Abstract
The cilium, once considered a vestigial structure, is a conserved, microtubule-based organelle critical for transducing extracellular chemical and mechanical signals that control cell polarity, differentiation, and proliferation. The cilium undergoes cycles of assembly and disassembly that are controlled by complex inter-relationships with the cytoskeleton. Microtubules form the core of the cilium, the axoneme, and are regulated by post-translational modifications, associated proteins, and microtubule dynamics. Although actin and septin cytoskeletons are not major components of the axoneme, they also regulate cilium organization and assembly state. Here, we discuss recent advances on how these different cytoskeletal systems affect cilium function, structure, and organization.
Keywords: actin, cilia, cytoskeleton, microtubule, organelle biogenesis, signaling
Introduction
Cilia are specialized, conserved organelles that are evolutionarily optimized for interaction with the world outside of the cell. They protrude into the extracellular space, are structurally resilient but also flexible and dynamic, and have unique mechanisms to tightly control their internal and membrane composition. These features ensure that the cilium is primed to perform highly regulated signaling, mechanosensory, and motile functions. In this review, we consider cilia mostly from the perspective of vertebrate animal cells, with additional information from the wide range of ciliated eukaryotes where appropriate.
There are two major types of cilia. Primary, or sensory, cilia are present in a single copy on most vertebrate cell types and sensory cells of many organisms. These cilia sense extracellular chemicals and mechanical force, and transduce critical signaling pathways. Motile cilia are found in specialized cells such as sperm and multiciliated epithelia in vertebrates, and on the surface of many aquatic unicellular organisms (e.g. Chlamydomonas and Tetrahymena). These cilia have highly regulated and co-ordinated beating dynamics. Differences between these cilia types are not universal in that some cilia, such as those of the developing node, are both motile and sensory. In all cases, the structure of the cilium is based on the axoneme, a columnar array of nine symmetrically arranged, stable, specialized microtubule (MT) doublets, of which the (+) end is oriented toward the cilium tip. In addition to these 18 outer axonemal microtubules (henceforth referred to as axoMTs), motile cilia usually feature an additional central pair of MTs in the axoneme lumen (Figure 1).
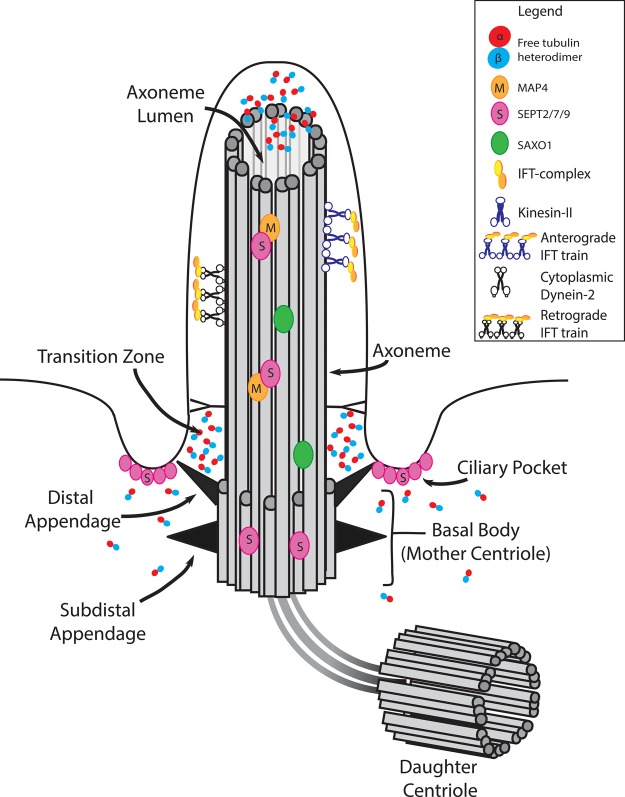
Figure 1. Elements of the microtubule cytoskeleton within the cilium.
The axoneme consists of nine bundles of MTs that are anchored in the basal body. Free tubulin diffuses through the axoneme lumen [1] and accumulates at the ciliary base, transition zone, and cilium tip. Exchange of tubulin between cytoplasmic and ciliary pools regulates axoneme length [2]. IFT motors, kinesin and dynein, carry cargo along the axoneme in different directions. Microtubule-associated proteins localize along the cilium and at the basal body, including SAXO1 (green), MAP4 (orange), and septin family proteins (pink), which also associate with the membrane of the ciliary pocket.
AxoMTs are templated directly by the mother centriole, often termed the basal body when supporting a cilium, which is located in the cytoplasm immediately beneath the plasma membrane (Figure 1). The basal body is a decorated mother centriole, a 500 × 250 nm cylinder comprised of stable MT triplets arranged in nine-fold symmetry [3]. A pair of centrioles, a mother and a daughter, form the core of the centrosome, which is the primary MT-organizing center (MTOC) in most animal cells. These two centrioles are embedded in a cloud of pericentriolar material containing the γ-TURC complex, which nucleates MTs, and MT-end anchoring proteins [4,5]. The centrosome nucleates and organizes arrays of MTs that are important in interphase cell organization and in cell division, but centrioles are not strictly required for MTOC function [6,7]. Rather, their essential function is to template the axoMTs of the cilium [8,9].
AxoMTs, like cytoplasmic MTs, bind motor proteins. The axoneme supports a specialized transport system, termed intraflagellar transport (IFT), that forms cargo-bearing trains powered by motors [plus (+) end MT motor kinesin-2 and minus (–) end MT motor cytoplasmic dynein] that travel bidirectionally within the cilium, depending on which motor is bound and active [10] (Figures 1 and 2). Protein translation does not occur within the cilium, and thus, the protein composition of the cilium is determined by controlling access from the cytoplasm, and transport throughout the cilium by IFT. Among these are tubulin subunits which replace tubulin that is disassembled from the axoneme tip. Tubulin dynamics and turnover in the axoneme regulate the state of cilium assembly and disassembly, and ciliary length [5,11] (Figure 1).
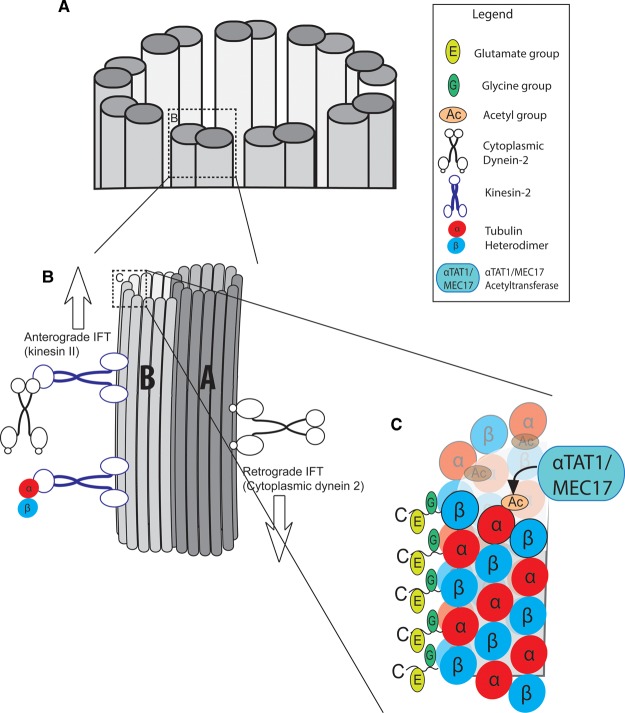
Figure 2. Composition and arrangement of axoneme microtubules.
(A) Cross-section of a generic non-motile axoneme is depicted, composed of nine microtubule doublets. (B) A doublet consists of a complete A-tubule and a partial B-tubule. The B-tubule binds the anterograde IFT motor protein kinesin-II, which transports cargos including tubulin subunits and the retrograde motor cytoplasmic dynein 2. The A-tubule binds the retrograde IFT motor dynein [12]. (C) AxoMTs are targets of microtubule PTMs, which affect MT stability and dynamics. K40 acetylation (orange) occurs on α-tubulin on the lumenal face of the tubule, catalyzed by αTAT1. Glycylation (green) and glutamylation (yellow) occur on the C-terminal tails of α- and β- tubulin.
The axoneme is surrounded by the ciliary membrane that is contiguous with the apical plasma membrane, but is compositionally distinct. The primary cilium membrane is especially enriched in specific signaling molecules including transmembrane receptors and signaling phosphoinositides that are critical for chemical sensing and signaling functions [13,14] (Figure 3). To specifically concentrate and regulate signaling molecules in the ciliary membrane, membrane cargos are trafficked in vesicles to the ciliary base by the Bardet–Biedl Syndrome (BBSome) coat complex [15]. Lateral entry into and exit from the ciliary membrane compartment is restricted by a diffusion barrier at the proximal (base) portion of the cilium, largely comprised of the transition zone [1,16,17].
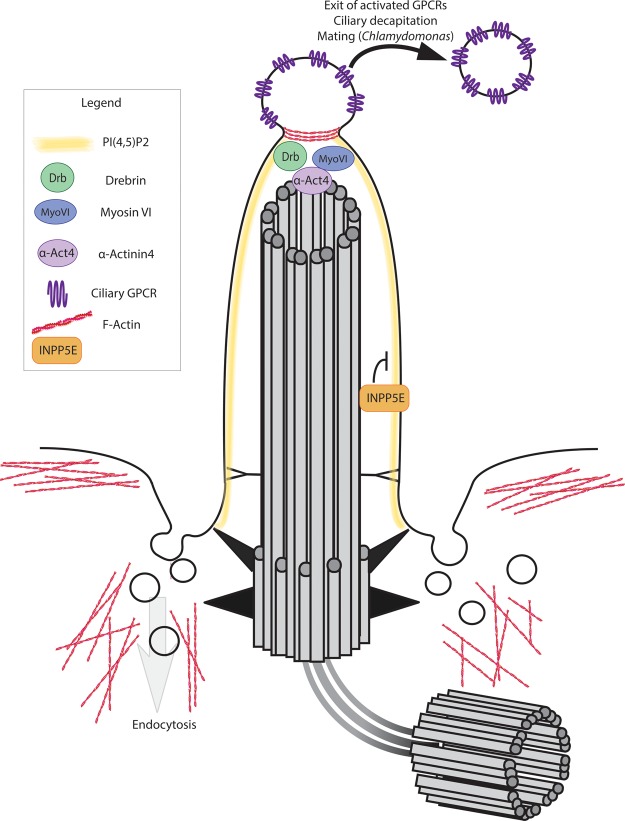
Figure 3. Functions of actin within and around cilia.
The ciliary pocket is a hub for actin dynamics and endocytosis. Ciliary ectosomes pinch off from the tip of the ciliary membrane in response to GPCR (purple) activation and blockage of intracellular retrieval machinery [18], prior to cilia disassembly [19], and in response to mating cues in Chlamydomonas [20]. Actin accumulates at the site of membrane pinching [19]. Actin-associated proteins drebrin (green), Myosin VI (blue), and α-actinin 4 (lavender) accumulate at the ciliary tip during excision [18].
Biogenesis of the cilium, or ciliogenesis, is a highly complex, elaborately regulated process involving many organelles, cellular machineries, and signaling pathways. Generally, the mother centriole, equipped with distal and subdistal appendages, associates with the membrane destined to become the ciliary membrane and supports the extension of the axoneme from the distal end. During this time, ciliary building blocks (tubulin) and resident proteins accumulate at the developing cilium. There are two main routes for ciliogenesis — the intracellular route, in which the basal body associates with a ciliary vesicle and likely begins axoneme elongation in the cytosol (Figure 5); and the extracellular route, in which the basal body docks at the apical membrane prior to axoneme growth [5,21,22]. Ciliary disassembly is less well understood, particularly in primary cilia, although critical regulators and pathways have recently been uncovered. The mitotic kinase Aurora A (AurA) is considered to be a key regulator of ciliary disassembly, and several pathways converging on AurA activation result in ciliary disassembly [23]. Upstream regulators include polo-like kinase 1 (Plk1), disheveled 2 (Dvl2), calcium/calmodulin signaling, and Nek2 [24]. AurA, in turn, activates many other factors contributing to ciliary dynamics, some of which are discussed in this review. Little is known regarding the physical events underlying primary ciliary disassembly, but other types of cilia, such as the flagella of Chlamydomonas, can disassemble either through resorption or excision [25–28]. Mechanisms of cilia assembly and disassembly are not a main focus of this review, and the reader is referred to other excellent reviews for details [10,13,22,23,27–29,196].
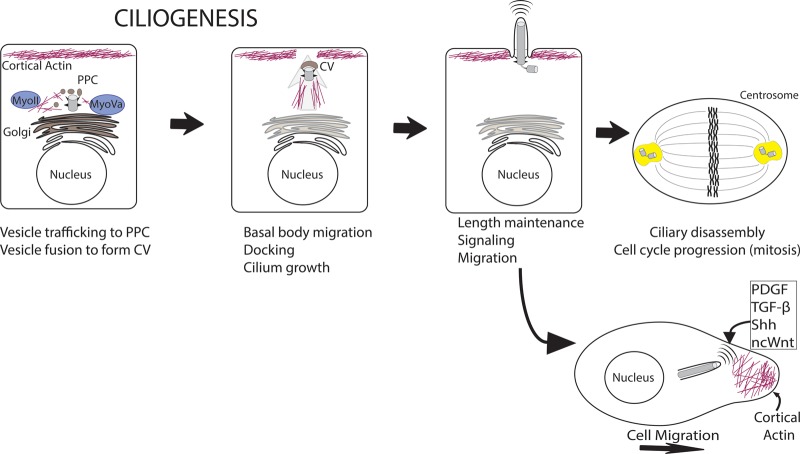
Figure 5. Diverse roles of cytoplasmic actin cytoskeleton in each stage of the ciliary life cycle.
Cellular actin and myosins mediate accumulation and fusion of vesicles at the PPC to form the ciliary vesicle (CV), basal body migration and docking at the apical surface, ciliary function and length maintenance, and disassembly. Ciliary signaling also regulates canonical functions of cytoplasmic actin, such as reorganization of actin and focal adhesions in cell migration.
In many cases, primary cilia are present on actively proliferating cells, and ciliary assembly and disassembly must be co-ordinated with the cell cycle. Disassembly is required to allow the centriole to detach from the plasma membrane, duplicate, and be segregated during cell division [22,30] (Figure 5). In most cycling animal cells, the primary cilium is present in G0/G1, when the centrosome is not required for mitotic functions, and its timely disassembly in S/G2 is required for mitotic entry and normal mitotic spindle function. The relationship between these two functions indicates that the primary cilium is not only dependent on cell cycle cues for assembly and disassembly, but also that the state of the cilium has direct consequences on cell cycle progression [2,13,26]. Therefore, cellular systems that control the cilium, including the elements of the cytoskeleton considered here, must act in coordination with the cell cycle to facilitate timely ciliary response to cell cycle cues, and vice versa.
The cellular functions of primary and specialized cilia translate to higher-order tissue and organism-level functions in development, mechanotransduction, and tissue homeostasis. Defects in the formation and function of primary cilia have been implicated in many developmental syndromes, collectively called ciliopathies, and misregulation of the cilia–cell cycle module has been linked to cancers. Ciliary defects also cause tissue-specific aberrations in tissues bearing specialized cilia types, including sensory tissues (photoreceptor cells in the retina, hair cells of the inner ear, and olfactory receptor neurons), fluid-clearing multiciliated tissues (trachea, bronchus and fallopian tube), and reproductive cells (sperm).
Thus, the primary cilium, once thought of as a vestigial organelle, has emerged as a potent regulatory hub for major cellular functions. Many recent studies have focused on the composition and structure of cilia in the context of transducing extracellular signals, and the reader is referred to recent reviews for more details [2,5,10,13,26,30]. On the intracellular side, cilia are anchored in, composed of, and regulated by the cytoskeleton, the master organizer and structural integrator of the cell. This review highlights new advances in the multifaceted relationship between the cilium and the microtubule, actin and septin cytoskeletal networks.
Cilia and the microtubule cytoskeleton
The axoneme — the microtubule-based core of the cilium
The axoneme is the stable, mechanically resilient MT-based structural core of the cilium. It supports the shape of the cilium and the ciliary membrane, and acts as a scaffold for motor-driven bidirectional transport of cargo between the cytoplasm and the cilium. AxoMTs form the structural core of the cilium and are essential for the transport of cargo into and out of the cilium by IFT (Figure 1). The axoneme is one of the most stable MT-based structures we know of, as axoMTs are resistant to nocodazole and cold, which induce complete cytoplasmic MT depolymerization [31]. Many features of axoMTs may underlie this stability, including the diversity of tubulin isotype and post-translational modification of axoMTs, the arrangement of axoMTs within the axoneme, and ciliary microtubule-associated proteins (MAPs). Many accessory proteins, in addition to motor proteins, regulate the structure and function of axoMTs, and together they affect the assembly/disassembly cycle of MTs and the cilium as a whole (Figure 1). These features are discussed below.
Axoneme structure
The axoneme has a unique, highly conserved structure with a striking circular symmetry of MTs. In most ciliated organisms, the axoneme has nine radially arranged MT doublets (consisting of a full A-tubule that scaffolds a partial B-tubule), which extend from the nine-fold MT triplets of the basal body (bearing a third partial C tubule) (Figure 2). Primary cilia have an empty axoneme lumen (designated 9 + 0), while motile cilia have a central pair of microtubules (designated 9 + 2). An intriguing question is whether the 9 + 0 and 9 + 2 organization of axoMTs, and hence immotile vs. motile cilia, are interchangeable. Leishmania mexicana is a model organism well suited to address this question, as it forms a 9 + 2 motile cilium during the promastigote life stage and a 9 + 0 immotile cilium during the amastigote stage. During transition between promastigote and amastigote stages, the 9 + 2 cilium is capable of direct conversion to a 9 + 0 organization through degradation of the central microtubule pair and several other structural proteins [32]. Thus, the basal body may not be committed to form one type of axoneme conformation, and the 9 + 2 and 9 + 0 cilia share fundamentally similar structural features.
Recent advances in ultrastructural methods have revealed new details about axoneme architecture. A cryo-electron tomography study of Tetrahymena axonemes revealed that the positioning of individual tubulin heterodimers in both MTs within the axoneme doublet is arranged as a B-type lattice — that is, αβ-tubulin heterodimers interact with each other vertically, similarly to most cytoplasmic MTs (Figure 2). MT inner proteins, including radial spoke proteins and inner-arm dyneins, promote connections between MT protofilaments of the axoMT doublets [33]. These interactions provide the basis for how the axoneme of motile cilia achieves a balance between structural stability and mechanical plasticity that is required for ciliary beating and sliding. Additional structural components of the axoneme and the basal body have been identified by cryoEM but have yet to be characterized, and may further contribute to axoneme stability and function [4,34].
The axoneme lumen is the internal compartment of the axoMT doublet cylinder and has largely been ignored until quite recently (Figure 1). Super-resolution single-particle tracking (SPEED microscopy) revealed the diffusion of the anterograde IFT motor kinesin-17 and α-tubulin in the axoneme lumen [35], consistent with an earlier study showing that kinesin-17 diffuses to the ciliary base following dissociation from the axoneme at the ciliary tip [10,36]. The role of tubulin diffusion in the axoneme lumen to the ciliary tip for ciliary growth seems to be an inefficient mechanism, and it may be more likely that this pool of tubulin is a product of axoMT depolymerization or for loading onto IFT complexes at the ciliary base for delivery to the ciliary tip. Nevertheless, that α-tubulin diffused in the axoneme lumen raises many interesting questions: is tubulin turnover at the axoneme tip strictly catalyzed by IFT motors, or can free tubulin associate and dissociate from the lumenal pool; can tubulin and other cargo proteins be modified within the lumen; do other diffusing ciliary proteins in the cilium mainly take the lumenal route, and could the central pair of motile cilia gate lumenal diffusion?
The entry of proteins into the axoneme lumen may occur passively, since exogenously expressed GFP diffuses within the lumen [35]. This is in contrast with the strictly regulated entry of proteins into the cilium via IFT [1,37–42]. However, it remains possible that diffusion of proteins into the axoneme lumen could be regulated by their size, charge, or identity, perhaps by a diffusion barrier at the base of the cilium [1,43] or regulation by resident axoneme lumen proteins.
The tubulin code — tubulin isotypes and post-translational modifications of axonemal microtubules
The ‘tubulin code’ [44,45] posits that MTs are regulated by tunable properties involving α- and β-tubulin isotypes and post-translational modifications (PTMs) of tubulin. The tubulin code is particularly relevant in the context of axoMTs, which have unique tubulin structures and isotypes, and are enriched in many tubulin PTMs. The tubulin code has been described in detail in recent reviews [26,44–47], and several major points will be covered here.
Role of tubulin isotype in the axoneme
The two best-characterized members of the tubulin superfamily are alpha (α)- and beta (β)-tubulin. Together, αβ-tubulin forms a heterodimer, the basic unit of a microtubule (Figure 2). There are a diversity of isotypes for each of these tubulins; humans, for example, have five α-tubulins and eight β-tubulins. Tubulin isotypes mainly vary in length, amino acid composition, and PTM affinity of the disordered C-terminal tails oriented toward the MT exterior [47]. Isotype expression is complex and variable, and differs between tissues and species, affecting MT stability and motor protein affinity and processivity [48]. Recently, a study of the axoMT tubulin isotype composition in Caenorhabditis elegans sensory cilia revealed a potentially important structural and functional regulation mechanism for the axoneme: loss of the α-tubulin isotype tba-6 results in abnormal axoneme ultrastructure, impaired motor protein distribution and cargo transport, and loss of secretory vesicle release [49].
Different tubulin isotypes regulate motor protein-binding affinities. For example, kinesin-2, which binds the α-tubulin C-terminus in vitro, has different binding affinities to tubulin isotype mixtures specific to different tissues. Overexpression of the kinesin-2 tail domain causes ciliogenesis defects [50], indicating that limiting motor protein affinity on the basis of tubulin isotype may function in regulating ciliary stability. Intriguingly, detailed correlative live cell imaging and EM in Chlamydomonas flagella demonstrated that anterograde IFT occupies B tubules, while retrograde IFT prefers A tubules of the same axoneme doublet; thus, a single doublet bears bidirectional cargo transport [51] (Figure 2). Future studies should examine whether, in addition to axoMT tubulin isotype composition, there are differences in PTMs, MAPs, or other features of adjacent tubules of the same doublet that confer differential binding affinity for specific IFT motors. This would contribute to the developing model that properties of axoMTs are finely tuned to allow strict functional regulation, potentially down to the level of individual axoneme doublets.
Post-translational modification of axonemal microtubules
AxoMTs are targets for a range of PTMs, including acetylation, glutamylation, and glycylation (Figure 2). In general, axoMT PTM levels increase during cilia assembly, distinguishing assembling cilia from mature cilia [36,52]. Of these PTMs, MT acetylation is the best understood, although conclusions about its function have been contradictory.
Acetylation is a marker of stable microtubules, such as axonemes, mid-bodies, and mitotic spindles. Most tubulin acetylation is catalyzed by the acetyltransferase αTAT1 (also referred to as ATAT1/MEC17) and occurs at lysine 40 within the lumen of individual polymerized MTs [36] (Figure 2). Although MT acetylation is a relatively abundant modification, it does not appear to be required for MT stability per se, nor does it affect the structure of dynamic MTs [12]. In fact, αTAT1 seems to confer both MT stability and dynamic MT growth independently of its acetylation activity [53]. Interestingly, tubulin acetylation promotes the binding of and sensitivity to the MT-severing enzyme katanin [54], which may also directly contribute to MT destabilization. αTAT1-mediated acetylation of axoMTs may promote, but is not required for ciliogenesis, and seems to be important for ciliary mechanosensation [55,56]. AxoMTs, and possibly cytoplasmic MTs, are stabilized by acetylation during ciliogenesis [57–59]. Finally, MT deacetylation contributes to ciliary disassembly [23,60–62], but whether directly on the axoneme or indirectly on cytoplasmic MTs has not been determined.
MT glycylation and glutamylation on the externally exposed C-terminal tail of tubulin subunits promote MT polymerization (Figure 2). Although less well studied than acetylation, glycylation and glutamylation can occur as single-residue or chain modifications on specific amino acids of the C-terminus and appear to contribute to ciliary structure and function [36]. Glycylation positively regulates cilia, as mutation of glycylases reduces the length and number of cilia, and causes cell cycle defects [63,64]. Glycylation also marks and stabilizes long cilia, and seems to be enriched at proximal regions of long cilia in epithelial cells [65]. Glutamylation, in contrast, may negatively regulate ciliary growth, as loss of cilia-specific glutamylases causes elongated cilia [66]. The amount of axoMT glutamylation seems to be critical for ciliary protein localization, kinesin motor speed, and sensory cilia function in C. elegans exosome-releasing neurons, as mutation of either the glutamylase TTLL-11 or the deglutamylase CCPP-1 impairs cilia severing and vesicle release [67]. TTLL-11 is also a ciliary cargo, indicating that axoMT glutamylation may be self-regulated by simultaneously influencing, and depending on ciliary motor function [67]. Finally, a specific dosage of glutamylation chains (8-Glu) is required for optimal MT severing by spastin in vitro [68]. Thus, regulation of axoMT polymerization or disassembly by glycylation and glutamylation may be tuned by the overall abundance, localization, and chain length of these PTMs.
Glycylation and glutamylation of axoMTs not only have opposite effects on cilia growth, but inversely affect each other. In mouse photoreceptor connecting cilia, loss of glycylation results in increased glutamylation levels and short, functionally defective cilia [69]. Mutation of the basal body and axonemal protein Bug22 reduces axoMT glycylation in Drosophila spermatids and impairs fertility, and Bug22 overexpression in RPE1 cells reduces axoMT glutamylation and results in elongated cilia [70]. Since glycylation and glutamylation can occur at the same residues of the tubulin C-terminal tail, an emerging model is that these PTMs compete and balance each other. A similar inter-relationship may also occur between acetylation and glutamylation, although these PTMs do not compete for a binding site, since acetylation or glutamylation of axoMTs in Chlamydomonas flagella increased and decreased axonemal dynein speed, respectively [71]. Perhaps, glutamylation, which is a bulky positively charged group, creates a greater physical hindrance to MT motor processivity than glycylation, which is small and neutral.
The regulation of axoMT modifications is complex and nuanced, and more studies are needed to fully understand their role in ciliary function and dysfunction. Many questions remain: do PTMs of the axoneme, independently or synergistically, contribute to axonemal stability and cargo transport, cilium motility, and signaling functions of the cilium; how are PTMs regulated during various stages of the cilium cycle, or in response to different ciliary assembly/disassembly cues; can differences between species, cell types, or even within a single cilium inform functional specificity, and are glutamylation and glycylation regulated by tubulin isoform diversity in the axoneme? A common caveat to these studies is that it is difficult to determine whether changes in MT modifications is causative or correlative to ciliary biology, or whether modification of cytoplasmic MTs, axoMTs, or both are at play in each case.
Microtubule motor proteins and microtubule-associated proteins in the axoneme
Microtubule motor proteins are bound to the axoneme [10]. These proteins include the IFT motors: kinesin-2 which mediates anterograde IFT (ciliary base to the tip) and cytoplasmic dynein which mediates retrograde IFT (ciliary tip to the base) (Figures 1 and 2). The reader is referred to recent reviews that discuss IFT motor composition, activity, and roles in human health [10,40,72–74].
Anterograde IFT is mediated by two kinesin-2 family proteins: the heterotrimeric Kif3 (referred to as kinesin-II in C. elegans and FLA-10 in Chlamydomonas) and the homodimer Kif17 (OSM-3 in C. elegans). KIF3 consists of motor domains Kif3A and either Kif3B or 3C, as well as the accessory protein KAP3. KAP3 function is incompletely understood, but it has been proposed to localize the complex to the ciliary base [75]. Heterotrimeric kinesin-2 is required for ciliary assembly [40,76], while the function of Kif17 seems to vary in different organisms and specialized cilia types. For instance, in Kif17, knockdown or dominant-negative inhibition in zebrafish does not affect overall ciliogenesis, but causes structural and developmental defects in photoreceptor cilia [76–78]. In rat olfactory sensory neurons, Kif17 directly binds cyclic nucleotide-gated channel CNGB1b and is required for ciliary localization of exogenously expressed CNGB1b in MDCK cells [79]. Therefore, KIF17 may regulate localization of ciliary cargo in specialized cell types.
Kif3 and Kif17 may act cooperatively when both expressed in the same cilium. This is supported by studies in C. elegans sensory neuron cilia, which feature a proximal region of doublet axonemal microtubules and a distal region of singlets. Both kinesin complexes are present in these cilia, but carry out transport at different rates — kinesin-II at ∼0.7 μm/s and OSM-3 at 1.3 μm/s [80]. Mutational studies of KAP3 and OSM-3 as well as analysis of transport dynamics within the sensory cilium revealed that kinesin-II is mainly active at the proximal doublet region, while OSM-3 is localized to the distal singlet regions, and that the motor complexes are involved in assembly of their respective axoneme regions [80,81]. Interestingly, there is a gradient of motor activity along the axoneme, such that slow IFT transport is carried out by kinesin-II at the proximal cilium, followed by recycling to the ciliary base, while faster IFT motion is carried out by OSM-3 at the distal cilium, followed by recycling to the ‘handover zone’ — the site between kinesin-II and OSM-3 enrichment [81]. A study which used an optical trap system to probe motor processivity revealed that Kif17/OSM-3 maintains processivity under either hindering or assisting mechanical load, while Kif3AB is more likely to dissociate from the axoneme [82]. Thus, a model for Kif3/Kif17 cooperativity is that Kif3 facilitates ciliogenesis and IFT entry across the transition zone, prior to dissociation from the axoneme, while Kif17 picks up cargo at a ‘handover zone’ for faster transport to the ciliary tip and singlet extension.
These motors bind to different parts of the axoMT doublet: kinesin-2 binds the B-tubule [51] and cytoplasmic dynein binds the A-tubule [83]. Single-particle tracking and correlative cryoEM techniques have begun to address a poorly understood problem — how are IFT motors transported along the axoneme, and how do they contribute to the composition and size of IFT trains? First, IFT trains fuse, fragment, and stop within cilia, demonstrating that IFT train size and velocity are not constant [51,83]. Second, kinesin-2 and cytoplasmic dynein have different modes of movement along the axoneme. Kinesin-2 moves processively along the axoneme, but dissociates at the ciliary tip and diffuses back to the base [83], possibly through the axoneme lumen [35] (Figure 2). Cytoplasmic dynein, which is a kinesin-2 IFT cargo in the anterograde direction, binds axoMTs at the tip and walks in the retrograde direction [83].
Additional motor proteins and their components, such as dynein light chain Tctex-1 and MT-depolymerizing and -polymerizing proteins, localize to cilia and affect ciliary assembly and size, perhaps independently of IFT activity [27,84–87]. Tctex-1 is a component of cytoplasmic dynein, but upon phosphorylation functions independently of dynein. Phospho-Tctex-1 is required for cilia disassembly and cilia-specific S-phase entry [84]. It is not clear, however, whether phospho-Tctex-1 is also a component of the retrograde IFT dynein complex, as its basal body/transition zone localization and phospho-activation suggest an IFT-independent role. Consistently with these observations, a recent study demonstrated that a direct interaction between Tctex-1 and actin regulators is necessary for ciliary disassembly by promoting endocytosis at the ciliary pocket [88] (Figure 4). The kinase upstream of phospho-Tctex-1 in ciliary disassembly has not been identified.
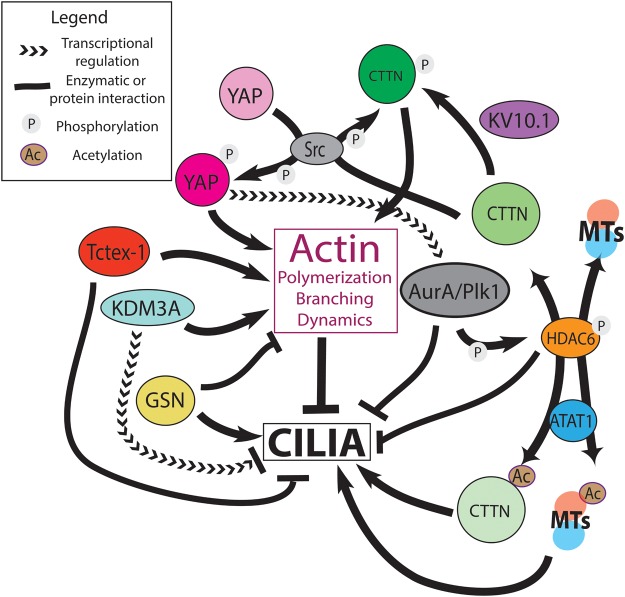
Figure 4. Regulators of cilia and the actin cytoskeleton.
Actin polymerization and dynamics generally inhibit ciliogenesis/promote cilia disassembly. Several cytoskeletal elements, kinases, and critical signaling pathways are involved in the link between cilia and actin. See the text for details. Abbreviations: ATAT-1, α-tubulin acetylatransferase 1; AurA, Aurora A; CTTN, cortactin; GSN, gelsolin; HDAC6, histone deacetylase 1; KDM3A, lysine demethylase 3A; KV10.1, voltage-dependent potassium channel 10.1; MTs, microtubules; Plk1, polo-like kinase 1; YAP, yes-associated protein.
Several MT-depolymerizing motor proteins localize to the cilium. Kinesin-13 in Chlamydomonas and Tetrahymena as well as the mammalian homolog Kif24 promote ciliary disassembly, perhaps by depolymerizing the distal (+) ends of axoMTs [27,87,89]. Interestingly, Kin13 is activated by CALK, an Aurora kinase homolog, while Kif24 is activated by Nek2, independently of the AurA signaling module [27,87]. In RPE1 cells, Kif24 recruits the Cp110/Cep97 centriole capping complex, an event that prevents axoneme growth and generally marks the completion of ciliary disassembly. However, Kif24 has low cytoplasmic MT-depolymerizing activity, suggesting an axoMT-specific role [90]. Thus, Kif24 may negatively regulate cilia by actively depolymerizing the axoneme and participating in machinery to block further cilia growth. In Tetrahymena, kinesin-13B and C isoforms regulate the polymerization, depolymerization, and post-translational modification of cytoplasmic and ciliary tubulin. Conditional overexpression of kinesin-13B causes axoMTs to depolymerize rapidly, but a kinesin-13B knockout mutant also has shorter cilia, as well as a lower level of the soluble pool of PTM-bearing tubulin in the cilium [91]. Therefore, kinesin-13/Kif24 appears to play a multifaceted role in maintaining a balance between cilia assembly and disassembly, by regulating both cytoplasmic MTs and ciliary axoMTs. Kif2a, another MT-depolymerizing kinesin, is also required for ciliary disassembly and acts mainly at the basal body to depolymerize centrosomal MTs [85]. Kif2a activity is regulated by the kinases Plk1 and AurA, both of which promote the ciliary disassembly pathway in addition to other critical cell cycle functions. Interestingly, phosphorylation of Kif2a by Plk1 promotes its MT-depolymerizing activity and is required at the basal body for ciliary disassembly [85,92], while phosphorylation by AurA has an inhibitory effect on Kif2a [92]. Whether Kif2a functions similarly to, and possibly in concert with, Kin-13, remains to be investigated.
MAPs regulate the stability, dynamics, and trafficking function of all MTs in the cell by binding directly to MTs. MAP4 localizes along the axoneme, and siRNA silencing leads to elongation of cilia and recruitment of SEPT7, a ciliogenesis promoter, and a member of the septin family of GTPases [93], indicating that MAP4 acts as a break to regulate cilia length (Figure 1). However, fibroblasts from patients with reduced expression of an MAP4 variant had centriole over-duplication and fewer, shorter cilia [94].
SAXO1, an MT-stabilizing protein specific to cilia in human primary cells and spermatozoa, promotes ciliary length in RPE1 cells [95] (Figure 1), indicating a role of SAXO in the establishment of stable axoneme microtubules during cilia growth. Interestingly, the SAXO homologs FAP203 and FAP256 were up-regulated in a proteomic screen of flagellar proteins during flagellar disassembly in Chlamydomonas [28].
MT-end-binding (EB) proteins associate with growing MT (+) ends, modulate MT dynamics, and recruit MT (+) end tip-interacting proteins and other regulatory proteins. In RPE1 cells, overexpression of EB1 or EB3 promotes ciliogenesis [96], and both proteins localize to the tips of motile cilia in bronchial epithelia and in Chlamydomonas [96,97] (Figure 1). In Chlamydomonas, EB1 recruitment to flagellar tips is independent of IFT and axonemal tubulin turnover, and is up-regulated in growing flagella [97].
In summary, a few MAPs have been identified in the axoneme, but they have not been studied in detail and the existing results are somewhat contradictory. Further studies are needed to identify additional ciliary MAPs and their interactions with the axoneme and with each other, and to understand their role in cilia organization and function. Specifically, how are MAPs incorporated into the axoneme; what upstream regulators control the ciliary localization and binding of each MAP; and does axoneme MAP composition depend on ciliary type, PTM, and stage of the ciliary cycle?
Axonemal microtubules and the cytoplasmic microtubule cytoskeleton
Although axoMTs are compartmentalized and structurally distinct from the cytoplasmic MT network, they can be viewed as an extension of, and therefore regulated by, the cytoplasmic MT network. The turnover of tubulin in axoMTs depends on pools of soluble cytoplasmic tubulin concentrated at the ciliary base and tip. Soluble tubulin is transported into cilia as IFT cargo [42,98–100] and possibly by diffusion in the axoneme lumen [35,99] (Figure 1).
Maintaining the balance between polymerized and free tubulin in the cytoplasm is critical for ciliary growth. In RPE1 cells, taxol treatment, which sequesters tubulin into polymerized MTs and depletes the cytoplasmic soluble pool, destabilizes and shortens cilia, while nocodazole-mediated depolymerization of cytoplasmic MTs increases ciliary number and length [31]. Regulators of MT polymerization have effects on cilia stability, including MT-depolymerizing kinesins (discussed in ‘Microtubule motor proteins and microtubule-associated proteins in the axoneme’; [91]) and MT-severing enzymes such as katanin. In Tetrahymena, depletion of katanin results in elevated levels of tubulin polymer, reduced levels of soluble tubulin, and short cilia [18]. Therefore, pools of soluble tubulin appear to be shared between the cytoplasm and cilia, and must be maintained at levels sufficient to support ciliary growth and maintenance. Since katanin preferentially associates with acetylated microtubules [54], a promising hypothesis may be that microtubule-severing activity at stable microtubules creates a balance between polymerized and soluble tubulin in the cytoplasm. However, this notion remains to be directly tested.
Connections between the ciliary basal body, the centrosome, and the cytoplasmic microtubule network
The cilium interfaces with the cytoplasmic MT cytoskeleton through the basal body, which is structurally related to the centrosome and both functions as an MTOC for the cilium and the cytoplasm, respectively. The centrosome comprises two centrioles, termed a daughter and a mother centriole. The mother centriole is modified for basal body function, primarily through the addition of distal and subdistal appendage structures that anchor the basal body to the membrane and the cytoskeleton, respectively [6]. Subdistal appendages (primary cilia) or basal feet (multiciliated cells) anchor cytoplasmic MTs [101]. It is not fully understood whether the interaction between subdistal appendages and microtubules is necessary primarily for MTOC activity, or whether it also plays a structural role in supporting the cilium. The latter is likely as the subdistal appendage is a marker of a mature ciliation-competent centriole, and depletion of a core subdistal appendage protein, ninein, impairs ciliogenesis [20]. The MT-subdistal appendage connection may mediate cilia-targeted vesicle trafficking toward the ciliary base, or act as a site of force generation upon the basal body in its migration during ciliary assembly or disassembly. In multiciliated cells, the basal feet are required for positioning and aligning basal bodies at the apical surface. In primary and multiciliated cells, distal appendages are required for basal body docking to the membrane [102], but the precise role of the MT–basal foot interaction is still poorly understood.
An intriguing observation uncovered a novel interaction between the primary cilium and the mid-body, a cytoplasmic structure of compacted spindle MTs that forms a bridge between daughter cells in the late stages of cell division. In IMCD3 and MDCK cells, a remnant of the mid-body is retained after cytokinesis and performs a novel non-mitotic process: it undergoes Rab8-mediated migration along the apical cell surface toward the centrosome, where it forms an MT-based connection to the basal body during ciliogenesis. Strikingly, removal of the mid-body by patch-clamp suction prevents cilium formation [19]. Thus, the inheritance of this mid-body remnant promotes ciliogenesis in the daughter cell, possibly by delivering ciliary proteins to the nascent cilium. The daughter cell that does not inherit the remnant of the mid-body may form a cilium by an alternate pathway. We suggest that the significance of this finding is three-fold: it (1) demonstrates a new role for the mid-body, (2) identifies novel mechanisms involved in ciliogenesis, and (3) illustrates a phenomenon directly underlying the cilia–cell cycle tug-of-war — a canonical mitotic structure is repurposed to aid in formation of the primary cilium, a negative regulator of the cell cycle. Many questions remain: when and how does the mid-body accumulate ciliary components; what is the basis of the MT link between the mid-body and the centrosome; how is the mid-body degraded after initiation of ciliogenesis and is mid-body inheritance to one of the daughter cells random, and if not, what regulates this?
Cilia and the actin cytoskeleton
In contrast with the wealth of knowledge on ciliary axoMT structure, regulation, and function, much less is known about the role of the actin cytoskeleton in the cilium. In part, this is because it has been difficult to detect actin in the cilium, and there is no distinct actin structure in the cilium compared with the MT-based axoneme. However, the actin cytoskeleton plays diverse roles in almost every stage of ciliogenesis and many aspects of ciliary function (Figures 3 and 4).
Roles of actin on ciliary organization and function
Historically, primary cilia are thought of as actin-deficient organelles. Nevertheless, the presence of actin in some specialized cilia has been noted [103,104]. The actin motor protein myosin VIIa localizes within the cilia of different tissues and co-sedimentates with the axonemal fraction of retinal photoreceptor outer segments [105]. Actin is distributed at the proximal portion of quail oviduct cilia and de-membranated ciliary fractions, indicating that actin is directly associated with the axoneme [106].
New evidence further indicates supporting roles of actin and actin-associated proteins within and surrounding the primary ciliary structure. The relationship between actin dynamics and cilia is generally one of negative regulation — actin depolymerization promotes ciliary assembly and elongation, while polymerization or branching of actin is associated with cilia disassembly or inhibited ciliogenesis [2,26,88]. Cytochalasin D (CytoD), a small molecule that depolymerizes F-actin, rapidly induces primary cilia formation and over-elongation of cilia in conditions that normally promote ciliary disassembly, such as serum stimulation of cultured cells [107,108]. CytoD also prevents mitotic entry, perhaps as a consequence of retention of the cilium during the cell cycle [84,87,107,109].
Although the relationship between actin cytoskeleton dynamics and cilia in tissue culture cells is generally considered to be antagonistic, in vivo studies indicate a more nuanced relationship. In quail oviduct epithelia, CytoD impairs estradiol benzoate-induced ciliogenesis and generates defects in basal body migration, apical docking, and cilium elongation and structure [110]. In Chlamydomonas, disruption of actin cytoskeleton structure and dynamics by CytoD and the actin-null mutant ida5 result in flagellar shortening, regeneration abnormalities, and defects in IFT train entry and size [25]. CytoD in combination with the MT-depolymerizing agent colchicine results in complete flagellar disassembly. The mutant pf18, which lacks a central MT doublet, has a stronger response to CytoD and colchicine than the wild type, indicating that the structural stability of the axoneme is important for resisting fluctuations in the assembly state of the actin cytoskeleton. Interestingly, CytoD not only shortens flagella, but also causes a greater variability in flagellar lengths, producing both abnormally shorter and longer flagella [111]. Similar effects occur in a null mutation of the histone demethylase KDM3A in mice, in which decreased expression and activity of actin components correlate with the formation of long, unstable primary cilia with bulging tips and abnormal IFT distribution. However, KDM3A-null RPE1 and MEF cells have an abnormally wide range of ciliary lengths (both shorter and longer) compared with normal cells due to instability and breakage of long cilia [112]. Thus, these effects may be due to the loss of an actin cytoskeleton at the ciliary base that may regulate the entry of ciliary cargo, including IFT particles [112].
Although actin has not been directly visualized in primary cilia, recent observations of ectosome shedding activity from primary cilia suggest a direct role for actin in ciliary membrane dynamics [13] (Figure 3). In IMCD3 cells with a primary cilium, activated GPCRs are packaged and released from the ciliary membrane when cargo retrieval mechanisms are blocked. This process requires the actin-binding proteins α-actinin 4, myosin VI, and drebrin. Myosin VI and drebrin also accumulate at ciliary tips upon stimulation in ectosome-forming conditions [113]. In differentiating neuronal precursors, ejection of the primary ciliary membrane was described upon actin accumulation at the ciliary base and subsequent apical abscission, but whether the axoneme is present in the shed fragment was not demonstrated [114]. Chlamydomonas flagella also secrete bioactive ectosomes during mating, but whether their release involves actin has not been determined [115,116] (Figure 3).
Perhaps by a similar process, IMCD3, RPE1, and 3T3 cells exhibit pinching and detachment, termed ‘decapitation’, of the ciliary tip at the onset of ciliary disassembly in serum-stimulated cells. This process involves regulation of inositol polyphosphate-5-phosphatase E INPP5E, which localizes to and stabilizes primary cilia. INPP5E mutations result in ciliopathy phenotypes in humans and mice [117]. INPP5E interacts with several regulators of ciliary dynamics including AurA, Arl13b, and Cep164 [118,119]. Phua et al. [120] demonstrated that, early in ciliary disassembly, INPP5E is displaced from the membrane, allowing accumulation of phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2) in the proximal ciliary membrane, followed by rapid accumulation of F-actin at the site of pinching (Figure 3). Whether actin-mediated ciliary membrane pinching is sufficient to disassemble the primary cilium is an intriguing possibility. While ciliary decapitation seems to occur as an early step to cilium loss, it does not account for the complete disassembly of the cilium since tubulin was not found in the decapitated ciliary fragments [120]. Therefore, the axoneme must be disassembled by other means, such as resorption into the cell or depolymerization by MT-depolymerizing kinesins. It would be interesting to test whether kinesin-13 or other depolymerizing kinesins are activated or localized to cilia in conjunction with ciliary decapitation events in an INPP5E-dependent manner.
The nature of actin availability for rapid influx during pinching events has not been studied. One possibility is that a pool of actin monomer exists at the ciliary base or proximal PI(4,5)P2-positive portion of the cilium. In RPE1 cells, actin filaments and cables have been identified at the base of the cilium and ciliary pocket, a site of active endocytosis, which seems to play an important role in ciliary disassembly [88,121]. Whether this actin network is associated with the cilium in other cell types, including those that do not have a ciliary pocket, and whether it can act also as a dynamic pool for ciliary membrane pinching in ectosome release, remains to be addressed. In general, mechanisms of axoneme disassembly in parallel with membrane removal, through release and/or endocytosis, remain to be investigated. Together, these observations indicate that the difficulty of localizing actin within primary cilia could be due to the transient nature of ciliary actin polymerization in cilia during rapid membrane-pinching events. Perhaps, actin-associated proteins localize stably to cilia, while F-actin polymerization and localization are tightly co-ordinated with the response to stimuli such as GPCR activation and cell cycle-related ciliary disassembly cues.
Functions of actin-associated proteins in ciliary organization
Although a direct relationship between actin and cilia remains poorly understood, many actin-associated proteins and signaling molecules that directly regulate, or are regulated by, actin dynamics have a role regulating cilia (Figure 4). The actin-binding protein cortactin (CTTN) is a prominent example of this relationship. CTTN functions in lamellipodia formation and cell migration, and promotes F-actin polymerization and branching by recruiting Arp2/3 to the cortical actin network [122,123]. CTTN is activated by Src kinase-dependent phosphorylation and is inactivated by acetylation [124,125]. HDAC6, a critical component of the cilia disassembly signaling cascade [23], is a deacetylase of CTTN which thereby activates CTTN and its actin polymerization activity to promote cilia loss [60]. Conversely, Missing-in-Metastasis promotes ciliogenesis by antagonizing CTTN phosphorylation by Src [107] (Figure 4). CTTN also directly interacts with the gated potassium channel Kv10.1, which promotes ciliary disassembly when overexpressed. Intriguingly, siRNA depletion of Kv10.1 results in increased levels of phospho-CTTN [126]. The spatial regulation of the interaction between Kv10.1 which localizes in the ciliary membrane, and CTTN which associates with cortical actin, needs further study to elucidate their roles in cilia regulation.
F-actin may further regulate primary cilia transcriptionally through the Hippo pathway effector YAP (Figure 4). Jasplakinolide-induced F-actin aggregation leads to YAP inactivation, cell rounding, and ciliogenesis [127]. A role for YAP may depend on its nuclear localization, and thus its transcription factor activity, since nuclear exclusion of YAP results in an increase in cilia number [108]. YAP depletion in RPE1 cells results in an increase in ciliogenesis and negatively regulates expression of the cilia disassembly factors Aurora A and Plk1. The inhibition or depletion of several actin remodeling factors (LIMK2, cofilin 1, and TESK1) led to a reduction in nuclear YAP and, coincidently, increased ciliogenesis [108]. An inverse relationship between primary cilia formation and YAP signaling indicates that Hippo-dependent cell proliferation requires the absence of primary cilia. However, teasing apart the precise role of actin dynamics, YAP localization and function will be complex. Kim et al. [108] suggested a two-fold mechanism — F-actin suppresses ciliogenesis by halting trafficking of ciliogenic vesicles to the basal body and supports cilia disassembly by activating a transcription factor (YAP/TAZ) upstream of disassembly machinery proteins (AurA/Plk1).
Taken together, these results illustrate the complexity in the relationship between cilium organization and the actin cytoskeleton (Figure 4). Overall, it seems that either complete polymerization or depolymerization of actin results in abnormalities in cilia length and dynamics. It is possible that differences between model organisms and ciliary type (i.e. motile 9 + 2 axoneme vs. immotile 9 + 0 axoneme) may result in opposing responses to actin perturbation. For example, treatment of RPE1 cells with either actin-depolymerizing CytoD or actin-polymerizing Jaspakinolide resulted in elongated cilia [31]. These seemingly contradictory effects of CytoD on primary cilia may be explained by concentration of the drug: low doses (0.5–1 μM), which cap and thereby inhibit growth and depolymerization of actin filaments, result in elongated cilia, whereas higher doses (10 μM) which cause F-actin depolymerization result in abnormally short and fewer cilia [128]. Nevertheless, any generalized relationship between cilia and actin is likely an oversimplification — artificially polymerizing or depolymerizing all cellular F-actin will not accurately convey what happens to the actin cytoskeleton during the cycles of cilium assembly and disassembly.
Role of actin in centrosome migration and docking
The association between the centrosomes/centrioles and the actin cytoskeleton is well described. In human polymorphonuclear leukocytes, centriole migration and splitting require an intact actin cytoskeleton [129–132]. In both primary- and multiciliated cells, basal body migration toward the cell surface during ciliogenesis is dependent on actin contractility and actin-binding motor proteins [130,131]. Myosin X plays a role in primary ciliogenesis [133,134], while in ependymal cells with multiple clustered motile cilia, a non-muscle myosin complex is required [132]. Interestingly, ciliogenesis is not the only context for actin-mediated centriole migration and docking at the plasma membrane. In cytotoxic T-lymphocytes, the actomyosin cytoskeleton transports the centrosome to the plasma membrane at the immune synapse. Docking of the mother centriole through the distal appendages may orient MTs toward the synapse, which is required for the proper secretion of lytic granules toward the target cell [135]. The many parallel features between this process and ciliogenesis lead to an attractive hypothesis that the immune synapse is a ciliary analog [135], and that there may be other contexts in which conserved ciliary machinery and mechanisms are repurposed for non-ciliary functions.
In addition to acting as an MTOC, the centrosome was also proposed to act as a potential organizing center for F-actin. Knockdown of Arp2/3 and the WASH complex abolished the actin nucleation by the centrosome, while inhibition of formins had no effect, indicating that the centrosome nucleates actin filaments through branching rather than formin-generated parallel filaments [136]. The identification of the centrosome as an actin-organizing center indicates that the centrosome and ciliary basal body are propelled or pulled by the actin cytoskeleton through the cytoplasm. However, this potentially illuminating function of the centrosome requires extensive validation in other cell types and in vivo.
The interaction between actin and the basal body/centrosomes is critical in most major steps of ciliogenesis, including (1) docking, or association of the basal body with the plasma membrane, either in the form of a preciliary vesicle (intracellular ciliogenesis) or with the apical plasma membrane (extracellular ciliogenesis); (2) delivery of ciliary components (membrane and cargo) to a growing cilium; and (3) migration of a cilium or basal body to the plasma membrane (Figure 5). The docking step occurs either before or after the migration step, depending on whether an intracellular or extracellular ciliogenesis pathway, respectively, is employed. Docking of the primary cilium basal body to a ciliary vesicle requires CCDC41 and Golgi localization [137], Chibby recruitment of Rab8 [138], interaction of Rac1 and Ezrin with the DOCK1/ELMO complex, and distal appendage components such as Ttbk2 and C2cd3 [139], Cep164 [140], and others [102]. Chibby also plays a role in facilitating membrane curvature of the nascent ciliary membrane [141].
Multiciliated epithelial cells (MCCs) are a powerful model for studying basal body/centriole duplication, migration, and ciliogenesis in the context of the actin cytoskeleton. Mouse tracheal epithelial cells produce hundreds of motile cilia, all of which are arranged in parallel along the apical surface. A subapical cytoplasmic actin meshwork is formed during ciliogenesis and is required for the mass migration of basal bodies toward the apical cell surface during ciliogenesis [130–132] (Figure 5). This process requires Rho GTPase, planar cell polarity (PCP) pathway proteins, and the MCC-specific transcription factor Foxj1 [131]. Interestingly, PCP proteins, such as Disheveled, interact with the exocyst complex, which together with Arl13b [142] are involved in vesicle trafficking and plasma membrane fusion during ciliogenesis [143].
Beneath the apical surface of MCCs are hundreds of basal bodies enmeshed between two actin networks, apical and subapical, which are required for maintaining the proper orientation and spacing of basal bodies. Interestingly, a ciliary adhesion complex consisting of focal adhesion proteins was shown to localize to basal bodies at the appendage and rootlet, conferring connections with the apical and subapical cytoskeletons. Disruption of this complex led to docking and spacing defects, resulting in clustered basal bodies [144]. It is likely that mechanisms involving the actin cytoskeleton in basal body migration and ciliogenesis are conserved between primary cilia and MCC motile cilia, but further studies are needed.
Role of actin in the transport of membrane and proteins to and from the cilium
Ciliogenesis requires the efficient production and transport of ciliary proteins and membrane to the site of the nascent, growing cilium. Ciliary proteins are packaged in Golgi-derived vesicles that are targeted to a tubulo-vesicular pericentrosomal preciliary compartment (PPC) at the basal body during cilia growth [145]. Many regulators of ciliary membrane delivery and biogenesis have been identified, including the BAR domain protein FAM92 and Chibby [141], Arl13b and the exocyst [142], the BBSome [146], and the membrane condensation protein MAL [147], and have been reviewed elsewhere [109].
The actin cytoskeleton facilitates the transport of vesicular cargo throughout the cell, driven by myosin motors along actin cables. During ciliogenesis, myosin II and myosin Va deliver the membrane in the form of vesicles, IFT components, and other cargo to growing cilia. Myosin Va transports preciliary vesicles to the basal body during the earliest stages of ciliogenesis and is recruited to artificially elongated cilia in CytoD-treated cells, implying a role in modulating the length of mature as well as assembling cilia [128,148,149]. Interestingly, myosin Va is also recruited during early stages of cilia disassembly [149], suggesting a parallel role in removing the ciliary membrane.
Assuming that myosin-mediated delivery of ciliary cargo requires polymerized actin, the model that actin polymerization negatively regulates primary cilia is difficult to understand. Currently, the prevailing model is that F-actin filament polymerization counteracts vesicle trafficking to the growing cilium [39,108,109,145]. Drug-induced actin depolymerization arrests vesicle transport, resulting in the accumulation of vesicles at the PPC, and rearranges actin into nodes that preferentially associate with, and facilitate ciliary membrane cargo delivery to the ciliary vesicle during ciliogenesis [39].
In summary, the actin cytoskeleton plays several critical roles in ciliogenesis, but many significant gaps in our understanding of mechanisms remain. Furthermore, whether these roles are duplicated during ciliary disassembly has not been studied directly. Actin could play a role in facilitating the transport of ciliary proteins and membrane away from the cilium for recycling or degradation and in driving migration of the basal body basally from the plasma membrane. Whether these processes require polymerization or depolymerization of actin is not understood.
Intersections between primary cilia, signaling, and canonical roles of cytoplasmic actin
The actin cytoskeleton confers critical function of ciliary dynamics, structure, and function (Figures 3–5). Conversely, the primary cilium may regulate canonical roles of actin in cellular processes that include cell migration, shape, and interactions with neighboring cells and the extracellular matrix. Furthermore, the primary cilium, like other actin-associated adhesion structures, is a marker of cell polarity, as it generally forms on the apical surface in polarized cells. As such, the regulation of the cilium and other polarity markers may be similar.
Cell migration
Migrating cells are polarized between the front and rear of the cell, and the primary cilium is a strong regulator of cell polarity upstream of cell migration [150] (Figure 5). The cilium, which co-ordinates multiple migration-related signaling pathways, orients along the axis of migration [151] and in most migratory cell types the centrosome is positioned between the nucleus and the leading edge, where it is thought to co-ordinate cytoskeletal dynamics. In general, defects in ciliary organization and function result in impaired cell migration, wound repair, development, and morphogenesis [150,152].
In neuronal development, ciliary signaling is required for proper cell migration. Joubert syndrome, a ciliopathy characterized by defects in neuronal migration and brain development such as microcephaly, results from disruption of ciliary signaling [152]. The ciliary membrane protein Arl13b is required for correct distribution of many migratory cue receptors in the ciliary membrane of interneurons, and its deletion impairs axon migration [153]. In ciliated breast cancer cells, knockdown of the estrogen receptor co-repressor SPEN results in loss of the primary cilium and impaired cell migration [154]. In MDCK cells, the canonical ciliary protein IFT88 may play cilium-independent roles by involvement in targeting protein to the leading edge during cell migration [155]. In the corneal endothelium, the basal body of primary cilia transiently repositions away from the nucleus and toward the leading edge of cells during wound repair; upon completion of wound closure, cilia are lost and centrosomes migrate toward the nucleus [156].
Primary ciliary signaling appears to regulate cell migration through modulating actin dynamics, targeting of polarity proteins to the leading edge, and cell–substrate focal adhesion formation [150,152] (Figure 5). The best-characterized ciliary signaling pathway in cell migration is the PDGF-AA/PDGFRα pathway [150]. PDGF-AA ligand activates its receptor PDGFRα at the ciliary membrane and at focal adhesions. Downstream, AKT/PKB is phosphorylated and activates the MEK1/2–ERK1/2–p90RTK cascade, which in turn activates leading edge-specific proteins such as the sodium hydrogen exchanger NHE1 which regulates intracellular pH [157]. In MEFs and NIH3T3 cells, loss of primary cilia abolishes PDGFRα signaling, NHE1 activation, and cell migration [158–160].
Turnover of integrin-based focal adhesions is critical for migration. In endothelial cells, primary cilia and Hsp27, a downstream target of PDGF signaling [161], are required for activation of focal adhesion kinase, actin remodeling, and focal adhesion turnover. Disruption of this pathway reduces cell migration and endothelial barrier integrity [162]. The retinitis pigmentosa protein RPGR localizes to primary cilia and is required for primary cilia, actin dynamics, and focal adhesion turnover [163], although a migration defect was not reported.
Many other migration-regulating signaling pathways are co-ordinated through the primary cilium. TGF-β signaling, another pathway upstream of cell migration, also seems to function through primary cilia. In MEFs, keratinocytes, and cancer cells, silencing or inhibition of the ceramide synthase CerS4 promotes trafficking of TGF-βRI/II to primary cilia membranes which activates Shh signaling and cell migration [164].
Wnt signaling is a ubiquitous pathway involved in cell migration and development and is also associated with primary cilia. Canonical Wnt pathways do not require the primary cilium [165], but some effectors localize at the basal body, including β-catenin and APC [166]. Rather, the cilium participates in noncanonical Wnt (ncWnt) signaling. Inversin, also referred to as nephrocystin-2, localizes to a specific region distal to the transition zone. This ‘inversin compartment’ modulates ncWnt signaling via negative regulation of Disheveled 1 (Dvl1), leading to actin reorganization, NHE1 targeting to the LE, and cell migration. Inversin mutants have impaired Wnt signaling, misoriented cilia, and loss of migration proteins targeted to the LE [167]. Other ncWnt proteins have been identified in association with cilia [166] (Figure 5).
Several inconsistencies arise from these studies of roles of the actin cytoskeleton in ciliogenesis and cell migration. For example, primary cilia tend to assemble and elongate when actin is depolymerized, and cell migration is inhibited. In cell culture, serum starvation induces ciliogenesis, but reduces lamellipodia formation and cell migration [150,168]. If cilia are formed when actin is depolymerized, how do primary cilia co-ordinate processes of actin polymerization and dynamics? Perhaps, ciliogenesis is a time of transient actin depolymerization, and actin filaments only assemble after the cilium is mature and ciliogenesis machinery has been inactivated. These nuances require further investigation.
Cell–cell junctions
Primary cilia and cell–cell junctions, including the adherens and tight junctions, have several unifying features — they are markers of cell polarity in epithelia, sites of mechanosensation, transducers of signals, and directly interface with, and are regulated by the actin cytoskeleton. Currently, the relationship between these structures seems to be correlative and indirect [169]. Fluid shear stress in the renal proximal tubule epithelium causes a loss of polarity markers, including cilia and proteins of adherens and tight junctions [170]. Mutation of the calcium-activated chloride channel TMEM16a leads to a desensitization of cells to chloride and calcium fluxes, abnormal distribution of surface PI(4,5P)2, disorganized cell–cell junction proteins, and short cilia [171]. Calcium-dependent deciliation of MDCK monolayers results in tight junction remodeling and increased tight junction permeability [172]. Finally, the ciliary calcium channel polycystin-2 also localizes to cell–cell contacts and promotes recruitment of α-actinin to adherens junctions [173]. Since a member of the α-actinin family was also identified within cilia during ciliary ectosome release [113], calcium signaling and α-actinin may represent two examples of co-regulation of ciliary dynamics and junctions. Thus, there are intriguing links between the assembly state and organization of the primary cilium, and the function of cell–cell junctions, but further studies are needed to define whether there are direct interactions, or whether the relationship is based in parallel, unconnected pathways.
Cilia and the septin cytoskeleton
Septins, a family of GTP-binding proteins, localize to cilia and basal bodies [174] (Figure 1). Septins assemble into filaments and rings, and contribute structural and scaffolding roles in many cellular processes [175]. Septins associate with, and regulate the dynamics of actin and MT cytoskeletons, and localize to sites of micron-scale membrane curvature throughout the cytoplasm and cell cortex [175].
Members of the septin family seem to localize at the ciliary base/transition zone, axoneme, or both. Each location implies a different potential role. A septin ring at the ciliary base, marked by SEPT2 in IMCD3 cells [43] and SEPT7 in frog embryos [176], is important for ciliary length and function. Importantly, septins act as a barrier to diffusing membrane proteins in the yeast mother-bud neck, the primary cilium [43], and at the sperm flagellum annulus. These studies have led to a model in which septins localize to the ciliary base, a site of membrane curvature, and form a barrier restricting the free movement of membrane proteins between the ciliary membrane and the apical plasma membrane (Figure 1). More work is needed to investigate the interaction between septin, BBSome, and transition zone modules in collectively mediating selective ciliary membrane protein entry, confinement, and exit [15,177].
Stable pools of SEPT2 and SEPT7, as well as SEPT9 were also found localized at the axoneme [93]. Septins influence MT dynamics by directly binding microtubules, as well as by binding and inhibiting MT-stabilization activity of MAP4. SEPT7 competes with MAP4 at the axoneme and positively regulates cilia length ([93], see ‘The tubulin code — tubulin isotypes and post-translational modifications of axonemal microtubules’). Thus, septins seem to play a stabilizing role as structural components of the axoneme.
Septins interact with regulators of ciliogenesis including Rab8, the exocyst, and PCP proteins, and promote tubulo-vesicular formation in vitro [174]. Septins may play specialized cytoskeletal roles at multiple points in ciliogenesis, by targeting and fusion of ciliogenic vesicles at the PPC, delivery into a growing axoneme, and formation of a ring at the nascent ciliary membrane to regulate membrane protein compartmentalization. However, each of these functions requires further, detailed studies.
Cross-talk between different cytoskeletal systems and implications for the cilium
The actin and MT cytoskeletons perform diverse functions in the cell, including organizing cell shape and structure, establishing polarity, interacting with and responding to extracellular cues, trafficking vesicles and organelles, and modulating signaling cascades. In most of these roles, both cytoskeletal networks are required and are co-ordinated with each other [178,179] (Figure 4).
Microtubule–actin cross-talk at the basal body
The centrosome or ciliary basal body is a hub for cross-talk between actin and microtubule networks. The relationship between the centrosome and microtubules is obvious, since the centrosome acts as the cell MTOC. However, the centrosome also interacts with actin and is capable of acting as an actin nucleator [136] (see ‘Cilia and the actin cytoskeleton’).
Cilia are subject to external forces, commonly bending by fluid shear or active beating, as well as internal forces from dynamics of the cytoplasmic cytoskeleton, and function as a mechanosensor and mechanotransducer [180,181]. Even in the absence of fluid flow, the primary cilium displays spontaneous pivoting and flexing originating from the rigid base, likely mediated by the actin cytoskeleton surrounding the basal body [182]. These ciliary dynamics may be important for mechanosensation, resilience to extracellular stresses, and orientation during cell migration and other specialized cellular processes. The basal body is a focal point of intersection of internal forces generated by the actin and MT cytoskeletons. In multiciliated cells, hundreds of basal bodies are embedded in dense, subapical plasma membrane actin networks, and the basal foot of each basal body is directly anchored to MTs. Both cytoskeletal connections are required for proper basal body alignment and positioning, and therefore for ciliary motility [183].
The basal body must migrate through the cytoplasm to dock with, or disengage from, the plasma membrane during cilia assembly and disassembly, respectively, both of which depend on actin polymerization. During ciliogenesis, actin reorganization occurs simultaneously with MT polymerization, stabilization, and modification. Disruption of actomyosin contraction reduces the number of stable MTs [57], indicating that actin assembly and actomyosin contraction promote MT stabilization in cells preparing to form a cilium. Indeed, taxol-induced stabilization of MTs in RPE1 cells rescues ciliary elongation induced by actin depolymerization in the presence of CytoD [31]. Thus, actin polymerization-mediated cilia growth requires both free tubulin for incorporation into the nascent axoneme and stabilized MTs to support apical migration of the basal body. How MT stabilization is regulated during ciliogenesis to ensure a sufficient pool of soluble tubulin is an important, outstanding question.
HDAC6 and ATAT1 — co-regulation of tubulin acetylation and cortactin at the cilium
An interface between actin and microtubule cytoskeletons and the cilium is mediated by the deacetylase HDAC6 (Figure 4), the only non-nuclear member of the histone deacetylase family, and a critical regulator of primary cilia [184–186]. Inhibition of HDAC6 function by tubacin [187] blocks primary cilia disassembly in RPE1 cells, downstream of Aurora A [23]. A recent screen for flagellar disassembly proteins in Chlamydomonas also identified HDAC6 homologs [28]. HDAC6 defects impair cilia disassembly and cell migration, and have been implicated in several cancers [188,189], adipocyte differentiation [190], and other human health contexts, making it a promising pharmaceutical target [185,191].
Both α-tubulin and cortactin are major HDAC6 substrates [60,124,186]. It was originally hypothesized that HDAC6 deacetylates axoMTs, resulting in axoMT destabilization at the onset of primary cilia disassembly [23,61]. SIRT2, another MT deacetylase, directly binds to HDAC6 and also mediates ciliary disassembly [192]. However, currently there is little evidence to show that HDAC6 activity directly induces axoMT disassembly, or even that HDAC6 is present within cilia [52,60]. Furthermore, there is no evidence that deacetylation destabilizes axoMTs, as acetylation seems to be a marker of stable MTs rather than a destabilizing modification. Finally, the HDAC6 null mouse is viable, and loss of acetylation of mouse sperm of axoMTs does impair sperm development or fertility [193]. Therefore, the role of HDAC6 in ciliary disassembly may be more indirect, perhaps through deacetylation of cytoplasmic α-tubulin and cortactin (see below), both of which are required for ciliary disassembly [60].
The role of tubulin acetylation on MT structure and dynamics is summarized above (see section ‘Post-translational modification of axonemal microtubules’). More globally, the acetylation state of cytoplasmic MTs has diverse effects in the cell, including affecting actin dynamics and functions. The MT acetyltransferase Mec17/ATAT1 is required for the ciliogenesis function of the actin motor Myh10 [134]. Tubulin deacetylation contributes to actin cytoskeletal functions including focal adhesion dynamics and cell migration [56,194]. ATAT1 also mediates localization of the actin scaffolding protein Merlin to MTs, is required for cell–cell and cell–substrate adhesion, and acts upstream of density-dependent YAP signaling inhibition [56].
The other major substrate of HDAC6 is CTTN. Src-mediated phosphorylation of CTTN modulates actin polymerization and dynamics upstream of ciliary disassembly [60,107]. Acetylated CTTN, however, is restricted to the nucleus [124,125]. Deacetylation of CTTN by HDAC6 is activated by ERK, which also promotes cell migration [195]. Other work showed that cell migration promotes the maintenance, not disassembly, of the primary cilium through signal transduction. Interestingly, ATAT1-induced acetylation of α-tubulin promotes the formation of invadopodia in breast cancer cells and hence cell migration. Furthermore, ATAT1 binds to CTTN, and therefore, ATAT1 may acetylate CTTN in addition to α-tubulin [188], indicating an overlap between the roles of acetylated α-tubulin and cortactin. This could establish an elegant model in which α-tubulin and cortactin are co-regulated through acetylation by ATAT1 and deacetylation by HDAC6, in order to co-operate in ciliary and cytoskeletal functions. It would be interesting to test whether both α-tubulin and cortactin are acetylated by ATAT1 during ciliogenesis, while HDAC6 is down-regulated.
Future directions
The last decade has seen significant advances in understanding the relationship between cilia and the cytoskeleton, which has major implications for basic cell biology, including cross-talk between cytoskeletal systems and organelles, intracellular signaling dynamics, cell cycle control, and cell migration. Recent advances in super-resolution microscopy have begun to yield new insights into ciliary ultrastructure, dynamics, and cargo transport with unprecedented spatiotemporal resolution. ‘Omic’ screens are leading to the identification of new players in ciliary assembly and disassembly, signaling, and cell cycle regulation. These and other newly developed tools can be used to address some of the questions raised throughout this review, such as the nature of actin within cilia, the dynamic regulation of axoneme PTMs, and how ciliary actin interacts with and regulates axoMTs. Septins, another cytoskeletal protein family that also interacts with both the actin and MT cytoskeletons, seem to play an important role in cilia biology, but have barely begun to be studied in depth [174]. Thus, the merging fields of cilia biology and the cytoskeleton will yield many significant discoveries that will affect our understanding of ciliary biology and have promising implications for the treatment of ciliopathies and cancer.
Acknowledgements
We thank Jenn Wang for comments on the manuscript. We thank Jackson Liang and Miguel Garcia for comments and assistance with figures. The content is solely the responsibility of the authors and does not necessarily represent the official view of the National Institutes of Health.
Abbreviations
- γ-TURC
gamma-tubulin ring complex
- αTAT1
alpha-tubulin acetylatransferase
- AurA
Aurora A
- BBSome
Bardet–Biedl Syndrome
- CTTN
cortactin
- CytoD
Cytochalasin D
- EB
end-binding
- GCPR
G-protein coupled receptor
- IFT
IntraFlagellar Transport
- HDAC6
histone deacetylase 6
- MAPs
microtubule-associated proteins
- MCCs
multiciliated epithelial cells
- MT
microtubule
- MDCK
Madin-Darby Canine Kidney
- MTOC
MT-organizing center
- ncWnt
noncanonical Wnt
- INPP5E
inositol polyphosphate-5-phosphatase E
- PCP
planar cell polarity
- PDGF
platelet-derived growth factor
- PI(4,5)P2
phosphatidylinositol 4,5-bisphosphate
- Plk1
polo-like kinase 1
- PPC
pericentrosomal preciliary compartment
- PTMs
post-translational modifications
Funding
M.M. was supported by the National Institute of General Medical Sciences of the National Institutes of Health under award number T32GM007276; T.S., NIH R01GM121424; and W.J.N., R35GM118064.
Competing Interests
The Authors declare that there are no competing interests associated with the manuscript.
References
- 1.Breslow D.K., Koslover E.F., Seydel F., Spakowitz A.J. and Nachury M.V. (2013) An in vitro assay for entry into cilia reveals unique properties of the soluble diffusion barrier. J. Cell Biol. 203, 129–147 10.1083/jcb.201212024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keeling J., Tsiokas L. and Maskey D. (2016) Cellular mechanisms of ciliary length control. Cells 5, 6 10.3390/cells5010006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Linck R.W., Chemes H. and Albertini D.F. (2016) The axoneme: the propulsive engine of spermatozoa and cilia and associated ciliopathies leading to infertility. J. Assist. Reprod. Genet. 33, 141–156 10.1007/s10815-016-0652-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta A. and Kitagawa D. (2018) Ultrastructural diversity between centrioles of eukaryotes. J. Biochem. 164, 1–8 10.1093/jb/mvy031 [DOI] [PubMed] [Google Scholar]
- 5.Bernabé-Rubio M. and Alonso M.A. (2017) Routes and machinery of primary cilium biogenesis. Cell. Mol. Life Sci. 74, 4077–4095 10.1007/s00018-017-2570-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Banterle N. and Onczy P. (2017) Centriole biogenesis: from identifying the characters to understanding the plot. Annu. Rev. Cell. Dev. Biol. 33, 23–49 10.1146/annurev-cellbio-100616-060454 [DOI] [PubMed] [Google Scholar]
- 7.Bazzi H. and Anderson K.V. (2014) Acentriolar mitosis activates a p53-dependent apoptosis pathway in the mouse embryo. Proc. Natl Acad. Sci. U.S.A. 111, E1491–E1500 10.1073/pnas.1400568111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stevens N.R., Raposo A.A.S.F., Basto R., St Johnston D. and Raff J.W. (2007) From stem cell to embryo without centrioles. Curr. Biol. 17, 1498–1503 10.1016/j.cub.2007.07.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Avidor-Reiss T., Ha A. and Basiri M.L. (2017) Transition zone migration: a mechanism for cytoplasmic ciliogenesis and postaxonemal centriole elongation. Cold Spring Harb. Perspect. Biol. 9, a028142 10.1101/cshperspect.a028142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishikawa H. and Marshall W.F. (2017) Intraflagellar transport and ciliary dynamics. Cold Spring Harb. Perspect. Biol. 9, a021998 10.1101/cshperspect.a021998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marshall W.F. and Rosenbaum J.L. (2001) Intraflagellar transport balances continuous turnover of outer doublet microtubules. J. Cell Biol. 155, 405–414 10.1083/jcb.200106141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Howes S.C., Alushin G.M., Shida T., Nachury M.V. and Nogales E. (2014) Effects of tubulin acetylation and tubulin acetyltransferase binding on microtubule structure. Mol. Biol. Cell 25, 257–266 10.1091/mbc.e13-07-0387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hsu K.S., Chuang J.Z. and Sung C.H. (2017) The biology of ciliary dynamics. Cold Spring Harb. Perspect. Biol. 9, a027904 10.1101/cshperspect.a027904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benmerah A. (2013) The ciliary pocket. Curr. Opin. Cell Biol. 25, 78–84 10.1016/j.ceb.2012.10.011 [DOI] [PubMed] [Google Scholar]
- 15.Ye F., Nager A.R. and Nachury M.V. (2018) BBSome trains remove activated GPCRs from cilia by enabling passage through the transition zone. J. Cell Biol. 217, 1847–1868 10.1083/jcb.201709041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia-Gonzalo F.R. and Reiter J.F. (2017) Open sesame: how transition fibers and the transition zone control ciliary composition. Cold Spring Harb. Perspect. Biol. 9, a028134 10.1101/cshperspect.a028134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gonçalves J. and Pelletier L. (2017) The ciliary transition zone: finding the pieces and assembling the gate. Mol. Cells 40, 243–253 10.14348/molcells.2017.0054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharma N., Bryant J., Wloga D., Donaldson R., Davis R.C., Jerka-Dziadosz M. et al. (2007) Katanin regulates dynamics of microtubules and biogenesis of motile cilia. J. Cell Biol. 178, 1065–1079 10.1083/jcb.200704021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bernabé-Rubio M., Andrés G., Casares-Arias J., Fernández-Barrera J., Rangel L., Reglero-Real N. et al. (2016) Novel role for the midbody in primary ciliogenesis by polarized epithelial cells. J. Cell Biol. 214, 259–273 10.1083/jcb.201601020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Graser S., Stierhof Y.-D., Lavoie S.B., Gassner O.S., Lamla S., Le Clech M. et al. (2007) Cep164, a novel centriole appendage protein required for primary cilium formation. J. Cell Biol. 179, 321–330 10.1083/jcb.200707181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu Q., Insinna C., Ott C., Stauffer J., Pintado P.A., Rahajeng J. et al. (2015) Early steps in primary cilium assembly require EHD1/EHD3-dependent ciliary vesicle formation. Nat. Cell Biol. 17, 228–240 10.1038/ncb3109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sánchez I. and Dynlacht B.D. (2016) Cilium assembly and disassembly. Nat. Cell Biol. 18, 711–717 10.1038/ncb3370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pugacheva E.N., Jablonski S.A., Hartman T.R., Henske E.P. and Golemis E.A. (2007) HEF1-dependent aurora A activation induces disassembly of the primary cilium. Cell 129, 1351–1363 10.1016/j.cell.2007.04.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Korobeynikov V., Deneka A.Y. and Golemis E.A. (2017) Mechanisms for nonmitotic activation of Aurora-A at cilia. Biochem. Soc. Trans. 45, 37–49 10.1042/BST20160142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Avasthi P. and Marshall W.F. (2013) Ciliary regulation: disassembly takes the spotlight. Curr. Biol. 23, R1001–R1003 10.1016/j.cub.2013.09.052 [DOI] [PubMed] [Google Scholar]
- 26.Liang Y., Meng D., Zhu B. and Pan J. (2016) Mechanism of ciliary disassembly. Cell. Mol. Life Sci. 73, 1787–1802 10.1007/s00018-016-2148-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu Z., Liang Y., He W. and Pan J. (2015) Cilia disassembly with two distinct phases of regulation. Cell Rep. 10, 1803–1810 10.1016/j.celrep.2015.02.044 [DOI] [PubMed] [Google Scholar]
- 28.Wang L., Gu L., Meng D., Wu Q., Deng H. and Pan J. (2017) Comparative proteomics reveals timely transport into cilia of regulators or effectors as a mechanism underlying ciliary disassembly. J. Proteome Res. 16, 2410–2418 10.1021/acs.jproteome.6b01048 [DOI] [PubMed] [Google Scholar]
- 29.Cao M., Li G. and Pan J. (2009) Regulation of cilia assembly, disassembly, and length by protein phosphorylation In Methods in Cell Biology (Roger D. Sloboda, ed.), 1st edn, vol. 94, pp. 333–346, Elsevier; 10.1016/S0091-679X(08)94017-6 [DOI] [PubMed] [Google Scholar]
- 30.Goto H., Inaba H. and Inagaki M. (2017) Mechanisms of ciliogenesis suppression in dividing cells. Cell. Mol. Life Sci. 74, 881–890 10.1007/s00018-016-2369-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharma N., Kosan Z.A., Stallworth J.E., Berbari N.F. and Yoder B.K. (2011) Soluble levels of cytosolic tubulin regulate ciliary length control. Mol. Biol. Cell 22, 806–816 10.1091/mbc.e10-03-0269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wheeler R.J., Gluenz E. and Gull K. (2015) Basal body multipotency and axonemal remodelling are two pathways to a 9+0 flagellum. Nat. Commun. 6, 8964 10.1038/ncomms9964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maheshwari A., Obbineni J.M., Bui K.H., Shibata K., Toyoshima Y.Y. and Ishikawa T. (2015) α- and β-tubulin lattice of the axonemal microtubule doublet and binding proteins revealed by single particle cryo-electron microscopy and tomography. Structure 23, 1584–1595 10.1016/j.str.2015.06.017 [DOI] [PubMed] [Google Scholar]
- 34.Wang J. and Stearns T. (2018) The ABCs of centriole architecture: the form and function of triplet microtubules. Cold Spring Harb. Symp. Quant. Biol. 82, 034496 10.1101/sqb.2017.82.034496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luo W., Ruba A., Takao D., Zweifel L.P., Lim R.Y.H., Verhey K.J. et al. (2017) Axonemal lumen dominates cytosolic protein diffusion inside the primary cilium. Sci. Rep. 7, 15793 10.1038/s41598-017-16103-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wloga D., Joachimiak E., Louka P. and Gaertig J. (2016) Posttranslational modifications of tubulin and cilia. Cold Spring Harb. Perspect. Biol. 9, a028159 10.1101/cshperspect.a028159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ye F., Breslow D.K., Koslover E.F., Spakowitz A.J., James Nelson W. and Nachury M.V. (2013) Single molecule imaging reveals a major role for diffusion in the exploration of ciliary space by signaling receptors. eLife 2013, 1–16 10.7554/eLife.00654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wingfield J.L., Mengoni I., Bomberger H., Jiang Y.-Y., Walsh J.D., Brown J.M. et al. (2017) IFT trains in different stages of assembly queue at the ciliary base for consecutive release into the cilium. eLife 6, e26609 10.7554/eLife.26609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fu W., Wang L., Kim S., Li J. and Dynlacht B.D. (2016) Role for the IFT-A complex in selective transport to the primary cilium. Cell Rep. 17, 1505–1517 10.1016/j.celrep.2016.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pedersen L.B. and Rosenbaum J.L. (2008) Chapter two: intraflagellar transport (IFT): role in ciliary assembly, resorption and signalling In Current Topics in Developmental Biology (Bradley K. Yoder, ed.), 1st edn, vol. 85, pp. 23–61, Elesvier Inc; 10.1016/S0070-2153(08)00802-8 [DOI] [PubMed] [Google Scholar]
- 41.Williams C.L., Mcintyre J.C., Norris S.R., Jenkins P.M., Zhang L., Pei Q. et al. (2014) Direct evidence for BBSome-associated intraflagellar transport reveals distinct properties of native mammalian cilia. Nat. Commun. 5, 5813 10.1038/ncomms6813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lechtreck K.F., Van De Weghe J.C., Harris J.A. and Liu P. (2017) Protein transport in growing and steady-state cilia. Traffic 18, 277–286 10.1111/tra.12474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hu Q., Milenkovic L., Jin H., Scott M.P., Nachury M.V., Spiliotis E.T. et al. (2011) A septin diffusion barrier at the base of the primary cilium maintains ciliary membrane protein distribution. Science 329, 436–439 10.1126/science.1191054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Verhey K.J. and Gaertig J. (2007) The tubulin code. Cell Cycle 6, 2152–2160 10.4161/cc.6.17.4633 [DOI] [PubMed] [Google Scholar]
- 45.Yu I., Garnham C.P. and Roll-Mecak A. (2015) Writing and reading the tubulin code. J. Biol. Chem. 290, 17163–17172 10.1074/jbc.R115.637447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gadadhar S., Bodakuntla S., Natarajan K. and Janke C. (2017) The tubulin code at a glance. J. Cell Sci. 130, 1347–1353 10.1242/jcs.199471 [DOI] [PubMed] [Google Scholar]
- 47.Janke C. (2014) The tubulin code: molecular components, readout mechanisms, and functions. J. Cell Biol. 206, 461–472 10.1083/jcb.201406055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sirajuddin M., Rice L.M. and Vale R.D (2014) Regulation of microtubule motors by tubulin isotypes and posttranslational modifications. Nat. Cell Biol. 16, 335–344 10.1038/ncb2920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Silva M., Morsci N., Nguyen K.C.Q., Rizvi A., Rongo C., Hall D.H. et al. (2017) Cell-specific α-tubulin isotype regulates ciliary microtubule ultrastructure, intraflagellar transport, and extracellular vesicle biology. Curr. Biol. 27, 968–980 10.1016/j.cub.2017.02.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Girotra M., Srivastava S., Kulkarni A., Barbora A., Bobra K., Ghosal D. et al. (2017) The C-terminal tails of heterotrimeric kinesin-2 motor subunits directly bind to α-tubulin1: possible implications for cilia-specific tubulin entry. Traffic 18, 123–133 10.1111/tra.12461 [DOI] [PubMed] [Google Scholar]
- 51.Stepanek L. and Pigino G. (2016) Microtubule doublets are double-track railways for intraflagellar transport trains. Science 352, 721–724 10.1126/science.aaf4594 [DOI] [PubMed] [Google Scholar]
- 52.Gaertig J. and Wloga D. (2008) Chapter 4: ciliary tubulin and its post-translational modifications In Current Topics in Developmental Biology (Bradley K. Yoder, ed.), 1st edn, vol. 85, pp. 83–113, Elesvier Inc; 10.1016/S0070-2153(08)00804-1 [DOI] [PubMed] [Google Scholar]
- 53.Kalebic N., Martinez C., Perlas E., Hublitz P., Bilbao-Cortes D., Fiedorczuk K. et al. (2013) Tubulin acetyltransferase αTAT1 destabilizes microtubules independently of its acetylation activity. Mol. Cell. Biol. 33, 1114–1123 10.1128/MCB.01044-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sudo H. and Baas P.W. (2010) Acetylation of microtubules influences their sensitivity to severing by katanin in neurons and fibroblasts. J. Neurosci. 30, 7215–7226 10.1523/JNEUROSCI.0048-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shida T., Cueva J.G., Xu Z., Goodman M.B. and Nachury M.V. (2010) The major -tubulin K40 acetyltransferase TAT1 promotes rapid ciliogenesis and efficient mechanosensation. Proc. Natl Acad. Sci. U.S.A. 107, 21517–21522 10.1073/pnas.1013728107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aguilar A., Becker L., Tedeschi T., Heller S., Iomini C. and Nachury M.V. (2014) α-Tubulin K40 acetylation is required for contact inhibition of proliferation and cell-substrate adhesion. Mol. Biol. Cell 25, 1854–1866 10.1091/mbc.e13-10-0609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pitaval A., Senger F., Letort G., Gidrol X., Guyon L., Sillibourne J. et al. (2017) Microtubule stabilization drives 3D centrosome migration to initiate primary ciliogenesis. J. Cell Biol. 216, 3713–3728 10.1083/jcb.201610039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.L'Hernault S.W. and Rosenbaum J.L. (1985) Chlamydomonas α-tubulin is posttranslationally modified by acetylation on the epsilon-amino group of a lysine. Biochemistry 24, 473–478 10.1021/bi00323a034 [DOI] [PubMed] [Google Scholar]
- 59.L'Hernault S.W. and Rosenbaum J.L. (1983) Chlamydomonas α-tubulin is posttranslationally modified in the flagella during assembly. J. Cell Biol. 97, 258–263 10.1083/jcb.97.1.258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ran J., Yang Y., Li D., Liu M. and Zhou J. (2015) Deacetylation of α-tubulin and cortactin is required for HDAC6 to trigger ciliary disassembly. Sci. Rep. 5, 12917 10.1038/srep12917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hernault S.W.L. and Rosenbaum J.L. (1985) Reversal of the posttranslational modification on Chlamydomonas flagellar alpha-tubulin occurs during flagellar resorption. J. Cell Biol. 30, 457–462 PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Portran D., Schaedel L., Xu Z., Théry M. and Nachury M.V. (2017) Tubulin acetylation protects long-lived microtubules against mechanical ageing. Nat. Cell Biol. 19, 391–398 10.1038/ncb3481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rocha C., Papon L., Cacheux W., Marques Sousa P., Lascano V., Tort O. et al. (2014) Tubulin glycylases are required for primary cilia, control of cell proliferation and tumor development in colon. EMBO J. 33, 2735 10.15252/embj.201490279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wloga D., Webster D.M., Rogowski K., Bré M.-H., Levilliers N., Jerka-Dziadosz M. et al. (2009) TTLL3 is a tubulin glycine ligase that regulates the assembly of cilia. Dev. Cell 16, 867–876 10.1016/j.devcel.2009.04.008 [DOI] [PubMed] [Google Scholar]
- 65.Gadadhar S., Dadi H., Bodakuntla S., Schnitzler A., Bièche I., Rusconi F. et al. (2017) Tubulin glycylation controls primary cilia length. J. Cell Biol. 216, 2701–2713 10.1083/jcb.201612050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kubo T., Hirono M., Aikawa T., Kamiya R. and Witman G.B. (2016) Reduced tubulin polyglutamylation suppresses flagellar shortness in Chlamydomonas. 13, 3215–3225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.O'Hagan R., Silva M., Nguyen K.C.Q., Zhang W., Bellotti S., Ramadan Y.H. et al. (2017) Glutamylation regulates transport, specializes function, and sculpts the structure of cilia. Curr. Biol. 27, 3430–3441.e6 10.1016/j.cub.2017.09.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Valenstein M.L. and Roll-Mecak A. (2016) Graded control of microtubule severing by tubulin glutamylation. Cell 164, 911–921 10.1016/j.cell.2016.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bosch Grau M., Masson C., Gadadhar S., Rocha C., Tort O., Marques Sousa P. et al. (2017) Alterations in the balance of tubulin glycylation and glutamylation in photoreceptors leads to retinal degeneration. J. Cell Sci. 130, 938–949 10.1242/jcs.199091 [DOI] [PubMed] [Google Scholar]
- 70.Mendes Maia T., Gogendeau D., Pennetier C., Janke C. and Basto R. (2014) Bug22 influences cilium morphology and the post-translational modification of ciliary microtubules. Biol. Open 3, 138–151 10.1242/bio.20146577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Alper J.D., Decker F., Agana B. and Howard J. (2014) The motility of axonemal dynein Is regulated by the tubulin code. Biophys. J. 107, 2872–2880 10.1016/j.bpj.2014.10.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rajagopalan V., D'Amico J.P. and Wilkes D.E. (2013) Cytoplasmic dynein-2: from molecules to human diseases. Front. Biol. 8, 119–126 10.1007/s11515-012-1242-y [DOI] [Google Scholar]
- 73.Prevo B., Scholey J.M. and Peterman E.J.G. (2017) Intraflagellar transport: mechanisms of motor action, cooperation, and cargo delivery. FEBS J. 284, 2905–2931 10.1111/febs.14068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Morthorst S.K., Christensen S.T. and Pedersen L.B. (2018) Regulation of ciliary membrane protein trafficking and signalling by kinesin motor proteins. FEBS J. 1–30 10.1111/febs.14583 [DOI] [PubMed] [Google Scholar]
- 75.Mueller J., Perrone C.A., Bower R., Cole D.G. and Porter M.E. (2005) The FLA3 KAP subunit is required for localization of kinesin-2 to the site of flagellar assembly and processive anterograde intraflagellar transport. Mol. Biol. Cell 16, 1341–1354 10.1091/mbc.e04-10-0931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Malicki J. and Besharse J. (2012) Kinesin-2 family motors in the unusual photoreceptor cilium. Vision Res. 75, 33–36 10.1016/j.visres.2012.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Insinna C., Pathak N., Perkins B., Drummond I. and Besharse J.C. (2008) The homodimeric kinesin, Kif17, is essential for vertebrate photoreceptor sensory outer segment development. Dev. Biol. 316, 160–170 10.1016/j.ydbio.2008.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Insinna C. and Besharse J.C. (2008) Intraflagellar transport and the sensory outer segment of vertebrate photoreceptors. Dev. Dyn. 237, 1982–1992 10.1002/dvdy.21554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jenkins P.M., Hurd T.W., Zhang L., McEwen D.P., Brown R.L., Margolis B. et al. (2006) Ciliary targeting of olfactory CNG channels requires the CNGB1b subunit and the kinesin-2 motor protein, KIF17. Curr. Biol. 16, 1211–1216 10.1016/j.cub.2006.04.034 [DOI] [PubMed] [Google Scholar]
- 80.Snow J.J., Ou G., Gunnarson A.L., Walker M.R.S., Zhou H.M., Brust-Mascher I. et al. (2004) Two anterograde intraflagellar transport motors cooperate to build sensory cilia on C. elegans neurons. Nat. Cell Biol. 6, 1109–1113 10.1038/ncb1186 [DOI] [PubMed] [Google Scholar]
- 81.Prevo B., Mangeol P., Oswald F., Scholey J.M. and Peterman E.J.G. (2015) Functional differentiation of cooperating kinesin-2 motors orchestrates cargo import and transport in C. elegans cilia. Nat. Cell Biol. 17, 1536–1545 10.1038/ncb3263 [DOI] [PubMed] [Google Scholar]
- 82.Milic B., Andreasson J.O.L., Hogan D.W. and Block S.M. (2017) Intraflagellar transport velocity is governed by the number of active KIF17 and KIF3AB motors and their motility properties under load. Proc. Natl Acad. Sci. U.S.A. 114, E6830–E6838 10.1073/pnas.1708157114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chien A., Shih S.M., Bower R., Tritschler D., Porter M.E. and Yildiz A. (2017) Dynamics of the IFT machinery at the ciliary tip. eLife 6, e28606 10.7554/eLife.28606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li A., Saito M., Chuang J.-Z., Tseng Y.-Y., Dedesma C., Tomizawa K. et al. (2011) Ciliary transition zone activation of phosphorylated tctex-1 controls ciliary resorption, S-phase entry and fate of neural progenitors. Nat. Cell Biol. 13, 402–411 10.1038/ncb2218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Miyamoto T., Hosoba K., Ochiai H., Royba E., Izumi H., Sakuma T. et al. (2015) The microtubule-depolymerizing activity of a mitotic kinesin protein KIF2A drives primary cilia disassembly coupled with cell proliferation. Cell Rep. 10, 664–673 10.1016/j.celrep.2015.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Inaba H., Goto H., Kasahara K., Kumamoto K., Yonemura S., Inoko A. et al. (2016) Ndel1 suppresses ciliogenesis in proliferating cells by regulating the trichoplein-Aurora A pathway. J. Cell Biol. 212, 409–423 10.1083/jcb.201507046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kim S., Lee K., Choi J.H., Ringstad N. and Dynlacht B.D. (2015) Nek2 activation of Kif24 ensures cilium disassembly during the cell cycle. Nat. Commun. 6, 8087 10.1038/ncomms9087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Saito M., Otsu W., Hsu K., Chuang J., Yanagisawa T., Shieh V. et al. (2017) Tctex-1 controls ciliary resorption by regulating branched actin polymerization and endocytosis. EMBO Rep. 18, 1460–1472 10.15252/embr.201744204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Piao T., Luo M., Wang L., Guo Y., Li D., Li P. et al. (2009) A microtubule depolymerizing kinesin functions during both flagellar disassembly and flagellar assembly in Chlamydomonas. Proc. Natl Acad. Sci. U.S.A. 106, 4713–4718 10.1073/pnas.0808671106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kobayashi T., Tsang W.Y., Li J., Lane W. and Dynlacht B.D. (2011) Centriolar kinesin Kif24 interacts with CP110 to remodel microtubules and regulate ciliogenesis. Cell 145, 914–925 10.1016/j.cell.2011.04.028 [DOI] [PubMed] [Google Scholar]
- 91.Vasudevan K.K., Jiang Y.-Y., Lechtreck K.F., Kushida Y., Alford L.M., Sale W.S. et al. (2014) Kinesin-13 regulates the quantity and quality of tubulin inside cilia In Molecular Biology of the Cell (Wallace Marshall, ed.), vol. 26, pp. 478–494 Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4310739&tool=pmcentrez&rendertype=abstract%5Cnhttp://www.ncbi.nlm.nih.gov/pubmed/25501369%5Cnhttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC4310739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jang C.-Y., Coppinger J.A., Seki A., Yates J.R. and Fang G. (2009) Plk1 and Aurora A regulate the depolymerase activity and the cellular localization of Kif2a. J. Cell Sci. 122, 1334–1341 10.1242/jcs.044321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ghossoub R., Hu Q., Failler M., Rouyez M.-C., Spitzbarth B., Mostowy S. et al. (2013) Septins 2, 7 and 9 and MAP4 colocalize along the axoneme in the primary cilium and control ciliary length. J. Cell Sci. 126, 2583–2594 10.1242/jcs.111377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zahnleiter D., Hauer N.N., Kessler K., Uebe S., Sugano Y., Neuhauss S.C.F. et al. (2015) MAP4-dependent regulation of microtubule formation affects centrosome, cilia, and Golgi architecture as a central mechanism in growth regulation. Hum. Mutat. 36, 87–97 10.1002/humu.22711 [DOI] [PubMed] [Google Scholar]
- 95.Dacheux D., Roger B., Bosc C., Landrien N., Roche E., Chansel L. et al. (2015) Human FAM154A (SAXO1) is a microtubule-stabilizing protein specific to cilia and related structures. J. Cell Sci. 128(7): 1294–1307 10.1242/jcs.155143 [DOI] [PubMed] [Google Scholar]
- 96.Schroder J.M., Larsen J., Komarova Y., Akhmanova A., Thorsteinsson R.I., Grigoriev I. et al. (2011) EB1 and EB3 promote cilia biogenesis by several centrosome-related mechanisms. J. Cell Sci. 124, 2539–2551 10.1242/jcs.085852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Harris J.A., Liu Y., Yang P., Kner P. and Lechtreck K.F. (2016) Single-particle imaging reveals intraflagellar transport-independent transport and accumulation of EB1 in Chlamydomonas flagella. Mol. Biol. Cell 27, 295–307 10.1091/mbc.e15-08-0608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bhogaraju S., Cajanek L., Fort C., Blisnick T., Weber K., Taschner M. et al. (2013) Molecular basis of tubulin transport within the cilium by IFT74 and IFT81. Science 341, 1009–1012 10.1126/science.1240985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hao L., Thein M., Brust-Mascher I., Civelekoglu-Scholey G., Lu Y., Acar S. et al. (2011) Intraflagellar transport delivers tubulin isotypes to sensory cilium middle and distal segments. Nat. Cell Biol. 13, 790–798 10.1038/ncb2268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Craft J.M., Harris J.A., Hyman S., Kner P. and Lechtreck K.F. (2015) Tubulin transport by IFT is upregulated during ciliary growth by a cilium-autonomous mechanism. J. Cell Biol. 208, 223–237 10.1083/jcb.201409036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Garcia-Gonzalo F.R., Corbit K.C., Sirerol-Piquer M.S., Ramaswami G., Otto E.A., Noriega T.R. et al. (2011) A transition zone complex regulates mammalian ciliogenesis and ciliary membrane composition. Nat. Genet. 43, 776–784 10.1038/ng.891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tanos B.E., Yang H.-J., Soni R., Wang W.J., Macaluso F.P., Asara J.M. et al. (2013) Centriole distal appendages promote membrane docking, leading to cilia initiation. Genes Dev. 27, 163–168 10.1101/gad.207043.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chaitin M.H. (1991) Actin filaments in the photoreceptor cilium of the rds mutant mouse. Exp. Eye Res. 53, 107–113 10.1016/0014-4835(91)90152-5 [DOI] [PubMed] [Google Scholar]
- 104.Chaitin M.H., Carlsen R.B. and Samara G.J. (1988) Immunogold localization of actin in developing photoreceptor cilia of normal and rds mutant mice. Exp. Eye Res. 47, 437–446 10.1016/0014-4835(88)90054-1 [DOI] [PubMed] [Google Scholar]
- 105.Wolfrum U., Liu X., Schmitt A., Udovichenko I.P. and Williams D.S. (1998) Myosin VIIa as a common component of cilia and microvilli. Cell Motil. Cytoskeleton 40, 261–271 [DOI] [PubMed] [Google Scholar]
- 106.Chailley B., Bork K., Gounon P. and Sandoz D. (1986) Immunological detection of actin in isolated cilia from quail oviduct. Biol. Cell 58, 43–52 10.1111/j.1768-322X.1986.tb00487.x [DOI] [PubMed] [Google Scholar]
- 107.Bershteyn M., Atwood S.X., Woo W.-M., Li M. and Oro A.E. (2010) MIM and cortactin antagonism regulates ciliogenesis and hedgehog signaling. Dev. Cell 19, 270–283 10.1016/j.devcel.2010.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kim J., Jo H., Hong H., Kim M.H., Kim J.M., Lee J.K. et al. (2015) Actin remodelling factors control ciliogenesis by regulating YAP/TAZ activity and vesicle trafficking. Nat. Commun. 6, 6781 10.1038/ncomms7781 [DOI] [PubMed] [Google Scholar]
- 109.Yan X. and Zhu X. (2013) Branched F-actin as a negative regulator of cilia formation. Exp. Cell Res. 319, 147–151 10.1016/j.yexcr.2012.08.009 [DOI] [PubMed] [Google Scholar]
- 110.Boisvieux-Ulrich E., Lainé M.-C. and Sandoz D. (1990) Cytochalasin D inhibits basal body migration and ciliary elongation in quail oviduct epithelium. Cell Tissue Res. 259, 443–454 10.1007/BF01740770 [DOI] [PubMed] [Google Scholar]
- 111.Dentler W.L. and Adams C. (1992) Flagellar microtubule dynamics in Chlamydomonas: cytochalasin D induces periods of microtubule shortening and elongation; and colchicine induces disassembly of the distal, but not proximal, half of the flagellum. J. Cell Biol. 117, 1289–1298 10.1083/jcb.117.6.1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yeyati P.L., Schiller R., Mali G., Kasioulis I., Kawamura A., Adams I.R. et al. (2017) KDM3A coordinates actin dynamics with intraflagellar transport to regulate cilia stability. J. Cell Biol. 216, 999–1013 10.1083/jcb.201607032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nager A.R., Goldstein J.S., Herranz-Pérez V., Portran D., Ye F., Garcia-Verdugo J.M. et al. (2017) An actin network dispatches ciliary GPCRs into extracellular vesicles to modulate signaling. Cell 168, 252–263.e14 10.1016/j.cell.2016.11.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Das R.M. and Storey K.G. (2014) Apical abscission alters cell polarity and dismantles the primary cilium during neurogenesis. Science 343, 200–204 10.1126/science.1247521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cao M., Ning J., Hernandez-Lara C.I., Belzile O., Wang Q., Dutcher S.K. et al. (2015) Uni-directional ciliary membrane protein trafficking by a cytoplasmic retrograde IFT motor and ciliary ectosome shedding. eLife 4, e05242 10.7554/eLife.05242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wood C.R. and Rosenbaum J.L. (2014) Proteins of the ciliary axoneme are found on cytoplasmic membrane vesicles during growth of cilia. Curr. Biol. 24, 1114–1120 10.1016/j.cub.2014.03.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jacoby M., Cox J.J., Gayral S., Hampshire D.J., Ayub M., Blockmans M. et al. (2009) INPP5E mutations cause primary cilium signaling defects, ciliary instability and ciliopathies in human and mouse. Nat. Genet. 41, 1027–1031 10.1038/ng.427 [DOI] [PubMed] [Google Scholar]
- 118.Humbert M.C., Weihbrecht K., Searby C.C., Li Y., Pope R.M., Sheffield V.C. et al. (2012) ARL13B, PDE6D, and CEP164 form a functional network for INPP5E ciliary targeting. Proc. Natl Acad. Sci. U.S.A. 109, 19691–19696 10.1073/pnas.1210916109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Plotnikova O.V., Seo S., Cottle D.L., Conduit S., Hakim S., Dyson J.M. et al. (2015) INPP5E interacts with AURKA, linking phosphoinositide signaling to primary cilium stability. J. Cell Sci. 128, 364–372 10.1242/jcs.161323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Phua S.C., Chiba S., Suzuki M., Su E., Roberson E.C., Pusapati G.V. et al. (2017) Dynamic remodeling of membrane composition drives cell cycle through primary cilia excision. Cell 168, 264–279.e15 10.1016/j.cell.2016.12.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Molla-Herman A., Ghossoub R., Blisnick T., Meunier A., Serres C., Silbermann F. et al. (2010) The ciliary pocket: an endocytic membrane domain at the base of primary and motile cilia. J. Cell Sci. 123, 1785–1795 10.1242/jcs.059519 [DOI] [PubMed] [Google Scholar]
- 122.Bryce N.S., Clark E.S., Leysath J.L., Currie J.D., Webb D.J. and Weaver A.M. (2005) Cortactin promotes cell motility by enhancing lamellipodial persistence. Curr. Biol. 15, 1276–1285 10.1016/j.cub.2005.06.043 [DOI] [PubMed] [Google Scholar]
- 123.He Y., Ren Y., Wu B., Decourt B., Lee A.C., Taylor A. et al. (2015) Src and cortactin promote lamellipodia protrusion and filopodia formation and stability in growth cones. Mol. Biol. Cell 26, 3229–3244 10.1091/mbc.e15-03-0142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhang X., Yuan Z., Zhang Y., Yong S., Salas-Burgos A., Koomen J. et al. (2007) HDAC6 modulates cell motility by altering the acetylation level of cortactin. Mol. Cell 27, 197–213 10.1016/j.molcel.2007.05.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ito T., Shimazu S., Shah A., Tsunoda T., Iemura S.-I., Natsume T. et al. (2015) The subcellular localization and activity of cortactin is regulated by acetylation and interaction with Keap1. Sci. Signal. 8, ra120 10.1126/scisignal.aad0667 [DOI] [PubMed] [Google Scholar]
- 126.Sánchez A., Urrego D. and Pardo L.A. (2016) Cyclic expression of the voltage-gated potassium channel KV10.1 promotes disassembly of the primary cilium. 17, 708–723 10.15252/embr.201541082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nagai T. and Mizuno K. (2017) Jasplakinolide induces primary cilium formation through cell rounding and YAP inactivation. PLoS ONE 12, e0183030 10.1371/journal.pone.0183030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wu C.-T., Chen H.-Y. and Tang T.K. (2018) Myosin-Va is required for preciliary vesicle transportation to the mother centriole during ciliogenesis. Nat. Cell Biol. 20, 175–185 10.1038/s41556-017-0018-7 [DOI] [PubMed] [Google Scholar]
- 129.Euteneuer U. and Schliwa M. (1985) Evidence for an involvement of actin in the positioning and motility of centrosomes. J. Cell Biol. 101, 96–103 10.1083/jcb.101.1.96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dawe H.R., Farr H. and Gull K. (2006) Centriole/basal body morphogenesis and migration during ciliogenesis in animal cells. J. Cell Sci. 120, 7–15 10.1242/jcs.03305 [DOI] [PubMed] [Google Scholar]
- 131.Vladar E.K. and Axelrod J.D. (2008) Dishevelled links basal body docking and orientation in ciliated epithelial cells. Trends Cell Biol. 18, 517–520 10.1016/j.tcb.2008.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Spassky N. and Meunier A. (2017) The development and functions of multiciliated epithelia. Nat. Publ. Gr. 18, 423–436 10.1038/nrm.2017.21 [DOI] [PubMed] [Google Scholar]
- 133.Hong H., Kiim J. and Kim J. (2015) Myosin heavy chain 10 (MYH10) is required for centriole migration during the biogenesis of primary cilia. Biochem. Biophys. Res. Commun. 461, 180–185 10.1016/j.bbrc.2015.04.028 [DOI] [PubMed] [Google Scholar]
- 134.Rao Y., Hao R., Wang B. and Yao T.P. (2014) A Mec17-Myosin II effector axis coordinates microtubule acetylation and actin dynamics to control primary cilium biogenesis. PLoS ONE 9, e114087 10.1371/journal.pone.0114087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Stinchcombe J.C., Randzavola L.O., Angus K.L., Mantell J.M., Verkade P. and Griffiths G.M. (2015) Mother centriole distal appendages mediate centrosome docking at the immunological synapse and reveal mechanistic parallels with ciliogenesis. Curr. Biol. 25, 3239–3244 10.1016/j.cub.2015.10.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Farina F., Gaillard J., Guérin C., Couté Y., Sillibourne J., Blanchoin L. et al. (2016) The centrosome is an actin-organizing centre. Nat. Cell Biol. 18, 65–75 10.1038/ncb3285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Joo K., Kim C.G., Lee M.-S., Moon H.-Y., Lee S.-H., Kim M.J. et al. (2013) CCDC41 is required for ciliary vesicle docking to the mother centriole. Proc. Natl Acad. Sci. U.S.A. 110, 5987–5992 10.1073/pnas.1220927110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Burke M.C., Li F.-Q., Cyge B., Arashiro T., Brechbuhl H.M., Chen X. et al. (2014) Chibby promotes ciliary vesicle formation and basal body docking during airway cell differentiation. J. Cell Biol. 207, 123–137 10.1083/jcb.201406140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ye X., Zeng H., Ning G., Reiter J.F. and Liu A. (2014) C2cd3 is critical for centriolar distal appendage assembly and ciliary vesicle docking in mammals. Proc. Natl Acad. Sci. U.S.A. 111, 2164–2169 10.1073/pnas.1318737111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Schmidt K.N., Kuhns S., Neuner A., Hub B., Zentgraf H. and Pereira G. (2012) Cep164 mediates vesicular docking to the mother centriole during early steps of ciliogenesis. J. Cell Biol. 199, 1083–1101 10.1083/jcb.201202126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Li F.-Q., Chen X., Fisher C., Siller S.S., Zelikman K., Kuriyama R. et al. (2016) BAR domain-containing FAM92 proteins interact with Chibby1 to facilitate ciliogenesis. Mol. Cell Biol. 36, 2668–2680 10.1128/MCB.00160-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Seixas C., Choi S.Y., Polgar N., Umberger N.L., East M.P., Zuo X. et al. (2016) Arl13b and the exocyst interact synergistically in ciliogenesis. Mol. Biol. Cell 27, 308–320 10.1091/mbc.e15-02-0061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Park T.J., Mitchell B.J., Abitua P.B., Kintner C. and Wallingford J.B. (2009) Dishevelled controls apical docking and planar polarization of basal bodies in ciliated epithelial cells. Nat. Genet. 40, 871–879 10.1038/ng.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Antoniades I., Stylianou P. and Skourides P.A. (2014) Making the connection: ciliary adhesion complexes anchor basal bodies to the actin cytoskeleton. Dev. Cell 28, 70–80 10.1016/j.devcel.2013.12.003 [DOI] [PubMed] [Google Scholar]
- 145.Kim J., Lee J.E., Heynen-Genel S., Suyama E., Ono K., Lee K. et al. (2010) Functional genomic screen for modulators of ciliogenesis and cilium length. Nature 464, 1048–1051 10.1038/nature08895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Nachury M.V. (2011) Trafficking to the ciliary membrane. Annu. Rev. Cell Dev. Biol. 26, 59–87 10.1146/annurev.cellbio.042308.113337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Reales E., Bernabé-Rubio M., Casares-Arias J., Rentero C., Fernández-Barrera J., Rangel L. et al. (2015) The MAL protein is critical for proper membrane condensation at the ciliary base required for primary cilium elongation. J. Cell Sci. 128, 2261–2270 10.1242/jcs.164970 [DOI] [PubMed] [Google Scholar]
- 148.Assis L.H.P., Silva-Junior R.M.P., Dolce L.G., Alborghetti M.R., Honorato R.V., Nascimento A.F.Z. et al. (2017) The molecular motor Myosin Va interacts with the cilia-centrosomal protein RPGRIP1L. Sci. Rep. 7, 43692 10.1038/srep43692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kohli P., Höhne M., Jüngst C., Bertsch S., Ebert L.K., Schauss A.C. et al. (2017) The ciliary membrane-associated proteome reveals actin-binding proteins as key components of cilia. EMBO Rep. 18, 1521–1535 10.15252/embr.201643846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Christensen S.T., Pedersen S.F., Satir P., Veland I.R. and Schneider L. (2008) The primary cilium coordinates signaling pathways in cell cycle control and migration during development and tissue repair. Curr. Top. Dev. Biol. 85, 261–301 10.1016/S0070-2153(08)00810-7 [DOI] [PubMed] [Google Scholar]
- 151.Albrecht-Buehler G. and Bushnell A. (1979) The orientation of centrioles in migrating 3T3 cells. Exp. Cell Res. 120, 111–118 10.1016/0014-4827(79)90542-1 [DOI] [PubMed] [Google Scholar]
- 152.Veland I.R., Lindbæk L. and Christensen S.T. (2014) Linking the primary cilium to cell migration in tissue repair and brain development. Bioscience 64, 1115–1125 10.1093/biosci/biu179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Higginbotham H., Eom T.-Y., Mariani L.E., Bachleda A., Hirt J., Gukassyan V. et al. (2012) Arl13b in primary cilia regulates the migration and placement of interneurons in the developing cerebral cortex. Dev. Cell 23, 925–938 10.1016/j.devcel.2012.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Légaré S., Chabot C. and Basik M. (2017) SPEN, a new player in primary cilia formation and cell migration in breast cancer. Breast Cancer Res. 19, 104 10.1186/s13058-017-0897-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Boehlke C., Janusch H., Hamann C., Powelske C., Mergen M., Herbst H. et al. (2015) A cilia independent role of Ift88/Polaris during cell migration. PLoS ONE 10, e0140378 10.1371/journal.pone.0140378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Blitzer A.L., Panagis L., Gusella G.L., Danias J., Mlodzik M. and Iomini C. (2011) Primary cilia dynamics instruct tissue patterning and repair of corneal endothelium. Proc. Natl Acad. Sci. U.S.A. 108, 2819–2824 10.1073/pnas.1016702108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Stock C. and Pedersen S.F. (2017) Roles of pH and the Na+/H+ exchanger NHE1 in cancer: from cell biology and animal models to an emerging translational perspective? Semin. Cancer Biol. 43, 5–16 10.1016/j.semcancer.2016.12.001 [DOI] [PubMed] [Google Scholar]
- 158.Schneider L., Cammer M., Lehman J., Sonja K., Guerra C.F., Veland I.R. et al. (2010) Directional cell migration and chemotaxis in wound healing response to PDGF-AA are coordinated by the primary cilium in fibroblasts. Cell Physiol. Biochem. 25, 279–292 10.1159/000276562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Schneider L., Stock C.-M., Dieterich P., Jensen B.H., Pedersen L.B., Satir P. et al. (2009) The Na+/H+ exchanger NHE1 is required for directional migration stimulated via PDGFR-a in the primary cilium. J. Cell Biol. 185, 163–176 10.1083/jcb.200806019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Clement D.L., Mally S., Stock C., Lethan M., Satir P. and Schwab A. (2012) PDGFR a signaling in the primary cilium regulates NHE1-dependent fibroblast migration via coordinated differential activity of MEK1/2–ERK1/2–p90 RSK and AKT signaling pathways. J. Cell Sci. 126, 953–965 10.1242/jcs.116426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hedges J.C., Dechert M.A., Yamboliev I.A., Martin J.L., Hickey E., Weber L.A. et al. (1999) A role for p38(MAPK)/HSP27 pathway in smooth muscle cell migration. Biochemistry 274, 24211–24219 PMID: [DOI] [PubMed] [Google Scholar]
- 162.Jones T.J., Adapala R.K., Geldenhuys W.J., Bursley C., Aboualaiwi W.A., Nauli S.M. et al. (2011) Primary cilia regulate the directional migration and barrier integrity of endothelial cells through the modulation of Hsp27-dependent actin cytoskeletal organization. J. Cell Physiol. 227, 70–76 10.1002/jcp.22704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Gakovic M., Shu X., Kasioulis I., Carpanini S., Moraga I. and Wright A.F. (2018) The role of RPGR in cilia formation and actin stability. Hum. Mol. Genet. 20, 4840–4850 10.1093/hmg/ddr423 [DOI] [PubMed] [Google Scholar]
- 164.Gencer S., Oleinik N., Kim J., Selvam S.P., De Palma R., Dany M. et al. (2017) TGF-b receptor I/II trafficking and signaling at primary cilia are inhibited by ceramide to attenuate cell migration and tumor metastasis. Sci. Signal. 10, eaam7464 10.1126/scisignal.aam7464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ocbina P.J.R., Tuson M. and Anderson K.V. (2009) Primary cilia are not required for normal canonical Wnt signaling in the mouse embryo. PLoS ONE 4, e6839 10.1371/journal.pone.0006839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.May-Simera H.L. and Kelley M.W. (2012) Cilia, Wnt signaling, and the cytoskeleton. Cilia 1, 17 10.1186/2046-2530-1-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Veland I.R., Montjean R., Eley L., Pedersen L.B., Schwab A., Goodship J. et al. (2013) Inversin/nephrocystin-2 is required for fibroblast polarity and directional cell migration. PLoS ONE 8, e60193 10.1371/journal.pone.0060193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Machesky L.M. and Hall A. (1997) Role of actin polymerization and adhesion to extracellular matrix in Rac- and Rho-induced cytoskeletal reorganization. J. Cell Biol. 138, 913–926 10.1083/jcb.138.4.913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Collins M.M. and Ryan A.K. (2014) Are there conserved roles for the extracellular matrix, cilia, and junctional complexes in left-right patterning? Genesis 52, 488–502 10.1002/dvg.22774 [DOI] [PubMed] [Google Scholar]
- 170.Maggiorani D., Dissard R., Belloy M., Saulnier-Blache J.-S., Casemayou A., Ducasse L. et al. (2015) Shear stress-induced alteration of epithelial organization in human renal tubular cells. PLoS ONE 10, e0131416 10.1371/journal.pone.0131416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.He M., Ye W.-J., Wang W., Sison E.S., Nung Y. and Yeh L. (2017) Cytoplasmic Cl− couples membrane remodeling to epithelial morphogenesis. Proc. Natl Acad. Sci. U.S.A. 114, E11161–E11169 10.1073/pnas.1714448115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Overgaard C., Sanzone K., Spiczka K., Sheff D., Sandra A. and Yeaman C. (2009) Deciliation is associated with dramatic remodeling of epithelial cell junctions and surface domains. Mol. Biol. Cell 20, 102–113 10.1091/mbc.e08-07-0741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Bhoonderowa L., Hameurlaine F., Arbabian A. and Faqir F. (2016) Polycystins and intercellular mechanotransduction : a precise dosage of polycystin 2 is necessary for alpha-actinin reinforcement of junctions upon mechanical stimulation. Exp. Cell Res. 348, 23–35 10.1016/j.yexcr.2016.08.021 [DOI] [PubMed] [Google Scholar]
- 174.Palander O., El-Zeiry M. and Trimble W.S. (2017) Uncovering the roles of septins in cilia. Front. Cell Dev. Biol. 5, 36 10.3389/fcell.2017.00036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Spiliotis E.T. and Nelson W.J. (2006) Here come the septins: novel polymers that coordinate intracellular functions and organization. J. Cell Sci. 119, 4–10 10.1242/jcs.02746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Kim S.K., Shindo A., Park T.J., Oh E.C., Ghosh S., Gray R.S. et al. (2010) Planar cell polarity acts through septins to control collective cell movement and ciliogenesis. Science 329, 1337–1340 10.1126/science.1191184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Milenkovic L., Weiss L.E., Yoon J., Roth T.L., Su Y.S., Sahl S.J. et al. (2015) Single-molecule imaging of Hedgehog pathway protein smoothened in primary cilia reveals binding events regulated by Patched1. Proc. Natl Acad. Sci. U.S.A. 112, 8320–8325 10.1073/pnas.1510094112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Huber F., Boire A., López M.P. and Koenderink G.H. (2015) Cytoskeletal crosstalk: when three different personalities team up. Curr. Opin. Cell Biol. 32, 39–47 10.1016/j.ceb.2014.10.005 [DOI] [PubMed] [Google Scholar]
- 179.Lopez M.P., Huber F., Grigoriev I., Steinmetz M.O., Akhmanova A., Koenderink G.H. et al. (2014) Actin-microtubule coordination at growing microtubule ends. Nat. Commun. 5, 4778 10.1038/ncomms5778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Orr A.W., Helmke B.P., Blackman B.R. and Schwartz M.A. (2006) Mechanisms of mechanotransduction. Dev. Cell 10, 11–20 10.1016/j.devcel.2005.12.006 [DOI] [PubMed] [Google Scholar]
- 181.Lee K.L., Guevarra M.D., Nguyen A.M., Chua M.C., Wang Y. and Jacobs C.R. (2015) The primary cilium functions as a mechanical and calcium signaling nexus. Cilia 4, 7 10.1186/s13630-015-0016-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Battle C., Ott C.M., Burnette D.T., Lippincott-Schwartz J. and Schmidt C.F. (2015) Intracellular and extracellular forces drive primary cilia movement. Proc. Natl Acad. Sci. U.S.A. 112, 1410–1415 10.1073/pnas.1421845112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Clare D.K., Magescas J., Piolot T., Dumoux M., Vesque C., Pichard E. et al. (2014) Basal foot MTOC organizes pillar MTs required for coordination of beating cilia. Nat. Commun. 5, 4888 10.1038/ncomms5888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Li Y., Shin D. and Kwon S.H. (2013) Histone deacetylase 6 plays a role as a distinct regulator of diverse cellular processes. FEBS J. 280, 775–793 10.1111/febs.12079 [DOI] [PubMed] [Google Scholar]
- 185.Yu F., Ran J. and Zhou J. (2016) Ciliopathies: does HDAC6 represent a new therapeutic target? Trends Pharmacol. Sci. 37, 114–119 10.1016/j.tips.2015.11.002 [DOI] [PubMed] [Google Scholar]
- 186.Valenzuela-Fernández A., Cabrero J.R., Serrador J.M. and Sánchez-Madrid F. (2008) HDAC6: a key regulator of cytoskeleton, cell migration and cell-cell interactions. Trends Cell Biol. 18, 291–297 10.1016/j.tcb.2008.04.003 [DOI] [PubMed] [Google Scholar]
- 187.Haggarty S.J., Koeller K.M., Wong J.C., Grozinger C.M. and Schreiber S.L. (2003) Domain-selective small-molecule inhibitor of histone deacetylase 6 (HDAC6)-mediated tubulin deacetylation. Proc. Natl Acad. Sci. U.S.A. 100, 4389–4394 10.1073/pnas.0430973100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Castro-Castro A., Janke C., Montagnac G., Paul-Gilloteaux P. and Chavrier P. (2012) ATAT1/MEC-17 acetyltransferase and HDAC6 deacetylase control a balance of acetylation of alpha-tubulin and cortactin and regulate MT1-MMP trafficking and breast tumor cell invasion. Eur. J. Cell Biol. 91, 950–960 10.1016/j.ejcb.2012.07.001 [DOI] [PubMed] [Google Scholar]
- 189.Gradilone S.A., Radtke B.N., Bogert P.S., Huang B.Q., Gajdos G.B. and LaRusso N.F. (2013) HDAC6 inhibition restores ciliary expression and decreases tumor growth. Cancer Res. 73, 2259–2270 10.1158/0008-5472.CAN-12-2938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Forcioli-Conti N., Lacas-Gervais S., Dani C. and Peraldi P. (2015) The primary cilium undergoes dynamic size modifications during adipocyte differentiation of human adipose stem cells. Biochem. Biophys. Res. Commun. 458, 117–122 10.1016/j.bbrc.2015.01.078 [DOI] [PubMed] [Google Scholar]
- 191.Dhanyamraju P.K., Holz P.S., Finkernagel F., Fendrich V. and Lauth M. (2015) Histone deacetylase 6 represents a novel drug target in the oncogenic hedgehog signaling pathway. Mol. Cancer Ther. 14, 727–739 10.1158/1535-7163.MCT-14-0481 [DOI] [PubMed] [Google Scholar]
- 192.Zhou X., Fan L.X., Li K., Ramchandran R., Calvet J.P. and Li X. (2014) SIRT2 regulates ciliogenesis and contributes to abnormal centrosome amplification caused by loss of polycystin-1. Hum. Mol. Genet. 23, 1644–1655 10.1093/hmg/ddt556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Zhang Y., Kwon S., Yamaguchi T., Cubizolles F., Rousseaux S., Kneissel M. et al. (2008) Mice lacking histone deacetylase 6 have hyperacetylated tubulin but are viable and develop normally. Mol. Cell Biol. 28, 1688–1701 10.1128/MCB.01154-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Tran A.D.-A., Marmo T.P., Salam A.A., Che S., Finkelstein E., Kabarriti R. et al. (2007) HDAC6 deacetylation of tubulin modulates dynamics of cellular adhesions. J. Cell Sci. 120(Pt 8), 1469–1479 10.1242/jcs.03431 [DOI] [PubMed] [Google Scholar]
- 195.Williams K.A., Zhang M., Xiang S., Hu C., Wu J.-Y., Zhang S. et al. (2013) Extracellular signal-regulated kinase (ERK) phosphorylates histone deacetylase 6 (HDAC6) at serine 1035 to stimulate cell migration. J. Biol. Chem. 288, 33156–33170 10.1074/jbc.M113.472506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Seeley E.S. and Nachury M.V. (2010) The perennial organelle: assembly and disassembly of the primary cilium. J. Cell Sci. 123, 511–518 10.1242/jcs.061093 [DOI] [PMC free article] [PubMed] [Google Scholar]