Abstract
The bacterium Haemophilus parasuis (H. parasuis) is the primary cause of Glässer’s disease. Currently, there are no effective vaccines that can confer protection against all H. parasuis serovars. Therefore, the present study aimed to investigate the effect of tea polyphenols on growth, expression of virulence-related factors, and biofilm formation of H. parasuis, as well as to evaluate their protective effects against H. parasuis challenge. Our findings demonstrated that tea polyphenols can inhibit H. parasuis growth in a dose-dependent manner and attenuate the biofilm formation of H. parasuis. In addition, tea polyphenols exerted inhibitory effects on the expression of H. parasuis virulence-related factors. Moreover, tea polyphenols could confer protection against a lethal dose of H. parasuis and can reduce pathological tissue damage induced by H. parasuis. In summary, our findings demonstrated the promising use of tea polyphenols as a novel treatment for H. parasuis infection in pigs.
Keywords: biofilm, Haemophilus parasuis, protection, tea polyphenols, virulence-related factor
Haemophilus parasuis (H. parasuis) is a bacterial pathogen that colonizes the upper respiratory tract of pigs, thereby causing Glässer’s disease [15]. Glässer’s disease is characterized by fibrinous polyserositis, polyarthritis, and meningitis, which can lead to sudden death [4, 19]. In total, 15 H. parasuis serovars have been identified to date, with serovars 4 and 5 being the most prevalent worldwide, although more than 20% of the isolates remain to be serotyped [3, 7]. The pathogenesis of H. parasuis infection remains poorly understood and represents a challenge for the development of preventative and control measures, making Glässer’s disease a significant problem in the pig industry.
Tea is the most popular beverage in the world and comprises an aqueous infusion of dried leaves from the plant Camellia sinensis [13]. In particular, green tea is rich in polyphenols, which have been shown to have numerous important biological functions [29]. Previous research has shown that oral administration of green tea polyphenols can prevent the progression of abdominal aortic aneurysm mediated by its anti-inflammatory effects [21] and they can inhibit colorectal tumorigenesis in azoxymethane-treated F344 rats [8]. H. parasuis has been demonstrated to be capable of biofilm formation [11]. In addition, tea polyphenols can inhibit the growth of Escherichia coli by promoting endogenous oxidative stress [24], attenuating the expression of virulence factors, and preventing biofilm formation of Fusobacterium nucleatum [2]. Tea polyphenols can reduce disease pathogenesis and suppress inflammatory responses in interleukin-2-deficient mouse models for chronic inflammatory disease [23]. In addition, tea polyphenols were demonstrated to attenuate the pathogenicity of Pseudomonas aeruginosa [26]. However, the effects of tea polyphenols on H. parasuis infection remain to be fully understood.
The present study aimed to evaluate the influence of tea polyphenols on H. parasuis, including their effects on cell integrity, virulence-related factors, and biofilm formation. Our findings demonstrated that tea polyphenols could inhibit growth, reduce biofilm formation, and downregulate the expression of virulence-related factors in H. parasuis SH0165. In addition, tea polyphenols were demonstrated to confer protection against a lethal dose of H. parasuis and reduce pathological tissue damage induced by H. parasuis. Therefore, the use of tea polyphenols could serve as a novel strategy to control and treat pig infections caused by H. parasuis.
MATERIALS AND METHODS
Bacterial strain, growth conditions, and tea polyphenols
The H. parasuis SH0165 strain was isolated from the lung of a commercial pig with arthritis, fibrinous polyserositis, hemorrhagic pneumonia, and meningitis [6]. The SH0165 strain was grown in tryptic soy broth (TSB; Difco Laboratories, Detroit, MI, U.S.A.) or tryptic soy agar (TSA; Difco Laboratories) supplemented with 10 µg/ml NAD (Sigma, St. Louis, MO, U.S.A.) and 10% newborn calf serum (Gibco, New York, NY, U.S.A.) at 37°C.
Tea polyphenols were obtained from the National Institute for Food and Drug Control (Beijing, China). A stock solution of tea polyphenols was prepared by dissolving 160 mg of powder in 1 ml of ethanol (160 mg/ml). The working solution was prepared by diluting the stock solution with culture medium at the appropriate ratio.
Determination of minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC)
The MIC and MBC of tea polyphenols against H. parasuis were determined as previously described [9, 20]. The MIC of tea polyphenols was defined as the minimum concentration at which no bacterial growth can be observed. To determine the MBC, 10-µl aliquots from each well of the plates with no visible growth were spread on TSA plates for 48 hr at 37°C. The MBC of the tea polyphenols were determined by measuring the lowest concentration at which no colony formation of H. parasuis was detected.
Determination of the kinetics of the bactericidal effect of tea polyphenols against H. parasuis
The kinetics of the bactericidal effects of tea polyphenols against H. parasuis was explored as previously described [25]. Briefly, H. parasuis (1 × 108 CFU/ml) and tea polyphenols (0, 40, 80, 160 and 320 µg/ml) were co-cultured in tubes at 37°C. Bacterial samples were collected at 0, 1, 2, 3, 4 and 5 hr. Samples were diluted in sterile phosphate buffered saline (PBS) and subsequently plated onto TSA plates. All plates were incubated for 24 hr at 37°C. The number of colonies was counted, and kinetic curves were constructed by plotting the log10 CFU/ml vs. time over a 5-hr period.
Biofilm susceptibility assay
The effects of tea polyphenols on biofilm formation by H. parasuis were determined by performing glass tube biofilm assay as previously described with some modifications [11]. Briefly, borosilicate tubes were filled with 5 ml of TSB containing 10 µg/ml NAD, 10% newborn calf serum, and 1% inoculum. Tubes were then incubated with tea polyphenols at concentrations of 0, 80, 160 or 320 µg/ml at 37°C for 16 hr with circular agitation at a speed of 150 rpm/min. Afterwards, the contents of each tube were removed, and the tubes were stained with 1% Hucker crystal violet solution at 37°C for 15 min. Then, the dye solution was removed from the glass tubes, and the glass tubes were washed with distilled water for 10 min until no color was observed in the flowing water. Excess water was removed from the glass tubes with tissue paper. In addition, the effects of tea polyphenols on biofilm formation were examined by performing a quantitative assay [17]. Briefly, we evaluated the effects of tea polyphenols on biofilm formation by treatment of H. parasuis in the wells of a 96-well microplate with tea polyphenols (80, 160 or 320 µg/ml) for 2 hr at 37°C. Wells treated with PBS were used as controls. Following treatment with tea polyphenols, H. parasuis was cultured for 16 hr, and the resulting culture was diluted with TSB to obtain an OD660 of 0.1. The samples (200 µl) were added to treated wells of the 96-well microplate. After incubation for 16 hr at 37°C, free-floating bacteria and spent media were removed using a 26-g needle. The wells were washed thrice with PBS, after which the H. parasuis biofilms were stained with 0.05% crystal violet (100 µl) for 30 min. Afterwards, wells were washed five times with PBS to remove unbound crystal violet dye and subsequently dried for 3 hr at 37°C. Afterwards, 100 µl of 95% (v/v) ethanol was added to each well, and the plate was shaken for 30 min. The absorbance at 550 nm was measured.
Analysis of H. parasuis cellular integrity by transmission electron microscopy (TEM)
The cellular integrity of H. parasuis was examined by transmission electron microscopy as previously described with minor modifications [17]. Briefly, H. parasuis was grown in TSB and harvested by centrifugation at 12,000 rpm for 10 min and subsequently washed thrice with sterile PBS. Bacteria were then suspended in sterile PBS to obtain an OD of 0.6 and incubated with tea polyphenols at a concentration of 160 µg/ml at 37°C for 5 hr. Cells were fixed with 0.1 M cacodylate buffer (pH 7) containing 5% glutaraldehyde and 0.15% ruthenium red at 37°C for 5 hr. Then, bacteria were reacted with polycationic ferritin (1 mg/ml). Thin sections were observed under a Tecnai G2 20 TWIN transmission electron microscope (FEI, Portland, OR, U.S.A.).
Determination of virulence related factor expression by quantitative RT-PCR
To explore the effects of tea polyphenols on the expression of virulence-related factors, H. parasuis was grown to the early-log phase. Then, tea polyphenols were added at 1/2 MIC (160 µg/ml) and co-cultured at 37°C for 5 hr. Bacterial cells were collected, and total RNA was extracted using the GenElute Total RNA Purification Kit (Sigma) according to the manufacturer’s instructions. Total RNA from H. parasuis was reverse-transcribed into cDNA using reverse transcriptase (TaKaRa). Expression levels were quantified using the SYBR Green PCR Kit (TaKaRa) according to the manufacturer’s instructions. The 16S rRNA gene was used as the internal control. Primer sequences used for quantitative RT-PCR are listed in Table 1.
Table 1. Primers used for qRT-PCR.
| Gene | Nucleotide sequence (5ʹ–3ʹ) | Tm (°C) |
|---|---|---|
| 16S RNA | Forward TGAAGTCGGAATCGCTAGTA | 53.4 |
| Reverse CCTACGGTTACCTTGTTACG | 55.4 | |
| ArcA | Forward GGAGGAGGAGGAAGAGGAGATA | 54.0 |
| Reverse AGGACTGAGAAGATGCCACTAC | 57.7 | |
| ClpP | Forward ACAACAAACCAGCACTGCAC | 52.1 |
| Reverse CTGCCTGGTACTGCTCTTCC | 55.8 | |
| RfaE | Forward GAAGGAGAAGAGGAGGCTGTT | 55.8 |
| Reverse AGATTGTGAACCTGTGGAGAGT | 54.0 | |
| RfaD | Forward TCTGCATGAGCTTTGTGCAAG | 54.0 |
| Reverse ACAGGGCAGACTCGAATTCAAC | 54.0 | |
| OmpP2 | Forward AGTAACCATCTCTGTGCAGTGT | 59.5 |
| Reverse TCTTATCATCATGTCCAGGAAC | 50.2 | |
| OmpP5 | Forward CGCTCTTCTGCCTACTGCACTTC | 55.8 |
| Reverse CTGTCCCTCGGCTTTGACATT | 52.1 | |
| GalU | Forward CCAGGAACCCAGCTATGAAC | 57.7 |
| Reverse CTGCACAGCCTCGACATT | 52.1 | |
| GalE | Forward CAGAGCCAGGAAGAGACT | 52.1 |
| Reverse GACCAGCACAGGAATGAG | 57.7 |
Determination of the protective effects of tea polyphenols against H. parasuis challenge
The present study was carried out in strict accordance with the recommendations by China’s Regulations for the Administration of Affairs Concerning Experimental Animals 1988, and Hubei Regulations for the Administration of Affairs Concerning Experimental Animals 2005. The protocol was approved by China Hubei Province Science and Technology Department [permit number SYXK (ER) 2010-0029]. All experimental animals were euthanized at the end of the experiments.
Female BALB/c mice (five to six weeks old) were purchased from the Laboratory Animal Center of the Center for Disease Control of Hubei Province (Wuhan, China). The mice were divided randomly into four treatment groups (12 mice per group). Three groups received were intragastrically administered with tea polyphenols at concentrations of 80, 160 and 320 µg/ml. The fourth group served as the negative control group and received PBS alone. After 30 min, all mice were challenged intraperitoneally with a lethal dose of 1.0 × 109 CFU of the H. parasuis SH0165 strain. All mice were monitored for 7 days after the challenge. The morbidity and mortality rates were recorded.
Histopathology
The lungs and brains of all mice that survived the H. parasuis SH0165 challenge were collected and fixed by immersion in 10% neutral-buffered formalin and embedded in paraffin. The 4-µm-wide tissue sections were stained with hematoxylin-eosin (H&E) based on a standard protocol and examined using light microscopy.
Statistical analysis
Experimental data were expressed as mean ± SD. Differences between two groups was analyzed using the two-tailed Student’s t-test. Survival analysis was performed using the log-rank test. P<0.05 was considered statistically significant. *,P<0.05 and **, P<0.01.
RESULTS
Tea polyphenols inhibit the growth of H. parasuis in vitro
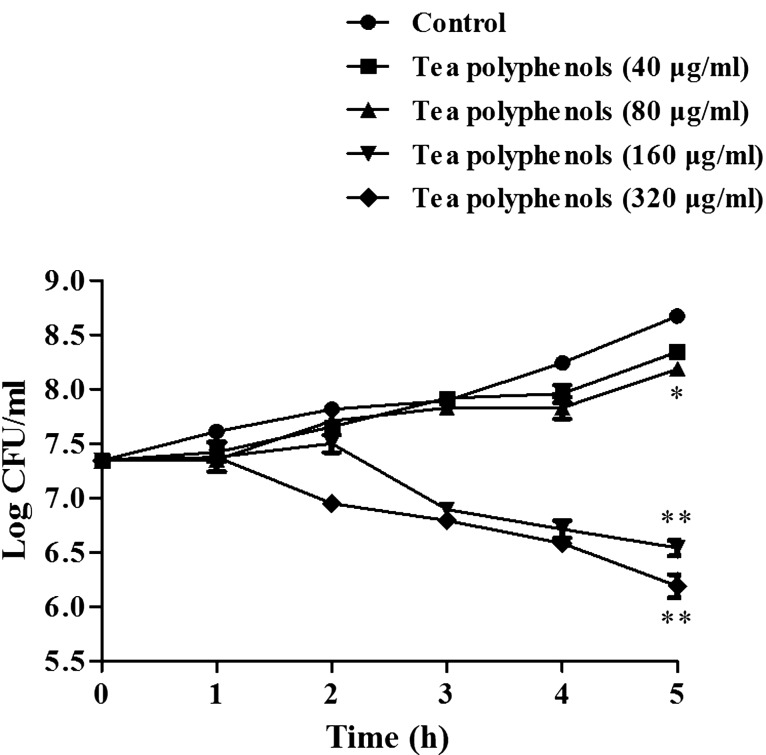
The results demonstrated that the tea polyphenols exhibited antibacterial activity against H. parasuis SH0165 (Table 2). The tea polyphenols had MIC and MBC values of 160 µg/ml and 320 µg/ml, respectively (Table 2). The kinetics of the antimicrobial effects of tea polyphenols against H. parasuis SH0165 indicated that tea polyphenols exerted a strong dose-dependent bactericidal effect against H. parasuis SH0165. In particular, H. parasuis SH0165 growth was significantly inhibited at a concentration of 160 µg/ml tea polyphenols over the 5-hr incubation period relative to the original inoculum (P<0.05) (Fig. 1).
Table 2. Minimal inhibitory concentration (MIC) and minimal bactericidal concentration (MBC) values of tea polyphenols for H. parasuis.
| Medium | Compound | MIC (µg/ml) | MBC (µg/ml) |
|---|---|---|---|
| TSB | Tea polyphenols | 160 | 320 |
Fig. 1.
Kinetics of the antimicrobial effects of tea polyphenols on H. parasuis. H. parasuis and tea polyphenols (0, 40, 80, 160 and 320 µg/ml) were co-cultured for 0, 1, 2, 3, 4 and 5 hr. The number of colonies was counted, and kinetics curves were constructed. Results are expressed as the mean ± SD of three independent experiments. ‘*’ indicates statistical significance at P<0.05 (vs. Control). ‘**’ indicates statistical significance at P<0.01 (vs. Control).
Tea polyphenols inhibit the formation of H. parasuis biofilms
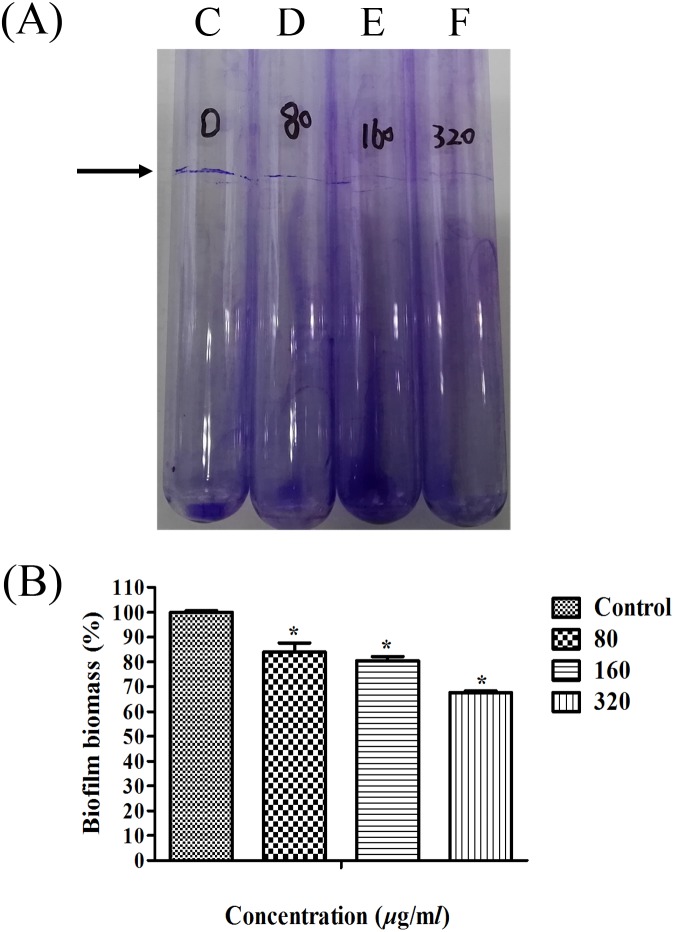
The effects of tea polyphenols on H. parasuis biofilm formation were evaluated by performing glass tube biofilm assay and the quantitative assay. Results indicated that tea polyphenols significantly reduced the formation of H. parasuis biofilms at concentrations of 80, 160 and 320 µg/ml relative to the control (0 µg/ml) (P<0.05) in a dose-dependent manner (Fig. 2).
Fig. 2.
Effect of tea polyphenols on H. parasuis biofilm formation based on glass tube biofilm assay (A) and quantitative assay (B). A: H. parasuis was incubated with tea polyphenols at concentrations of 0, 80, 160 and 320 µg/ml, and the tubes were stained with crystal violet solution. C: Control; D: H. parasuis treated with 80 µg/ml tea polyphenols; E: H. parasuis treated with 160 µg/ml tea polyphenols; F: H. parasuis treated with 320 µg/ml tea polyphenols. Arrow indicates biofilm formation at the air-liquid interface. B: A relative value of 100% was assigned to control. Data are expressed as the mean ± SD of three independent experiments. ‘*’ indicates statistical significance at P<0.05.
Tea polyphenols affect the cellular integrity of H. parasuis
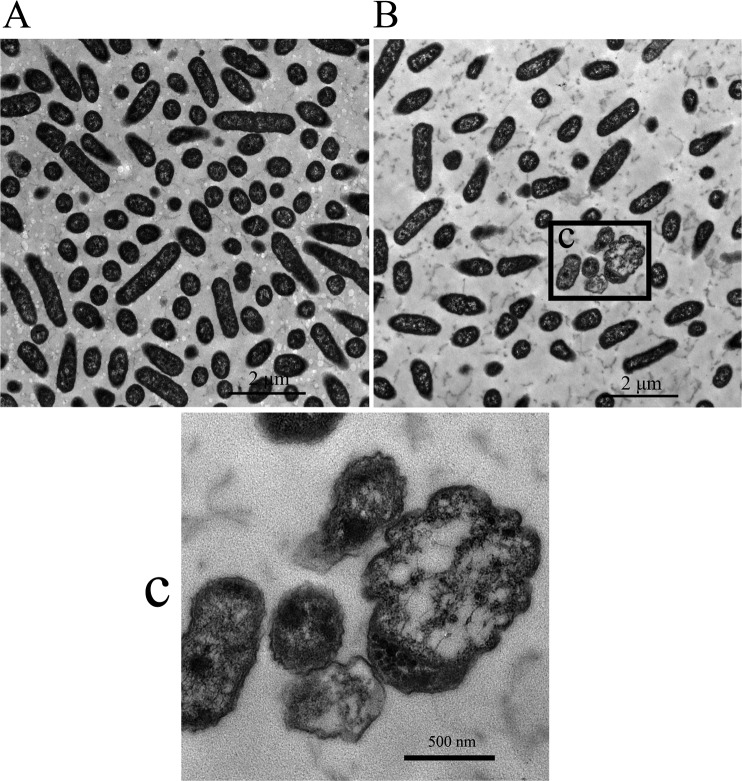
Cell ultrastructure was analyzed by transmission electron microscopy to determine the effect of tea polyphenols on the cellular integrity of H. parasuis. H. parasuis cells treated with tea polyphenols at a concentration of 160 µg/ml showed evident damage to the cell ultrastructure relative to those of negative control cells (Fig. 3). Treated cells displayed severe cellular damage, which led to disruption of the cell wall and cytoplasmic membrane, accompanied by the leakage of cytoplasmic inclusions (Fig. 3B and 3C).
Fig. 3.
Analysis of H. parasuis cellular integrity by transmission electron microscopy. (A) Control: untreated bacteria. (B) Bacteria treated with tea polyphenols (160 µg/ml). (C): High-magnification image of the indicated area.
Tea polyphenols inhibit the expression of virulence-related factors of H. parasuis in vitro
To determine the effect of tea polyphenols on virulence-related factors, we evaluated the expression of the virulence related factors ArcA, ClpP, RfaE, RfaD, OmpP2, OmpP5, GalU and GalE in H. parasuis SH0165. The results showed that tea polyphenols at 80 µg/ml downregulated the expression of ArcA, ClpP, RfaE, OmpP2, OmpP5 and GalE by greater than 40% relative to the untreated control in TSB medium (P<0.05) (Fig. 4). Furthermore, tea polyphenols significantly inhibited the expression of GalU by around 16% (Fig. 4). However, tea polyphenols significantly upregulated RfaD expression relative to the untreated control (P<0.05) (Fig. 4).
Fig. 4.
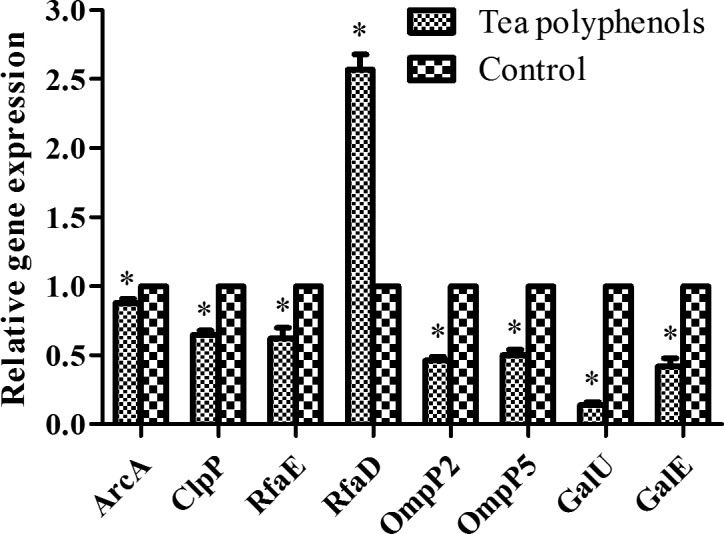
Effect of tea polyphenols on virulence-related factors of H. parasuis. H. parasuis was treated with tea polyphenols at 1/2 MIC (160 µg/ml), and the expression of virulence-related factors (ArcA, ClpP, RfaE, RfaD, OmpP2, OmpP5, GalU, and GalE) of H. parasuis were determined. Data are expressed as the mean ± SD of three independent experiments. ‘*’ indicates significance at P<0.05.
Tea polyphenols exert protective effects against H. parasuis challenge
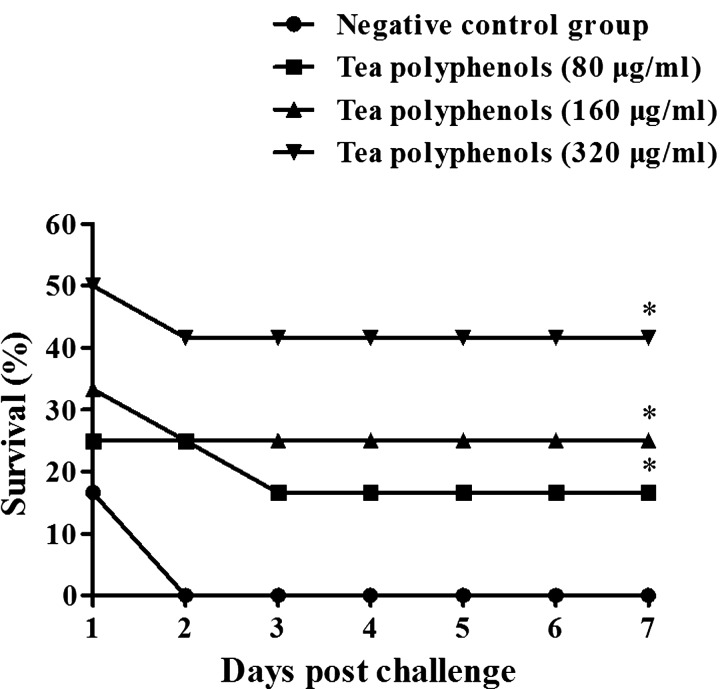
The protective effects of tea polyphenols were assessed by evaluating survival in a mouse model following lethal dose challenge with H. parasuis. After intragastrical administration of tea polyphenols for 30 min, mice were challenged with a lethal dose of 1.0 × 109 CFU H. parasuis SH0165. Results showed that mice administered with tea polyphenols had higher survival rates compared to mice in the negative control group (P<0.05) (Fig. 5). No protective effects were observed in the negative control group (Fig. 5), and all mice died within 48 hr of the challenge. Mice administered with 80 or 160 µg/ml tea polyphenols presented mild clinical symptoms associated with H. parasuis infection. However, the mice administered with 320 µg/ml tea polyphenols displayed no clinical symptoms (data not shown).
Fig. 5.
Survival rates of mice administered with tea polyphenols (80, 160, and 320 µg/ml) via intragastrical administration following H. parasuis SH0165 challenge. ‘*’ indicates statistical significance at P<0.05 (vs. Negative control group).
Histopathology analysis
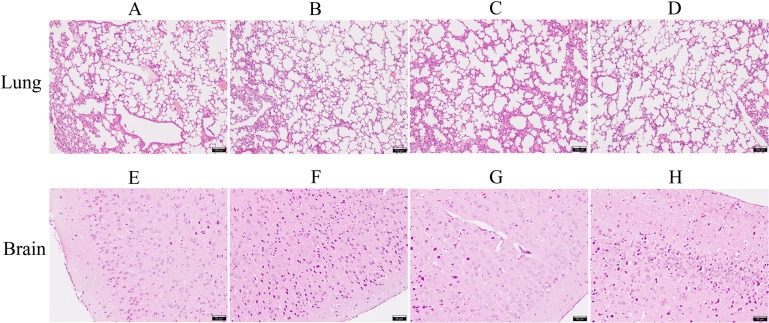
To examine the protective effects of tea polyphenols against the development of disease, we analyzed tissue pathologies of the lung and brain samples. Results showed that lung tissues from the negative control group presented extensive edema with fibroblast proliferation and connective tissue formation (Fig. 6A). Brain tissues from the negative control group exhibited meningitis with meningeal edema and detachment, as well as inflammatory cell exudation (Fig. 6E). However, only minor pathological changes were detected in the lung and brain tissues of animals treated with tea polyphenols at the concentrations of 80, 160 and 320 µg/ml (Fig. 6B, 6C, 6D, 6F, 6G and 6H).
Fig. 6.
Representative lung and brain sections of mice from different treatment groups. (A) and (E): negative control group. (B) and (F): mice administered with 80 µg/ml tea polyphenols. (C) and (G): mice administered with 160 µg/ml tea polyphenols. (D) and (H): mice administered with 320 µg/ml tea polyphenols. Bars=100 µm.
DISCUSSION
In recent years, H. parasuis has emerged as one of the most serious respiratory bacterial pathogens that infect livestock worldwide, and H. parasuis infections have caused substantial economic loss [7]. In the present study, tea polyphenols were demonstrated to confer protective effects against a lethal dose of H. parasuis, thereby suggesting that administration with tea polyphenols could serve as a novel strategy for disease control and treatment of H. parasuis infections.
In the present study, tea polyphenols were dissolved in ethanol, thereby raising concern on the potential effects of ethanol in the experiments. Consistent with previous reports [14, 22], the working concentration of tea polyphenols was obtained by diluting the stock solution with very high dilutions using the culture medium. Therefore, the effects of ethanol on the bacterial were considered negligible.
Biofilms are sessile communities of bacterial cells enclosed in a self-generated extracellular polysaccharide matrix, which subsequently conglutinates to a biotic or abiotic surface [11]. In recent years, bacterial biofilms produced by microbial pathogens have become an important focus of scientific research [18]. Previous studies showed that Staphylococcus aureus causes chronic infections through biofilm formation [16]. Candida albicans causes catheter-related bloodstream infections via the formation of biofilms, which exhibit increased tolerance to antimicrobials [1]. H. parasuis serovar 5 has been reported to exhibit a high degree of biofilm formation [11] and are considered highly virulent [12]. The H. parasuis SH0165 strain is classified under serovar 5 [3]. In a recent study, we used H. parasuis SH0165 as the model organism because of its ability to form biofilms [11]. Our findings showed that tea polyphenols can inhibit H. parasuis biofilm formation and can thus be effective for the treatment of chronic infections caused by H. parasuis.
Virulence-related factors play important roles in bacterial pathogenesis. Previous studies showed that deletion of the ArcA gene increased bacterial sensitivity to porcine serum and produced lower biofilm mass [5]. The ClpP gene is essential for stress tolerance and acts a negative regulator of biofilm formation in H. parasuis [10]. Deletion of the RfaE gene can attenuate serum resistance, adherence, and invasion of porcine kidney epithelial cells [28]. RfaD overexpression in the ΔompP2 mutant of H. parasuis could enhance adherence and invasion to PUVEC and PK-15 cells [27]. Deletion of GalU resulted in impaired biofilm formation, whereas GalE mutants produced more biofilm mass compared with wild-type H. parasuis [30]. Therefore, we investigated the mRNA expression of these important virulence-related factors (ArcA, ClpP, RfaE, RfaD, OmpP2, OmpP5, GalU and GalE) following treatment with tea polyphenols. Our results showed that tea polyphenols could downregulate the mRNA expression of ArcA, ClpP, RfaE, OmpP2, OmpP5, GalU and GalE to different degrees. Notably, RfaD mRNA levels were significantly upregulated by treatment with tea polyphenols. The mechanism by which tea polyphenols induce RfaD upregulation warrants further study.
In this study, mice were administered with tea polyphenols via intragastrical administration for 30 min, after which increased plasma concentrations of the tea polyphenols were detected. Therefore, we selected 30 min as the treatment period for H. parasuis SH0165 challenge. Piglets were used as the infection model and were challenged by intranasal inoculation to mimic the natural infection route [6]. Mice were selected as the infection model for evaluating the protective effects of tea polyphenols. Consistent with the methods of our previous research, mice were challenged via intraperitoneal injection [7].
Our results demonstrated that tea polyphenols can inhibit growth, attenuate biofilm formation, and downregulate the expression of virulence-related factors of H. parasuis SH0165. In addition, tea polyphenols can confer protection against H. parasuis challenge and reduce infection-associated tissue damage. Our findings suggested that tea polyphenols exhibit potential as a novel anti-bacterial compounds for the prevention and control of H. parasuis infection.
Acknowledgments
We thank Pei Zhang and Anna Du from the core facility and technical support at the Wuhan Institute of Virology for their help in generating the TEM micrographs. This research is supported by science and technology research program of Hubei Provincial Department of Education (No. Q20171703).
REFERENCES
- 1.Akbari F., Kjellerup B. V.2015. Elimination of Bloodstream Infections Associated with Candida albicans Biofilm in Intravascular Catheters. Pathogens 4: 457–469. doi: 10.3390/pathogens4030457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ben Lagha A., Haas B., Grenier D.2017. Tea polyphenols inhibit the growth and virulence properties of Fusobacterium nucleatum. Sci. Rep. 7: 44815. doi: 10.1038/srep44815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cai X., Chen H., Blackall P. J., Yin Z., Wang L., Liu Z., Jin M.2005. Serological characterization of Haemophilus parasuis isolates from China. Vet. Microbiol. 111: 231–236. doi: 10.1016/j.vetmic.2005.07.007 [DOI] [PubMed] [Google Scholar]
- 4.Correa-Fiz F., Galofre-Mila N., Costa-Hurtado M., Aragon V.2017. Identification of a surface epitope specific of virulent strains of Haemophilus parasuis. Vet. Microbiol. 198: 116–120. doi: 10.1016/j.vetmic.2016.12.015 [DOI] [PubMed] [Google Scholar]
- 5.Ding L., Wen X., He L., Yan X., Wen Y., Cao S., Huang X., Wu R., Wen Y.2016. The arcA gene contributes to the serum resistance and virulence of Haemophilus parasuis serovar 13 clinical strain EP3. Vet. Microbiol. 196: 67–71. doi: 10.1016/j.vetmic.2016.10.011 [DOI] [PubMed] [Google Scholar]
- 6.Fu S., Zhang M., Xu J., Ou J., Wang Y., Liu H., Liu J., Chen H., Bei W.2013. Immunogenicity and protective efficacy of recombinant Haemophilus parasuis SH0165 putative outer membrane proteins. Vaccine 31: 347–353. doi: 10.1016/j.vaccine.2012.11.003 [DOI] [PubMed] [Google Scholar]
- 7.Guo L., Xu L., Wu T., Fu S., Qiu Y., Hu C. A., Ren X., Liu R., Ye M.2017. Evaluation of recombinant protein superoxide dismutase of Haemophilus parasuis strain SH0165 as vaccine candidate in a mouse model. Can. J. Microbiol. 63: 312–320. doi: 10.1139/cjm-2016-0671 [DOI] [PubMed] [Google Scholar]
- 8.Hao X., Xiao H., Ju J., Lee M. J., Lambert J. D., Yang C. S.2017. Green Tea Polyphenols Inhibit Colorectal Tumorigenesis in Azoxymethane-Treated F344 Rats. Nutr. Cancer 69: 623–631. doi: 10.1080/01635581.2017.1295088 [DOI] [PubMed] [Google Scholar]
- 9.Holla G., Yeluri R., Munshi A. K.2012. Evaluation of minimum inhibitory and minimum bactericidal concentration of nano-silver base inorganic anti-microbial agent (Novaron®) against streptococcus mutans. Contemp. Clin. Dent. 3: 288–293. doi: 10.4103/0976-237X.103620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang J., Wang X., Cao Q., Feng F., Xu X., Cai X.2016. ClpP participates in stress tolerance and negatively regulates biofilm formation in Haemophilus parasuis. Vet. Microbiol. 182: 141–149. doi: 10.1016/j.vetmic.2015.11.020 [DOI] [PubMed] [Google Scholar]
- 11.Jin H., Zhou R., Kang M., Luo R., Cai X., Chen H.2006. Biofilm formation by field isolates and reference strains of Haemophilus parasuis. Vet. Microbiol. 118: 117–123. doi: 10.1016/j.vetmic.2006.07.009 [DOI] [PubMed] [Google Scholar]
- 12.Kielstein P., Rapp-Gabrielson V. J.1992. Designation of 15 serovars of Haemophilus parasuis on the basis of immunodiffusion using heat-stable antigen extracts. J. Clin. Microbiol. 30: 862–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Y. F., Ouyang S. H., Chang Y. Q., Wang T. M., Li W. X., Tian H. Y., Cao H., Kurihara H., He R. R.2017. A comparative analysis of chemical compositions in Camellia sinensis var. puanensis Kurihara, a novel Chinese tea, by HPLC and UFLC-Q-TOF-MS/MS. Food Chem. 216: 282–288. doi: 10.1016/j.foodchem.2016.08.017 [DOI] [PubMed] [Google Scholar]
- 14.Lombardo Bedran T. B., Morin M. P., Palomari Spolidorio D., Grenier D.2015. Black tea extract and its theaflavin derivatives inhibit the growth of periodontopathogens and modulate interleukin-8 and β-defensin secretion in oral epithelial cells. PLoS One 10: e0143158. doi: 10.1371/journal.pone.0143158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Macedo N., Cheeran M. C., Rovira A., Holtcamp A., Torremorell M.2017. Effect of enrofloxacin on Haemophilus parasuis infection, disease and immune response. Vet. Microbiol. 199: 91–99. doi: 10.1016/j.vetmic.2016.12.032 [DOI] [PubMed] [Google Scholar]
- 16.Moormeier D. E., Bayles K. W.2017. Staphylococcus aureus biofilm: a complex developmental organism. Mol. Microbiol. 104: 365–376. doi: 10.1111/mmi.13634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morin M. P., Bedran T. B., Fournier-Larente J., Haas B., Azelmat J., Grenier D.2015. Green tea extract and its major constituent epigallocatechin-3-gallate inhibit growth and halitosis-related properties of Solobacterium moorei. BMC Complement. Altern. Med. 15: 48. doi: 10.1186/s12906-015-0557-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Percival S. L.2017. Importance of biofilm formation in surgical infection. Br. J. Surg. 104: e85–e94. doi: 10.1002/bjs.10433 [DOI] [PubMed] [Google Scholar]
- 19.Prüller S., Turni C., Blackall P. J., Beyerbach M., Klein G., Kreienbrock L., Strutzberg-Minder K., Kaspar H., Meemken D., Kehrenberg C.2016. Towards a standardized method for broth microdilution susceptibility testing of haemophilus parasuis. J. Clin. Microbiol. 55: 264–273. doi: 10.1128/JCM.01403-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sandalakis V., Chochlakis D., Goniotakis I., Tselentis Y., Psaroulaki A.2014. Minimum inhibitory concentration distribution in environmental Legionella spp. isolates. J. Water Health 12: 678–685. doi: 10.2166/wh.2014.217 [DOI] [PubMed] [Google Scholar]
- 21.Setozaki S., Minakata K., Masumoto H., Hirao S., Yamazaki K., Kuwahara K., Ikeda T., Sakata R.2017. Prevention of abdominal aortic aneurysm progression by oral administration of green tea polyphenol in a rat model. J. Vasc. Surg. 65: 1803–1812.e2. doi: 10.1016/j.jvs.2016.06.003 [DOI] [PubMed] [Google Scholar]
- 22.Tshivhandekano I., Ntushelo K., Ngezimana W., Tshikalange T. E., Mudau F. N.2014. Chemical compositions and antimicrobial activities of Athrixia phylicoides DC. (bush tea), Monsonia burkeana (special tea) and synergistic effects of both combined herbal teas. Asian Pac. J. Trop. Med. 7S1: S448–S453. doi: 10.1016/S1995-7645(14)60273-X [DOI] [PubMed] [Google Scholar]
- 23.Varilek G. W., Yang F., Lee E. Y., deVilliers W. J., Zhong J., Oz H. S., Westberry K. F., McClain C. J.2001. Green tea polyphenol extract attenuates inflammation in interleukin-2-deficient mice, a model of autoimmunity. J. Nutr. 131: 2034–2039. doi: 10.1093/jn/131.7.2034 [DOI] [PubMed] [Google Scholar]
- 24.Xiong L. G., Chen Y. J., Tong J. W., Huang J. A., Li J., Gong Y. S., Liu Z. H.2017. Tea polyphenol epigallocatechin gallate inhibits Escherichia coli by increasing endogenous oxidative stress. Food Chem. 217: 196–204. doi: 10.1016/j.foodchem.2016.08.098 [DOI] [PubMed] [Google Scholar]
- 25.Xu X., Zhou X. D., Wu C. D.2011. The tea catechin epigallocatechin gallate suppresses cariogenic virulence factors of Streptococcus mutans. Antimicrob. Agents Chemother. 55: 1229–1236. doi: 10.1128/AAC.01016-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yin H., Deng Y., Wang H., Liu W., Zhuang X., Chu W.2015. Tea polyphenols as an antivirulence compound disrupt quorum-sensing regulated pathogenicity of pseudomonas aeruginosa. Sci. Rep. 5: 16158. doi: 10.1038/srep16158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang B., Xu C., Zhang L., Zhou S., Feng S., He Y., Liao M.2013. Enhanced adherence to and invasion of PUVEC and PK-15 cells due to the overexpression of RfaD, ThyA and Mip in the ΔompP2 mutant of Haemophilus parasuis SC096 strain. Vet. Microbiol. 162: 713–723. doi: 10.1016/j.vetmic.2012.09.021 [DOI] [PubMed] [Google Scholar]
- 28.Zhang B., Yu Y., Zeng Z., Ren Y., Yue H.2014. Deletion of the rfaE gene in Haemophilus parasuis SC096 strain attenuates serum resistance, adhesion and invasion. Microb. Pathog. 74: 33–37. doi: 10.1016/j.micpath.2014.07.006 [DOI] [PubMed] [Google Scholar]
- 29.Zou L. Q., Liu W., Liu W. L., Liang R. H., Li T., Liu C. M., Cao Y. L., Niu J., Liu Z.2014. Characterization and bioavailability of tea polyphenol nanoliposome prepared by combining an ethanol injection method with dynamic high-pressure microfluidization. J. Agric. Food Chem. 62: 934–941. doi: 10.1021/jf402886s [DOI] [PubMed] [Google Scholar]
- 30.Zou Y., Feng S., Xu C., Zhang B., Zhou S., Zhang L., He X., Li J., Yang Z., Liao M.2013. The role of galU and galE of Haemophilus parasuis SC096 in serum resistance and biofilm formation. Vet. Microbiol. 162: 278–284. doi: 10.1016/j.vetmic.2012.08.006 [DOI] [PubMed] [Google Scholar]