1442. [DOI] [PubMed] [Google Scholar]
- Larson KF, Limberg JK, Baker SE, Joyner MJ & Curry TB (2017). Intact blood pressure, but not sympathetic, responsiveness to sympathoexcitatory stimuli in a patient with unilateral carotid body resection. Physiol Rep 5, e13212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limberg JK, Curry TB, Prabhakar NR & Joyner MJ (2014). Is insulin the new intermittent hypoxia? Med Hypotheses 82, 730–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limberg JK, Johnson BD, Holbein WW, Ranadive SM, Mozer MT & Joyner MJ (2016). Interindividual variability in the dose‐specific effect of dopamine on carotid chemoreceptor sensitivity to hypoxia. J Appl Physiol (1985) 120, 138–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limberg JK, Mozer MT, Holbein WW, Johnson BD, Prabhakar NR, Curry TB & Joyner MJ (2017). Low‐dose dopamine, but not acute hyperoxia, attenuates the sympathoexcitatory response to hyperinsulinemia in healthy humans. FASEB J 31(Suppl), Abstract 867.4. http://www.fasebj.org/doi/10.1096/fasebj.31.1_supplement.867.4. [Google Scholar]
- Limberg JK, Taylor JL, Mozer MT, Dube S, Basu A, Basu R, Rizza RA, Curry TB, Joyner MJ & Wehrwein EA (2015). Effect of bilateral carotid body resection on cardiac baroreflex control of blood pressure during hypoglycemia. Hypertension 65, 1365–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusina SJ, Kennedy PM, Inglis JT, McKenzie DC, Ayas NT & Sheel AW (2006). Long‐term intermittent hypoxia increases sympathetic activity and chemosensitivity during acute hypoxia in humans. J Physiol 575, 961–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQueen DS & Eyzaguirre C (1974). Effects of temperature on carotid chemoreceptor and baroreceptor activity. J Neurophysiol 37, 1287–1296. [DOI] [PubMed] [Google Scholar]
- Meslier N, Gagnadoux F, Giraud P, Person C, Ouksel H, Urban T & Racineux JL (2003). Impaired glucose‐insulin metabolism in males with obstructive sleep apnoea syndrome. Eur Respir J 22, 156–160. [DOI] [PubMed] [Google Scholar]
- Narkiewicz K, Ratcliffe LE, Hart EC, Briant LJ, Chrostowska M, Wolf J, Szyndler A, Hering D, Abdala AP, Manghat N, Burchell AE, Durant C, Lobo MD, Sobotka PA, Patel NK, Leiter JC, Engelman ZJ, Nightingale AK & Paton JF (2016). Unilateral carotid body resection in resistant hypertension: a safety and feasibility trial. JACC Basic Transl Sci 1, 313–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natalino MR, Zwillich CW & Weil JV (1977). Effects of hyperthermia on hypoxic ventilatory response in normal man. J Lab Clin Med 89, 564–572. [PubMed] [Google Scholar]
- Newhouse LP, Joyner MJ, Curry TB, Laurenti MC, Man CD, Cobelli C, Vella A & Limberg JK (2017). Three hours of intermittent hypoxia increases circulating glucose levels in healthy adults. Physiol Rep 5, e13106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niewinski P, Janczak D, Rucinski A, Tubek S, Engelman ZJ, Piesiak P, Jazwiec P, Banasiak W, Fudim M, Sobotka PA, Javaheri S, Hart EC, Paton JF & Ponikowski P (2017). Carotid body resection for sympathetic modulation in systolic heart failure: results from first‐in‐man study. Eur J Heart Fail 19, 391–400. [DOI] [PubMed] [Google Scholar]
- Pardal R & Lopez‐Barneo J (2002). Low glucose‐sensing cells in the carotid body. Nat Neurosci 5, 197–198. [DOI] [PubMed] [Google Scholar]
- Paton JF, Sobotka PA, Fudim M, Engelman ZJ, Hart EC, McBryde FD, Abdala AP, Marina N, Gourine AV, Lobo M, Patel N, Burchell A, Ratcliffe L & Nightingale A (2013). The carotid body as a therapeutic target for the treatment of sympathetically mediated diseases. Hypertension 61, 5–13. [DOI] [PubMed] [Google Scholar]
- Ribeiro MJ, Sacramento JF, Gonzalez C, Guarino MP, Monteiro EC & Conde SV (2013). Carotid body denervation prevents the development of insulin resistance and hypertension induced by hypercaloric diets. Diabetes 62, 2905–2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin MK, Yao Q, Jun JC, Bevans‐Fonti S, Yoo DY, Han W, Mesarwi O, Richardson R, Fu YY, Pasricha PJ, Schwartz AR, Shirahata M & Polotsky VY (2014). Carotid body denervation prevents fasting hyperglycemia during chronic intermittent hypoxia. J Appl Physiol (1985) 117, 765–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit AA, Timmers HJ, Wieling W, Wagenaar M, Marres HA, Lenders JW, van Montfrans GA & Karemaker JM (2002). Long‐term effects of carotid sinus denervation on arterial blood pressure in humans. Circulation 105, 1329–1335. [DOI] [PubMed] [Google Scholar]
- Tumminello G, Guazzi M, Lancellotti P & Pierard LA (2007). Exercise ventilation inefficiency in heart failure: pathophysiological and clinical significance. Eur Heart J 28, 673–678. [DOI] [PubMed] [Google Scholar]
- Ward DS, Voter WA & Karan S (2007). The effects of hypo‐ and hyperglycaemia on the hypoxic ventilatory response in humans. J Physiol 582, 859–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrwein EA, Basu R, Basu A, Curry TB, Rizza RA & Joyner MJ (2010). Hyperoxia blunts counterregulation during hypoglycaemia in humans: possible role for the carotid bodies? J Physiol 588, 4593–4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrwein EA, Limberg JK, Taylor JL, Dube S, Basu A, Basu R, Rizza RA, Curry TB & Joyner MJ (2015). Effect of bilateral carotid body resection on the counterregulatory response to hypoglycaemia in humans. Exp Physiol 100, 69–78. [DOI] [PubMed] [Google Scholar]
- Xie A, Skatrud JB, Puleo DS & Morgan BJ (2001). Exposure to hypoxia produces long‐lasting sympathetic activation in humans. J Appl Physiol (1985) 91, 1555–1562. [DOI] [PubMed] [Google Scholar]


