Abstract
Purpose
To identify risk factors for VNS-associated arrhythmia.
Methods
A literature review identified 14 papers with 21 patients. We compared patients with VNS associated arrhythmia (arrhythmia group, n = 22) and patients without VNS associated arrhythmia (control group of our VNS implanted patients, n = 29).
Results
New onset syncopal events following VNS placement were seen in the arrhythmia group (p < 0.001).
Conclusion
Even though arrhythmia could be symptomatic, most cases associated with syncope were treated as new-onset epileptic seizures with adjustment of anti-seizure drugs. To detect cardiac asystole during VNS treatment, clinicians should be alert to the possibility of new onset syncopal events that differ from habitual seizures.
Keywords: Vagus nerve stimulation (VNS) therapy, Cardiac pacemaker, Bradycardia, New onset syncopal events, Psychotic disorder
Highlights
-
•
VNS induced bradycardia was misdiagnosed as new onset epileptic seizure.
-
•
Post-VNS new onset syncopal events should be monitored carefully.
-
•
Cardiac pacemaker treatment resolved bradycardia.
1. Introduction
Vagus nerve stimulation (VNS) therapy has been widely used. The most common stimulation-associated side effects are voice alteration, hoarseness, throat and neck pain, headache, cough, and dyspnea [1]. The most severe side effects of VNS therapy are bradycardia and cardiac asystole [2]. We encountered a patient with symptomatic bradycardia due to VNS who underwent cardiac pacemaker (PM) implantation and resolved symptomatic bradycardia. We hypothesized that risk factors for such bradycardia may exist, identification of which would help prevent VNS-induced critical arrhythmia.
2. Methods
A search for medical papers on PubMed and Google Scholar using the key words “vagus nerve stimulation”, “arrhythmia”, and “bradycardia” revealed 14 papers with 21 patients, not including our case.
We compared patients with VNS-induced arrhythmia (arrhythmia group, n = 22) and patients without such arrhythmia (control group, n = 29). The control group comprised all other patients who underwent VNS implantation in our facility between 2011 and 2014 and had not developed any VNS-induced arrhythmia for more than four years. Inclusion criteria for the control group were: 1) > 4 years follow-up; and 2) surgery performed by the same surgeon (AF).
We statistically compared each factor between groups, using the Mann–Whitney rank-sum test and Fisher's exact test as appropriate. Statistical significance was set at p < 0.05. All analyses were performed using Sigma Plot 14 software (Systat Software, San Jose, CA).
3. Case report
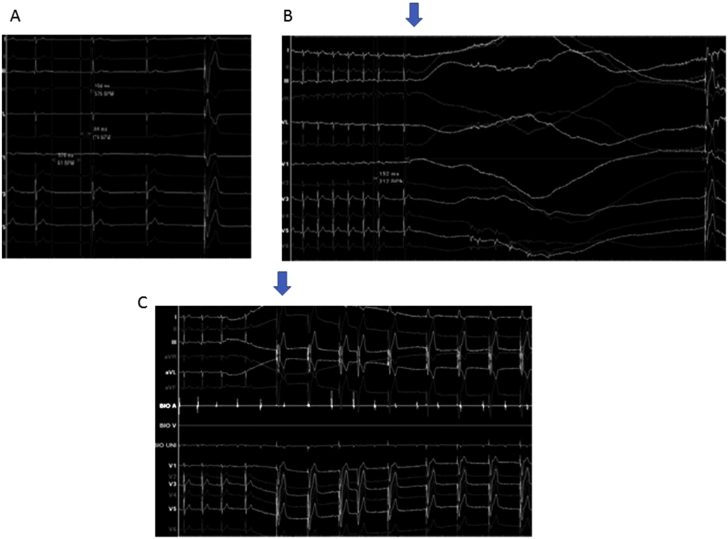
A 43-year-old, right-handed man was admitted to our hospital due to loss of consciousness (LOC). At 17-years-old, he experienced a right temporal lobe contusion in a motor cycle accident. The patient started to exhibit focal impaired awareness seizures and focal to bilateral tonic-clonic seizures at 27 years old, followed by psychiatric symptoms and accompanying delusions and hallucinations from his early thirties. Temporal lobe epilepsy and epileptic psychosis were diagnosed in his early thirties and he was followed by a local psychiatrist. As his epilepsy had been drug-resistant, he was referred to our hospital. He was on topiramate 200 mg/day, carbamazepine (CBZ) 400 mg/day and risperidone 4 mg/day when he visited our hospital. He underwent long-term video-electroencephalography (VEEG), brain magnetic resonance imaging (MRI), 123I-iomazenil single-photon emission computed tomography (IMZ-SPECT), 2-[18F]fluoro-2-deoxy-d-glucose positron emission tomography (FDG-PET), and neuropsychiatric tests. Interictal VEEG showed frequent epileptiform discharges in the right frontotemporal area, while ictal VEEG right frontotemporal rhythmic activities with short periods of loss of awareness and mouthing movements followed by postictal confusion. MRI, FDG-PET and IMZ-SPECT were all concordant, showing a right mesial temporal seizure onset zone. We diagnosed right temporal lobe epilepsy. At 36 years old, he underwent right frontotemporal invasive monitoring. The invasive monitoring showed a seizure onset area arising from the mesial temporal lobe, quickly spreading to the right frontal area. We therefore performed right temporal lobectomy. Weekly seizures reduced to monthly. VNS treatment was then started at 37-years-old for residual seizures. Intraoperative VNS stimulation did not induce any electrocardiographic (ECG) changes. Generalized seizures disappeared, but from 40 years old he sometimes exhibited sudden LOC, differing in character from the habitual seizures. At first, we regarded these sudden LOC as epileptic seizures and continued to adjust the regimen of anti-seizure drugs (ASDs). As he exhibited sudden LOC and fell weekly at this point, he was placed under VEEG monitoring, which revealed bradycardia for several seconds during sleep. As VNS reproducibly caused arrhythmia including bradycardia, atrioventricular (AV) block, and a short period of cardiac arrest (Fig. 1), symptomatic bradyarrhythmia with third-degree AV block induced by VNS was diagnosed and a PM was implanted. When implanting the PM, we conducted an electrophysiological study while changing the output for VNS and decided to position the pacemaker where it would not over-sense stimulation from VNS.
Fig. 1.
A) Critical atrioventricular (AV) block did not occur when vagus nerve stimulation (VNS) was turned off, according to pressure in bilateral carotid arteries. B) With the 1.75-mA output current of VNS, cardiac arrest (arrow) lasted 15 s. C) Complete AV block (arrow) was also detected.
At this time, the patient was on levetiracetam (LEV) 3000 mg/day, CBZ 400 mg/day and risperidone 4 mg/day. Since implantation of the PM, the patient has remained free of sudden LOC.
3.1. Ethics approval
Written informed consent for publication of case details was obtained from our patient. This study was approved by the ethics committee at Seirei Hamamatsu General Hospital.
4. Results
All clinical data are shown in Table 1. The 22 patients [1], [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14] in the arrhythmia group comprised of 8 females and 16 males (mean age, 39 years; range, 8–59 years), while the 29 patients in the control group comprised of 11 females and 18 males (mean age, 31 years; range, 9–52 years). The arrhythmia group was significantly older than the control group (p = 0.038). Epilepsy onset occurred at an older age in the arrhythmia group (mean age, 35.8 years; range, 2–59 years) than in the control group (mean age, 8.6 years; range 0.3–43 years; p < 0.001). The output current of VNS was significantly lower in the arrhythmia group (median, 1.0 mA) than in the control group (median, 2.0 mA; p < 0.001). The duty cycle of VNS was lower in the arrhythmia group (median, 10%) than in the control group (median, 15%; P = 0.028). No significant difference in the number of times an ASD was used (p = 0.097) was apparent between groups. However, concomitant psychiatric disorder (p = 0.034) and usage of psychotropic drugs (p < 0.001) were both significantly more frequent in the arrhythmia group than in the control group. New onset syncopal events differing in character from the habitual seizures were seen only in the arrhythmia group (p < 0.001).
Table 1.
Reviewed patients and our case. Onset, epileptic seizure onset; VNS, vagus nerve stimulation; CPS, complex partial seizure; GTC, generalized tonic–clonic seizure; sGTC, secondary GTC; LOC, loss of consciousness; n.a, not available; ASD, anti-epilepsy drug; LEV, levetiracetam; PER, perampanel; CBZ, carbamazepine; OXC, oxcarbazepine; LTG, lamotrigine; CLB, clobazam; CLZ, clonazepam; PB, phenobarbital; PRM, primidone; VPA, valproate; TPM, topiramate; PHT, phenytoin; FMB, felbamate; BZP, benzodiazepine; GBP, gabapentin; ZNS, zonisamide; DM, diabetes mellitus; AV block, atrioventricular block.
| Author | Age [years] | Sex | Onset [years] | Age at VNS [years] | Main seizure | VNS induced symptoms | VNS output | Duty cycle | AED | Other medication | Concomitant symptoms | VNS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tatum 1999 | 38 | F | n.a | 38 | CPS | n.a | 1 mA | n.a | PHT/lorazepam/tiagabine | n.a | Anxiety | Deactivation |
| 57 | M | n.a | 57 | Partial seizure | n.a | 1 mA | n.a | LTG/PRM | Olanzapine/sertraline | Encephalopathy/depression/alcohol abuse/ | Deactivation | |
| 38 | M | n.a | 38 | Partial seizure | n.a | 1 mA | n.a | CBZ/VPA/vigabatrin | n.a | Left encephalomalacia | Deactivation | |
| 42 | M | n.a | 42 | Partial seizure | n.a | > 1 mA | n.a | TPM/FMB/PHT/PB | Trifluoperazine | Encephalopathy/multiple handicaps/autism | Dose decreased | |
| Asconape 1999 | 56 | M | n.a | 56 | CPS | n.a | 1 mA | n.a | LTG/PRM | Olanzapine/sertraline | Encephalopathy/pulmonary disease/hypertension | Deactivation |
| Ali 2004 | 53 | M | 4 | 53 | GTC/atypical absence | n.a | 1 mA | n.a | CBZ/VPA | n.a | Encephalopathy | Deactivation |
| 40 | M | n.a | 40 | GTC/CPS/myoclonus | n.a | 1 mA | n.a | CBZ/VPA | n.a | Encephalopathy | Deactivation | |
| 42 | F | n.a | 42 | CPS | n.a | 1.25 mA | n.a | GBP/CZP | DM/vasodilator | Diabetes/hypertension/1st degree AV block | Dose decreased | |
| Srinivasan 2004 | 40 | F | n.a | 40 | Partial seizure | Nausea/vomiting/lightheadedness/palpitation | n.a | n.a | n.a | n.a | n.a | Continued |
| Adresh 2007 | 32 | F | 14 | 32 | CPS | n.a | 1 mA | n.a | OXC/FMB | n.a | n.a | Continued |
| 52 | M | n.a | 52 | Partial seizure | n.a | 1 mA | n.a | PHT/TPM | n.a | Depression/1st-degree AV block | Continued | |
| 59 | F | 2 | 59 | GTC | n.a | 1 mA | n.a | CBZ/VPA/CLB | n.a | Bitemporal sclerosis | Continued | |
| Koeing 2008 | 8 | F | 3 m | 8 | Multiple types | Improved | n.a | n.a | VPA/FMB/BZP | n.a | Respiratory sinus arrhythmia | Continued |
| Amark 2008 | 17 | M | 5 m | 15 | CPS | Sudden LOC uncontrollable fall | 1.75 mA | 10% | VPA/GBP/acetazolamide | n.a | n.a | Deactivation |
| Borusiak 2009 | 13 | M | 5 | 7 | CPS without sGTC | Significant increase in seizure frequency | 2.25 mA | 10% | PB/FMB/ZNS | Calcium/vitamin D | n.a | Deactivation |
| Irarte 2009 | 47 | F | 12 | 38 | CPS/status epilepticus | New events of dizziness, unsteadiness | 1.75 mA | 10% | PGB/CZP/LEV | n.a | Psychogenic non-epileptic spell | Deactivation |
| Clark 2012 | 13 | M | 2 | 2 | GTC | Syncope/obtundation | 1.25 mA | 8% | CZP/ZNS/RFM | n.a | Septo-optic dysplasia | Revision/continued |
| Shanker 2013 | 55 | M | n.a | 47 | Partial seizure/sGTC/drop attack | Sudden increase in frequency of atonic spells | 2.25 mA | 16% | LEV/PGB | n.a | Encephalopathy/cerebral anoxic brain | Deactivation |
| Schevchuck 2014 | 40 | M | n.a | 39 | CPS without sGTC | New type of seizure | n.a | 10% | LCM/LTG/LEV/PGB | Encephalopathy | Continued | |
| Cantarin 2016 | 13 | F | 2 | 3 | Drop attack/myoclonus | New attack with sudden fall/LOC | 1.25 mA | 10% | VPA | n.a | n.a | Dose decreased |
| Pascual 2015 | 56 | M | 9 | 42 | CPS | Syncope/lightheadedness/LOC | 2.75 mA | 12% | LEV/TPM | Alprazolam | Anxiety | Deactivation |
| Our case | 47 | M | 27 | 37 | CPS | Sudden LOC with uncontrollable fall | 1.75 mA | 10% | LEV/CBZ | Risperidone | Epileptic psychiatric disorder | Continued with pacemaker |
F, female; M, male; m, months; Onset, epileptic seizure onset; VNS, vagus nerve stimulation; CPS, complex partial seizure focal impaired awareness seizure; GTC, generalized tonic–clonic seizure; sGTC, secondary GTC (focal to bilateral tonic-clonic seizure); LOC, loss of consciousness; n.a, not available; AED, anti-epilepsy drug; LEV, levetiracetam; PER, perampanel; CBZ, carbamazepine; OXC, oxcarbazepine; LTG, lamotrigine; CLB, clobazam; CLZ, clonazepam; PB, phenobarbital; PRM, primidone; VPA, valproate; TPM, topiramate; PHT, phenytoin; FMB, felbamate; BZP, benzodiazepine; GBP, gabapentin; ZNS, zonisamide; DM, diabetes mellitus; A-V block, atrioventricular block.
5. Discussion
From our data and another study assessing the relationship between VNS and sudden unexpected death in epilepsy [15], the VNS dose and duty cycle themselves did not appear directly linked to arrhythmia. This stimulation might not directly affect cardiac conduction, but indirect stimulation of the central nervous system involving the cardiac conduction system might cause bradycardia. If the bradycardia had been induced by direct stimulation, the control group (which showed both higher output current and higher duty cycle) would have been expected to show a higher prevalence of arrhythmia. Ali et al. [5] speculated that activation of the afferent pathway for the left vagal nerve has wide-ranging effects on multiple systems and pathways, and may result in activation of multiple other synaptic pathways with influences on cardiac rhythm. This might be the mechanism underlying arrhythmia.
In this study, the arrhythmia group was significantly older than the control group. Arrhythmia associated with vasovagal syncope is reportedly seen most often in the adult population [16], [17]. Increased hypersensitivity of the vagus nerve induced symptomatic arrhythmia even without relatively stronger stimulation in our case. We therefore suggest patients with VNS be monitored the long term. In the case of repetitive syncopal events seen, not only an ECG but also prolonged ECG using a Holter ECG or loop recorder are recommended [18].
Future work is needed to validate these findings from a multi-center reports with a greater number of patients.
6. Conclusion
Even though arrhythmia could become symptomatic, most cases were treated as new-onset epileptic seizures with adjustment of ASDs. To interrupt prolonged cardiac asystole in VNS treatment patients, clinicians should be alert to the possibility of new-onset syncopal events when they differ from habitual seizures.
Author contributions
Pacemaker implantation: HK and RS. Neurosurgical operation: AF. Acquisition of data: KI, HK and AF. Analysis and interpretation of data: AF, TO, and HE.
Conflict of interest
No funding was received for this research.
References
- 1.Pascual F.T. Vagus nerve stimulation and late-onset bradycardia and asystole: case report. Seizure. 2015;26:5–6. doi: 10.1016/j.seizure.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 2.Amark P., Stodberg T., Wallstedt L. Late onset bradyarrhythmia during vagus nerve stimulation. Epilepsia. 2007;48:1023–1024. doi: 10.1111/j.1528-1167.2007.01023.x. [DOI] [PubMed] [Google Scholar]
- 3.Asconape J.J., Moore D.D., Zipes D.P., Hartman L.M., Duffell W.H., Jr. Bradycardia and asystole with the use of vagus nerve stimulation for the treatment of epilepsy: a rare complication of intraoperative device testing. Epilepsia. 1999;40:1452–1454. doi: 10.1111/j.1528-1157.1999.tb02019.x. [DOI] [PubMed] [Google Scholar]
- 4.Tatum WOt, Moore D.B., Stecker M.M., Baltuch G.H., French J.A., Ferreira J.A. Ventricular asystole during vagus nerve stimulation for epilepsy in humans. Neurology. 1999;52:1267–1269. doi: 10.1212/wnl.52.6.1267. [DOI] [PubMed] [Google Scholar]
- 5.Ali I.I., Pirzada N.A., Kanjwal Y., Wannamaker B., Medhkour A., Koltz M.T. Complete heart block with ventricular asystole during left vagus nerve stimulation for epilepsy. Epilepsy Behav. 2004;5:768–771. doi: 10.1016/j.yebeh.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 6.Srinivasan B., Awasthi A. Transient atrial fibrillation after the implantation of a vagus nerve stimulator. Epilepsia. 2004;45:1645. doi: 10.1111/j.0013-9580.2004.31004.x. [DOI] [PubMed] [Google Scholar]
- 7.Ardesch J.J., Buschman H.P., van der Burgh P.H., Wagener-Schimmel L.J., van der Aa H.E., Hageman G. Cardiac responses of vagus nerve stimulation: intraoperative bradycardia and subsequent chronic stimulation. Clin Neurol Neurosurg. 2007;109:849–852. doi: 10.1016/j.clineuro.2007.07.024. [DOI] [PubMed] [Google Scholar]
- 8.Koenig S.A., Longin E., Bell N., Reinhard J., Gerstner T. Vagus nerve stimulation improves severely impaired heart rate variability in a patient with Lennox-Gastaut-syndrome. Seizure. 2008;17:469–472. doi: 10.1016/j.seizure.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 9.Borusiak P., Zilbauer M., Cagnoli S., Heldmann M., Jenke A. Late-onset cardiac arrhythmia associated with vagus nerve stimulation. J Neurol. 2009;256:1578–1580. doi: 10.1007/s00415-009-5162-y. [DOI] [PubMed] [Google Scholar]
- 10.Iriarte J., Urrestarazu E., Alegre M., Macias A., Gomez A., Amaro P. Late-onset periodic asystolia during vagus nerve stimulation. Epilepsia. 2009;50:928–932. doi: 10.1111/j.1528-1167.2008.01918.x. [DOI] [PubMed] [Google Scholar]
- 11.Clark A.J., Kuperman R.A., Auguste K.I., Sun P.P. Intractable episodic bradycardia resulting from progressive lead traction in an epileptic child with a vagus nerve stimulator: a delayed complication. J Neurosurg Pediatr. 2012;9:389–393. doi: 10.3171/2011.12.PEDS11124. [DOI] [PubMed] [Google Scholar]
- 12.Shankar R., Olotu V.O., Cole N., Sullivan H., Jory C. Case report: vagal nerve stimulation and late onset asystole. Seizure. 2013;22(4):312. doi: 10.1016/j.seizure.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 13.Schevchuck A., West M.B. Late-onset advanced heart block due to vagal nerve stimulation. Am J Ther. 2014;21:545–547. doi: 10.1097/MJT.0b013e31828df0c7. [DOI] [PubMed] [Google Scholar]
- 14.Cantarin-Extremera V., Ruiz-Falco-Rojas M.L., Tamariz-Martel-Moreno A., Garcia-Fernandez M., Duat-Rodriguez A., Rivero-Martin B. Late-onset periodic bradycardia during vagus nerve stimulation in a pediatric patient. A new case and review of the literature. Eur J Paediatr Neurol. 2016;20:678–683. doi: 10.1016/j.ejpn.2016.02.014. [DOI] [PubMed] [Google Scholar]
- 15.Annegers J.F., Coan S.P., Hauser W.A., Leestma J., Duffell W., Tarver B. Epilepsy, vagal nerve stimulation by the NCP system, mortality, and sudden, unexpected, unexplained death. Epilepsia. 1998;39:206–212. doi: 10.1111/j.1528-1157.1998.tb01360.x. [DOI] [PubMed] [Google Scholar]
- 16.Sutton R. Carotid sinus syndrome: progress in understanding and management. Glob Cardiol Sci Pract. 2014;2014:1–8. doi: 10.5339/gcsp.2014.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berger T.M., Porter C.-B.J. Mayo Clinic Proceedings. Elsevier; 1993. Carotid sinus syndrome and wrestling; pp. 366–369. [DOI] [PubMed] [Google Scholar]
- 18.Shen W.K., Sheldon R.S., Benditt D.G., Cohen M.I., Forman D.E., Goldberger Z.D. 2017 ACC/AHA/HRS guideline for the evaluation and management of patients with syncope: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2017;136:e25–e59. doi: 10.1161/CIR.0000000000000498. [DOI] [PubMed] [Google Scholar]