Abstract
Experts have previously postulated a linkage between lupus associated vascular pathology and abnormal brain barriers in the immunopathogenesis of neuropsychiatric lupus. Nevertheless, there are some discrepancies between the experimental evidence, or its interpretation, and the working hypotheses prevalent in this field; specifically, that a primary contributor to neuropsychiatric disease in lupus is permeabilization of the blood brain barrier. In this commonly held view, any contribution of the other known brain barriers, including the blood-cerebrospinal fluid and meningeal barriers, is mostly excluded from the discussion. In this review we will shed light on some of the blood brain barrier hypotheses and try to trace their roots. In addition, we will suggest new research directions to allow for confirmation of alternative interpretations of the experimental evidence linking the pathology of intra-cerebral vasculature to the pathogenesis of neuropsychiatric lupus.
Keywords: Blood-brain-barrier, neuropsychiatric lupus, SLE, blood-CSF barrier, choroid plexus, glymphatics
1. Introduction
Systemic lupus erythematosus (SLE) is a complex, multifactorial autoimmune disease with diverse potential targets, including the kidneys, skin, and brain. Much progress has been made to clarify many of the immunopathogenic mechanisms underlying the development of end organ disease in SLE. These often involve complex immunological cascades, including humoral mediated immunity such as autoantibody and complement deposition, with subsequent leukocyte and cytokine driven tissue damage [1]. While this autoimmune dynamic is quite evident in certain manifestations of SLE, including nephritis, there remain many unanswered questions regarding the etiology of neuropsychiatric disease in SLE patients (NPSLE).
NPSLE is broadly defined as one of two sets of presentations: focal and diffuse disease [2]. Generally speaking, focal disease in the form of stroke or focal seizures is closely related to lupus associated coagulopathies, including the antiphospholipid syndrome. Diffuse disease, however, is quite variable between patients, and may include depression, anxiety, memory impairment, and general cognitive decline [3]. Furthermore, there likely are several distinct pathways that may yield any or all of these latter symptoms, including neurotoxic autoantibodies [4], cytokine mediated inflammation (both from the periphery as well as within the central nervous system (CNS)), and cell mediated inflammation.
Due to the relative scarcity of human brain tissue available for research purposes, an obvious necessity in the study of many neurological conditions is the use of model organisms. To that end, there are several mouse models of NPSLE with particular strengths and weaknesses. For example, passive transfer of NPSLE associated neurotoxic autoantibodies (including anti-N-methyl-D-aspartate receptor (NMDAR) and anti-ribosomal P antibodies), either directly into the CNS or through peripheral intravenous injection following experimental disruption of the blood brain barrier (BBB), has been shown to reproduce specific features of diffuse NPSLE such as memory deficits and depression [5–7]. A problem with these models, however, is an underlying assumption that circulating autoantibodies in SLE typically have a means of entry into the CNS. Furthermore, these models may not optimally represent the subtle and progressive interplay between humoral and cell mediated autoimmunity over time which is likely operative in human disease.
Several spontaneous models of SLE with various CNS manifestations exist, including NZB/W-F1, BXSB, and MRL/faslpr/lpr (MRL/lpr) mice [8, 9]. Of these, the MRL/lpr mouse has proven to be the most useful spontaneous model of NPSLE, for several reasons. Both the NZB/W-F1 and BXSB strains are confounded by neuroanatomical anomalies [10], while in BXSB only males are affected, which is inconsistent with the human SLE 9:1 female to male ratio. The MRL/lpr mouse, however, besides a strong female bias, has a very similar overall disease pattern to human SLE including renal and cutaneous manifestations [11, 12], as well as a neuropsychiatric profile consistent with the diffuse manifestations of NPSLE including depression-like behavior and memory deficits [13, 14]. There is a significant and growing body of research into manifestations of NPSLE in MRL/lpr mice, including extensive behavioral characterization and brain tissue evaluation. Furthermore, several means of immunomodulation, including gene knockout and pharmacological interventions, have been applied to MRL/lpr mice, clarifying the relative importance of several cytokine and autoantibody contributors [15–22].
An overarching topic in the discussion of neuroimmune interactions is the unique nature of the brain vasculature. In particular, the CNS has long been believed to be immunoprivileged with limited routine exposure to systemic immune mediators, owing in large part to the highly restrictive and selective nature of CNS endothelium, known as the BBB. There has been much progress in our understanding of the neuroimmune interface in recent years, including identification of novel routes of leukocyte migration in and out of the CNS which bypass the BBB, and a unique mechanism of interstitial fluid clearance [23–25]. In this review, we will carefully explore the long-standing belief in BBB disruption in SLE, and address the potential role other brain barriers may play in the pathogenesis of NPSLE.
2. Vascular pathology and NPSLE
Studies geared toward understanding vascular involvement in SLE, primarily focusing on peripheral tissues outside the brain, suggest that circulating autoreactive antibodies may interact with the endothelial lumen and only subsequently with parenchymal determinants. In most lupus target organs, there is believed to be a vascular component, including (albeit uncommonly) overt vasculitis [26]. From this perspective, it is therefore not surprising that NPSLE manifestations in some patients may have a vascular component. Indeed, when evaluating the focal manifestations of NPSLE, the direct contributions of vascular abnormalities are clear. Multifocal microinfarcts consistent with thrombotic disease, including macro and microthrombosis, have been found in lupus patients post mortem, mainly associated with anti-phospholipid antibodies [27].
A role for a vascular component in the diffuse manifestation of NPSLE (e.g. depression, anxiety, headaches) is less obvious, but microinfarcts, microhemorrhages, and vasculopathy have been reported in autopsies of patients with diffuse NPSLE [27]. Additionally, MRI evaluation of NPSLE patients supports the notion that vascular disease is a hallmark of NPSLE, despite variations in the actual brain regions affected. One such study examining a newly diagnosed NSPLE cohort (108 patients within 6 months of NPSLE diagnosis, with data collected over 9 years [28]), found imaging evidence for large vessel disease (brain infarcts in large arterial supply territories) as well as small vessel disease (small subcortical infarcts, lacunar infarcts, and microbleeds).
Capillaries are the vascular element that allows for all metabolic exchanges between blood and brain. Consequently, an insult to CNS capillaries can yield dramatic effects in brain function. Moreover, from a vascular biology perspective, it is well appreciated that the CNS capillaries are not only the functional unit in the vascular tree responsible for blood-tissue molecular influx and efflux, they are also physically the major interface between the vasculature and the parenchyma (far bigger in total surface area than large and medium diameter vessels). While both post-mortem and MRI data in lupus emphasize the vascular pathology at the level of large and small vessels (arteries and penetrating arterioles), information regarding an important, if not most central component of the vascular tree, the CNS capillary bed, is distinctly missing. In section 3, we will explore some of the unique characteristics of CNS capillaries in their role as the BBB.
3. The BBB is one of three brain barriers
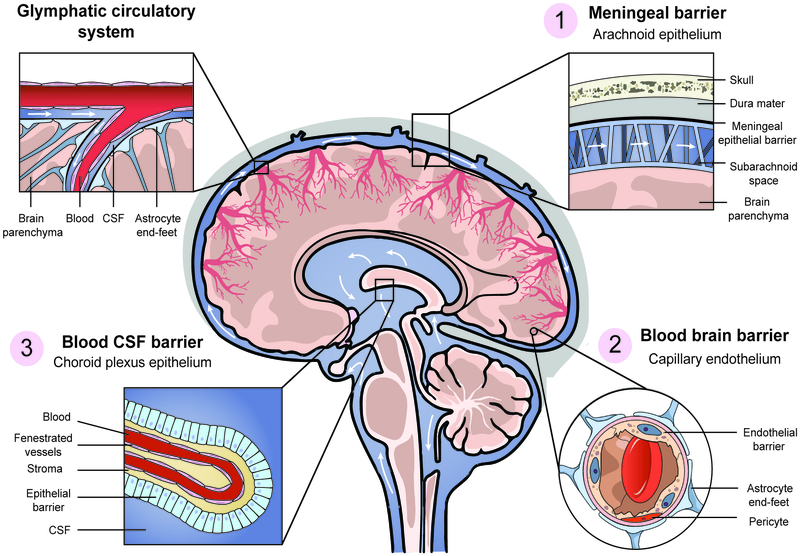
The BBB is the defining feature of brain vasculature. It insulates the brain from unwanted blood borne materials, provides for the special metabolic needs of the brain, and defines the stable environment crucial for brain homeostasis. The function of blood vessels in the brain is very different from that of peripheral blood vessels: brain capillaries primarily isolate the brain from the blood, and only then allow for influx/efflux of materials through the capillary wall in a tightly regulated manner. CNS capillaries are lined by a single layer of endothelial cells and therefore the physical barrier starts with the luminal plasma membrane and extends through the scant cytoplasm of the endothelial cell, the basolateral plasma membrane, and a specialized basal lamina (extracellular matrix produced by both endothelial cells and surrounding astrocytes). As such, it is the functional characteristics of CNS endothelial cells that serve as the foundation of the unique physiological phenomenon that is the BBB (Figure 1) [29]. Conceptually extending beyond BBB endothelial cells, the neurovascular unit (NVU) also consists of pericytes, astrocytic end-feet, and neuronal termini that contribute to the modifiable functions of the BBB. NVU cells provide continuous signaling to the endothelium that facilitates the maintenance of its barrier properties but also actively participate in barrier function, including regulation of water homeostasis, solute transport, and contribution to basement membrane composition [30].
Figure 1. Coordinated function of three brain barriers maintains the brain hemostatic environment.
Regions filled with CSF are highlighted in blue, with white arrows representing CSF flow.
(1) The meningeal barrier separates the outer surface of the brain from the surrounding tissue. The dura mater is a thick and stabilizing envelope of fibrous tissue with permeable vasculature. Specialized epithelial cells, lining the floor of the dura mater/roof of the arachnoid, separate peripheral interstitial fluid from CSF flowing in the subarachnoid space.
(2) The blood brain barrier (BBB) separates blood from the brain parenchyma. Endothelial cells composing this highly selective barrier are continuous, without fenestra, have specialized tight junctions, and a very low rate of transcytosis. Pericytes share a basement membrane with BBB endothelial cells and a sleeve of astrocytic end-feet covering the entire vasculature.
(3) The blood cerebrospinal fluid barrier (BCSFB) at the choroid plexus (CP) separates the blood from the ventricular system. The capillaries within the CP stroma are fenestrated and highly permeable. Cuboidal epithelium at the CP perimeter generates the BCSFB, as well as synthesize and secrete CSF into the ventricles. CSF flows between the four connected ventricles to the subarachnoid space and into the perivascular space, and intermix with brain parenchymal interstitial fluid (also known as the “glymphatic system”).
In their widely referenced review, Abbott, Mendonca, and Dolman extensively describe how BBB pathology may be involved in the development of NPSLE [27]. Though these authors emphasized the importance of the two other major brain barriers, the blood cerebrospinal fluid barrier (BCSFB) and the meningeal barrier, there is a surprising dearth of experimental data or even discussion about these latter barriers in the broader NPSLE literature. One reason for this may be that, when discussing brain barriers in SLE, authors have tended to consider all brain barriers collectively under a single BBB umbrella definition. However, while both the BCSFB and the meningeal barrier are made of specialized epithelial cells that share some common isolating properties with BBB endothelium, these barriers differ in transport mechanisms, resulting in different degrees of selectivity.
Of the three brain barriers, the most obvious yet most overlooked is the meningeal barrier in the arachnoid mater (Figure 1). This outer barrier separates the brain from surrounding tissues, which are rich with fenestrated vasculature making peripheral interstitial fluid very different in composition from the brain interstitial and cerebrospinal fluid (CSF). Moreover, the highly gyrated structure of the brain maintains its contact with meningeal tissues within these folds, thereby increasing the brain surface in close proximity to this outer, non-homeostatic environment. Interestingly, a case-report observation found leptomeningeal abnormality on Gd-DTPA enhanced MRI imaging which might reflect meningeal barrier abnormalities [31], though there is little more about this structure found in the literature.
The second, and far more challenging to comprehend, is the barrier from within: the BBB (Figure 1). In order to appreciate this barrier’s location, it is first necessary to understand the vascular tree of the brain. Originating with the large meningeal blood vessels, bifurcating penetrating arteries are the first to “dive” into brain parenchymal tissue. A vast vascular network composed of arterioles are further distributed into capillaries, which then drain into post capillary venules and then veins leaving the brain parenchyma back into the large meningeal veins. The entire vascular tree, once surrounded by brain parenchyma, has demonstrable barrier properties, and separates brain tissue from blood flowing inside these vessels, i.e., the BBB.
The third barrier separates the blood from the ventricular system: i.e. the BCSFB (Figure 1). The lateral, third and fourth ventricles of the brain are interconnected cisterns filled with CSF, which travels down the central canal of the spinal cord and subsequently connects with the sub-arachnoid space. CSF is produced and secreted into the ventricles by tissue called the choroid plexus (CP). The CP is an elongated sheet-like structure comprised of cuboidal epithelium surrounding a highly vascularized stromal core. It originates in the meningeal folia adjacent to the ventricle, in which CP tissue “floats” in a bath of CSF. The polarized cuboidal epithelium that builds the outer walls of the CP produces and secretes the CSF by selectively absorbing CSF solutes and water from the basolateral, stromal side, and subsequently secreting these constituents at the apical side into the ventricles. The capillary plexus at the center of the CP structure is highly permeable owing to extensive fenestrations, originating and terminating with the vasculature at the base of the CP. Much like the BBB endothelium, CP epithelial properties determine the selectivity of the BCSFB, by utilizing selective and polarized transporters embedded in the plasma membrane in lieu of paracellular and vesicular transport [32]. In this sense, the CP epithelium determines CSF composition just as the BBB endothelium determines CNS interstitial fluid composition.
The metabolic demands of the brain resemble no other organ in the body; proper chemical composition for synaptic transmission and other brain functions requires a tightly regulated environment, as well as insuring that it is free of toxins and pathogens. This environment is maintained by coordinated function of all three barriers described above. Gross examination of fluid movement in the adult brain can provide insight into this dynamic: blood is “filtered” through the BBB, contributing to interstitial fluid in brain parenchyma. Blood is also filtered through the BCSFB, generating CSF in the ventricles. Ependymal cells lining the ventricles have low barrier properties (an embryonic barrier that loses selectivity after birth), and therefore there is a certain degree of CSF penetration in the sub-ventricular zones (surrounding the ventricles), mixing with interstitial fluid. Separately, CSF from the sub-arachnoid space is thought to penetrate along the perivascular space formed between large/medium BBB arteries and their surrounding sheaths of astrocyte end-feet (Figure 1). CSF then diffuses into the parenchyma, again mixing with interstitial fluids before being collected into the perivascular space around veins back into the sub-arachnoid space. This pathway is termed the “glymphatic system” [23], which is speculated to participate in removal of brain waste products as a substitute for lymphatics, which are not distributed throughout the CNS.
BBB breakdown has recently been shown to be associated with various neurological disorders, including Alzheimer’s disease, amyotrophic lateral sclerosis, multiple sclerosis, and stroke [33, 34]. Similarly, in the NSPLE literature, it is widely accepted that of the three brain barriers, BBB breakdown is present. In the remainder of this section, we will attempt to identify the basis for this belief, as well as suggest alternative interpretations to the data that supported this conclusion.
3.1. Possible origins of the NPSLE related BBB dysfunction dogma
Diagnostic tools for NPSLE patients are limited [35] since currently there are no in vivo imaging biomarkers providing direct evidence for BBB dysfunction. Contrast agents in CT and MRI that typically cannot traverse the BBB can provide indications of abnormal leakage [36]. However, the current spatial resolution of CT and MRI scans is low and it is not clear if small leakage at the capillary level can be identified at this resolution. Even if leakage can be identified, given the three barriers described above contrast enhancement is not likely to have sufficient specificity to identify which barrier is leaking. Moreover, NPSLE patients are not routinely evaluated by these imaging methodologies. Several new and emerging MRI methodologies may provide additional insights regarding brain barrier function. Diffusion MRI is one promising approach that is sensitive to the micron scale displacement of water molecules [37]. Specific diffusion models attempt to quantify the contribution of perfusion in the capillary bed on intra-voxel incoherent motion of water molecules that constitutes the diffusion MRI signal [38]. Other models combine diffusion MRI with additional MR contrasts such as dynamic contrast-enhanced MRI (DCE-MRI) [39], or arterial spin labeling [40], to model subtle dynamics in the capillary bed that might be indicative of leakage. Additional promising approaches attempt to quantify the amount of extracellular free-water as a possible marker of leakage [41], or develop new MR contrast agents such as ultra-small superparamagnetic particles [42, 43], which are potentially small enough to identify leakage in capillaries as well.
A long standing approach to evaluate possible barrier dysfunction in both human subjects and NPSLE mouse models is to measure the amount of endogenous serum molecules that usually remain intravascular (such as albumin or IgG) within the CNS, most commonly by sampling CSF. Indeed, an elevated albumin quotient (Qalb) and IgG index have been used as surrogate markers to demonstrate barrier dysfunction in both mouse models and human NPSLE [44–47]. However, because evaluation of the “brain side” is done by sampling CSF, could not the presence of albumin or IgG indicate disruption of the BCSFB, rather than the BBB? While abnormal CSF composition could be a consequence of BBB dysfunction, without an accurate method to distinguish between possible entry routes of these surrogate molecules, relating increased albumin or IgG to BBB dysfunction may be unjustified. For example, CSF taken from the cisternae magna of mice or by lumbar puncture in humans may reflect the function of meningeal barrier at the arachnoid, whereas direct sampling from the ventricular system may be more indicative of CP dysfunction. Moreover, there is extensive and ongoing mixing between interstitial fluid and CSF, making it impossible to ascertain the source of any leakage products. An alternative approach to evaluate BBB dysfunction may be the presence of CNS markers in the serum as a surrogate indicator. This approach assumes enhanced perivascular cell death in the CNS with barrier dysfunction, and abnormal clearance of the marker of interest into the serum. For example, serum levels of S100B (an astrocyte marker) were found to be increased in NPSLE, which was taken as a sign of BBB dysfunction [48]. Again, whether this indeed indicated a breach (since S100B is not exclusively expressed by CNS cells; it is found in adipocytes, peripheral nerves and other tissues as well), and of which barrier, is not clear.
The tendency to group all brain barriers under the umbrella of the BBB likely led to the conclusion that the evidence presented thus far is truly indicative of BBB dysfunction at the level of brain capillaries. The discovery of autoantibodies that cross-react with the NMDA-receptor in NPSLE patients and mouse models highlighted their potential CNS pathogenicity in NPSLE [49]. Diamond and her group created an extensive body of work on this topic, including demonstrating the harmful effects of anti-NMDAR antibodies inducing neuronal death in vitro [5], as well as showing that introducing these antibodies directly into the brain of non-autoimmune mice will cause the same effects [50]. Nevertheless, it is apparent that circulating pathogenic antibodies in these studies do not gain access into the brain through the functioning BBB under baseline conditions. Rather, a pharmacological breach of the BBB was triggered by intravenous administration of lipopolysaccharide or epinephrine (both of which disrupt the BBB) to facilitate access of the pathogenic autoantibodies into the brain to exert their neurotoxic effects. In fact, it seems that the experimental manipulation of BBB integrity was never intended to be linked to specific NPSLE pathologies in and of themselves, but rather was used as a tool to demonstrate that circulating anti-NMDAR antibodies are capable of damaging neurons. Another important aspect of these studies is noteworthy as well: the identification of these autoantibodies in the CSF of SLE patients, absent overt BBB disruption, may further support possible dysfunction of the BCSFB in NPSLE.
3.2. NPSLE related changes in BBB endothelium
Traditionally, inflammatory stimulation of endothelia and its downstream consequences have been collectively described as “activation”. This tissue-variable process may include adhesion molecule expression, as well as overt engagement in the inflammatory microenvironment through secretion of cytokines and chemokines. Brain endothelium is an important focus in the study of NPSLE, as it represents the first line of defense facing blood borne neuropathic molecules. Brain reactive autoantibodies, for example, have been found in the sera of SLE patients without neuropsychiatric disease, suggesting that barrier function can be variably affected between patients [51, 52]. Additionally, changed expression of cellular adhesion molecules may be important to the recruitment of leukocytes to the CNS, as in the case of multiple sclerosis and its rodent model experimental autoimmune encephalomyelitis (EAE) [53].
There have been several studies of the potential role of brain endothelium in the pathogenesis of NPSLE. One such study of particular interest by Yoshio, et al. [54] describes the ability of anti-NMDAR antibodies to activate human endothelium in culture via NF-κB signaling, upregulating expression of various cytokines, such as IL-6 and IL-8, as well as several adhesion molecules. Their findings are of added importance in accounting for the imperfect correlation between anti-NMDAR seropositivity and manifestations of NPSLE. A similar dynamic has been described in relation to anti-ribosomal-P antibodies, which have been found to be endothelial cell auto-reactive and induce IL-6 expression in endothelia as well [55].
Evaluation of MRL/lpr mouse brains for adhesion molecules found increased expression of ICAM-1 and E-selectin. Interestingly, while E-selectin was elevated on the vasculature, changed ICAM-1 expression was found on astrocytes and microglia [20]. Separately, in a study by James, et al. evaluating the role of ICAM-1 [56], ICAM-1 knockout MRL/lpr mice paradoxically had increased cellular infiltration. The fact that the site of infiltration in these mice was primarily the choroid plexus, coupled with the report that ICAM-1 expression is predominately on the apical surface of choroid plexus epithelium [57], indicate that increased lymphocyte counts within the stroma may represent poor trafficking of lymphocytes in the CSF space.
Complement breakdown products are typically elevated in SLE, due to immune complex formation, autoantibody deposition, and subsequent complement mediated inflammation. One such product, C5a, not only induced disruption of an in-vitro model of an SLE BBB, but also induced caspase-mediated endothelial cell apoptosis in MRL/lpr mice [58, 59]. Additionally, antibody-antigen immune complexes may activate endothelial cells directly via NF-κB signaling, resulting in elevated cytokine and adhesion molecule expression [60], though their role in NPSLE has not been evaluated.
The cytokine TNF-like weak inducer of apoptosis (TWEAK) has been found to play a role in the pathogenesis of NPSLE (and other neuropathologies, such as stroke and EAE) [16, 61–65]. Evaluation of human cerebral microvascular endothelium in culture found that TWEAK signaling induced increased expression of cytokines, including IL-6 and IL-8, as well as ICAM-1 and E-selectin [66]. Intracerebroventricular injection of TWEAK in B6 mice resulted in increased brain permeability to serum IgG, as well as an increase in aquaporin 4 positivity around cerebral vessels [61]. Finally, evaluation of TWEAK receptor (Fn14) knockout MRL/lpr mice found decreased fibronectin in brain parenchyma, a surrogate marker for vascular leakage, as well as decreased expression of several adhesion molecules [16].
Collectively, these data provide evidence that endothelial activation may be a component of NPSLE. Lupus serum autoantibodies may interact with the endothelial vascular lumen, causing downstream changes in expression resulting in proinflammatory outcomes. MRL/lpr mice display changes in adhesion molecule expression, as well as complement protein C5a mediated endothelial cell apoptosis. Finally, interruption of TWEAK/Fn14 signaling improved outcomes in MRL/lpr mice, including measures of NVU function. Interestingly, in all of these studies, there is little explicit evidence of BBB disruption. Rather, these studies provide evidence suggesting that inflammatory functions of brain endothelia, including increased secretion of inflammatory cytokines and adhesion molecule upregulation, may occur even without loss of barrier properties (i.e. tight junction stability).
3.3. Evidence supporting BCSFB pathology
Thus far, we have suggested that BBB dysfunction in NPSLE is not the only explanation supported by the available data, and that in fact there is no direct evidence for BBB dysfunction. Could the leakage evidence represent primarily BCSFB dysfunction? What evidence is available to support such an hypothesis?
Interpretations of Qalb and IgG index data may in fact provide stronger support of BCSFB dysfunction then of BBB dysfunction, merely by the virtue of CSF (and not interstitial fluid) being the sampled substrate. Revisiting previous murine studies (mouse models of NPSLE), in which evidence for BBB deficits was provided by extravascular deposition of albumin, fibronectin or IgG depositing around BBB vasculature inside brain parenchyma (e.g. [15, 17]), shows that these proteins appeared to be found around medium-large vascular profiles. In light of the recently discovered glymphatic system described here in section 3, an alternative interpretation could therefore be that this deposition may originate from leaks through the BCSFB, with proteins subsequently transported through the subarachnoid space and to the perivascular space around large vessels and further penetrating into the CNS by bypassing the astrocytic cuff. Such an interpretation could explain the apparent bias toward deposition around medium-large vessels. This hypothesis needs to be further evaluated by tracking experiments with exogenous tracers in NPSLE mouse models.
Inflammation at the CP is overtly evident in MRL/lpr mice, and has been suggested in SLE patients as well. In the MRL/lpr mouse, there is a severe and progressive infiltration of leukocytes, mostly B and T cells [67]. The degree of this infiltration is associated with NPSLE severity [62], though the functional properties of this infiltrate remain unclear as the majority of these lymphocytes remain within the stroma of the CP. Additionally, evaluation of the expression of various adhesion molecules in the CP of the MRL/lpr mouse identifies inflammatory changes as well, further highlighting the role of this centrally located tissue in leukocyte trafficking. Interestingly, these changes are not exclusively seen on the basolateral (vascular) side, but on the apical surface of epithelia as well, suggesting a role in intrathecal leukocyte migration [57, 68].
In SLE patients, there have been several findings which similarly suggest choroid plexus involvement. These studies presented transmission electron microscopy data of patient choroid plexus tissue, identifying electron dense deposits in the epithelial basement membrane remarkably similar to those found in lupus glomerulonephritis [69–71]. Additionally, several case reports describing MRI evaluation of SLE patients with neuropsychiatric disease revealed choroid plexus abnormalities in anatomical and contrast enhanced sequences with concurrent aseptic pleiocytosis of the CSF, suggesting that the choroid plexus may have been a route of leukocyte entry in these patients [72]. Importantly, normalization of the choroid plexus MRI findings following treatment was associated with an improved neuropsychiatric profile. Finally, it is noteworthy that the choroid plexus has been identified as a central region for normal immunosurveillance in the CNS [73], and is an essential starting point in the development of EAE, during which Th17 cells infiltrate through the choroid plexus initiating subsequent demyelinating events [74]. Between what is already known about the potential role of the BCSFB in the pathogenesis of NPSLE, coupled with its role in other neuroimmunopathologies and basal neurosurveillance, the choroid plexus appears to be an attractive research target that demands further attention in the study of NPSLE.
4. What is the value of identifying the affected brain barrier in NPSLE ?
If normal brain function relies on proper function of all three brain barriers, what is the significance of identifying the source of leakage in the etiology of NPSLE? Such knowledge may influence future research directions, including searching for therapeutics to restore the function of specific barriers, designing of drug delivery to the CNS in NPSLE, as well as having broader implications for other neuroimmunopathologies. If barrier dysfunction is indeed primarily at the CP, pathogenic autoantibodies would be predicted to affect periventricular brain regions. If an inflammatory process is responsible for BCSFB perturbations, efforts should be made to find the specific inflammatory initiator and find CP targeted therapeutics that would restore BCSFB function. Finally, if the BBB is not as severely compromised as previously thought, drug permeability across brain vasculature must be considered when treating NPSLE patients.
4.1. Emerging approaches in the study of cerebral vasculature in NPSLE
A possible link has been previously proposed between the general vascular pathology of SLE and specific BBB dysfunction. Thrombus formation in brain microvasculature results in local ischemia, which in turn causes BBB disruption [28, 44]. A recent study has shown that the biphasic time-course of increased BBB permeability, known for many years in stroke, is translated into alteration of two distinct cell biological properties of BBB endothelium [75]. The first phase is increased transcytosis and the second is disassembly of tight junctions, eventually culminating in local hemorrhage. With new imaging modalities and molecular markers for these cell biological properties, the potential temporal link found in models of stroke could be revisited using NPSLE mouse models. In a similar fashion, new insights into the molecular mechanisms and signaling pathways that control functional properties of BBB endothelium (e.g SHH, Wnt, MFSD2A, transport mechanisms, etc.) should be revisited in NPSLE as well. Additionally, while the current technology of diagnostic MRI studies may provide only limited opportunities to investigate the possible changes in the vascular network in humans, mouse models could provide insights into overall vascular network patterning defects up to the level of single capillaries.
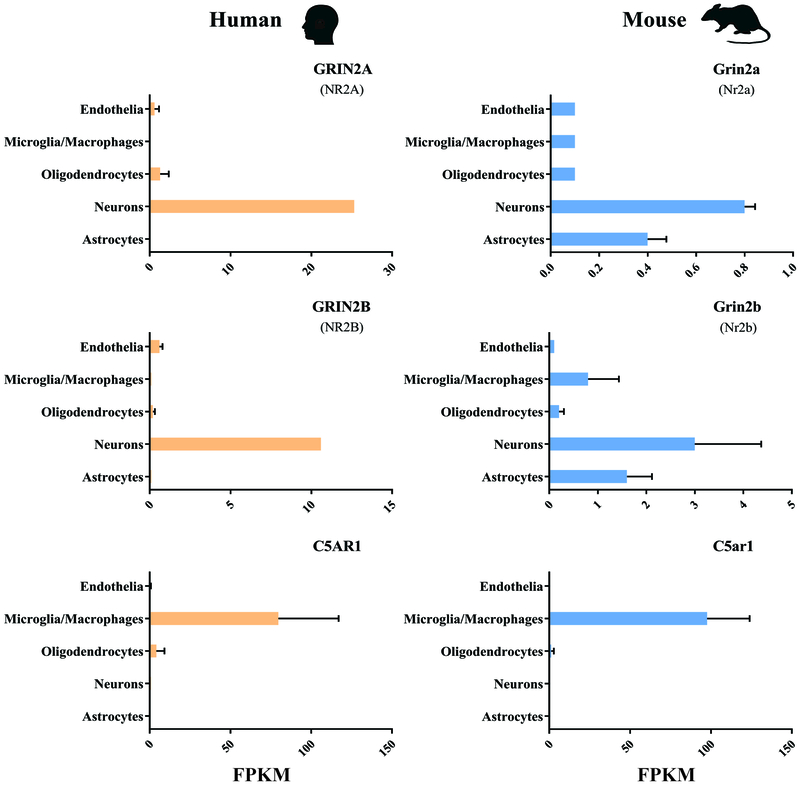
Finally, autoantibodies against BBB endothelial cells and activation of the complement pathways were both suggested above in section 3.2. as harmful events in the etiology of NPSLE. To the best of our knowledge, the majority of experiments supporting this suggestion involve in vitro challenges to cultured endothelial cells, and those in vivo only provide surrogate markers of BBB leakage without direct evaluation. This experimental approach makes the interpretation somewhat limited because BBB endothelial cells lose their specific properties in a de-differentiation process when removed from the brain environment and cultured in vitro [76]. Attempting to recapitulate barrier properties by in vitro BBB models has become increasingly complex, including mixed cultures using neuronal/glial/pericyte/endothelial cell types, stem cell derived cells, three-dimensional and artificial blood flow, none of which have been applied to the study of NPSLE. For these reasons, BBB research is almost exclusively focused on studies in live animal models. Nevertheless, recent studies with genomic and proteomic techniques permit large-scale identification of BBB molecular components. Some of these studies have led to the creation of publicly available databases, which provide an opportunity to test the in vivo relevance of proposed BBB endothelial insults in NPSLE [77]. For example, ‘An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex’ [78] and ‘Purification and characterization of progenitor and mature human astrocytes reveals transcriptional and functional differences with mouse’ [79] are databases that identify the expression value of genes of interest (reflected by their steady state mRNA levels), in particular cell populations isolated from either mouse or human brains. Using these tools, one can find that NMDA receptor subunits, whose activation by autoantibodies have been implicated in activation of human umbilical vein endothelial cells, have negligible transcripts in BBB endothelial cells in vivo but are predictably abundant in neurons (see Figure 2, FPKM of 0.1 represents noise threshold of the data and FPKM>10 is readily detectable by qPCR). Similarly, C5a, which was found to induce apoptosis in endothelial cells in vitro, presumably transduces its effects through the C5AR1 receptor, whose transcript is not detectable in BBB endothelial cells in vivo but is rather abundant in microglia.
Figure 2. RNA-sequencing transcriptome database of glia, neurons, and vascular cells of the cerebral cortex:
NMDAR subunits (Grin2a (Nr2a) & Grin2b (Nr2b)) or the C5a complement receptor (C5ar1) have been implicated in NPSLE related endothelial activation/apoptosis, based on in vitro studies. However, low to negligible transcript levels in BBB endothelial cells in vivo have been seen. As expected, these gene transcripts are most abundant in neurons and microglia, respectively (FPKM of 0.1 represents noise threshold of the data and FPKM>10 is readily detectable by qPCR). This observation is true for both human and mouse BBB endothelial cells. Adapted from [82].
5. Conclusions
The existing collective body of data has suggested overt dysfunction of the BBB in NPSLE. However, formulating this particular understanding of lupus neuropathology requires a set of assumptions that have been questioned in recent years, including the significance of albumin leakage as marker of capillary tight junction disruption, the translatability of culture based in vitro models of the BBB to in vivo disease progression, and the immune privileged nature of the CNS. Additionally, our improved understanding of the choroid plexus as an important potential focus of the neuro-immune interface in NPSLE strongly suggests that this unique structure should be more extensively studied. It is possible that previous NPSLE studies, with a particular emphasis on neuropathic autoantibodies and their entry into the CNS, may warrant reinterpretation in light of the multiple brain barriers described herein. Novel and specific experimental modalities that are now being tested and developed may help to further advance our imperfect understanding of the pathogenesis of NPSLE - crucial progress, which may have important therapeutic implications.
Take-home messages.
The blood brain barrier is one of three major barriers in the central nervous system;
While circulating molecules, including autoantibodies, may enter the central nervous system in neuropsychiatric lupus, this may occur through the meningeal arachnoid and/or blood-cerebrospinal fluid barriers;
New experimental modalities specific for each of the three brain barriers should be pursued;
Advances in magnetic resonance imaging may prove powerful in translating information from model organisms and systems to better understanding of human neuropsychiatric lupus;
The choroid plexus appears to be a particularly attractive region on which to focus.
Acknowledgements
These studies were supported by training grant T32-GM007288 to A. Stock from the NIH; research grants from the National Institute of Mental Health (MH108574) and the National Institute of Biomedical Imaging and Bioengineering (EB015902) to O. Pasternak; research grants from the Abisch-Frenkel Foundation (15/H1) and the Helmsley Foundation to A. Ben-Zvi; and a research grant from the National Institute of Arthritis and Musculoskeletal Diseases (AR065594) to C. Putterman.
Abbreviations
- SLE
Systemic lupus erythematosus
- NPSLE
Neuropsychiatric Systemic Lupus Erythematosus
- CNS
Central Nervous System
- NMDAR
N-methyl-D-aspartate receptor
- BBB
Blood brain barrier
- NVU
Neurovascular unit
- BCSFB
Blood cerebrospinal fluid barrier
- CSF
Cerebrospinal fluid
- CP
Choroid Plexus
- EAE
Experimental autoimmune encephalomyelitis
Footnotes
Disclosures
None.
References:
- [1].Hahn BH, Chapter 3 - The Pathogenesis of SLE, Dubois’ Lupus Erythematosus and Related Syndromes (Eighth Edition), W.B. Saunders, Philadelphia, 2013, pp. 25–34. [Google Scholar]
- [2].Govoni M, Bortoluzzi A, Padovan M, Silvagni E, Borrelli M, Donelli F, Ceruti S and Trotta F, The diagnosis and clinical management of the neuropsychiatric manifestations of lupus, J. Autoimmun 74 (2016) 41–72. [DOI] [PubMed] [Google Scholar]
- [3].Sciascia S, Bertolaccini ML, Baldovino S, Roccatello D, Khamashta MA and Sanna G, Central nervous system involvement in systemic lupus erythematosus: Overview on classification criteria, Autoimmun. Rev 12 (2013) 426–9. [DOI] [PubMed] [Google Scholar]
- [4].Rekvig OP, Putterman C, Casu C, Gao HX, Ghirardello A, Mortensen ES, Tincani A and Doria A, Autoantibodies in lupus: culprits or passive bystanders?, Autoimmun. Rev 11 (2012) 596–603. [DOI] [PubMed] [Google Scholar]
- [5].Kowal C, Degiorgio LA, Lee JY, Edgar MA, Huerta PT, Volpe BT and Diamond B, Human lupus autoantibodies against NMDA receptors mediate cognitive impairment, Proc. Natl. Acad. Sci. U. S. A 103 (2006) 19854–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kowal C and Diamond B, Aspects of CNS lupus: mouse models of anti-NMDA receptor antibody mediated reactivity, Methods Mol. Biol 900 (2012) 181–206. [DOI] [PubMed] [Google Scholar]
- [7].Segovia-Miranda F, Serrano F, Dyrda A, Ampuero E, Retamal C, Bravo-Zehnder M, Parodi J, Zamorano P, Valenzuela D, Massardo L, van Zundert B, Inestrosa NC and Gonzalez A, Pathogenicity of lupus anti-ribosomal P antibodies: role of cross-reacting neuronal surface P antigen in glutamatergic transmission and plasticity in a mouse model, Arthritis Rheum. 67 (2015) 1598–610. [DOI] [PubMed] [Google Scholar]
- [8].Arabo A, Costa O, Dubois M, Tron F and Caston J, Effects of systemic lupus erythematosus on spatial cognition and cerebral regional metabolic reactivity in BxSB lupus-prone mice, Neuroscience 135 (2005) 691–702. [DOI] [PubMed] [Google Scholar]
- [9].Vogelweid CM, Wright DC, Johnson JC, Hewett JE and Walker SE, Evaluation of memory, learning ability, and clinical neurologic function in pathogen-free mice with systemic lupus erythematosus, Arthritis Rheum. 37 (1994) 889–97. [DOI] [PubMed] [Google Scholar]
- [10].Sherman GF, Galaburda AM, Behan PO and Rosen GD, Neuroanatomical anomalies in autoimmune mice, Acta Neuropathol. 74 (1987) 239–42. [DOI] [PubMed] [Google Scholar]
- [11].Doerner JL, Wen J, Xia Y, Paz KB, Schairer D, Wu L, Chalmers SA, Izmirly P, Michaelson JS, Burkly LC, Friedman AJ and Putterman C, TWEAK/Fn14 Signaling Involvement in the Pathogenesis of Cutaneous Disease in the MRL/lpr Model of Spontaneous Lupus, J. Invest. Dermatol (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Chalmers SA, Wen J, Shum J, Doerner J, Herlitz L and Putterman C, CSF-1R inhibition attenuates renal and neuropsychiatric disease in murine lupus, Clin. Immunol (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sakic B, The MRL model: an invaluable tool in studies of autoimmunity-brain interactions, Methods Mol. Biol 934 (2012) 277–99. [DOI] [PubMed] [Google Scholar]
- [14].Gulinello M and Putterman C, The MRL/lpr mouse strain as a model for neuropsychiatric systemic lupus erythematosus, J. Biomed. Biotechnol 2011. (2011) 207504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wen J, Doerner J, Chalmers S, Stock A, Wang H, Gullinello M, Shlomchik MJ and Putterman C, B cell and/or autoantibody deficiency do not prevent neuropsychiatric disease in murine systemic lupus erythematosus, J. Neuroinflammation 13 (2016) 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wen J, Doerner J, Weidenheim K, Xia Y, Stock A, Michaelson JS, Baruch K, Deczkowska A, Gulinello M, Schwartz M, Burkly LC and Putterman C, TNF-like weak inducer of apoptosis promotes blood brain barrier disruption and increases neuronal cell death in MRL/lpr mice, J. Autoimmun 60 (2015) 40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Stock AD, Wen J, Doerner J, Herlitz LC, Gulinello M and Putterman C, Neuropsychiatric systemic lupus erythematosus persists despite attenuation of systemic disease in MRL/lpr mice, J. Neuroinflammation 12 (2015) 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hasegawa H, Chemokine blockade for lupus model mice. Front Biosci. 1 (2008) 13:2900–8. [DOI] [PubMed] [Google Scholar]
- [19].Williams S, Stafford P and Hoffman SA, Diagnosis and early detection of CNS-SLE in MRL/lpr mice using peptide microarrays, BMC Immunol. 15 (2014) 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Stielke S, Keilhoff G, Kirches E, Mertens PR, Neumann KH, Tsokos GC and Mawrin C, Adhesion molecule expression precedes brain damages of lupus-prone mice and correlates with kidney pathology, J. Neuroimmunol 252 (2012) 24–32. [DOI] [PubMed] [Google Scholar]
- [21].Marcinko K, Parsons T, Lerch JP, Sled JG and Sakic B, Effects of prolonged treatment with memantine in the MRL model of CNS lupus, Clin Exp Neuroimmunol 3 (2012) 116–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Jeltsch-David H and Muller S, Neuropsychiatric systemic lupus erythematosus and cognitive dysfunction: the MRL-lpr mouse strain as a model, Autoimmun. Rev 13 (2014) 963–73. [DOI] [PubMed] [Google Scholar]
- [23].Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS, Harris TH and Kipnis J, Structural and functional features of central nervous system lymphatic vessels, Nature 523 (2015) 337–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Yang L, Kress BT, Weber HJ, Thiyagarajan M, Wang B, Deane R, Benveniste H, Iliff JJ and Nedergaard M, Evaluating glymphatic pathway function utilizing clinically relevant intrathecal infusion of CSF tracer, J. Transl. Med 11 (2013) 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Engelhardt B, Vajkoczy P and Weller RO, The movers and shapers in immune privilege of the CNS, Nat. Immunol 18 (2017) 123–131. [DOI] [PubMed] [Google Scholar]
- [26].Szekanecz Z and Koch AE, Vascular involvement in rheumatic diseases: ‘vascular rheumatology’, Arthritis Res. Ther 10 (2008) 224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Abbott NJ, Mendonça LLF and Dolman DEM, The blood–brain barrier in systemic lupus erythematosus, Lupus 12 (2003) 908–915. [DOI] [PubMed] [Google Scholar]
- [28].Sarbu N, Alobeidi F, Toledano P, Espinosa G, Giles I, Rahman A, Yousry T, Capurro S, Jager R, Cervera R and Bargallo N, Brain abnormalities in newly diagnosed neuropsychiatric lupus: systematic MRI approach and correlation with clinical and laboratory data in a large multicenter cohort, Autoimmun. Rev 14 (2015) 153–9. [DOI] [PubMed] [Google Scholar]
- [29].Reese TS and Karnovsky MJ, Fine structural localization of a blood-brain barrier to exogenous peroxidase, J. Cell Biol 34 (1967) 207–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].McConnell HL, Kersch CN, Woltjer RL and Neuwelt EA, The translational significance of the neurovascular unit, J. Biol. Chem 292 (2017) 762–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Okano R, Kuroki M, Otsuka M, Yamada A and Ueki A, [Leptomeningeal abnormality on Gd-DTPA enhanced MRI in a case of SLE presenting diffuse organic brain syndrome], Rinsho Shinkeigaku. 33 (1993) 78–82. [PubMed] [Google Scholar]
- [32].Johanson CE, Stopa EG and McMillan PN, The blood-cerebrospinal fluid barrier: structure and functional significance, Methods Mol. Biol 686 (2011) 101–31. [DOI] [PubMed] [Google Scholar]
- [33].Zhao Z, Nelson AR, Betsholtz C and Zlokovic BV, Establishment and dysfunction of the blood-brain barrier, Cell 163 (2015) 1064–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Zlokovic BV, The blood-brain barrier in health and chronic neurodegenerative disorders, Neuron 57 (2008) 178–201. [DOI] [PubMed] [Google Scholar]
- [35].Zardi EM, Taccone A, Marigliano B, Margiotta DP and Afeltra A, Neuropsychiatric systemic lupus erythematosus: tools for the diagnosis, Autoimmun. Rev 13 (2014) 831–9. [DOI] [PubMed] [Google Scholar]
- [36].Weiner HL and Selkoe DJ, Inflammation and therapeutic vaccination in CNS diseases, Nature 420 (2002) 879–84. [DOI] [PubMed] [Google Scholar]
- [37].Assaf Y and Pasternak O, Diffusion tensor imaging (DTI)-based white matter mapping in brain research: a review, J. Mol. Neurosci 34 (2008) 51–61. [DOI] [PubMed] [Google Scholar]
- [38].Le Bihan D, Diffusion, confusion and functional MRI, Neuroimage 62 (2012) 1131–6. [DOI] [PubMed] [Google Scholar]
- [39].Montagne A, Barnes SR, Sweeney MD, Halliday MR, Sagare AP, Zhao Z, Toga AW, Jacobs RE, Liu CY, Amezcua L, Harrington MG, Chui HC, Law M and Zlokovic BV, Blood-brain barrier breakdown in the aging human hippocampus, Neuron 85 (2015) 296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Gulati G, Jones JT, Lee G, Altaye M, Beebe DW, Meyers-Eaton J, Wiley K, Brunner HI and DiFrancesco MW, Altered blood-brain barrier permeability in patients with systemic lupus erythematosus: a novel imaging approach, Arthritis Care Res. (Hoboken) 69 (2017) 299–305. [DOI] [PubMed] [Google Scholar]
- [41].Pasternak O, Kubicki M and Shenton ME, In vivo imaging of neuroinflammation in schizophrenia, Schizophr. Res 173 (2016) 200–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Deddens LH, Van Tilborg GA, Mulder WJ, De Vries HE and Dijkhuizen RM, Imaging neuroinflammation after stroke: current status of cellular and molecular MRI strategies, Cerebrovasc. Dis 33 (2012) 392–402. [DOI] [PubMed] [Google Scholar]
- [43].Tourdias T and Dousset V, Neuroinflammatory imaging biomarkers: relevance to multiple sclerosis and its therapy, Neurotherapeutics 10 (2013) 111–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Stock AD, Wen J and Putterman C, Neuropsychiatric lupus, the blood brain barrier, and the TWEAK/Fn14 pathway, Front. Immunol 4 (2013) 484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Ganrot K and Laurell CB, Measurement of IgG and albumin content of cerebrospinal fluid, and its interpretation, Clin. Chem 20 (1974) 571–3. [PubMed] [Google Scholar]
- [46].McLean BN, Miller D and Thompson EJ, Oligoclonal banding of IgG in CSF, blood-brain barrier function, and MRI findings in patients with sarcoidosis, systemic lupus erythematosus, and Behcet’s disease involving the nervous system, J. Neurol. Neurosurg. Psychiatry 58 (1995) 548–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Ho RC, Thiaghu C, Ong H, Lu Y, Ho CS, Tam WW and Zhang MW, A meta-analysis of serum and cerebrospinal fluid autoantibodies in neuropsychiatric systemic lupus erythematosus, Autoimmun. Rev 15 (2016) 124–38. [DOI] [PubMed] [Google Scholar]
- [48].Kapural M, Krizanac-Bengez L, Barnett G, Perl J, Masaryk T, Apollo D, Rasmussen P, Mayberg MR and Janigro D, Serum S-100beta as a possible marker of blood-brain barrier disruption, Brain Res. 940 (2002) 102–4. [DOI] [PubMed] [Google Scholar]
- [49].DeGiorgio LA, Konstantinov KN, Lee SC, Hardin JA, Volpe BT and Diamond B, A subset of lupus anti-DNA antibodies cross-reacts with the NR2 glutamate receptor in systemic lupus erythematosus, Nat. Med 7 (2001) 1189–93. [DOI] [PubMed] [Google Scholar]
- [50].Kowal C, DeGiorgio LA, Nakaoka T, Hetherington H, Huerta PT, Diamond B and Volpe BT, Cognition and immunity; antibody impairs memory, Immunity 21 (2004) 179–88. [DOI] [PubMed] [Google Scholar]
- [51].Lauvsnes MB and Omdal R, Systemic lupus erythematosus, the brain, and anti-NR2 antibodies, J. Neurol 259 (2012) 622–9. [DOI] [PubMed] [Google Scholar]
- [52].Karassa FB, Afeltra A, Ambrozic A, Chang DM, De Keyser F, Doria A, Galeazzi M, Hirohata S, Hoffman IE, Inanc M, Massardo L, Mathieu A, Mok CC, Morozzi G, Sanna G, Spindler AJ, Tzioufas AG, Yoshio T and Ioannidis JP, Accuracy of anti-ribosomal P protein antibody testing for the diagnosis of neuropsychiatric systemic lupus erythematosus: an international meta-analysis, Arthritis Rheum. 54 (2006) 312–24. [DOI] [PubMed] [Google Scholar]
- [53].Legroux L and Arbour N, Multiple sclerosis and T lymphocytes: an entangled story, J. Neuroimmune Pharmacol 10 (2015) 528–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Yoshio T, Okamoto H, Hirohata S and Minota S, IgG anti-NR2 glutamate receptor autoantibodies from patients with systemic lupus erythematosus activate endothelial cells, Arthritis Rheum. 65 (2013) 457–63. [DOI] [PubMed] [Google Scholar]
- [55].Yoshio T, Hirata D, Onda K, Nara H and Minota S, Antiribosomal P protein antibodies in cerebrospinal fluid are associated with neuropsychiatric systemic lupus erythematosus, J. Rheumatol 32 (2005) 34–9. [PubMed] [Google Scholar]
- [56].James WG, Hutchinson P, Bullard DC and Hickey MJ, Cerebral leucocyte infiltration in lupus-prone MRL/MpJ-fas lpr mice--roles of intercellular adhesion molecule-1 and P-selectin, Clin. Exp. Immunol 144 (2006) 299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Steffen BJ, Breier G, Butcher EC, Schulz M and Engelhardt B, ICAM-1, VCAM-1, and MAdCAM-1 are expressed on choroid plexus epithelium but not endothelium and mediate binding of lymphocytes in vitro, Am. J. Pathol 148 (1996) 1819–38. [PMC free article] [PubMed] [Google Scholar]
- [58].Mahajan SD, Tutino VM, Redae Y, Meng H, Siddiqui A, Woodruff TM, Jarvis JN, Hennon T, Schwartz S, Quigg RJ and Alexander JJ, C5a induces caspase-dependent apoptosis in brain vascular endothelial cells in experimental lupus, Immunology 148 (2016) 407–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Mahajan SD, Parikh NU, Woodruff TM, Jarvis JN, Lopez M, Hennon T, Cunningham P, Quigg RJ, Schwartz SA and Alexander JJ, C5a alters blood-brain barrier integrity in a human in vitro model of systemic lupus erythematosus, Immunology 146 (2015) 130–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Sun W, Jiao Y, Cui B, Gao X, Xia Y and Zhao Y, Immune complexes activate human endothelium involving the cell-signaling HMGB1-RAGE axis in the pathogenesis of lupus vasculitis, Lab. Invest 93 (2013) 626–38. [DOI] [PubMed] [Google Scholar]
- [61].Wen J, Chen CH, Stock A, Doerner J, Gulinello M and Putterman C, Intracerebroventricular administration of TNF-like weak inducer of apoptosis induces depression-like behavior and cognitive dysfunction in non-autoimmune mice, Brain. Behav. Immun 54 (2016) 27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Wen J, Xia Y, Stock A, Michaelson JS, Burkly LC, Gulinello M and Putterman C, Neuropsychiatric disease in murine lupus is dependent on the TWEAK/Fn14 pathway, J. Autoimmun 43 (2013) 44–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Yepes M, Brown SA, Moore EG, Smith EP, Lawrence DA and Winkles JA, A soluble Fn14-Fc decoy receptor reduces infarct volume in a murine model of cerebral ischemia, Am. J. Pathol 166 (2005) 511–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Desplat-Jego S, Creidy R, Varriale S, Allaire N, Luo Y, Bernard D, Hahm K, Burkly L and Boucraut J, Anti-TWEAK monoclonal antibodies reduce immune cell infiltration in the central nervous system and severity of experimental autoimmune encephalomyelitis, Clin. Immunol 117 (2005) 15–23. [DOI] [PubMed] [Google Scholar]
- [65].Desplat-Jego S, Varriale S, Creidy R, Terra R, Bernard D, Khrestchatisky M, Izui S, Chicheportiche Y and Boucraut J, TWEAK is expressed by glial cells, induces astrocyte proliferation and increases EAE severity, J. Neuroimmunol 133 (2002) 116–123. [DOI] [PubMed] [Google Scholar]
- [66].Stephan D, Sbai O, Wen J, Couraud PO, Putterman C, Khrestchatisky M and Desplat-Jego S, TWEAK/Fn14 pathway modulates properties of a human microvascular endothelial cell model of blood brain barrier, J. Neuroinflammation 10 (2013) 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Ma X, Foster J and Sakic B, Distribution and prevalence of leukocyte phenotypes in brains of lupus-prone mice, J. Neuroimmunol 179 (2006) 26–36. [DOI] [PubMed] [Google Scholar]
- [68].Zameer A and Hoffman SA, Increased ICAM-1 and VCAM-1 expression in the brains of autoimmune mice, J. Neuroimmunol 142 (2003) 67–74. [DOI] [PubMed] [Google Scholar]
- [69].Atkins CJ, Kondon JJ, Quismorio FP and Friou GJ, The choroid plexus in systemic lupus erythematosus, Ann. Intern. Med 76 (1972) 65–72. [DOI] [PubMed] [Google Scholar]
- [70].Sher JH and Pertschuk LP, Immunoglobulin G deposits in the choroid plexus of a child with systemic lupus erythematosus, J. Pediatr 85 (1974) 385–7. [DOI] [PubMed] [Google Scholar]
- [71].Boyer RS, Sun NC, Verity A, Nies KM and Louie JS, Immunoperoxidase staining of the choroid plexus in systemic lupus erythematosus, J. Rheumatol 7 (1980) 645–50. [PubMed] [Google Scholar]
- [72].Duprez T, Nzeusseu A, Peeters A and Houssiau FA, Selective involvement of the choroid plexus on cerebral magnetic resonance images: a new radiological sign in patients with systemic lupus erythematosus with neurological symptoms, J. Rheumatol 28 (2001) 387–91. [PubMed] [Google Scholar]
- [73].Kunis G, Baruch K, Rosenzweig N, Kertser A, Miller O, Berkutzki T and Schwartz M, IFN-gamma-dependent activation of the brain’s choroid plexus for CNS immune surveillance and repair, Brain 136 (2013) 3427–40. [DOI] [PubMed] [Google Scholar]
- [74].Reboldi A, Coisne C, Baumjohann D, Benvenuto F, Bottinelli D, Lira S, Uccelli A, Lanzavecchia A, Engelhardt B and Sallusto F, C-C chemokine receptor 6-regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE, Nat. Immunol 10 (2009) 514–23. [DOI] [PubMed] [Google Scholar]
- [75].Knowland D, Arac A, Sekiguchi KJ, Hsu M, Lutz SE, Perrino J, Steinberg GK, Barres BA, Nimmerjahn A and Agalliu D, Stepwise recruitment of transcellular and paracellular pathways underlies blood-brain barrier breakdown in stroke, Neuron 82 (2014) 603–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Lyck R, Ruderisch N, Moll AG, Steiner O, Cohen CD, Engelhardt B, Makrides V and Verrey F, Culture-induced changes in blood-brain barrier transcriptome: implications for amino-acid transporters in vivo, J. Cereb. Blood Flow Metab 29 (2009) 1491–502. [DOI] [PubMed] [Google Scholar]
- [77].Huntley MA, Bien-Ly N, Daneman R and Watts RJ, Dissecting gene expression at the blood-brain barrier, Front. Neurosci 8 (2014) 355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O’Keeffe S, Phatnani HP, Guarnieri P, Caneda C, Ruderisch N, Deng S, Liddelow SA, Zhang C, Daneman R, Maniatis T, Barres BA and Wu JQ, An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex, J. Neurosci 34 (2014) 11929–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Zhang Y, Sloan SA, Clarke LE, Caneda C, Plaza CA, Blumenthal PD, Vogel H, Steinberg GK, Edwards MS, Li G, Duncan JA 3rd, Cheshier SH, Shuer LM, Chang EF, Grant GA, Gephart MG and Barres BA, Purification and characterization of progenitor and mature human astrocytes reveals transcriptional and functional differences with mouse, Neuron 89 (2016) 37–53. [DOI] [PMC free article] [PubMed] [Google Scholar]