Abstract
Soil-transmitted helminths infect 1.5 billion people worldwide. Treatment with anthelminthics is the key intervention but interactions between anthelminthic agents and the gut microbiota have not yet been studied. In this study, the effects of four anthelminthic drugs and combinations (tribendimidine, tribendimidine plus ivermectin, tribendimidine plus oxantel-pamoate, and albendazole plus oxantel-pamoate) on the gut microbiota were assessed. From each hookworm infected adolescent, one stool sample was collected prior to treatment, 24 h post-treatment and 3 weeks post-treatment, and a total of 144 stool samples were analyzed. The gut bacterial composition was analyzed using 16S rRNA gene sequencing. Tribendimidine given alone or together with oxantel-pamoate, and the combination of albendazole and oxantel pamoate were not associated with any major changes in the taxonomic composition of the gut microbiota in this population, at both the short-term post-treatment (24 h) and long-term post-treatment (3 weeks) periods. A high abundance of the bacterial phylum Bacteroidetes was observed following administration of tribendimidine plus ivermectin 24 h after treatment, due predominantly to difference in abundance of the families Prevotellaceae and Candidatus homeothermaceae. This effect is transient and disappears three weeks after treatment. Higher abundance of Bacteroidetes predicts an increase in metabolic pathways involved in the synthesis of B vitamins. This study highlights a strong relationship between tribendimidine and ivermectin administration and the gut microbiota and additional studies assessing the functional aspects as well as potential health-associated outcomes of these interactions are required.
Keywords: Soil-transmitted helminths, Gut microbiota, Anthelminthics, Microbiome-drug interaction
Graphical abstract
Highlights
-
•
Tribendimidine in combination with ivermectin modulate the gut microbiota.
-
•
Bacteroidetes phylum is more abundant 24 h after treatment.
-
•
B vitamins biosynthesis pathways are over-represented 24 h post-treatment.
-
•
Tribendimidine/ivermectin effect is transient and disappears within 3 weeks.
1. Introduction
Recent estimates suggest that the worldwide prevalence of soil-transmitted helminths (STHs), including infection with Ascaris lumbricoides, Trichuria trichuris and hookworm, is 1.5 billion people (Hotez et al., 2009; Pullan et al., 2014; Jourdan et al., 2017). The total estimated burden due to STHs is 3.4 million disability-adjusted life years (GBD, 2015 DALYs and HALE Collaborators, 2016). The symptoms associated with STH infection are not specific and are usually more severe and debilitating in school-aged children and in the elderly. Children who are chronically infected can display malnutrition and developmental delay while elderly people infected with STHs often display reduced work-related productivity (Bethony et al., 2006).
Four drugs (albendazole, mebendazole, pyrantel pamoate, levamisole) are on the World Health Organization's list of essential medicines for the treatment of STH infections (WHO, 2002). The two benzimidazoles (albendazole and mebendazole) are most commonly used drugs in preventive chemotherapy programs (WHO, 2002; Keiser and Utzinger, 2008; Abou-Zeid et al., 2012). Ivermectin has a broad spectrum of activity against different parasites ranging from nematodes such as Strongyloides stercoralis or A. lumbricoides to filarial parasites, and arthropods and even affects the Anopheline vectors of malaria (Marti et al., 1996; Wen et al., 2008; Grayson et al., 2010; Ōmura and Crump, 2017). Oxantel has high activity against T. trichuris (Zaman and Sabapathy, 1975; Horton, 2003; Speich et al., 2014). Finally, tribendimidine has been shown to have a similar spectrum to that of albendazole, and could be an alternative to the latter in case of emergence of benzimidazole resistance (Xiao et al., 2013). There is growing consensus to use these drugs in combination to increase efficacy and decrease the risk of drug resistance.
Recent advancements in high-throughput sequencing technologies enable the characterization of the human microbiome (Turnbaugh et al., 2007; Arumugam et al., 2011; Human Microbiome Project Consortium, 2012), which was not possible at the time those drugs were introduced. Thus, the interactions of these anthelminthic drugs and drug combinations with the human gut microbiota composed of a variety of microorganisms, including other eukaryotic parasites, protozoa, viruses, fungi and most importantly bacteria (Nicholson et al., 2005; Lozupone et al., 2012; Schneeberger et al., 2016), can now be studied. Drug-microbiota interactions can modulate the bioavailability and activity of drugs and hence modulate their efficacy (Nicholson et al., 2005; Clayton et al., 2009; Wilson and Nicholson, 2009; Cheng et al., 2013; Maurice et al., 2013). STHs share the same environmental niche as the gut bacterial microbiota, but potential interactions between the gut microbiota and anthelminthic agents have not been assessed to date. The aim of this study was to identify potential interactions between these treatments and the non-target microbiota.
2. Methods
2.1. Sample collection and ethics statement
Samples were collected in the framework of a randomized, controlled, single blind, non-inferiority trial in the Agboville district in Côte d’Ivoire. Hookworm positive adolescents (age 15 to 18), confirmed by quadruplicate Kato-Katz thick smears, were randomly assigned to four treatment arms, including tribendimidine (400 mg), tribendimidine (400 mg) plus ivermectin (200 μg/kg), tribendimidine (400 mg) plus oxantel pamoate (25 mg/kg) and albendazole (400 mg) plus oxantel pamoate (25 mg/kg). Details about the study are presented elsewhere (Moser et al., 2017). From each adolescent, one stool sample was collected prior to treatment, 24 h post-treatment and 3 weeks post-treatment, and a total of 144 stool samples were analyzed (Table 1).
Table 1.
Summary of volunteers investigated in this study. From each adolescent of each of the four treatments, a sample was collected before, 24 h after and 3 weeks after treatment. EPG = Hookworm eggs per gram of stool, FU = follow-up.
| ID | Samples | EPG (baseline) | EPG (FU) | ID | Samples | EPG (baseline) | EPG (FU) |
|---|---|---|---|---|---|---|---|
|
Treatment arm 1 |
Treatment arm 2 |
||||||
| P- 5 | D6, E6, F6 | 318 | 426 | P- 6 | D8, E7, F7 | 144 | 0 |
| P- 7 | D9, E8, F8 | 30 | 0 | P- 17 | D21, E18, F18 | 12 | 0 |
| P- 9 | D11, E10, F10 | 96 | 132 | P- 19 | D23, E20, F20 | 84 | 0 |
| P- 10 | D12, E11, F11 | 36 | 228 | P- 23 | D27, E24, F24 | 1002 | 0 |
| P- 11 | D13, E12, F12 | 198 | 108 | P- 27 | D31, E28, F28 | 72 | 0 |
| P- 14 | D16, E15, F15 | 78 | 0 | P- 33 | D42, E30, F30 | 534 | 0 |
| P- 22 | D26, E23, F23 | 450 | 78 | P- 34 | D43, E31, F31 | 78 | 0 |
| P- 26 | D30, E27, F27 | 36 | 0 | P- 35 | D44, E44, F44 | 42 | 0 |
| P- 29 | D34, E40, F40 | 102 | 6 | P- 43 | D78, E45, F45 | 30 | 0 |
| P- 32 | D39, E43, F43 | 336 | 54 | P- 46 | D91, E37, F37 | 18 | 0 |
| P- 38 | D55, E33, F33 | 78 | 0 | P- 48 | D95, E34, F34 | 4740 | 0 |
| P- 41 |
D76, E38, F38 |
240 |
0 |
||||
|
Treatment arm 3 |
Treatment arm 4 |
||||||
| P- 2 | D3, E3, F3 | 450 | 66 | P- 1 | D1, E1, F1 | 42 | 36 |
| P- 3 | D4, E4, F4 | 966 | 0 | P- 4 | D5, E5, F5 | 96 | 288 |
| P- 8 | D10, E9, F9 | 186 | 210 | P- 15 | D18, E16, F16 | 534 | 0 |
| P- 12 | D14, E13, F13 | 126 | 0 | P- 20 | D24, E21, F21 | 66 | 0 |
| P- 13 | D15, E14, F14 | 492 | 108 | P- 21 | D25, E22, F22 | 204 | 90 |
| P- 16 | D20, E17, F17 | 630 | 516 | P- 24 | D28, E25, F25 | 426 | 24 |
| P- 18 | D22, E19, F19 | 696 | 282 | P- 25 | D29, E26, F26 | 72 | 108 |
| P- 28 | D33, E29, F29 | 1824 | 0 | P- 31 | D37, E41, F41 | 222 | 204 |
| P- 30 | D35, E42, F42 | 1698 | 1302 | P- 37 | D52, E46, F46 | 258 | 48 |
| P- 36 | D50, E47, F47 | 222 | 24 | P- 40 | D62, E36, F36 | 912 | 0 |
| P- 39 | D58, E48, F48 | 60 | 12 | P- 42 | D77, E39, F39 | 582 | 0 |
| P- 44 | D79, E2, F2 | 270 | 0 | P- 47 | D94, E32, F32 | 2580 | 60 |
| P- 45 | D86, E35, F35 | 168 | 0 | ||||
Ethical approval was obtained from the Comité National d’Ethique et de la Recherche in Côte d’Ivoire (083/MSHP/CNER-kp) and the Ethics Committee of North-western and Central Switzerland (EKNZ UBE-15/35). The trial was registered with ISRCTN (number 14373201).
2.2. Sample collection
For DNA isolation, 150–250 mg of stool sample was diluted in 500 μl of AVL buffer (Qiagen, Darmstadt, Germany) and subsequently homogenized using a soil-grinding SK38 kit on the Precellys 24 system (Bertin Technologies, Saint-Quentin, France). Homogenized samples were further extracted on a Magna Pure 96 system (Roche, Basel, Switzerland) using the DNA and Viral NA Large Volume kit (Roche, Basel, Switzerland) according to the manufacturers' protocol.
2.3. 16S amplicon PCR
2.5 μl of isolated DNA was used to perform amplification of the V3-V4 region using the following primer pair:
Forward primer = 5′-TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGCCTACGGGNGGCWGCAG
Reverse primer = 5′-GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGACTACHVGGGTATCTAATCC
The polymerase chain reaction (PCR) was performed in 25 μl reaction volumes using the 2X KAPA HiFi HotStar ReadyMix (KAPA Biosystems; Boston, MA, USA). The thermocycler was set as follows: 95 °C for 3 min, 25 cycles of 95 °C (30 s), 55 °C (30 s) and 72 °C (30 s), one additional step at 72 °C for 5 min and finally set on hold indefinitely at 4 °C. The quality of the amplified product was controlled visually on a 1% agarose gel. The amplicons were purified using an AMPure XP beads (Beckman-Coulter; Fullerton, CA, USA) protocol.
2.4. Sequencing
We used a Nextera XT Index kit (Illumina, San Diego, CA, United States of America) to perform the index PCR and generate barcoded pools of 96 samples. The amplification reaction was conducted on a thermocycler under the following condition: 95 °C for 3 min, 20 cycles of 95 °C for 30 s, 55 °C for 30 s, 72 °C for 30 s, one additional step at 72 °C for 5 min and a final step at 4 °C until further processing. Amplicons were cleaned with AMPure XP beads according to Illuminas' library preparation guide. The quality of the product was assessed using a 1% agarose gel and the quantification was performed using a Qubit Fluorimeter (Life Technologies, Carlsbad, CA, United States of America) and the corresponding High-sensitivity DNA assays (Life Technologies, USA). The 96 amplicon samples were pooled together in an equimolar way and loaded on a cartridge on the Illumina Miseq sequencing platform (Illumina, USA). We used V3 reagents (2*300bp) (Illumina, USA) for this experiment in 2*300 base pair mode.
2.5. Data processing and statistical analysis
Raw Fastq files were filtered using a quality score (Phred) above Q20. Filtered reads were fed in the QIIME pipeline (Caporaso et al., 2010) v 1.9.1, which was configured for standard OTU picking (closed reference, Greengenes database v.13_8). Shannon diversity index was computed with QIIME using a rarefaction depth of 19500 sequences and principal component analysis (PCA) plots were generated with the STAMP statistical analysis package (Parks et al., 2014) using Euclidean distance matrices. Taxonomic profiles were further analyzed with the PICRUSt suite (Langille et al., 2013), in order to predict the functional content of the gut microbiota based on the abundance of 16S rRNA genes. To compare groups (treatment arms), we performed an analysis of variance using the STAMP software (Parks et al., 2014), both for taxonomical and functional profiles. Benjamini-Hochberg correction (Benjamini and Hochberg, 1995) was applied to the uncorrected statistics, to control for multiple testing bias. Statistical significance is reached with a corrected p-value (q-value) below 0.1.
3. Results
3.1. Overall comparison of the taxonomic composition between treatment arms
Pre-processing, including filtering and denoising of the sequence datasets resulted in the analysis of a total of 2,311,784 high quality reads for the 48 pre-treatment samples (median = 55,000), 2,261,185 reads for the 48 samples collected 24 h after treatment (median = 56,826), and 2,580,596 filtered reads for the 48 samples collected in the follow-up period of 3 weeks (median = 59,889). We compared the overall microbiota diversity of all samples, grouped by treatment arm, using a principal component analysis and the Shannon diversity index, as shown in Fig. 1. Complete taxonomic profiles stratified by treatment arm are shown in Supplementary Fig. 1.
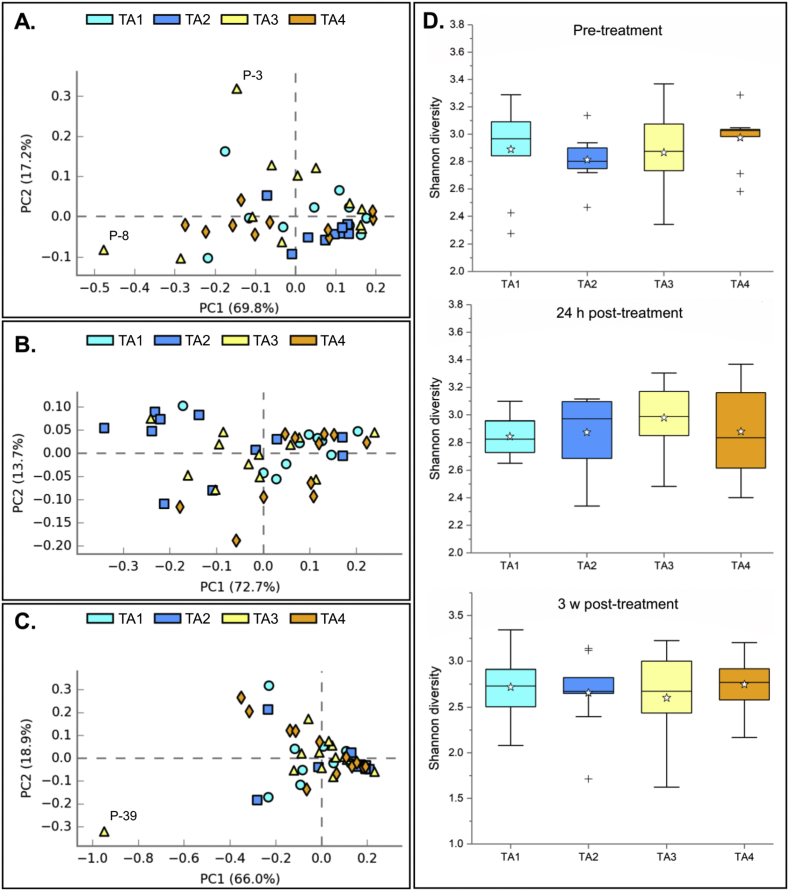
Fig. 1.
Overall diversity comparison of taxonomic composition at the phylum level, stratified by treatment arm. TA1 = Tribendimidine; TA2 = Tribendimidine plus ivermectin; TA3 = Tribendimidine plus oxantel pamoate; TA4 = Albendazole plus oxantel pamoate. Panel A: pre-treatment. Panel B: 24 h post-treatment. Panel C: 3 weeks post-treatment. Panel D shows the Shannon diversity index at each time point. h = hours; w = weeks.
Treatment groups were not associated with significant differences in taxonomic composition or Shannon diversity at any time-point. Although two samples (P-3 and P-8) from treatment arm 3 (tribendimidine plus oxantel pamoate) differed significantly from the rest of the population before treatment, there was no observable difference in gut bacterial composition of these two patients both at 24 h post-treatment and 3 weeks post treatment.
At 24 h after drug administration, the principal component analysis showed a homogeneous population and the SDI across all treatment arms was not significantly different. However, there was a noticeable spread (∼0.35–0.2) of samples belonging to treatment arm 2 over component 1 (x-axis), while samples from this group all clustered tightly before treatment (within coordinates −0.1 and ∼0.1 on both axis).
Finally, the observation of a generally homogeneous bacterial composition of the gut microbiota at the phylum level in the studied population was confirmed in the samples collected 3 weeks after administration of the treatments. According to the PCA, the sample collected from patient 39 differed from the rest of the population, at this sampling time. The SDI for this sample was low (SDI = 1.72) compared to the rest of the group.
3.2. Differences in taxonomic composition after administration of the four treatments
In order to assess the potential effect of each drug/drug combination tested in this study on the gut microbiota, we compared abundance means of each bacterial taxon between groups, at different taxonomic levels (phylum and family). At baseline, there was no phylum which was differentially abundant between the four treatment arms. 24 h after treatment, there was a significantly higher abundance (q-value < 0.1) of species from the Bacteroidetes phylum in samples from treatment arm 2. The relative abundance of Bacteroidetes was greater in treatment arm 2 than all other groups 24 h after treatment, but not at baseline nor at 3 weeks.
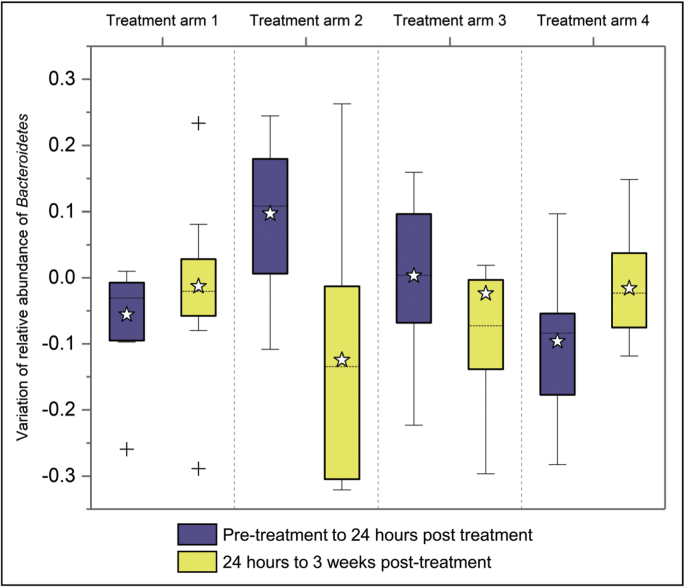
In order to quantify the degree of variation of Bacteroidetes over time, we compared those from pre-to 24 h post-treatment samples and those from 24 h post-to 3 weeks post-treatment samples, as shown in Fig. 2.
Fig. 2.
Box plot showing the abundance variation of the phylum Bacteroidetes. This plot summarizes variations of abundance of Bacteroidetes between two sampling times (from pre-treatment to 24 h post-treatment and 24 h to 3 weeks post-treatment), by treatment arm. Treatment arm 1 = Tribendimidine; Treatment arm 2 = Tribendimidine plus ivermectin; Treatment arm 3 = Tribendimidine plus oxantel pamoate; Treatment arm 4 = Albendazole plus oxantel pamoate.
In order to further characterize potential drug-bacteria interactions, we screened lower taxonomic levels and found two families, which follow a similar abundance pattern (A) to that of Bacteroidetes (ATA2>ATA1 = ATA3 = ATA4), namely S24_7 (recently renamed to Candidatus homothermaceae (Ormerod et al., 2016),) and Prevotellaceae. However, for both families, the significance for inter-group difference was not reached (q-value > 0.1).
3.3. Prediction of metabolic pathways
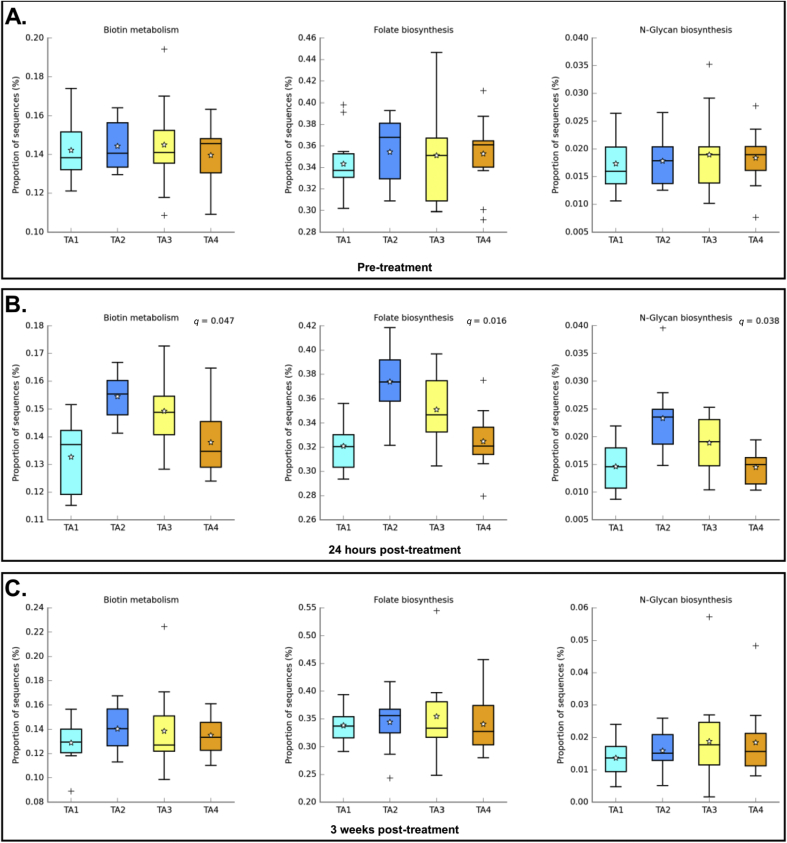
In order to highlight possible interactions, we compared the predicted abundance of metabolic pathways obtained with PICRUSt between the four treatment arms at each sampling time (Fig. 3).
Fig. 3.
Box plot comparing the abundance of predicted metabolic pathways at the different sampling times. TA1 = Tribendimidine; TA2 = Tribendimidine plus ivermectin; TA3 = Tribendimidine plus oxantel pamoate; TA4 = Albendazole plus oxantel pamoate. Panel A shows the abundance of biotin metabolism, folate biosynthesis and N-glycan biosynthesis pathways before treatment, by treatment arm. Panel B shows the abundance of the three metabolic pathways, 24 h after administration of treatment. Panel C highlights the abundance of the three metabolic pathways, 3 weeks after administration of treatment. Significance is achieved with a q-value < 0.1. q-values are shown only for comparisons which are significantly different.
In samples collected at baseline, the abundance of sequences related to biotin metabolism, folate, and N-glycan biosynthesis was the same across all treatment groups. The situation changed 24 h after treatment when these metabolic pathways became significantly more abundant in the tribendimidine-ivermectin treatment arm (q-values = 0.047, 0.016, and 0.038, for biotin metabolism, folate biosynthesis, and N-glycan biosynthesis, respectively). The abundance pattern was the same for each of the three pathways, the highest abundance being associated to treatment with tribendimidine in combination with ivermectin (treatment arm 2) and being lower for the three other groups. The situation after 3 weeks was similar to pre-treatment, and no differentially abundant pathway could be identified.
4. Discussion
There is a growing interest in associations between the human microbiome and drug therapy, which has been called “pharmacomicrobiomics” (Rizkallah et al., 2010; Wang et al., 2011; Klatt et al., 2017; Wilson and Nicholson, 2017). This study is the first investigating effects of different anthelminthic drugs and their combinations on the human gut microbiota of adolescents infected with hookworms in a tropical setting.
Several studies have shown that the effects of various parasitic infections on the gut microbiota are relatively modest (Cantacessi et al., 2014; Kay et al., 2015; Schneeberger et al., 2018), yet, there is only scarce information about the impact, direct (= drug effect on bacterial composition) or indirect (= change in bacterial composition is due to clearance of the worm), of drugs used to treat parasitic infections on the gut microbiota. Cross-reaction of drugs designed to target eukaryotic parasites between the gut microbiota and various treatments (e.g. anti-cancerous drugs) has been shown (Alexander et al., 2017; Roy and Trinchieri, 2017), and given the fact STHs and gut bacteria share the same environment these effects are possible for anthelminthic drugs as well.
Our analyses indicate that tribendimidine given alone or together with oxantel pamoate, and the combination of albendazole and oxantel pamoate is not associated with any major changes in the taxonomic composition of the gut microbiota in this population, at both the short-term post-treatment (24 h) and long-term post-treatment (3 weeks) periods. However, treatment with tribendimidine in combination with ivermectin was found to be associated with shifts in the relative abundance of Bacteroidetes. This phylum increased in relative abundance in the first 24 h post-administration and decreased over the 3 weeks post-treatment. Within the Bacteroidetes phylum, two bacteria families, Prevotellaceae and Candidatus Homeothermaceae (former S24_7 (Ormerod et al., 2016)), accounted for most of the variation observed in the abundance of Bacteroidetes.
We assessed the potential metabolic significance of this taxonomic shift using PICRUSt prediction, which accurately infers metabolic gene content from taxonomic composition (Lindgreen et al., 2016). Of all predicted metabolic pathways, three were significantly more abundant in samples from treatment arm 2, namely, biotin metabolism, folate biosynthesis, and N-glycan biosynthesis, and all are involved at some point in the biosynthesis of B vitamins. While the statistical proof of higher abundance of Prevotellaceae and Candidatus Homeothermaceae in patients from treatment arm 2 (tribendimidine plus ivermectin) is somewhat weaker to that of Bacteroidetes, it has been shown that both groups present characteristics highly relevant to each of the three differentially abundant metabolic pathways. Indeed, and while acknowledging that it does not encompass the complete range of their activities in the gut, members from the Prevotellaceae family almost ubiquitously harbor the genes associated with B vitamin biosynthesis (Magnúsdóttir et al., 2015). Similarly, one of the purposes of Candidatus Homeothermaceae in the gut of mammals is to regulate the synthesis of B vitamins (Ormerod et al., 2016). Of note, a possible cause for the lack of statistical power for findings observed in our study for these two taxa is the intrinsically weak taxonomic resolution of 16S rRNA gene identification, which frequently does not allow categorizing sequences at lower taxonomic levels, in this case, the family level. High level of biotin, or vitamin B7, in rat livers, has been associated with decreased absorption of orally administered vitamin B12 (Puddu and Marchetti, 1965). For folate (vitamin B9), decreased concentrations in combinations with low B12 levels have been associated with various symptoms, including anemia (Morris et al., 2007; Selhub et al., 2009), a common consequence of ivermectin administration (Ndyomugyenyi et al., 2008). However, in order to validate these observations and to understand these interactions in detail, additional studies investigating other parameters (e.g. monitoring of B vitamins levels after treatment) and tests with higher taxonomic resolution (e.g. strain identification with shotgun metagenomics) are required.
This study has limitations. Indeed, while the study design in its current form enables the identification of significant differences in the composition of the gut microbiota between different treatment groups, it does not allow distinguishing between direct or indirect effects. Future studies might be considered including treatment of uninfected patients and patients who received a placebo, although this might be challenging from an ethical point of view. However, it is worth highlighting that the observed effects (higher abundance of Bacteroidetes and specific metabolic pathways) are distributed homogeneously across all samples analyzed from the tribendimidine-ivermectin group, except for one sample measured 24 h post-treatment. Additionally, stable abundance of Bacteroidetes and the metabolic pathways is observed homogeneously across all samples from the other treatment groups, except for two samples. Both facts support the hypothesis of a re-compositional event triggered by the administration of tribendimidine plus ivermectin and rule out observed effects driven mainly by random outlying samples.
5. Conclusion
In conclusion, our study revealed several key findings of antihelminthic drugs/microbiota interactions. Tribendimidine, tribendimidine plus oxantel pamoate and albendazole plus oxantel-pamoate do not cause microbiome-specific effects and hence are unlikely to cause variability in response. On the other hand, treatment with a combination of tribendimidine and ivermectin triggers a re-compositional event of the gut microbiota, which was not observed for the other treatments studied. Moreover, a higher abundance of Bacteroidetes, and to some extent, of bacteria from the Prevotellaceae and Candidatus Homeothermaceae families as well as an overrepresentation of metabolic pathways related to B vitamins biosynthesis, was observed in patients treated with tribendimidine and ivermectin, 24 h after treatment. Importantly, the observed effects are transient as they disappear within three weeks after administration of this anthelminthic treatment.
Funding information
JK is grateful to the Swiss National Science Foundation for financial support (number 320030_14930/1).
Competing interests
The authors declare that they have no competing interests.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ijpddr.2018.07.001.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
References
- Abou-Zeid A.H., Abkar T.A., Mohamed R.O. Schistosomiasis and soil-transmitted helminths among an adult population in a war affected area, Southern Kordofan state, Sudan. Parasites Vectors. 2012;5:133. doi: 10.1186/1756-3305-5-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander J.L., Wilson I.D., Teare J., Marchesi J.R., Nicholson J.K., Kinross J.M. Gut microbiota modulation of chemotherapy efficacy and toxicity. Nat. Rev. Gastroenterol. Hepatol. 2017;14:356–365. doi: 10.1038/nrgastro.2017.20. [DOI] [PubMed] [Google Scholar]
- Arumugam M., Raes J., Pelletier E., Le Paslier D., Yamada T., Mende D.R., Fernandes G.R., Tap J., Bruls T., Batto J.M., Bertalan M., Borruel N., Casellas F., Fernandez L., Gautier L., Hansen T., Hattori M., Hayashi T., Kleerebezem M., Kurokawa K., Leclerc M., Levenez F., Manichanh C., Nielsen H.B., Nielsen T., Pons N., Poulain J., Qin J., Sicheritz-Ponten T., Tims S., Torrents D., Ugarte E., Zoetendal E.G., Wang J., Guarner F., Pedersen O., de Vos W.M., Brunak S., Dore J., Meta H.I.T.C., Antolin M., Artiguenave F., Blottiere H.M., Almeida M., Brechot C., Cara C., Chervaux C., Cultrone A., Delorme C., Denariaz G., Dervyn R., Foerstner K.U., Friss C., van de Guchte M., Guedon E., Haimet F., Huber W., van Hylckama-Vlieg J., Jamet A., Juste C., Kaci G., Knol J., Lakhdari O., Layec S., Le Roux K., Maguin E., Merieux A., Melo Minardi R., M'Rini C., Muller J., Oozeer R., Parkhill J., Renault P., Rescigno M., Sanchez N., Sunagawa S., Torrejon A., Turner K., Vandemeulebrouck G., Varela E., Winogradsky Y., Zeller G., Weissenbach J., Ehrlich S.D., Bork P. Enterotypes of the human gut microbiome. Nature. 2011;473:174–180. doi: 10.1038/nature09944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. Controlling the false discovery rate - a practical and powerful approach to multiple testing. J. Roy. Stat. Soc. B. 1995;57:289–300. [Google Scholar]
- Bethony J., Brooker S., Albonico M., Geiger S.M., Loukas A., Diemert D., Hotez P.J. Soil-transmitted helminth infections: ascariasis, trichuriasis, and hookworm. Lancet. 2006;367:1521–1532. doi: 10.1016/S0140-6736(06)68653-4. [DOI] [PubMed] [Google Scholar]
- Cantacessi C., Giacomin P., Croese J., Zakrzewski M., Sotillo J., McCann L., Nolan M.J., Mitreva M., Krause L., Loukas A. Impact of experimental hookworm infection on the human gut microbiota. J. Infect. Dis. 2014;210:1431–1434. doi: 10.1093/infdis/jiu256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso J.G., Kuczynski J., Stombaugh J., Bittinger K., Bushman F.D., Costello E.K., Fierer N., Pena A.G., Goodrich J.K., Gordon J.I. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X.-Y., Tian X.-L., Wang Y.-S., Lin R.-M., Mao Z.-C., Chen N., Xie B.-Y. Metagenomic analysis of the pinewood nematode microbiome reveals a symbiotic relationship critical for xenobiotics degradation. Sci. Rep. 2013;3:1869. doi: 10.1038/srep01869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton T.A., Baker D., Lindon J.C., Everett J.R., Nicholson J.K. Pharmacometabonomic identification of a significant host-microbiome metabolic interaction affecting human drug metabolism. Proc. Natl. Acad. Sci. U. S. A. 2009;106:14728–14733. doi: 10.1073/pnas.0904489106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GBD 2015 DALYs and HALE Collaborators Global, regional, and national disability-adjusted life-years (DALYs) for 315 diseases and injuries and healthy life expectancy (HALE), 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1603–1658. doi: 10.1016/S0140-6736(16)31460-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayson M.L., Crowe S.M., McCarthy J.S., Mills J., Mouton J.W., Norrby S.R., Paterson D.L., Pfaller M.A. CRC Press; 2010. Kucers' the Use of Antibiotics Sixth Edition: a Clinical Review of Antibacterial, Antifungal and Antiviral Drugs. [Google Scholar]
- Horton J. Global anthelmintic chemotherapy programs: learning from history. Trends Parasitol. 2003;19:405–409. doi: 10.1016/s1471-4922(03)00171-5. [DOI] [PubMed] [Google Scholar]
- Hotez P.J., Fenwick A., Savioli L., Molyneux D.H. Rescuing the bottom billion through control of neglected tropical diseases. Lancet. 2009;373:1570–1575. doi: 10.1016/S0140-6736(09)60233-6. [DOI] [PubMed] [Google Scholar]
- Human Microbiome Project Consortium Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourdan P.M., Lamberton P.H.L., Fenwick A., Addiss D.G. Soil-transmitted helminth infections. Lancet. 2017;391:252–265. doi: 10.1016/S0140-6736(17)31930-X. [DOI] [PubMed] [Google Scholar]
- Kay G.L., Millard A., Sergeant M.J., Midzi N., Gwisai R., Mduluza T., Ivens A., Nausch N., Mutapi F., Pallen M. Differences in the faecal microbiome in Schistosoma haematobium infected children vs. uninfected children. PLoS Neglected Trop. Dis. 2015;9 doi: 10.1371/journal.pntd.0003861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keiser J., Utzinger J. Efficacy of current drugs against soil-transmitted helminth infections: systematic review and meta-analysis. JAMA. 2008;299:1937–1948. doi: 10.1001/jama.299.16.1937. [DOI] [PubMed] [Google Scholar]
- Klatt N.R., Cheu R., Birse K., Zevin A.S., Perner M., Noel-Romas L., Grobler A., Westmacott G., Xie I.Y., Butler J., Mansoor L., McKinnon L.R., Passmore J.S., Abdool Karim Q., Abdool Karim S.S., Burgener A.D. Vaginal bacteria modify HIV tenofovir microbicide efficacy in African women. Science. 2017;356:938–945. doi: 10.1126/science.aai9383. [DOI] [PubMed] [Google Scholar]
- Langille M.G., Zaneveld J., Caporaso J.G., McDonald D., Knights D., Reyes J.A., Clemente J.C., Burkepile D.E., Thurber R.L.V., Knight R. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 2013;31:814–821. doi: 10.1038/nbt.2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgreen S., Adair K.L., Gardner P.P. An evaluation of the accuracy and speed of metagenome analysis tools. Sci. Rep. 2016;6:19233. doi: 10.1038/srep19233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozupone C.A., Stombaugh J.I., Gordon J.I., Jansson J.K., Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489:220–230. doi: 10.1038/nature11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnúsdóttir S., Ravcheev D., de Crécy-Lagard V., Thiele I. Systematic genome assessment of B-vitamin biosynthesis suggests co-operation among gut microbes. Front. Genet. 2015;6:148. doi: 10.3389/fgene.2015.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti H., Haji H.J., Savioli L., Chwaya H.M., Mgeni A.F., Ameir J.S., Hatz C. A comparative trial of a single-dose ivermectin versus three days of albendazole for treatment of Strongyloides stercoralis and other soil-transmitted helminth infections in children. Am. J. Trop. Med. Hyg. 1996;55:477–481. doi: 10.4269/ajtmh.1996.55.477. [DOI] [PubMed] [Google Scholar]
- Maurice Corinne F., Haiser Henry J., Turnbaugh Peter J. Xenobiotics shape the physiology and gene expression of the active human gut microbiome. Cell. 2013;152:39–50. doi: 10.1016/j.cell.2012.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris M.S., Jacques P.F., Rosenberg I.H., Selhub J. Folate and vitamin B-12 status in relation to anemia, macrocytosis, and cognitive impairment in older Americans in the age of folic acid fortification. Am. J. Clin. Nutr. 2007;85:193–200. doi: 10.1093/ajcn/85.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser W., Coulibaly J.T., Ali S.M., Ame S.M., Amour A.K., Yapi R.B., Albonico M., Puchkov M., Huwyler J., Hattendorf J. Efficacy and safety of tribendimidine, tribendimidine plus ivermectin, tribendimidine plus oxantel pamoate, and albendazole plus oxantel pamoate against hookworm and concomitant soil-transmitted helminth infections in Tanzania and Côte d'Ivoire: a randomised, controlled, single-blinded, non-inferiority trial. Lancet Infect. Dis. 2017;17:1162–1171. doi: 10.1016/S1473-3099(17)30487-5. [DOI] [PubMed] [Google Scholar]
- Ndyomugyenyi R., Kabatereine N., Olsen A., Magnussen P. Efficacy of ivermectin and albendazole alone and in combination for treatment of soil-transmitted helminths in pregnancy and adverse events: a randomized open label controlled intervention trial in Masindi district, western Uganda. Am. J. Trop. Med. Hyg. 2008;79:856–863. [PubMed] [Google Scholar]
- Nicholson J.K., Holmes E., Wilson I.D. Gut microorganisms, mammalian metabolism and personalized health care. Nat. Rev. Microbiol. 2005;3:431–438. doi: 10.1038/nrmicro1152. [DOI] [PubMed] [Google Scholar]
- Ormerod K.L., Wood D.L.A., Lachner N., Gellatly S.L., Daly J.N., Parsons J.D., Dal'Molin C.G.O., Palfreyman R.W., Nielsen L.K., Cooper M.A., Morrison M., Hansbro P.M., Hugenholtz P. Genomic characterization of the uncultured Bacteroidales family S24-7 inhabiting the guts of homeothermic animals. Microbiome. 2016;4:36. doi: 10.1186/s40168-016-0181-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks D.H., Tyson G.W., Hugenholtz P., Beiko R.G. STAMP: statistical analysis of taxonomic and functional profiles. Bioinformatics. 2014;30:3123–3124. doi: 10.1093/bioinformatics/btu494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puddu P., Marchetti M. The effect of vitamin B(12) and biotin on the metabolism of vitamin B(12) in biotin-deficient rats. Biochem. J. 1965;96:24–27. doi: 10.1042/bj0960024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullan R.L., Smith J.L., Jasrasaria R., Brooker S.J. Global numbers of infection and disease burden of soil transmitted helminth infections in 2010. Parasites Vectors. 2014;7:37. doi: 10.1186/1756-3305-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizkallah M.R., Saad R., Aziz R.K. The Human Microbiome Project, personalized medicine and the birth of pharmacomicrobiomics. Curr. Pharmacogenomics Personalized Med. (CPPM) 2010;8:182–193. [Google Scholar]
- Roy S., Trinchieri G. Microbiota: a key orchestrator of cancer therapy. Nat. Rev. Canc. 2017;5:271–285. doi: 10.1038/nrc.2017.13. [DOI] [PubMed] [Google Scholar]
- Schneeberger P.H., Becker S.L., Pothier J.F., Duffy B., N'Goran E.K., Beuret C., Frey J.E., Utzinger J. Metagenomic diagnostics for the simultaneous detection of multiple pathogens in human stool specimens from Côte d'Ivoire: a proof-of-concept study. Infect. Genet. Evol. 2016;40:389–397. doi: 10.1016/j.meegid.2015.08.044. [DOI] [PubMed] [Google Scholar]
- Schneeberger P.H., Coulibaly J.T., Panic G., Daubenberger C., Gueuning M., Frey J.E., Keiser J. Investigations on the interplays between Schistosoma mansoni, praziquantel and the gut microbiome. Parasites Vectors. 2018;11:168. doi: 10.1186/s13071-018-2739-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selhub J., Morris M.S., Jacques P.F., Rosenberg I.H. Folate–vitamin B-12 interaction in relation to cognitive impairment, anemia, and biochemical indicators of vitamin B-12 deficiency. Am. J. Clin. Nutr. 2009;89:702S–706S. doi: 10.3945/ajcn.2008.26947C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speich B., Ame S.M., Ali S.M., Alles R., Huwyler J., Hattendorf J., Utzinger J., Albonico M., Keiser J. Oxantel pamoate–albendazole for Trichuris trichiura infection. N. Engl. J. Med. 2014;370:610–620. doi: 10.1056/NEJMoa1301956. [DOI] [PubMed] [Google Scholar]
- Turnbaugh P.J., Ley R.E., Hamady M., Fraser-Liggett C., Knight R., Gordon J.I. The human microbiome project: exploring the microbial part of ourselves in a changing world. Nature. 2007;449:804. doi: 10.1038/nature06244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- W. H. O. Expert Committee Prevention and control of schistosomiasis and soil-transmitted helminthiasis. World Health Organ. Tech. Rep. Ser. 2002;912:1–57. i-vi. [PubMed] [Google Scholar]
- Wang Z., Klipfell E., Bennett B.J., Koeth R., Levison B.S., Dugar B., Feldstein A.E., Britt E.B., Fu X., Chung Y.M., Wu Y., Schauer P., Smith J.D., Allayee H., Tang W.H., DiDonato J.A., Lusis A.J., Hazen S.L. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen L.-Y., Yan X.-L., Sun F.-H., Fang Y.-Y., Yang M.-J., Lou L.-J. A randomized, double-blind, multicenter clinical trial on the efficacy of ivermectin against intestinal nematode infections in China. Acta Trop. 2008;106:190–194. doi: 10.1016/j.actatropica.2008.03.007. [DOI] [PubMed] [Google Scholar]
- Wilson I.D., Nicholson J.K. The role of gut microbiota in drug response. Curr. Pharmaceut. Des. 2009;15:1519–1523. doi: 10.2174/138161209788168173. [DOI] [PubMed] [Google Scholar]
- Wilson I.D., Nicholson J.K. Gut microbiome interactions with drug metabolism, efficacy, and toxicity. Transl. Res. 2017;179:204–222. doi: 10.1016/j.trsl.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao S.-H., Utzinger J., Tanner M., Keiser J., Xue J. Advances with the Chinese anthelminthic drug tribendimidine in clinical trials and laboratory investigations. Acta Trop. 2013;126:115–126. doi: 10.1016/j.actatropica.2013.01.009. [DOI] [PubMed] [Google Scholar]
- Zaman V., Sabapathy N. Clinical trial with a new anti-Trichuris drug, trans-1, 4, 5, 6 tetrahydro-2-(3-hydroxystyryl)-l-methyl pyrimidine (CP-14,445) Southeast Asian J. Trop. Med. Publ. Health. 1975;6:103–105. [PubMed] [Google Scholar]
- Ōmura S., Crump A. Ivermectin and malaria control. Malar. J. 2017;16:172. doi: 10.1186/s12936-017-1825-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.