Abstract
A simple, non-alcoholic extraction method for measuring estrogen and progesterone metabolites in excreta using enzyme immunoassays (EIAs) was developed in Japanese macaques. The obtained detection limits of EIAs using estrone conjugates (E1C), pregnanediol glucuronide (PdG), and estriol glucuronide (E3G) polyclonal antibodies with cross-reactivity to urinary and fecal steroid metabolites were 6.6 pg/ml, 2.1 ng/ml and 0.35 ng/ml, respectively. These assays allowed the determination of E1C, PdG, and E3G from the excreta with good reproducibility and accuracy. Thereafter, urine and fecal samples of two menstrual cycles and six pregnancies from eight female Japanese macaques were assayed. A typical increase in urinary and fecal E1C in follicular phase and PdG in luteal phase were shown during non-conceptive menstrual cycles. Urinary E3G levels also showed a preovulatory increase; however, fecal E3G levels were very low throughout the non-conceptive menstrual cycles. Levels of E1C and PdG in the urine and feces of pregnant females were gradually increased until parturition, while fecal E3G levels were low and reached detectable levels after the mid-pregnancy period. Although the extraction rate of estrogen and progestogen metabolites by our method was lower compared to those of the previous extraction method using an alcohol-containing buffer, our method was simple, and the correlation coefficients for the relationship between two methods were found to be statistically significant. The results presented here are of great practical value for a non-invasive method of monitoring ovarian function and pregnancy in Japanese macaques.
Keywords: EIA, Japanese macaque, simple extraction method, steroid metabolite
The non-invasive measurement of sex steroids in captive and free-ranging animals provides valuable information for understanding their current reproductive status, and is widely applied to zoological research [5, 11, 13, 22]. At the beginning, the method was directed at measuring reproductive steroids in urine samples. However, in free-ranging species, collecting urine samples is difficult, and fecal samples are easily collected from the ground [20]. Consequently, the measurement of steroid hormones in fecal samples has been developed [5].
The measurements of urinary and fecal estrogen and progestogen metabolites as an alternative to blood have been developed to monitor concentrations of estrogen conjugates and progesterone metabolites, mainly in the form of pregnanediol-3-glucuronide (PdG), in free-ranging animals [12]. In most mammals, steroids are metabolized by the liver, and afterwards excreted via the kidneys into urine, or via the bile ducts into the gut [5]. Additionally, metabolic products can be conjugated to various degrees to glucuronides and/or sulfates [2]. A previous study reported that in general, circulating bioactive estradiol (E2) and progesterone (P4) were metabolized to their conjugated forms in urine, and unconjugated forms in feces [13]. Furthermore, Shideler et al. [20] administered 14C-steroids to macaques, and clarified that E2 was metabolized to estrone conjugates (E1C) in urine, but existed as free E1 form in feces. Additionally, they clarified that P4 did not metabolize to authentic pregnanediol conjugates, but to complex conjugated pregnane-like forms in urine and free pregnanediol in feces of macaques [19,20,21]. In the case of Japanese macaques, O’Neill [15] also reported that free E1 and pregnanediol forms were found in their feces. Thus, because the primary metabolite form is different between urine and feces, using appropriate antibodies is important for the effectiveness of the immunoassay.
Though fecal samples are easily to collect, the extraction process of feces is indispensable to measuring steroids by immunoassay. The fecal steroid metabolites of macaques have been measured by immunoassays with various extraction methods, commonly with high concentrated alcohol or volatile solvents [15, 16, 18, 25, 29]. Though these solvents allow for a high extraction rate, highly concentrated alcohol or volatile solvents can interfere with the enzymatic and/or immunometric reaction. Therefore, it is necessary to remove the blockage from the extraction. This complicated extraction process in fecal samples requires special equipment and can lead to some errors, which will then bring incorrect results. Consequently, a simpler method of obtaining accurate results from fecal extraction is desirable.
The present study described the establishment of 1) a simple and rapid method for fecal extraction without a large quantity of alcohol or volatile solvent, and 2) EIAs using appropriate polyclonal E1C, PdG, and E3G antibodies with cross-reactivity to urinary and fecal conjugated and unconjugated steroid metabolites in Japanese macaques. 3) Then, we measured these steroid metabolites using this method in both the urine and fecal samples of female Japanese macaques.
MATERIALS AND METHODS
Experimental subjects
Eight sexually mature female Japanese macaques (Macaca fuscata); with body weights ranging from 7.2–11.5 kg (mean=9.2 kg, SD ± 1.4) were used in this study. All females were housed individually at the Primate Research Institute (PRI), Kyoto University, Inuyama, Japan. They were provisioned commercial monkey chow daily, and supplemented with sweet potatoes. Water was available ad libitum. They were maintained in a natural lighting conditioned room at a temperature of approximately 20°C. All females were multiparous, and daily vaginal bleeding was monitored. After confirmation of the onset of menstruation, all females were mated with males between approximately 10–15 days from the beginning of menstruation. The use of laboratory subjects adhered to the Guide for Care and Use of Laboratory Primates (1986) of the Primate Research Institute, Kyoto University.
Sample collection
Urine and fecal samples were collected two times per week during the entire breeding season. Large collection trays were placed beneath the individual cages, and a stainless steel mesh was used to separate the feces from urine to minimize contamination. Morning urine samples were aspirated from the tray using a disposable syringe. Feces were collected and placed into plastic collection bags. All samples were immediately frozen at −30°C and stored until the assay.
At the end of the breeding season, six of eight females conceived and five pregnancies resulted in the birth of healthy offspring. However, one offspring died five days after birth by negligence. One other pregnant female had a premature stillbirth. The urine and fecal samples of pregnant females were collected over the entire gestation period. The menstrual cycles of the two remaining females that had not conceived were monitored until the end of July.
Sample preparation
Urinary samples. Urinary samples were unprocessed, and diluted with deionized water between 100–5,000, 1–50, 1–20, 50–100 and 1–20 times for the E1C, PdG, and E3G assays of pregnant females: and the E1C, and PdG assays of non-pregnant females, respectively. For the E3G assay, urinary samples were used without dilution.
Fecal samples. Fecal samples were thawed and dried using a vacuum drier at 50°C for approximately 12 hr. Dried samples were pulverized, and foreign substances such as rough fiber and seeds from the dried fecal powder were removed. A fecal sample of the powder representing 0.25 g was placed into a 14 ml polypropylene test tube (Stockwell Scientific Inc., Scottsdale, AZ, U.S.A.), and 2.5 ml of extraction buffer which contained 0.1 M phosphate buffer (pH 7.0) 0.1% BSA, with 0.05% Tween 20 and no alcohol were added, and then rotated on a test-tube rotator (Labinco, B.V., Breda, Netherlands) for 24 hr at room temperature. Following centrifugation at 4°C, 1,500 × g for 10 min, the supernatant was decanted into microtubes and stored at −30°C until the assays.
Enzyme Immunoassays
Antibodies. Previous studies have described that major estradiol (E2) metabolites were two types of estrone-monoconjugates (E1C) in urine and unconjugated estrone (E1) in feces. Progesterone (P4) metabolites were various 20α-hydroxy C21 compound-monoconjugates in urine and unconjugated pregnanediol in feces [6, 15, 21]. Moreover, during pregnancy, it has been reported that large quantities of E2 are produced from the placenta of humans and great apes [7, 17]. Therefore, we employed group-specific polyclonal antibodies against estrone-3-glucuronide-BSA, pregnanediol-3-glucuronide-BSA, and estriol-6-carboxymethyloxime-BSA raised in rabbits by Dr. A. Kambegawa.
Plate preparation. Anti-rabbit IgG (H+L) goat serum (Rockland Immunochemicals Inc., Limerick, PA, U.S.A.) was diluted to 0.15 µg/ml in pH 9.6, 0.05 M carbonate buffer, and 50 µl was added to microtiter plates (Maxisorp flat-bottom, Thermo Fisher Scientific, Waltham, MA, U.S.A.) for the E1C, PdG and E3G assays. Then plates were left for one or two nights at room temperature (<24°C). The plates were washed of the unbound anti-rabbit IgG with wash buffer (0.15 M NaCl, 0.05% Tween20), and blocked with blocking buffer (0.05 M borate buffer, pH 7.8, 0.1% BSA, 3% sucrose) overnight. The following day, the blocking buffer was discarded and the plates were dried, sealed, and stored in 4°C until the assays.
E1C EIA. The E1C polyclonal antibody was diluted with assay buffer (0.05 M borate buffer, pH 7.8, 0.2% BSA) at an appropriate dilution rate (1:8,000,000), and 50 µl of the antibody was added to IgG-coated plates and left overnight at room temperature. The following day, the plate was washed to remove the unbound first antibody, and all emptied wells were filled with 50 µl of assay buffer immediately. Afterwards, serially diluted 50 µl of standards (range 0.001–10 ng/ml), internal controls, and unknown samples were added to each well. Correspondingly, 50 µl of estrone 3-carboxymethyl ether (CME)-HRP diluted with assay buffer (1:300,000–700,000) was added to each well. The plate was sealed and incubated overnight at room temperature. The following day, the plate was washed, and 100 µl of substrate solution (0.2 M citrate buffer, pH 4.5, 0.05% o-phenylenediamin, 0.025% hydrogen peroxide solution) was added to each well. The plate was incubated on the reciprocating shaker for approximately 30 min at room temperature. After the plate color was developed, 50 µl of acid was added to each well to stop the enzyme reaction, and absorbance was measured at 492 nm on an automatic plate reader (Sunrise Rainbow; TECAN, Zurich, Switzerland). The concentrations of each sample were calculated by fitting the absorbance for a standard curve using a four-parameter logistic model (LS-PLATE Manager 2004; Wako Pure Chemical Industries, Ltd., Osaka, Japan).
PdG and E3G EIAs. The PdG and E3G antibodies were diluted at 1:200,000 and 1:55,000 with assay buffer, respectively. Other processes were the same as used for E1C EIA. Pregnanediol-3-glucuronide-HRP for PdG EIA (1:300,000–500,000), and estriol-3-calboxymethyl ether (CME)-HRP for E3G EIA (1:50,000) were used. Serially diluted standard ranges were 1–1,000 ng/ml for PdG and 0.03–300 ng/ml for E3G EIA.
Urinary steroid metabolites were compensated creatinine concentrations measured by means of the Jaffe reaction [24].
Assay validation
The cross reactivity of steroids and steroid metabolites structurally related to estrone, pregnanediol, and estriol were assessed with antibodies against estrone-3-glucuronide-BSA, pregnanediol-3-glucuronide-BSA, and estriol-6-carboxymethyloxime-BSA by EIA [1]. Sensitivity of the assays was estimated as the minimum detectable dose by mean −2 SD of the optical densities from the zero concentration of the standard. Linearity was assessed in the recovery rates by serially diluted urinary and fecal extractions from three randomly selected female Japanese macaques. Accuracy was examined by determining the recovery of a known amount of steroid added to three randomly selected urinary and fecal samples before their extraction. Precision of these EIAs was assessed in high and low controls of each hormone. The intra- and inter-assay coefficients of variation (CV) were calculated by urinary and fecal sample assays from eight females, and the high and low control values.
Comparison of two extraction methods
In order to evaluate the extraction method, hormonal profiles were compared between our extraction and the previous extraction method using 50% ethanol and diethyl ether [15, 23] with some modification. In brief, the dried fecal sample was divided into two portions, and the first 0.25 g of dried feces was extracted by our simple method (previously described). The remaining portion was extracted with 5 ml of 50% ethanol and the aliquot of 500 µl of supernatant was extracted with 5 ml of diethyl ether. Thereafter, the ether layer was decanted and reconstituted in the assay buffer.
RESULTS
Assay validation
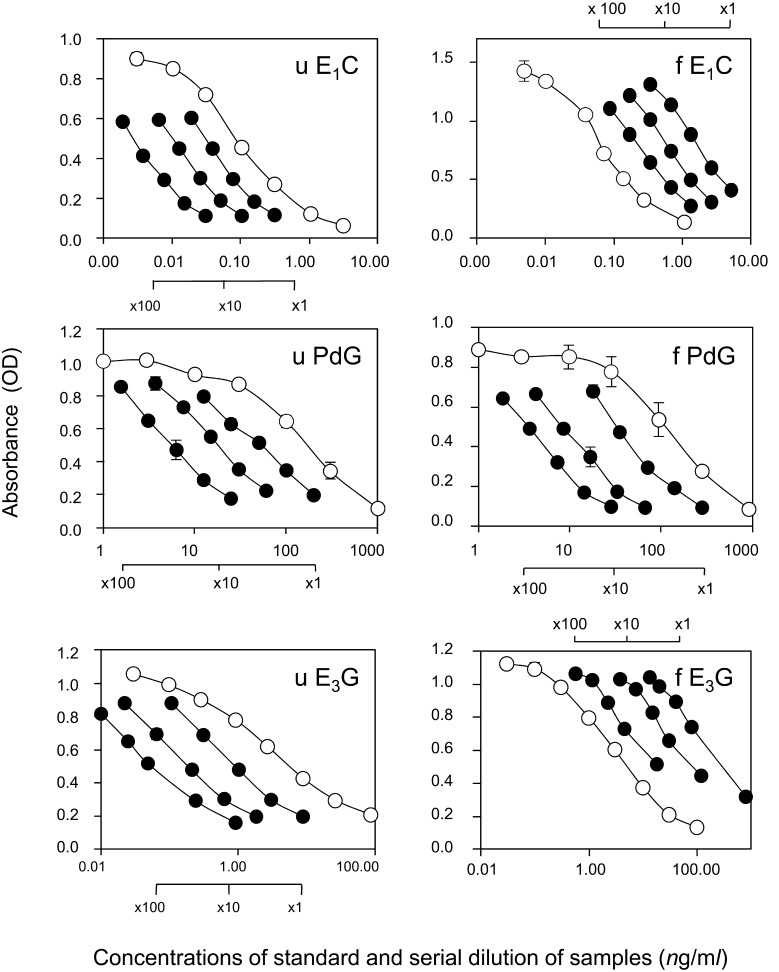
Specificity of the polyclonal antibodies against estrone-3-glucuronide-BSA and pregnanediol-3-glucuronide-BSA were tested. Cross-reactions of various steroids and their related metabolites with the E1C, PdG, and E3G antibodies are summarized in Table 1. Validations of the three EIAs were summarized in Table 2. The sensitivities of the EIAs for three steroids were 6.6 pg/ml, 2.1 ng/ml, and 0.35 ng/ml for E1C, PdG, E3G, respectively. Serial dilutions of urinary and fecal extractions from three randomly selected individuals produced displacement curves parallel to those of the standard curves in the EIAs (Fig. 1). The endogenous E3 levels of fecal extractions in female macaques were very low. Therefore, the displacement curves from fecal E3 extractions were parallel to those of the low dose-response standard curves (Fig. 1). The recoveries for the accuracy of each steroid from the urine or feces were assessed by determining the recovery of known amounts of added hormone. Average recoveries are listed in Table 2. Mean ± SD recovery values ranged from 94.1 ± 12.6% for urinary E1C and 108.0 ± 7.5% for fecal E1, 113.4 ± 23.8% for urinary PdG and 119.1 ± 25.4% for fecal pregnanediol, and 90.7 ± 11.7% for urinary E3G and 103.5 ± 18.0% for fecal E3. The intra-assay coefficients of variation for E1C, PdG, and E3G were 5.0, 5.4 and 6.2%, respectively. The inter-assay coefficients of variation for E1C, PdG, and E3G, with internal high and low controls, were 14.0 and 9.0% for E1C, 18.7 and 13.5% for PdG, and 18.2 and 13.6% for E3G, respectively.
Table 1. Crossreactivity (%) of relevant steroids in the three EIAs.
| E1C EIA |
PdG EIA |
E3G EIA |
|||
|---|---|---|---|---|---|
| Steroid | % | Steroid | % | Steroid | % |
| Estrone-3-glucuronide | 100.0 | Pregnanediol-3-glucuronide | 100.0 | Estriol-3-glucuronide | 100.0 |
| Estrone | 32.4 | Pregnanediol | 100.0 | Estriola) | 100.0 |
| Estrone-3-sulfatea) | 15.0 | 20α-OH-Progesterone | 74.7 | Estronea) | 5.5 |
| Estradiola) | 0.7 | Progesteronea) | 2.3 | Pregnenolonea) | 2.5 |
| Estradiol-3-glucuronidea) | 0.6 | 5β-Pregnane-3-α,20β-diola) | 0.8 | Testosteronea) | 1.2 |
| Cortisola) | 0.1 | 5β-Pregnane-3-β,20α-diola) | 0.2 | Estrone-3-sulfatea) | 0.7 |
| 5β-Pregnane-3-β-ol-20-onea) | 0.1 | ||||
| Pregnenolonea) | 0.1 | ||||
a) Data were provided by Dr. A. Kambegawa.
Table 2. Validation of three steroid EIAs.
| E1C | PdG | E3G | |||
|---|---|---|---|---|---|
| Sensitivitya) | 6.6 pg/ml | 2.1 ng/ml | 0.35 ng/ml | ||
| Linearity | Mean ± SD (%) | Urine | 89.0 ± 7.4 (1:20–320) | 112.8 ± 15.5 (1:2–32) | 86.6 ± 6.1 (1:6–50) |
| (dilution factor) | Feces | 107.9 ± 14.5 (1:1–32) | 95.5 ± 11.3 (1:1–8) | 94.4 ± 10.0 (1:1–60) | |
| Accuracy | Mean ± SD (%) | Urine | 94.1 ± 12.6 | 113.4 ± 23.8 | 90.7 ± 11.7 |
| y=ax + b (r2)b) | y=0.8276x + 0.0166 (r2=0.97) | y=0.9537x + 6.6217 (r2=0.93) | y=0.9564x − 0.2656 (r2=0.98) | ||
| Mean ± SD (%) | Feces | 108.0 ± 7.5 | 119.1 ± 25.4 | 103.5 ± 18.0 | |
| y=ax + b (r2)b) | y=0.9739x + 0.0084 (r2=1.00) | y=1.1837x + 0.7159 (r2=0.90) | y=1.187x − 0.4577 (r2=0.98) | ||
| Precision | Intra-assay CV (%) | 5.0 (n=712) | 5.4 (n=723) | 6.2 (n=452) | |
| Inter-assay CV (%) | Low control | 14.0 | 18.7 | 18.2 | |
| High control | 9.0 | 13.5 | 13.6 | ||
a) Determined from the mean-2SD of the absorbance (0 concentration). b) Observed amount=slope × added amount + intercept (r2).
Fig. 1.
Linearity of the three steroid metabolites. Standard curves and dose-response curves of serially diluted urine and feces. Each figure shows urinary E1C, fecal E1C, urinary PdG, fecal PdG, urinary E3G and fecal E3G. Closed circles (●) showed the displacement curves of serially diluted urine or fecal extracts from three randomly selected female Japanese macaques. They were generated parallel to the dose-response standard curves, which depicted open circles (○). Absorbance is depicted as means ± SD.
Evaluation of the simple extraction method
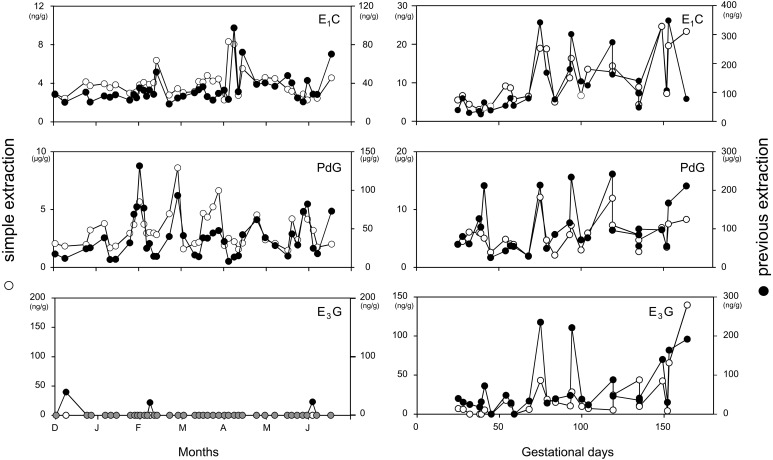
Our method was compared with the previous extraction method using 50% ethanol and diethyl ether, and the results are depicted in Fig. 2 (left, non-conceptive cycle; right, conceptive period). Fecal E1C and PdG levels could be measured using our simple extraction method within the detectable range. On the other hand, E3G levels in the fecal extract of the non-conceptive cycle and early pregnancy period were very low and almost undetectable in both extraction methods. Although the extraction rate of our method was 10–12% of the previous extraction method, the results of the assays showed a similar pattern in the E1C, PdG, and E3G profiles. The Spearman’s rank correlation coefficients ρ (r) for comparison of the two methods were 0.412 (df=43, P<0.01) for E1C, 0.632 (df=43, P<0.001) for PdG and 0.538 (df=16, P<0.05) for E3G from the fecal extraction of female Japanese macaques. The correlation coefficients for the relationship between two methods were found to be statistically significant.
Fig. 2.
Hormonal profiles of fecal E1C (top), PdG (middle) and E3G (bottom) during the non-conceptive menstrual cycle using the simple extraction method (○) and previous extraction method containing ethanol and diethyl ether (●) in a non-pregnant female Japanese macaque. Grey circles in the bottom panel indicate the overlapping of both data (left). Hormonal profiles of fecal E1C (top), PdG (middle), and E3G (bottom) during an entire pregnancy of a female Japanese macaque using the simple extraction method (○) and previous extraction method containing ethanol and diethyl ether (●) (right).
Hormonal profiles in urine and fecal samples
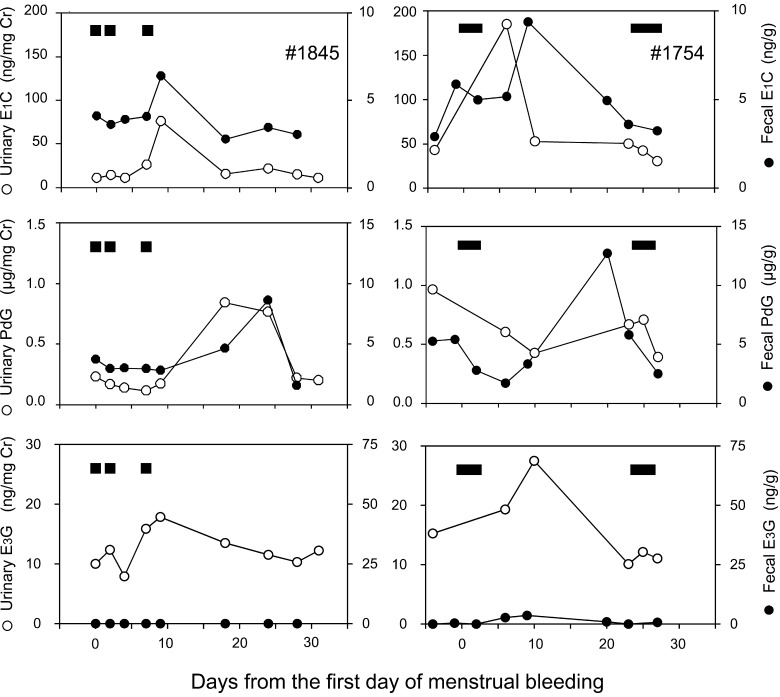
The representative profiles of urinary and fecal E1C, PdG, and E3G during a non-conceptive menstrual cycle of two females are illustrated in Fig. 3. Urinary E1C levels transitorily increased during the follicular phase, from baseline levels of 11.16–30.15 ng/mg creatinine (Cr) to preovulatory peak values of 75.93–184.87 ng/mg Cr. Then, urinary E1C levels decreased to the nadir. Urinary PdG levels were low during the follicular phase (0.10–0.25 µg/mg Cr), and increased after the E1C peak to reach their maximum levels of 0.71–1.40 µg/mg Cr during the mid-luteal phase. Thereafter, PdG levels decreased to their baseline levels. Urinary E3G levels gradually increased during the follicular phase from their baseline levels of 2.46–11.01 ng/mg Cr to their preovulatory peak values of 13.33–31.38 ng/mg Cr. Thereafter, urinary E3G levels decreased to their baseline levels.
Fig. 3.
Hormonal profiles of urinary (○) and fecal (●) E1C (top), PdG (middle) and E3G (bottom) during the non-conceptive menstrual cycles of two female Japanese macaques. The X-axis shows days from the first day of menstrual bleeding. Horizontal black bars at the upper part of each figure indicate menstrual bleeding.
Fecal E1C levels also increased 1.3 to 3-fold from their follicular phase levels of 1.92–3.60 ng/g to a preovulatory peak of 4.82–9.36 µg/g on approximately day 10, and thereafter gradually declined. Fecal PdG profiles showed a well-defined pattern throughout the menstrual cycle, with low levels of 1.02–3.02 µg/g during the follicular phase, and increased with a maximum of 5.43–12.69 µg/g in the luteal phase. Thus, the profiles of E1C and PdG showed similar fluctuating patterns between the urinary and fecal samples. On the other hand, fecal E3G levels were observed at low and barely detectable levels during the menstrual cycle.
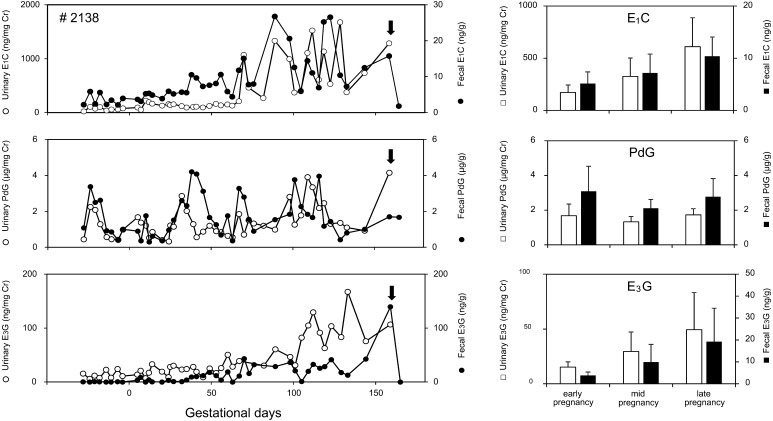
Profiles of E1C, PdG and E3G levels in urine and feces of one pregnant female during the entire gestation period are shown in Fig. 4 (left). Urinary and fecal E1C levels during the conceptive cycle gradually increased as gestation progressed until parturition. Urinary and fecal E3G levels were low during the early pregnancy period, particularly fecal E3G levels, which were barely detectable. Afterward, both urinary and fecal E3G levels were apparently increased during the late pregnancy until parturition. In comparison to E1C and E3G, urinary and fecal PdG showed a peak at early pregnancy, and then maintained a modest increase until parturition. Levels of all hormones decreased abruptly to the nadir within a few days of parturition. Figure 4 (right) illustrates the average urinary and fecal E1C, PdG and E3G levels during the early, mid, and late pregnancy in the six females. While urinary and fecal E1C and E3G increased as gestation progressed, PdG levels in urine and feces were maintained at nearly constant levels during gestation. The mean ± SD levels of urinary E1C during the early (E), mid (M) and late (L) pregnancy were 172.00 ± 72.36 (E), 326.34 ± 175.67 (M) and 611.28 ± 276.11 mg/mg Cr (L), and fecal E1C levels were 257.09 ± 112.05 (E), 359.33 ± 180.99 (M) and 518.85 ± 184.83 ng/g (L), (n=6), respectively. The mean urinary E3G levels were 15.27 ± 4.73 (E), 29.54 ± 17.82 (M) and 49.44 ± 33.85 ng/mg Cr (L), and fecal E3G levels were 7.72 ± 3.01 (E), 19.82 ± 16.23 (M) and 38.48 ± 30.53 ng/g (L), (n=6). The mean urinary PdG levels were 1.68 ± 0.68 (E), 1.32 ± 0.31 (M) and 1.73 ± 0.35 µg/mg Cr (L), and fecal PdG levels were 3.08 ± 1.45 (E), 2.11 ± 0.51 (M) and 2.78 ± 1.04 ng/g (L), (n=6), respectively.
Fig. 4.
Hormonal profiles of urinary (○) and fecal (●) E1C (top), PdG (middle) and E3G (bottom) during the full-term pregnancy of an individual female Japanese macaque. Arrows indicate the day of parturition. Day 0 indicates the estimated conception day (left). Mean (± SD) levels of urinary (□) and fecal (■) E1C (top), PdG (middle) and E3G (bottom) during the early, mid, and late pregnancy periods of six female Japanese macaques (right).
DISCUSSION
The present study described a simple and practical extraction method for measuring fecal steroid metabolites by EIAs in female Japanese macaques. As mentioned above (see Introduction), the concentrations of fecal steroid metabolites in non-human primates have been measured by immunoassays following their extraction, commonly with highly concentrated alcohol and volatile solvents [10, 15, 16, 18, 25, 29]. However, there is a risk that highly concentrated alcohol, detergent, or volatile solvents could interfere with the enzymatic and/or immunometric reaction. Therefore, Heistermann et al. [10] diluted the methanol extractant to avoid the risk before RIA. However, the complicated extraction process of fecal samples takes labor, and sometimes can lead to errors. To shorten the amount of labor involved by providing an easy procedure would decrease the artificial error and improve the working efficacy. Our extraction method, using aqueous buffer to measure fecal hormone metabolite, reduced the amount of steps for extraction and gave us accurate results.
Studies on Old World monkeys have focused exclusively on measuring steroids in the unconjugated form in excreta [4, 26]. Profiles of fecal extractions in the previous HPLC study suggest that there are at least 8–10 fecal P4 metabolites, and many of them contribute to the immunoreactive profile [20]. The previous study also suggested that to assess the C21 PdG-like metabolites provide useful information when applied to solubilized macaque feces [20]. Because the steroid metabolite form in excreta is not only single and different between urine and feces, using the appropriate antibody is important for the efficiency of the immunoassay. It is known that major E2 metabolites are two types of estrone monoconjugates (E1C) in urine and unconjugated E1 in feces. Progesterone (P4) metabolites were various 20α-hydroxy C21 compound-monoconjugates in urine and unconjugated pregnanediol in feces [6, 15, 21]. Therefore, we employed group-specific polyclonal antibodies against estrone-3-glucuronide-BSA, pregnanediol-3-glucuronide-BSA, and estriol-6-carboxymethyloxime-BSA for the EIAs raised by Dr. A. Kambegawa.
In the present study, the results of sensitivity, linearity, accuracy, and precision were quite satisfactory in the E1C, PdG and E3G EIAs. The profiles of E1C and PdG levels in the urine and feces showed a similar trend. The important difference between the urinary and fecal profiles was the large amount of P4 metabolites in the feces. The previous study described that the fecal profiles were superior in their correspondence to serum P4 when compared to that in urinary PdG, presumably because the fecal compartment contained the majority of hormone metabolites, and consequently, the fecal measurements were more accurate [21].
Because of our low sampling frequency, the lag times between hormone levels in urine and feces could not be detected; however, fecal peaks appeared slightly later than urinary peaks. Shideler et al. [20] performed an experiment to characterize the time course and metabolic rate of circulating E2 and P4 in their in vivo study of 14C labeled steroids. The observed lag times between hormone levels in feces and blood of macaques were 1.0–2.3 days behind in E2 and 1.3–2.0 days behind in P4 [21]. Likewise, in their results, lag times between testosterone levels in feces and blood of Japanese macaques showed, approximately, a 48 hr delay [3]. Wasser et al. also reported similar results in baboons [27, 28]. Similar to macaques, urinary PdG and E1C levels lagged behind that of serum by an average of one day in women [14].
Pregnanediol-3-glucuronide is the primary progesterone metabolite excreted by humans, and accounts for approximately 30% of P4 in blood [8]. In comparison to humans, macaques excrete very low levels of pregnanediol. The major P4 metabolite in macaques is a compound immunoreactively similar to 20α-OH-progesterone, which is believed to reflect the same ovarian activity as that of pregnanediol in other anthropoids [8].
Furthermore, the urinary and fecal E3G levels were low and barely detectable in the non-conceived cycle and early pregnancy period of Japanese macaques. It is not elucidated in the present study, but this may be because these hormones are released from the fetal zone and are of adrenal origin. Unlike most mammals, primates possess a fetal adrenal composed of a definitive zone and a fetal zone. A previous study reported that the fetal zone produces the precursor of E3, and the placenta then converts this precursor to E3. Estriol was the major estrogen during the last month of gestation, since the formation of E3 in anthropoid primates is largely dependent upon 16α-hydroxylase activities on fetal dehydroepiandrosterone before aromatization [9]. In this study, E3 levels increased during the late pregnancy period until parturition. Although further study is needed to assess this in more detail, the measurement of E3 levels in urine or feces can be considered as a reliable pregnancy diagnosis method in Japanese macaques.
In conclusion, the present study demonstrates a new extraction method using aqueous buffer for measuring fecal E1C, PdG, and E3G in female Japanese macaques. The method presented here is simple and practical, and has reliable applications to detect the reproductive status of Japanese macaques, and probably that of other non-human primates.
Acknowledgments
We are gratefully indebted to Dr. A. Kambegawa for providing helpful technical assistance to develop this method. We wish to thank the staff of the Center for Human Evolution Modeling Research, Primate Research Institute, Kyoto University, for supporting in sample collections and for their technical assistance: and Prof. T. Furuichi, Prof. C. Hashimoto, and Prof. M. Myowa for their constant encouragement that made this study possible. This study was financed by a Grant-in-Aid for Scientific Research to K.S. (19040012) from the Ministry of Education, Science, Sports, and Culture, Japan.
REFERENCES
- 1.Abraham G. E.1969. Solid-phase radioimmunoassay of estradiol-17 β. J. Clin. Endocrinol. Metab. 29: 866–870. doi: 10.1210/jcem-29-6-866 [DOI] [PubMed] [Google Scholar]
- 2.Bahr N. I., Palme R., Möhle U., Hodges J. K., Heistermann M.2000. Comparative aspects of the metabolism and excretion of cortisol in three individual nonhuman primates. Gen. Comp. Endocrinol. 117: 427–438. doi: 10.1006/gcen.1999.7431 [DOI] [PubMed] [Google Scholar]
- 3.Barrett G. M., Shimizu K., Bardi M., Mori A.2002. Fecal testosterone immunoreactivity as a non-invasive index of functional testosterone dynamics in male Japanese macaques (Macaca fuscata). Primates 43: 29–39. doi: 10.1007/BF02629574 [DOI] [PubMed] [Google Scholar]
- 4.Bamberg E., Mostl E., Patzl M., King G.1991. Pregnancy diagnosis by enzyme immunoassay of estrogens in feces from nondomestic species. J. Zoo Wildl. Med. 22: 73–77. [Google Scholar]
- 5.Behringer V., Deschner T.2017. Non-invasive monitoring of physiological markers in primates. Horm. Behav. 91: 3–18. doi: 10.1016/j.yhbeh.2017.02.001 [DOI] [PubMed] [Google Scholar]
- 6.Braasch H. V., Frederiksen M. C., Chatterton R. T., Jr.1988. Metabolism and pharmacokinetics of progesterone in the cynomolgus monkey (Macaca fascicularis). Steroids 52: 279–294. doi: 10.1016/0039-128X(88)90009-8 [DOI] [PubMed] [Google Scholar]
- 7.Czekala N. M., Benirschke K., McClure H., Lasley B. L.1983. Urinary estrogen excretion during pregnancy in the gorilla (Gorilla gorilla), orangutan (Pongo pygmaeus) and the human (Homo sapiens). Biol. Reprod. 28: 289–294. doi: 10.1095/biolreprod28.2.289 [DOI] [PubMed] [Google Scholar]
- 8.Czekala N. M., Shedelar S. E., Lasley B. L.1988. Comparisons of female reproductive hormone patterns in the Hominoids. pp. 117–122. In: Orang Utan Biology (Schuwartz, J. H. ed.), Oxford University Press, Oxford. [Google Scholar]
- 9.Faiman C., Reyes F. I., Winter J. S. D., Hobson W. C.1981. Endocrinology of pregnancy in apes. pp. 45–68. In: Reproductive Biology of the Great Apes (Graham, C. E. ed.), Academy Press, New York. [Google Scholar]
- 10.Heistermann M., Tari S., Hodges J. K.1993. Measurement of faecal steroids for monitoring ovarian function in New World primates, Callitrichidae. J. Reprod. Fertil. 99: 243–251. doi: 10.1530/jrf.0.0990243 [DOI] [PubMed] [Google Scholar]
- 11.Hodges J. K., Heistermann M.2011. Field endocrinology: monitoring hormonal changes in free-ranging primates. pp. 353–370. In: Field and Laboratory Methods in Primatology. A Practical Guide. 2nd ed. (Setchell, J. M. and Curtis, D. J. eds.), Cambridge University Press, Cambridge. [Google Scholar]
- 12.Lasley B. L.1985. Methods for evaluating reproductive function in exotic species. Adv. Vet. Sci. Comp. Med. 30: 209–228. [PubMed] [Google Scholar]
- 13.Lasley B. L., Kirkpatrick J. F.1991. Monitoring ovarian function in captive and free-ranging wildlife by means of urinary and fecal steroids. J. Zoo Wildl. Med. 22: 23–31. [Google Scholar]
- 14.O’Connor K. A., Brindle E., Holman D. J., Klein N. A., Soules M. R., Campbell K. L., Kohen F., Munro C. J., Shofer J. B., Lasley B. L., Wood J. W.2003. Urinary estrone conjugate and pregnanediol 3-glucuronide enzyme immunoassays for population research. Clin. Chem. 49: 1139–1148. doi: 10.1373/49.7.1139 [DOI] [PubMed] [Google Scholar]
- 15.O’Neill A. C., Fedigan L. M., Ziegler T. E.2004. Relationship between ovarian cycle phase and sexual behavior in female Japanese macaques (Macaca fuscata). Am. J. Phys. Anthropol. 125: 352–362. doi: 10.1002/ajpa.20024 [DOI] [PubMed] [Google Scholar]
- 16.Palme R., Touma C., Arias N., Dominchin M. F., Lepschy M.2013. Steroid extraction: Get the best out of fecal samples. Wien Tierrarztl Monat-Vet Med Austria 100: 238–246. [Google Scholar]
- 17.Reyes F. I., Winter J. S., Faiman C., Hobson W. C.1975. Serial serum levels of gonadotropins, prolactin and sex steroids in the nonpregnant and pregnant chimpanzee. Endocrinology 96: 1447–1455. doi: 10.1210/endo-96-6-1447 [DOI] [PubMed] [Google Scholar]
- 18.Risler L., Wasser S. K., Sackett G. P.1987. Measurement of excreted steroids in macaca nemestrina. Am. J. Primatol. 12: 91–100. doi: 10.1002/ajp.1350120105 [DOI] [PubMed] [Google Scholar]
- 19.Shideler S. E., Haggerty M. A., Lasley B. L.1989. The excretory time course and metabolic fate of ovarian and adrenal steroids in Macaca mulatta. Biol. Reprod. 40: 105. [Google Scholar]
- 20.Shideler S. E., Ortuño A. M., Morán F. M., Moorman E. A., Lasley B. L.1993. Simple extraction and enzyme immunoassays for estrogen and progesterone metabolites in the feces of Macaca fascicularis during non-conceptive and conceptive ovarian cycles. Biol. Reprod. 48: 1290–1298. doi: 10.1095/biolreprod48.6.1290 [DOI] [PubMed] [Google Scholar]
- 21.Shideler S. E., Shackleton C. H. L., Moràn F. M., Stauffer P., Lohstroh P. N., Lasley B. L.1993. Enzyme immunoassays for ovarian steroid metabolites in the urine of Macaca fascicularis. J. Med. Primatol. 22: 301–312. [PubMed] [Google Scholar]
- 22.Shimizu K.2005. Studies on reproductive endocrinology in non-human primates: application of non-invasive methods. J. Reprod. Dev. 51: 1–13. doi: 10.1262/jrd.51.1 [DOI] [PubMed] [Google Scholar]
- 23.Strier K. B., Ziegler T. E.1997. Behavioral and endocrine characteristics of the reproductive cycle in wild muriqui monkeys, Brachyteles arachnoides. Am. J. Primatol. 42: 299–310. [DOI] [PubMed] [Google Scholar]
- 24.Taussky H. H.1954. A microcolorimetric determination of creatine in urine by the Jaffe reaction. J. Biol. Chem. 208: 853–861. [PubMed] [Google Scholar]
- 25.Wallner B., Aspernig D., Millesi E., Machatschke I. H.2011. Non-lactating versus lactating females: a comparison of sex steroids, sexual coloration, and sexual behavior in Japanese macaques. Primates 52: 69–75. doi: 10.1007/s10329-010-0221-7 [DOI] [PubMed] [Google Scholar]
- 26.Wasser S. K., Monfort S. L., Wildt D. E.1991. Rapid extraction of faecal steroids for measuring reproductive cyclicity and early pregnancy in free-ranging yellow baboons (Papio cynocephalus cynocephalus). J. Reprod. Fertil. 92: 415–423. doi: 10.1530/jrf.0.0920415 [DOI] [PubMed] [Google Scholar]
- 27.Wasser S. K., Monfort S. L., Southers J., Wildt D. E.1994. Excretion rates and metabolites of oestradiol and progesterone in baboon (Papio cynocephalus cynocephalus) faeces. J. Reprod. Fertil. 101: 213–220. doi: 10.1530/jrf.0.1010213 [DOI] [PubMed] [Google Scholar]
- 28.Wasser S. K., Thomas R., Nair P. P., Guidry C., Southers J., Lucas J., Wildt D. E., Monfort S. L.1993. Effects of dietary fibre on faecal steroid measurements in baboons (Papio cynocephalus cynocephalus). J. Reprod. Fertil. 97: 569–574. doi: 10.1530/jrf.0.0970569 [DOI] [PubMed] [Google Scholar]
- 29.Ziegler T. E., Wittwer D. J.2005. Fecal steroid research in the field and laboratory: improved methods for storage, transport, processing, and analysis. Am. J. Primatol. 67: 159–174. doi: 10.1002/ajp.20175 [DOI] [PubMed] [Google Scholar]