Abstract
Mammals must continuously regulate the levels of O2 and CO2, which is particularly important for the brain. Failure to maintain adequate O2/CO2 homeostasis has been associated with numerous disorders including sleep apnoea, Rett syndrome and sudden infant death syndrome. But, O2/CO2 homeostasis poses major regulatory challenges, even in the healthy brain. Neuronal activities change in a differentiated, spatially and temporally complex manner, which is reflected in equally complex changes in O2 demand. This raises important questions: is oxygen sensing an emergent property, locally generated within all active neuronal networks, and/or the property of specialized O2‐sensitive CNS regions? Increasing evidence suggests that the regulation of the brain's redox state involves properties that are intrinsic to many networks, but that specialized regions in the brainstem orchestrate the integrated control of respiratory and cardiovascular functions. Although the levels of O2 in arterial blood and the CNS are very different, neuro‐glial interactions and purinergic signalling are critical for both peripheral and CNS chemosensation. Indeed, the specificity of neuroglial interactions seems to determine the differential responses to O2, CO2 and the changes in pH.
Keywords: preBötzinger Complex, retrotrapezoid nucleus, carotid body, glomus cells, astrocytes, cardiorespiratory coupling
Introduction
Endothermy gave mammals and birds distinct evolutionary advantages. It allowed them to move quickly and over long distances irrespective of their surrounding environmental temperatures. This enabled them to conquer novel ecological niches. However, endothermy came with a substantially higher metabolic demand (Clarke & Pörtner, 2010). This demand is best met by aerobic metabolism, since oxygen releases substantial energy per electron transfer (Ramirez et al. 2007). Aerobic metabolism is particularly important to maintain the brain's state of persistent activity (Raichle et al. 2001; Raichle, 2015; Mitra & Raichle, 2016).
The dependency on aerobic metabolism is challenging for two important reasons. Firstly, O2 cannot effectively be stored; consequently, mammals and birds cannot survive a prolonged cessation of breathing and heartbeat. Secondly, the dependency on the molecule with the largest energy release per electron transfer poses major regulatory challenges because too little oxygen is as detrimental as too much oxygen, a topic of great clinical significance (Semenza & Prabhakar, 1985; Haddad & Jiang, 1997; D'Agostino et al. 2007; Huang et al. 2012; Popa‐Wagner et al. 2013; Igbal & Eftekharpour, 2017). Indeed, the within the CNS is maintained within a narrow range of approximately 1–4% O2 (Mulkey et al. 2001). This suggests that neuronal microcircuits in the brain must maintain persistent activity in an oxidative microenvironment that is only slightly higher than the threshold for aerobic metabolism, which lies around 1% O2 (Clemens et al. 2001; Hill et al. 2011). Thus, maintaining a stable redox state requires precise and dynamic O2 sensing and response mechanisms, which is achieved through neurovascular coupling involving neurons, smooth muscle cells, astrocytes (Filosa & Blanco, 2007; Ndunuizu & LaManna, 2007; Kim et al. 2016; Iadecola, 2017; Kisler et al. 2017) and possibly oligodendrocytes (Roth & Núñez, 2016).
The neuronal responses to hypoxia are differentiated, and involve various mechanisms (Haddad & Jiang, 1994; Bickler & Donohoe, 2002; Björklund et al. 2008). In general, acute exposure to hypoxia leads to a rapid decrease in neuronal activity and synaptic depression in many regions of the brain (Garcia et al. 2010a, b ; Mukandala et al. 2016). While this may be protective, it also leads to the loss of synaptic plasticity (Lyubkin et al. 1997) and learning deficits (Row et al. 2003). During anoxia this homeostatic response breaks down as neurons depolarize within minutes until they lose their ionic gradients across the membranes (Haddad & Jiang, 1993; Fung & Haddad, 1997; Folklow et al. 2008). Interestingly, diving mammals have developed specialized neuroglial adaptations to withstand prolonged periods of anoxia (Folklow et al. 2008; Mitz et al. 2009; Ramirez et al. 2011; Czech‐Damal et al. 2014).
The neuronal responses to hyperoxia are as differentiated (Garcia et al. 2010a, b ). The reactive oxygen species (ROS) superoxide anion and H2O2 serve neuromodulatory functions. In midbrain dopaminergic neurons, H2O2 activates KATP channels to reduce neuronal excitability (Avshalumov et al. 2005). At the neuromuscular junction, H2O2 differentially modulates presynaptic Ca2+ entry (Giniatullin & Giniatullin, 2003). The superoxide anion facilitates phrenic and hypoglossal motor outputs (MacFarlane & Mitchell, 2008; MacFarlane et al. 2008), and can induce plasticity (Kamsler & Segal, 2003; MacFarlane & Mitchell, 2008). Similar modulatory effects have been described for the carotid body (CB) (Peng et al. 2003, 2009).
The response to changes in oxygen is of critical importance in areas that are responsible for controlling O2 supply. The preBötzinger complex (preBötC) is a microcircuit critical for different forms of inspiration that range from normal breathing to sighing and gasping (Smith et al. 1991; Lieske et al. 2000; Hayes et al. 2012; Wang et al. 2014). This network is located within the medulla (Smith et al. 1991; Schwarzacher et al. 2011) and is essential for breathing (Ramirez, 1998; Gray et al. 2001; Tan et al. 2008) (Fig. 1). Neuronal and glial functions within this network are responsive to hypoxia even when the network is isolated in a slice preparation (Peña & Ramirez, 2004; Peña et al. 2004; Tryba et al. 2006; Gourine et al. 2010; Huckstepp et al. 2010a; Hill et al. 2011; Nieto‐Posadas et al. 2014; Rivera‐Angula & Peña‐Ortega, 2014; Angelova et al. 2015; Lorea‐Hernandez et al. 2016; Peña‐Ortega, 2017). Hypoxia evokes a biphasic response: a rapid augmentation with the generation of sighs is followed by a respiratory depression (Fig. 2; Wilken et al. 1998; Ramirez et al. 1998b b; Telgkamp & Ramirez, 1999; Lieske et al. 2000; Thoby‐Brisson & Ramirez, 2000; Telgkamp et al. 2002; Peña & Ramirez, 2005). This hypoxic sensitivity of the preBötC neurons was also demonstrated in vivo (Solomon et al. 2000). Hypoxia also evokes increased activity in hypoglossal (XII) neurons (Donnelly et al. 1992, 2009; Jiang et al. 1992; Jiang & Haddad, 1994; Telgkamp & Ramirez, 1999) and in pre‐sympathetic neurons of the rostral ventrolateral medulla (RVLM) (Sun et al. 1992; Sun & Reis, 1994), while hypoxia hyperpolarizes the dorsal vagal motor nucleus (Trapp & Ballanyi, 1995; Kulik et al. 2002; Ballanyi, 2004; Balfour & Trapp, 2007). The central responses to hypoxia within the preBötC, XII, presympathetic and parasympathetic neurons will likely contribute to an increased respiratory and sympathetic drive and a decreased parasympathetic drive (Dyavanapalli et al. 2014). These examples of sensitivity to hypoxia within brainstem respiratory circuits illustrate that central oxygen‐sensitive mechanisms exist and locally regulate the activity of microcircuits in an adaptive manner.
Figure 1. Anatomical schematic representation of medullary network involved in chemosensitivity.
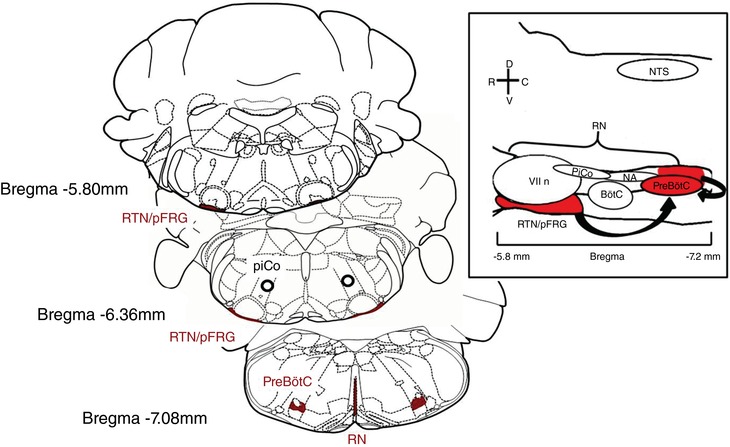
Sagittal view of ventral medullary respiratory group and the raphe nucleus (RN). The respiratory group consists of the retrotrapezoid nucleus (RTN)–parafacial respiratory group (pFRG) complex (RTN/pFRG), the Bötzinger complex (BötC), the postinspiratory complex (PiCo) and the pre‐Bötzinger complex (preBötC). Structures in red have been extensively studied and mentioned in this review contributing to chemosensitivity in the CNS. Black arrows suggest communication between medullary network, RTN/pFRG and RN signal the preBötC ultimately leading to changes in breathing.
Figure 2. The effect of hypoxia on the preBötC in medullary slice recording (population and single cell recordings).
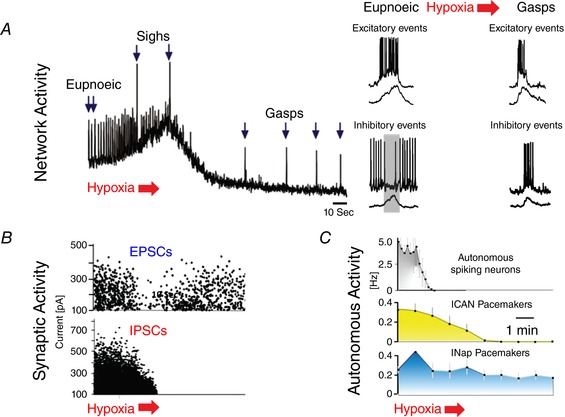
A, the respiratory network shows a biphasic response: an initial augmentation during which the respiratory frequency is enhanced and sighs are generated is followed by a depression during which the network reconfigures into gasping. B, synaptic changes occur during the hypoxia‐induced reconfiguration as exemplified by transient changes in synaptic excitation and a suppression of synaptic inhibition. C, hypoxia alters bursting properties: it inhibits bursting properties that depend on the Ca2+‐activated non‐specific cation current (I CAN), while bursting mechanisms that depend on the persistent sodium current (I Nap) remain relatively unaffected.
This raises an important question: are these neuronal responses controlled by discrete central oxygen sensors, such as the specialized cellular interactions within the CB (Prabhakhar, 2013; Nurse, 2014; Nanduri et al. 2015a; Prabhakhar & Peng, 2017; Rakoczy & Wyatt, 2018), or do these responses emerge from multiple oxygen sensitivities intrinsic to the networks themselves? Here we propose that the hypoxic response involves both emergent network properties and specialized chemosensitive neuroglial interactions. From a functional perspective the responses to changes in O2, CO2 and pH must be different. Indeed, there is increasing evidence that different networks seem to specialize in sensing primarily hypoxia or hypercapnia. Yet, the strikingly different network responses seem to rely on neuroglial interactions in which astrocytes are instrumental in differentiating chemosensory responses into specific O2 as well as CO2 sensitivities. Thus, although this review focuses on oxygen homeostasis and the hypoxic response of the CNS, we will consider the differential O2 and CO2 sensitivities when discussing the neuroglial interactions. Ultimately, the organism needs to respond to changes in both blood gases in a synergistic and adaptive manner.
Unravelling the network mechanisms underlying peripheral and central O2 sensing
To mount an effective response to changes in blood gases, O2 sensing mechanisms within the CNS must be tightly coordinated with inputs derived from peripheral chemosensory mechanisms (Basting et al. 2016; Wilson & Teppema, 2016; Guyenet et al. 2018). Exactly how these peripheral mechanisms are integrated within the central neuronal networks in the brainstem is not fully understood, and is a source of controversy (as reviewed in Smith et al. 2010). At the organismal level, the hypoxic ventilatory response (HVR) is biphasic (Bissonnette, 2000): an initial augmentation is followed by a ventilatory depression (Moss, 2000). The augmentation has been associated with an excitatory drive from the CB, the depression with central regulatory activity (Teppema & Dahan, 2010; Rajani et al. 2018). However, it is not quite this simple. Some experiments suggest that CB denervation eliminates the augmentation phase (Bureau et al. 1985; Wang et al. 1996; Izumizaki et al. 2004; Hill et al. 2011), while others suggest that the initial augmentation during hypoxia is preserved in peripherally chemoreceptor denervated animals (Moyer & Beecher, 1942; Miller & Tenney, 1975; Richter et al. 1991). Indeed elegant studies have convincingly demonstrated that specific CNS hypoxia stimulates ventilation during wakefulness and sleep (Engwall et al. 1985; Smith et al. 1993; Curran et al. 2000). This is consistent with surgical CB denervation, which does not lead to obvious catastrophic physiological consequences. Thus, CB denervation became a procedure performed on patients in instances of carotid sinus syndrome, asthma or pulmonary disease. (For more details on benefits and risks see Holton & Wood, 1965; Marschke et al. 1965; Wood et al. 1965; Toorop et al. 2010; Fitzgerald, 2014; Gourine & Funk, 2017; Iturriaga, 2018).
Limitations and caveats associated with studying hypoxic response
The discussion in the previous section is represents the challenges faced when exploring oxygen homeostasis and the neuronal response: O2 supply and delivery depend on experimental conditions that vary widely and any experimental manipulation can have complex ramifications that are difficult to control and often difficult to interpret. This is not only the case for in vivo studies, but also for studies that are performed in reduced preparations in which oxygen‐depth profiles differ, e.g. in the working heart–brainstem preparation (Wilson et al. 2001), the isolated brainstem spinal cord preparation (Brockhaus et al. 1993; Okada et al. 1993), as well as brain slices (Bingmann & Kolde, 1982; Mulkey et al. 2001; Garcia et al. 2010a; Hill et al. 2011). Oxygen profiles even differ within a given preparation, because oxygen levels depend on neuronal activity that varies between different regions of a slice (Bingmann & Kolde, 1982). Brain slices are typically studied at cooler temperatures. By decreasing metabolic consumption tissue oxygenation increases within the core, but the superficial layers are rendered hyperoxic. Thus, the neuronal networks will be exposed concurrently to hyperoxic and hypoxic conditions that will affect neuronal activity. Oxygenation is also influenced by the rate and method of superfusion, the exact composition of the artificial cerebrospinal fluid, as well as the ambient barometric pressure (Jiang et al. 1991; Mulkey et al. 2001; Fong et al. 2008). Experimental conditions also depend on the research questions. Studying the postnatal development of a network will be complicated by the fact that mature and neonatal slices vary in their oxygenation profile (Jiang et al. 1991; Mulkey et al. 2001; Hill et al. 2011). Characterizing network interactions between different regions also require slices to be cut in different thicknesses (D'Agostino et al. 2007; Ballanyi & Ruangkittisakul, 2009; Hill et al. 2011; Gourevitch & Mellen, 2014; Anderson & Ramirez, 2017).
Yet, to achieve a complete understanding of the central hypoxic response, different preparations and approaches have to be combined. The introduction of modern transgenic, optogenetic and molecular biological methods significantly increased the experimental repertoire and allows for more specific manipulations and characterizations of identified neuron classes in preparations that range from brain slices to alert and freely behaving animals (Angelova et al. 2015; Burke et al. 2015; Guyenet & Bayliss, 2015; Guyenet et al. 2016; Rajani et al. 2018).
Reconfiguration of the respiratory network during hypoxia
Much has been learned about the hypoxic response of the preBötC. The respiratory rhythm in this microcircuit depends on glutamatergic neurons that are primarily derived from progenitor cells characterized by the transcription factor Dbx1 (Bouvier et al. 2010; Gray et al. 2010; Cui et al. 2016), and inhibitory neurons that can be subdivided into glycinergic and GABAergic neurons (Ramirez et al. 1997; Janczewski et al. 2013; Sherman et al. 2015). These neurons possess a variety of intrinsic membrane properties. Upon synaptic isolation respiratory neurons are silent, tonically active or possess intrinsic bursting properties (Viemari & Ramirez, 2006; Carroll & Agarwal, 2010; Morgado‐Valle et al. 2010). These bursting properties are mediated by two principal inward currents: the persistent sodium current (I Nap) and the calcium‐dependent non‐specific cation current (I CAN) (Thoby‐Brisson & Ramirez, 2001; Peña & Ramirez, 2004; Crowder et al. 2007; Rubin et al. 2009; Del Negro et al. 2011; Dunmyre et al. 2011). Early during hypoxia, I CAN‐dependent bursting ceases but bursting persists in neurons that depend on I Nap (Fig. 2). This differential sensitivity impacts the network's dependency on these two properties. Rhythmogenesis persists when I Nap is blocked with riluzole in control, but it ceases when I Nap is blocked during hypoxia (Peña et al. 2004). At the concentration used, riluzole specifically blocked bursting, but not action potential generation (Peña et al. 2004), suggesting that the ‘bursting property’ is critical for rhythmogenesis in hypoxia. However, these pharmacological experiments cannot exclude that riluzole also altered other properties, such as synaptic transmission. Yet, modulators unrelated to riluzole had similar effects: blocking 5‐HT2A or α2‐adrenergic receptors blocked I Nap and respiratory activity during hypoxia, but not in controls (Tryba et al. 2006; Viemari et al. 2011). These data imply that the respiratory network changes from a ‘normoxic’ state that depends on multiple, heterogeneous membrane properties to a ‘hypoxic’ (i.e. gasping) state that is particularly sensitive to the blockade of I Nap (Peña & Ramirez, 2004; Paton et al. 2006). This hypoxic network state is characterized not only by an increased dependency on I Nap, but also by weakened connectivity between respiratory neurons (Nieto‐Posadas et al. 2014).
The selective dependency of the hypoxic state on the activation of the 5‐HT2A receptor subtype is interesting in the context of sudden infant death syndrome (SIDS). Children that die from SIDS breathe normally under normoxic conditions, but fail to gasp during hypoxia (Poets et al. 1999; Garcia et al. 2013). Various studies also demonstrated dysregulation of 5‐HT in SIDS (Broadbelt et al. 2012; Massey et al. 2013; Rognum et al. 2014; Haynes et al. 2016, 2017; Bright et al. 2017). Thus, infants with disturbed 5‐HT mechanisms might be protected under normal oxygenated conditions, but become vulnerable to genetic mutations that affect serotonergic neurons when the network transitions into a hypoxic state (Tryba et al. 2006; Garcia et al. 2013).
Importantly, the reconfiguration of the preBötC can only describe a small aspect of a wider network reconfiguration that will include additional microcircuits located rostral to the preBötC. This includes the retrotrapezoid nucleus (RTN)/parafacial respiratory group (pFRG), which is critical for the generation of active expiration (Janczewski & Feldman, 2006; Pagliardini et al. 2011; Huckstepp et al. 2016), and the postinspiratory complex (PiCo), which is critical for generating postinspiration (Anderson et al. 2016); since postinspiratory neurons lose their inhibitory input during the inspiratory phase, we suggest these neurons might synchronize with inspiratory activity (Schmidt et al. 1995; Ramirez et al. 1998a; Richter & Smith, 2014). This synchronization of the network can be an acute endogenous survival response to extreme environmental changes, such as hypoxia (Michiels, 2004; Peña‐Ortega, 2017).
The effect of intermittent hypoxia on the cardiorespiratory network and implications for obstructive sleep apnoea
Hypoxic conditions are often experienced in the form of intermittent hypoxia. It is a characteristic condition in patients suffering from obstructive sleep apnoea, familial dysautonomia (Weese‐Mayer et al. 2008a, b ; Carroll et al. 2012), Rett syndrome (Weese‐Mayer et al. 2006; Schüle et al. 2008; Janc et al. 2016), mitochondrial disease (Brown & Squier, 1996; Quintana et al. 2012; Herst et al. 2017), epilepsy (Cohen‐Gadol et al. 2004; Farrell et al. 2016) and many other disorders characterized as ‘dysautonomia’. These disorders are often associated with breathing disturbances, increased heart rate, decreased heart rate variability and other forms of disturbed cardiorespiratory coupling.
Chronic exposure to intermittent hypoxia (CIH) results in increased levels of hypoxia‐inducible factor (HIF) 1α and decreased HIF2, which cause an imbalance between the hypoxic and antioxidant system and a build‐up of reactive oxygen species (Semenza & Prabhakar, 2007; Nanduri et al. 2008; Nanduri et al. 2015b). CIH seems to act directly on the CB, which then affects CNS networks through the release of neurogenic ROS. This conclusion is based on the observation that CB lesioning abolishes many of the detrimental consequences associated with obstructive sleep apnoea (Semenza & Prabhakar, 1985; Prabhakhar & Semenza, 2016). CIH leads to an upregulation of haem oxygenase 1 (Sunderram et al. 2016) and to an increased desynchronization of the inspiratory neurons within the preBötC (Garcia et al. 2016; Garcia et al. 2017). Incompletely synchronized preBötC bursts fail to evoke a population burst within the XII motor nucleus (Garcia et al. 2016), which could contribute to a pharyngeal collapse (Ramirez et al. 2013). The transmission failures from the preBötC to the XII can be prevented with ROS scavengers, suggesting that the CIH‐induced changes involve a build‐up of ROS and oxidative stress within the brainstem (Fig. 3; Garcia et al. 2016). The CIH‐induced amplitude fluctuations in the preBötC are reminiscent of fluctuations also seen in an animal model of Rett syndrome (Fig. 4; Viemari et al. 2005). These mice and also human patients are characterized by increased oxidative stress (De Felice et al. 2012, 2014; Janc & Muller, 2014; Ciccoli et al. 2015; Filosa et al. 2015; Janc et al. 2016; Pecorelli et al. 2016). It is therefore conceivable that the oxidative stress seen after CIH also contributes to the breathing disturbances in Rett syndrome including the characteristic fluctuations in tidal volume (Fig. 4; Weese‐Mayer et al. 2006). However, CIH and ROS production affects not only the preBötC but also other CNS sites, including the nucleus tractus solitarii (NTS; Kline, 2010), where CIH alters neurotransmission, neuromodulation (de Paula et al. 2007; Kline et al. 2007; Zhang et al. 2008; Kline et al. 2009; Costa‐Silva et al. 2012; Shell et al. 2016), neuroprotection and plasticity by altering proteins such as TrkB and brain‐derived neurotrophic factor (Almado et al. 2012; Moreau & Ciriello, 2015). CIH also enhances sympathetic drive and alters the baroreflex by acting differentially on central respiratory neurons (Moraes et al. 2016, 20136; Souza et al. 2016, 2017; Machado et al. 2017). Taken together, these studies show the close interaction between the central respiratory and cardiovascular response; however, it is also important to take careful consideration concerning CIH studies, as paradigms can vary widely among experimenters. It seems that the changes in sympathetic discharge and the levels of arterial pressure are due to the changes in the central respiratory network (Machado et al. 2017). This interaction occurs via connections from the respiratory microcircuits to the brainstem neurons that control sympathetic, but also parasympathetic activity. How whether the recently discovered excitatory postinspiratory complex (Anderson et al. 2016) contributes to the integration of the sympathetic nervous system is still an open question, but a recent study indicates that there are anatomical interactions between PiCo, preBötC and the RVLM (Dempsey et al. 2017). For the preBötC it has been shown that it contributes to the activity of vagal neurons and parasympathetic control (Dergacheva et al. 2010) involving GABAergic neurons (Frank & Mendelowitz, 2012).
Figure 3. The effect of chronic intermittent hypoxia (CIH) on respiratory centres.
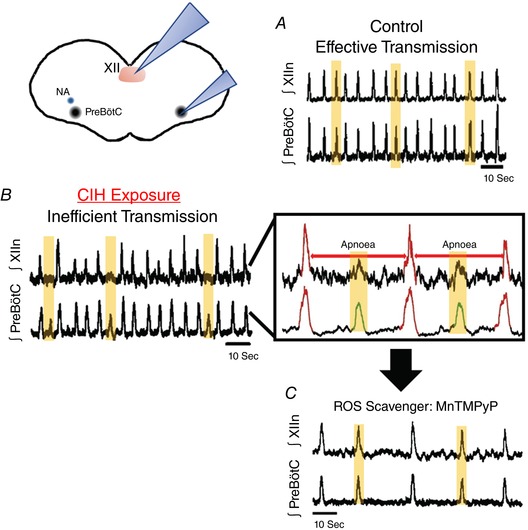
A and B, integrated population recordings from the hypoglossal (XII, upper traces) and preBötC (lower traces) indicate that CIH exposure results in transmission failures reflected in the XII output. C, These transmission failures translate in ‘XII apnoeas’ that are prevented by treatment with cell‐permeant SOD mimetic manganese(III) tetrakis(1‐methyl‐4‐pyridyl)porphyrin (MnTMPyP).
Figure 4.
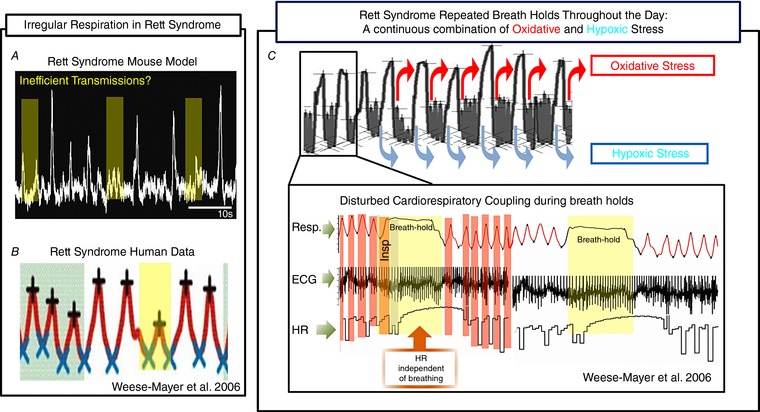
Respiratory irregularities in Rett syndrome
A, slices obtained from a MeCP2 KO male mouse, a model for Rett syndrome, show amplitude fluctuations in the integrated population recordings from the PreBötC that resemble those seen after CIH exposure. B, human data from Rett syndrome patients show large fluctuations in tidal volume. C, breath holds during Rett syndrome elicit oxidative and hypoxic stress. During these breath hold episodes cardiorespiratory coupling is compromised as the heart rate (HR) becomes independent from the breathing rhythm. Adapted with permission from Weese‐Mayer et al. 2006.
It is important to emphasize that the degree and specific pattern of hypoxia determine whether the consequences are detrimental or beneficial (Navarrete‐Opazo & Mitchell, 2014; Wilkerson et al. 2018), as intermittent hypoxia can decrease (Edge & O'Halloran, 2015) or increase long‐term facilitation (Fuller & Mitchell, 2017; Dougherty et al. 2018). Thus, under the right conditions, intermittent hypoxia has been successfully used to induce plasticity that is very beneficial during the rehabilitation following spinal cord injury (Trumbower et al. 2012; Dale et al. 2014; Fields & Mitchell, 2015; Gonzalez‐Rothi et al. 2015).
These studies also revealed a close link to inflammation, which can suppress some aspects of the plasticity evoked by intermittent hypoxia (Vinit et al. 2011; Huxtable et al. 2013; Huxtable et al. 2015), while other pathways that lead to facilitation are resistant to inflammation (Agosto‐Marlin et al. 2017). How hypoxia and inflammation interact within the CNS is an interesting and emerging area of research, with important implications for the respiratory system and the clinic (Gresham et al. 2011; Jafri et al. 2013; Lorea‐Hernandez et al. 2016; Ribeiro et al. 2017). A commonly used approach to study inflammation is the use of lipopolysaccharide (LPS; Gresham et al. 2011; Balan et al. 2012; Master et al. 2016; Ribeiro et al. 2017), which via the vagal nerve causes neuroinflammation (Balan et al. 2011; de La Serre et al. 2015; Le Maitre et al. 2017). The relationship between inflammation and CIH is particularly relevant for premature infants, which are susceptible to lung injuries and have unstable periodic breathing (Di Fiore et al. 2013). Both CIH and LPS‐induced inflammation modulate CB development with long‐lasting consequences (Abbott et al. 2011) for the control of breathing, including attenuated hypoxic and hyperoxic responses (Gauda et al. 2013; Master et al. 2016). Unravelling these interactions will be critical to understanding the relationship between respiratory infections and the resulting changes in breathing that are characteristic of small infants (Gresham et al. 2011; Balan et al. 2012).
There is increasing evidence that astrocytes play a central role in the response to inflammation and hypoxia. These cells are intrinsically sensitive to hypoxic insults, and they have been implicated in the inflammatory response (Bellaver et al. 2015; Forster & Reiser, 2016), the control of the cardiorespiratory responses and the modulation of sympathetic drive (Kasymov et al. 2013; Angelova et al. 2015; Marina et al. 2015, 2016a). The hypoxic environment increases ATP and lactate release by astrocytes, which is thought to lead to overexcitation of sympathetic circuits affecting cardiorespiratory control (Marina et al. 2016a, b ). A recent review by Marina and colleagues has detailed how researchers have tackled the astrocyte hypothesis by blocking ATP‐mediated signalling, which leads to slow progression of cardiac remodelling in rats and reduced systemic blood pressure in hypertensive rats (Marina et al. 2017). The role of glia and purinergic signalling will be discussed in more detail in the next section.
The role of purinergic signalling and neuroglial interaction in sensing O2 and CO2
There is an increasing consensus that neuroglial interactions play critical roles in sensing changes not only in but also in /H+. Indeed, it seems that specialized glial cells determine whether a given region, organ or network is sensitive to or /H+. These glial cells then communicate with neurons and other glia through transmitter release (Pascual et al. 2005; Gourine et al. 2010); in particular, ATP (Guthrie et al. 1999), d‐serine (Schnell et al. 1995; Beltrán‐Castillo et al. 2017) and glutamate (Parpura et al. 1994). The concept of specialized cell‐to‐cell interactions among neurons is emerging for astrocytes within the medulla, but also the cortex (Kasymov et al. 2013), and they may confer differential sensitivity to and /H+ depending on location within the ventral respiratory column (VRC) (Grass et al. 2004; Oku et al. 2016; Beltrán‐Castillo et al. 2017; Forsberg et al. 2017). Interestingly, the neuroglial interactions that seem to underlie //H+ sensitivity in the central nervous system are strikingly similar to those that occur peripherally in the carotid bodies (Figs 5 and 6) (Kumar & Prabhakar, 2012).
Figure 5.
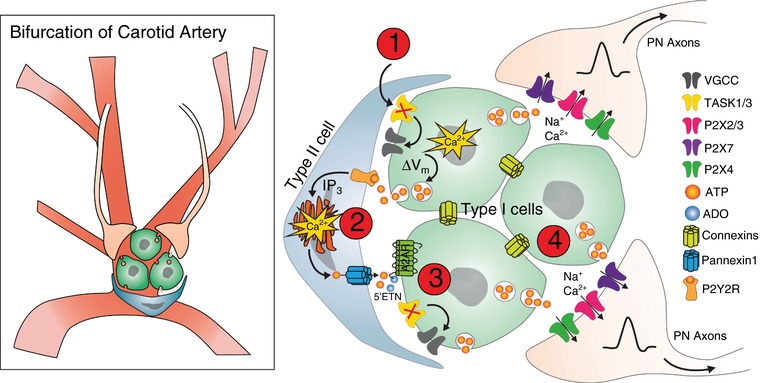
Illustration of the proposed mechanisms underlying chemosensitivity in the carotid bodies, located at the bifurcation of the aortic artery
Glomeruli are made up of type I cells ensheathed by type II cells, which relay changes in blood gas levels to the heart and brain through the petrosal ganglion and carotid sinus nerve. (1) Hypercapnia/H+ or hypoxia triggers a rise in [Ca2+]i in type I cells by inhibition of TASK1/3 K+ channels, which is followed by a secondary increase in [Ca2+]i in type II cells. (2) type I cells depolarize, again creating an increase in [Ca2+]i resulting in neuro‐/gliotransmitter release, primarily ATP, binding to P2Y2R receptors in type II cells and allowing ATP release through pannexin‐1 channels. ATP is broken down through 5′‐endonucleotidase activity and converted to ADO, which (3) binds to A2ARs on type 1 cells. (4) This cascade creates a positive feedback loop, followed by Na+/Ca2+ release from type I cells that activates afferent axons in the petrosal axons through a variety of P2X channels (Nurse & Piskuric, 2013). It has been hypothesized that the connexin family of gap junction channels may also play a role in facilitating electrical coupling (Murali et al. 2014; Nurse, 2014; Murali & Nurse, 2016). VGCC, voltage‐gated calcium channel.
Figure 6. Illustration of the proposed mechanisms underlying sensitivity of the VRC to changes in blood gases.
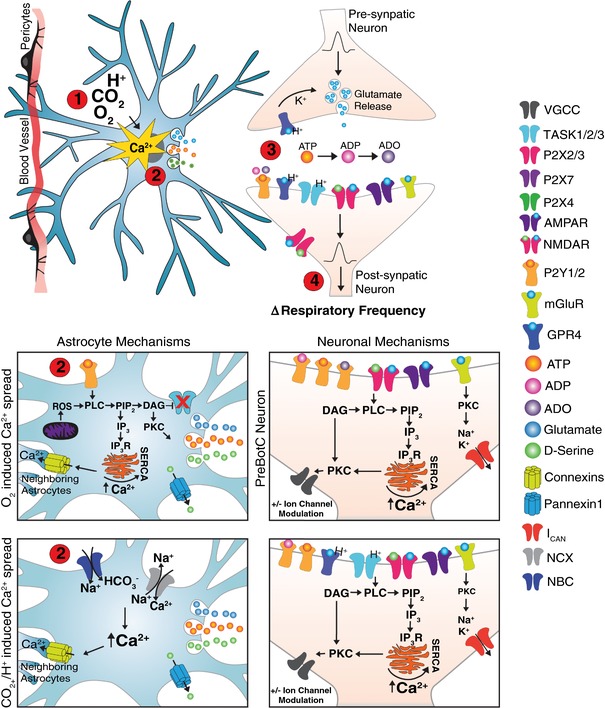
(1) Hypercapnia/H+ or hypoxia triggers a cascade of events through which mitochondrial release of ROS or NBC/NCX transporters leads to a rise in [Ca2+]i in astrocytes close to the ventral medullary surface near blood vessels (Gourine et al. 2010; Angelova et al. 2015; Turovsky et al. 2016; Rajani et al. 2018). (2) Increase in astrocytic [Ca2+]i results in vesicular release of gliotransmitters, such as ATP (Guthrie et al. 1999; Angelova et al. 2015; Holloway et al. 2015), glutamate (Huxtable et al. 2010; Holloway et al. 2015) and d‐serine (Beltrán‐Castillo et al. 2017). ATP release has been proposed to be facilitated by connexin hemichannels (Huckstepp et al. 2010a a, b ,,). (3) ATP and its derivatives (ADP and ADO; Burnstock, 2006; Robson et al. 2006; Funk, 2013), glutamate and d‐serine are released and bind to respective receptors on neurons (or ATP to P2YRs on other astrocytes, facilitating [Ca2+]i spread) (Kumar et al. 2015; Beltrán‐Castillo et al. 2017; Rajani et al. 2018). Different mechanisms have been proposed for responses of the preBötC and RTN/pFRG, where the RTN appears to respond to changes in astrocytic Ca2+ through TASK2/P1YR activation (Mulkey et al. 2004, 2006, 2007b b; Gestreau et al. 2009; Wang et al. 2013b) and preBötC neurons may respond by releasing glutamate that binds to NMDAR/AMPAR/mGluRs on the postsynaptic neuron or onto P2YRs on astrocytes. (4) Neuronal [Ca2+]i release is then mediated by diacylglycerol (DAG)/inositol 1,4,5,‐trisphosphate (IP3), further activating protein kinase C (PKC) to modulate ion channels, thereby altering respiratory frequency (Mulkey et al. 2006; Lorier et al. 2008). GPR4, G‐protein‐coupled receptor 4; SERCA, sarco/endoplasmic reticulum Ca2+‐ATPase; VGCC, voltage‐gated calcium channel.
Therefore, the CB could provide critical insights into our understanding of how the CNS responds to hypoxia and CO2. The CB consists of two primary cell types: type I (glomus) and type II (sustentacular) cells. These cells are bundled tightly in groups, and located in close contact with capillary beds. Afferent sensory nerves leading to the carotid sinus nerve receive autonomic innervation from the petrosal ganglion, and connect to the NTS to control breathing (Housley et al. 1987; López‐Barneo et al. 2008; Kumar & Prabhakar, 2012; Prabhakhar et al. 2015). Type I cells are of neural origin (Duchen et al. 1988; López‐Barneo et al. 2008; Pakkarato et al. 2015) and there are proposed to be several subtypes (McDonald & Mitchell, 1975; Chen & Yates, 1984; Prabhakhar et al. 2012). The responsiveness of type I cells to changes in //H+ may therefore be representative of the heterogeneous hypoxic responses of central respiratory neurons (St John & Wang, 1977; Richter et al. 1991; Ballanyi et al. 1994; Peña et al. 2004; Hill et al. 2011; Beltrán‐Castillo et al. 2017). In contrast, type II cells are glial‐like and are located in close proximity to groups of type I cells, where they unsheathe type I cells with thin proccesses and are arranged into glomeruli (Fig. 5; Kumar & Prabhakar, 2012).
Both CB cell types possess distinct electrophysiological properties (Clarke & de Burgh Daly, 1981; Duchen et al. 1988), and there is significant crosstalk between them. ATP released from type I cells during hypoxia or hypercapnia leads to a rise in intracellular Ca2+ ([Ca2+]i), followed by a delayed, secondary [Ca2+]i increase in proximal type II cells (Murali et al. 2014; Murali & Nurse, 2016). A depolarization of type I cells results in sensory output to the petrosal ganglion and on to the carotid sinus nerve, mediating the cardiorespiratory response in the NTS (Housley et al. 1987; Iturriaga & Alcayaga, 2004). The primary transmitter, ATP, activates P2X2/3 receptors on afferent nerve terminals (Wood et al. 1965; Prasad et al. 2001; Murali & Nurse, 2016), possibly through pannexin‐1 channels, which release ATP after activation of P2Y2 receptors (Prabhakhar, 2013; Murali et al. 2014; Prabhakhar & Joyner, 2015; Prabhakhar & Semenza, 2015).
Mechanisms proposed to underlie chemoreception in the RTN/pFRG, raphe nucleus (RN), NTS, preBötC and other areas of the respiratory network similarly rely on purinergic signalling involving astrocytes (Guthrie et al. 1999; Gourine et al. 2010; Huxtable et al. 2010; Huda et al. 2012; Turovsky et al. 2016; Gourine & Funk, 2017). Much has been learned about the RTN/pFRG as an important site for and /H+ sensing, but there are additional areas with varying sensitivity to hypoxia and hypercapnia in the VRC (Gourine et al. 2005). Purinergic signalling also plays a critical role in the central control of the cardiovascular system (Burnstock, 2006; Hawkins et al. 2017; Nishimura et al. 2017).
In principle, the astrocytic response to local changes in //H+ results in elevated levels of [Ca2+]i, which then leads to release of ATP, which further propagates the astrocytic Ca2+ signal in a feedforward manner. This in turn increases the activation of chemosensitive neuron populations that are directly activated by the release of ATP (Fig. 6; Hartel et al. 2009; Gourine et al. 2010; Okada et al. 2012; Wang et al. 2013b b). Depending on the microcircuits in which these neuroglial interactions occur, there will be different responses at the organismic level. For example, the RTN or preBötC will mount different aspects of the systems‐level responses to changes in CO2 and O2. The direct effect on astrocytes seems relatively clear, as blocking neuronal responses or injection of current does not alter the astrocytic Ca2+ response (Gourine et al. 2010). Additionally, ATP antagonists diminished pH‐induced [Ca2+]i signals (Gourine et al. 2010). The Ca2+ spread is partially due to gap junctions (Gourine et al. 2010), and is mediated by release of gliotransmitters (for review of Ca2+ spread in astrocytes, see Chemes & Ciaume, 2006). Interestingly, acidosis of the cortex and dorsal areas of the brainstem caused no change in the astrocytic [Ca2+]i response, supporting the hypothesis that central chemoreception is localized to specific area(s) within the respiratory network (Gourine et al. 2010; Turovsky et al. 2016).
Precise mechanisms behind astrocytic chemosensitivity and the role of various P2X and P1/2Y receptors in modulating respiratory frequency have been partially revealed for the preBötC (Lorier et al. 2007, 2008; Huxtable et al. 2009; Zwicker et al. 2011; Rajani et al. 2018). These mechanisms are possibly similar for the RTN/pFRG. However, this is still debated as the sensitivity of the RTN/pFRG to ATP does not appear to be dependent on P2‐related mechanisms (Fig. 6; Mulkey et al. 2006; Wenker et al. 2012, 2013).
In slices, P2Y1 receptor (P2Y1R) activation in the preBötC by ATP creates a 2‐ to 4‐fold increase in the frequency of fictive inspiratory burst activity (Lorier et al. 2007). ATP released by astrocytes in the preBötC during the HVR also mediates an increase in inspiratory frequency and reduces the secondary depression phase through activation of P2Y1Rs (Rajani et al. 2018). Astrocytes in the preBötC sense changes in and release [Ca2+]i, translating into release of ATP (and possibly other gliotransmitters), and activation of P2Y1Rs on neurons (Rajani et al. 2018). It is hypothesized that astrocytes detect changes in blood gas levels through mitochondria, relaying this information through a ROS and phosphatidylinositol 4,5‐bisphosphate (PIP2)‐mediated cascade that leads to the commonly detected increase in [Ca2+]i (Angelova et al. 2015). As mentioned above, the effect of P2Y1R activation in the RTN/pFRG is somewhat unclear. Several experiments have shown that although P2Y1Rs are expressed in RTN/pFRG neurons, they may only play a partial role in modulating CO2‐ (Mulkey et al. 2004) or pH‐ (Mulkey et al. 2006) driven excitation, as these responses show experimental cell to cell variablity in vitro versus in vivo. However, with local application of P2Y1R antagonists during hypercapnia, there is a resultant increase in amplitude and frequency of phrenic nerve output (Burnstock, 2006; Wenker et al. 2012). Further downstram effects of receptor activation occur to create changes in respiratory output. Breakdown of ATP results in neuroactive metabolites, such as ADP and adenosine (ADO), which are agonists of P2YRs and P1YRs, respectively (Robson et al. 2006; Funk, 2013). Both of these byproducts have additional effects on respiratory frequency, as ADP is excitatory (Lorier et al. 2007) while ADO may have an inhibitory effect in neonates (Fig. 6; Herlenius, 2011; for review on P2Y1Rs in respiration, see Rajani et al. 2016).
An emergent concept is that astrocytes form different functional subpopulations. This is best illustrated by astrocytes that exist in the RTN/pFRG and preBötC performing different functions in modulating respiratory network activity (Grass et al. 2004; Schnell et al. 2011; Oku et al. 2016; Forsberg et al. 2017). Forsberg et al. recently produced novel evidence of two astrocyte subtypes within the RTN/pFRG and preBötC, a portion of which exhibited rhythmic calcium oscillations, and another group that maintained a state of inactivity. They also found sensitivity differences in regions of the VRC. Selective activation of astrocytes in the RTN/pFRG and preBötC increased oscillatory activity; but RTN/pFRG astrocytes released prostaglandin E2, resulting in neural activation, whereas neurons in the preBötC had no response to an increase in calcium oscillations (Forsberg et al. 2017). However, as already discussed, the interpretation of findings like this needs to carefully consider experimental caveats. In normoxia, high levels of glutamate seem to be required to create astrocyte–neuron coupling. As hypothesized, these non‐physiological levels of glutamate could potentially only occur during hypoxia or when blocking neuronal glutamate uptake (Schnell et al. 2011). Regardless of these uncertainties, it appears that each respiratory microcircuit is sensitive to changes in //H+ and that multiple astrocytic subtypes may have different support functions, which is reminiscent of the situation in the CBs (Kasymov et al. 2013). This concept has been raised for networks throughout the CNS, not only the respiratory network (Ben Haim & Rowitch, 2017).
Further support for the concept of astrocyte subtypes and differential sensitivity in the respiratory networks comes from evidence that astrocytes also respond to changes in CO2 by releasing d‐serine (Beltrán‐Castillo et al. 2017). It is thought that d‐serine in the RN and VRC, but not NTS, increases respiratory frequency under control conditions and hypercapnia through NMDAR‐dependent mechanisms (Papouin & Oliet, 2014; Beltrán‐Castillo et al. 2017). Astrocytes appear to interact not only with neurons, but also with pericytes. Pericytes are responsive to lactate, which results in contraction and relaxation under normal and hypoxic conditions, respectively. Lactate has been shown to be released by astrocytes located in the respiratory groups (Erlichman et al. 2008, 2010; Lazarenko et al. 2009; Erlichman & Leiter, 2010; Funk et al. 2015; Marina et al. 2015).
Astrocytes versus neurons as the primary target in oxygen sensing
As discussed above, there is support for the notion that astrocytes are directly targeted by changes in //H+, while neurons are indirectly controlled by the astrocytes. Specifically, it is hypothesized that changes in blood gases are detected by astrocytes (Angelova et al. 2015), which in turn elicit an increase in [Ca2+]i, a subsequent release of gliotransmitters and neuronal activation (Hartel et al. 2009). However, most likely, this hypothesized mode of chemosensory transmission is much more complex, and there is still much to be learned when it comes to specific neuroglial interactions. Moreover, as discussed before, the responsiveness and mechanisms may vary for different regions.
However, can neurons be intrinsically sensitive to changes in O2 or CO2? In the preBötC, synaptically isolated pacemaker neurons respond to hypoxia with transient increases in rhythmicity, which is followed by cessation of the endogenous rhythm during extended exposure to hypoxia, indicating that pacemakers play a direct role in the hypoxic response (Thoby‐Brisson & Ramirez, 2000). While these hypoxic responses persist after synaptic isolation, it is important to emphasize that this study does not exclude a possible involvement of gliotransmitter involving purinergic signalling. Thus, it will be necessary to demonstrate that the hypoxic responses are maintained when physically isolated, as has been shown for Raphe neurons and RVLM neurons for the CO2 response (Wang et al. 1998; Wang & Richerson, 2000; D'Agostino et al. 2009; Sunderram et al. 2016). For the preBötC and C1 region, it has been shown that cells express haem oxygenase (HO‐1), but these cells were anatomically identified, and it is not clear whether they play a role in the hypoxic response (Mazza et al. 2001; D'Agostino et al. 2009). Some neurons in the RTN/pFRG have also been reported to respond directly to changes in /H+ (Guyenet & Bayliss, 2015). These neurons are purported to detect via TASK2 receptors and G‐protein‐coupled receptor 4 (Fig. 6; Gestreau et al. 2009; Guyenet & Bayliss, 2015; Kumar et al. 2015; Ruffault et al. 2015). Yet, these neurons seem to obtain this information also from surrounding astrocytes and peripheral chemoreceptors (Gourine et al. 2010). In this study, blocking activity of RTN/pFRG neurons had no effect on the astrocytic Ca2+ increase (Gourine et al. 2010). It has also been reported that inward currents in preBötC astrocytes occurring in phase with rhythmic neuronal oscillations under normoxia are due to neuronal release of K+ and glutamate (Schnell et al. 2011).
Thus, while neurons may have intrinsic sensitivity to O2 and CO2, there seems to be more evidence to support the notion that astrocytes are the primary sensors for pH and hypoxic conditions. Moreover, astrocytes within the medulla are found to be in close proximity to blood vessels (Gourine et al. 2010) and exhibit radial processes that are in contact with vessels (Wenker et al. 2010). They have reversal potentials near K+ equilibrium potential (E K), and are blocked with barium and desipramine, a blocker of Kir4.1 channels; this has led to later experiments that have helped to uncover specific channels that astrocytes use in sensing changes in oxygen or pH (Wenker et al. 2010).
Ion channels and the mechanisms of O2 and CO2 sensing
The studies from the previous section cohesively define the role of astrocytes within the ventrolateral medulla as chemosensitive units, but specific mechanisms underlying //H+ sensing and what role each specific area of the respiratory network plays in chemosensitivity have yet to be completely uncovered. Our limited understanding is partly rooted in the aforementioned experimental challenges in isolating independent mechanisms in slices or in vivo. This is exemplified by the amount and variety of ion channels tied to the oxygen sensing abilities of astrocytes and neurons. Clearly, multiple ion channels are involved in chemoreception. However, which channels and what particular role they play are dependent on cell type, anatomical location and experimental conditions (Lazarenko et al. 2010). Gap junction channels facilitate Ca2+ spread (Gourine et al. 2010), and several types of K+ channels contribute to chemotransduction pathways within both astrocytes and neurons in the respiratory groups, especially within the RTN/pFRG (Bayliss et al. 2001; Mulkey et al. 2007b; Gestreau et al. 2009; Lazarenko et al. 2009; Wang et al. 2013a; Rajani et al. 2016; Sobrinho et al. 2017). K+ channels such as the Kir4.1 inward rectifying channel have been implicated in regulation of the astrocyte resting membrane potential throughout the brain in several studies (Nwaobi et al. 2016), and could explain astrocytic activation through voltage‐dependent mechanisms (Olsen et al. 2015). Interestingly, the application of fluorocitrate had no effect on astrocytes when applied to the NTS or raphe neurons (Sobrinho et al. 2017). The possibility of TASK channels playing a role in chemoreception was proposed in 2001, as these channels are prominent in brainstem motor nuclei and exhibit high sensitivity to pH (Bayliss et al. 2001, 2014). However, the situation may be more differentiated, since TASK1/3 knock‐out mice seem to exhibit no signs of health issues (Mulkey et al. 2007a a), while the loss of TASK2 channels in the RTN/pFRG blunted the response to pH changes. Indeed, these three subunits are in different subgroups of the same family of K2P channels, and thus the intrinsic sensitivities of TASK1/3 channels and TASK2 channels are different. Subunits 1 and 3 both exhibit a very tight range of pH sensitivity, while TASK2 has a much broader sensitivity to changes in alkalinity (Lesage & Barhanin, 2011; Bayliss et al. 2014). These subtle differences could underlie different mechanisms of chemosensitivity within networks of the VRC, and also explain why knockout and mutation studies were not severely detrimental to respiratory activity. TASK1 channels have similarly been suggested to have a role in controlling the background current in the carotid bodies, as mRNA and protein expression data show that they are expressed within the atrium and ventricles of heart tissue (Jones et al. 2002; O'Connell et al. 2002; Buckler, 2010).
It has been well established in recent years that ATP plays a major role in astrocytic detection of changing //H+ levels. However, it would be an oversimplification to imply that purinergic signalling is the only mechanism involved. Indeed, some studies indicate that sensing O2 and CO2/H+ could involve entirely different pathways within astrocytes. Turovsky et al. demonstrated that the Na+–HCO3 − cotransport (NBC) and the Na+/Ca2+ exchanger (NCX) are required for increases in calcium fluctuations in astrocytes in response to changes in pH (Fig. 6; Turovsky et al. 2016). It will be interesting to learn whether mechanisms of mitochondrial activation and activation by NBC/NCX can occur in one astrocyte population, or if specialized subtypes exist that are specific to detecting O2 and CO2/H+ changes.
As already mentioned, the mechanisms for sensing changes in //H+ levels in both the carotid bodies and the CNS are surprisingly similar. For the astrocytes in the RN and VRC it has been proposed that CO2‐evoked d‐serine release is due to gap junction hemichannels, specifically pannexin1 (Beltrán‐Castillo et al. 2017), similar to type II cells in the CB (Murali et al. 2014). Moreover, NMDARs are expressed in CB glomus cells and CNS astrocytes. Thus, the role of d‐serine in the VRC (Liu et al. 2009) may bear semblance to its role in the CB. Connexins, specifically connexin 26, respond to increases in CO2 and mediate ATP release (Huckstepp et al. 2010a, b ), while connexins 36 and 43 are expressed in the carotid bodies and myenteric plexus in mice and are known to play a role in CO2 detection (Chen et al. 2001; Frinchi et al. 2013).
Taken together, there is accumulating evidence for a central chemosensory component to the HVR that acts through mechanisms not dissimilar from those proposed in the carotid bodies. With the understanding that the ventrolateral medulla plays a role in the HVR, we postulate that localized responsiveness to O2 or CO2/H+ in the preBötC and RTN/pFRG, respectively, emerge through specialized neuroglial interactions. Each of these regions exhibits a high sensitivity to either O2 or CO2/H+, which involves regionalized astrocytes with specialized sensitivity to either O2 or CO2/H+. Thus, it seems that the differences between the RTN/pFRG and preBötC are a function of the proportion of each astrocyte subtype that exists in each location (Fig. 6).
Summary
This review discussed the necessity and complexity involved in chemosensation in the CNS. We highlighted the evolutionary importance of aerobic metabolism and how our bodies have developed impeccable mechanisms to maintain homeostasis between O2 and CO2, which is particularly critical for normal brain function. Tightly regulated networks in the medulla that include the NTS, RN, RTN/pFRG and preBötC are primarily responsible for the homeostatic response and the regulation of blood gases. The preBötC, involved in inspiration, responds to changes in O2 in a biphasic manner by rapidly increasing neuronal activity followed by respiratory depression that leads to gasping. This biphasic response to O2 is attributed to a dynamic reconfiguration involving changes in ionic channel dependencies, excitatory and inhibitory conductances and the synchronization of respiratory neurons. Abnormalities in this and other medullary networks lead to disorders that affect cardiorespiratory coupling and elicit inflammatory responses exacerbating these conditions. At the core of these central chemosensory network responses are highly differentiated neuroglial interactions involving purinergic signalling. Although the chemosensitive processes in RTN/pFRG and preBötC involve neuroglial interactions including several receptors and neuromodulators that are strikingly similar to those described for the CB, it is also important to emphasize their differences, as astrocytic subtypes imbue different regions with different response properties. Unravelling the differential roles of astrocytes as the primary target in O2 and CO2 sensing is a riveting process, and is a departure from the ideas that (1) astrocytes are all similar and (2) neurons are the primary foci of study in the CNS. While this review emphasizes the importance of chemosensitivity, we also wanted to highlight the need for more research that will be required to unravel how the body controls its most necessary function, respiration.
Additional information
Competing interests
The authors have no competing interests to declare.
Author contributions
All authors have read and approved the final version of this manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
Work in this lab is supported by grants from the NIH (R01 HL126523, P01 HL 090554).
Biographies
Jan Marino (Nino) Ramirez is a Professor of Neurological Surgery and Director of the Center for Integrative Brain Research at the Seattle Children's Research Institute. He has a general research interest in the neural control of rhythmic activity. He studies neural mechanisms involved in the generation of respiratory rhythms, neocortical activity and epilepsy. His translational work has explored the neuronal mechanisms underlying erratic breathing in Rett syndrome and familial dysautonomia, and the mechanisms leading to sleep apnoea and sudden infant death syndrome. His current work is focused on hypoxic effects on mammalian respiratory neural networks.

Liza J. Severs is a graduate student in the Physiology and Biophysics Graduate Program at The University of Washington in Seattle and will be conducting her PhD research in the lab of Nino Ramirez at the Seattle Children's Research Institute. Her research centres on the mechanisms underlying rhythmic activity in the respiratory groups of the ventrolateral medulla, with a focus on how these networks are pharmacologically modulated and how neuronal connectivity shapes oscillatory behaviour. She is also interested in astrocyte biology in the context of oxygen sensing in the respiratory column and the role these cells play in the central component of the hypoxic ventilatory response.
Edited by Harold Schultz and Gregory Funk
J. M. Ramirez and L. Severs contributed equally to this article.
This review was presented at the symposium ‘Advances in cellular and integrative control of oxygen and carbon dioxide homeostasis’, which took place at the XX ISAC meeting, Baltimore, MD, USA, 2327 July 2017.
References
- Abbott SB, Stornetta RL, Coates MB & Guyenet PG (2011). Phox2b‐expressing neurons of the parafacial region regulate breathing rate, inspiration, and expiration in conscious rats. J Neurosci 31, 16410–16422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agosto‐Marlin I, Nichols N & Mitchell G (2017). Adenosine‐dependent phrenic motor facilitation is inflammation resistant. J Neurophysiol 117, 836–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almado C, Machado B & Leão R (2012). Chronic intermittent hypoxia depresses afferent neurotransmission in NTS neurons by a reduction in the number of active synapses. J Neurosci 32, 16736–16746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson TM, Garcia AJ 3rd, Baertsch NA, Pollak J, Bloom JC, Wei AD, Rai KG & Ramirez JM (2016). A novel excitatory network for the control of breathing. Nature 536, 76–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson TM & Ramirez JM (2017). Respiratory rhythm generation: triple oscillator hypothesis. F1000Res 6, 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelova PR, Kasymov V, Christie I, Sheikhbahaei S, Turovsky E, Marina N, Korsak A, Zwicker J, Teschemacher AG, Ackland GL, Funk GD, Kasparov S, Abramov AY & Gourine AV (2015). Functional oxygen sensitivity of astrocytes. J Neurosci 35, 10460–10473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avshalumov MV, Chen BT, Koos T, Tepper JM & Rice ME (2005). Endogenous hydrogen peroxide regulates the excitability of midbrain dopamine neurons via ATP‐sensitive potassium channels. J Neurosci 25, 4222–4231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balan KV, Kc P, Hoxha Z, Mayer CA, Wilson CG & Martin RJ (2011). Vagal afferents modulate cytokine‐mediated respiratory control at the neonatal medulla oblongata. Respir Physiol Neurobiol 178, 458–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balan KV, Kc P, Mayer CA, Wilson CG, Belkadi A & Martin RJ (2012). Intrapulmonary lipopolysaccharide exposure upregulates cytokine expression in the neonatal brainstem. Acta Paediatr 101, 466–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balfour R & Trapp S (2007). Ionic currents underlying the response of rat dorsal vagal neurons to hypoglycaemia and chemical anoxia. J Physiol 15, 691–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballanyi K (2004). Protective role of neuronal KATP channels in brain hypoxia. J Exp Biol 207, 3201–3212. [DOI] [PubMed] [Google Scholar]
- Ballanyi K & Ruangkittisakul A (2009). Structure‐function analysis of rhythmogenic inspiratory pre‐Botzinger complex networks in “calibrated” newborn rat brainstem slices. Respir Physiol Neurobiol 168, 158–178. [DOI] [PubMed] [Google Scholar]
- Ballanyi K, Volker A & Richter D (1994). Anoxia induced functional inactivation of neonatal respiratory neurones in vitro Neuroreport 6, 165–168. [DOI] [PubMed] [Google Scholar]
- Basting T, Abe C, Viar K, Stornetta R & Guyenet P (2016). Is plasticity within the retrotrapezoid nucleus responsible of the set‐point after carotid body denervation in rats? J Physiol 594, 3371–3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayliss DA, Barhanin J, Gestreau C & Guyenet PG (2014). The role of pH‐sensitive TASK channels in central respiratory chemoreception. Pflugers Arch 467, 917–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayliss DA, Talley EM, Sirois JE & Lei Q (2001). TASK‐1 is a highly modulated pH‐sensitive ‘leak’ K+ channel expressed in brainstem respiratory neurons. Resp Physiol 129, 159–174. [DOI] [PubMed] [Google Scholar]
- Bellaver B, Souza DG, Bobermin LD, Goncalves CA, Souza DO & Quincozes‐Santos A (2015). Guanosine inhibits LPS‐induced pro‐inflammatory response and oxidative stress in hippocampal astrocytes through the heme oxygenase‐1 pathway. Purinergic Signal 11, 571–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltrán‐Castillo S, Olivares MJ, Contreras RA, Zúñiga G, Llona I, von Bernhardi R & Eugenín JL (2017). D‐serine released by astrocyte in brainstem regulates breathing response to CO2 levels. Nat Commun 8, 838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Haim L & Rowitch DH (2017). Functional diversity of astrocytes in neural circuit regulation. Nat Rev Neurosci 18, 31–41. [DOI] [PubMed] [Google Scholar]
- Bickler P & Donohoe P (2002). Adaptive responses of vertebrate neurons to hypoxia. J Exp Biol 205, 3579–3586. [DOI] [PubMed] [Google Scholar]
- Bingmann D & Kolde G (1982). ‐profiles in hippocampal slices of the guinea pig. Exp Brain Res 48, 89–96. [DOI] [PubMed] [Google Scholar]
- Bissonnette JM (2000). Mechanisms regulating hypoxic respiratory depression during fetal and postnatal life. Am J Physiol Regul Integr Comp Physiol 278, R1391–R1400. [DOI] [PubMed] [Google Scholar]
- Björklund O, Shang M, Tonazzini I, Daré E & Fredholm B (2008). Adenosine A1 and A3 receptors protect astrocytes from hypoxic damage. Eur J Pharmacol 596, 6–13. [DOI] [PubMed] [Google Scholar]
- Bouvier J, Thoby‐Brisson M, Renier N, Dubreuil V, Ericson J, Champagnat J, Pierani A, Chedotal A & Fortin G (2010). Hindbrain interneurons and axon guidance signaling critical for breathing. Nat Neurosci 13, 1066–1074. [DOI] [PubMed] [Google Scholar]
- Bright FM, Byard RW, Vink R & Paterson DS (2017). Medullary serotonin neuron abnormalities in an australian cohort of sudden infant death syndrome. J Neuropathol Exp Neurol 76, 864–873. [DOI] [PubMed] [Google Scholar]
- Broadbelt K, Rivera K, Paterson D, Duncan J, Trachtenberg F, Paulo J, Stapels M, Borenstein N, Belliveau R, Haas E, Stanley C, Krous H, Steen H & Kinney H (2012). Brainstem deficiency of the 14‐3‐3 regulator of serotonin synthesis: a proteomics analysis in the sudden infant death syndrome. Mol Cell Proteomics 11, M111.009530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockhaus J, Ballanyi K, Smith JC & Richter DW (1993). Microenvironment of respiratory neurons in the in vitro brainstem‐spinal cord of neonatal rats. J Physiol 462, 421–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown G & Squier M (1996). Neuropathology and pathogenesis of mitochondrial diseases. J Inherit Metab Dis 19, 553–572. [DOI] [PubMed] [Google Scholar]
- Buckler K (2010). Two‐pore domain K+ channels and their role in chemoreception. Adv Exp Med Biol 661, 15–30. [DOI] [PubMed] [Google Scholar]
- Bureau MA, Lamarche J, Foulon P & Dalle D (1985). Postnatal maturation of respiration in intact and carotid body‐chemodenervated lambs. J Appl Physiol 59, 869–874. [DOI] [PubMed] [Google Scholar]
- Burke P, Kanbar R, Basting T, Hodges W, Viar K, Stornetta R & Guyenet P (2015). State‐dependent control of breathing by the retrotrapezoid nucleus. J Physiol 593, 2909–2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G (2006). Purinergic signalling. Br J Pharmacol 147, S172–S181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JL & Agarwal A (2010). Development of ventilatory control in infants. Paediatr Respir Rev 11, 199–207. [DOI] [PubMed] [Google Scholar]
- Carroll MS, Kenny AS, Patwari PP, Ramirez JM & Weese‐Mayer DE (2012). Respiratory and cardiovascular indicators of autonomic nervous system dysregulation in familial dysautonomia. Pediatr Pulmonol 47, 682–691. [DOI] [PubMed] [Google Scholar]
- Chemes E & Ciaume C (2006). Astrocyte calcium waves: what they are and what they do. Glia 54, 716–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen I & Yates R (1984). Two types of glomus cells in the rat carotid body as revealed by alpha‐bungarotoxin binding. J Neurocytol 13, 281–302. [DOI] [PubMed] [Google Scholar]
- Chen J, He L, Dinger B, Stensaas L & Fidone S (2001). Chronic hypoxia upregulates connexin43 expression in rat carotid body and petrosal ganglion. J Appl Physiol 92, 1480–1486. [DOI] [PubMed] [Google Scholar]
- Ciccoli L DFC, Leoncini S, Signorini C, Cortelazzo A, Zollo G, Pecorelli A, Rossi M & Hayek J (2015). Red blood cells in Rett syndrome: oxidative stress, morphological changes and altered membrane organization. Biol Chem 396, 1233–1240. [DOI] [PubMed] [Google Scholar]
- Clarke A & Pörtner H (2010). Temperature, metabolic power and the evolution of endothermy. Biol Rev Camb Philos Soc 85, 703–727. [DOI] [PubMed] [Google Scholar]
- Clarke JA & de Burgh Daly M (1981). Distribution of carotid body type I cells and other periadventitial type I cells in the carotid bifurcation regions of the rabbit. Cell Tiss Res 216, 603–614. [DOI] [PubMed] [Google Scholar]
- Clemens S, Massabuau JC, Meyrand P & Simmers J (2001). A modulatory role for oxygen in shaping rhythmic motor output patterns of neuronal networks. Resp Physiol 128, 299–315. [DOI] [PubMed] [Google Scholar]
- Cohen‐Gadol A, DiLuna M & Spencer D (2004). Partial epilepsy presenting as episodic dyspnea: a specific network involved in limbic seizure propagation. Case report. J Neurosurg 100, 565–567. [DOI] [PubMed] [Google Scholar]
- Costa‐Silva J, Zoccal D & Machado B (2012). Chronic intermittent hypoxia alters glutamatergic control of sympathetic and respiratory activities in the commissural NTS of rats. Am J Physiol Regul Integr Comp Physiol 302, R785–R793. [DOI] [PubMed] [Google Scholar]
- Crowder E, Saha M, Pace R, Zhang H, Prestwich G & Del Negro C (2007). Phosphatidylinositol 4,5‐bisphosphate regulates inspiratory burst activity in the neonatal mouse preBötzinger complex. J Physiol 582, 1047–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, Kam K, Sherman D, Janczewski WA, Zheng Y & Feldman JL (2016). Defining preBotzinger complex rhythm‐ and pattern‐generating neural microcircuits in vivo. Neuron 91, 602–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran A, Rodman J, Eastwood P, Henderson K, Dempsey J & Smith C (2000). Ventilatory resonses to specific CNS hypoxia in sleeping dogs. J Appl Physiol 88, 1840–1852. [DOI] [PubMed] [Google Scholar]
- Czech‐Damal NU, Geiseler SJ, Hoff ML, Schliep R, Ramirez JM, Folkow LP & Burmester T (2014). The role of glycogen, glucose and lactate in neuronal activity during hypoxia in the hooded seal (Cystophora cristata) brain. Neuroscience 275, 374–383. [DOI] [PubMed] [Google Scholar]
- D'Agostino D, Mazza EJ & Neubauer JA (2009). Heme oxygenase is necessary for the excitatory response of cultured neonatal rat rostral ventrolateral medulla neurons to hypoxia. Am J Physiol Regul Integr Comp Physiol 298, R102–R118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Agostino DP, Putnam RW & Dean JB (2007). Superoxide production in CA1 neurons of rat hippocampal slices exposed to graded levels of oxygen. J Neurophysiol 98, 1030–1041. [DOI] [PubMed] [Google Scholar]
- Dale E, Ben Mabrouk F & Mitchell G (2014). Unexpected benefits of intermittent hypoxia: enhanced respiratory and nonrespiratory motor function. Physiology (Bethesda) 29, 39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Felice C, Della Ragione F, Signorini C, Leoncini S, Pecorelli A, Ciccoli L, Scalabrì F, Marracino F, Madonna M, Belmonte G, Ricceri L, De Filippis B, Laviola G, Valacchi G, Durand T, Galano J, Oger C, Guy A, Bultel‐Poncé V, Guy J, Filosa S, Hayek J & D'Esposito M (2014). Oxidative brain damage in Mecp2‐mutant murine models of Rett syndrome. Neurobiol Dis 68, 66–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Felice C, Signorini C, Leoncini S, Pecorelli A, Durand T, Valacchi G, Ciccoli L & Hayek J (2012). The role of oxidative stress in Rett syndrome: an overview. Ann N Y Acad Sci 1259, 121–135. [DOI] [PubMed] [Google Scholar]
- de La Serre CB, de Lartigue G & Raybould HE (2015). Chronic exposure to low dose bacterial lipopolysaccharide inhibits leptin signaling in vagal afferent neurons. Physiol Behav 139, 188–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Negro CA, Hayes JA & Rekling JC (2011). Dendritic calcium activity precedes inspiratory bursts in preBotzinger complex neurons. J Neurosci 31, 1017–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Paula P, Tolstykh G & Mifflin S (2007). Chronic intermittent hypoxia alters NMDA and AMPA‐evoked currents in NTS neurons receiving carotid body chemoreceptor inputs. Am J Physiol Regul Integr Comp Physiol 292, R2259–R2265. [DOI] [PubMed] [Google Scholar]
- Dempsey B, Le S, Turner A, Bokiniec P, Ramadas R, Bjaalie JG, Menuet C, Neve R, Allen AM, Goodchild AK & McMullan S (2017). Mapping and analysis of the connectome of sympathetic premotor neurons in the rostral ventrolateral medulla of the rat using a volumetric brain atlas. Front Neural Circuits 11, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dergacheva O, Griffioen K, Neff R & Mendelowitz D (2010). Respiratory modulation of premotor cardiac vagal neurons in the brainstem. Respir Physiol Neurobiol 174, 102–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Fiore JM, Martin RJ & Gauda EB (2013). Apnea of prematurity – perfect storm. Respir Physiol Neurobiol 189, 213–222. [DOI] [PubMed] [Google Scholar]
- Donnelly DF, Bavis RW, Kim I, Dbouk HA & Carroll JL (2009). Time course of alterations in pre‐ and post‐synaptic chemoreceptor function during developmental hyperoxia. Respir Physiol Neurobiol 168, 189–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly DF, Jiang C & Haddad G (1992). Comparative responses of brain stem and hippocampal neurons to O2 deprivation: in vitro intracellular studies. Am J Physiol 262, L549–L554. [DOI] [PubMed] [Google Scholar]
- Dougherty B, Terada J, Springborn S, Vinit S, MacFarlane P & Mitchell G (2018). Daily acute intermittent hypoxia improves breathing function with acute and chronic spinal injury via distinct mechanisms. Respir Physiol Neurobiol (in press; doi: 10.1016/j.resp.2017.05.004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchen MR, Caddy KWT, Kirby GC, Patterson DL, Ponte J & Biscoe TJ (1988). Biophysical studies of the cellular elements of the rabbit carotid body. Neuroscience 26, 291–311. [DOI] [PubMed] [Google Scholar]
- Dunmyre JR, Del Negro CA & Rubin JE (2011). Interactions of persistent sodium and calcium‐activated nonspecific cationic currents yield dynamically distinct bursting regimes in a model of respiratory neurons. J Comput Neurosci 31, 305–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyavanapalli J, Jameson H, Dergacheva O, Jain V, Alhusayyen M & Mendelowitz D (2014). Chronic intermittent hypoxia‐hypercapnia blunts heart rate responses and alters neurotransmission to cardiac vagal neurons. J Physiol 592, 2799–2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edge D & O'Halloran K (2015). Chronic intermittent hypoxia blunts the expression of ventilatory long term facilitation in sleeping rats. Adv Exp Med Biol 860, 335–342. [DOI] [PubMed] [Google Scholar]
- Engwall M, Smith C, Dempsey J & Bisgard G (1985). Ventilatory afterdischarge and central respiratory drive interactions in the awake goat. J Appl Physiol 76, 416–423. [DOI] [PubMed] [Google Scholar]
- Erlichman JS, Hewitt A, Damon TL, Hart M, Kurascz J, Li A & Leiter JC (2008). Inhibition of monocarboxylate transporter 2 in the retrotrapezoid nucleus in rats: a test of the astrocyte‐neuron lactate‐shuttle hypothesis. J Neurosci 28, 4888–4896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlichman JS & Leiter JC (2010). Glia modulation of the extracellular milieu as a factor in central CO2 chemosensitivity and respiratory control. J Appl Physiol (1985) 108, 1803–1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlichman JS, Leiter JC & Gourine AV (2010). ATP, glia and central respiratory control. Respir Physiol Neurobiol 173, 305–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell J, Gaxiola‐Valdez I, Wolff M, David L, Dika H, Geeraert B, Rachel Wang X, Singh S, Spanswick S, Dunn J, Antle M, Federico P & Teskey G (2016). Postictal behavioural impairments are due to a severe prolonged hypoperfusion/hypoxia event that is COX‐2 dependent. Elife 5, e19352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields D & Mitchell G (2015). Spinal metaplasticity in respiratory motor control. Front Neural Circuits 9, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filosa JA & Blanco VM (2007). Neurovascular coupling in the mammalian brain. Exp Physiol 92, 641–646. [DOI] [PubMed] [Google Scholar]
- Filosa S, Pecorelli A, D'Esposito M, Valacchi G & Hajek J (2015). Exploring the possible link between MeCP2 and oxidative stress in Rett syndrome. Free Radic Biol Med 88, 81–90. [DOI] [PubMed] [Google Scholar]
- Fitzgerald R (2014). Carotid body: a new target for rescuing neural control of cardiorespiratory balance in disease. Front Physiol 5, 304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folklow L, Ramirez J, Ludvigsen S, Ramirez N & Blix A (2008). Remarkable neuronal hypoxia tolerance in the deep‐diving adult hooded seal (Cystophora cristata). Neurosci Lett 446, 147–150. [DOI] [PubMed] [Google Scholar]
- Fong A, Corcoran A, Zimmer M, Andrade D & Milsom W (2008). Respiratory rhythm of brainstem‐spinal cord preparations: Effects of maturation, age, mass and oxygenation. Respir Physiol Neurobiology 164, 429–440. [DOI] [PubMed] [Google Scholar]
- Forsberg D, Ringstedt T & Herlenius E (2017). Astrocytes release prostaglandin E2 to modify respiratory network activity. Elife 6, e29566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster D & Reiser G (2016). Nucleotides protect rat brain astrocytes against hydrogen peroxide toxicity and induce antioxidant defense via P2Y receptors. Neurochem Int 94, 57–66. [DOI] [PubMed] [Google Scholar]
- Frank JG & Mendelowitz D (2012). Synaptic and intrinsic activation of GABAergic neurons in the cardiorespiratory brainstem network. PLoS One 7, e36459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frinchi M, Di Liberto V, Turimella S, D'Antoni F, Theis M, Belluardo N & Mudò G (2013). Connexin36 (Cx36) expression and protein detection in the mouse carotid body and myenteric plexus. Acta Histochemica 115, 252–256. [DOI] [PubMed] [Google Scholar]
- Fuller D & Mitchell G (2017). Respiratory neuroplasticity – Overview, significance and future directions. Exp Neurol 287, 144–152. [DOI] [PubMed] [Google Scholar]
- Fung M & Haddad G (1997). Anoxia‐induced depolarization in CA1 hippocampal neurons: role of Na+‐dependent mechanisms. Brain Res 762, 97–102. [DOI] [PubMed] [Google Scholar]
- Funk GD (2013). Neuromodulation: purinergic signaling in respiratory control. Compr Physiol 3, 331–363. [DOI] [PubMed] [Google Scholar]
- Funk GD, Rajani V, Alvares TS, Revill AL, Zhang Y, Chu NY, Biancardi V, Linhares‐Taxini C, Katzell A & Reklow R (2015). Neuroglia and their roles in central respiratory control; an overview. Comp Biochem Physiol A Mol Integr Physiol 186, 83–95. [DOI] [PubMed] [Google Scholar]
- Garcia AJ 3rd, Dashevskiy T, Khuu MA & Ramirez JM (2017). Chronic intermittent hypoxia differentially impacts different states of inspiratory activity at the level of the preBotzinger complex. Front Physiol 8, 571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia AJ 3rd, Koschnitzky JE & Ramirez JM (2013). The physiological determinants of Sudden Infant Death Syndrome. Respir Physiol Neurobiol 189, 288–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia AJ 3rd, Putnam RW & Dean JB (2010a). Hyperbaric hyperoxia and normobaric reoxygenation increase excitability and activate oxygen‐induced potentiation in CA1 hippocampal neurons. J Appl Physiol (1985) 109, 804–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia AJ 3rd, Putnam RW & Dean JB (2010b). Hyperoxic stimulation of synchronous orthodromic activity and induction of neural plasticity does not require changes in excitatory synaptic transmission. J Appl Physiol (1985) 109, 820–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia AJ 3rd, Zanella S, Dashevskiy T, Khan SA, Khuu MA, Prabhakar NR & Ramirez JM (2016). Chronic intermittent hypoxia alters local respiratory circuit function at the level of the preBotzinger complex. Front Neurosci 10, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauda EB, Shirahata M, Mason A, Pichard LE, Kostuk EW & Chavez‐Valdez R (2013). Inflammation in the carotid body during development and its contribution to apnea of prematurity. Respir Physiol Neurobiol 185, 120–131. [DOI] [PubMed] [Google Scholar]
- Gestreau C, Heitzmannb D, Thomasd J, Dubreuile V, Bandulikb S, Reicholdb M, Bendahhouf S, Piersonf P, Sternerb C, Peyronnet‐Rouxa J, Benfrihaa C, Tegtmeierb I, Ehnesb H, Georgieffd M, Lesageg F, Brunete JF, Goridise C, Warthb R & Barhaninf J (2009). Task 2 potassium channels set central respiratory CO2 and O2 sensitivity. Proc Natl Acad Sci U S A 107, 2325–2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giniatullin AR & Giniatullin RA (2003). Dual action of hydrogen peroxide on synaptic transmission at the frog neuromuscular junction. J Physiol 552, 283–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez‐Rothi E, Lee K, Dale E, Reier P, Mitchell G & Fuller D (2015). Intermittent hypoxia and neurorehabilitation. J Appl Physiol 119, 1455–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourevitch B & Mellen N (2014). The preBotzinger complex as a hub for network activity along the ventral respiratory column in the neonate rat. Neuroimage 98, 460–474. [DOI] [PubMed] [Google Scholar]
- Gourine AV & Funk GD (2017). On the existence of a central respiratory oxygen sensor. J Appl Physiol 123, 1344–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourine AV, Kasymov V, Marina N, Tang F, Figueiredo MF, Lane S, Teschemacher AG, Spyer KM, Deisseroth K & Kasparov S (2010). Astrocytes control breathing through pH‐dependent release of ATP. Science 329, 571–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourine AV, Llaudet E, Dale N & Spyer KM (2005). ATP is a mediator of chemosensory transduction in the central nervous system. Nature 436, 108–111. [DOI] [PubMed] [Google Scholar]
- Grass D, Pawlowski PG, Jirrlinger J, Papadopoulos N, Richter DW, Kirchhoff F & Hülsmann S (2004). Diversity of functional astroglial properties in the respiratory network. J Neurosci 24, 1358–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray PA, Hayes JA, Ling GY, Llona I, Tupal S, Picardo MC, Ross SE, Hirata T, Corbin JG, Eugenin J & Del Negro CA (2010). Developmental origin of preBotzinger complex respiratory neurons. J Neurosci 30, 14883–14895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray PA, Janczewski WA, Mellen N, McCrimmon DR & Feldman JL (2001). Normal breathing requires preBötzinger complex neurokinin‐1 receptor‐expressing neurons. Nat Neurosci 4, 927–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresham K, Boyer B, Mayer C, Foglyano R, Martin R & Wilson C (2011). Airway inflammation and central respiratory control: results from in vivo and in vitro neonatal rat. Respir Physiol Neurobiol 178, 414–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie P, Knappenberger J, Segal M, Bennett M, Charles A & Kater S (1999). ATP released from astrocytes mediates glial calcium waves. J Neurosci 19, 520–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG & Bayliss DA (2015). Neural control of breathing and CO2 homeostasis. Neuron 87, 946–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG, Bayliss DA, Stornetta RL, Kanbar R, Shi Y, Holloway BB, Souza G, Basting TM, Abbott SBG & Wenker IC (2018). Interdependent feedback regulation of breathing by the carotid bodies and the retrotrapezoid nucleus. J Physiol 596, 3029–3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet P, Bayliss D, Stornetta R, Ludwig M, Kumar N, Shi Y, Burke P, Kanbar R, Basting T, Holloway B & Wenker I (2016). Proton detection and breathing regulation by the retrotrapezoid nucleus. J Physiol 594, 1529–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad G & Jiang C (1993). Mechanisms of anoxia‐induced depolarization in brainstem neurons: in vitro current and voltage clamp studies in the adult rat. Brain Res 625, 261–268. [DOI] [PubMed] [Google Scholar]
- Haddad G & Jiang C (1994). Mechanisms of neuronal survival during hypoxia: ATP‐sensitive K+ channels. Biol Neonate 65, 160–165. [DOI] [PubMed] [Google Scholar]
- Haddad GG & Jiang C (1997). O2‐sensing mechanisms in excitable cells: role of plasma membrane K+ channels. Annu Rev Physiol 59, 23–42. [DOI] [PubMed] [Google Scholar]
- Hartel K, Schnell C & Hulsmann S (2009). Astrocytic calcium signals induced by neuromodulators via functional metabotropic receptors in the ventral respiratory group of neonatal mice. Glia 57, 815–827. [DOI] [PubMed] [Google Scholar]
- Hawkins VE, Takakura AC, Trinh A, Malheiros‐Lima MR, Cleary CM, Wenker IC, Dubreuil T, Rodriguez EM, Nelson MT, Moreira TS & Mulkey DK (2017). Purinergic regulation of vascular tone in the retrotrapezoid nucleus is specialized to support the drive to breathe. Elife 6, e25232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes JA, Wang X & Del Negro CA (2012). Cumulative lesioning of respiratory interneurons disrupts and precludes motor rhythms in vitro. Proc Natl Acad Sci U S A 109, 8286–8291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes R, Folkerth R, Paterson D, Broadbelt K, Dan Zaharie S, Hewlett R, Dempers J, Burger E, Wadee S, Schubert P, Wright C, Sens M, Nelsen L, Randall B, Tran H, Geldenhuys E, Elliott A, Odendaal H, Kinney H & Network P (2016). Serotonin receptors in the medulla oblongata of the human fetus and infant: the analytic approach of the international safe passage study. J Neuropathol Exp Neurol 75, 1048–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes R, Frelinger A, Giles E, Goldstein R, Tran H, Kozakewich H, Haas E, Gerrits A, Mena O, Trachtenberg F, Paterson D, Berry G, Adeli K, Kinney H & Michelson A (2017). High serum serotonin in sudden infant death syndrome. Proc Natl Acad Sci U S A 114, 7695–7700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herlenius E (2011). An inflammatory pathway to apnea and autonomic dysregulation. Respir Physiol Neurobiol 178, 449–457. [DOI] [PubMed] [Google Scholar]
- Herst P, Rowe M, Carson G & Berridge M (2017). Functional mitochondria in health and disease. Front Endocrinol (Lausanne) 8, 296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill AA, Garcia A Jr, Zenella S & Upadhyaya R (2011). Graded reductions in oxygenation evoke graded reconfiguration of the isolated respiratory network. J Neurophysiol 105, 625–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway BB, Viar KE, Stornetta RL & Guyenet PG (2015). The retrotrapezoid nucleus stimulates breathing by releasing glutamate in adult conscious mice. Eur J Neurosci 42, 2271–2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holton P & Wood JB (1965). The effects of bilateral removal of the carotid bodies and denervation of the carotid sinuses in two human subjects. J Physiol 553, 3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Housley GD, Martin‐Body R, Dawson N & Sincair J (1987). Brain stem projections of the glossopharyngeal nerve and its carotid sinus branch in the rat. J Neurosci 22, 237–250. [DOI] [PubMed] [Google Scholar]
- Huang T, Zou Y & Corniola R (2012). Oxidative stress and adult neurogenesis—effects of radiation and superoxide dismutase deficiency. Semin Cell Dev Biol 23, 738–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huckstepp RT, Eason R, Sachdev A & Dale N (2010a). CO2‐dependent opening of connexin 26 and related β connexins. J Physiol 588, 3921–3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huckstepp RT, Henderson LE, Cardoza KP & Feldman JL (2016). Interactions between respiratory oscillators in adult rats. Elife 5, e14203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huckstepp RT, id Bihi R, Eason R, Spyer KM, Dicke N, Willecke K, Marine N, Gourine AV & Dale N (2010b). Connexin hemichannel‐mediated CO2‐dependent release of ATP in the medulla oblongata contributes to central respiratory chemosensitivity. J Physiol 588, 3901–3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huda R, Pollema‐Mays SL, Chang Z, Alheid GF, McCrimmon DR & Martina M (2012). Acid‐sensing ion channels contribute to chemosensitivity of breathing‐related neurons of the nucleus of the solitary tract. J Physiol 590, 4761–4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxtable A, Smith S, Peterson T, Watters J & Mitchell G (2015). Intermittent hypoxia‐induced spinal inflammation impairs respiratory motor plasticity by a spinal p38 MAP kinase‐dependent mechanism. J Neurosci 35, 6871–6880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxtable AG, Smith S, Vinit S, Watters J & Mitchell GS (2013). Systemic LPS induces spinal inflammatory gene expression and impairs phrenic long‐term facilitation following acute intermittent hypoxia. J Appl Physiol (1985) 114, 879–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxtable AG, Zwicker JD, Alvares TS, Ruangkittisakul A, Fang X, Hahn LB, Posse de Chaves E, Baker GB, Ballanyi K & Funk GD (2010). Glia contribute to the purinergic modulation of inspiratory rhythm‐generating networks. J Neurosci 30, 3947–3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxtable AG, Zwicker JD, Poon BY, Pagliardini S, Vrouwe SQ, Greer JJ & Funk GD (2009). Tripartite purinergic modulation of central respiratory networks during perinatal development: the influence of ATP, ectonucleotidases, and ATP metabolites. J Neurosci 29, 14713–14725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C (2017). The neurovascular unit coming of age: a journey through neurovascular coupling in health and disease. Neuron 96, 17–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igbal M & Eftekharpour E (2017). Regulatory role of redox balance in determination of neural precursor cell fate. Stem Cells Int 2017, 9209127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iturriaga R (2018). Translating carotid body function into clinical medicine. J Physiol 596, 3067–3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iturriaga R & Alcayaga J (2004). Neuotransmission in the carotid body: transmitters and modulators between glomus cells and petrosal ganglion nerve terminals. Brain Res Rev 47, 46–53. [DOI] [PubMed] [Google Scholar]
- Izumizaki M, Pokorski M & Homma I (2004). Role of the carotid bodies in chemosensory ventilatory responses in the anesthetized mouse. J Appl Physiol 97, 1401–1407. [DOI] [PubMed] [Google Scholar]
- Jafri A, Belkadi A, Zaidi S, Getsy P, Wilson C & Martin R (2013). Lung inflammation induces IL‐1β expression in hypoglossal neurons in rat brainstem. Respir Physiol Neurobiol 188, 21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janc O, Hüser M, Dietrich K, Kempkes B, Menzfeld C, Hülsmann S & Müller M (2016). Systemic radical scavenger treatment of a mouse model of Rett syndrome: merits and limitations of the vitamin E derivative Trolox. Front Cell Neurosci 10, 266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janc OA & Muller M (2014). The free radical scavenger Trolox dampens neuronal hyperexcitability, reinstates synaptic plasticity, and improves hypoxia tolerance in a mouse model of Rett syndrome. Front Cell Neurosci 8, 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janczewski W & Feldman JL (2006). Distinct rhythm generators for inspiration and expiration in the juvenile rat. J Physiol 570, 407–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janczewski WA, Tashima A, Hsu P, Cui Y & Feldman JL (2013). Role of inhibition in respiratory pattern generation. J Neurosci 33, 5454–5465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C, Agulian S & Haddad G (1991). O2 tension in adult and neonatal brain slices under several experimental conditions. Brain Res 568, 159–164. [DOI] [PubMed] [Google Scholar]
- Jiang C, Agulian S & Haddad G (1992). Cl− and Na+ homeostasis during anoxia in rat hypoglossal neurons: intracellular and extracellular in vitro studies. J Physiol 448, 697–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C & Haddad G (1994). A direct mechanism for sensing low oxygen levels by central neurons. Proc Natl Acad Sci U S A 91, 7198–7201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SA, Morton MJ, Hunter M & Boyett MR (2002). Expression of TASK‐1, a pH‐sensitivie twin‐pore domain K+ channel, in rat myocytes. Am J Physiol Heart Circ Physiol 283, H181–H185. [DOI] [PubMed] [Google Scholar]
- Kamsler A & Segal M (2003). Hydrogen peroxide modulation of synaptic plasticity. J Neurosci 23, 269–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasymov V, Larina O, Castaldo C, Marina N, Patrushev M, Kasparov S & Gourine AV (2013). Differential sensitivity of brainstem versus cortical astrocytes to changes in pH reveals functional regional specialization of astroglia. J Neurosci 33, 435–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Ramiro Diaz J, Iddings J & Filosa J (2016). Vasculo‐neuronal coupling: retrograde vascular communication to brain neurons. J Neurosci 36, 12624–12639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisler K, Nelson A, Montagne A & Zlokovic B (2017). Cerebral blood flow regulation and neurovascular dysfunction in Alzheimer disease. Nat Rev Neurosci 18, 419–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline D (2010). Chronic intermittent hypoxia affects integration of sensory input by neurons in the nucleus tractus solitarii. Respir Physiol Neurobiol 174, 29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline D, Hendricks G, Hermann G, Roggers R & Kunze D (2009). Dopamine inhibits N‐type channels in visceral afferents to reduce synaptic transmitter release under normoxic and chronic intermittent hypoxic conditions. J Neurophysiol 101, 2270–2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline D, Ramirez‐Navarro A & Kunze D (2007). Adaptive depression in synaptic transmission in the nucleus of the solitary tract after in vivo chronic intermittent hypoxia: evidence for homeostatic plasticity. J Neurosci 27, 4663–4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulik A, Brockhaus J, Pedarzani P & Ballanyi K (2002). Chemical anoxia activates ATP‐sensitive and blocks Ca2+ dependent K+ channels in rat dorsal vagal neurons in situ. Neuroscience 110, 541–554. [DOI] [PubMed] [Google Scholar]
- Kumar NN, Velic A, Soliz J, Shi Y, Li K, Wang S, Weaver JL, Sen J, Abbott SB, Lazarenko RM, Ludwig M‐G, Perez‐Reyes E, Mohebbi N, Bettoni C, Gassmann M, Suply T, Sewen K, Guyenet PG, Wagner CA & Bayliss DA (2015). Regulation of breathing CO2 requires the proton‐activated receptor GPR4 in retrotrapezoid nucleus neurons. Science 348, 1255–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P & Prabhakar NR (2012). Peripheral chemoreceptros: function and plasticity of the carotid body. Comp Physiol 2, 141–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarenko R, Fortuna M, Shi Y, Mulkey D, Takakura A, Moreira T, Guyenet PG & Bayliss DA (2010). Anesthetic activation of central respiratory chemoreceptor neurons involves inhibition of a THIK‐1‐like background K+ current. J Neurosci 30, 9324–9334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarenko R, Mlilner T, Depuy S, Stornetta R, GH W, Kievits J & Bayliss DA (2009). Acid sensitivity and ultrastructure of the retrotrazezoid nucleus in Phox2b‐EGFP mice. J Comp Neurol 517, 69–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Maitre E, Revathikumar P, Estelius J & Lampa J (2017). Increased recovery time and decreased LPS administration to study the vagus nerve stimulation mechanisms in limited inflammatory responses. J Vis Exp, doi: 10.3791/54890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesage F & Barhanin J (2011). Molecular physiology of pH‐sensitive background K2P channels. Physiology (Bethesda) 26, 424–437. [DOI] [PubMed] [Google Scholar]
- Lieske S, Thoby‐Brisson M, Telgkamp P & Ramirez J (2000). Reconfiguration of the neural network controlling multiple breathing patterns: eupnea, sighs and gasps. Nat Neurosci 3, 600–607. [DOI] [PubMed] [Google Scholar]
- Liu Y, Ji E‐S, Xiang S, Ramisier R, Tong J, Huang J & Weiss JW (2009). Exposure to cyclic intermittent hypoxia increases expression of functional NMDA receptors in the rat carotid body. J Appl Physiol 106, 259–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López‐Barneo J, Ortega‐Sáenz P, Pardal R, Pascual A & Piruat JI (2008). Carotid body oxygen sensing. Eur Resp J 32, 1386–1398. [DOI] [PubMed] [Google Scholar]
- Lorea‐Hernandez JJ, Morales T, Rivera‐Angulo AJ, Alcantara‐Gonzalez D & Pena‐Ortega F (2016). Microglia modulate respiratory rhythm generation and autoresuscitation. Glia 64, 603–619. [DOI] [PubMed] [Google Scholar]
- Lorier AR, Huxtable AG, Robinson DM, Lipski J, Housley GD & Funk GD (2007). P2Y1 receptor modulation of the pre‐bötzinger complex inspiratory rhythm generating network in vitro. J Neurosci 27, 993–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorier AR, Lipski J, Housley GD, Greer J & Funk GD (2008). ATP sensitivity of preBötzinger complex neurones in neonatal rat in vitro: mechanism underlying a P2 receptor‐mediated increase in inspiratory frequency. J Physiol 586, 1429–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyubkin M, Durand D & Haxhiu M (1997). Interaction between tetanus long‐term potentiation and hypoxia‐induced potentiation in the rat hippocampus. J Neurophysiol 78, 2475–2482. [DOI] [PubMed] [Google Scholar]
- MacFarlane PM & Mitchell GS (2008). Respiratory long‐term facilitation following intermittent hypoxia requires reactive oxygen species formation. Neuroscience 152, 189–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacFarlane PM, Wilkerson JE, Lovett‐Barr MR & Mitchell GS (2008). Reactive oxygen species and respiratory plasticity following intermittent hypoxia. Respir Physiol Neurobiol 164, 263–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado B, Zoccal D & Moraes D (2017). Neurogenic hypertension and the secrets of respiration. Am J Physiol Regul Integr Comp Physiol 312, R864–R872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marina N, Ang R, Machhada A, Kasymov V, Karagiannis A, Hosford PS, Mosienko V, Teschemacher AG, Vihko P, Paton JF, Kasparov S & Gourine AV (2015). Brainstem hypoxia contributes to the development of hypertension in the spontaneously hypertensive rat. Hypertension 65, 775–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marina N, Kasymov V, Ackland GL, Kasparov S & Gourine AV (2016a). Astrocytes and brain hypoxia. Adv Exp Med Biol 903, 201–207. [DOI] [PubMed] [Google Scholar]
- Marina N, Teschemacher AG, Kasparov S & Gourine AV (2016b). Glia, sympathetic activity and cardiovascular disease. Exp Physiol 101, 565–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marina N, Turovsky E, Christie IN, Hosford PS, Hadjihambi A, Korsak A, Ang R, Mastitskaya S, Sheikhbahaei S, Theparambil SM & Gourine AV (2017). Brain metabolic sensing and metabolic signaling at the level of an astrocyte. Glia 66, 1185–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marschke G, Beall GN & Stern EW (1965). Carotid‐body removal in asthma. JAMA 191, 397. [DOI] [PubMed] [Google Scholar]
- Massey C, Kim G, Corcoran A, Haynes R, Paterson D, Cummings K, Dymecki S, Richerson G, Nattie E, Kinney H & Commons K (2013). Development of brainstem 5‐HT1A receptor‐binding sites in serotonin‐deficient mice. J Neurochem 126, 749–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Master ZR, Porzionato A, Kesavan K, Mason A, Chavez‐Valdez R, Shirahata M & Gauda EB (2016). Lipopolysaccharide exposure during the early postnatal period adversely affects the structure and function of the developing rat carotid body. J Appl Physiol (1985) 121, 816–827. [DOI] [PubMed] [Google Scholar]
- Mazza E, Thakkar‐Varia S, Tozzi CA & Neubauer JA (2001). Expression of heme oxygenase in the oxygen‐sensing regions of the rostral ventrolateral medulla. J Appl Physiol (1985) 91, 379–385. [DOI] [PubMed] [Google Scholar]
- McDonald DM & Mitchell RA (1975). The innervation of glomus cells, ganglion cells and blood vessels in the rat carotid body: a quantitative ultrastructural analysis. J Neurocytol 4, 177–230. [Google Scholar]
- Michiels C (2004). Physiological and pathological responses to hypoxia. Am J Pathol 164, 1875–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M & Tenney S (1975). Hypoxia‐induced tachypnea in carotid‐deafferented cats. Resp Physiol 23, 31–39. [DOI] [PubMed] [Google Scholar]
- Mitra A & Raichle M (2016). How networks communicate: propagation patterns in spontaneous brain activity. Philos Trans R Soc Lond B Biol Sci 371, 20150546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitz SA, Reuss S, Folkow LP, Blix AS, Ramirez JM, Hankeln T & Burmester T (2009). When the brain goes diving: glial oxidative metabolism may confer hypoxia tolerance to the seal brain. Neuroscience 163, 552–560. [DOI] [PubMed] [Google Scholar]
- Moraes D, Bonagamba L, da Silva M, Mecawi A, Antunes‐Rodrigues J & Machado B (2016). Respiratory network enhances the sympathoinhibitory component of baroreflex of rats submitted to chronic intermittent hypoxia. Hypertension 68, 1021–1030. [DOI] [PubMed] [Google Scholar]
- Moraes D, da Silva M, Bonagamba L, Mecawi A, Zoccal D, Antunes‐Rodrigues J, Varanda W & Machado B (2013). Electrophysiological properties of rostral ventrolateral medulla presympathetic neurons modulated by the respiratory network in rats. J Neurosci 33, 19223–19237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau J & Ciriello J (2015). Chronic intermittent hypoxia induces changes in expression of synaptic proteins in the nucleus of the solitary tract. Brain Res 1622, 300–307. [DOI] [PubMed] [Google Scholar]
- Morgado‐Valle C, Baca SM & Feldman JL (2010). Glycinergic pacemaker neurons in preBotzinger complex of neonatal mouse. J Neurosci 30, 3634–3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss IR (2000). Respiratory responses to single and episodic hypoxia during development: mechanisms of adaption. Resp Physiol 121, 185–197. [DOI] [PubMed] [Google Scholar]
- Moyer C & Beecher H (1942). Effects of barbiturate anesthesia (evipal and penthothal sodium) upon the integration of respiratory control mechanisms. A study directed toward improvement of methods for the preclinical evaluation of anesthetic agents. J Clin Invest 21, 429–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukandala G, Tynan R, Lanigan S & O'Connor J (2016). The effects of hypoxia and inflammation on synaptic signaling in the CNS. Brain Sci 6, E6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey DK, Henderson RA, Olson JE, Putnam RW & Dean JB (2001). Oxygen measurements in brain stem slices exposed to normobaric hyperoxia and hyperbaric oxygen. J Appl Physiol 90, 1887–1899. [DOI] [PubMed] [Google Scholar]
- Mulkey DK, Mistry AM, Guyenet PG & Bayliss DA (2006). Purinergic P2 receptors modulate excitability but do not mediate pH sensitivity of RTN respiratory chemoreceptors. J Neurosci 26, 7230–7233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey D, Rosin D, West G, Takakura A, Moreira T, Bayliss DA & Guyenet PG (2007a). Serotonergic neurons activate chemosensitive retrotrapezoid nucleus neurons by a pH‐independent mechanism. J Neurosci 27, 14128–14138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey DK, Stornetta RL, Weston MC, Simmons JR, Parker A, Bayliss DA & Guyenet PG (2004). Respiratory control by ventral surface chemoreceptor neurons in rats. Nat Neurosci 7, 1360–1369. [DOI] [PubMed] [Google Scholar]
- Mulkey DK, Talley EM, Stornetta RL, Siegel AR, West GH, Chen X, Sen N, Mistry AM, Guyenet PG & Bayliss DA (2007b). TASK channels determine pH sensitivity in select respiratory neurons but do not contribute to central respiratory chemosensitivity. J Neurosci 27, 14049–14058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murali S & Nurse CA (2016). Purinergic signaling mediates bidirectional crosstalk between chemoreceptor type I and glial‐like type II cells of the rat carotid body. J Physiol 594, 391–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurse CA & Piskuric NA (2013). Signal processing at mammalian carotid body chemoreceptors. Semin Cell Dev Biol 24, 22–30. [DOI] [PubMed] [Google Scholar]
- Murali S, Zhang M & Nurse CA (2014). Angiotensin II mobilizes intracellular calcium and activates pannexin‐1 channels in rat carotid body type II cells via AT1 receptors. J Physiol 592, 4747–4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanduri J, Peng YJ, Yuan G, Kumar GK & Prabhakar NR (2015a). Hypoxia‐inducible factors and hypertension: lessons from sleep apnea syndrome. J Mol Med (Berl) 93, 473–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanduri J, Reddy Vaddi D, Khan SA, Wang N, Makarenko V, Semenza GL & Prabhakar NR (2015b). HIF‐1α activation by intermittent hypoxia requires NADPH oxidase stimulation by xanthine oxidase. PLoS One 10, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanduri J, Yuan G, Kumar GK, Semenza GL & Prabhakar NR (2008). Transcriptional responses to intermittent hypoxia. Respir Physiol Neurobiol 164, 277–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarrete‐Opazo A & Mitchell G (2014). Therapeutic potential of intermittent hypoxia: a matter of dose. Am J Physiol Regul Integr Comp Physiol 307, R1181–R1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndunuizu O & LaManna JC (2007). Brain Tissue Oxygen Concentration Measurements. Antioxid Redox Signal 9, 1207–1219. [DOI] [PubMed] [Google Scholar]
- Nieto‐Posadas A, Flores‐Martinez E, Lorea‐Hernandez JJ, Rivera‐Angulo AJ, Perez‐Ortega JE, Bargas J & Pena‐Ortega F (2014). Change in network connectivity during fictive‐gasping generation in hypoxia: prevention by a metabolic intermediate. Front Physiol 5, 265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Fleisher LA, Jneid H, Mack MJ, McLeod CJ, O'Gara PT, Rigolin VH, Sundt TM 3rd & Thompson A (2017). 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 70, 252–289. [DOI] [PubMed] [Google Scholar]
- Nurse C (2014). Synaptic and paracrine mechanisms at carotid body arterial chemoreceptors. J Physiol 592, 3419–3426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nwaobi SE, Cuddapah VA, Patterson KA, Randolph AC & Olsen ML (2016). The role of glial‐specific Kir4.1 in normal and pathological states of the CNS. Acta Neuropathol 132, 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell AD, Morton MJ & Hunter M (2002). Two‐pore domain K+ channels—molecular sensors. Biochim Biophys Acta 1566, 152–161. [DOI] [PubMed] [Google Scholar]
- Okada Y, Mückenhoff K & Scheid P (1993). Hypercapnia and medullary neurons in the isolated brain stem‐spinal cord of the rat. Resp Physiol 93, 327–336. [DOI] [PubMed] [Google Scholar]
- Okada Y, Sasaki T, Oku Y, Takahashi N, Seki M, Ujita S, Tanaka KF, Matsuki N & Ikegaya Y (2012). Preinspiratory calcium rise in putative pre‐Botzinger complex astrocytes. J Physiol 590, 4933–4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oku Y, Fresemann J, Miwakeichi F & Hülsmann S (2016). Respiratory calcium fluctuations in low‐frequency oscillating astrocytes in the pre‐Bötzinger complex. Respir Physiol Neurobiol 226, 11–17. [DOI] [PubMed] [Google Scholar]
- Olsen ML, Khakh BS, Skatchkov SN, Zhou M, Justin Lee C & Rouach N (2015). New insights on astrocyte ion channels: critical for homeostasis and neuron‐glia signaling. J Neurosci 35, 13827–13835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliardini S, Janczewski WA, Tan W, Dickson CT, Deisseroth K & Feldman JL (2011). Active expiration induced by excitation of ventral medulla in adult anesthetized rats. J Neurosci 31, 2895–2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakkarato S, Chomphoo S, Kagawa Y, Owada U, Mothong W, Iamsaard S, Sawatpanich T, Kondo H & Hipkaeo W (2015). Immunohistochemical analysis of sustentacular cells in the adrenal medulla, carotid body and sympathetic ganglion of mice using an antibody against brain‐type fatty acid binding protein (B‐FABP). J Anat 226, 348–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papouin T & Oliet SH (2014). Organization, control and function of extrasynaptic NMDA receptors. Philos Trans R Soc Lond B Biol Sci 369, 20130601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parpura V, Basarsky TA, Liu F, Jeftinija K, Jeftinija S & Haydon PG (1994). Glutamate‐mediated astrocyte‐neuron signaling. Nat Neurosci 369, 744–747. [DOI] [PubMed] [Google Scholar]
- Pascual O, Casper KB, Kubera C, Zhang J, Revilla‐Sanchez R, Sul J‐Y, Takano H, Moss SJ, McCarthy K & Haydon PG (2005). Astrocytic purinergic signaling coordinates synaptic networks. Science 310, 113–116. [DOI] [PubMed] [Google Scholar]
- Paton JF, Abdala AP, Koizumi H, Smith JC & St‐John WM (2006). Respiratory rhythm generation during gasping depends on persistent sodium current. Nat Neurosci 9, 311–313. [DOI] [PubMed] [Google Scholar]
- Pecorelli A, Cervellati C, Hayek J & Valacchi G (2016). OxInflammation in Rett syndrome. Int J Biochem Cell Biol 81, 246–253. [DOI] [PubMed] [Google Scholar]
- Peña F, Parkis MA, Tryba AK & Ramirez JM (2004). Differential contribution of pacemaker properties to the generation of respiratory rhythms during normoxia and hypoxia. Neuron 43, 105–117. [DOI] [PubMed] [Google Scholar]
- Peña F & Ramirez JM (2004). Substance P‐mediated modulation of pacemaker properties in the mammalian respiratory network. J Neurosci 24, 7549–7556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña F & Ramirez JM (2005). Hypoxia‐induced changes in neuronal network properties. Mol Neurobiol 32, 251–283. [DOI] [PubMed] [Google Scholar]
- Peña‐Ortega F (2017). Neural network reconfigurations: changes of the respiratory network by hypoxia as an example. Adv Exp Med Biol 1015, 217–237. [DOI] [PubMed] [Google Scholar]
- Peng YJ, Nanduri J, Yuan G, Wang N, Deneris E, Pendyala S, Natarajan V, Kumar GK & Prabhakar NR (2009). NADPH oxidase is required for the sensory plasticity of the carotid body by chronic intermittent hypoxia. J Neurosci 29, 4903–4910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng YJ, Overholt JL, Kline D, Kumar GK & Prabhakar NR (2003). Induction of sensory long‐term facilitation in the carotid body by intermittent hypoxia: implications for recurrent apneas. Proc Natl Acad Sci U S A 100, 10073–10078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poets C, Meny R, Chobanian M & Bonofigio R (1999). Gasping and other cardiorespiratory patterns during sudden infant deaths. Pediatr Res 45, 350–354. [DOI] [PubMed] [Google Scholar]
- Popa‐Wagner A, Mitran S, Sivanesan S, Chang E & Buga A (2013). ROS and brain disease: the good, the bad, and the ugly. Oxid Med Cell Longev 2013, 963520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakhar NR ( 2013). Sensing hypoxia: physiology, genetics and epigenetics. J Physiol 591, 2245–2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakhar NR, Kumar GK & Peng YJ (2012). Sympatho‐adrenal activation by chronic intermittent hypoxia. J Appl Physiol (1985) 113, 1304–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakhar NR & Joyner MJ (2015). Tasting arterial blood: what do the carotid chemoreceptors sense? Front Physiol 5, 524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakhar NR & Peng YJ (2017). Oxygen sensing by the carotid body: past and present. Adv Exp Med Biol 977, 3–8. [DOI] [PubMed] [Google Scholar]
- Prabhakhar NR, Peng YJ, Kumar GK & Nanduri J (2015). Peripheral chemoreception and arterial pressure responses to intermittent hypoxia. Compr Physiol 5, 561–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakhar NR & Semenza G (2015). Oxygen sensing and homeostasis. Physiology (Bethesda) 30, 340–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakhar NR & Semenza GL (2016). Regulation of carotid body oxygen sensing by hypoxia‐inducible factors. Pflugers Arch 468, 71–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad M, Fearon IM, Zhang M, Laing M, Vollmer C & Nurse CA (2001). Expression of P2X2 and P2X3 receptor subunits in rat carotid body afferent neurones: role in chemosensory signaling. J Physiol 537, 667–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana A, Zanella S, Koch H, Kruse SE, Lee D, Ramirez JM & Palmiter RD (2012). Fatal breathing dysfunction in a mouse model of Leigh syndrome. J Clin Invest 122, 2359–2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME (2015). The restless brain: how intrinsic activity organizes brain function. Philos Trans R Soc Lond B Biol Sci 370, 20140172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA & Shulman GL (2001). A default mode of brain function. Proc Natl Acad Sci U S A 98, 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajani V, Zhang Y, Jalubula V, Rancic V, SheikhBahaei S, Zwicker JD, Pagliardini S, Dickson CT, Ballanyi K, Kasparov S, Gourine AV & Funk GD (2018). Release of ATP by preBotzinger complex astrocytes contributes to the hypoxic ventilatory response via a Ca2+‐dependent P2Y1 receptor mechanism. J Physiol 596, 3245–3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajani V, Zhang Y, Revill A & Funk GD (2016). The role of P2Y1 receptor signaling in central respiratory control. Respir Physiol Neurobiol 226, 3–10. [DOI] [PubMed] [Google Scholar]
- Rakoczy R & Wyatt C (2018). Acute oxygen sensing by the carotid body: a rattlebag of molecular mechanisms. J Physiol 596, 2969–2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez JM (1998). Reconfiguration of the respiratory network at the onset of locust flight. J Neurophysiol 80, 3137–3147. [DOI] [PubMed] [Google Scholar]
- Ramirez JM, Folkow LP & Blix A (2007). Hypoxia tolerance in mammals and birds: from the wilderness to the clinic. Annu Rev Physiol 69, 113–143. [DOI] [PubMed] [Google Scholar]
- Ramirez JM, Folkow LP, Ludvigsen S, Ramirez PN & Blix AS (2011). Slow intrinsic oscillations in thick neocortical slices of hypoxia tolerant deep diving seals. Neuroscience 177, 35–42. [DOI] [PubMed] [Google Scholar]
- Ramirez JM, Garcia AJ III, Anderson T, Koschnitzky JE, Peng Y, Kumar GK & Prabhakar NR (2013). Central and peripheral factors contributing to obstructive sleep apneas. Respir Physiol Neurobiol 189, 344–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez JM, Quellmalz UJ & Wilken B (1997). Developmental changes in the hypoxic response of the hypoglossus respiratory motor output in vitro. J Neurophysiol 78, 383–392. [DOI] [PubMed] [Google Scholar]
- Ramirez JM, Quellmalz UJ, Wilken B & Richter DW (1998a). The hypoxic response of neurones within the in vitro mammalian respiratory network. J Physiol 507, 571–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez JM, Schwarzacher SW, Pierrefiche O, Olivera BM & Richter DW (1998b). Selective lesioning of the cat pre‐Botzinger complex in vivo eliminates breathing but not gasping. J Physiol 507, 895–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro A, Mayer C, Wilson C, Martin R & MacFarlane P (2017). Intratracheal LPS administration attenuates the acute hypoxic ventilatory response: Role of brainstem IL‐1β receptors. Respir Physiol Neurobiol 242, 45–51. [DOI] [PubMed] [Google Scholar]
- Richter DW, Bischoff A, Anders K, Bellingham M & Windhorst U (1991). Response of the medullary respiratory network of the cat to hypoxia. J Physiol 443, 231–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter DW & Smith JC (2014). Respiratory rhythm generation in vivo. Physiology (Bethesda) 29, 58–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera‐Angula A & Peña‐Ortega F (2014). Isocitrate supplementation promotes breathing generation, gasping, and autoresuscitation in neonatal mice. J Neurosci Res 92, 375–388. [DOI] [PubMed] [Google Scholar]
- Robson SC, Sevigny J & Zimmermann H (2006). The E‐NTPDase family of ectonucleotidases: Structure function relationships and pathophysiological significance. Purinergic Signal 2, 409–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rognum I, Tran H, Haas E, Hyland K, Paterson D, Haynes R, Broadbelt K, Harty B, Mena O, Krous H & Kinney H (2014). Serotonin metabolites in the cerebrospinal fluid in sudden infant death syndrome. J Neuropathol Exp Neurol 73, 115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth A & Núñez M (2016). Oligoendrocytes: functioning in a delicate balance between high metabolic requirements and oxidative damage. Adv Exp Med Biol 949, 167–181. [DOI] [PubMed] [Google Scholar]
- Row B, Liu R, Xu W, Kheirandish L & Gozal D (2003). Intermittent hypoxia is associated with oxidative stress and spatial learning deficits in the rat. Am J Respir Crit Care Med 167, 1548–1553. [DOI] [PubMed] [Google Scholar]
- Rubin JE, Hayes JA, Mendenhall JL & Del Negro CA (2009). Calcium‐activated nonspecific cation current and synaptic depression promote network‐dependent burst oscillations. Proc Natl Acad Sci U S A 106, 2939–2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffault P, D'Autréaux F, Hayes J, Nomaksteinsky M, Autran S, Fujiyama T, Hoshino M, Hägglund M, Kiehn O, Brunet J, Fortin G & Goridis C (2015). The retrotrapezoid nucleus neurons expressing Atoh1 and Phox2b are essential for the respiratory response to CO2 . Elife 4, e07051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt C, Bellingham MC & Richter DW (1995). Adenosinergic modulation of respiratory neurones and hypoxic responses in the anaesthetized cat. J Physiol 483, 769–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell C, Fresemann J & Hulsmann S (2011). Determinants of functional coupling between astrocytes and respiratory neurons in the pre‐Bötzinger complex. PLoS One 6, e26309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell MJ, Molliver ME & Synder SH (1995). D‐Serine, an endogenous synpatic modulator: Localization to astrocytes and glutamate‐stimulated release. Proc Natl Acad Sci U S A 92, 3948–3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schüle B, Armstrong D, Vogel H, Oviedo A & Francke U (2008). Severe congenital encephalopathy caused by MECP2 null mutations in males: central hypoxia and reduced neuronal dendritic structure. Clin Genet 74, 116–126. [DOI] [PubMed] [Google Scholar]
- Schwarzacher SW, Rub U & Deller T (2011). Neuroanatomical characteristics of the human pre‐Bötzinger complex and its involvement in neurodegenerative brainstem diseases. Brain 134, 24–35. [DOI] [PubMed] [Google Scholar]
- Semenza G & Prabhakar N (1985). Neural regulation of hypoxia‐inducible factors and redox state drives the pathogenesis of hypertension in a rodent model of sleep apnea. J Appl Physiol 119, 1152–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza G & Prabhakar NR (2007). HIF‐1‐dependent respiratory, cardiovascular, and redox responses to chronic intermittent hypoxia. Antioxid Redox Signal 9, 1391–1396. [DOI] [PubMed] [Google Scholar]
- Shell B, Faulk K & Cunningham JT (2016). Neural control of blood pressure in chronic intermittent hypoxia. Curr Hypertens Rep 18, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman D, Worrell J, Cui Y & Feldman J (2015). Optogenetic perturbation of preBötzinger complex inhibitory neurons modulates respiratory pattern. Nat Neurosci 18, 408–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C, Engwall M, Dempsey J & Bisgard G (1993). Effects of specific carotid body and brain hypoxia on respiratory muscle control in the awake goat. J Physiol 460, 623–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C, Forster H, Blain G & Dempsey J (2010). An interdependent model of central/peripheral chemoreception: evidence and implications for ventilatory control. Respir Physiol Neurobiol 173, 288–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Ellenberger HH, Ballanyi K, Richter DW & Feldman JL (1991). Pre‐Bötzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science 254, 726–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobrinho CR, Goncalves CM, Takakura AC, Mulkey DK & Moreira TS (2017). Fluorocitrate‐mediated depolarization of astrocytes in the retrotrapezoid nucleus stimulates breathing. J Neurophysiol 118, 1690–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon IC, Edelman NH & Neubauer JA (2000). Pre‐Bötzinger complex functions as a central hypoxia chemosensor for respiration in vivo. J Neurophysiol 83, 2854–2868. [DOI] [PubMed] [Google Scholar]
- Souza G, Amorim MR, Moraes DJA & Machado BH (2017). Sex differences in the respiratory‐sympathetic coupling in rats exposed to chronic intermittent hypoxia. Respir Physiol Neurobiol (in press; doi: 10.1016/j.resp.2017.09.003). [DOI] [PubMed] [Google Scholar]
- Souza G, Bonagamba L, Amorim M, Moraes D & Machado B (2016). Inspiratory modulation of sympathetic activity is increased in female rats exposed to chronic intermittent hypoxia. Exp Physiol 101, 1345–1358. [DOI] [PubMed] [Google Scholar]
- St John W & Wang S (1977). Alteration from apneusis to more regular rhythmic respiration in decerebrate cats. Resp Physiol 59, 1201–1207. [DOI] [PubMed] [Google Scholar]
- Sun MK, Jeske IT & Reis DJ (1992). Cyanide excites medullary sympathoexcitatory neurons in rats. Am J Physiol 262, R182–R189. [DOI] [PubMed] [Google Scholar]
- Sun MK & Reis DJ (1994). Dopamine or transmitter release from rat carotid body may not be essential to hypoxic chemoreception. Am J Physiol 267, R1632–R1639. [DOI] [PubMed] [Google Scholar]
- Sunderram J, Semmlow J, Patel P, Rao H, Chun G, Agarwala P, Bhaumik M, Le‐Hoang O, Lu SE & Neubauer JA (2016). Heme oxygenase‐1‐dependent central cardiorespiratory adaptations to chronic intermittent hypoxia in mice. J Appl Physiol (1985) 121, 944–952. [DOI] [PubMed] [Google Scholar]
- Tan W, Janczewski W, Yang P, Shao X, Callaway E & Feldman J (2008). Silencing preBötzinger complex somatostatin‐expressing neurons induces persistent apnea in awake rat. Nat Neurosci 11, 538–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telgkamp P, Cao YQ, Basbaum AI & Ramirez JM (2002). Long‐term deprivation of substance P in PPT‐A mutant mice alters the anoxic response of the isolated respiratory network. J Neurophysiol 88, 206–213. [DOI] [PubMed] [Google Scholar]
- Telgkamp P & Ramirez JM (1999). Differential responses of respiratory nuclei to anoxia in rhythmic brain stem slices of mice. J Neurophysiol 82, 2163–2170. [DOI] [PubMed] [Google Scholar]
- Teppema LJ & Dahan A (2010). The ventilatory response to hypoxia in mammals: mechanisms, measurement, and analysis. Physiol Rev 90, 675–754. [DOI] [PubMed] [Google Scholar]
- Thoby‐Brisson M & Ramirez JM (2000). Role of inspiratory pacemaker neurons in mediating the hypoxic response of the respiratory network in vitro. J Neurosci 20, 5858–5866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoby‐Brisson M & Ramirez JM (2001). Identification of two types of inspiratory pacemaker neurons in the isolated respiratory neural network of mice. J Neurophysiol 86, 104–112. [DOI] [PubMed] [Google Scholar]
- Toorop RJ, Scheltinga MR, Huige MC & Moll FL (2010). Clinical results of carotid denervation by adventitial stripping in carotid sinus syndrome. Eur J Vasc Endovasc Surg 39, 146–152. [DOI] [PubMed] [Google Scholar]
- Trapp S & Ballanyi K (1995). KATP channel mediation of anoxia‐induced outward current in rat dorsal vagal neurons in vitro. J Physiol 15, 37–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trumbower RD, Jayaraman A, Mitchell GS & Rymer WZ (2012). Exposure to acute intermittent hypoxia augments somatic motor function in humans with incomplete spinal cord injury. Neurorehabil Neural Repair 26, 163–172. [DOI] [PubMed] [Google Scholar]
- Tryba AK, Peña F & Ramirez J‐M (2006). Gasping activity in vitro: A rhythm dependent on 5‐HT2A receptors. J Neurosci 26, 2623–2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turovsky E, Theparambil SM, Kasymov V, Deitmer JW, Del Arroyo AG, Ackland GL, Corneveaux JJ, Allen AN, Huentelman MJ, Kasparov S, Marina N & Gourine AV (2016). Mechanisms of CO2/H+ sensitivity of astrocytes. J Neurosci 36, 10750–10758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viemari JC, Garcia AJ 3rd, Doi A & Ramirez JM (2011). Activation of alpha‐2 noradrenergic receptors is critical for the generation of fictive eupnea and fictive gasping inspiratory activities in mammals in vitro. Eur J Neurosci 33, 2228–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viemari J‐C & Ramirez J‐M (2006). Norepinheprine differentially modulates different types of respiratory pacemaker and nonpacemaker neurons. J Neurophysiol 95, 2070–2082. [DOI] [PubMed] [Google Scholar]
- Viemari J‐C, Roux J‐C, Tryba AK, Saywell V, Burnet H, Peña F, Zanella S, Bévengut M, Barthelemy‐Requin, Herzing LB, Moncla A, Mancini J, Ramirez J‐M, Villard L & Hilaire G (2005). Mecp2 deficiency disrupts norepinephrine and respiratory systems in mice. J Neurosci 25, 11521–11530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinit S, Windelborn J & Mitchell G (2011). Lipopolysaccharide attenuates phrenic long‐term facilitation following acute intermittent hypoxia. Respir Physiol Neurobiol 176, 130–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Bejamer N, Zanella S, Kumar NN, Shi Y, Bévengut M, Penton D, Guyenet PG, Lesage F, Gestreau C, Narhanin J & Bayliss DA (2013a). TASK‐2 channels contribute to ph sensitivity of retrotrapezoid nucleus chemoreceptor neurons. J Neurosci 33, 16033–16044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Shi Y, Shu S, Guyenet PG & Bayliss DA (2013b). Phox2b‐expressing retrotrapezoid neurons are intrinsically responsive to H+ and CO2 . J Neurosci 33, 7756–7761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Fund M, Darnall R & St John W (1996). Characterizations and comparisons of eupnoea and gasping in neonatal rats. J Physiol 490, 277–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Pizzonia JH & Richerson GB (1998). Chemosensitivity of rat medullary raphe neurones in primary tissue culture. J Physiology 511, 433–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W & Richerson GB (2000). Chemosensitivity of non‐respiratory rat CNS neurons in tissue culture. Brain Res 860, 119–129. [DOI] [PubMed] [Google Scholar]
- Wang X, Hayes J, Revill A, Song H, Kottick A, Vann N, LaMar M, Picardo M, Akins V, Funk G & Del Negro C (2014). Laser ablation of Dbx1 neurons in the pre‐Bötzinger complex stops inspiratory rhythm and impairs output in neonatal mice. Elife 3, e03427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weese‐Mayer DE, Kenny AS, Bennett HL, Ramirez JM & Leurgans SE (2008a). Familial dysautonomia: frequent, prolonged and severe hypoxemia during wakefulness and sleep. Pediatr Pulmonol 43, 251–260. [DOI] [PubMed] [Google Scholar]
- Weese‐Mayer D, Lieske S, Boothby C, Kenny A, Bennett H & Ramirez J (2008b). Autonomic dysregulation in young girls with Rett Syndrome during nighttime in‐home recordings. Pediatr Pulmonol 43, 1045–1060. [DOI] [PubMed] [Google Scholar]
- Weese‐Mayer DE, Lieske SP, Boothby CM, Kenny AS, Bennett HL, Silvestri JM & Ramirez JM (2006). Autonomic nervous system dysregulation: breathing and heart rate perturbation during wakefulness in young girls with Rett syndrome. Pediatric Research 60, 443–449. [DOI] [PubMed] [Google Scholar]
- Wenker I, Kréneisz O, Nishiyama A & Mulkey D (2010). Astrocytes in the retrotrapezoid nucleus sense H+ by inhibition of a Kir4.1‐Kir5.1‐like current and may contribute to chemoreception by a purinergic mechanism. J Neurophysiol 104, 3042–3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenker IC, Sobrinho CR, Takakura AC, Moreira TS & Mulkey DK (2012). Regulation of ventral surface CO2/H+‐sensitive neurons by purinergic signaling. J Physiol 590, 2137–2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenker IC, Sobrinho CR, Takakura AC & Mulkey DK (2013). P2Y1‐receptors expressed by C1 neurons determine peripheral chemoreceptor modulation of breathing, sympathetic activity and blood pressure. Hypertension 62, 263–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilken B, Ramirez JM, Probst I, Richter DW & Hanefeld F (1998). Creatine protects the central respiratory network of mammals under anoxic conditions. Pediatr Res 43, 8–14. [DOI] [PubMed] [Google Scholar]
- Wilkerson J, Devinney M & Mitchell G (2018). Intermittent but not sustained moderate hypoxia elicits long‐term facilitation of hypoglossal motor output. Respir Physiol Neurobiol (in press; doi: 10.1016/j.resp.2017.10.005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R, Remmers J & Paton J (2001). Brain stem PO2 and pH of the working heart‐brain stem preparation during vascular perfusion with aqueous medium. Am J Physiol Regul Integr Comp Physiol 281, R528–R538. [DOI] [PubMed] [Google Scholar]
- Wilson R & Teppema L (2016). Integration of central and peripheral respiratory chemoreflexes. Compr Physiol 6, 1005–10041. [DOI] [PubMed] [Google Scholar]
- Wood JB, Frankland AW & Eastcott HH (1965). Bilateral removal of carotid bodies for asthma. Thorax 20, 570–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Carreño F, Cunningham J & Mifflin S (2008). Chronic sustained and intermittent hypoxia reduce function of ATP‐sensitive potassium channels in nucleus of the solitary tract. Am J Physiol Integr Comp Physiol 295, R1555–R1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwicker J, Rajani V, Hahn L & Funk GD (2011). Purinergic modulation of preBötzinger complex inspiratory rhythm in rodents: the interaction between ATP and adenosine. J Physiol 589, 4583–4600. [DOI] [PMC free article] [PubMed] [Google Scholar]


