Abstract
The object is to determine the neuroprotective and antioxidative effects of submicron and blended Lycium barbarum (LB) on retinal degeneration as evaluated by ERG, retinal histopathology and assays of antioxidant (total GSH) and peroxidant (MDA) in the retina. A rat model of light-induced retinal degeneration was used to assess the protective effect of different forms of Lycium barbarum (LB) on retinal degeneration. Rats were divided into four experimental groups, normal control, light-induced untreated, submicron LB and blended LB treated. The rats of submicron and blended groups were treated with 250 mg/kg LB orally once daily for 54 days, followed by induction of retinal degeneration. Retinal function was assessed by electroretinography (ERG). Enzyme-linked immunosorbent assay of the retina lysates was measured for the levels of antioxidants, reduced glutathione and glutathione disulfide, and peroxidants, malondialdehyde, in the retina. The ERG results showed a protective effect in LB treated groups with a greater effect observed in submicron LB treated group than the blended LB treated group. There were higher levels of GSH plus GSSG and lower MDA in submicron LB treated group than other groups. In conclusion, LB provided protective and antioxidative effects on the rat retina with light-induced retinal degeneration. Submicron LB protected degenerative retina better than blended LB. LB is effective against oxidative stress in the degenerative retina.
Keywords: Antioxidative, Lycium barbarum, neuroprotective, retinal degeneration
Aged-related macular degeneration (AMD) is the leading cause of visual impairment and blindness in the world among people over age 55 years with the prevalence as 10–15% [2]. One of the major causes of aging is oxidative stress which increases production of free radicals, reactive species, and oxidant-related reactions resulting in cell damage [1, 23]. It is widely accepted that disorganizing free radical reactions linked to oxygen metabolism or oxidative stress play an important role not only in normal aging but also in progression of retinal degeneration [1, 5, 23].
Lycium barbarum (LB) extract from the fruits contains carotenoids such as zeaxanthin dispalmitate and polysaccharides which are important antioxidants as well as free radical scavengers [14]. Zeaxanthin dispalmitate is as the predominant carotenoid in the fruits of the lycium species confirmed by high performance liquid chromatography-photo diode array detection-mass spectrometery (HPLC-DAD-MS) method [13]. As an antioxidants, LB has been reported to decrease DNA damage in non-insulin dependent diabetes mellitus rats possibly via a decrease in oxidative stress levels [30], protect retinal ganglion cells from pressure-induced losing in a rat OH model [6], decrease the oxidative stress in skeletal muscle tissue of rats induced by the exhaustive exercise [18], rescue photoreceptors in rd1 mice [17], inhibit malondialdehyde formation in rat liver homogenate, and superoxide anion scavenging and anti-superoxide formation activities [31] and restore photoreceptors in experimental light-induced phototoxicity and macular degeneration [33].
Much progress has been achieved in nanobiotechnology in recent decades. Nanotechnology has improved pharmacokinetics by alterations in nanometric size ranges, improved permeability, resistance to proteolytic and hydrolytic degradation, and enhanced uptake based on functional surface ligands [19]. Top-down approach is used to extract Lycium barbarum by homogenizer “POLYTRON PT3100” (Kinemativa AG, Littau-Luzern, Swizerland) and “Minipur” (Netzsch-Feinmahltechnik GmbH, Belb, Germany) the laboratory mill to obtain the submicron Lycium barbarum with the particle size as 100 ± 70 nm. The blended Lycium barbarum is made by general juice blender with the particle size as 3.58 ± 3.8 µm.
Light-induced retinal degeneration (Noell et al. 1966) [20] in experimental animal has been studied for 50 years as a model for visual cell loss arising from retinal degeneration disease like canine retinal degeneration and human age-related macular degeneration. It has been suggested that intensive light induces oxidative stress in the retina leading to photoreceptors degeneration. According to that, the treatment increased glutathione peroxidase activity and glutathione level exists a high correlation with rescuing photoreceptors [17]. Lipid peroxidants may induced by light damage in retina as malondialdehyde (MDA) and antioxidant enzymatic system as glutathione (GSH) and glutathione disulfide (GSSG) has been assayed in rat retina as a function of age [7]. It seems GSH and GSSG directly protect against oxidative stress by scavenging free radicals and other reactive species like MDA. Retinal function and histopathology were evaluated following light-induced retinal degeneration to evaluate the protective effect of two forms of LB in the rat [27].
MATERIALS AND METHODS
Animals
Male Sprague-Dawley rats (8–10 weeks old, 200–300 g) were housed in a temperature-controlled animal room subjected to a 12-hr light/12-hr dark cycle and provided with appropriate food and water The rats were kept in the same row of the steel cages that the cages were exposed equally to the light between 50–100 lux measured by photometer [22]. All of the rats were examined by biomicroscopy, indirect ophthalmoscopy, and electroretinography (ERG) prior to study initiation to ensure they were free from ophthalmic abnormalities. All animal protocols were in accordance with and approved by the Institutional Animal Care. Use Committee (IACUC) of the National Taiwan University and the IACUC approval number of the study: NTU-98- EL-15. All protocols were in accordance with the ARVO’s Statement for the Use of Animals in Ophthalmic and Vision Research.
Experimental design
Animals were divided into four groups, treatment Group A (n=7): diet supplemented with 250 mg/kg submicron LB, treatment Group B (n=7): supplemented with 250 mg/kg blended LB, untreated Group C (n=9) and control Group D (n=3) were fed with a normal diet. All were housed and fed for a period of 54 days. All rats were free to have their food and water at any time during the whole experiment. White cool light was chosen for the induction of retinal degeneration with the intensity between 1,400–1,500 lux and exposure for 2 days subjected to a 12-hr light/12-hr dark cycle [11, 21]. Groups A, B and C were exposed to the increased light while Group D served as an unexposed control. The light-induced damage began after feeding LB for 54 days and following exposure rats were returned to normal housing for 7 days. On the 7th day all rats were examined by biomicroscopy, indirect ophthalmoscopy and ERG are according to the original light induced retinal degeneration model established by Noell in 1966.
Preparation of Lycium barbarum extract
Five g dried fruits of Lycium barbarum was prepared by boiling in 500 ml distilled water then blended with high speed homogenizer stirrer (Polytron PT 3100) to obtain the blended aqueous extract with the particle size of 3.58 ± 3.8 µm. In addition, the blended Lycium barbarum was milled with Minipur to obtain a submicron extract of LB with a particle size of 100 ± 70 nm. The particle sizes were all measured by Flow CytoMetry to make sure the quality of the test material was consistent.
Electroretinography
Retinal function was evaluated using an electroretinogram (BPM-200 Control Program System, ERG/VEP v5.50, RetinoGraphics Inc., Norwalk, CT, U.S.A.) on the 49th and 63rd day after feeding Lycium barbarum extract. The chosen date is exactly one week before and one week after the light-induced damage procedure. The data were recorded following general anesthesia with isoflurane (AttaneTM) and corneal anesthesia with 0.5% proparacaine (Alcaine, Alcon). All manipulations were performed under dim red illumination (λmax=659 nm) following overnight dark adaptation (>12 hr). Flash ERG was performed after pupillary dilation with 0.5% tropicamide (Mydriacyl®, Alcon) using an LED light source and responses were recorded and using a contact lens electrode (ERG-jet®, Universo Plastique Inc., Le Crêt-du-Locle, Switzerland) referenced to a subcutaneous needle electrode (platinum subcutaneous needle electrode F-E2, Grass-Telefactor Division, Astro-Med Inc., West Warwick, RI, U.S.A.) and a ground electrode on the ear. Two ERG responses from the right and the left eyes were elicited at 10 sec intervals and were averaged [12].
Histopathology examination
After ERG examination, the animals were sacrificed by intracardiac injection of thiamylal sodium (Citosol®, Shinlin Sinseng, Taipei, Taiwan) administered under deep anesthesia by inhaled isoflurane 7 days after light-induced damage. Immediately following death, the eyes were enucleated and fixed in phosphate-buffered saline containing 4% paraformaldehyde for 1 hr then the cornea was excised and the globe fixed for 48 hr. After graded ethanol serial dehydration, the bilateral globes were transversely dissected from optic disc into two half spheres separately and embedded in paraffin. Tissue sections were cut at 4 µm thickness and mounted on slides and stained with hematoxylin and eosin. The retinal thickness, including inner plexiform layer (IPL), inner nuclear layer (INL), outer plexiform layer (OPL), outer nuclear layer (ONL), photoreceptor segment layer (PSL), and photoreceptor segment layer−inner limiting membrane (PSL-ILM), were measured under light microscopy. In total, six spots from each eye were chosen for examination and measurement, beginning at the optic disc and moving peripherally in 250 µm intervals for each measurement [27].
Measurement of retinal levels of total glutathione (GSH + GSSG) after light exposure
Glutathione assay kit (CS0260, Sigma-Aldrich Inc., St. Louis, MO, U.S.A.) was used to measure the level of total glutathione in retinal homogenate of the left eye. The retina was excised immediately and was washed twice with PBS before flash freezing in liquid nitrogen. The flash freezing retina was ground in a pestle and molar with liquid nitrogen to prepare a fine powder. Took an aliquot powder and added 5% 5-sulfosalicyic acid and vortex. Homogenated the prepared sample with a polytetrafluoroethylene (PTFE) pestle in glass tube until an even suspension was achieved. Left the suspension at 4°C for 10 min and then centrifuged at 10,000 rpm for 10 min. Measured the supernatant and used it as the original sample volume in the calculation for glutathione determination. The total glutathione levels was gauged by spectrophotometer with the absorbance at 410 nm as the prescription of the assay kit [8].
Measurement of MDA levels in the retina
A biomarker of lipid peroxidation MDA, was determined by 2-thiobarbituric acid (TBA, T5500, Sigma-Aldrich Inc.) colorimetric method described by Richter et al. [26], and Winkler et al. [32]. TBA solution (100 g/l) was added to the retinal homogenate in a centrifuge tube which was placed in boiling water for 15 min. After cooling with running water, the tube was subjected to 10 min centrifugation at 1,000 rpm, and then TBA (6.7 g/l) was added into the supernatant and the tube was placed into boiling water for another 15 min. After cooling, the samples were read at absorbance of 532 nm by standard spectrophotometer and the concentration of MDA was calculated as MDA-TBA complex. TMP (1,1,3,3-Tetramethoxypropane, T1642, Sigma-Aldrich Inc.) was used as an external standard, and level of lipid peroxides was expressed as nM/g of MDA.
Statistical analysis
The results are expressed as mean ± standard deviation (SD). The statistical analyses were carried out using one-way ANOVA. In all cases, a P-value of ≤0.05 was considered significant.
RESULTS
Histopathological examination and determination of retinal thickness
Morphological retinal changes are shown in Table 1 and Fig. 1. The retinal thickness (µm) in Groups A including PSL-ILM (163.09 ± 21.58 µm) and IPL (68.94 ± 4.4 µm) was significantly thicker than that in Group C (120.80 ± 10.17 µm, 58.41 ± 7.18 µm) (P<0.05). ONL and PSL thickness in Groups A (24.00 ± 2.79 µm, 27.97 ± 6.19 µm) and B (19.13 ± 3.54 µm, 18.35 ± 10.29 µm) were significantly thicker than that in Group C (6.26 ± 2.17 µm, 5.87 ± 2.04 µm) (P<0.001) (Figs. 2, 3, 4, 5 and Table 1). These results indicated that photo-toxicity caused significant retinal degeneration predominantly in the outer segments. LB exhibited a protective effect in these retinal segments although there was no difference between the submicron and blended LB groups.
Table 1. Retinal thickness (µm) of all experimental groups.
| Layer thickness groups | PSL-ILM | IPL | INL | ONL | PSL |
|---|---|---|---|---|---|
| Group A | 163.09 ± 21.58a) | 68.94 ± 4.40b) | 26.37 ± 5.58 | 24.00 ± 2.79a,c) | 27.97 ± 6.19a) |
| Group B | 139.39 ± 19.38 | 65.75 ± 6.92 | 25.10 ± 6.43 | 19.13 ± 3.54a) | 18.35 ± 10.29a) |
| Group C | 120.80 ± 10.17 | 58.41 ± 7.18 | 20.19 ± 2.51 | 6.26 ± 2.17 | 5.87 ± 2.04 |
| Group D | 195.24 ± 13.95a) | 83.66 ± 9.01a) | 27.30 ± 3.86 | 40.51 ± 8.12a,c) | 47.15 ± 7.83a) |
The data were presented as Mean ± SD (Group A, B, C, D, n=7, 7, 9, 3) and evaluation by one way ANOVA followed by the Student’s t-test to detect inter-group differences. Differences were considerate to be statistically significant if P<0.05. a) P<0.001, compared with normal control group (C). b) P<0.05, compared with normal control group (C). c) P<0.05, compared with blended LB treated group (B).
Fig. 1.
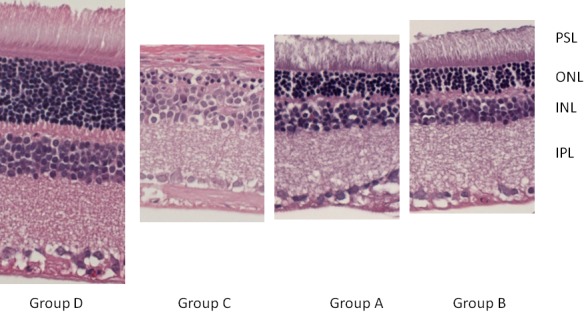
Histopathology thickness of retina. The section was excised from the central retina. The ONL and PSL were almost disappeared in Group A, B, C. The loss of OPL and IPL especially in Group C makes ONL and INL barely seen. Group A and B seems to preserve more layer of cells than Group C. IPL=inner plexiform laye, OPL=outer plexiform layer, PSL=photoreceptor segment layer, ONL=outer nucleus layer, INL=inner nucleus layer.
Fig. 2.
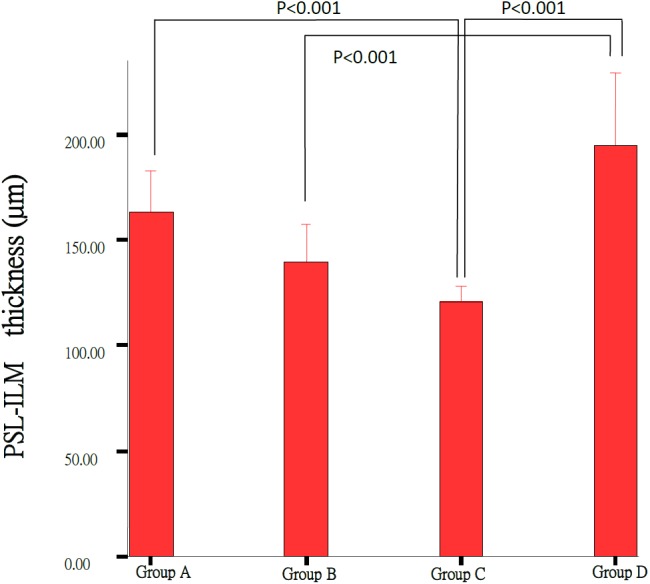
Comparison of the PSL-ILM thickness of the retinas between Group A (n=7), Group B (n=7), Group C (n=9) and Control Group D (n=3); each group result represents average ± SD. Significant difference (P<0.001) was noted between Group C and Group D, Group D and control Group D.
Fig. 3.
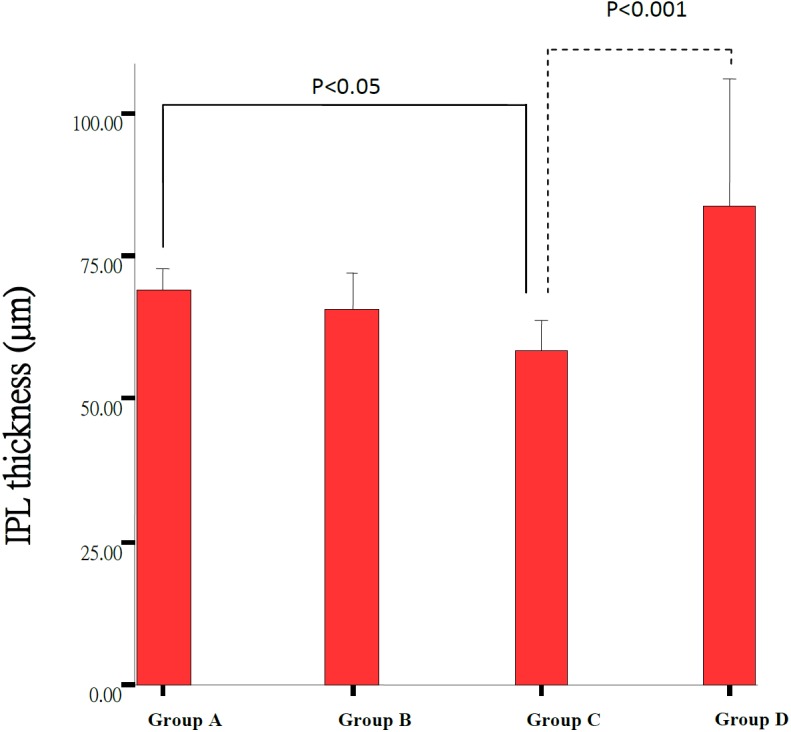
Comparison of the IPL thickness of the retinas between Group A (n=7), Group B (n=7), Group C (n=9) and control Group D (n=3). Each group result presents Mean ± SD. Significant difference (P<0.05) was noted between Group C and Group A.
Fig. 4.
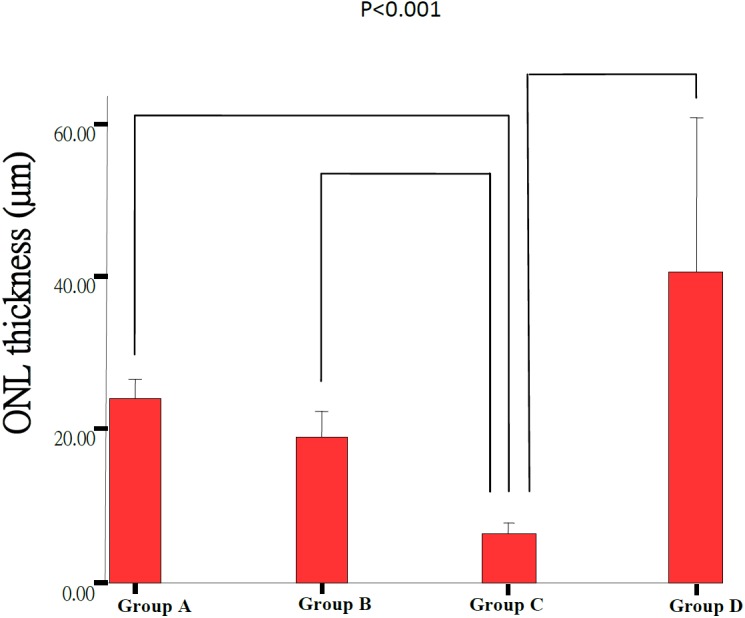
Comparison of the ONL thickness of the retinas between Group A (n=7), Group B (n=7), Group C (n=9) and Group D (n=3). Each group result presents Mean ± SD. Significant difference (P<0.001) was noted between all groups except between Group A and Group B.
Fig. 5.
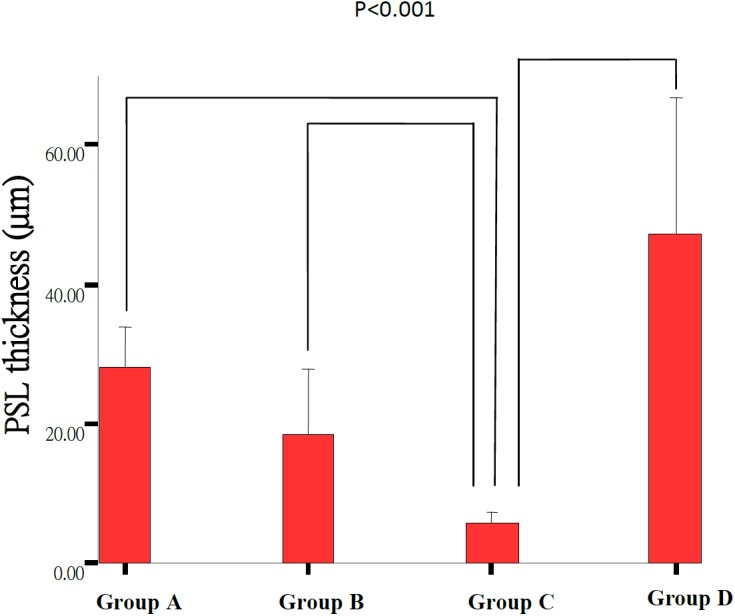
Comparison of the PSL thickness of the retinas between Group A (n=7), Group B (n=7), Group C (n=9) and Group D (n=3). Each group result presents Mean ± SD. Significant difference (P<0.001) was noted between Group C and any other groups as lines indicated.
Electroretinography
The changes of retinal function following light damage were examined by electroretinogram (ERG) are shown in Table 2 and Fig. 6. Light-induced retinal damage groups (A–C) showed a significant decrease in a- and b-wave ratio (31.59 ± 6%, 16.1 ± 5%, 5.10 ± 2% and 33.39 ± 11%, 28.69 ± 6%, 6.5 ± 2%) as compared to the control group (Group D) (97.46 ± 2% and 98.59 ± 1%). The a- and b-wave ratio in Groups A and B were significantly (31.59 ± 6%, 16.1 ± 5% and 33.39 ± 11%, 28.69 ± 6%) better preserved as compared to the untreated control, Group C (5.10 ± 2% and 6.5 ± 2%) (P<0.001). The ERG of Group A was better preserved in both a- and b-wave (31.59 ± 6% and 33.39 ± 11%) than Group B (16.1 ± 5% and 28.69 ± 6%) (P<0.001, P<0.05). These results are consistent with the histopathology except that there was a significant difference between the submicron and blended LB groups.
Table 2. ERG wave ratio of all experimental groups.
| Groups ERG presevation | Group A | Group B | Group C | Group D |
|---|---|---|---|---|
| A wave (%) | 31.59 ± 6a,c) | 16.1 ± 5a) | 5.10 ± 2 | 97.46 ± 2a,c) |
| B wave (%) | 33.39 ± 11b,d) | 28.69 ± 6b) | 6.5 ± 2 | 98.59 ± 1b,d) |
The data were presented as Mean ± SD (Group A, B, C, D, n=7, 7, 9, 3) and evaluation by one way ANOVA followed by the Student’s t-test to detect inter-group differences. Differences were considerate to be statistically significant if P<0.05. a) P<0.001, compared with normal control group (C). b) P<0.05, compared with normal control group (C). c) P<0.001, compared with blended LB treated group (B). d) P<0.05, compared with blended LB treated group (B).
Fig. 6.
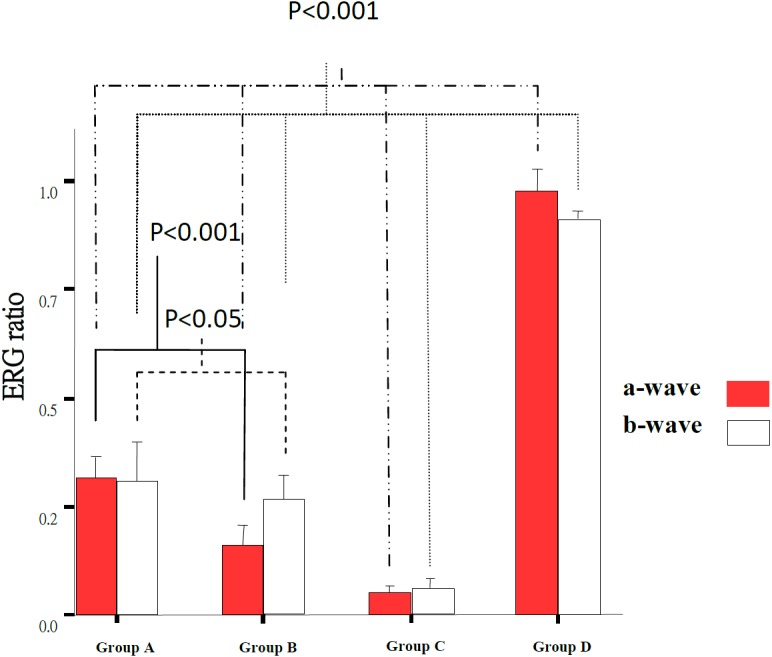
ERG wave ratio in 4 experimental groups. The dark bars present a-wave and blank bars present b-wave ratio. There was significant difference in a-wave ratio between all groups (P<0.001). There was significant difference in b-wave ratio between all groups (P<0.001) from Group C and Group D to any others. There was significant difference in a- and b-wave between the Group A and Group B (P<0.001, P<0.05).
Determination of total glutathione and MDA levels in the retina
The MDA level in rat retina homogenate increased significantly in Group C (52.06 ± 5.11 nM/g) compared to that in other groups (Group A, B, D: 25.68 ± 3.00 nM/g, 24.53 ± 11.23 nM/g, 30.68 ± 5.94 nM/g) (P<0.05). MDA concentration in Group A (25.68 ± 3.00 nM/g) was slightly decreased compared to untreated rats (Group D, 30.68 ± 5.94 nM/g) (Table 3, Fig. 8). It showed the lowest MDA level in the rats of feeding submicron LB group. Even though the MDA level was lower in feeding blended LB comparing to no treatment group, the average of MDA level was higher than the control group.
Table 3. Retinal GSSG+GSH and MDA concentration in experimental groups.
| Groups | Group A | Group B | Group C | Group D |
|---|---|---|---|---|
| GSSG + GSH (nmol/ml) | 23.49 ± 3.87a,b) | 12.75 ± 0.72a) | 1.26 ± 0.25 | 15.96 ± 2.24a,b) |
| MDA (nM/g) | 25.68 ± 3.00c) | 24.53 ± 11.23c) | 52.06 ± 5.11 | 30.68 ± 5.94c) |
The data were presented as Mean ± SD (Group A, B, C, D, n=7, 7, 9, 3) and evaluation by one way ANOVA followed by the Student’s t-test to detect inter-group differences. Differences were considerate to be statistically significant if P<0.05. a) P<0.001, compared with normal control group (C). b) P<0.001, compared with blended LB treated group (B). c) P<0.05, compared with normal control group (C).
Fig. 8.
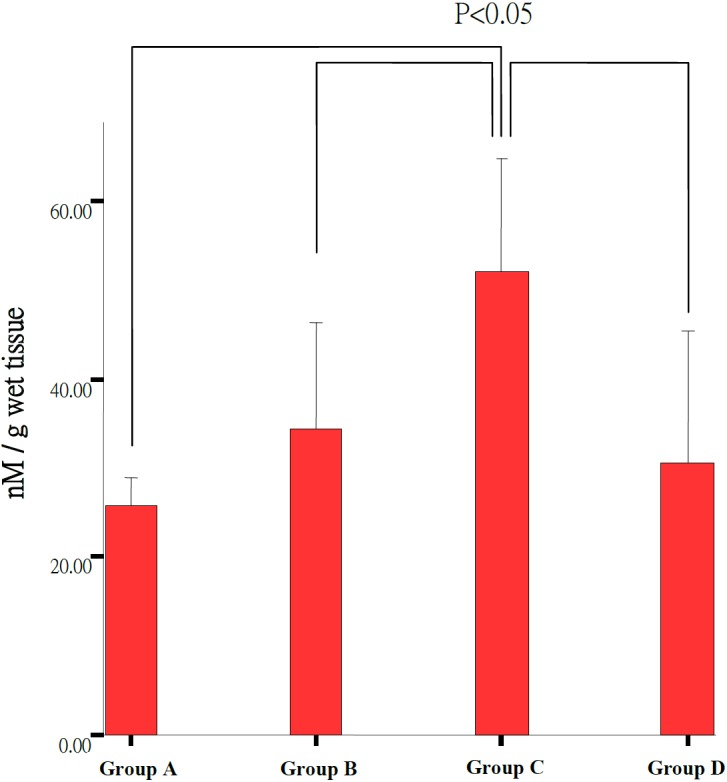
Malonylidialdehyde (MDA) concentrations in all experimental groups. The Group C (n=3) showed significant higher level of MDA concentration than Group A (n=6), Group B (n=6) and Group D (n=3) (P<0.05).
The total glutathione level was significantly higher in the treatment Group A and B (23.49 ± 3.87 nmol/ml, 12.75 ± 0.72 nmol/ml) than untreated control Group C (1.26 ± 0.25 nmol/ml) (Table 3, Fig. 7). There was higher total glutathione level in the submicron LB Group A (23.49 ± 3.87 nmol/ml) as compared to the blended LB Group B (12.75 ± 0.72 nmol/ml) but no different between blended LB Group B (12.75 ± 0.72 nmol/ml) and normal control Group D (15.96 ± 2.24 nmol/ml).
Fig. 7.
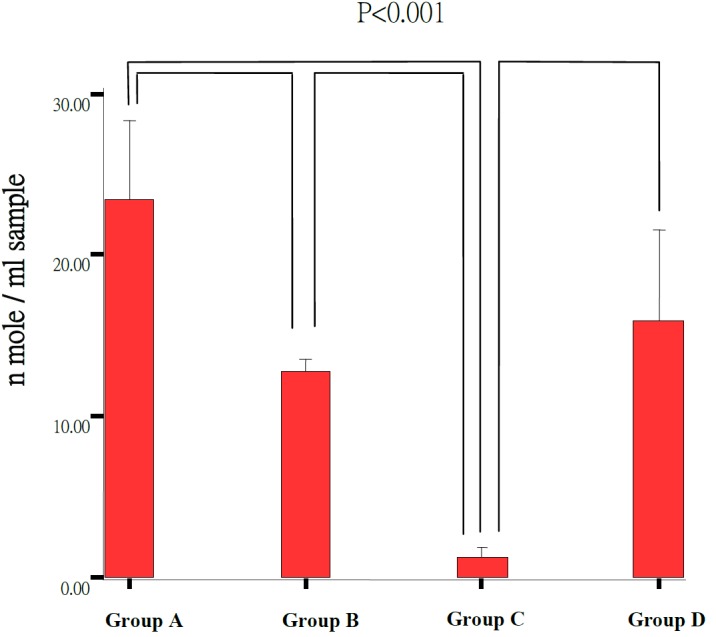
Retinal GSH and GSSG level in experimental groups. The Group C (n=3) showed significant lower concentration than the Group A (n=5), Group B (n=6) and Group D (n=3) (P<0.001). The Group A showed significant higher concentration of total glutathione than Group B (P<0.001).
DISCUSSION
Prophylactic treatment for retinal degeneration in human and animals is based on the capacity of various antioxidants to alleviate or reduce the detrimental effects of reactive oxygen species in response to noxious stimuli.
Lipid peroxidation has been defined as “the oxidation deterioration of polyunsaturated lipids that can reduce membrane fluidity, inactive membrane-bound proteins and decompose into cytotoxic aldehydes such as malondialdehyde” [32], which is just as the theory when light reaches retinal photoreceptor outer segments inducing peroxidation [23, 28].
The retina of experimental rats can be affected irreversibly by visible light. Primary damage to the outer nuclear cells rather than the inner nuclear cells was caused by constant light exposure by a previous study [28]. One class of the type I light damage is produced by a short-term exposure to low-intensity illumination. This type of damage is characterized by diffuse degeneration of the retina, especially the loss of photoreceptor cells [22, 28]. In this study, the retinal thickness was reduced most significantly in ONL and PSL indicating more severe damage in the outer retina as compared with the inner retina. Light-induced damage involves loss of outer segment membrane and photoreceptors [21, 28] followed by degeneration of the undifferentiated first retinal neuron layer [1, 27] but the detailed mechanism remains to be investigated.
The emphasis of aging and retinal degeneration is placed on the generation of oxidants and free radicals contributing oxidative stress to the outer retina and photoreceptors [4, 17, 23, 29]. MDA as secondary oxidation products influence the gene expression and protein synthesis leading to further damage by crosslinking of proteins. It is a good biomarker that is fast and easy to measure but less specific [8]. The level of malondialdehyde and hydroxynonenal- conjugated collagen protein increases with age in rat tissues. We have also observed an increase in the levels of MDA, a marker of lipid peroxidation [7]. In our study, the two LB treatment groups (Group A and B) exhibited significantly lower MDA levels than that in the untreated group (Group C) suggesting the LB treatment might decrease the generation or increased elimination of MDA.
Oxidative damage may occur and accumulate throughout life as a major contributory factor in retinal aging resulting in loss of retinal cells. A range of potent antioxidants such as endogenous and exogenous glutathione and carotenoids restrict the degree of oxidative damage [9, 23]. GSH is the major free thiol in most living cells and is the key antioxidant in animal tissue. In this study, the total glutathione level was higher in both LB treatment groups (Group A and B) as compared with the untreated control and control groups (Group C and D). Furthermore, there was a higher level of total glutathione in the group fed submicron LB (Group A) than that in the group fed blended LB (Group B). These results are consistent with the ERG findings. MDA levels indicate the protective effect of submicron LB may be more potent than the blended one.
The submicron particles are close to nanomaterials that are thought to improve the pharmacokinetics and absorption. This allows penetration through blood-ocular barrier and delivery to specific regions [10, 24].
Fruit of Lycium barbarum is a well-known traditional Chinese herb with a variety of beneficial effects, such as reducing blood glucose and serum lipids, anti-aging, immune-modulating, anti-fatigue [15]. There are various antioxidants such as carotein, riboflavin, betaine, thiamine, nicotinic acid, cryptoxanthin, coumarin, ascorbic acid, and riboflavin, which may have some interactions and synergistic effects for antioxidant properties [3, 16]. There are various carious chemical constituents found in Lycium barbarum fruit with a reddish-orange color which is derived from carotenoids prominently as zeaxanthin [25]. This composition was confirmed by Inbaraj [13] and the serum levels with macular density of zeaxanthin were raised by feeding a carotenoid-containing fraction of fruit lycium, which is a good dietary source of zeaxanthin supplement [3, 25]. Despite of all these beneficial effects of LB, it is still not recommended for some populations to take LB, such as pregnant women and people with underlying diseases like diarrhea, fever, and arthritis [25]. The detailed mechanism of antioxidation by LB requires more investigations.
CONFLICT OF INTEREST
No competing financial interests exist.
Acknowledgments
We would like to thank Drs. Nien-Chen Lin and An-I Yeh for their assistance of LB preparation. The study was supported by research grants 98 Agri-3.1.3-Food-Z2, and 96-2313-B-002-044-MY3 (National Science Council Taiwan). There is no potential conflict relative to this work.
REFERENCES
- 1.Algvere P. V., Marshall J., Seregard S.2006. Age-related maculopathy and the impact of blue light hazard. Acta Ophthalmol. Scand. 84: 4–15. doi: 10.1111/j.1600-0420.2005.00627.x [DOI] [PubMed] [Google Scholar]
- 2.Algvere P. V., Seregard S.2002. Age-related maculopathy: pathogenetic features and new treatment modalities. Acta Ophthalmol. Scand. 80: 136–143. doi: 10.1034/j.1600-0420.2002.800204.x [DOI] [PubMed] [Google Scholar]
- 3.Amagase H., Nance D. M.2008. A randomized, double-blind, placebo-controlled, clinical study of the general effects of a standardized Lycium barbarum (Goji) Juice, GoChi. J. Altern. Complement. Med. 14: 403–412. doi: 10.1089/acm.2008.0004 [DOI] [PubMed] [Google Scholar]
- 4.Büchi E. R.1992. Cell death in the rat retina after a pressure-induced ischaemia-reperfusion insult: an electron microscopic study. I. Ganglion cell layer and inner nuclear layer. Exp. Eye Res. 55: 605–613. doi: 10.1016/S0014-4835(05)80173-3 [DOI] [PubMed] [Google Scholar]
- 5.Cukras C., Fine S. L.2007. Classification and grading system for age-related macular degeneration. Int. Ophthalmol. Clin. 47: 51–63. doi: 10.1097/IIO.0b013e31802bd785 [DOI] [PubMed] [Google Scholar]
- 6.Chan H. C., Chang R. C., Koon-Ching Ip A., Chiu K., Yuen W. H., Zee S. Y., So K. F.2007. Neuroprotective effects of Lycium barbarum Lynn on protecting retinal ganglion cells in an ocular hypertension model of glaucoma. Exp. Neurol. 203: 269–273. doi: 10.1016/j.expneurol.2006.05.031 [DOI] [PubMed] [Google Scholar]
- 7.Castorina C., Campisi A., Di Giacomo C., Sorrenti V., Russo A., Vanella A.1992. Lipid peroxidation and antioxidant enzymatic systems in rat retina as a function of age. Neurochem. Res. 17: 599–604. doi: 10.1007/BF00968789 [DOI] [PubMed] [Google Scholar]
- 8.Camera E., Picardo M.2002. Analytical methods to investigate glutathione and related compounds in biological and pathological processes. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 781: 181–206. doi: 10.1016/S1570-0232(02)00618-9 [DOI] [PubMed] [Google Scholar]
- 9.Erden-Inal M., Sunal E., Kanbak G.2002. Age-related changes in the glutathione redox system. Cell Biochem. Funct. 20: 61–66. doi: 10.1002/cbf.937 [DOI] [PubMed] [Google Scholar]
- 10.El-Ansary A., Al-Daihan S.2009. On the toxicity of therapeutically used nanoparticles: an overview. J. Toxicol. 2009: 754810. doi: 10.1155/2009/754810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gordon W. C., Casey D. M., Lukiw W. J., Bazan N. G.2002. DNA damage and repair in light-induced photoreceptor degeneration. Invest. Ophthalmol. Vis. Sci. 43: 3511–3521. [PubMed] [Google Scholar]
- 12.Green D. G., Kapousta-Bruneau N. V.1999. A dissection of the electroretinogram from the isolated rat retina with microelectrodes and drugs. Vis. Neurosci. 16: 727–741. doi: 10.1017/S0952523899164125 [DOI] [PubMed] [Google Scholar]
- 13.Inbaraj B. S., Lu H., Hung C. F., Wu W. B., Lin C. L., Chen B. H.2008. Determination of carotenoids and their esters in fruits of Lycium barbarum Linnaeus by HPLC-DAD-APCI-MS. J. Pharm. Biomed. Anal. 47: 812–818. doi: 10.1016/j.jpba.2008.04.001 [DOI] [PubMed] [Google Scholar]
- 14.Li X. M., Ma Y. L., Liu X. J.2007. Effect of the Lycium barbarum polysaccharides on age-related oxidative stress in aged mice. J. Ethnopharmacol. 111: 504–511. doi: 10.1016/j.jep.2006.12.024 [DOI] [PubMed] [Google Scholar]
- 15.Luo Q., Cai Y., Yan J., Sun M., Corke H.2004. Hypoglycemic and hypolipidemic effects and antioxidant activity of fruit extracts from Lycium barbarum. Life Sci. 76: 137–149. doi: 10.1016/j.lfs.2004.04.056 [DOI] [PubMed] [Google Scholar]
- 16.Leung I., Tso M., Li W., Lam T.2001. Absorption and tissue distribution of zeaxanthin and lutein in rhesus monkeys after taking Fructus lycii (Gou Qi Zi) extract. Invest. Ophthalmol. Vis. Sci. 42: 466–471. [PubMed] [Google Scholar]
- 17.Miranda M., Arnal E., Ahuja S., Alvarez-Nölting R., López-Pedrajas R., Ekström P., Bosch-Morell F., van Veen T., Romero F. J.2010. Antioxidants rescue photoreceptors in rd1 mice: Relationship with thiol metabolism. Free Radic. Biol. Med. 48: 216–222. doi: 10.1016/j.freeradbiomed.2009.10.042 [DOI] [PubMed] [Google Scholar]
- 18.Niu A. J., Wu J. M., Yu D. H., Wang R.2008. Protective effect of Lycium barbarum polysaccharides on oxidative damage in skeletal muscle of exhaustive exercise rats. Int. J. Biol. Macromol. 42: 447–449. doi: 10.1016/j.ijbiomac.2008.02.003 [DOI] [PubMed] [Google Scholar]
- 19.Nguyen P., Meyyappan M., Yiu S. C.2010. Applications of nanobiotechnology in ophthalmology—Part I. Ophthalmic Res. 44: 1–16. doi: 10.1159/000279436 [DOI] [PubMed] [Google Scholar]
- 20.Noell W. K., Walker V. S., Kang B. S., Berman S.1966. Retinal damage by light in rats. Invest. Ophthalmol. 5: 450–473. [PubMed] [Google Scholar]
- 21.Naash M. L., Peachey N. S., Li Z. Y., Gryczan C. C., Goto Y., Blanks J., Milam A. H., Ripps H.1996. Light-induced acceleration of photoreceptor degeneration in transgenic mice expressing mutant rhodopsin. Invest. Ophthalmol. Vis. Sci. 37: 775–782. [PubMed] [Google Scholar]
- 22.Organisciak D. T., Jiang Y. L., Wang H. M., Pickford M., Blanks J. C.1989. Retinal light damage in rats exposed to intermittent light. Comparison with continuous light exposure. Invest. Ophthalmol. Vis. Sci. 30: 795–805. [PubMed] [Google Scholar]
- 23.Organisciak D. T., Darrow R. M., Barsalou L., Darrow R. A., Kutty R. K., Kutty G., Wiggert B.1998. Light history and age-related changes in retinal light damage. Invest. Ophthalmol. Vis. Sci. 39: 1107–1116. [PubMed] [Google Scholar]
- 24.Prow T. W.2010. Toxicity of nanomaterials to the eye. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2: 317–333. doi: 10.1002/wnan.65 [DOI] [PubMed] [Google Scholar]
- 25.Potterat O.2010. Goji (Lycium barbarum and L. chinense): Phytochemistry, pharmacology and safety in the perspective of traditional uses and recent popularity. Planta Med. 76: 7–19. doi: 10.1055/s-0029-1186218 [DOI] [PubMed] [Google Scholar]
- 26.Richter C.1987. Biophysical consequences of lipid peroxidation in membranes. Chem. Phys. Lipids 44: 175–189. doi: 10.1016/0009-3084(87)90049-1 [DOI] [PubMed] [Google Scholar]
- 27.Tawara A.1995. [Histopathological studies on the rds mouse retina--relationship between photoreceptor and pigment epithelial cells]. Nippon Ganka Gakkai Zasshi 99: 277–285 (in Japanese). [PubMed] [Google Scholar]
- 28.Vaughan D. K., Coulibaly S. F., Darrow R. M., Organisciak D. T.2003. A morphometric study of light-induced damage in transgenic rat models of retinitis pigmentosa. Invest. Ophthalmol. Vis. Sci. 44: 848–855. doi: 10.1167/iovs.02-0709 [DOI] [PubMed] [Google Scholar]
- 29.Voss P., Siems W.2006. Clinical oxidation parameters of aging. Free Radic. Res. 40: 1339–1349. doi: 10.1080/10715760600953859 [DOI] [PubMed] [Google Scholar]
- 30.Wu H., Guo H., Zhao R.2006. Effect of Lycium barbarum polysaccharide on the improvement of antioxidant ability and DNA damage in NIDDM rats. Yakugaku Zasshi 126: 365–371. doi: 10.1248/yakushi.126.365 [DOI] [PubMed] [Google Scholar]
- 31.Wu S. J., Ng L. T., Lin C. C.2004. Antioxidant activities of some common ingredients of traditional chinese medicine, Angelica sinensis, Lycium barbarum and Poria cocos. Phytother. Res. 18: 1008–1012. doi: 10.1002/ptr.1617 [DOI] [PubMed] [Google Scholar]
- 32.Winkler B. S., Boulton M. E., Gottsch J. D., Sternberg P.1999. Oxidative damage and age-related macular degeneration. Mol. Vis. 5: 32–59. [PMC free article] [PubMed] [Google Scholar]
- 33.Yu M. S., Leung S. K., Lai S. W., Che C. M., Zee S. Y., So K. F., Yuen W. H., Chang R. C.2005. Neuroprotective effects of anti-aging oriental medicine Lycium barbarum against beta-amyloid peptide neurotoxicity. Exp. Gerontol. 40: 716–727. doi: 10.1016/j.exger.2005.06.010 [DOI] [PubMed] [Google Scholar]


