Abstract
Background
A ketogenic diet (KD) may have a role in treating patients in super-refractory status epilepticus (SRSE). Sodium-glucose cotransporter 2 (SGLT2) inhibitors have a risk of ketoacidosis that could facilitate induction of KD.
Case summary
A 42-year-old with a history of drug resistant epilepsy developed SRSE requiring several pharmacological interventions during her hospital course including the initiation of KD that failed. SGLT2 inhibitor therapy was initiated in a successful attempt to augment ketone production.
Conclusion
SGLT2 inhibitors may have a therapeutic value in SRSE patients who cannot achieve ketosis with KD alone.
Keywords: Ketogenic diet, Ketosis, Ketoacidosis, Status epilepticus, Refractory status epilepticus, Super-refractory status epilepticus, Sodium-glucose cotransporter 2 inhibitor, Epilepsy, Seizure, Pentobarbital
Highlights
-
•
Super-refractory status epilepticus (SRSE) carries a high risk of morbidity and mortality despite both pharmacologic and non-pharmacologic interventions.
-
•
The ketogenic diet can play an important role as an adjunct treatment for these patients, but delaying ketosis could negate those benefits.
-
•
Sodium-glucose cotransporter 2 inhibitors can carry an increased risk of ketoacidosis that may benefit patients in SRSE who do not achieve ketosis on the ketogenic diet alone.
1. Introduction
Super-refractory status epilepticus (SRSE) is defined as status epilepticus (SE) that continues for more than 24 h despite general anesthetic agents, including recurrent SE upon reduction or withdrawal of anesthesia [1]. SRSE carries a high risk of morbidity and a high mortality rate ranging from 23 to 57% [2], [3]. Current SE guidelines only offer pharmacological recommendations likely due to the lack of evidence for initiating other therapies for refractory status epilepticus [4]. The optimal management of SRSE is unknown, but a growing body of evidence suggests that initiating a KD in these patients may be effective in terminating SE [5], [6], [7], [8], [9], [10], [11], [12]. The KD is a high fat, low carbohydrate, sufficient protein diet that mimics a starvation state provoking fat metabolism for energy and ketone body production. Diet modification therapies including KD and Modified Atkins Diet can be effective and safe when used as an adjunct therapy for patients with epilepsy, regardless of their age [13].
Sodium-glucose cotransporter 2 (SGLT2) inhibitors are a relatively novel class of oral glucose-lowering therapies for managing type 2 diabetes. SGLT2 inhibitors control glucose levels by preventing reabsorption of glucose at the proximal renal tubules thereby enhancing urinary glucose excretion. This insulin-independent mechanism of glycemic control creates a reduction in circulating insulin and increased glucagon levels. As a result, a metabolic shift towards lipolysis and hepatic ketogenesis may occur leading to ketoacidosis that is often euglycemic in nature [14]. One of several risk factors for SGLT2 inhibitor related ketoacidosis is low carbohydrate intake [14], [15], [16], [17], [18]. We present a case with a patient in SRSE who experienced an inability to sustain ketosis with KD alone, but after the addition of an SGLT2 inhibitor, consistent ketosis was achieved.
2. Case
A 42-year-old female with a long standing history of drug resistant epilepsy in the setting of cerebral palsy. Based on previous records, her seizures were only described as her waking up with tongue trauma and bladder or bowel incontinence, but were overall controlled with phenytoin monotherapy. Previous work-ups have included an MRI of her brain with and without contrast which was reportedly negative in her early to mid-thirties. A routine EEG was abnormal due to continuous background and generalized theta slowing and the occurrence of frequent, nearly continuous atypical absence seizures.
She developed refractory SE after transitioning from phenytoin to a combination of levetiracetam and lamotrigine because of concern of phenytoin associated long-term side effects. She presented to an outside hospital with clusters of breakthrough seizures in the setting of hyponatremia, hypoglycemia, and subtherapeutic serum levetiracetam levels. Intravenous levetiracetam was loaded and her outpatient dosage was continued with the addition of intravenous lacosamide. The patient's seizures initially improved for several days before reverting back to refractory SE. She was intubated, sedated with propofol, and loaded with fosphenytoin. A bedside electroencephalograph (EEG) was performed off propofol and interpreted as an onset of rhythmic 1–2 Hz sharp activity emanating from the right fronto-temporal region synchronous with observed rhythmic left jaw twitching, then limb twitching, and finally lower extremity twitching. This soon became bilateral synchronous polyspike-like discharges confirming refractory SE at which time propofol was restarted. She was transferred to our level 4 Comprehensive Epilepsy Center for further evaluation and treatment.
While monitored with continuous video-EEG, the patient was maintained on scheduled fosphenytoin, lacosamide, levetiracetam, and continuous propofol. An MRI of her brain reported advanced, diffuse cerebellar atrophy, but no acute intracranial process. After four days of maintaining burst-suppression, attempts to wean propofol led to recurrent SE associated with facial twitching. This was associated with electrographic changes over the vertex and posterior head regions. Despite increasing lacosamide and maintaining optimized serum phenytoin levels, she failed to wean from propofol over the next week prompting the addition of a continuous midazolam infusion to maintain burst-suppression.
Over the next month, multiple anti-seizure drugs, including a trial of ketamine, were added without significant improvement. Despite these interventions, SRSE returned with every propofol or midazolam wean attempt. As a result of propofol-induced hypertriglyceridemia and midazolam tachyphylaxis, a pentobarbital infusion was started to replace the latter two infusions. Moreover, failure to wean off these tertiary SRSE therapies lead to an enrollment in a phase II trial evaluating allopregnanolone for SRSE treatment. However, the patient never received, nor was exposed to allopregnanolone as it was later halted and not initiated due to study closure [19].
Approximately 12 weeks after unsuccessful attempts to control the patient's SRSE without infusions of anesthetic agents, the ketogenic diet was initiated with daily monitoring of serum beta hydroxybutyrate (BHB) and serum glucose levels (Fig. 1). The patient was placed in a fasting state for 24 h prior to starting KD. KetoCal® 4:1 was started with a goal of 1780 kcals per day which consisted of 175.5 g (g) of fat, 36.5 g of protein, and 20.5 g of carbohydrates. All dextrose containing intravenous admixtures were changed to normal saline and enteral solutions were adjusted to minimize carbohydrate intake.
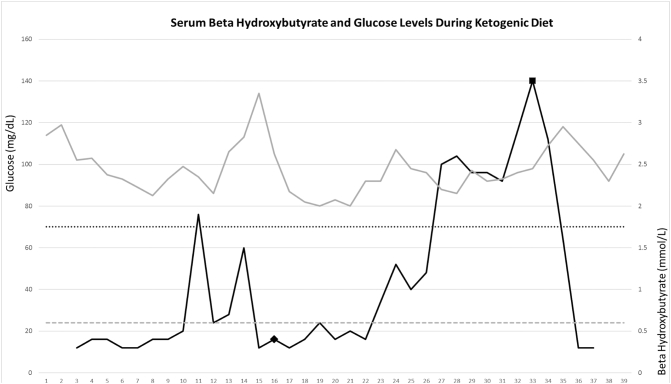
Fig. 1.
Serum beta hydroxybutyrate and glucose levels recorded over time (days) during KD. Serum glucose plotted from the left y-axis values with 70 mg/dL (hypoglycemia threshold) represented with black dotted line. Serum beta hydroxybutyrate levels plotted from right y-axis values with 0.6 mmol/L (upper limit of normal) represented with grey dashed line. SGLT2 inhibitor introduced on day 16 post KD represented by the black diamond with ketosis occuring in 7 days. Initiation of lorazepam infusion (propylene glycol source) is represented by a black square on day 33 post KD with subsequent normalization of beta hydroxybutyrate.
After about a week without consistent ketosis, her macronutrient intake was adjusted to account for the metabolic impact of 40% propylene glycol additive in pentobarbital [20]. KetoCal® 4:1 was reduced to 1068 kcals per day and the remaining nutritional goal was corrected with enteral medium chain triglyceride (MCT) oil. Over the next week, intermittent elevation in serum BHB occurred, but sustained ketosis was not achieved.
16 days after starting KD, dapagliflozin (SGLT2 inhibitor) 10 mg daily via nasal gastric tube was added in attempt to augment ketone production while maintaining euglycemia. After one week, the patient entered a consistent state of ketosis which allowed the pentobarbital infusion to be weaned off for the first time in 65 days. With the absence of propylene glycol, the MCT oil was discontinued and the original KD formula was resumed.
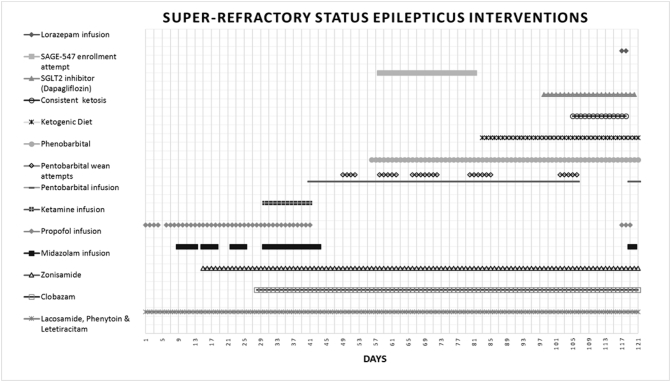
Despite undetectable serum pentobarbital levels 72 h after its discontinuation, the patient remained severely encephalopathic. Ten days after pentobarbital discontinuation, SRSE returned prompting reintroduction of propofol, lorazepam, and eventually pentobarbital infusions. This occurred despite maintaining ketosis during this time. Shortly after lorazepam infusion began, ketosis was lost likely due its high propylene glycol content. A multidisciplinary discussion took place resulting in a final decision of comfort care. The patient expired after 120 days of SRSE management summarized in Fig. 2.
Fig. 2.
Representation of SRSE interventions over 121 day course in the neuroscience intensive care unit. Tertiary anesthetic agents were used including pentobarbital titrated for burst suppression starting on day 40. Several pentobarbital weaning attempts are noted with black diamonds with eventual discontinuation on day 105 following consistent ketosis obtainment. Pentobarbital was resumed on day 119 due to relapse of SRSE.
3. Discussion
KD for seizure control was first described in the literature in 1921 yet the exact mechanism is unknown and seems to be complex [21], [22]. Although KD is more commonly used for drug resistant epilepsy primarily in children, it is emerging as an adjunctive treatment option for adult patients in SRSE. One challenge for treating patients in SRSE who do not respond to pharmacological therapy is that there are no current guidelines that recommend the use or specify the timing of KD.
The ability of KD to control SRSE has been reported in several case reports, small case series, and a small prospective multicenter trial [5], [6], [7], [8], [9], [10], [11], [12]. A retrospective case review of 10 patients from 4 medical centers utilizing KD for SRSE described a 90% ketosis obtainment with SE resolution [11] The median time to KD initiation was 21.5 days and the time to ketosis ranged from one to seven days (median of three days). Minimal side effects were reported including increased triglycerides in two patients and acidosis in one patient. More recently, Cervenka and colleagues performed a prospective multicenter observational trial to investigate the feasibility, safety, and efficacy of KD for SRSE [12]. Fifteen patients were enrolled and all patients achieved ketosis. The median time to KD initiation was ten days and the time to ketosis ranged from 0 to 16 days (median of two days). SRSE resolved in 73% of patients with minimal side effects. The authors concluded that this study provided preliminary evidence that KD is a feasible, safe, and effective adjunctive treatment for SRSE management with the need for comparative randomized placebo-controlled trials to assess outcomes. Combining the two previously described studies, the median time to initiate KD after SRSE onset was only 17 days with a range of 2–60 days [11], [12]. The one patient who failed to achieve ketosis in these studies did not undergo KD initiation until day 60.
Our case was challenging in that consistent ketosis was not achieved after more than two weeks of KD despite multidisciplinary efforts to limit carbohydrate intake. It is unknown why this patient was resistant to KD, but several exogenous factors may have contributed. One possibility is that delaying initiation of KD may allow metabolic changes that delay induction of ketosis. For our patient, KD was not initiated until 82 days after admission. This delay may have allowed for accumulation of glycogen storage postponing fat-dependent metabolism.
Another potential factor for delayed ketosis involves propylene glycol which is typically used as a solvent for several intravenous medications including benzodiazepines and pentobarbital. The patient described above underwent a prolonged pentobarbital infusion with significant amounts of propylene glycol which may have provided an energy source contributing to delayed ketosis. This significant exposure to a propylene glycol energy source may have factored into delayed ketosis. Additionally, there remains the possibility that unrecognized additives were given that may have negatively impacted induction of ketosis. Despite the lack of human studies investigating propylene glycol on delaying ketosis, there is a wide array of literature within the cattle industry outlining the successful use of propylene glycol as a treatment for ketosis. For example, Jenkins and colleagues demonstrated that cows with subclinical ketosis were likely to be cured after treatment with propylene glycol [24].
After exhausting multiple treatment modalities and considering the patient's severity of illness, we decided to initiate an SGLT2 inhibitor in an attempt to augment ketogenesis given that we were not able to achieve sustained ketosis with the KD alone. SGLT2 inhibitors have the potential to induce significant ketosis as demonstrated by a large claims database analysis concluding about a two-fold risk of developing ketoacidosis with a rate of 4.9 vs 2.3 events per 1000 person years [15]. In 2015 the Food and Drug Administration released a drug safety communication regarding the potential risk for ketosis with SGLT2 inhibitors and reports have highlighted increased risk when combined with a low carbohydrate diet [14], [15], [16], [17], [18], [23]. In a small (N = 23) randomized open-label exploratory study, Yabe and colleagues evaluated ketone production in Japanese diabetic patients receiving luseogliflozin (SGLT2 inhibitor) 2.5 mg while consuming 55% of total energy source from carbohydrates compared to 40% (low carbohydrate arm) [18]. Participants were started on their protocol diet for 14 days with SGLT2 inhibitor initiation on days 8–15. Blood samples were drawn on day 1, 8 and 15 to assess ketone production. Ketone bodies on day 15 (7 days after SGLT2 inhibitor initiation) were significantly higher in the low carbohydrate group. The authors concluded that strict low carbohydrate consumption while on SGLT2 inhibitors should be avoided due to increased risk for ketoacidosis.
To our knowledge, this is the first SRSE patient that achieved consistent ketosis within 7 days post SGLT2 initiation while on KD without hypoglycemic events (Fig. 1). Although SGLT2 inhibitors do not directly induce hypoglycemia, serum glucose should be monitored closely as KD alone can lead to hypoglycemia [14]. An obvious limitation in this report is the lack of any control group and therefore, it is uncertain whether the addition of an SGLT2 inhibitor augmented ketone production or if consistent ketosis was from KD and more time alone. As such, use of SGLT2 inhibitors for this indication should be reserved for patients who do not readily achieve ketosis with KD alone. If data continues to support KD initiation for adjunctive treatment of SRSE, then there may be clinical utility in formally evaluating the safety and efficacy of SGLT2 inhibitor augmentation of KD and whether it leads to faster ketosis and potentially faster SRSE resolution.
4. Conclusion
Despite recommendations to avoid low carbohydrate diets when taking a SGLT2 inhibitors due to risk of ketoacidosis in normal circumstances, there could be therapeutic value in SRSE patients that are unable to achieve consistent ketosis with KD alone. Our case suggests that initiation of a SGLT2 inhibitor is a safe adjunct that may augment the production of ketones in patients on a KD. It remains to be shown whether such intervention conclusively enhances ketone production compared to KD alone and whether this impact has any effect on morbidity or mortality in this critically ill population. In addition, the consideration of early KD initiation should be contemplated when unable to wean from general anesthetic therapies. By doing so, potential factors that may delay ketosis can be identified and corrected in order to attain the best outcome.
Ethical statement
The following article is of original work and complies with all submission instructions to the best of our knowledge. All authorship requirements have been met and the final manuscript was approved by all authors. This article has not been published elsewhere and is not under consideration for publication elsewhere. No source funding took place and there were no conflicts of interest or ethical dilemmas involved with any of the following authors.
Conflict of interest statement
The authors involved declare no conflict of interest.
References
- 1.Hocker S.E., Britton J.W., Mandrekar J.N., Wijdicks E.F., Rabinstein A.A. Predictors of outcome in refractory status epilepticus. JAMA Neurol. 2013;70:72–77. doi: 10.1001/jamaneurol.2013.578. [DOI] [PubMed] [Google Scholar]
- 2.Ferlisi M., Shorvon S. The outcome of therapies in refractory and super-refractory convulsive status epilepticus and recommendations for therapy. Brain. 2012;135:2314–2328. doi: 10.1093/brain/aws091. [DOI] [PubMed] [Google Scholar]
- 3.Hocker S., Tatum W.O., LaRoche S., Freeman W.D. Refractory and super-refractory status epilepticus: an update. Curr Neurol Neurosci Rep. 2014;14:452. doi: 10.1007/s11910-014-0452-x. [DOI] [PubMed] [Google Scholar]
- 4.Brophy G.M., Bell R., Claassen J., Alldredger B., Bleck T.P., Glauser T. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care. 2012;17:3–23. doi: 10.1007/s12028-012-9695-z. [DOI] [PubMed] [Google Scholar]
- 5.Bodenant M., Moreau C., Sejourne C., Auvin S., Delval A., Cuisset J.M. Interest of the ketogenic diet in a refractory status epilepticus in adults. Rev Neurol. 2008;164:194–199. doi: 10.1016/j.neurol.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 6.Cervenka M.C., Hartman A.L., Venkatesan A., Geocadin R.G., Kossoff E.H. The ketogenic diet for medically and surgically refractory status epilepticus in the neurocritical care unit. Neurocrit Care. 2011;15:519–524. doi: 10.1007/s12028-011-9546-3. [DOI] [PubMed] [Google Scholar]
- 7.Nabbout R., Mazzuca M., Hubert P., Peudennier S., Allaire C., Flurin V. Efficacy of ketogenic diet in severe refractory status epilepticus initiating fever induced refractory epileptic encephalopathy in school age children (FIRES) Epilepsia. 2010;51:2033–2037. doi: 10.1111/j.1528-1167.2010.02703.x. [DOI] [PubMed] [Google Scholar]
- 8.Nam S.H., Lee B.L., Lee C.G., Yu H.J., Joo E.Y., Lee J. The role of ketogenic diet in the treatment of refractory status epilepticus. Epilepsia. 2011;52:e181–e184. doi: 10.1111/j.1528-1167.2011.03289.x. [DOI] [PubMed] [Google Scholar]
- 9.Wusthoff C.J., Kranick S.M., Morley J.F., Christina Bergqvist A.G. The ketogenic diet in treatment of two adults with prolonged nonconvulsive status epilepticus. Epilepsia. 2010;51:1083–1085. doi: 10.1111/j.1528-1167.2009.02388.x. [DOI] [PubMed] [Google Scholar]
- 10.Strzelczyk A., Reif P.S., Bauer S., Belke M., Oertel W.H., Knake S. Intravenous initiation and maintenance of ketogenic diet: proof of concept in super-refractory status epilepticus. Seizure. 2013;22:581–583. doi: 10.1016/j.seizure.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 11.Thakur K.T., Probasco J.C., Hocker S.E., Roehl K., Henry B., Kossoff E.H. Ketogenic diet for adults in super-refractory status epilepticus. Neurology. 2014;82:665–670. doi: 10.1212/WNL.0000000000000151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cervenka M.C., Hocker S., Koenig M., Bar B., Henry-Barron B., Kossoff E.H. Phase I/II multicenter ketogenic diet study for adult superrefractory staus epilepticus. Neurology. 2017;88:938–943. doi: 10.1212/WNL.0000000000003690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kossoff E.H., Zupec-Kania B.A., Amark P.E., Ballaban-Gil K.R., Christina Bergqvist A.G., Blackford R. Optimal clinical management of children receiving the ketogenic diet: recommendations of the International Ketogenic Diet Study Group. Epilepsia. Feb 2009;50(2):304–317. doi: 10.1111/j.1528-1167.2008.01765.x. [DOI] [PubMed] [Google Scholar]
- 14.Ogawa W., Sakaguchi K. Euglycemic diabetic ketoacidosis induced by SGLT2 inhibitors: possible mechanism and contributing factors. J Diabetes Investig. 2016;7:135–138. doi: 10.1111/jdi.12401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fralick M., Schneeweiss S., Patorno E. Risk of diabetic ketoacidosis after initiation on an SGLT2 inhibitor. N Engl J Med. 2017;376:2300–2302. doi: 10.1056/NEJMc1701990. [DOI] [PubMed] [Google Scholar]
- 16.Handelsman Y., Henry R.R., Bloomgarden Z.T., Dagogo-Jack S., DeFronzo R.A., Einhorn D. American association of clinical endocrinologists and American college of endocrinology position statement on the association of SGLT-2 inhibitors and diabetic ketoacidosis. Endocr Pract. 2016;22:753–762. doi: 10.4158/EP161292.PS. [DOI] [PubMed] [Google Scholar]
- 17.Hayami T., Kato Y., Kamiya H., Kodo M., Naito E., Sugiura Y. Case of ketoacidosis by sodium-glucose cotransporter 2 inhibitor in a diabetic patient with low carbohydrate diet. J Diabetes Investig. 2015;6:587–590. doi: 10.1111/jdi.12330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yabe D., Iwasaki M., Kuwata H., Haraguchi T., Hamamoto Y., Kurose T. Sodium-glucose cotransporter 2 inhibitor use and dietary carbohydrate intake in Japanese individuals with type 2 diabetes: a randomized, open-label, 3-arm parallel comparative, exploratory study. Diabetes Obes Metab. 2017;19:739–743. doi: 10.1111/dom.12848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosenthal E.S., Claassen J., Wainwright M.S., Husain A.M., Vaitkevicius H., Raines S. Brexanolone as adjunctive therapy in super-refractory status epilepticus. Ann Neurol. 2017;82:342–352. doi: 10.1002/ana.25008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nembutal (pentobarbital) [prescribing information]. Lake Forest, IL Oak Pharmaceuticals. November 2013. [Google Scholar]
- 21.Masino S.A., Rho J.M. Mechanisms of ketogenic diet action. In: Noebels J.L., Avoli M., Rogawski M.A., editors. Jasper's basic mechanisms of the epilepsies [internet] 4th ed. National Center for biotechnology information (US); Bethesda (MD): 2012. [Google Scholar]
- 22.Geyelin H. Fasting as a method for treating epilepsy. Med Rec. 1921;99:1037–1039. [Google Scholar]
- 23.FDA/CEDR Resources Page Food and drug administration web site. https://www.fda.gov/drugs/drugsafety/ucm475463.htm
- 24.Jenkins N.T., Pena G., Risco C., Barbosa C.C., Vieira-Neto A., Galvao K.N. Utility of inline milk fat and protein ratio to diagnose subclinical ketosis and to assign propylene glycol treatment in lactating dairy cows. Can Vet J. 2015;56:850–854. [PMC free article] [PubMed] [Google Scholar]