Abstract
Key points
Carotid body dysfunction is recognized as a cause of hypertension in a number of cardiorespiratory diseases states and has therefore been identified as a potential therapeutic target.
Purinergic transmission is an important element of the carotid body chemotransduction pathway.
We show that inhibition of ecto‐5′‐nucleotidase (CD73) in vitro reduces carotid body basal discharge and responses to hypoxia and mitochondrial inhibition.
Additionally, inhibition of CD73 in vivo decreased the hypoxic ventilatory response, reduced the hypoxia‐induced heart rate elevation and exaggerated the blood pressure decrease in response to hypoxia.
Our data show CD73 to be a novel regulator of carotid body sensory function and therefore suggest that this enzyme may offer a new target for reducing carotid body activity in selected cardiovascular diseases.
Abstract
Augmented sensory neuronal activity from the carotid body (CB) has emerged as a principal cause of hypertension in a number of cardiovascular related pathologies, including obstructive sleep apnoea, heart failure and diabetes. Development of new targets and pharmacological treatment strategies aiming to reduce CB sensory activity may thus improve outcomes in these key patient cohorts. The present study investigated whether ecto‐5′‐nucleotidase (CD73), an enzyme that generates adenosine, is functionally important in modifying CB sensory activity and cardiovascular respiratory responses to hypoxia. Inhibition of CD73 by α,β‐methylene ADP (AOPCP) in the whole CB preparation in vitro reduced basal discharge frequency by 76 ± 5% and reduced sensory activity throughout graded hypoxia. AOPCP also significantly attenuated elevations in sensory activity evoked by mitochondrial inhibition. These effects were mimicked by antagonism of adenosine receptors with 8‐(p‐sulfophenyl) theophylline. Infusion of AOPCP in vivo significantly decreased the hypoxic ventilatory response (Δ E control 74 ± 6%, Δ E AOPCP 64 ± 5%, P < 0.05). AOPCP also modified cardiovascular responses to hypoxia, as indicated by reduced elevations in heart rate and exaggerated changes in femoral vascular conductance and mean arterial blood pressure. Thus we identify CD73 as a novel regulator of CB sensory activity. Future investigations are warranted to clarify whether inhibition of CD73 can effectively reduce CB activity in CB‐mediated cardiovascular pathology.
Keywords: adenosine, carotid body, ecto‐5′‐nucleotidase
Key points
Carotid body dysfunction is recognized as a cause of hypertension in a number of cardiorespiratory diseases states and has therefore been identified as a potential therapeutic target.
Purinergic transmission is an important element of the carotid body chemotransduction pathway.
We show that inhibition of ecto‐5′‐nucleotidase (CD73) in vitro reduces carotid body basal discharge and responses to hypoxia and mitochondrial inhibition.
Additionally, inhibition of CD73 in vivo decreased the hypoxic ventilatory response, reduced the hypoxia‐induced heart rate elevation and exaggerated the blood pressure decrease in response to hypoxia.
Our data show CD73 to be a novel regulator of carotid body sensory function and therefore suggest that this enzyme may offer a new target for reducing carotid body activity in selected cardiovascular diseases.
Abbreviations
- ABP
arterial blood pressure
- AOPCP
α,β‐methylene ADP
- CB
carotid body
- CD39
ectonucleoside triphosphate diphosphohydrolyase 1
- CD73
ecto‐5′‐nucleotidase
- CSN
carotid sinus nerve
- ENT
equilibrative nucleotide transporter
- fR
respiratory frequency
- FVC
femoral vascular conductance
- HR
heart rate
- HVR
hypoxic ventilatory response
- MABP
mean arterial blood pressure
- NBTI
nitrobenzylthioinosine
- NO2−
nitrite
- 8‐SPT
8‐(p‐sulfophenyl) theophylline
- E
minute ventilation
- Vt
tidal volume
Introduction
Chronic carotid body (CB) overactivation has emerged as an important driver of hypertension in a number of cardiovascular related diseases, including heart failure, sleep disordered breathing and diabetes (Ribeiro et al. 2013; Schultz et al. 2015; Del Rio et al. 2016; Prabhakar, 2016). Thus, the development of novel treatment strategies that target the CB either pharmacologically or surgically may improve outcomes in these large patient populations. Indeed, some preliminary data suggest that bilateral CB resection decreases sympathetic outflow and improves exercise tolerance in human heart failure patients (Niewinski et al. 2017). In a small cohort of patients with drug resistant hypertension, unilateral CB resection caused a reduction in ambulatory blood pressure in ∼50% of patients studied (Narkiewicz et al. 2016). However, in view of the potential safety concerns of bilateral resection, as indicated by a substantial reduction in O2 saturation during sleep (Niewinski et al. 2017), as well as the variable efficacy of unilateral resection (Narkiewicz et al. 2016), pharmacological dampening of chemoreceptor activity may still offer a more viable treatment alternative.
Carotid sinus nerve (CSN) discharge frequency, transmitted into the CNS, is the neuronal signal that promotes ventilatory and cardiovascular reflex responses originating from the CB, most notably in response to systemic hypoxia (Kumar & Prabhakar, 2012). Modification of CSN outflow is controlled by a number of important neurotransmitters and neuromodulators, including ATP, ACh, dopamine, serotonin, adrenaline and adenosine (Nurse, 2010) (McQueen & Ribeiro, 1986; Fitzgerald et al. 1999; Zhang et al. 2000; Conde et al. 2006, 2012; Hauton et al. 2013; Thompson et al. 2016). Adenosine is an established CB chemostimulant in both animals (Runold et al. 1990; Vandier et al. 1999; Conde et al. 2006, 2012; Xu et al. 2006) and humans (Tubek et al. 2016). Better characterization of the physiological relevance of adenosine and its generation and/or signalling pathways may provide useful information for identifying new potential targets that reduce CB chemoafferent activity.
Synaptic adenosine may be generated following extracellular breakdown of ATP; a neurotransmitter tonically released from the CB type 1 cell (Piskuric & Nurse, 2013). Conversion of ATP to adenosine requires both membrane bound ectonucleoside triphosphate diphosphohydrolyase 1 (CD39) and ecto‐5′‐nucleotidase (CD73) (Bianchi & Spychala, 2003). The expression of these two enzymes has been confirmed in extracts of the whole rat CB (Salman et al. 2016). Adenosine causes chemostimulation by either increasing type 1 cell excitability (via A2A and/or A2B receptors) (Conde et al. 2006, 2008; Xu et al. 2006; Livermore & Nurse, 2013) or by directly activating postsynaptic sensory fibres (via A2A receptor stimulation) (Conde et al. 2006), with both mechanisms acting to increase cAMP (Nunes et al. 2014; Holmes et al. 2015). Alternatively, it has been proposed that adenosine may be formed in the type 1 cell and then is released directly into the synapse through the bidirectional equilibrative nucleotide transporter (ENT) (Cass et al. 1998).
The present study investigated whether pharmacological targeting of either CD73 or ENT effectively reduces CSN discharge frequency under normoxic or hypoxic conditions. Furthermore, we examined whether ventilation, heart rate, blood pressure and vascular conductance responses to hypoxia were modified by targeting CD73 in vivo. The data obtained suggest that antagonism of CD73 but not ENT inhibits basal CSN frequency and blunts the response to hypoxia. Inhibition of CD73 also attenuates ventilatory and cardiovascular responses to hypoxia. Thus, we propose that CD73e is a novel modulator of CB chemoafferent outflow.
Methods
Ethical approval
The procedures conducted on animals were approved and carried out in line with the current Home Office (UK) and University of Birmingham guidelines on ethical use of animals. Adult male Wistar rats (n = 32) were used for the study and were supplied by Charles River Laboratories (Margate, UK). Animals were housed in individually ventilated cages in the Biomedical Services Unit at the University of Birmingham. Food and water was available ad libitum.
Extracellular recordings of chemoafferent neurones
Intact carotid bifurcations containing the CSN and CB were isolated from adult male Wistar rats (100–200 g) under inhalation anaesthesia (2–4% isoflurane in O2, 3 L min−1). Following tissue procurement, animals were immediately killed by exsanguination. Connective tissue and surrounding structures were excised and the CSN was sectioned exposing nerve fibres and axons. To facilitate the extracellular neuronal recordings, the whole tissue was partially digested by incubation in enzyme Krebs solution (0.075 mg ml−1 collagenase type II, 0.0025 mg ml−1 dispase type I; Sigma‐Aldrich, St Louis, MO, USA), at 37°C, for 20–30 min.
Extracellular recordings of chemoafferent activity were made from the cut end of the CSN as described previously (Holmes et al. 2014, 2016). The superfusate was continuously measured using an O2 electrode (ISO2; World Precision Instruments, Sarasota, FL, USA) and O2 meter (OXELP; World Precision Instruments). The and chemoafferent derived voltage were both recorded using a CED micro1401 (Cambridge Electronic Design, Cambridge, UK) and visualized on a personal computer via Spike2, version 7.1 (Cambridge Electronic Design). Chemoafferent voltage signal was sampled at 15 000 Hz and the at 100 Hz. Single fibres were used for the analysis. Electrical activity originating from a single chemoafferent fibre was determined by its unique ‘wavemark’ signature based on frequency, shape and amplitude.
Throughout experimentation, whole CBs were continuously superfused with a standard bicarbonate buffered Krebs solution containing (in mm): 115 NaCl, 4.5 KCl, 1.25 NaH2PO4, 5 Na2SO4, 1.3 MgSO4, 24 NaHCO3, 2.4 CaCl2 and 11 d‐glucose at 37°C (pH 7.4). For normoxia/hyperoxia, the superfusate was maintained throughout at a level that kept spontaneous chemodischarge between 0.25 and 1.0 Hz (Holmes et al. 2014). was maintained at 40 mmHg. For hypoxia, the superfusate was gradually reduced, at constant , until discharge was elevated above 10 Hz. This was repeated in the presence of pharmacological agents used to target adenosinergic signalling pathways: α,β‐methylene ADP (AOPCP) (100 μm) (Conde & Monteiro, 2004; Holmes et al. 2015); 8‐(p‐sulfophenyl) theophylline (8‐SPT) (300 μm) (Wyatt et al. 2007; Holmes et al. 2015); and nitrobenzylthioinosine (NBTI) (10 μm) (Conde et al. 2012) and again after drug washout.
The single fibre chemoafferent discharge frequency was plotted against the superfusate , and fitted to an exponential decay curve with offset, y = a + be −cx, where, y is the single fibre discharge frequency in Hz, x is the superfusate (in mmHg), a is the discharge frequency as the tends to infinity (offset), b is the discharge frequency when the is 0 mmHg (minus the offset) and c is the exponential rate constant. In addition, for any given discharge frequency, the corresponding could be calculated using the inverse function of the exponential decay curve, x = −(Ln[(y – a)/b)]/c, where x is the (in mmHg), y is the single fibre discharge frequency in Hz and a, b and c are constants as above. The at a frequency of 5 Hz was used to quantify a shift in the hypoxic response curve. A value of 5 Hz was chosen because it lies on the exponential region of the hypoxic response curve but is not of a magnitude at which the discharge is probably has started to diminish.
To evaluate chemoafferent responses to mitochondrial inhibition, the rapidly reversible mitochondrial inhibitor nitrite (NO2 −, 10 mm) was used to induce moderate elevations in chemoafferent discharge (Holmes et al. 2016). Responses to NO2 − were measured under control conditions, in the presence of pharmacological agents targeting adenosinergic signalling pathways, as described above, and again after drug washout.
In vivo ventilatory and cardiovascular responses to hypoxia
Adult male Wistar rats (Charles River Laboratories) were initially anaesthetized with 3–4% isoflurane in O2 at 3–4 L min−1 (Merial Animal Health Ltd, Woking, UK). Following cannulation of the right jugular vein, isoflurane was removed and anaesthesia was maintained with i.v. Alfaxan® (Vétoquinol UK Ltd, Buckingham, UK), at 17–20 mg kg−1 h−1 with 0.1 ml boluses as necessary. Core body temperature was maintained at 37°C with a homeothermic heat pad system (Harvard Apparatus, Cambridge, MA, USA).
The trachea was cannulated and a spirometer was attached to measure airflux; respiratory frequency (f R), tidal volume (V t) and minute ventilation ( E = f R × V t) were derived from this. The tracheal cannula was connected to a system of rotameters in a gas proportioner frame (CP Instruments Co. Ltd, London, UK) allowing variation of inspiratory gases. Animals breathed room air as the normoxic control. For hypoxia, animals inspired hypoxic gas mixture for 2 min periods over the range of 20% to 8% O2. An arterial blood gas sample was taken at each inspired O2. The response to hypoxia was calculated as the mean of the last minute of the exposure to hypoxia.
Arterial blood pressure (ABP) was measured from the right brachial artery and heart rate (HR) was derived. Femoral blood flow (Transonic Systems Inc., Ithica, NY, USA) was measured from the left femoral artery. Arterial blood samples were taken from the right femoral artery, utilizing a looped cannula technique to reduce blood loss when sampling pure mixed arterial blood. Drug infusions of AOPCP (160 μg kg−1, i.v.) were given via the right femoral vein at a dose known to reduce vasodilation evoked by ATP infusion in similar in vivo studies (Skinner & Marshall, 1996).
Statistical analysis
Values are expressed as the mean ± SEM unless otherwise stated. Statistical analysis was performed using (i) a paired two‐tailed Student's t test or (ii) repeated measures one‐way ANOVA with Bonferroni or Dunnett's post hoc analysis where appropriate (StatView, version 5; SAS Institute Inc., Cary, NC, USA; or Prism, version 6; GraphPad Software Inc., San Diego, CA, USA). P < 0.05 was considered statistically significant.
Results
CD73 regulates peripheral chemoreceptor activity in normoxia and hypoxia
Inhibition of CD73 using 100 μm AOPCP (Conde & Monteiro, 2004; Holmes et al. 2015) reduced basal single fibre activity by 76 ± 5% (Fig. 1 A–C). AOPCP also significantly attenuated the CB chemoafferent response to hypoxia. A characteristic example of a single fibre hypoxic response is shown in Fig. 1(A and B) and demonstrates a characteristic leftward shift in the hypoxia response curve caused by AOPCP. The inhibition was rapidly reversible and the original response to hypoxia was almost fully recovered after removal of the agent from the superfusate (Fig. 1 A and B). Mean chemoafferent discharge frequency was significantly depressed over a range of hypoxic superfusate values from 175 to 100 mmHg (Fig. 1 D). Grouped paired measurements showed that AOPCP significantly decreased the superfusate required to elicit a discharge frequency of 5 Hz by a mean value of ∼30 mmHg, which is consistent with blunted hypoxic sensitivity (Fig. 1 E).
Figure 1. CD73 inhibition reduces CB sensory activity in hypoxia.
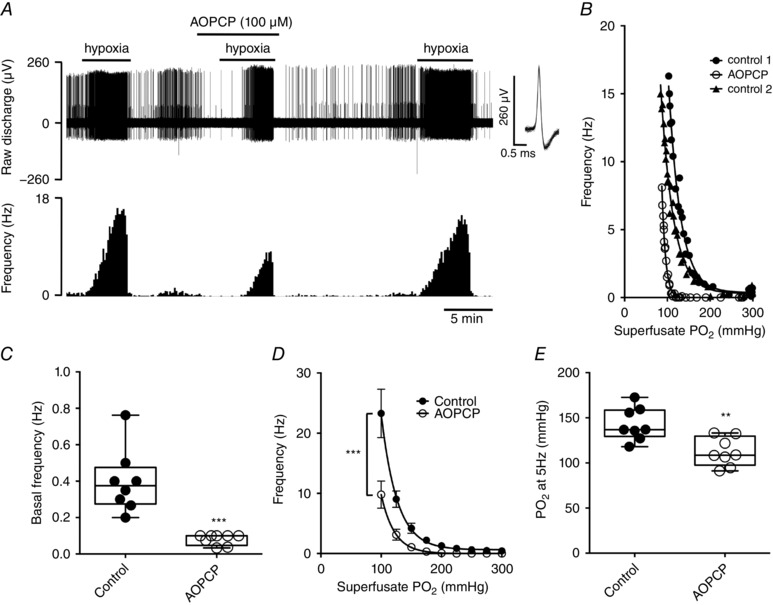
A, characteristic example of CB chemoafferent response to graded hypoxia (300–100 mmHg ) recorded in the presence and absence of 100 μm AOPCP. Overdrawn action potentials demonstrate single fibre discrimination. B, hypoxic response curves for the corresponding fibre recorded in (A). C, mean basal single fibre frequency ± AOPCP. D, mean frequency ± AOPCP during graded hypoxia. E, values required to stimulate a discharge frequency of 5 Hz during hypoxia ± AOPCP. (C) and (E) ** P < 0.01 and *** P < 0.001 AOPCP vs. control (n = 8 fibres, N = 5 CB preparations).
A similar reduction in basal frequency was observed in the presence of the adenosine receptor antagonist 8‐SPT (300 μm) (Wyatt et al. 2007; Holmes et al. 2015)) (Fig. 2 A and B). 8‐SPT decreased chemoafferent activity at all levels, although its inhibitory effect was enhanced during hypoxia (Fig. 2 A). In all fibres tested, the addition of 8‐SPT reduced CB hypoxic sensitivity, as indicated by evoking a leftward shift in the chemoafferent hypoxic response curve, with a mean superfusate reduction at 5 Hz measuring ∼40 mmHg (Fig. 2 B). To determine whether the neuromodulatory actions of adenosine are dependent on release through the ENT, additional hypoxic responses were performed in the presence of NBTI (10 μm, an inhibitor of ENT) (Conde et al. 2012). NBTI did not alter basal single fibre discharge frequency and had no effect on the chemoafferent response to hypoxia (Fig. 2 C and D). NBTI did not significantly alter the required to generate a 5 Hz frequency and produced no change of chemoafferent activity throughout exposure of the CB to graded hypoxia (Fig. 2 C and D).
Figure 2. Inhibition of adenosine receptors but not the ENT blunts CB discharge frequency in hypoxia.
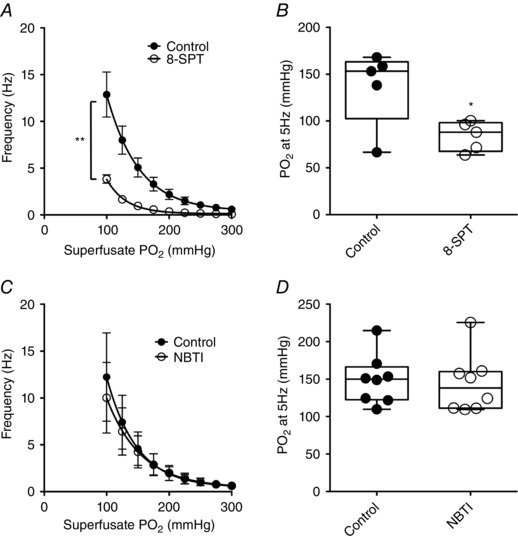
A, mean single fibre frequency ± 8‐SPT (300 μm; adenosine receptor antagonist) during graded hypoxia. B, values at 5 Hz during hypoxia ± 8‐SPT. * P < 0.05 and ** P < 0.01 8‐SPT vs. control (n = 6 fibres, N = 4 CB preparations). C, mean single fibre frequency ± NBTI (10 μm; ENT antagonist) during graded hypoxia. D, values at 5 Hz during hypoxia ± NBTI (n = 8 fibres, N = 4 CB preparations).
CD73 and adenosine mediate chemoreceptor responses to mitochondrial inhibitors
CB activation by hypoxia is commonly ascribed to be a direct consequence of depletion in mitochondrial energy metabolism (Buckler & Turner, 2013; Holmes et al. 2016). Thus, AOPCP and 8‐SPT should have similar effects on CB responses to mitochondrial inhibitors. An example trace demonstrating the impact of 100 μm AOPCP on the response to mitochondrial inhibition using NO2 − is shown in Fig. 3(A). AOPCP almost completely abolished basal frequency and the response to NO2 − (Fig. 3 B). The absolute elevation in chemoafferent activity evoked by NO2 − (NO2 − – basal) was significantly decreased in the presence of AOPCP, measuring only ∼0.4% of control (Fig. 3 C). 8‐SPT (300 μm) also significantly diminished frequency responses to NO2 − (Fig. 3 D and E). The rise in discharge frequency elicited by NO2 − in the presence of 8‐SPT was reduced in each fibre studied, with the mean NO2 −‐induced frequency elevation in the presence of 8‐SPT measuring only ∼20% of control (Fig. 3 D). Thus, AOPCP and 8‐SPT are effective in attenuating CB responses to both hypoxia and mitochondrial inhibition.
Figure 3. Carotid body stimulation by mitochondrial inhibition is attenuated by antagonism of CD73 and adenosine receptors.
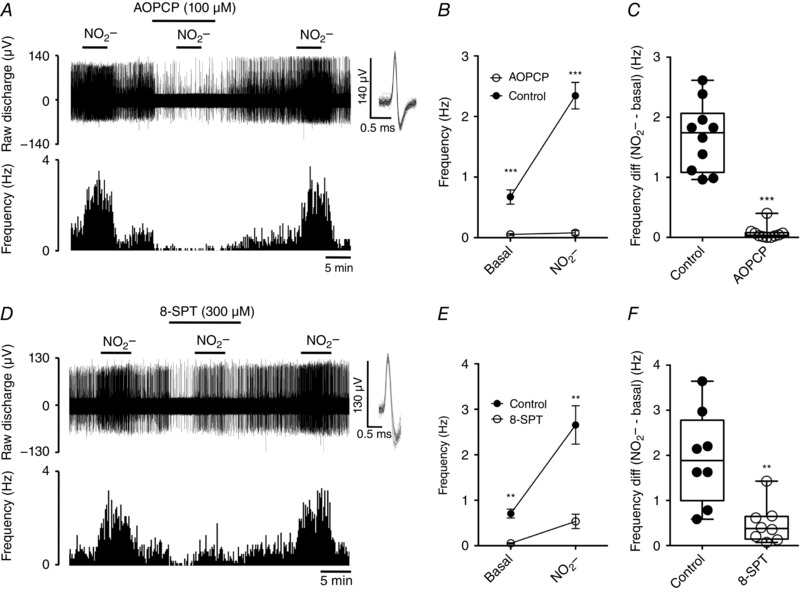
A, example recording of the CB sensory response to the mitochondrial inhibitor NO2 − (10 mm) in the presence and absence of AOPCP (100 μm). Overdrawn action potentials demonstrate single fibre discrimination. B, mean discharge frequencies recorded under basal conditions and following addition of NO2 − ± AOPCP. C, frequency differences (NO2 − – basal) for each fibre in the presence and absence of AOPCP. *** P < 0.001 AOPCP vs. control (n = 10 fibres, N = 5 CB preparations). D, recording of the CB sensory response to NO2 − in the presence and absence of 8‐SPT (300 μm). E, mean discharge frequencies recorded under basal conditions and following addition of NO2 − 8‐SPT. F, frequency differences (NO2 − – basal) for each fibre in the presence and absence of 8‐SPT. ** P < 0.01 8‐SPT vs. control (n = 8 fibres, N = 5 CB preparations).
CD73 inhibition reduces hypoxic ventilatory sensitivity
During normoxic breathing, AOPCP elevated baseline minute ventilation (control 187 ± 10; AOPCP 195 ± 10 ml min−1, n = 9), an effect that was the result of an increase in tidal volume but not respiratory frequency (Fig. 4 A–C). Accordingly, under these normoxic conditions, AOPCP significantly reduced (control 45 ± 1; AOPCP 39 ± 1 mmHg, P < 0.05, paired two‐tailed Student's t test, n = 9), consistent with hyperventilation. Graded hypoxia increased minute ventilation, respiratory frequency and tidal volume (Fig. 4 A–C) and reduced (Fig. 5 A) in the control and in the presence of AOPCP. However, AOPCP attenuated the magnitude of the hypoxic ventilatory response (HVR) (e.g. at 40 mmHg ; Δ E control 74 ± 6%, Δ E AOPCP 64 ± 5%, n = 9) (Fig. 4 F). This attenuation in HVR was dependent on a reduction in the hypoxia‐induced elevation in tidal volume but not respiratory frequency (Fig. 4 D–F). AOPCP also reduced the hypoxic exponential ventilatory rate constant (control 0.032 ± 0.0044; AOPCP 0.022 ± 0.003, n = 9, P < 0.01, paired t test), signifying a decrease in hypoxic ventilatory sensitivity. Blood gas analysis revealed that the magnitude of the decrease in caused by hypoxia was also attenuated by AOPCP (Fig. 5 A and B). Collectively, these results suggest that, although systemic pharmacological inhibition of CD73 causes a baseline increase in ventilation, it also significantly attenuates the HVR.
Figure 4. Ventilatory responses to hypoxia are reduced by antagonism of CD73.
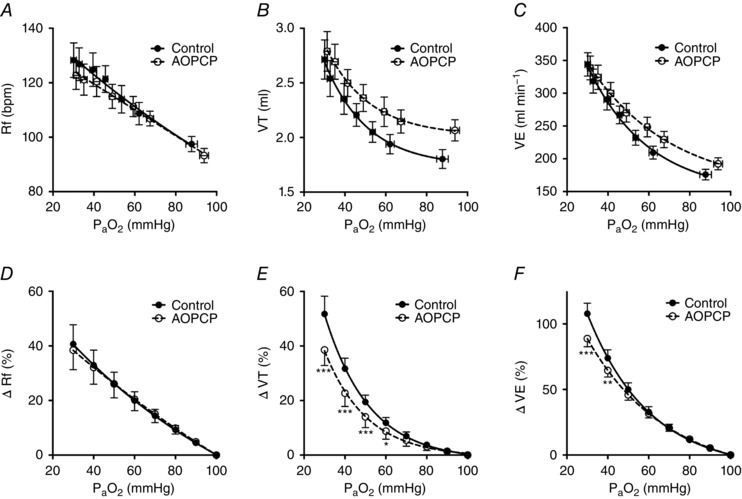
A–C, f R, V t and E at baseline and in response to graded hypoxia ± AOPCP (160 μg.kg−1, i.v.). D and E, percentage changes in f R, V t and E at different calculated compared to 100 mmHg baseline for control and during AOPCP infusion. (D) to (F) * P < 0.05, ** P < 0.01 and *** P < 0.001 AOPCP vs. control (N = 9).
Figure 5. Hypoxia induced fall in is blunted by inhibition of CD73.
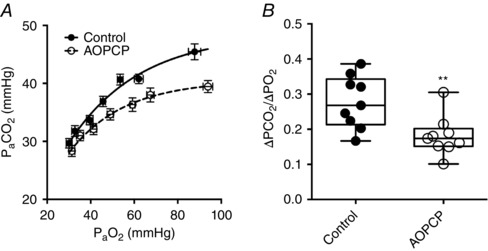
A, absolute changes in during graded hypoxia in the presence and absence of AOPCP. B, calculated reduction in per mmHg fall in , in the presence and absence of AOPCP. ** P < 0.01 (N = 9).
CD73 mediates cardiovascular responses to hypoxia
Consistent with the baseline hyperventilation, AOPCP increased heart rate (control 441 ± 12; AOPCP 460 ± 10 beats min–1, n = 9) and reduced femoral vascular conductance (control 0.016 ± 0.001; AOPCP 0.012 ± 0.001 ml min−1 mmHg−1, n = 8) during normoxic air breathing, although this did not cause an increase in mean arterial blood pressure (MABP) (Fig. 6 A–C). AOPCP attenuated the hypoxia induced rise in HR (e.g. at 40 mmHg ; ΔHR control 15 ± 4%, ΔHR AOPCP 7 ± 3%, n = 9), enhanced the hypoxic increase in femoral vascular conductance (FVC) (e.g. at 40 mmHg ; ΔFVC control 11 ± 5 %, ΔFVC AOPCP 29 ± 5 %, n = 8) and exaggerated the fall in MABP (e.g. at 40 mmHg ; ΔMABP control −19 ± 3%, ΔMABP AOPCP –26 ± 4%, n = 9) (Fig. 6 D–F).
Figure 6. Cardiovascular responses to hypoxia are modified by inhibition of CD73.
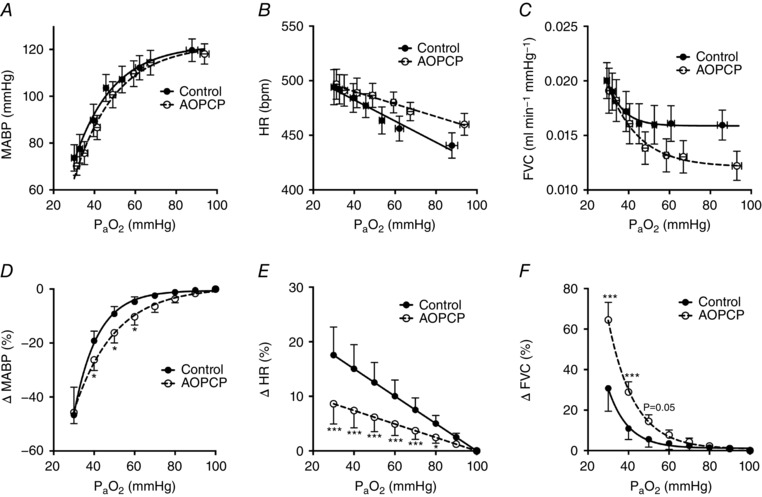
A–C, MABP, HR and FVC during air breathing and in response to graded hypoxia ± AOPCP (160 μg kg−1, i.v.). D–E, percentage changes in MABP, HR and FVC at different calculated compared to 100 mmHg baseline for control and AOPCP infusion. (D) to (F) * P < 0.05 and *** P < 0.001 AOPCP vs. control [N = 9 (MABP and HR), N = 8 FVC].
Discussion
The present study identifies CD73 as a novel functional protein that contributes to CB and whole body cardiorespiratory responses to acute hypoxia. Inhibition of CD73 activity dampens CB sensory neuronal discharge in vitro, both under basal conditions and during stimulation by hypoxia. In addition, pharmacological inhibition of CD73 in vivo reduces the HVR and attenuates the hypoxia‐induced rise in HR. Future investigations could evaluate whether targeting CB CD73 activity may be effective in reducing persistent CB hyperactivity in selected pathologies.
CD73 is a novel regulator of CB sensory discharge in normoxia and hypoxia
Emerging evidence suggests that a chronic increase in CB sensory output in normoxia and hypoxia contributes significantly to hypertension and cardiac arrhythmia in patients and animal models of sleep disordered breathing, heart failure and diabetes (Ribeiro et al. 2013; Schultz et al. 2015; Del Rio et al. 2016; Prabhakar, 2016). This is a result of the CB driving a chronic rise in sympathetic neuronal outflow to the vasculature and heart, thereby leading to persistent vasoconstriction and pro‐arrhythmic cardiac autonomic imbalance (Narkiewicz et al. 1998; Peng et al. 2003; Del Rio et al. 2016; Linz et al. 2016). A number of recent small‐scale clinical studies suggest that surgical uni‐ or bilateral CB ablation may be beneficial in reducing sympathetic outflow and arterial blood pressure in some patient cohorts (Narkiewicz et al. 2016; Niewinski et al. 2017). However, widespread use as a first‐line treatment option for CB mediated hypertension may be limited because of potential safety concerns of complete loss of CB sensory activity, such as exaggerated O2 desaturation during sleep (Niewinski et al. 2017). This safety concern may be particularly relevant in patients with obstructive sleep apnoea who rely on CB sensory activity to cause arousal and recovery of airway patency. Furthermore, uni‐lateral CB resection appears to confer a relatively short‐term (up to 12 months) reduction in blood pressure and is effective in only ∼50% of hypertensive patients (Narkiewicz et al. 2016). Thus, identifying new potential drug targets in the CB that can be targeted to reduce (but not abolish) chemosensory activity, may offer a safer and more applicable treatment for this form of CB mediated hypertension.
In the present study, we provide the first functional evidence indicating that CD73 mediates the CB sensory discharge frequency in hypoxia. CD73 catalyses the formation of adenosine from AMP, following the initial breakdown of ATP and ADP by CD39 (Bianchi & Spychala, 2003). In the CB, a significant synaptic ATP concentration is a result of the tonic vesicular neurosecretion from the type 1 cell (Zhang & Nurse, 2004) and also through ATP release from type 2 cells via pannexin‐1 channels (Murali & Nurse, 2016). The attenuation of sensory discharge by inhibiting CD73 is therefore most probably the result of a reduction in adenosine formation. Indeed, we show that antagonism of adenosine receptors reduced normoxic and hypoxic CB sensory activity by an amount that was similar to inhibition of the CD73. Furthermore, antagonism of CD73 and adenosine receptors decreases the threshold required for CB activation during hypoxia, which is consistent with a comparable reduction in CB hypoxic sensitivity.
Adenosine generated from CD73 has the potential to modulate CB hypoxic sensitivity via activation of A2A and A2B receptors on the type 1 cell (Conde et al. 2006, 2008; Xu et al. 2006; Livermore & Nurse, 2013) or by stimulating the nerve ending upon binding to postsynaptic A2A receptors (Conde et al. 2006). Both of these mechanisms are probable given that CD73 will increase extracellular adenosine in the type 1 cell‐chemoafferent synapse, thus having access to both pre‐ and postsynaptic receptors. Despite 8‐SPT potentially also targeting A1 receptors, a functional role is improbable in our studies given the absence of A1 mRNA expression in the rat CB (Gauda, 2000; Kobayashi et al. 2000).
Importantly, we also demonstrate that pharmacological antagonism of CD73 reduces chemoafferent activity in hypoxia without completely abolishing it. Thus, effective targeting of CD73 would still allow for transmission of a definitive hypoxic neuronal signal into the CNS, albeit it being significantly reduced. Demonstration of significant CD73 mRNA expression in the rat CB has been confirmed (Salman et al. 2016). However, the precise localization of CD73 and CD39 remains to be defined. Given the known co‐localization between CD73 and A2A receptors in striatal neurones in the CNS (Augusto et al. 2013), we suggest that CD73 in the CB is probably located similarly (i.e. on type 1 cells and nerve endings).
By contrast, inhibitory targeting of the ENT had no effect on the normoxic or hypoxic discharge frequency, suggesting that insufficient quantities of adenosine are released through ENT in normoxia and hypoxia to impact directly on hypoxic neuronal discharge. This is consistent with earlier observations demonstrating that extracellular adenosine recovery in normoxia was independent of ENT activity (Conde & Monteiro, 2004; Conde et al. 2012).
A direct up‐regulation of CD73 activity in the CB in chronic or chronic intermittent hypoxia remains to be confirmed. However, recent studies suggest that ATP and 5‐HT can both augment ATP release from type 2 cells, thereby amplifying the synaptic ATP concentration (Murali & Nurse, 2016; Murali et al. 2017). Our data suggests that increased CD73 activity driven by this increased availability of ATP would act to augment CB O2 sensitivity, which may contribute to the exaggerated chemoafferent responses observed in chronic hypoxia or chronic intermittent hypoxia.
Our data also show that inhibition of CD73 and adenosine receptors attenuates sensory discharge frequency in response to mitochondrial inhibition. This is important not only because a run‐down in mitochondrial energy metabolism is considered to be a necessary step in coupling hypoxia with type 1 cell stimulation (Duchen & Biscoe, 1992; Buckler & Turner, 2013; Holmes et al. 2016), but also in view of the evidence that chronic impairment of mitochondrial function is associated with the increase in CB sensory activity in animal models of obstructive sleep apnoea (Peng et al. 2003). Thus, our data support the idea that functional inhibition of CD73 inhibits basal and hypoxic discharge frequency and responses to stimuli relevant to CB pathology.
CD73 reduces HVR and modifies cardiovascular responses to hypoxia
Intravenous infusion of AOPCP to inhibit CD73 in vivo caused an inhibition of HVR (Fig. 4) and evoked a smaller reduction in during hypoxia (Fig. 5), suggestive of a less marked hyperventilation. We also calculated that the hypoxic ventilatory exponential rate constant is depressed in the presence of AOPCP. These observations are in agreement with our in vitro data and suggest that AOPCP effectively blunts CB hypoxic sensitivity in vivo. Previous work has also show that 8‐SPT (a compound that does not cross the blood–brain barrier) also inhibits the acute phase of the HVR in rats (Lee et al. 2005), an action that our data implies is a result of adenosine being important in establishing the CB sensitivity to hypoxia (Fig. 2).
AOPCP also reduced the HR response to hypoxia and exaggerated rise in FVC and fall in MABP. These data are consistent with a reduced elevation in sympathetic outflow to the heart and vasculature in hypoxia caused by CD73 inhibition. In view of the positive correlation between CB chemodischarge and sympathetic outflow during hypoxic stimulation, the reduction in sympathetic activity is probably a result of reduced CB stimulation. In addition, this could also be the result of a lower level of hyperventilation and lung stretch receptor activation, which again is a consequence of blunted CB hypoxic chemodischarge and respiratory drive. If similar findings are validated in humans, then pharmacological inhibition of CB CD73 may offer a novel and important means of reducing cardiovascular sympathetic outflow in CB related pathology.
Nevertheless, AOPCP also evoked a significant hyperventilation under normoxic conditions, as indicated by a significant decrease in . It also provoked a rise in baseline HR and reduced FVC in normoxia. CD73 is known to be expressed in brain regions where it regulates adenosine generation (Chu et al. 2014). Furthermore, A1 receptor activation depresses central respiratory drive (Montandon et al. 2007). Although speculative, we therefore suggest that hyperventilation under normoxic conditions is a consequence of a reduction in adenosine concentration in the central respiratory brain regions following the inhibition of cerebral CD73. Such data raise the intriguing possibility that CD73 may also have an important functional role in mediating central respiratory drive under normoxic conditions. These findings also suggest that selective targeting of the CB CD73 or the development of nucleotidase inhibitors that do not cross the blood–brain barrier could be necessary to selectively reduce CB sensory activity.
Limitations
The present studies were performed in rodents and the validation of a functional role for CB CD73 is necessary in humans. Our in vivo experiments were performed specifically using Alfaxan®, an anaesthetic that improves preservation of cardiovascular reflexes. However, we cannot rule out that there would be some suppression of chemoreceptor function, although we speculate that, if this is the case, then our estimates of a reduction in HVR caused by CD73 may be an underestimate. Furthermore, our experiments used a pharmacological approach to probe the importance of CD73 in CB function and HVR. We targeted CD73 with AOPCP because this is known to be selective for membrane‐bound CD73 and has been used as such previously to distinguish the activities of membrane bound and cytosolic nucleotidases (Sala‐Newby et al. 2003). However, to confirm our findings, it would be interesting to perform similar experiments in mice deficient in CD73, ideally restricted to CB or tyrosine hydroxylase positive cells. Finally, in view of the present findings, an evaluation of the expression levels of CB CD73 and the contribution to CB sensory activity in diseases such as obstructive sleep apnoea, heart failure and diabetes is warranted.
Conclusions
CD73 is a novel mediator of CB chemoafferent activity. Inhibition of CD73 reduces basal CB sensory neuronal activity and attenuates responses to hypoxia. Inhibition of CD73 in vivo blunts HVR and modifies cardiovascular changes in hypoxia, including reduced HR elevation. Future studies will be important to assess whether CD73 may be a feasible target for reducing CB activity and sympathetic outflow in CB related cardiovascular pathology.
Additional information
Competing interests
The authors declare that they have no competing interests.
Author contributions
PK, APH, CJR and AMC devised the experimental concepts and were responsible for the study design. APH, CJR, SAP and AMC collected data. APH, CJR, AMC and PK interpreted and analysed data. APH carried out the original drafting of the manuscript, which was edited by all of the authors. All authors approve of the final version of the manuscript, and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved, and all persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
This work was supported financially by the College of Medical and Dental Sciences, University of Birmingham, UK. APH was supported by the A. E. Hills Scholarship awarded by the Postgraduate School, University of Birmingham, UK.
References
- Augusto E, Matos M, Sevigny J, El‐Tayeb A, Bynoe MS, Muller CE, Cunha RA & Chen JF (2013). Ecto‐5′‐nucleotidase (CD73)‐mediated formation of adenosine is critical for the striatal adenosine A(2A) receptor functions. J Neurosci 33, 11390–11399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi V & Spychala J (2003). Mammalian 5′‐nucleotidases. J Biol Chem 278, 46195–46198. [DOI] [PubMed] [Google Scholar]
- Buckler KJ & Turner PJ (2013). Oxygen sensitivity of mitochondrial function in rat arterial chemoreceptor cells. J Physiol 591, 3549–3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cass CE, Young JD & Baldwin SA (1998). Recent advances in the molecular biology of nucleoside transporters of mammalian cells. Biochem Cell Biol 76, 761–770. [DOI] [PubMed] [Google Scholar]
- Chu S, Xiong W & Parkinson FE (2014). Effect of ecto‐5′‐nucleotidase (eN) in astrocytes on adenosine and inosine formation. Purinergic Signal 10, 603–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde SV, Gonzalez C, Batuca JR, Monteiro EC & Obeso A (2008). An antagonistic interaction between A2B adenosine and D2 dopamine receptors modulates the function of rat carotid body chemoreceptor cells. J Neurochem 107, 1369–1381. [DOI] [PubMed] [Google Scholar]
- Conde SV & Monteiro EC (2004). Hypoxia induces adenosine release from the rat carotid body. J Neurochem 89, 1148–1156. [DOI] [PubMed] [Google Scholar]
- Conde SV, Monteiro EC, Rigual R, Obeso A & Gonzalez C (2012). Hypoxic intensity: a determinant for the contribution of ATP and adenosine to the genesis of carotid body chemosensory activity. J Appl Physiol 112, 2002–2010. [DOI] [PubMed] [Google Scholar]
- Conde SV, Obeso A, Vicario I, Rigual R, Rocher A & Gonzalez C (2006). Caffeine inhibition of rat carotid body chemoreceptors is mediated by A2A and A2B adenosine receptors. J Neurochem 98, 616–628. [DOI] [PubMed] [Google Scholar]
- Del Rio R, Andrade DC, Lucero C, Arias P & Iturriaga R (2016). Carotid body ablation abrogates hypertension and autonomic alterations induced by intermittent hypoxia in rats. Hypertension 68, 436–445. [DOI] [PubMed] [Google Scholar]
- Duchen MR & Biscoe TJ (1992). Relative mitochondrial membrane potential and [Ca2+]i in type I cells isolated from the rabbit carotid body. J Physiol 450, 33–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald RS, Shirahata M & Wang HY (1999). Acetylcholine release from cat carotid bodies. Brain Res 841, 53–61. [DOI] [PubMed] [Google Scholar]
- Gauda EB (2000). Expression and localization of A2a and A1‐adenosine receptor genes in the rat carotid body and petrosal ganglia. A2a and A1‐adenosine receptor mRNAs in the rat carotid body. Adv Exp Med Biol 475, 549–558. [PubMed] [Google Scholar]
- Hauton D, Holmes A, Ziff O & Kumar P (2013). The impact of acute and chronic catecholamines on respiratory responses to hypoxic stress in the rat. Pflügers Arch 465, 209–219. [DOI] [PubMed] [Google Scholar]
- Holmes AP, Nunes AR, Cann MJ & Kumar P (2015). Ecto‐5′‐nucleotidase, adenosine and transmembrane adenylyl cyclase signalling regulate basal carotid body chemoafferent outflow and establish the sensitivity to hypercapnia In Arterial Chemoreceptors in Physiology and Pathophysiology, eds Peers C, Kumar P, Wyatt CN, Gauda E, Nurse CA. & Prabhakar N, pp. 279–289. Springer‐Verlag Berlin, Berlin. [DOI] [PubMed] [Google Scholar]
- Holmes AP, Turner PJ, Buckler KJ & Kumar P (2016). Moderate inhibition of mitochondrial function augments carotid body hypoxic sensitivity. Pflügers Arch 468, 143–155. [DOI] [PubMed] [Google Scholar]
- Holmes AP, Turner PJ, Carter P, Leadbeater W, Ray CJ, Hauton D, Buckler KJ & Kumar P (2014). Glycogen metabolism protects against metabolic insult to preserve carotid body function during glucose deprivation. J Physiol 592, 4493–4506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S, Conforti L & Millhorn DE (2000). Gene expression and function of adenosine A(2A) receptor in the rat carotid body. Am J Physiol Lung Cell Mol Physiol 279, L273–L282. [DOI] [PubMed] [Google Scholar]
- Kumar P & Prabhakar NR (2012). Peripheral chemoreceptors: function and plasticity of the carotid body. Compr Physiol 2, 141–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SD, Nakano H & Farkas GA (2005). Adenosinergic modulation of ventilation in obese zucker rats. Obes Res 13, 545–555. [DOI] [PubMed] [Google Scholar]
- Linz D, Linz B, Hohl M & Bohm M (2016). Atrial arrhythmogenesis in obstructive sleep apnea: therapeutic implications. Sleep Med Rev 26, 87–94. [DOI] [PubMed] [Google Scholar]
- Livermore S & Nurse CA (2013). Enhanced adenosine A2b receptor signaling facilitates stimulus‐induced catecholamine secretion in chronically hypoxic carotid body type I cells. Am J Physiol Cell Physiol 305, C739–C750. [DOI] [PubMed] [Google Scholar]
- McQueen DS & Ribeiro JA (1986). Pharmacological characterization of the receptor involved in chemoexcitation induced by adenosine. Br J Pharmacol 88, 615–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montandon G, Kinkead R & Bairam A (2007). Disruption of adenosinergic modulation of ventilation at rest and during hypercapnia by neonatal caffeine in young rats: role of adenosine A(1) and A(2A) receptors. Am J Physiol Regul Integr Comp Physiol 292, R1621–R1631. [DOI] [PubMed] [Google Scholar]
- Murali S & Nurse CA (2016). Purinergic signalling mediates bidirectional crosstalk between chemoreceptor typeI and glial‐like typeII cells of the rat carotid body. J Physiol‐London 594, 391–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murali S, Zhang M & Nurse CA (2017). Evidence that 5‐HT stimulates intracellular Ca2+ signalling and activates pannexin‐1 currents in type II cells of the rat carotid body. J Physiol 595, 4261–4277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narkiewicz K, Ratcliffe LE, Hart EC, Briant LJ, Chrostowska M, Wolf J, Szyndler A, Hering D, Abdala AP, Manghat N, Burchell AE, Durant C, Lobo MD, Sobotka PA, Patel NK, Leiter JC, Engelman ZJ, Nightingale AK & Paton JF (2016). Unilateral carotid body resection in resistant hypertension: a safety and feasibility trial. JACC Basic Transl Sci 1, 313–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narkiewicz K, van de Borne PJH, Montano N, Dyken ME, Phillips BG & Somers VK (1998). Contribution of tonic chemoreflex activation to sympathetic activity and blood pressure in patients with obstructive sleep apnea. Circulation 97, 943–945. [DOI] [PubMed] [Google Scholar]
- Niewinski P, Janczak D, Rucinski A, Tubek S, Engelman ZJ, Piesiak P, Jazwiec P, Banasiak W, Fudim M, Sobotka PA, Javaheri S, Hart EC, Paton JF & Ponikowski P (2017). Carotid body resection for sympathetic modulation in systolic heart failure: results from first‐in‐man study. Eur J Heart Fail 19, 391–400. [DOI] [PubMed] [Google Scholar]
- Nunes AR, Holmes AP, Conde SV, Gauda EB & Monteiro EC (2014). Revisiting cAMP signaling in the carotid body. Front Physiol 5, 406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurse CA (2010). Neurotransmitter and neuromodulatory mechanisms at peripheral arterial chemoreceptors. Exp Physiol 95, 657–667. [DOI] [PubMed] [Google Scholar]
- Peng YJ, Overholt JL, Kline D, Kumar GK & Prabhakar NR (2003). Induction of sensory long‐term facilitation in the carotid body by intermittent hypoxia: implications for recurrent apneas. Proc Natl Acad Sci USA 100, 10073–10078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piskuric NA & Nurse CA (2013). Expanding role of ATP as a versatile messenger at carotid and aortic body chemoreceptors. J Physiol 591, 415–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakar NR (2016). Carotid body chemoreflex: a driver of autonomic abnormalities in sleep apnoea. Exp Physiol 101, 975–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro MJ, Sacramento JF, Gonzalez C, Guarino MP, Monteiro EC & Conde SV (2013). Carotid body denervation prevents the development of insulin resistance and hypertension induced by hypercaloric diets. Diabetes 62, 2905–2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runold M, Cherniack NS & Prabhakar NR (1990). Effect of adenosine on isolated and superfused cat carotid body activity. Neurosci Lett 113, 111–114. [DOI] [PubMed] [Google Scholar]
- Sala‐Newby GB, Freeman NVE, Curto MA & Newby AC (2003). Metabolic and functional consequences of cytosolic 5′‐nucleotidase‐IA overexpression in neonatal rat cardiomyocytes. Am J Physiol Heart Circul Physiol 285, H991–H998. [DOI] [PubMed] [Google Scholar]
- Salman S, Vollmer C & Nurse CA (2016). Characterization of ectonucleotidase expression in the rat carotid body: potential regulation by hypoxia? FASEB J 30, 983.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz HD, Marcus NJ & Del Rio R (2015). Mechanisms of carotid body chemoreflex dysfunction during heart failure. Exp Physiol 100, 124–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner MR & Marshall JM (1996). Studies on the roles of ATP, adenosine and nitric oxide in mediating muscle vasodilatation induced in the rat by acute systemic hypoxia. J Physiol 495, 553–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson EL, Ray CJ, Holmes AP, Pye RL, Wyatt CN, Coney AM & Kumar P (2016). Adrenaline release evokes hyperpnoea and an increase in ventilatory CO2 sensitivity during hypoglycaemia: a role for the carotid body. J Physiol 594, 4439–4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tubek S, Niewinski P, Reczuch K, Janczak D, Rucinski A, Paleczny B, Engelman ZJ, Banasiak W, Paton JF & Ponikowski P (2016). Effects of selective carotid body stimulation with adenosine in conscious humans. J Physiol 594, 6225–6240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandier C, Conway AF, Landauer RC & Kumar P (1999). Presynaptic action of adenosine on a 4‐aminopyridine‐sensitive current in the rat carotid body. J Physiol 515, 419–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt CN, Mustard KJ, Pearson SA, Dallas ML, Atkinson L, Kumar P, Peers C, Hardie DG & Evans AM (2007). AMP‐activated protein kinase mediates carotid body excitation by hypoxia. J Biol Chem 282, 8092–8098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F, Xu J, Tse FW & Tse A (2006). Adenosine stimulates depolarization and rise in cytoplasmic [Ca2+] in type I cells of rat carotid bodies. Am J Physiol Cell Physiol 290, C1592–C1598. [DOI] [PubMed] [Google Scholar]
- Zhang M & Nurse CA (2004). CO2/pH chemosensory signaling in co‐cultures of rat carotid body receptors and petrosal neurons: role of ATP and ACh. J Neurophysiol 92, 3433–3445. [DOI] [PubMed] [Google Scholar]
- Zhang M, Zhong H, Vollmer C & Nurse CA (2000). Co‐release of ATP and ACh mediates hypoxic signalling at rat carotid body chemoreceptors. J Physiol 525, 143–158. [DOI] [PMC free article] [PubMed] [Google Scholar]


