Abstract
The increasing number of incidental intracranial aneurysms creates a dilemma of which aneurysms to treat and which to observe. Clinical scoring systems consider risk factors for aneurysm rupture however objective parameters for assessment of aneurysms stability are needed. We retrospectively analysed contrast enhancing behaviour of un-ruptured aneurysms in the black blood magnetic resonance imaging (MRI) in N=71 patients with 90 aneurysms and assessed correlation between aneurysm wall contrast enhancement (AWCE) and aneurysm anatomy and clinical scoring systems. AWCE is associated with aneurysm height and height to width ratio in ICA aneurysms. AWCE is correlated to larger aneurysms in every anatomical location evaluated. However the mean size of the contrast enhancing aneurysms is significantly different between anatomical localizations indicating separate analyses for every artery. Clinical scoring systems like PHASES and UIATS correlate positively with AWCE in black blood MRI. MRI aneurysm wall contrast enhancement is a positive predictor for aneurysm instability and should be routinely assessed in follow up of incidental aneurysms. Aneurysms smaller than 7 mm with AWCE should be followed closely with focus on growth, as they may be prone to growth and rupture.
Key words: Black blood MRI, intracranial aneurysm, aneurysm wall inflammation, unruptured intracranial aneurysm
Introduction
Aneurysms in the adult population are increasingly identified incidentally and have a prevalence of 3%.1,2 Identification of asymptomatic intracranial aneurysms is a neurosurgical dilemma as the treatment risk must be weighed against the natural behaviour of the aneurysms, which is not completely predictable. Scoring systems such as the un-ruptured intracranial aneurysm treatment score (UIATS) or PHASES, which estimates the absolute 5- year risk of aneurysm rupture based on data pooled from prospective cohort studies attempt to predict aneurysm rupture risk.3,4 These scoring systems incorporate different factors, which suggested to increase the risk of rupture such as age, hypertension, maximum diameter, morphology, location of the aneurysm, history of previous subarachnoid haemorrhage to name a few. However, objective factors for rupture prediction remain debatable. Visualization of contrast enhancement in the aneurysm wall by double inversion recovery black blood MRI sequence, has been shown to correlate with aneurysm wall thickness and inflammation, and therefore may represent a new tool for prediction of aneurysm rupture.5,6 It is accepted that hemodynamic stress in the aneurysm wall leads to endothelial reorganisation, inflammation, aneurysm growth and eventually rupture.5 These processes can be visualized by black blood MRI however the remaining question is still the same: Can unstable aneurysms be identified by contrast enhancement behaviour in the black blood MRI? We conducted the present retrospective study to evaluate correlation of aneurysm wall contrast enhancement (AWCE) in the black blood MRI and established risk factors of unstable aneurysms. Additionally we compared black blood MRI results with UIATS and PHASES scores in order to assess the role of black blood MRI as an objective predictive method of aneurysm rupture.
Materials and Methods
Data analysis
We retrospectively evaluated the charts of patients with intracranial aneurysms examined in our department form January 1st until July 30th, 2017 who received a black blood MRI. Patients <18 years old were excluded. Although black blood MRI analysis had been performed in 5 patients with ruptured aneurysms these patients were excluded from further analysis. Only patients with un-ruptured aneurysms were included in the study. All patients seen from January 1st have a black blood MRI. There is no selection bias. Correlation between AWCE in the black blood MRI and the following parameters was analysed: age, gender, aneurysm localisation, aneurysm shape, length (max. diameter in coronar section of CTA), width (max. diameter in transverse section of CTA), height (neck-dome), height/width ratio (hwr), width/neck ratio (bottleneck), height/neck ratio (aspect ratio), UIATS scores and PHASES scores. Black blood MRIs were evaluated by a neuroradiologist and the rest of the data were inserted into an excel database by a neurosurgeon who was blinded to the black blood MRI results in order to avoid any bias. Aneurysm shape and geometrical values were evaluated by CT angiography (CTA).
Black blood MRI sequence
The cranial MRI was performed on a 3T MR scanner (Magnetom Skyra, Siemens, Erlangen) with a 20-channel head coil. The protocol included a 3 D T1 space sequence with fat saturation (SPAIR) and blood suppression (field of view 179*230, repetition time 693 ms, echo time 18 ms, matrix 256x256, spatial resolution 0.9x0.9x0.9 mm) before and after administration of gadolinium (0.2 mL/kg/BW, maximum 20 mL; ProHance, Bracco Imaging, Germany). Total scan time was 7:55 min.
Statistics
All statistical analyses were performed using the R statistical computing package, R version 3.2.2 as released on 2015-08-14 (https://r-project.org/). A manual analysis with students t test for the significant pairs confirmed the results. Statistical significance was defined after Bonferonis correction P≤0.0071 for multiple parameters and as P≤0.01 for paired analysis (localization of aneurysms and size in contrast enhancing and non-enhancing aneurysms).
Results
Patients
We retrospectively evaluated N=71 patients with N=90 un-ruptured aneurysms who received black blood MRIs (Figure 1A and C).
Figure 1.
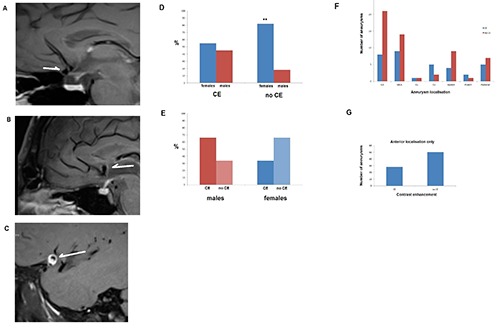
A) Black blood MRI sequence in a non-contrast enhancing AcomA aneurysm (un-ruptured). 58 y.o. female patient (sagittal section). B) Another black blood MRI showing a contrast enhanced un-ruptured AcomA aneurysm wall. 62 y.o. female patient. C) A black blood MRI showing an un-ruptured MCA aneurysm with a strong contrast enhancement in its wall in a 28 y.o. male patient. D) Gender distribution of contrast enhancing aneurysms. Contrast enhancing aneurysms are almost equally distributed between females and males. However 82% of non-contrast enhancing aneurysms were present in female patients and only 18% in males. This difference between non-contrast enhancing aneurysms in male and females was statistically significance at P≤0.01, although the difference depends also on the difference in prevalence of aneurysms between females and males. E) In males the contrast enhanced/non-contrast enhanced aneurysm distribution was 2:1, whereas in females it was 1:2. Aneurysms in males are twice as common prone to be unstable (contrast enhanced) than stable. In females the aneurysms, which are stable, are twice as often as the unstable ones. F) Absolute number of aneurysms (N=90) in the studied population of 71 patients and their localization. Most aneurysms were localized in the ICA. G) Excluding the 12 aneurysms of the posterior localization shows the distribution of contrast vs non-contrast enhancement of the remaining 78 aneurysms in the anterior circulation of the circle of Willis (absolute numbers).
Age and gender
The mean age of the female patients with AWCE was 57.6 years (SD: 13.2 years) and 57.8 years (SD: 14.7 years). In females without AWCE. For male patients the mean age was 49.2 years (SD: 18.7 years) in the AWCE group and 62 years (SD: 16.8 years) in the non-AWCE. The female/male percentage distribution was 55% vs 45% in the AWCE and 82% vs 18% in the non-AWCE (Figure 1D). Overall the female to male ratio of aneurysms was 4:1. Interestingly the ratio of AWCE to non- AWCE in males was 2:1 but 1:2 in females suggesting that in males contrast enhancement of aneurysms is twice as common (Figure 1E). We had a female to male ratio of 4:1 for un-ruptured aneurysms in our population. We know from our own patient collective of ruptured aneurysms, as well as from the literature that the female/male ratio in ruptured aneurysms is about 1.45-1.66 (7, 20), which shows a clear difference to the 4:1 ratio of unruptured aneurysms. In an equation this can expressed as:
Female / male (non ruptured Aneurysms) x (x) = f : m in subarachnoid haemorrhage
Or
| 4 / 1 x = 1.45 to 1.66 |
and
x= 0.36 to 0.415, i.e. reduction of the f: m ratio from non ruptured aneurysm to ruptured of 2.4 – 2.77 times
The change of the female/male ratio of 2.6 times from 4 to about 1.5 may imply that although females have a higher prevalence of aneurysms, males are 2.4-2.77 x more prone to aneurysm rupture than females. The black blood MRI shows that in males the ratio of AWCE to non-AWCE is 2:1 implying that the aneurysms are inflamed and at risk to rupture. In females however this ratio is 1:2 suggesting more frequent stable aneurysms.
In the studied population N=90 intracranial aneurysms were identified. The localization of these aneurysms is illustrated in Figure 1F.
Excluding the posterior localized aneurysms because of their small number (vertebral artery, posterior inferior cerebellar artery (PICA), superior cerebellar artery (SUCA), posterior cerebral artery (PCA), Basilar artery) and adding together the anterior localized aneurysms [internal carotid artery (ICA), posterior communicating artery (PCOM), anterior cerebral artery precommunicans (A1), anterior communicating artery (AcomA), pericallosal artery (A2), medial cerebral artery (MCA) shows that 33% of aneurysms were contrast enhancing and 57% were non-contrast enhancing (Figure 1G).
PHASES scoring and black blood MRI contrast enhancement
Because the PHASES scoring system aims to predict an unstable aneurysm and because AWCE is suggested to reflect inflammation (which by itself may serve as a future objective parameter for aneurysm instability), one could expect a correlation between these two parameters. As shown in Figure 2 there is a correlation between PHASES scores and AWCE. A stronger correlation was seen in the interval scaled analysis between PHASES and height of the aneurysm (i.e. its size) (Figure 3A). The correlation towards height was highly significant (Table 1, P≤0.00…1).
Figure 2.
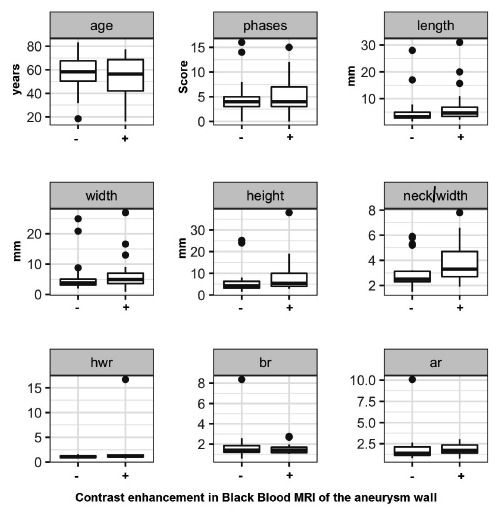
Analysis of the different parameters tested in contrast enhanced and nonenhanced aneurysms. At first sight the differences between the two groups have no statistical significance since aneurysms from any localization are added. -: non-contrast enhancing, +: AWCE, hwr: height to width ratio, br: bottleneck ratio, ar: aspect ratio.
Figure 3.
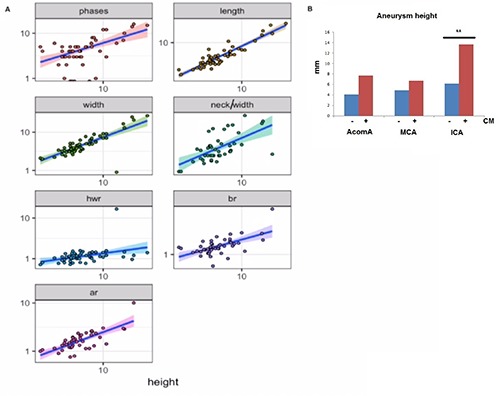
A) Explorative analysis on which parameter to choose for further evaluation shows that height is significantly correlating with all the other measured parameters. This means that height is positively correlating with higher PHASES scores, length, width etc., except height to width ratio. Further analysis between contrast enhancing and nonenhancing aneurysms will be based on these two parameters (height, height to width ratio), which stay in relatio9n to all other parameters. Should we proceed to test every parameter for every group separately the Bonferoni correction for significant p value would rise too high and our parameters would not reach any significance based on the number of studied aneurysms which would be too low. Therefore we proceeded on analysing height, which stays in correlation with the other parameters, or height to width ratio, which stays in not in relation with height. hwr: height to width ratio; br: bottleneck ratio; ar: aspect ratio. B) As shown in this diagram there are significant differences between contrast enhancing behaviour and aneurysm localization. Based on this observation it became obvious why there was no difference between the groups (Figure 4) when all aneurysms were analysed irrespective of their localization. The results of this diagram dictated to analyse the contrast and non-contrast groups separately for each localization.
Table 1.
Statistical correlation analysis between the variants.
| var1 | var2 | R | p | sig |
|---|---|---|---|---|
| height | PHASES | 0.7746223 | 0.0000000 | *** |
| height | length | 0.9512631 | 0.0000000 | *** |
| height | width | 0.9230655 | 0.0000000 | *** |
| height | Neck/width | 0.4902783 | 0.0007291 | ** |
| height | hwr | 0.1958549 | 0.1479979 | |
| height | br | 0.7529957 | 0.0000000 | *** |
| height | ar | 0.7982152 | 0.0000000 | *** |
hwr: height/width ratio; br: bottleneck ratio; ar: aspect ratio.
UIATS score and black blood MRI contrast enhancement
Correlation between AWCE and the UIATS score favouring treatment (therefore indicating an unstable aneurysm) was found to be statistically significant by Fischer’s exact test for count data with a P value of 0.02.
Aneurysm geometry and black blood contrast enhancement in the aneurysm wall
Figures 2 and 3A illustrate the results of AWCE in correlation with the given parameters for all aneurysms irrespective of their localization. No correlation between geometrical parameters and AWCE was found with exception of height, which shows a clear trend (Table 2). A subgroup analysis of AWCE, height and specific localization of aneurysms was performed and revealed that the mean height of aneurysms with AWCE was 13.6 mm for ICA aneurysms; 7.6 mm for AcomA aneurysms and 6.6 mm for MCA aneurysms. The ICA aneurysms with contrast enhancement were significantly greater in size than AcomA and MCA aneurysms (P<0.01, Students t test for independent variables, Figure 3B). Additionally ICA aneurysm size was significantly greater in aneurysms with AWCE than in non- AWCE (13.6 vs 6.1 mm) whereas in AcomA and MCA aneurysms no statistical significance in height between contrast and non-contrast aneurysms could be seen. To test all the parameters against their contrast enhancement correlation we first performed an explorative analysis testing for correlations between interval scaled variables and we could see that the variants tested correlated with height except the height/width ratio (significance level according to Bonferoni correction: 0.0071429) (Figure 3A and Table 1). In conclusion, all variables except height/width ratio are highly correlated with height. Therefore, it suffices to test the black blood enrichment as a function of one of these two variables and the localization. Since length and width represent the maximum diameter in coronar and transverse sections respectively there was not a significant difference between these values indicating that the aneurysms are more or less spherical or barrel shaped. Aneurysm irregularity was not significant in the present study.
Table 2.
Values of the studied parameters in the contrast enhancing and non-contrast enhancing aneurysm groups.
| Parameters | Contrast enhancing | Non-contrast enhancing |
|---|---|---|
| Age (years) | Minimum 18.1 | Minimum 18.4 |
| 1st quarter 42 | 1st quarter 50.3 | |
| Median 56.4 | Median 58.2 | |
| Mean 54.8 | Mean 57.5 | |
| 3rd quarter 68.6 | 3rd quarter 63.8 | |
| Maximum 77.3 | Maximum 83.2 | |
| Stand. Dev. 16.9 | Stand. Dev. 15.1 | |
| PHASES Score | Minimum 0 | Minimum 0 |
| 1st quarter 3 | 1st quarter 3 | |
| Median 4 | Median 4 | |
| Mean 5.7 | Mean 4.4 | |
| 3rd quarter 7 | 3rd quarter 5 | |
| Maximum 15 | Maximum 16 | |
| Stand. Dev. 3.6 | Stand. Dev. 3.4 | |
| Length (mm) | Minimum 2.1 | Minimum 1.5 |
| 1st quarter 3.3 | 1st quarter 2.8 | |
| Median 4.6 | Median 3.3 | |
| Mean 7.2 | Mean 5.0 | |
| 3rd quarter 6.8 | 3rd quarter 4.9 | |
| Maximum 31.7 | Maximum 28 | |
| Stand. Dev. 6.9 | Stand. Dev. 5.2 | |
| Width (mm) | Minimum 0.9 | Minimum 2 |
| 1st quarter 3.6 | 1st quarter 3.1 | |
| Median 4.9 | Median 3.8 | |
| Mean 6.6 | Mean 5.3 | |
| 3rd quarter 7 | 3rd quarter 5.0 | |
| Maximum 27 | Maximum 25 | |
| Stand. Dev. 5.4 | Stand. Dev. 4.9 | |
| Height (mm) | Minimum 2.9 | Minimum 1.5 |
| 1st quarter 4 | 1st quarter 3.2 | |
| Median 5.4 | Median 4.2 | |
| Mean 8.6 | Mean 5.7 | |
| 3rd quarter 10 | 3rd quarter 6.3 | |
| Maximum 38 | Maximum 25.2 | |
| Stand. Dev. 7.6 | Stand. Dev. 5.3 | |
| Neck/width ratio | Minimum 1.9 | Minimum 1.5 |
| 1st quarter 2.7 | 1st quarter 2.3 | |
| Median 3.3 | Median 2.5 | |
| Mean 3.7 | Mean 2.9 | |
| 3rd quarter 4.7 | 3rd quarter 3.1 | |
| Maximum 7.8 | Maximum 5.9 | |
| Stand. Dev. 1.7 | Stand. Dev. 1.2 | |
| Height/width ratio | Minimum 0.7 | Minimum 0.7 |
| 1st quarter 1.0 | 1st quarter 0.9 | |
| Median 1.1 | Median 1.0 | |
| Mean 1.8 | Mean 1.0 | |
| 3rd quarter 1.4 | 3rd quarter 1.2 | |
| Maximum 16.6 | Maximum 1.5 | |
| Stand. Dev. 3.1 | Stand. Dev. 0.2 | |
| Bottleneck ratio (width/neck ratio) | Minimum 1.0 | Minimum 0.5 |
| 1st quarter 1.1 | 1st quarter 1.2 | |
| Median 1.4 | Median 1.4 | |
| Mean 1.5 | Mean 1.7 | |
| 3rd quarter 1.7 | 3rd quarter 1.8 | |
| Maximum 2.7 | Maximum 8.3 | |
| Stand. Dev. 0.5 | Stand. Dev. 1.4 | |
| Aspect ratio (height/neck) | Minimum 0.7 | Minimum 0.8 |
| 1st quarter 1.4 | 1st quarter 1.1 | |
| Median 1.7 | Median 1.3 | |
| Mean 1.8 | Mean 1.9 | |
| 3rd quarter 2.3 | 3rd quarter 2.1 | |
| Maximum 3 | Maximum 10 | |
| Stand. Dev. 0.7 | Stand. Dev. 1.7 |
When the height/width ratio [which seemed not to correlate with height in the explorative analysis (Table 1)] was tested, it was found that the height/width ratio is a significant predictor for AWCE only for ICA aneurysms, but there was also a trend for A2 and PCA. The 50% probability of AWCE was achieved for the height/width ratio >1.0465216 for ACI, 0.8184623 (A2) and 1.201597 (PCA), respectively (Figure 4A).
Figure 4.
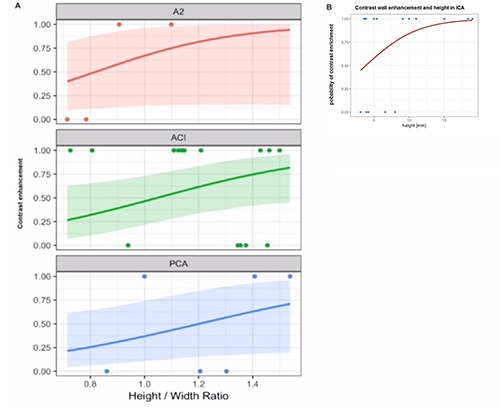
A) Separate analysis of contrast and non-contrast enhancing aneurysm for every localization (ICA, PComA, A1, AcomA, Posterior localization (PCA), A2) showed a positive correlation of height to width ratio in A2, ICA, and PCA aneurysms. Similar results are given in the text for aneurysm height and ICA. B) The 50% probability of contrast enhancement in the ICA was achieved for a height of > 3.95 mm establishing the strong association between size and contrast enhancement in the ICA.
In the inferential analysis of aneurysm height and specific localization (ICA, A2, AcomA, basilar artery, MCA, PCA) we could see that only in the ICA height was a significant predictor for contrast enhancement in black blood MRI. The 50% probability of contrast AWCE in the ICA was achieved for a height of >3.95 mm establishing the strong association between size and AWCE in the ICA (Figure 4B).
It is of interest that in 40% (5/12) aneurysms in the posterior circulation contrast enhancement could be seen along the vessel carrying the aneurysm whereas in the anterior circulation the artery carrying the aneurysm had contrast enhancement only in 2.5% (Figure 5A).
Figure 5.
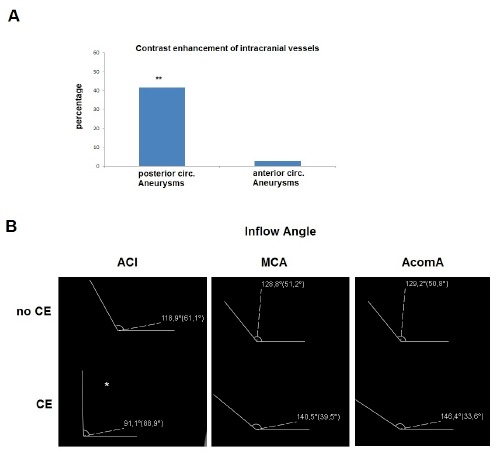
A) Contrast enhancement of the artery carrying the aneurysm. In the posterior circulation 40% of the arteries with aneurysms are contrast enhancing, whereas in the anterior circulation there are only 2.5% such cases (*P<0.001). B) Aneurysm inflow angle in AWCE and non-AWCE aneurysms. In the ICA aneurysms the inflow angle was significantly smaller for the AWCE aneurysms.
The inflow angle was significantly different only for the ICA aneurysms. The results of the inflow angles can be seen in Figure 5B.
Discussion
The rupture of an intracranial aneurysm is associated with high morbidity and mortality of about 30%.7 Since incidental aneurysms become more and more prevalent the dilemma emerges when and which aneurysm should be treated. Because a prospective study of natural aneurysm behaviour, would be unethical, scoring systems based on meta-analyses (PHASES) or expert opinion (UIATS) were developed.3,4 Parameters like age, hypertension, smoking, previous history of subarachnoid haemorrhage, aneurysm size, aneurysm location, geographical region etc. were taken into account. Nevertheless quantitative prediction of risk of aneurysm rupture, have yet to be established. The presence of contrast enhancement in the aneurysm wall reflecting inflammation and possibly aneurysm growth or at least some degree of instability could serve as an objective parameter for aneurysm rupture prediction.6 In the present study we see a positive correlation of AWCE, PHASES and UIATS scores, suggesting that aneurysms fulfilling the criteria of instability in the scoring systems are indeed contrast enhancing aneurysms. Of course the fact that AWCE correlates positively with size and size being considered as a negative prognostic factor in the scoring systems, establishes at least one common parameter in all 3 methods and predefines a positive correlation by size alone. Still, with exception of ICA aneurysms the mean size of aneurysms located elsewhere in the brain was close to 4-5 mm turning them from low risk aneurysms in the UIATS and PHASES scoring systems to unstable aneurysms in the AWCE MRI evaluation. Together with all other factors the aneurysms were deemed as high risk in the common scoring systems too, irrespective of their small size. Contrast enhancing in the black blood MRI, rendering the black blood MRI AWCE alone, was a risk factor of aneurysm rupture. Another interesting observation was, that although in every specific localization the AWCE aneurysms were larger in size compared to those without AWCE, there was a significant difference in size of AWCE aneurysms between the anatomical localizations. In other words, a 4 mm ICA aneurysm is less likely to be contrast enhancing and therefore is rendered as stable, whereas a 4 mm MCA aneurysm is more likely to be contrast enhancing and is rendered as unstable. This can explain why in the ISUIA study small aneurysms (<7 mm) seemed not to rupture but in clinical routine the majority of ruptured aneurysms is smaller than 7 mm.1,7,8 The majority of ruptured aneurysms are in the AComA and MCA arteries and as we show they are much less than 7 mm when they are contrast enhancing. On the other hand ICA aneurysms with AWCE are in the mean, much larger in size than 7 mm. Basing analyses on size only, therefore probably underestimates the risk of rupture of small aneurysms. Furthermore aneurysms in different localizations appear to be completely different in their contrast enhancing behaviour and also should not be evaluated as a group.
Underlying causes for the lack of correlation between AWCE and height, aspect ratio, bottleneck ratio, height to width ratio in MCA and AcomA aneurysms may be explained by smaller case number.
In our explorative analysis we identified a correlation of height to all other geometrical parameters except height to width ratio. Therefore we continued our further analysis with these two parameters and found height, as well as aspect ratio to correlate positively with AWCE in ICA aneurysms. Additionally, the height to width ratio appears to correlate with AWCE in the A2 and PCA aneurysms. These results are not surprizing, as they confirm previous data showing that the shape of an aneurysm and all its parameters indicating a change from a spherical to a barrel shape can be risk factors of aneurysms instability.9
Furthermore, there are inter-individual differences in aneurysm behaviour, which prevent us from making reliable predictions just by exploring aneurysm anatomy. Clinical factors, which are established in the above, mentioned scoring systems like hypertonia, smoking etc. contribute to aneurysm changes and initiation of inflammatory processes, which can be identified by the black blood MRI. Brinjikji et al, showed that larger and non-saccular aneurysms had a higher growth rate which is in accordance with our study showing that larger aneurysms and the ones with greater height to width ratio are the ones which show AWCE.10 Since it has been shown that aneurysms at growth are at significantly higher risk of rupture it remains to be evaluated whether AWCE is associated with aneurysm growth in further prospective studies.10 Inflammatory reactions of the aneurysm wall are well established risk factors for aneurysm rupture.11,12 The characterization of aneurysm wall atherosclerotic plaques and inflammation in opposite contrast MRA had a sensitivity of 88.9% and a specificity of 100% confirming the imaging results in 15 out of 16 operated specimens.13
AWCE could indicate wall damage as wall stress contributes to wall damage and is not only dependent on intrinsic parameters of the aneurysm but also on extrinsic parameters like blood pressure abnormalities. 14 Of course there are also limitations in the visualisation of the aneurysm wall with the black blood MRI, which have to be taken into consideration. There is still a significant spatial and contrast resolution limit, which does not allow us to observe the whole aneurysm wall with the risk to overlook inflamed thin wall areas. Park et al. report visualising aneurysms with a wall thickness of 0.5 mm but the measured wall thickness is close to the pixel size and therefore may lead to overestimation of wall thickness.6,15 Nevertheless there are encouraging results showing a positive correlation of contrast enhancement and aneurysm instability in aneurysms evaluated histologically after surgical resection which showed clear signs of inflammation.16 To avoid the risk of misinterpretation of aneurysm wall thickness we focused our analysis only on AWCE. Our results are in complete accordance with the results of Liu et al. who showed in a series of 48 patients and 61 aneurysms that aneurysm size was an independent risk factor associated with AWCE in the black blood MRI with an OR of 2.46 per mm with high prevalence of contrast enhancement in larger aneurysms but also a 12% contrast enhancing aneurysms with a size <7 mm.16 In addition we not only confirm these results in 90 studied aneurysms but we could show that there are significant differences in the contrast enhancing behaviour of aneurysms depending on their localization, which could be in correlation with the parent vessel diameter. Stivaros et al. have shown a relationship between parent vessel diameter and black blood contrast enhancement.17 Additionally, mathematically we hypothesize that even though females have a higher prevalence of intracranial aneurysms, it is males who have the higher risk of rupture. The change of the female/male ratio of about 2.5 times from 4 to about 1.5 could imply that although females have a higher prevalence of aneurysms, males are 2.5 x more prone of aneurysm rupture than females. The data are confirming previous works showing a f : m ratio for un-ruptured aneurysms of 2.57 to 4 for de novo aneurysm formation.18,19 The f : m ratio for ruptured aneurysms changed to 1.45-1.66 and shows a decrease of the ratio.20,21 The black blood MRI shows that males have a ratio of 2:1 of contrast enhancing/non contrast enhancing aneurysms, which implies that the aneurysms are inflamed and at risk to rupture, whereas in females this ratio is 1:2 indicating that the aneurysms in females are more often stable than not. Age of the patients on the other hand did not play a role in contrast enhancement of aneurysms. The significant difference in the inflow angle in the ICA aneurysms and AWCE could be related to flow dynamics, should be studied in an additional work.
The great limitation of the study is its retrospective character. However, based on the present results there is now an opportunity for a prospective study in which unruptured aneurysms with AWCE can be followed up to evaluate the possibility of growth in these aneurysms in time. The growing behaviour could be observed and in case of growth there would still be enough time to intervene before rupture avoiding an unethical study design.
Conclusions
The results of the present study show that black blood MRI contrast wall enhancement in the aneurysm is a predictive factor for aneurysm instability. We could show a positive correlation between AWCE and localization and size of aneurysms, and UIATS and PHASES scores.
References
- 1.Petridis AK, Kamp MA, Cornelius JF, et al. Aneurysmal subarachnoid haemorrhage. Dtsch Arztebl Int 2017;114:226-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thompson BG, Brown RD Jr, Amin-Hanjani S, et al. Guidelines for the management of patients with unruptured intracranial aneurysms: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke J Cereb Circ 2015;46:2368-400. [DOI] [PubMed] [Google Scholar]
- 3.Etminan N, Brown RD Jr, Beseoglu K, et al. The unruptured intracranial aneurysm treatment score. A multidisciplinary consensus. Neurology 2015;85:881-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greving JP, Wermer MJH, Brown RD, Jr, et al. Development of the PHASES score for prediction of the risk of rupture of intracranial aneurysms: a pooled analysis of six prospective cohort studies. Lancet Neurol 2014;13:59-66. [DOI] [PubMed] [Google Scholar]
- 5.Hu P, Yang Q, Wang D, et al. Wall enhancement on high-resolution magnetic resonance imaging may predict an unsteady state of an intracranial saccular aneurysm. Neuroradiol 2016;58:979-85. [DOI] [PubMed] [Google Scholar]
- 6.Park JK, Lee CS, Sim KB, et al. Imaging of the walls of saccular cerebral aneurysms with double inversion recovery black blood sequence. J Magn Res Imag 2009;30:1179-83. [DOI] [PubMed] [Google Scholar]
- 7.Matsushige T, Akiyama Y, Okazaki T, et al. Vascular wall imaging of unruptured cerebral aneurysms with a hybrid of opposite-contrast MR angiography. AJNR 2015;36:1507-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wiebers DO, Whisnant JP, Huston J 3rd, et al. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet 2003;362:103-10. [DOI] [PubMed] [Google Scholar]
- 9.Maslehaty H, Capone C, Frantsev R, et al. Predicitive anatomical factors for rupture in middle cerebral artery mirror bifurcation aneurysms. J Neurosurg 2017;25:1-9. [DOI] [PubMed] [Google Scholar]
- 10.Brinjikji W, Zhu YQ, Lanzino G, et al. Risk factors for growth of intracranial aneurysms: A systematic review and meta-analysis. AJNR 2016;37:615-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fennel VS, Kalani MYS, Atwal G, et al. Biology os saccular cerebral aneurysms: a review of current understanding and future directions. Front Surg 2016;3:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kilic T, Sohrabifar M, Kurtkaya O, et al. Expression of structural proteins and angiogenic factors in normal arterial and unruptured and ruptured aneurysm walls. Neurosurgery 2005;57:997-1007. [DOI] [PubMed] [Google Scholar]
- 13.Park JK, Lee CS, Sim KB, et al. Imaging of the walls of saccular cerebral aneurysms with double inversion recovery black.blood sequence. J Magn Res Imag 2009;30:1179-83. [DOI] [PubMed] [Google Scholar]
- 14.Cebral JR, Sheridan M, Putman CM. Hemodynamics and bleb formation in intracranial aneurysms: AJNR 2010;31:304-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stivaros SM, Harris JN, Adams W, Jackson A. Does black blood MRA have a role in the assessment of intracerebral aneurysms? Eur Radiol 2009;19:184-92. [DOI] [PubMed] [Google Scholar]
- 16.Maslehaty H, Ngando H, Meila D, et al. Estimated low risk of rupture of smallsized unruptured intracranial aneurysms (UIAs) in relation to intracranial aneurysms in patients with subarachnoid haemorrhage. Acta Neurochir (Wien) 2013;155:1095-100. [DOI] [PubMed] [Google Scholar]
- 17.Thompson BG, Brown RD, Jr, Amin-Hanjani S, et al. Guidelines for the management of patients with unruptured intracranial aneurysms: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke J Cereb Circ 2015;46:2368-400. [DOI] [PubMed] [Google Scholar]
- 18.Ghods AJ, Lopes D, Chen M. Gender differences in cerebral aneurysm location. Front Neurol 2012;3:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Juvela S, Poussa K, Porras M. Factors affecting formation and growth of intracranial aneurysms: a long-term follow- up study. Stroke 2001;32:485-91. [DOI] [PubMed] [Google Scholar]
- 20.Hamdan A, Bames J, Mitchell. Subarachnoid haemorrhage and the female sex: analysis of risk factors, aneurysm characteristics, and outcomes. J Neurosurg 2014;121:1367-73. [DOI] [PubMed] [Google Scholar]
- 21.Zhao L, Zhang L, Zhang X, et al. An analysis of 1256 cases of sporadic ruptured cerebral aneurysm in a single Chinese institution. PLoS One 2014;9:e85668. [DOI] [PMC free article] [PubMed] [Google Scholar]


