Abstract
Key points
Superposition of hypoxia on 21 day bed rest did not worsen the impairment of skeletal muscle oxidative function induced by bed rest alone.
A significant impairment of maximal oxidative performance was identified downstream of cardiovascular O2 delivery, involving both the intramuscular matching between O2 supply and utilization and mitochondrial respiration.
These chronic adaptations appear to be relevant in terms of exposure to spaceflights and reduced gravity habitats (Moon or Mars), as characterized by low gravity and hypoxia, in patients with chronic diseases characterized by hypomobility/immobility and hypoxia, as well as in ageing.
Abstract
Skeletal muscle oxidative function was evaluated in 11 healthy males (mean ± SD age 27 ± 5 years) prior to (baseline data collection, BDC) and following a 21 day horizontal bed rest (BR), carried out in normoxia ( = 133 mmHg; N‐BR) and hypoxia ( = 90 mmHg; H‐BR). H‐BR was aimed at simulating reduced gravity habitats. The effects of a 21 day hypoxic ambulatory confinement ( = 90 mmHg; H‐AMB) were also assessed. Pulmonary O2 uptake (), vastus lateralis fractional O2 extraction (changes in deoxygenated haemoglobin + myoglobin concentration, Δ[deoxy(Hb + Mb)]; near‐infrared spectroscopy) and femoral artery blood flow (ultrasound Doppler) were evaluated during incremental one‐leg knee‐extension exercise (reduced constraints to cardiovascular O2 delivery) carried out to voluntary exhaustion in a normoxic environment. Mitochondrial respiration was evaluated ex vivo by high‐resolution respirometry in permeabilized vastus lateralis fibres. decreased (P < 0.05) after N‐BR (0.98 ± 0.13 L min−1) and H‐BR (0.96 ± 0.17 L min−1) vs. BDC (1.05 ± 0.14 L min−1). In the presence of a decreased (by ∼6–8%) thigh muscle volume, normalized per unit of muscle mass was not affected by both interventions. Δ[deoxy(Hb + Mb)]peak decreased (P < 0.05) after N‐BR (65 ± 13% of limb ischaemia) and H‐BR (62 ± 12%) vs. BDC (73 ± 13%). H‐AMB did not alter or Δ[deoxy(Hb + Mb)]peak. An overshoot of Δ[deoxy(Hb + Mb)] was evident during the first minute of unloaded exercise after N‐BR and H‐BR. Arterial blood flow to the lower limb during both unloaded and peak knee extension was not affected by any intervention. Maximal ADP‐stimulated mitochondrial respiration decreased (P < 0.05) after all interventions vs. control. In 21 day N‐BR, a significant impairment of oxidative metabolism occurred downstream of cardiovascular O2 delivery, affecting both mitochondrial respiration and presumably the intramuscular matching between O2 supply and utilization. Superposition of H on BR did not worsen the impairment induced by BR alone.
Keywords: planetary habitats, microgravity, hypoxia, mitochondrial respiration, skeletal muscle oxidative metabolism
Key points
Superposition of hypoxia on 21 day bed rest did not worsen the impairment of skeletal muscle oxidative function induced by bed rest alone.
A significant impairment of maximal oxidative performance was identified downstream of cardiovascular O2 delivery, involving both the intramuscular matching between O2 supply and utilization and mitochondrial respiration.
These chronic adaptations appear to be relevant in terms of exposure to spaceflights and reduced gravity habitats (Moon or Mars), as characterized by low gravity and hypoxia, in patients with chronic diseases characterized by hypomobility/immobility and hypoxia, as well as in ageing.
Introduction
Bed rest (BR) studies are widely utilized as experimental models for evaluating the physiological consequences of prolonged muscle disuse and unloading (Pavy‐Le Traon et al. 2007), as experienced by astronauts in microgravity, as well as by individuals exposed to injuries, chronic diseases and ageing. Prolonged BR periods greatly affect oxidative metabolism by acting at several levels of the O2 pathway, with the skeletal muscles being one of these (Pavy‐Le Traon et al. 2007; Ade et al. 2015). In previous BR studies, by utilizing an exercise paradigm (one‐leg knee extension) characterized by markedly elevated blood flows (Richardson et al. 1993), in which any impairment of oxidative function intrinsic to skeletal muscle becomes fully manifest, we confirmed the presence of functional constraints to oxidative function ‘downstream’ of bulk cardiovascular O2 delivery (Porcelli et al. 2010; Salvadego et al. 2011).
Spaceflights and future reduced gravity habitats (on the Moon or Mars) are low gravity environments that will expose astronauts not only to a condition of muscle disuse/unloading, but also to a substantial hypoxic stimulus. Hypoxic spacecraft are planned to minimize the risk of decompression sickness and thus to facilitate extra‐vehicular activity on the Moon and Mars. Similar combined stressors of muscle disuse and hypoxia are also encountered in pulmonary, cardiovascular and metabolic diseases, as well as in ageing.
Hypoxia per se can enhance or deteriorate oxidative function, depending on factors such as time of exposure, degree of hypoxia, association with exercise and energy balance (Cerretelli & Hoppeler, 1996; Calbet et al. 2003; Jacobs et al. 2012, 2013). Thus, when associated with physical inactivity, the hypoxic stimulus could preserve or further impair oxidative function, with relevant consequences for exercise tolerance and the quality of life of subjects/astronauts/patients. In a previous study, we observed that the superposition of hypoxia ( = 0.141; corresponding to an altitude of ∼4000 m) during a 10‐day horizontal BR did not aggravate the functional impairment of oxidative metabolism in vivo induced by BR alone and also did not affect mitochondrial respiration ex vivo (Salvadego et al. 2016). Rather unexpectedly, superposition of hypoxia actually attenuated/prevented some of the impairments observed after BR alone (Salvadego et al. 2016). This raised questions regarding the potential interactive effects of hypoxia and bed rest during longer periods of exposure, with the aim of better relating the results to long‐term spaceflights and chronic adaptations to reduced gravity habitats.
The present ‘PlanHab: Planetary Habitat Simulation’ project was specifically designed to investigate the interaction between horizontal BR and hypoxia on human physiological systems over a longer‐term exposure. In accordance with a randomized cross‐over protocol, healthy young male subjects were exposed for 21 days to normoxic horizontal BR (N‐BR), hypoxia ( = 0.141; H) during upright ambulation (H‐AMB), and combined BR and hypoxia (H‐BR); more details concerning the experimental protocol are provided in Debevec et al. (2014). The hypoxic stimulus ( ∼90 mmHg), corresponding to ∼4000 m of altitude, is similar (actually slightly ‘stronger’) to that expected in future habitats on the Moon or Mars (David et al. 2006). A 3‐week time period characterizes medium‐term bed rest campaigns, in accordance with ESA standards, and is typically adopted for studying chronic adaptations to physical inactivity/unloading.
In another study deriving from the PlanHab project, Keramidas et al. (2016a) observed that a 21 day H‐BR induced a greater reduction in peak pulmonary () during exercise on a cycle ergometer than N‐BR. In the present study, the functional evaluation of oxidative metabolism in vivo was carried out during knee‐extensor exercise, a paradigm in which the functional evaluation is mainly focused downstream of cardiovascular O2 delivery (Andersen et al. 1985; Richardson et al. 1993; Salvadego et al. 2011, 2016), inside skeletal muscle. Vastus lateralis fractional O2 extraction and indices evaluating the intramuscular matching between O2 delivery and O2 consumption were investigated by near‐infrared spectroscopy (NIRS) (Grassi & Quaresima, 2016). Lower limb blood flow was determined in the femoral artery by ultrasound Doppler (Rådegran, 1997; Osada & Rådegran, 2009). In addition, mitochondrial respiratory function was evaluated ex vivo in permeabilized vastus lateralis fibres (Pesta & Gnaiger, 2012; Salvadego et al. 2016). We hypothesized that, over a 21 day exposure, the superposition of hypoxia on microgravity/unloading (bed rest) would exacerbate the impairment of skeletal muscle oxidative function, in vivo and ex vivo, compared to that observed following bed rest alone.
Materials and methods
Ethical approval
All of the participants of the study were informed about the aims, procedures and possible risks of the investigations and provided their written informed consent to participate in all of the experimental measurements. The study was approved by the National Committee for Medical Ethics at the Ministry of Health of the Republic of Slovenia, conformed with the European Space Agency recommendations for bed rest protocols (Standardization of bed rest study conditions 1.5, August 2009), as well as with the principles of the Declaration of Helsinki (2000), and was registered at http://ClinicalTrials.gov (NCT02293772).
Subjects
The present study was part of the ‘PlanHab: Planetary Habitat Simulation’ research program (Framework Program 7, European Commission) investigating the effects of a 21 day combined exposure to H and BR on cardiorespiratory, musculoskeletal, metabolic/nutritional, immunological, thermoregulatory and neurohumoral functions. The present study relates to muscular adaptations. Further details on the whole project are available elsewhere (Debevec et al. 2014; Keramidas et al. 2016a,b).
Eleven healthy recreationally active men (mean ± SD age 27 ± 5 years) took part in this investigation, as well as all of the investigations included in the PlanHab project; their main physical characteristics at baseline are given in Table 1.
Table 1.
Anthropometric and body composition characteristics of the subjects
| BDC | N‐BR | H‐BR | H‐AMB | |
|---|---|---|---|---|
| Body mass (kg) | 73.7 ± 9.9 | 70.6 ± 8.9* | 70.0 ± 9.4* | 72.0 ± 9.9 |
| BMI (kg m−2) | 22.9 ± 2.3 | 22.4 ± 2.3* | 21.9 ± 2.2* | 22.8 ± 2.7 |
| Lean body mass (kg) | 59.1 ± 7.1 | 56.9 ± 6.5* | 55.9 ± 6.1* | 56.6 ± 6.9* |
| Lean thigh mass (kg) | 7.1 ± 1.1 | 6.7 ± 1.1* | 6.5 ± 0.9*§ | 6.7 ± 1.1* |
| Fat body mass (kg) | 14.6 ± 4.0 | 13.8 ± 3.6 | 14.1 ± 4.3 | 15.4 ± 4.7 |
Data are expressed as the mean ± SD. BDC, baseline data collection; N‐BR, normoxic bed rest; H‐BR, hypoxic bed rest; H‐AMB, hypoxic ambulatory confinement. BMI, body mass index. *Significantly different vs. BDC (P < 0.05). §Significantly different vs. N‐BR (P < 0.05).
None of the participants was engaged in competitive sports activities or followed specific training programms before and throughout the study. All the participants were low altitude ( <500 m) residents. Participants’ recruitment procedure was based on the European Space Agency recommendations (Standardization of bed rest study conditions, version 1.5, August 2009). Exclusion criteria were: a medical history of respiratory, hematological or cardiovascular diseases; altitude exposure (>2000 m) in the last 2 months prior to the experiments; participation in dietary programmes during the last 6 months before the experiments; and the use of drugs and (or) medications (Debevec et al. 2014).
Experimental protocol
The experiments were carried out at the hypoxic facility of the Olympic Sports Centre (Planica‐Rateče, Slovenia) situated at an altitude of 940 m. The facility has the capability to induce/maintain simulated altitudes in an entire floor of the building, comprising 10 double rooms. The simulation of altitude was achieved by reducing the O2 fraction in the rooms/living areas using a Vacuum Pressure Swing Adsorption system (b‐Cat, Tiel, The Netherlands). The ambient O2 fraction was continuously monitored and adjusted to the target simulated altitude (see below) (Debevec et al. 2014).
Each participant underwent three 21 day campaigns in a randomized order: normobaric normoxic (fraction of inspired O2, = 0.209; = 133.1 ± 0.3 mm Hg) horizontal bed rest (N‐BR); hypoxic ( = 0.141; = 90.0 ± 0.4 mm Hg, target simulated altitude of ∼4000 m) horizontal bed rest (H‐BR); hypoxic ( = 0.141; = 90.0 ± 0.4 mm Hg) ambulatory confinement (H‐AMB). The interventions were separated by a 4‐month washout period to allow the effects of prior exposure to hypoxia and/or bed rest to be eliminated.
During the bed rest interventions (N‐BR and H‐BR), no deviations from the lying position, nor muscle stretching or static contractions, were permitted. Subjects in H‐AMB were allowed to move freely within the hypoxic area and were engaged in two 30 min bouts of low‐intensity exercise per day (stepping, cycling or dancing): one in the morning and one in the afternoon. Adherence to the assigned protocol was ensured using continuous closed‐circuit television surveillance and constant supervision by researchers and medical staff. Subjects consumed an individually tailored, standardized diet and were allowed to drink water and tea ad libitum. Details of the study design, physical activity and daily energy intakes during interventions are provided in Debevec et al. 2014. The environmental conditions within the facility remained stable throughout the experimental sessions (ambient temperature = 22.4 ± 2.1°C; relative humidity = 40 ± 11%; ambient pressure = 689 ± 6 mm Hg). All tests were conducted under close medical supervision and following standard safety procedures.
Before data collection, subjects were allowed time to gain familiarity with the investigators and experimental arrangement, and were familiarized with the exercise protocols by means of short preliminary practice runs. Subjects underwent to a very tight schedule of experimental tests before and after each intervention. For the purposes of the present study, subjects performed two incremental exercise protocols on a custom‐built knee‐extension ergometer (modified Monark cycle ergometer) (Andersen et al. 1985). Tests were performed 3 and 1 days before (baseline data collection; BDC) and 2 and 4 days after each intervention; their order was randomized among subjects and kept constant before and after all the interventions.
During knee extension, subjects were constrained on an adjustable seat by a safety belt, which anchored the angle of the hip at ∼90°. Subjects pushed on a padded bar attached to a lever arm connected to the crank of the cycle ergometer, allowing knee extension between ∼90 and ∼170°. This type of exercise confines muscle contractile activity to the quadriceps femoris muscle of one leg, which is activated during the extension phase. The return of the leg to the starting position is brought about passively by the momentum of the flywheel of the ergometer (Salvadego et al. 2011). After an initial 2 min of unloaded knee‐extension exercise, an incremental test was performed with the right leg. Work rate was increased by 6 W every minute, aiming to allow the subjects to reach voluntary exhaustion in ∼10 min. Work rate was applied by adjusting the tension of a strap around the ergometer flywheel, as in a mechanically braked cycle ergometer. Throughout the test, the active knee extension and passive knee flexion cycle was carried out at a cadence of ∼40 min−1, as imposed by a metronome. During each cycle (total duration of 1.5 s), knee extension lasted ∼1 s. In other words, muscle contraction corresponded to ∼65% of the duty cycle.
All exercises were conducted to voluntary exhaustion, which was defined as the inability to maintain the imposed work rate at the required frequency and through the full range of motion despite vigorous encouragement by the operators. Mean values of pulmonary ventilation, gas exchange, heart rate, and muscle and cerebral oxygenation indices (see below) were calculated during the last 30 s of each work rate; values obtained during the exhausting work rate were considered as peak values.
Measurements
Anthropometry
Body mass (BM), regional and whole body composition were assessed before and immediately after each campaign with dual‐energy X‐ray absorptiometry (DEXA) using a fan‐beam densitometer (Discovery W – QDR series; Hologic, Marlborough, MA, USA). More details on the DEXA technique and analysis are reported in Debevec et al. 2014. The lean mass of the lower limbs was measured as the sum of the fat‐free masses of the legs and thighs. Skinfold measurements were made by a caliper at the site of placement of the NIRS probe on the vastus lateralis muscle, aiming to estimate skin and subcutaneous adipose tissue thickness.
Cardiopulmonary and metabolic variables
Pulmonary ventilation (), tidal volume (V T), respiratory frequency (fR), O2 uptake () and CO2 output () were determined on a breath‐by‐breath basis by means of a metabolic unit (Quark CPET; Cosmed, Rome, Italy). Expiratory flow measurements were performed by a turbine flow meter calibrated before each experiment by a 3 L syringe at three different flow rates. Calibration of O2 and CO2 analysers was performed before each experiment by utilizing gas mixtures of known composition. The gas‐exchange ratio (R) was calculated as . values were expressed as L min−1 and normalized for the lean mass of the thigh.
Heart rate (HR) was determined using a heart rate monitor (RS 400; Polar, Kempele, Finland). Capillary oxyhaemoglobin saturation () was continuously monitored by pulse oximetry (MicrO2; Siemens Medical Systems, Danvers, MA, USA) at the finger. Ratings of perceived exertion (RPE) for dyspnoea and limb effort were obtained at rest and at every work rate of the incremental exercise using the Borg's modified CR10 scale (Borg, 1998). Peak power output was taken as an index of performance.
Skeletal muscle and cerebral oxygenation
Vastus lateralis muscle and cerebral (frontal cortex) oxygenation profiles during exercise were evaluated by NIRS. Principles, limitations and potential applications of this method have been discussed in detail for skeletal muscle (Grassi & Quaresima, 2016) and brain (Ferrari et al. 2016). NIRS measurements in muscle tissue have been shown to be well correlated with local venous O2 saturation. A limitation of the NIRS approach is related to the fact that only a relatively small and superficial portion of the tissue of interest can be investigated.
Cerebral oxygenation profiles were monitored with a continuous three‐wavelength (735, 810 and 850 nm) NIRS device (NIRO‐200NX; Hamamatsu Photonics, Shizuoka, Japan). The probe was positioned over the left prefrontal cortex at the midpoint between Fp1 and F3 landmarks of the international EEG 10–20 system. Data were recorded at 5 Hz, and expressed relative to the resting period of each trial. The Beer‐Lambert law was used to calculate micromolar (μm) changes in tissue oxygenation (Δ[oxy(Hb)] and Δ[deoxy(Hb)]) using received optical densities and a differential path‐length factor of 5.93. Measurements obtained during exercise were normalized as changes from an initial value arbitrarily defined as 0 μm. Total (Δ[oxy + deoxy(Hb)]) was taken as an index of changes in regional blood volume.
For muscle measurements, a portable NIR continuous‐wave instrument (PortaMon; Artinis, Elst, The Netherlands) was utilized. The instrument measures micromolar (μm) changes in oxygenated haemoglobin (Hb) + myoglobin (Mb) concentrations (∆[oxy(Hb + Mb)]), and in deoxygenated [Hb + Mb] (∆[deoxy(Hb + Mb)]), with respect to an initial value arbitrarily set equal to zero and obtained during the resting condition preceding the test. The sum of the two variables (∆[total(Hb + Mb)]) is related to changes in the total Hb volume in the muscle region of interest. Specific details on the method are provided in recent papers by our group (Salvadego et al. 2011, 2013).
In contracting muscle, ∆[deoxy(Hb + Mb)] is relatively insensitive to changes in blood volume and has been considered an estimate of skeletal muscle fractional O2 extraction (ratio between and O2 delivery []) (Grassi & Quaresima, 2016). A ‘physiological calibration’ of ∆[deoxy(Hb + Mb)] values was performed by inducing a transient ischaemia of the limb when the subject was sitting on the ergometer few minutes after the exercise. Data obtained during exercise were expressed as a percentage of the value of maximal muscle deoxygenation obtained in each subject by inflating a pressure cuff (at 300–350 mmHg) at the inguinal crease of the thigh for a few minutes, until the ∆[deoxy(Hb + Mb)] increase reached a plateau (Porcelli et al. 2012).
The presence of a deoxygenation ‘overshoot’ (transitory sharp increase in ∆[deoxy(Hb + Mb)] above the steady‐state (Grassi & Quaresima, 2016) was investigated during the first 2 min of constant work rate exercise (Porcelli et al. 2014). Data were first fitted by a mono‐exponential function of the type:
| (1) |
and parameter values (TDTot, τTot) were determined that yielded the lowest sum of squared residuals. In eqn (1), y BAS indicates the baseline, ATot is the amplitude of the response, TDTot is the time delay and τTot is the time constant of the function.
Data were then fitted by a double exponential function of the type:
| (2) |
In eqn (2) A u indicates the amplitude of the upward component between y BAS and the transient steady‐state value reached in the first seconds of the kinetics; TDu is the time delay; and τu is the time constant of the upward component of the function. A d, TDd and τd indicate, respectively, the amplitude, time delay and time constant of the downward (d) component.
The equation that best fit the experimental data was determined by an F test. The area under the best fitting curves was calculated by integration, after subtracting the resting values.
Femoral artery blood flow
Blood flow in the common femoral artery was estimated by measurements of mean blood flow velocity and vessel diameter distal to the inguinal ligament, 1 cm above the profound femoral branch, using an ultrasonographic equipment (Vivid 9; GE Healthcare, Horten, Norway) with a linear array multifrequency transducer (4.5–12 MHz). Two‐dimensional measurements of the arterial lumen were made from B‐mode image in longitudinal view; blood flow velocities were collected with the sample volume covering more than 75% of the arterial lumen, and with an angle correction always kept at 50°. Arterial blood flow was calculated by multiplying arterial cross‐sectional area by the time integral of the mean blood flow velocity over a period of 10–15 knee‐extension cycles, during the last 30 s of each sampling period (Radegran, 1997; Osada & Radegran, 2009).
Mitochondrial respiration ex vivo
Muscle samples were obtained from the vastus lateralis muscle of the non‐dominant leg in the morning after a night of fasting (≥10 h). The biopsy was taken, for all subjects, 1 day before each intervention and on the last day of each intervention; the baseline biopsy was taken in a normoxic environment (air), whereas the intervention biopsies were taken in the prevailing ambient gas environment. The subject was transferred to the laboratory in a supine position and a sample was obtained from the superficial mid‐portion of the vastus lateralis muscle. The site was anaesthetized by injecting 2–4 mL of 2% lidocaine hydrochloride. Following the application of the anaesthetic, a 1–1.5 cm incision was made to the skin, subcutaneous tissue and muscle fascia, and the muscle sample was removed using a Weil‐Blakesley rongeur biopsy forceps (Gebrüder Zepf Medizintechnik, Tuttlingen, Germany).
The muscle samples were divided into several portions. One portion (∼20 mg wet weight) was immediately frozen in liquid nitrogen and stored at –80°C until determination of citrate synthase (CS) protein expression (see below). Another portion (∼15 mg wet weight) was used to evaluate mitochondrial respiration ex vivo (Pesta & Gnaiger, 2012) and was immediately placed in an ice‐cold preservation solution (BIOPS; Oroboros Instruments, Innsbruck, Austria) containing: EGTA‐calcium buffer (10 mm) (free Ca2+ concentration 100 nmol L−1), imidazole (20 mm), taurine (20 mm), K+/4 morpholinoethanesulphonic acid (50 mm), dithiothreitol (0.5 mm), MgCl2 (6.56 mm), ATP (5.77 mm) and phosphocreatine (15 mm) (pH 7.1).
Fibre bundles were separated with sharp‐ended needles, leaving only small areas of contact, incubated in the above solution (4°C) containing 10% (w/v) fatty acid free BSA and 30% (v/v) DMSO, and snap frozen in liquid nitrogen. The samples were stored at –80°C until analysis, which was carried out within 1 month (Kuznetsov et al. 2003; Wüst et al. 2012; Salvadego et al. 2016).
For analysis, the fibre bundles were quickly thawed in a water bath at 37°C and washed in BIOPS containing 2 mg mL−1 BSA to remove any residual DMSO from the tissue. Fibres were then incubated in 5 mL of BIOPS (4 °C) containing 50 g mL−1 saponin for 30 min with continuous gentle stirring to ensure complete permeabilization. After being rinsed twice for 10 min in a respiration medium (MiR05l Oroboros Instruments; 0.5 mm EGTA, 60 mm potassium lactobionate, 3 mm MgCl2 6H2O, 20 mm taurine, 10 mm KH2PO4, 20 mm Hepes, 110 mm sucrose and 1 g L−1 BSA, pH 7.1), permeabilized fibres were measured for wet weight and immediately transferred into a respirometer (Oxygraph‐2k; Oroboros Instruments) for the analysis.
Mitochondrial respiratory function was evaluated by measuring O2 consumption polarographically by high‐resolution respirometry (Pesta & Gnaiger, 2012). Data were digitally recorded using DatLab4 software (Oroboros Instruments). The instrumentation allows for O2 consumption measurements with small amounts of sample in closed respiration chambers containing 3 mL of air‐saturated respiration medium at 37 °C; 3–5 mg of muscle fibres were used for the analysis. Standardized instrumental and chemical calibrations were performed to correct for back‐diffusion of O2 into the chamber from the various components (e.g. leak from the exterior, O2 consumption by the chemical medium and by the sensor O2) (Pesta & Gnaiger, 2012). The O2 concentration in the chamber was maintained between 300 and 400 μm (average O2 partial pressure ∼250 mmHg) to avoid O2 limitation of respiration. Intermittent reoxygenation steps were performed during the experiments by injections of 1–3 μl of 200 mm H2O2, which was instantaneously dismutated by catalase, already present in the medium, to O2 and H2O. All respirometric analyses were performed in duplicate.
A substrate‐uncoupler‐inhibitor‐titration protocol, with a substrate combination that matches physiological intracellular conditions, was applied (Pesta & Gnaiger, 2012; Salvadego et al. 2013, 2016). Non‐phosphorylating resting mitochondrial respiration was measured in the presence of malate (4 mm) and glutamate (10 mm) and in the absence of adenylates, so that O2 consumption was mainly driven by the back leakage of protons through the inner mitochondrial membrane (‘leak’ respiration). ADP‐stimulated mitochondrial respiration sustained by Complex I (state 3 respiration) was measured by stepwise additions of ADP (2.5 mm) as phosphate acceptor, with malate and glutamate as substrates. Succinate (10 mm) was added to support convergent electron flow into the Q‐junction through complexes I and II. Maximal ADP‐stimulated mitochondrial respiration was then determined in the presence of saturating [ADP] (5 mm). The addition of cytochrome c (10 μm) had no significant additive effects on respiration, with minor increases of ∼5%, thereby confirming the integrity of the outer mitochondrial membrane. We also examined electron transport system capacity by stepwise addition of the chemical uncoupler protonophore carbonylcyanide‐p‐trifluoromethoxyphenylhydrazone (FCCP) to optimum concentration (1.25 μm). Rotenone (1 μm) and anti‐mycin A (2.5 μm) were added to inhibit complexes I and III, providing a measure of residual O2 consumption, indicative of non‐mitochondrial O2 consumption. Mitochondrial respiration was then corrected for O2 flux as a result of residual O2 consumption. The degree of coupling of oxidative phosphorylation for a specific substrate supply (glutamate and malate in this case) was determined by calculating the ratio between state 3 respiration minus leak respiration and state 3 respiration [(state 3 – leak)/state 3] (Pesta & Gnaiger, 2012).
CS content
Frozen muscle samples were pulverized and re‐suspended in a lysis buffer [20 mm Tris‐HCl, 1% Triton X‐100, 10% glycerol, 150 mm NaCl, 5 mm EDTA, 100 mm NaF and 2 mm NaPPi supplemented with protease and phosphatase inhibitors (Sigma‐Aldrich, St Louis, MO, USA) and 1 mm phenylmethanesulphonyl fluoride]. The obtained homogenate was centrifuged at 18.000 g for 20 min at 4°C. Muscle extracted proteins (15 μg) were loaded on gradient precast gels (AnyKd) purchased from Bio‐Rad (Hercules, CA, USA) and were electrotransferred to nitrocellulose membranes at 100 V for 2 h at 4 °C. The membranes were incubated with the anti‐CS primary antibodies (Abcam, Cambridge, MA, USA) overnight. Thereafter, membranes were blocked in 5% milk and then incubated in anti‐rabbit IgG HRP‐conjugated secondary antibody (Cell Signaling Technology, Beverly, MA, USA) for 1 h. The protein bands were visualized by an enhanced chemiluminescence method. The content of CS protein was assessed by determining the brightness–area product of the protein band and normalizing on actin content, as described previously (Brocca et al. 2012).
Statistical analysis
The results were expressed as the mean ± SD. To evaluate the effects of N‐BR and H‐BR, linear mixed effect (LME) models were constructed with subject as random effect and with the H‐AMB data excluded. For all variables, time (Before vs. After), condition (N‐BR vs. H‐BR) and their interaction were used as fixed effects. Data from the baseline collection phases were not significantly different and were lumped together (BDC). The H‐AMB data were then analysed via LME with time as random effect and subject as fixed effect. LME models were optimized according to Akaike's information criterion. Data were Box‐Cox transformed when non‐linear quantile‐quantile plots or heteroscedasticity were found. Models for statistical testing of the primary hypothesis were simplified in a stepwise manner. First, the time × condition interaction term was discarded where justified by non‐significance and Akaike's criterion, and condition term was discarded in the next step. Any significant effects were followed up with treatment contrasts, using BDC and N‐BR as reference. Data fitting by exponential functions was performed using the least squares residuals method. P < 0.05 was considered statistically significant. Statistical analyses were carried out using software packages (Prism, version 5.0; GraphPad Software Inc., San Diego, CA, USA; SPSS, version 13.0.1; SPSS Inc., Chicago, IL, USA).
Results
Values of BM, body mass index, lean body mass, fat body mass and thigh lean mass are reported in Table 1. BM decreased significantly (by ∼5%) after N‐BR and H‐BR. Lean body mass and the lean mass of the thigh decreased significantly (by ∼6–8%) after all interventions. Fat body mass did not change after the interventions. The mean values of skin and adipose tissue thickness measured at the site of placement of the NIRS probe ranged between 11.0 and 11.6 mm and were not altered by any intervention.
Peak values of the main cardiovascular, ventilatory and gas‐exchange variables determined at exhaustion are presented in Table 2. , RPE‐dyspnoea and HR peak values suggest that, as expected, the exercise was not maximal from a cardiorespiratory perspective; HR peak, in particular, was ∼60–65% of the age‐predicted maximum. No capillary oxyhaemoglobin desaturation (see peak values) was observed following any intervention. RPE‐leg and R peak values (∼1.15–1.21 under all conditions) suggest that the exercise was maximal for the involved muscles. Peak pulmonary (L min−1) was lower after both N‐BR (−7%) and H‐BR (−9 %) vs. BDC. As for H‐AMB, values were not significantly different vs. BDC. When pulmonary was normalized for the lean mass of the thigh, values were not different (vs. BDC) after both N‐BR and H‐BR, whereas they were significantly higher after H‐AMB.
Table 2.
Peak values of the main investigated variables determined at voluntary exhaustion during the incremental knee‐extension exercise
| BDC | N‐BR | H‐BR | H‐AMB | |
|---|---|---|---|---|
| Power outputpeak (W) | 49.1 ± 9.4 | 47.8 ± 6.1 | 45.7 ± 12.2 | 50.4 ± 14.2 |
| (L min−1) | 45.6 ± 14.5 | 49.0 ± 24.7 | 48.2 ± 13.8 | 43.0 ± 7.2 |
| (L) | 1.55 ± 0.38 | 1.59 ± 0.55 | 1.62 ± 0.57 | 1.48 ± 0.40 |
| fRpeak (br min−1) | 29.6 ± 9.1 | 30.8 ± 9.2 | 29.9 ± 5.7 | 29.0 ± 7.2 |
| (L min−1) | 1.06 ± 0.15 | 0.98 ± 0.13* | 0.96 ± 0.17* | 1.05 ± 0.16 |
| /TM mass (mL kg−1 min−1) | 150.0 ± 22.8 | 148.1 ± 23.5 | 147.2 ± 30.5 | 161.3 ± 30.8* |
| (L min−1) | 1.23 ± 0.21 | 1.19 ± 0.19* | 1.12 ± 0.23* | 1.21 ± 0.20 |
| R peak | 1.18 ± 0.16 | 1.21 ± 0.15 | 1.18 ± 0.15 | 1.15 ± 0.11 |
| (mmHg) | 104.6 ± 6.1 | 105.9 ± 6.2 | 107.8 ± 4.1 | 104.1 ± 2.6 |
| (mmHg) | 31.0 ± 5.0 | 30.7 ± 4.2 | 28.7 ± 2.9 | 30.0 ± 2.0 |
| HRpeak (b min−1) | 119 ± 18 | 132 ± 19 | 125 ± 19 | 118 ± 13 |
| (%) | 98.6 ± 1.3 | 98.3 ± 1.3 | 98.2 ± 1.4 | 98.5 ± 1.4 |
| RPE‐dyspnoea scores (0–10) | 2.8 ± 1.8 | 3.2 ± 1.7 | 3.4 ± 2.0 | 2.1 ± 1.6 |
| RPE‐leg effort scores (0–10) | 9.1 ± 1.4 | 9.6 ± 0.9 | 9.9 ± 0.3 | 9.5 ± 1.2 |
Data are the mean ± SD. BDC, baseline data collection; N‐BR, normoxic bed rest; H‐BR, hypoxic bed rest; H‐AMB, hypoxic ambulatory confinement.
, pulmonary ventilation, V T, tidal volume; fR, respiratory frequency; , O2 uptake; BM, body mass; TM: thigh muscle mass; , CO2 output; R, gas‐exchange ratio, , O2 end‐tidal pressure; , CO2 end‐tidal pressure; HR, heart rate; : arterial blood O2 saturation; RPE, ratings of perceived exertion. *Significantly different from BDC (P < 0.05).
No differences among conditions were observed for the other peak variables, with the exception of pulmonary , which was lower (vs. BDC) in N‐BR and H‐BR.
Mean ± SD values of the NIRS‐obtained cerebral (frontal cortex) oxygenation data are reported as a function of work rate in Fig. 1. ∆[oxy(Hb)] and ∆[oxy + deoxy(Hb] increased from the baseline at every work rate, reaching the highest values at peak exercise, whereas ∆[deoxy(Hb)] slightly decreased from baseline reaching a plateau at ∼50% of peak exercise. These patterns, suggesting an enhanced cerebral oxygenation during exercise, were not affected by any intervention.
Figure 1. Brain (frontal cortex) oxygenation data, as a function of work rate, obtained by NIRS during incremental knee‐extension exercise in BDC, after N‐BR, H‐BR and H‐AMB.
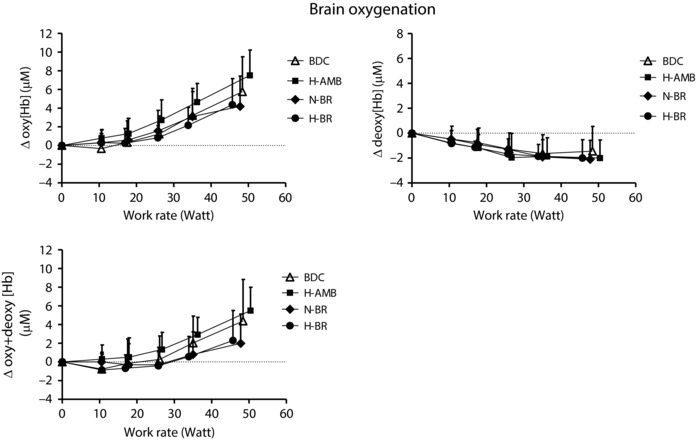
Mean ± SD values of changes in concentration of oxy‐haemoglobin (Δoxy[Hb]), deoxy‐haemoglobin (Δdeoxy[Hb]) and total (oxy + deoxy) haemoglobin (Δ[oxy + deoxyHb]) are shown. For further details, see text.
Mean ± SD peak values of vastus lateralis ∆[deoxy(Hb + Mb)], which was taken as an estimate of muscle fractional O2 extraction, are shown in Fig. 2. Data are expressed as a percentage of those obtained during a transient limb ischaemia. Values were significantly lower after N‐BR (by ∼10%) and H‐BR (by ∼12%) vs. BDC. As for H‐AMB, no difference vs. BDC was observed. In The data for peak pulmonary (L min−1 and normalized per unit of thigh muscle mass) and of peak femoral artery blood flow (L min−1 and normalized per unit of thigh muscle mass) are also shown in Fig. 2. Only qualitative inferences can be drawn by the analysis of these data because both (determined at the pulmonary level) and blood flow (determined in the femoral artery) were not specifically measured at the level of the exercising muscle (the quadriceps of one leg); moreover, thigh muscle mass and not quadriceps muscle mass was determined. Nonetheless, it appears that, when blood flow and are expressed per unit of muscle mass, the impaired O2 extraction in N‐BR and H‐BR is compensated for by an increased blood flow (by ∼20%), yielding an unchanged . The compensation does not occur when blood flow and are expressed in absolute terms, in which case blood flow values were unchanged across conditions and lower values were observed in N‐BR and H‐BR.
Figure 2. Peak values of the main variables related to O2 delivery and O2 utilization.
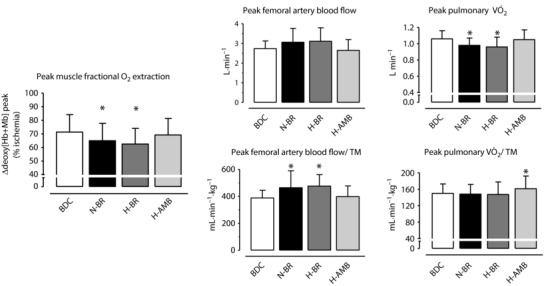
Mean ± SD peak values of NIRS‐obtained vastus lateralis muscle deoxygenation (∆[deoxy(Hb + Mb)]). Left: variable estimating fractional O2 extraction. Data are expressed as a percentage of values obtained during a transient limb ischaemia at the end of the test. Middle: mean ± SD values of peak arterial flow (expressed in L min−1 and in mL min−1 kg−1 of thigh muscle mass) are shown. Right: mean ± SD values of peak pulmonary (expressed in L min−1 and in mL min−1 kg−1 of thigh muscle mass) are shown. Data are presented for BDC, N‐BR, H‐BR and H‐AMB. For further details, see text. * P < 0.05 vs. BDC.
Figure 3 shows the mean ± SD values of ∆[deoxy(Hb + Mb)] during the first 2 min of unloaded exercise. An ‘overshoot’ (see Materials and methods) of ∆[deoxy(Hb + Mb)] was evident in both N‐BR and H‐BR, whereas no overshoot was observed in BDC and H‐AMB. The mean ± SD values of the amplitude of the overshoot, the area under the overshoot and the area under the total ∆[deoxy(Hb + Mb)] response are given in Table 3. The amplitude and the area of the overshoot were greater after N‐BR and H‐BR vs. BDC, whereas no differences between conditions were observed for the area under the total ∆[deoxy(Hb + Mb)] response.
Figure 3. Muscle deoxygenation data.
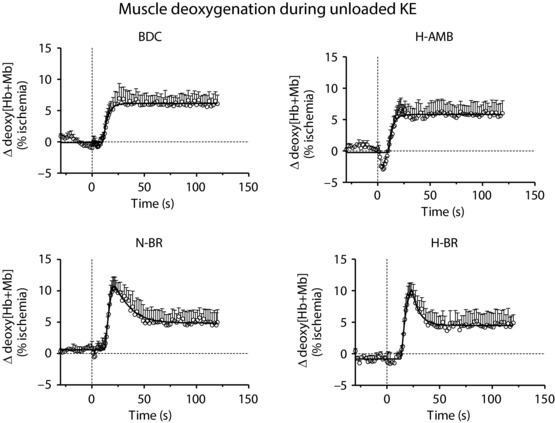
Mean ± SD values of ∆[deoxy(Hb + Mb)] during the first 2 min of unloaded knee‐extension exercise in BDC, after N‐BR, H‐BR and H‐AMB. For further details, see text.
Table 3.
Muscle deoxygenation overshoot during the first minutes of knee‐extension exercise
| BDC | N‐BR | H‐BR | H‐AMB | |
|---|---|---|---|---|
| Amplitude Δ[deoxy(Hb + Mb)] overshoot (% ischaemia) | 0.3 ± 1.0 | 4.3 ± 3.5* | 4.4 ± 4.2* | 0.3 ± 1.2 |
| Area under Δ[deoxy(Hb + Mb)] overshoot (% s) | 3.3 ± 10.5 | 104.8 ± 85.0* | 93.2 ± 107.7* | 3.5 ± 11.4 |
| Area under Δ[deoxy(Hb + Mb)] response (% s) | 696 ± 392 | 715 ± 308 | 701 ± 418 | 747 ± 357 |
| Δ[deoxy(Hb + Mb)] overshoot (% total response) | 0.5 ± 1.8 | 26.4 ± 17.6* | 24.9 ± 22.1* | 0.4 ± 1.4 |
Data are the mean ± SD. BDC, baseline data collection; N‐BR, normoxic bed rest; H‐BR, hypoxic bed rest; H‐AMB, hypoxic ambulatory confinement. Amplitude and area under muscle deoxygenation (Δ [deoxy(Hb + Mb)]) overshoot (expressed as a percentage of the maximal muscle deoxygenation during transient limb ischaemia) were measured during the first 2 min of constant work rate knee extension.
The ‘overshoot component’ was calculated as the area of the deoxygenation overshoot relative to the overall area of the Δ[deoxy(Hb + Mb)] response. *Significantly different from BDC.
Mean ± SD values of femoral artery blood flow determined every 40 s over the first 2 min of unloaded knee extension are shown in Fig. 4. Although the relatively long time‐resolution of the measurement was not ideal for this type of analysis and may attenuate transient overshoots or undershoots of the response, the mean ± SD data strongly suggest that the blood flow data reached a steady‐state at 20–30 s of exercise. Values at rest and during exercise were similar among conditions.
Figure 4. Arterial flow at the femoral artery.
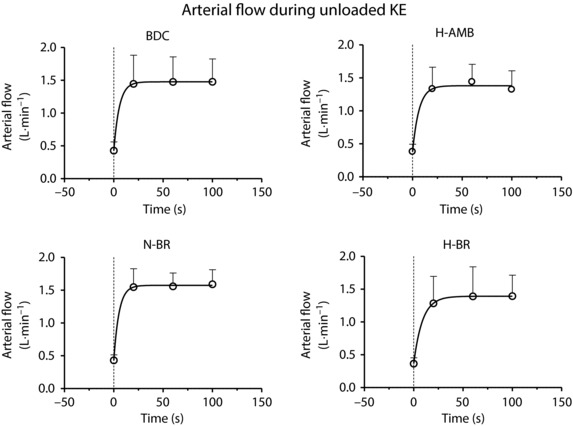
Mean ± SD values of arterial flow at the femoral artery during the first 2 min of unloaded knee‐extension exercise in BDC, after N‐BR, H‐BR and H‐AMB. Data were collected at three different time periods (0–40, 40–80 and 80–120 s). For further details, see text.
CS expression, taken as an estimate of mitochondrial content, was not significantly different among the experimental conditions (0.10 ± 0.03 arbitrary units in BDC; 0.10 ± 0.02 in N‐BR; 0.10 ± 0.01 in H‐BR; 0.09 ± 0.03 in H‐AMB). The main data related to mitochondrial respiration determined ex vivo in isolated permeabilized fibres are shown in Fig. 5. Maximal ADP‐stimulated mitochondrial respiration and the maximal capacity of the electron transport system, as induced by the chemical uncoupler FCCP, were significantly lower under all experimental conditions vs. BDC. Mitochondrial leak respiration was higher in N‐BR, whereas no significant differences were observed in H‐BR or H‐AMB. The degree of coupling of oxidative phosphorylation [(State 3 – Leak)/State 3] at a specific substrate supply (glutamate and malate), was lower in N‐BR vs. BDC, whereas no significant differences were observed between H‐BR or H‐AMB and BDC.
Figure 5. Variables of mitochondrial respiratory function, expressed per unit of tissue mass (wet weight), measured by high‐resolution respirometry in permeabilized muscle fibres.
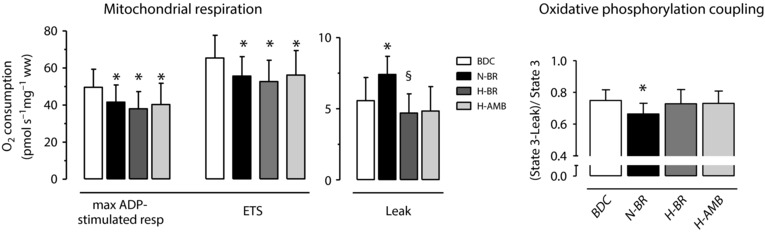
Left: maximal ADP‐stimulated mitochondrial respiration, maximal capacity of the electron transport system (ETS) and ‘Leak’ respiration. Right: oxidative phosphorylation coupling [(State 3 – Leak)/State 3]. Values are presented as the mean ± SD. For further details, see text. * P < 0.05 vs. BDC. § P < 0.05 vs. N‐BR.
Discussion
The present study was part of the PlanHab project, investigating the effects of a 21 day exposure to bed rest (BR, taken as a proxy of microgravity) and normobaric hypoxia (H) ( = 0.141), alone and combined, on different human physiological systems. The data of the present study deal with a functional evaluation of skeletal muscle oxidative metabolism, both in vivo and ex vivo.
Refuting our hypothesis, superposition of H on BR did not worsen the expected BR‐induced impairment of skeletal muscle oxidative function in vivo, as evaluated by , peak skeletal muscle fractional O2 extraction and temporal profiles of O2 extraction during constant work‐rate exercise. The measurements were carried out during dynamic one‐leg knee‐extension exercise, in which central cardiovascular constraints are significantly attenuated as a consequence of the relatively small muscle mass involved in the exercise (the quadriceps of one leg) and any impairment of oxidative function intrinsic to skeletal muscle becomes fully manifest (Andersen et al. 1985; Richardson et al. 1993; Salvadego et al. 2011, 2016). In the present study, no systemic limitations to exercise performance were observed, both in terms of cardiorespiratory function (e.g. , , R, RPE‐dyspnoea) and in terms of cerebral oxygenation. This confirms the adequacy of the employed exercise protocol in specifically investigating oxidative metabolism at the skeletal muscle level.
Percentage‐wise, the decreases in pulmonary (L∙min−1) (by ∼8%) and in ∆[deoxy(Hb + Mb)]peak (by ∼10%) observed after N‐BR were similar to those obtained using the same exercise paradigm following a BR exposure of shorter duration (10 days) (Salvadego et al. 2016). This suggests that, after an initial decrease occurring during the first 10 days of exposure to N‐BR, these variables do not present a further significant decrease during the following 10 days of exposure. Moreover, the similar impairments in pulmonary and ∆[deoxy(Hb + Mb)]peak suggest that the decreased maximal aerobic performance was substantially the result of a decreased capacity of O2 extraction by skeletal muscles. Confirming this concept (Fig. 2), peak femoral artery blood flow values were not different across conditions and, when expressed per unit of thigh muscle mass, they were actually higher in N‐BR and in H‐BR vs. BDC.
Also in our previous study, the addition of H to BR (H‐BR) did not exacerbate the reduction of and ∆[deoxy(Hb + Mb)]peak during knee extension observed following BR alone (Salvadego et al. 2016). By contrast to our previous study, however, H did not attenuate the impairment of muscle oxidative function induced by BR alone in the present study. It could be hypothesized that, following a relatively prolonged exposure, any ‘protective’ effect of H on muscle O2 delivery (e.g. increased [Hb]) would be counterbalanced, at least to some extent, by some impairments at other levels, such as mitochondrial respiration (see below). It is interesting to observe that the decreases observed in the present study after H‐BR were somewhat less pronounced (in particular in terms of ∆[deoxy(Hb + Mb)]peak) than those reported by Keramidas et al. (2016a) for the same subjects and the same environmental conditions, during cycle ergometer exercise, an exercise paradigm in which central cardiovascular constraints contribute to limit oxidative metabolism and aerobic performance.
In the present study, a 6–8% decrease in thigh muscle mass was observed under all experimental conditions, notwithstanding a tightly controlled energy balance (Debevec et al. 2014). When pulmonary was normalized per unit of thigh muscle mass, no significant differences were observed between BDC and N‐BR or H‐BR. This observation suggests that the observed changes in skeletal muscle oxidative metabolism (in terms of ) were substantially quantitative, in other words related to the decreased muscle mass. This may appear in contradiction with the observed decrease of peak fractional O2 extraction at the level of the vastus lateralis muscle and with the impairment of mitochondrial respiration observed in isolated fibres of the same muscle (see below). The apparent contradiction might be reconciled by hypothesizing that these functional impairments were masked by an elevated muscle blood flow, which is typical of this type of exercise (Andersen et al. 1985; Richardson et al. 1993). As noted above, in the present study, peak blood flow in the femoral artery was higher in N‐BR and H‐BR vs. BDC when the variable was expressed per unit of thigh muscle mass, whereas it was unchanged (confirming a previous observation by Weber et al. 2014 in subjects undergoing limb unloading) vs. BDC when it was expressed in absolute terms.
The dynamic profiles of ∆[deoxy(Hb + Mb)] during the first 2 min of unloaded knee extension were analysed to evaluate parameters related to the intramuscular matching between O2 delivery and O2 consumption in the vastus lateralis muscle (Porcelli et al. 2010, 2014, 2016; Grassi & Quaresima, 2016; Salvadego et al. 2016). After both N‐BR and H‐BR, the time‐course of ∆[deoxy(Hb + Mb)] was characterized by a transient overshoot, which was not evident in BDC or after H‐AMB. This overshoot implies an impaired/delayed matching between intramuscular O2 delivery and metabolic demand, and has the same meaning as the undershoot of microvascular described by phosphorescence quenching under different experimental conditions (Koga et al. 2014; Heinonen et al. 2015). A lowering of microvascular , albeit transitory, would determine a decreased driving pressure for blood‐myocyte O2 flux, thereby impairing oxidative metabolism (Koga et al. 2014; Heinonen et al. 2015). Similar ∆[deoxy(Hb + Mb)] responses during metabolic transitions were observed after 10 days (Salvadego et al. 2016) and 35 days (Porcelli et al. 2010) of N‐BR and, to a lesser extent, after 10 days of H‐BR (Salvadego et al. 2016). Also for this variable, in the present study the superposition of H on BR did not affect the response induced by BR alone.
During the transition from rest to unloaded knee extension, we evaluated also the dynamics of arterial blood flow to the lower limb. As shown in Fig. 4, the time‐course of this variable was substantially identical across experimental conditions: a fast exponential increase, reaching a steady‐state (whose values were not different across conditions) in ∼20 s. Notwithstanding, the time resolution of measurements was not ideal for discriminating kinetics patterns, the unchanged values over time in all experimental conditions suggest the absence of any overshoot or undershoot in arterial flow with metabolic transitions, and thus an unchanged vascular function, at least at the level of the major feeding vessels. This response should be analysed in conjunction with that of ∆[deoxy(Hb + Mb)] (O2 extraction), described above. Because the overshoots in O2 extraction observed after N‐BR and H‐BR were probably not associated with overshoots/undershoots of blood flow in the main artery supplying the muscle, an impairment of the matching between O2 delivery and O2 consumption was presumably responsible for the extraction overshoot and presumably affected peripheral O2 diffusion and mitochondrial energetics. The mismatch could derive from a suboptimal nitric oxide signalling within the muscle, which would ‘uncouple’ the heterogeneous microvascular blood flow increase from the presumably heterogeneous O2 consumption increase (Poole et al. 2012), and it has also been described in patients with chronic heart failure (Sperandio et al. 2009), as well as in patients with metabolic myopathies (Porcelli et al. 2014, 2016). According to another possibility, the transient overshoot in O2 extraction may be the consequence of an accumulation of blood in skeletal muscle at rest, which has been hypothesized to occur during bed rest (Rittweger et al. 2013). In this situation, the muscle may transiently increase O2 extraction from the increased blood reservoir in the muscle. This scenario, however, does not appear to be compatible with the apparent unchanged adjustment of arterial blood flow described in the present study under all conditions. The mechanistic hypotheses mentioned above could not be directly tested.
In the present study, mitochondrial oxidative function was evaluated ex vivo in permeabilized vastus lateralis fibres by high‐resolution respirometry (Pesta & Gnaiger, 2012). Different from the 10 day study (Salvadego et al. 2016), in which no changes were observed, in the present study, maximal ADP‐stimulated mitochondrial respiration, which is the maximal respiration in isolated non‐contracting permeabilized fibres, in the presence of saturating ADP levels and unlimited substrates and O2 availability, was significantly impaired (∼30% decreases) following all interventions. Also for this variable, the impairment observed following BR alone was not aggravated by H. In short, relatively long (20 days) exposures impaired mitochondrial respiratory function, whereas shorter exposures (10 days) did not (Salvadego et al. 2016).
Similar to that observed in the 10 day study (Salvadego et al. 2016), mitochondrial content of the tissue, as estimated by CS expression, was not affected by any intervention. Thus, the observed changes in mitochondrial respiration were qualitative. It should also be considered that, in the presence of a decreased muscle mass, an unchanged mitochondrial content is presumably associated with a decreased muscle mitochondrial mass.
‘Leak’ respiration (‘dissipation’ of the H+ gradient across the inner mitochondrial membrane, not associated with rephosphorylation of ADP) was enhanced following N‐BR, whereas this effect was reversed following H‐BR and H‐AMB. The enhanced leak respiration in N‐BR was associated with a reduced coupling between oxidation and phosphorylation, suggesting a lower efficiency of oxidative phosphorylation. These findings are consistent with an upregulation of muscle protein UCP‐3 following prolonged muscle unloading (Mazzatti et al. 2008), which would protect cells against an excessive mitochondrial reactive oxygen species formation, albeit at the cost of an increased energy dissipation.
As far as the molecular mechanisms responsible for the observed changes, some hypotheses can be proposed. Skeletal muscle atrophy following disuse/unloading is the result of decreased protein synthesis and increased protein degradation, which are triggered by oxidative stress (Powers et al. 2012) and/or altered mitochondrial function and structure (Cannavino et al. 2014, 2015). In this perspective, mitochondrial alterations induced by inactivity/microgravity would be responsible for the impairment of mitochondrial respiration and could be one of the factors leading to muscle atrophy. In hypoxia, a down‐regulation of electron transport chain complexes (possibly aimed at mitigating the increase in reactive oxygen species production) would be induced by decreased levels of peroxisome proliferative activated receptor‐γ coactivator‐α (Murray & Horscroft, 2016) and would explain the impaired maximal mitochondrial respiration described in the present study. We recognize that further biochemical, molecular and structural studies would be needed to gain more mechanistic insights into the impairment of mitochondrial function.
In the present study, the respiratory impairment observed in H‐AMB at the fibre level did not translate into a functional impairment in vivo during small muscle mass exercise, as also observed in the study by Calbet et al. 2009. It can be hypothesized that this observation, together with the absence of further functional impairments following H‐BR, compared to N‐BR, could be attributed, at least in part, to the increased blood [Hb] occurring in hypoxia (Keramidas et al. 2016b).
The hypoxic stimulus utilized in the present study, corresponding to 4000 m of altitude, is similar (albeit slightly ‘stronger’) than that expected in future habitats on the Moon or Mars (David et al. 2006). Such a stimulus is sufficiently severe to induce a down‐regulation of the oxidative processes inside the skeletal muscle (Horscroft & Murray, 2014) and a significant erythropoietin response, as indeed demonstrated by Keramidas et al. (2016b) in the same subjects of the present study.
Microgravity (bed rest) impairs peripheral O2 diffusional conductance (Ade et al. 2015). However, it is not known whether superposition of hypoxia on microgravity further impairs this function, although indirect data obtained in the present study and in a previous study by Salvadego et al. (2016) suggest that this is not the case. No structural analyses (muscle fibre diameter, capillarization, etc.) could be performed on the muscle samples obtained in the present study. Thus, we cannot speculate how the observed muscle atrophy could have affected peripheral O2 diffusion and cellular hypoxia under the different conditions.
It should be acknowledged that the results of the present study were obtained during knee‐extension exercise, carried out after the 3‐week period of exposure to the different experimental conditions, but in a normoxic environment. Our main aim, however, was to compare chronic adaptations to bed rest vs. chronic adaptations to bed rest + hypoxia, and, accordingly, measurements carried out in a hypoxic environment could represent a confounding factor.
Moreover, the results of the present study relate only to men and future investigations should focus on whether similar responses are observed in women. Longer periods of exposure to the experimental conditions would also be of interest.
In conclusion, in the present study, after 21 days of normoxic bed rest and hypoxic bed rest (where the latter condition was aimed at simulating future planetary habitats), a significant impairment of peak oxidative metabolism ‘downstream’ of cardiovascular O2 delivery was revealed. Superposition of hypoxia to bed rest did not aggravate the impairment observed following bed rest alone. The impaired oxidative function in vivo was at least in part attributable to an impaired mitochondrial respiratory function (which was not detected in a previous study with a shorter exposure) and was associated with signs of altered/delayed intramuscular matching between O2 delivery and O2 utilization. In terms of the chronic adaptations to spaceflights and future reduced gravity habitats (on the Moon or Mars), characterized by low‐gravity and hypoxia, as well as conditions characterized by immobility/hypomobility and hypoxia (e.g. ageing, cardiovascular and respiratory diseases), the impairment of skeletal muscle oxidative function appears mainly attributable to microgravity/immobility/hypomobility, whereas the role of hypoxia is relatively marginal.
Additional information
Author contributions
The experiments were carried out at the Olympic Sports Centre Planica, Slovenia. IBM, OE and BG conceived the study and obtained financial support. IBM was responsible for the recruitment of the subjects and for the logistics of the study. BG and DS contributed to the design of the experiments. Data were collected and analysed by DS, MEK, RK, LB, SL, OE and JR. IM contributed to the high‐resolution respirometry analyses. DS and BG wrote the first draft of the manuscript. All authors contributed to the critical revision of the manuscript and approved the final version submitted for publication.
Funding
Financial support provided by the European Union Programme FP7 (PlanHab project: grant no. 284438), European Space Agency, Programme for European Cooperating States (Contract No. 40001043721/11/NL/KML: Planetary Habitat Simulation) and Slovene Research Agency (Contract No. L3‐3654) is acknowledged.
Acknowledgements
The authors thank the all staff of the PlanHab project, Drs Shawnda Morrison and Tadej Debevec (Jožef Stefan Institute, Ljubljana) for the co‐ordination in loco of the project, all the assistants for the excellent technical collaboration and supervision of the subjects during the experimental campaigns, and the subjects who enthusiastically participated in the study. Special thanks are extended to Mr Miro Vrhovec (Jožef Stefan Institute, Ljubljana) and Mr Ranieri Burelli (Department of Medicine, University of Udine) for providing technical assistance.
Biography
Desy Salvadego is a Postdoctoral Fellow in the Department of Medicine at the University of Udine where she completed a PhD programme in Exercise Physiology. Her research activity focuses on the functional evaluation of oxidative metabolism by an integrative approach linking skeletal muscle mitochondria to the respiratory, cardiovascular and skeletal muscle systems, aiming to detect the possible site(s) of exercise limitation in healthy subjects exposed to environmental stressors and in patients affected by chronic diseases.

PlanHab*: Planetary Habitat Simulation
Edited by: Kim Barrett & Harold Schultz
Linked articles This article is highlighted by a Perspective by Lewis. To read this Perspective, visit https://doi.org/10.1113/JP276033.
References
- Ade CJ, Broxterman RM & Barstow TJ (2015). and microgravity exposure: convective versus diffusive O2 transport. Med Sci Sports Exerc 47, 1351–1361. [DOI] [PubMed] [Google Scholar]
- Andersen P, Adams RP, SjØgaard G, Thorboe A & Saltin B (1985). Dynamic knee extension as model for study of isolated exercising muscle in humans. J Appl Physiol 59, 1647–1653. [DOI] [PubMed] [Google Scholar]
- Borg G (1998). Borg's perceived exertion and pain scales. Human Kinetics, Champaign, IL. [Google Scholar]
- Brocca L, Cannavino J, Coletto L, Biolo G, Sandri M, Bottinelli R & Pellegrino MA (2012). The time course of the adaptations of human muscle proteome to bed rest and the underlying mechanisms. J Physiol 590, 5211–5230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calbet JA, Boushel R, Rådegran G, Søndergaard H, Wagner PD & Saltin B (2003). Why is after altitude acclimatization still reduced despite normalization of arterial O2 content? Am J Physiol Regul Integr Comp Physiol 284, R304–R316. [DOI] [PubMed] [Google Scholar]
- Calbet JA, Rådegran G, Boushel R & Saltin B (2009). On the mechanisms that limit oxygen uptake during exercise in acute and chronic hypoxia: role of muscle mass. J Physiol 587, 477–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannavino J, Brocca L, Sandri M, Bottinelli R & Pellegrino MA (2014). PGC‐1α over‐expression prevents metabolic alterations and soleus muscle atrophy in hindlimb unloaded mice. J Physiol 592, 4575–4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannavino J, Brocca L, Sandri M, Grassi B, Bottinelli R & Pellegrino MA (2015). The role of alterations in mitochondrial dynamics and PGC‐1α over‐expression in fast muscle atrophy following hindlimb unloading. J Physiol 593, 1981–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerretelli P & Hoppeler H (1996). Morphologic and metabolic response to chronic hypoxia: the muscle system In: Handbook of Physiology, Section 4, Environmental Physiology, Vol. 4, ed Fregly MJ. & Blatteis CM, pp. 1155–1181. Oxford University Press, New York, NY. [Google Scholar]
- David B, Paul E & Kenneth B (2006). A human lunar surface base and infrastructure solution In: Space 2006, (ed) SPACE conferences and exposition . American Institute of Aeronautics and Astronautics, Reston, VA: 10.2514/6.2006-7336. [DOI] [Google Scholar]
- Debevec T, Bali TC, Simpson EJ, Macdonald IA, Eiken O & Mekjavic IB (2014). Separate and combined effects of 21‐day bed rest and hypoxic confinement on body composition. Eur J Appl Physiol 114, 2411–2425. [DOI] [PubMed] [Google Scholar]
- Ferrari M, Culver JP, Yoshi W & Wabnitz H (2016). Clinical near‐infrared spectroscopy and imaging of the brain. J Biomed Optics 21, 091301. [DOI] [PubMed] [Google Scholar]
- Grassi B & Quaresima V (2016). Near‐infrared spectroscopy and skeletal muscle oxidative function in vivo in health and disease: a review from an exercise physiology perspective. J Biomed Optics 21, 091313. [DOI] [PubMed] [Google Scholar]
- Heinonen I, Koga S, Kalliokoski KK, Musch TI & Poole DC (2015). Heterogeneity of muscle blood flow and metabolism: influence of exercise, aging, and disease states. Exerc Sport Sci Rev 43, 117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horscroft JA & Murray AJ (2014). Skeletal muscle energy metabolism in environmental hypoxia: climbing towards consensus. Extrem Physiol Med 3, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs RA, Boushel R, Wright‐Paradis C, Calbet JA, Robach P, Gnaiger E & Lundby C (2013). Mitochondrial function in human skeletal muscle following high‐altitude exposure. Exp Physiol 98, 245–255. [DOI] [PubMed] [Google Scholar]
- Jacobs RA, Siebenmann C, Hug M, Toigo M, Meinild AK & Lundby C (2012). Twenty‐eight days at 3454‐m altitude diminishes respiratory capacity but enhances efficiency in human skeletal muscle mitochondria. FASEB J 26, 5192–5200. [DOI] [PubMed] [Google Scholar]
- Keramidas ME, Kölegård R, Mekjavic IB & Eiken O (2016a). PlanHab: hypoxia exaggerates the bed‐rest‐induced reduction in peak oxygen uptake during upright cycle ergometry. Am J Physiol Heart Circ Physiol 311, H453–H464. [DOI] [PubMed] [Google Scholar]
- Keramidas ME, Mekjavic IB, Kolegard R, Chouker A, Strewe C & Eiken O (2016b). PlanHab: hypoxia counteracts the erythropoietin suppression, but seems to exaggerate the plasma volume reduction induced by 3 weeks of bed rest. Physiol Rep 4, e12760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga S, Rossiter HB, Heinonen I, Musch TI & Poole DC (2014). Dynamic heterogeneity of exercising muscle blood flow and O2 utilization. Med Sci Sports Exerc 46, 860–876. [DOI] [PubMed] [Google Scholar]
- Kuznetsov AV, Kunz WS, Saks V, Usson Y, Mazat JP, Letellier T, Gellerich FN & Margreiter R (2003). Cryopreservation of mitochondria and mitochondrial function in cardiac and skeletal muscle fibers. Anal Biochem 319, 296–303. [DOI] [PubMed] [Google Scholar]
- Mazzatti DJ, Smith MA, Oita RC, Lim FL, White AJ & Reid MB (2008). Muscle unloading‐induced metabolic remodeling is associated with acute alterations in PPARdelta and UCP‐3 expression. Physiol Genomics 34, 149–161. [DOI] [PubMed] [Google Scholar]
- Murray AJ & Horscroft JA (2016). Mitochondrial function at extreme high altitude. J Physiol 594, 1137–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osada T & Rådegran G (2009). Femoral artery blood flow and its relationship to spontaneous fluctuations in rhythmic thigh muscle workload. Clin Physiol Funct Imaging. 29, 277–292. [DOI] [PubMed] [Google Scholar]
- Pavy‐Le Traon A, Heer M, Narici MV, Rittweger J & Vernikos J (2007). From space to Earth: advances in human physiology from 20 years of bed rest studies (1986‐2006). Eur J Appl Physiol 101, 143–194. [DOI] [PubMed] [Google Scholar]
- Pesta D & Gnaiger E (2012). High‐resolution respirometry. OXPHOS protocols for human cell cultures and permeabilized fibers from small biopsies of human muscle. Methods Mol Biol 810, 25–58. [DOI] [PubMed] [Google Scholar]
- Poole DC, Hirai DM, Copp SW & Musch TI (2012). Muscle oxygen transport and utilization in heart failure: implications for exercise (in)tolerance. Am J Physiol Heart Circ Physiol 302, H1050–H1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcelli S, Marzorati M, Belletti M, Bellistri G, Morandi L & Grassi B (2014). The ‘second wind’ in McArdle's disease patients during a second bout of constant work rate submaximal exercise. J Appl Physiol 116, 1230–1237. [DOI] [PubMed] [Google Scholar]
- Porcelli S, Marzorati M, Lanfranconi F, Vago P, Pišot R & Grassi B (2010). Role of skeletal muscles impairment and brain oxygenation in limiting oxidative metabolism during exercise after bed rest. J Appl Physiol 109, 101–111. [DOI] [PubMed] [Google Scholar]
- Porcelli S, Marzorati M, Morandi M & Grassi B (2016). Home‐based aerobic exercise training improves skeletal muscle oxidative metabolism in patients with metabolic myopathies. J Appl Physiol 121, 699–708. [DOI] [PubMed] [Google Scholar]
- Porcelli S, Marzorati M, Pugliese L, Adamo S, Gondin J, Bottinelli R & Grassi B (2012). Lack of functional effects of neuromuscular electrical stimulation on skeletal muscle oxidative metabolism in healthy humans. J Appl Physiol 113, 1101–1109. [DOI] [PubMed] [Google Scholar]
- Powers SK, Smuder AJ & Judge AR (2012). Oxidative stress and disuse muscle atrophy: cause or consequence? Curr Opin Clin Nutr Metab Care 15, 240–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rådegran G (1997). Ultrasound Doppler estimates of femoral artery blood flow during dynamic knee extensor exercise in humans. J Appl Physiol 83, 1383–1388. [DOI] [PubMed] [Google Scholar]
- Richardson RS, Poole DC, Knight DR, Kurdak SS, Hogan MC, Grassi B, Johnson EC, Kendrick KF, Erickson BK & Wagner PD (1993). High muscle blood flow in man: is maximal O2 extraction compromised? J Appl Physiol 75, 1911–1916. [DOI] [PubMed] [Google Scholar]
- Rittweger J, Möller K, Bareille MP, Felsenberg D & Zange J (2013). Muscle X‐ray attenuation is not decreased during experimental bed rest. Muscle Nerve 47, 722–730. [DOI] [PubMed] [Google Scholar]
- Salvadego D, Domenis R, Lazzer S, Porcelli S, Rittweger J, Rizzo G, Mavelli I, Simunic B, Pisot R & Grassi B (2013). Skeletal muscle oxidative function in vivo and ex vivo in athletes with marked hypertrophy from resistance training. J Appl Physiol 114, 1527–1535. [DOI] [PubMed] [Google Scholar]
- Salvadego D, Keramidas ME, Brocca L, Domenis R, Mavelli I, Rittweger J, Eiken O, Mekjavic IB & Grassi B (2016). Separate and combined effects of a 10‐d exposure to hypoxia and inactivity on oxidative function in vivo and mitochondrial respiration ex vivo in humans. J Appl Physiol 121, 154–163. [DOI] [PubMed] [Google Scholar]
- Salvadego D, Lazzer S, Marzorati M, Porcelli S, Rejc E, Simunic B, Pisot R, di Prampero PE & Grassi B (2011). Functional impairment of skeletal muscle oxidative metabolism during knee extension exercise after bed rest. J Appl Physiol 111, 1719–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperandio PA, Borghi‐Silva A, Barroco A, Nery LE, Almeida DR & Neder JA (2009). Microvascular oxygen delivery‐to‐utilization mismatch at the onset of heavy‐intensity exercise in optimally treated patients with CHF. Am J Physiol Heart Circ Physiol 297, H1720–H1728. [DOI] [PubMed] [Google Scholar]
- Weber T, Ducos M, Mulder E, Å Beijer, Herrera F, Zange J, Degens H, Bloch W & Rittweger J (2014). The relationship between exercise‐induced muscle fatigue, arterial blood flow and muscle perfusion after 56 days local muscle unloading. Clin Physiol Funct Imaging 34, 218–229. [DOI] [PubMed] [Google Scholar]
- Wüst RC, Myers DS, Stones R, Benoist D, Robinson PA, Boyle JP, Peers C, White E & Rossiter HB (2012). Regional skeletal muscle remodeling and mitochondrial dysfunction in right ventricular heart failure. Am J Physiol Heart Circ Physiol 302, H402–H411. [DOI] [PMC free article] [PubMed] [Google Scholar]


