Abstract
Hypoxia‐inducible factors mediate adaptive responses to reduced O2 availability. In patients with obstructive sleep apnoea, repeated episodes of hypoxaemia and reoxygenation (intermittent hypoxia) are sensed by the carotid body (CB). The ensuing CB chemosensory reflex activates the sympathetic nervous system and increased secretion of catecholamines by the adrenal medulla, resulting in hypertension and breathing abnormalities. In the CB, intermittent hypoxia induces the formation of reactive oxygen species (ROS) and increased intracellular Ca2+ levels, which drive increased expression of hypoxia‐inducible factor (HIF) 1α and a decrease in the levels of HIF‐2α. Intermittent hypoxia increases HIF‐1α‐dependent expression of Nox2, encoding the pro‐oxidant enzyme NADPH oxidase 2, and decreased HIF‐2α‐dependent expression of Sod2, encoding the anti‐oxidant enzyme superoxide dismutase 2. These changes in gene expression drive persistently elevated ROS levels in the CB, brainstem, and adrenal medulla that are required for the development of hypertension and breathing abnormalities. The ROS generated by dysregulated HIF activity in the CB results in oxidation and inhibition of haem oxygenase 2, and the resulting reduction in the levels of carbon monoxide leads to increased hydrogen sulfide production, triggering glomus cell depolarization. Thus, the pathophysiology of obstructive sleep apnoea involves the dysregulation of O2‐regulated transcription factors, gasotransmitters, and sympathetic outflow that affects blood pressure and breathing.
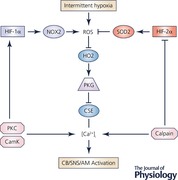
Keywords: HIF‐1, HIF‐2, Redox State, NADPH oxidase, Superoxide dismutase, sleep apnea
Oxygen homeostasis and hypoxia‐inducible factors
Oxygen must be constantly delivered to and consumed by most cells in the human body in order to maintain their viability. O2 delivery is affected by the combined action of the respiratory and circulatory systems under the control of chemo‐ and baroreceptors as well as the central nervous system. O2 consumption is determined by cellular metabolism, principally the relative rates of cellular respiration and glycolysis. Hypoxia‐inducible factors (HIFs) serve as master regulators to maintain oxygen homeostasis in every cell of the body by balancing O2 supply and demand (Prabhakar & Semenza, 2012). HIFs are heterodimeric transcription factors composed of HIF‐α (HIF‐1α, HIF‐2α or HIF‐3α) and HIF‐1β subunits. HIF‐1α is present in nucleated cells of all metazoan species, whereas HIF‐2α and HIF‐3α are expressed only in certain cell types and only in vertebrate species. The HIF‐α subunits are subject to O2‐dependent hydroxylation, ubiquitination and proteasomal degradation under normoxic conditions, whereas the proteins rapidly accumulate under hypoxic conditions (Kaelin & Ratcliffe, 2008). The HIF‐α and HIF‐1β subunits dimerize and bind to the consensus DNA sequence 5′‐RCGTG‐3′ (R = A or G), which is present in hypoxia response elements that are located in or near target genes (Semenza et al. 1996; Semenza, 2014). HIF binding leads to the recruitment of co‐activator proteins that increase transcription of the target gene (Luo et al. 2011, 2012).
Over 2500 direct HIF target genes have been identified. Only a subset of HIF target genes is transactivated in any given cell, allowing each cell to respond to hypoxia in a unique manner (Kelly et al. 2003; Semenza, 2014). HIF target genes include EPO, which encodes erythropoietin, the hormone that is released from the kidney and controls red blood cell production (Semenza & Wang, 1992), and VEGF, which encodes vascular endothelial growth factor, which controls angiogenesis (Forsythe et al. 1996). These two proteins are critical for systemic and local responses to hypoxia, respectively. HIFs also regulate the expression of genes encoding glycolytic enzymes (Semenza et al. 1994, 1996; Iyer et al. 1998) and pyruvate dehydrogenase kinase 1, which mediates a switch from oxidative to glycolytic metabolism in response to hypoxia (Kim et al. 2006). Analysis of knockout mice revealed that complete HIF‐1α deficiency leads to embryonic lethality at mid‐gestation (embryonic day 10.5) with cardiac defects, vascular regression and impaired erythropoiesis, indicating that all three components of the circulatory system are dependent on HIF‐1 for their normal development (Iyer et al. 1998; Yoon et al. 2006).
Carotid body‐mediated reflex responses to hypoxaemia
A decrease in arterial O2 levels increases the sensory nerve activity of the carotid body (CB), a chemoreceptor organ located at the bifurcation of the internal and external carotid arteries that was shown to play a critical role in oxygen sensing nearly a century ago (DeCastro, 1926; Heymans & Heymans, 1927; Kumar & Prabhakar, 2012). Afferent axons from the CB course through the carotid sinus nerve, a branch of the glossopharyngeal (IXth cranial nerve), to the nucleus tractus solitarius (NTS) in the caudal medulla. Interneurons project from the NTS to the rostral ventral respiratory group and the nucleus ambiguus, which project via the phrenic nerve to the diaphragm to regulate respiratory rate (Fig. 1). A third group of interneurons projects from the NTS to the rostral ventrolateral medulla (RVLM) and stimulates neurons whose axons course through the corticospinal tract and stimulate neurons that project via the sympathetic ganglion and adrenal sympathetic nerve to the adrenal medulla (AM), leading to increased release of catecholamines (adrenaline and noradrenaline), which cause an increase in heart rate and arterial vasoconstriction that increases blood pressure (BP) (Fig. 1). Thus, the chemosensory reflex arising from the CB is a major regulator of breathing and sympathetic nerve activity.
Figure 1. The chemosensory reflex pathway mediates rapid cardiovascular and respiratory responses to acute hypoxia.
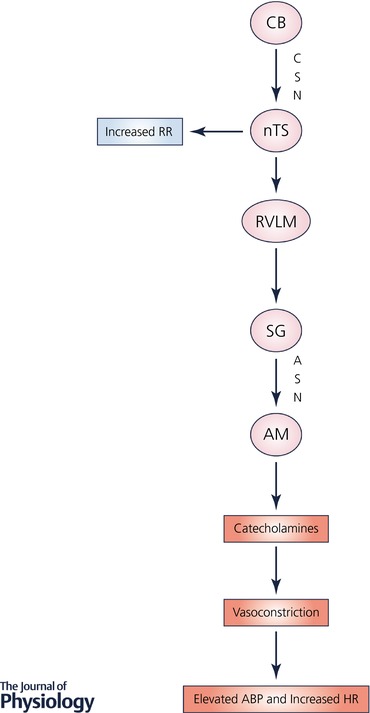
Abbreviations: ABP, arterial blood pressure; AM, adrenal medulla; ASN, adrenal sympathetic nerve; CB, carotid body; CSN, carotid sinus nerve, a branch of IX cranial nerve; HR, heart rate; nTS, nucleus tractus solitarius; RR, respiratory rate; RVLM, rostral ventrolateral medulla; SG, sympathetic ganglion.
Emerging evidence implicates the gasotransmitters carbon monoxide (CO) and hydrogen sulfide (H2S) in hypoxic sensing by the CB. Glomus cells, the primary hypoxia sensing cells in the CB, express haem oxygenase 2 (HO2) and cystathionine‐γ‐lyase (CSE), which are enzymes that produce CO and H2S, respectively (Prabhakar et al. 1995; Williams et al. 2004; Peng et al. 2010). Under normoxic conditions, CO inhibits CSE from producing H2S through protein kinase G‐dependent signalling (Yuan et al. 2015). HO2 has a high K m for O2, such that decreased O2 concentration in glomus cells is sufficient to decrease the production of CO (Yuan et al. 2015). During hypoxia, reduced CO production from HO2 leads to disinhibition of CSE and thereby increases H2S production. H2S, in turn increases the sensory nerve activity by depolarizing glomus cells (Fig. 2).
Figure 2. O2 sensing in carotid body glomus cells involves the generation of carbon monoxide and hydrogen sulfide gasotransmitters.
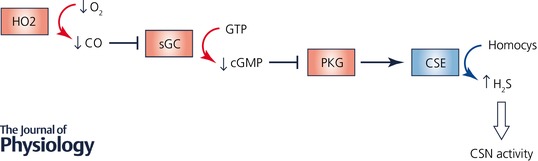
Symbols: arrow, stimulation; blocked arrow, inhibition; curved arrow, substrate/product. Abbreviations: CO, carbon monoxide; CSE, cystathionine‐γ‐lyase; CSN, carotid sinus nerve; H2S, hydrogen sulfide; HO2, haem oxygenase 2; PKG, protein kinase G; sGC, soluble guanylate cyclase.
Obstructive sleep apnoea and systemic hypertension
Obstructive sleep apnoea (OSA) is a highly prevalent respiratory disorder associated with morbidity and mortality (Dempsey et al. 2010). Since the initial study by Young et al. (1993), the reported incidence of OSA has increased partly due to improved screening of sleep‐disordered breathing. According to a recent report (Peppard et al. 2013) OSA affects 3% of 30‐ to 49‐year‐old women, 9% of 50‐ to 70‐year‐old women, 10% of 30‐ to 49‐year‐old men, and 17% of 50‐ to 70‐year‐old men in a community‐based study of 1520 Wisconsin adults. Predisposing factors include micrognathia, retrognathia and, most commonly, obesity. In OSA, when affected individuals fall asleep, the loss of muscle tone causes the upper airway to become occluded by pharyngeal soft tissue, leading to apnoea (cessation of breathing) for 10–40 s, which causes hypoxaemia followed by arousal, clearing of the airway and reoxygenation; the patient falls asleep again and the process is repeated dozens or even hundreds of times per night. OSA causes increased BP and is the leading cause of treatment‐resistant hypertension (Wang, 2014; de Abreu‐Silva & Beltrami‐Moreira, 2014). In addition, OSA patients have breathing abnormalities, such as irregular breathing and central apnoeas. Patients are treated with continuous positive airway pressure (CPAP) to maintain airway patency. CPAP‐treated patients have lower BP than non‐treated patients but it is still elevated compared to the general population. Furthermore, CPAP does not reduce the risk of acute coronary syndrome or stroke in OSA patients (Yu et al. 2017). Thus, novel therapies are urgently needed to prevent cardiovascular sequelae.
Intermittent hypoxia increases ROS production in the CB
Despite the fact that apnoea results in both intermittent hypoxia (IH) and intermittent hypercarbia, exposure of rodents to IH is sufficient to cause hypertension that is dependent on O2 sensing by the CB and the resulting reflex activation of the sympathetic nervous system leading to catecholamine secretion from the AM into the systemic circulation (Fletcher et al. 1995; Lesske et al. 1997). Fletcher and co‐workers examined the effects of combined IH and CO2 on BP responses (Fletcher et al. 1995). Rats were subjected to 35 days with either hypocapnic hypoxia (no added CO2), or eucapnic hypoxia (7–10% inspired CO2 fraction; ), or hypercarbic hypoxia (11–14% ). They found that chronic intermittent hypocapnic hypoxia increased BP by 11 mmHg, but neither episodic eucapnic hypoxia nor intermittent asphyxia had any additional effect beyond this, suggesting that the neurohumoral systems involved in the chronic diurnal blood pressure response to IH are already maximally stimulated by hypocapnic hypoxia.
In one model of OSA, rodents are placed in a chamber in which the O2 concentration is rapidly decreased to 5%, held for 15 s and then rapidly increased to 21%, and held for 5 min. This cycle of hypoxia and reoxygenation is repeated for 8 h during the animal's sleep cycle and results in hypertension within 10 days (Prabhakar, 2001). The process of hypoxia and reoxygenation results in the generation of reactive oxygen species (ROS), which is critical for the pathogenesis of hypertension because administration of the superoxide scavenger manganese (III) tetrakis (1‐methyl‐4‐pyridyl) porphyrin (MnTMPyP) during the 10‐day exposure to IH blocks the development of hypertension (Peng et al. 2006). Increased production of ROS seems to occur during reoxygenation rather than the hypoxic phase of IH (Yuan et al. 2004). Hydrogen peroxide (H2O2) generated by the superoxide anion was identified as a major reactive oxygen species mediating the effects of IH (Peng et al. 2009).
Intermittent hypoxia dysregulates HIF activity in the CB
IH induces the expression of HIF‐1α protein in the CB and central nervous system, which is blocked by MnTMPyP administration (Peng et al. 2006; Yuan et al. 2011). Mice that are heterozygous for a knockout allele at the Hif1a locus are protected from the development of hypertension in response to IH and have no increase in ROS (Peng et al. 2006). The finding that HIF‐1α was both upstream and downstream of ROS suggested a positive feedback mechanism in which ROS‐induced HIF‐1 activity led to the expression of a protein that further increased ROS. This protein turned out to be NADPH oxidase 2 (NOX2), which is a major source of superoxide production (Yuan et al. 2011). The NOX2‐dependent increase in ROS in IH‐exposed PC12 rat pheochromocytoma cells triggered increased intracellular Ca2+ levels and activation of protein kinase C and mammalian target of rapamycin (mTOR), as well as inhibition of HIF prolyl hydroxylase activity, leading to increased synthesis and stability of HIF‐1α (Yuan et al. 2008). Whereas the inhibition of prolyl hydroxylase activity rapidly resolved after cessation of IH, mTOR activity and HIF‐1α protein levels remained increased for 90 min, which parallels the persistent hyper‐reactivity of CBs taken from animals after cessation of IH (Peng et al. 2006; Yuan et al. 2008).
Both HIF‐1α and HIF‐2α are expressed within the O2‐sensing glomus cells of the CB (Roux et al. 2005; Peng et al. 2014). However, HIF‐1α levels are low in the CB under normoxic conditions and induced by IH, whereas HIF‐2α levels are high in the CB under normoxic conditions and extinguished by IH (Nanduri et al. 2009). Degradation of HIF‐2α by IH is mediated by calpains, which are Ca2+‐dependent proteases. Remarkably, whereas HIF‐1α is a positive regulator of Nox2 gene expression, HIF‐2α activates transcription of the Sod2 gene, which encodes the mitochondrial enzyme that converts superoxide to hydrogen peroxide (Nanduri et al. 2009). Treatment of rodents with N‐acetyl‐l‐leucyl‐l‐leucyl‐l‐norleucinal, a calpain inhibitor, blocked HIF‐2α degradation, maintained Sod2 expression, blocked the increase in ROS and prevented the development of hypertension in mice exposed to IH (Nanduri et al. 2009).
Hif1a+/− mice were completely resistant to the development of oxidative stress, sympathetic nervous system activation and hypertension in response to IH (Peng et al. 2006). In contrast, Hif2a+/− mice were found to have hypertension and respiratory instability under basal conditions in normoxia and their CBs were hyper‐reactive when subjected to acute hypoxia, similar to CBs from wild‐type mice that have been exposed to IH for 10 days (Peng et al. 2011). In addition to decreased Sod2 expression, Hif2a+/− mice display increased Nox2 expression as well as oxidative stress in the CB and AM due to increased HIF‐1α expression under normoxic conditions (Yuan et al. 2013). Conversely, in CB and AM of Hif1a+/− mice, HIF‐2α and Sod2 expression were increased. As a consequence of increased anti‐oxidant enzyme level, Hif1a+/− mice exhibit a reduced cellular oxidative (redox) state. Most dramatically, Hif1a+/−;Hif2a+/− mice that were heterozygous for knockout alleles at both loci were completely normal with respect to redox state in the CB and AM, CB response to acute hypoxia, breathing and BP (Yuan et al. 2013).
The changes in HIF‐1α and HIF‐2α expression (as well as Nox2 and Sod2 expression) and redox state that were observed in the CB and AM in response to IH were also observed in the NTS and RVLM, but not in nearby brainstem regions that do not receive input from the CB (Peng et al. 2014). Furthermore, CB ablation blocked all of these responses to IH in the NTS, RVLM and AM, indicating that responses to IH were initiated solely in the CB and were subsequently transduced as responses of the efferent neural pathway.
Effect of intermittent hypoxia on oxygen sensing
Thus far, the role of gasotransmitters in acute oxygen sensing and of hypoxia‐inducible factors in the response to chronic IH have been presented. There is a connection between these two pathways and it is through ROS, which are generated during IH and amplified by the dysregulation of HIF‐1α and HIF‐2α expression. Oxidation of cysteine residue 265 in HO2 inhibits its catalytic activity, thereby decreasing the production of CO, which in turn, leads to increased production of H2S and increased glomus cell depolarization (Yuan et al. 2016). The increase in BP associated with chronic IH was not observed in Cse−/− mice or wild‐type mice treated with a CSE inhibitor, which lack H2S production in the CB (Peng et al. 2017). In contrast, Ho2−/− mice have irregular breathing and frequent hypopnea and apnoea episodes, which include both central and obstructive events (Peng et al. 2017). Remarkably, all of these abnormalities were corrected by treatment with a CO donor or by treatment with a CSE inhibitor (Peng et al. 2017). Hence, it is possible to integrate the regulatory circuits governed by gasotransmitters and O2‐regulated transcription factors (Abstract figure).
Conclusions and implications
Taken together, these studies indicate the existence of mutual antagonism between HIF‐1α and HIF‐2α in the CB and downstream neural components of the chemosensory reflex, and that the balance between HIF‐1α‐dependent pro‐oxidant and HIF‐2α‐dependent anti‐oxidant activity determines the redox state of the CB, which in turns determines the set point of the sympathetic nervous system and cardiorespiratory homeostasis. This balance can be disturbed by environmental (OSA‐induced IH) or genetic (Hif2a null allele) causes, leading to increased sympathetic activation and the development of hypertension. Drugs that selectively inhibit HIF‐1 or HIF‐2 might prevent the development of hypertension in OSA patients or induce hypertension in non‐OSA patients, respectively. For example, HIF‐2α selective inhibitors, currently under evaluation as anti‐cancer therapy (Chen et al. 2016; Cho et al. 2016), might lead to an imbalance between HIF‐1 and HIF‐2 activity, resulting in hypertension and breathing abnormalities (Yuan et al. 2013). Based on these findings, we hypothesize that in addition to OSA, systemic hypertension due to other causes may also involve disturbance of the balance between HIF‐1α and HIF‐2α, leading to oxidative stress in the CB and AM leading to sympatho‐adrenal activation.
Additional information
Competing interests
The authors declare they have no competing interests.
Author contributions
G.L.S. and N.R.P. wrote the manuscript. Both authors have approved the final version of the manuscript and agree to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
Sleep apnoea research in the authors’ laboratories is supported by Public Health Service grant P01‐HL‐90554 from the National Institutes of Health.
Biographies
Gregg L. Semenza received his MD and PhD from the University of Pennsylvania, Philadelphia, USA. He is currently C. Michael Armstrong Professor and Director, Vascular Biology Program, Institute for Cell Engineering at the Johns Hopkins University, School of Medicine, Baltimore, USA. He is known for the discovery of HIF‐1 and establishing its role in oxygen homeostasis.

Nanduri R. Prabhakar received PhD in Physiology from Baroda, India and DSc in Biology from Ruhr‐University, Germany. He is currently Harold H. Hines Professor and Inaugural Director of the Institute for Integrative Physiology and Center for Systems Biology of O2 sensing at the University of Chicago, USA. He is recognized for his studies on O2 sensing mechanisms by the carotid body and physiological consequences of hypoxia.
Edited by: Kim Barrett & Harold Schultz
Contributor Information
Gregg L. Semenza, Email: gsemenza@Jhmi.edu.
Nanduri R. Prabhakar, Email: nprabhak@medicine.bsd.uchicago.edu.
References
- Chen W, Hill H, Christie A, Kim MS, Holloman E, Pavia‐Jimenez A, Homayoun F, Ma Y, Patel N, Yell P, Hao G, Yousuf Q, Joyce A, Pedrosa I, Geiger H, Zhang H, Chang J, Gardner KH, Bruick RK, Reeves C, Hwang TH, Courtney K, Frenkel E, Sun X, Zojwalla N, Wong T, Rizzi JP, Wallace EM, Josey JA, Xie Y, Xie XJ, Kapur P, McKay RM & Brugarolas J (2016). Targeting renal cell carcinoma with a HIF‐2 antagonist. Nature 539, 112–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H, Du X, Rizzi JP, Liberzon E, Chakraborty AA, Gao W, Carvo I, Signoretti S, Bruick RK, Josey JA, Wallace EM & Kaelin WG (2016). On‐target efficacy of a HIF‐2α antagonist in preclinical kidney cancer models. Nature 539, 107–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Abreu‐Silva EO & Beltrami‐Moreira M (2014). Sleep apnea: an underestimated cause of resistant hypertension. Curr Hypertens Rev 10, 2–7. [DOI] [PubMed] [Google Scholar]
- De Castro F (1926). Sur la structure et l'innervation de la glande intercarotidienne (glomus caroticum) de l'homme et des mammiferes et sur un nouveau systeme de l'innervation autonome du nerf glossopharyngien. Trav Lab Rech Biol 24, 365–432. [Google Scholar]
- Dempsey JA, Veasey SC, Morgan BJ & O'Donnell CP (2010). Pathophysiology of sleep apnea. Physiol Rev 90, 47–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher EC, Bao G & Miller CC (1995). Effect of recurrent episodic hypocapnic, eucapnic, and hypercapnic hypoxia on systemic blood pressure. J Appl Physiol 78, 1516–1521. [DOI] [PubMed] [Google Scholar]
- Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD & Semenza GL (1996). Activation of vascular endothelial growth factor gene transcription by hypoxia‐inducible factor 1. Mol Cell Biol 16, 4604–4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heymans J & Heymans C (1927). Sur les modifications directes et sur la regulation reflexe de l'activitie du centre respiratory de la tete isolee du chien. Arch Int Pharmacodyn Ther 33, 273–372. [Google Scholar]
- Iyer NV, Kotch LE, Agani F, Leung SW, Laughner E, Wenger RH, Gassmann M, Gearhart JD, Lawler AM, Yu AY & Semenza GL (1998). Cellular and developmental control of O2 homeostasis by hypoxia‐inducible factor 1α. Genes Dev 12, 149–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaelin WG Jr & Ratcliffe PJ (2008). Oxygen sensing in metazoans: the central role of the HIF hydroxylase pathway. Mol Cell 30, 393–402. [DOI] [PubMed] [Google Scholar]
- Kelly BD, Hackett SF, Hirota K, Oshima Y, Cai Z, Berg‐Dixon S, Rowan A, Yan Z, Campochiaro PA & Semenza GL (2003). Cell type‐specific regulation of angiogenic growth factor gene expression and induction of angiogenesis in nonischemic tissue by a constitutively active form of hypoxia‐inducible factor 1. Circ Res 93, 1074–1081. [DOI] [PubMed] [Google Scholar]
- Kim JW, Tchernyshyov I, Semenza GL & Dang CV (2006). HIF‐1‐mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab 3, 177–185. [DOI] [PubMed] [Google Scholar]
- Kumar P & Prabhakar NR (2012). Peripheral chemoreceptors: function and plasticity of the carotid body. Compr Physiol 2, 141–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesske J, Fletcher EC, Bao G & Unger T (1997). Hypertension caused by chronic intermittent hypoxia – influence of chemoreceptors and sympathetic nervous system. J Hypertens 15, 1593–1603. [DOI] [PubMed] [Google Scholar]
- Luo W, Chang R, Zhong J, Pandey A & Semenza GL (2012). Histone demethylase JMJD2C is a coactivator for hypoxia‐inducible factor 1 that is required for breast cancer progression. Proc Natl Acad Sci USA 109, E3367–E3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W, Hu H, Chang R, Zhong J, Knabel M, O'Meally R, Cole RN, Pandey A & Semenza GL (2011). Pyruvate kinase M2 is a PHD3‐stimulated coactivator for hypoxia‐inducible factor 1. Cell 145, 732–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanduri J, Wang N, Yuan G, Khan SA, Souvannakitti D, Peng YJ, Kumar GK, Garcia JA & Prabhakar NR (2009). Intermittent hypoxia degrades HIF‐2α via calpains resulting in oxidative stress: implications for recurrent apnea‐induced morbidities. Proc Natl Acad Sci USA 106, 1199–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng YJ, Nanduri J, Khan SA, Yuan G, Wang N, Kinsman B, Vaddi DR, Kumar GK, Garcia JA, Semenza GL & Prabhakar NR (2011). Hypoxia‐inducible factor 2α (HIF‐2α) heterozygous‐null mice exhibit exaggerated carotid body sensitivity to hypoxia, breathing instability, and hypertension. Proc Natl Acad Sci USA 108, 3065–3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng YJ, Nanduri J, Raghuraman G, Souvannakitti D, Gadalla MM, Kumar GK, Snyder SH & Prabhakar NR (2010). H2S mediates O2 sensing in the carotid body. Proc Natl Acad Sci USA 107, 10719–10724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng YJ, Nanduri J, Yuan G, Wang N, Deneris E, Pendyala S, Natarajan V, Kumar GK & Prabhakar NR (2009). NADPH oxidase is required for the sensory plasticity of the carotid body by chronic intermittent hypoxia. J Neurosci 29, 4903–4910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng YJ, Yuan G, Khan S, Nanduri J, Makarenko VV, Reddy VD, Vasavda C, Kumar GK, Semenza GL & Prabhakar NR (2014). Regulation of hypoxia‐inducible factor‐α isoforms and redox state by carotid body neural activity in rats. J Physiol 592, 3841–3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng YJ, Yuan G, Ramakrishnan D, Sharma SD, Bosch‐Marce M, Kumar GK, Semenza GL & Prabhakar NR (2006). Heterozygous HIF‐1α deficiency impairs carotid body‐mediated systemic responses and reactive oxygen species generation in mice exposed to intermittent hypoxia. J Physiol 577, 705–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng YJ, Zhang X, Gridina A, Chupikova I, McCormick DL, Thomas RJ, Scammell TE, Kim G, Vasavda C, Nanduri J, Kumar GK, Semenza GL, Snyder SH & Prabhakar NR (2017). Complementary roles of gasotransmitters CO and H2S in sleep apnea. Proc Natl Acad Sci USA 114, 1413–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peppard PE, Young T, Barnet JH, Palta M, Hagen EW & Hla KM (2013). Increased prevalence of sleep‐disordered breathing in adults. Am J Epidemiol 177, 1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakar NR (2001). Oxygen sensing during intermittent hypoxia: cellular and molecular mechanisms. J Appl Physiol 90, 1986–1994. [DOI] [PubMed] [Google Scholar]
- Prabhakar NR, Dinerman JL, Agani FH & Snyder SH (1995). Carbon monoxide: a role in carotid body chemoreception. Proc Natl Acad Sci USA 92, 1994–1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakar NR & Semenza GL (2012). Adaptive and maladaptive cardiorespiratory responses to continuous and intermittent hypoxia mediated by hypoxia‐inducible factors 1 and 2. Physiol Rev 92, 967–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux JC, Brismar H, Aperia A & Lagercranz H (2005). Developmental changes in HIF transcription factor in carotid body: relevance for O2 sensing by chemoreceptors. Pediatr Res 58, 53–57. [DOI] [PubMed] [Google Scholar]
- Semenza GL (2014). Hypoxia‐inducible factor 1 and cardiovascular disease. Annu Rev Physiol 76, 39–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza GL, Jiang BH, Leung SW, Passantino R, Concordet JP, Maire P & Giallongo A (1996). Hypoxia response elements in the aldolase A, enolase 1, and lactate dehydrogenase A gene promoters contain essential binding sites for hypoxia‐inducible factor 1. J Biol Chem 271, 32529–32537. [DOI] [PubMed] [Google Scholar]
- Semenza GL, Roth PH, Fang HM & Wang GL (1994). Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia‐inducible factor 1. J Biol Chem 269, 23757–23763. [PubMed] [Google Scholar]
- Semenza GL & Wang GL (1992). A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol 12, 5447–5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon D, Pastore YD, Divoky V, Liu E, Mlodnicka AE, Rainey K, Ponka P, Semenza GL, Schumacher A & Prchal JT (2006). Hypoxia‐inducible factor‐1 deficiency results in dysregulated erythropoiesis signaling and iron homeostasis in mouse development. J Biol Chem 281, 25703–25711. [DOI] [PubMed] [Google Scholar]
- Young T, Palta M, Dempsey J, Skatrud J, Weber S & Badr S (1993). The occurrence of sleep‐disordered breathing among middle‐aged adults. N Engl J Med 328, 1230–1235. [DOI] [PubMed] [Google Scholar]
- Yu J, Zhou Z, McEvoy RD, Anderson CS, Rodgers A, Perkovic V & Neal B (2017). Association of positive airway pressure with cardiovascular events and death in adults with sleep apnea: a systematic review and meta‐analysis. JAMA 318, 156–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan G, Adhikary G, McCormick AA, Holcroft JJ, Kumar GK & Prabhakar NR (2004). Role of oxidative stress in intermittent hypoxia‐induced immediate early gene activation in rat PC12 cells. J Physiol 557, 773–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan G, Khan SA, Luo W, Nanduri J, Semenza GL & Prabhakar NR (2011). Hypoxia‐inducible factor 1 mediates increased expression of NADPH oxidase‐2 in response to intermittent hypoxia. J Cell Physiol 226, 2925–2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan G, Nanduri J, Khan S, Semenza GL & Prabhakar NR (2008). Induction of HIF‐1α expression by intermittent hypoxia: involvement of NADPH oxidase, Ca2+ signaling, prolyl hydroxylases, and mTOR. J Cell Physiol 217, 674–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan G, Peng YJ, Khan SA, Nanduri J, Singh A, Vasavda C, Semenza GL, Kumar GK, Snyder SH & Prabhakar NR (2016). H2S production by reactive oxygen species in the carotid body triggers hypertension in a rodent model of sleep apnea. Sci Signal 9, ra80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan G, Peng YJ, Reddy VD, Makarenko VV, Nanduri J, Khan SA, Garcia JA, Kumar GK, Semenza GL & Prabhakar NR (2013). Mutual antagonism between hypoxia‐inducible factors 1α and 2α regulates oxygen sensing and cardio‐respiratory homeostasis. Proc Natl Acad Sci USA 110, E1788–E1796. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Yuan G, Vasavda C, Peng YJ, Makarenko VV, Raghuraman G, Nanduri J, Gadalla MM, Semenza GL, Kumar GK, Snyder SH & Prabhakar NR (2015). Protein kinase G‐regulated production of H2S governs oxygen sensing. Sci Signal 8, ra37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang AY (2014). Sleep disordered breathing and resistant hypertension. Semin Nephrol 34, 520–531. [DOI] [PubMed] [Google Scholar]
- Williams SE, Wootton P, Mason HS, Bould J, Iles DE, Riccardi D, Peers C & Kemp PJ (2004). Hemoxygenase‐2 is an oxygen sensor for a calcium‐sensitive potassium channel. Science 306, 2093–2007. [DOI] [PubMed] [Google Scholar]


