Abstract
Study Design
Retrospective study.
Purpose
We aimed to determine the utility of transcranial motor evoked potential (TcMEP) monitoring for the detection of intraoperative nerve root injury.
Overview of Literature
Intraoperative neuromonitoring is important for the prediction of neurological injuries or postoperative paralysis. Nerve root injury can develop as a complication of adult spinal deformity (ASD) surgery.
Methods
We analyzed 295 patients who underwent ASD surgery using multi-channel TcMEP monitoring between 2010 and 2016 (58 men, 237 women; median age, 68 years; follow-up period ≥1 year). We defined the alarm point as a TcMEP amplitude <30% of that at baseline, and nerve root injury as meeting the focal TcMEP alerts shortly following surgical procedures with the presence of postoperative motor deficits in the selected muscles. Patients were classified into two groups, as those with nerve root injury and those with true-negatives.
Results
Seven patients (2.4%) exhibited neurological events related to nerve root injury, comprising six true-positive and one false-negative cases. TcMEP monitoring from multiple myotomes was effective in detecting nerve root injury. Compared to the 248 true-negative cases, the seven cases of nerve root injury were associated with significantly different preoperative pelvic tilt (PT) values, sacral slope values, and degree of change in PT. The cutoff for the degree of change in PT for predicting nerve root injury, with the best sensitivity and specificity, was 17.5°. Multivariate logistic analyses revealed that a change of >17.5° in PT (odds ratio, 17.5; 95% confidence interval, 1.994–153.560; p =0.010) was independently associated with intraoperative nerve root injury.
Conclusions
Multi-channel TcMEP monitoring may be useful for detecting nerve root injuries. A change in PT of >17.5° may be a significant risk factor for neurological events related to intraoperative nerve root injury.
Keywords: Spinal nerve roots, Intraoperative complication, Intraoperative monitoring
Introduction
Intraoperative neuromonitoring (IONM) is important for predicting spinal cord injuries and postoperative paralysis [1,2]. However, it is debatable whether IONM can detect isolated nerve root injury [3]. IONM includes the monitoring of somatosensory evoked potentials (SSEPs), transcranial motor evoked potentials (TcMEPs), and spinal cord motor evoked potentials; spontaneous electromyography (EMG); and triggered EMG. In particular, TcMEP monitoring is currently used with high sensitivity and specificity to detect postoperative paralysis [1,4,5].
Thoracolumbar nerve root injuries may result in transient or permanent motor weakness in up to 30% of adult spinal deformity (ASD) cases [5,6]. They can occur during the correction of fixed sagittal imbalance with osteotomies, lumbar fusion, or decompression [5-7]. IONM has been effectively used for the detection and prevention of spinal cord injuries during cervical and thoracolumbar surgeries [8,9]; however, these methods have a low efficacy for detecting isolated lumbar nerve root injuries [6]. Moreover, studies have reported nerve root injuries associated with intraoperative TcMEP changes [5,10].
With respect to intraoperative nerve root injury, the utility of TcMEP monitoring during ASD surgery has been reported in few trials [5,10]; however, to our knowledge, no studies have explored the contributing factors or the mechanisms. Therefore, we designed this study with the aim of evaluating the utility of TcMEP monitoring for detecting nerve root injuries during ASD surgery and to clarify the risk factors using characteristic, surgical, and radiographic data.
Materials and Methods
1. Participants or study subjects
The study protocol was approved by the Institutional Review Board of Hamamatsu University School of Medicine (research approval no., 14-096) and adhered to the principles of the Declaration of Helsinki. We monitored the nerves intraoperatively based on the TcMEPs in 295 ASD patients who gave informed consent to participate and no history of spinal operations between January 2010 and June 2016. Most patients were women (237 out of 295, 80%), with a median age of 68 years (range, 18–84 years) (Table 1). ASD was defined as the presence of at least one of the following indicators: degenerative or idiopathic scoliosis with spinal curvature >20° in the coronal plane, a C7 sagittal vertical axis (SVA) >50 mm, pelvic tilt (PT) >25°, and thoracic kyphosis (TK) >60°. The inclusion criteria were age ≥18 years, presence of at least four fuzed vertebral segments, and provision of informed consent for study participation. All the patients were followed up for at least 1 year. Complete datasets that included information regarding demographic, operative, and postoperative parameters were available for all patients, and sufficient radiographic data for analyses were available for most patients.
Table 1.
Baseline characteristics of 295 patients with adult spinal deformity
| Characteristic | Value |
|---|---|
| Age (yr) | 68 (55–74) |
| Male sex | 58 (19.7) |
| Height (cm) | 150.5±10.1 |
| Body weight (kg) | 51.1±10.8 |
| Body mass index (kg/m2) | 22.6±3.8 |
| American Society of Anesthesiologist classification score | 1.9±0.5 |
| Preoperative Oswestry Disability Index score | 41.4±21.1 |
| Surgical factor | |
| Length of surgery (min) | 367±95 |
| Estimated blood loss (mL) | 1,460±1,120 |
| No. of levels fused | 9.6±2.5 |
| Multi-stage operation | 72 (24.4) |
| Type of surgery | |
| Posterior corrective surgery without osteotomies | 40 (13.6) |
| Posterior corrective surgery with posterior column resections | 83 (28.1) |
| Posterior corrective surgery with 3-column osteotomies | 114 (38.6) |
| Posterior corrective surgery after lumbar lateral interbody fusion | 58 (19.7) |
Values are presented as mean (range), number (%), or mean±standard deviation.
2. Variables, data sources, and bias
Patient characteristics and medical and demographic details were obtained by performing a review of the medical records. The anesthetic records were reviewed to collect surgical data, including the length of surgery, estimated blood loss (EBL), number of levels fuzed, use of 3-column osteotomies, use of single- or multi-stage operations, combined use of lumbar lateral interbody fusion, and type of surgery. The preoperative general status of each patient was estimated as per the American Society of Anesthesiologists classification system, as judged by an anesthesiologist. The measured radiographic parameters were the SVA, T5–T12 TK, L1–S1 lumbar lordosis (LL), PT, pelvic incidence, sacral slope (SS), Cobb angle between the most tilted vertebrae in the anteroposterior radiograph view (Cobb angle), and C7 central sacral vertical line. The Oswestry Disability Index (ODI) was used to quantify the disability associated with low back pain or clinical symptoms.
3. Alarm point and definition of nerve root injury
The baseline control TcMEP amplitude was defined as that observed at the time of incision or that prior to decompression, depending on each individual patient. Thereafter, we defined our alarm point as a TcMEP amplitude <30% of that at baseline. This definition was based on a previous study by the Monitoring Committee of the Japanese Society for Spine Surgery and Related Research [4]. We defined nerve root injury as the meeting of the focal TcMEP alerts soon after the surgical procedures and the presence of postoperative motor deficits in the selected muscles. When an alarm point was achieved, several interventions were performed. Depending on the case, these could include the termination of the surgical procedure, reversal of the precipitating procedures, additional decompression, or administration of steroids. We defined neurological complications as a decrease in the patient’s muscle strength by at least 2 grades on the manual muscle test.
4. Anesthesia management and intraoperative monitoring
Total intravenous anesthesia was administered during IONM. General anesthesia was maintained with pumpcontrolled intravenous infusions of propofol, fentanyl, and remifentanil. A single bolus of non-depolarizing muscle relaxant (rocuronium; 0.6 mg・kg−1) was administered at induction to facilitate intubation and ventilation. Fewer than 20 transcranial stimuli were delivered in 5-stimulus trains with 200-mA intensities, a 2-ms inter-stimulus interval, a 50- to 1,000-Hz filter, and a 100-ms recording time. The stimulator was placed 2 cm anterior and 5 cm lateral to Cz (international 10–20 system) over the motor cortex. TcMEPs were recorded from the peripheral limbs via needle electrodes using a Neuromaster MEE 1232 Stimulator (Nihon-Kohden, Tokyo, Japan). The evoked muscles were the bilateral deltoid, abductor digiti minimi, quadriceps femoris, hamstring, tibialis anterior, and gastrocnemius. We measured the TcMEP amplitudes as peak-to-peak voltages. Conventional unilateral lower limb SSEPs were obtained, mainly from the left side, by the electrical stimulation of the posterior tibial nerve at the ankle. The cathode was placed proximally, halfway between the Achilles tendon and the medial malleolus, and the anode was placed 2–3 cm distally. Bilateral interleaving square-wave pulse stimuli were applied with 500-μs durations at 4.3 Hz. The stimulus intensity was individually adjusted; however, at least 20 mA. The SSEP-recording electrodes were positioned 2 cm posterior to Cz and Fz for the P38/N46 responses generated within the sensory cortex [11]. The recording parameters were a 20- to 1,500-Hz bandpass filter with a 10-μs analysis time and a 10-μV/div gain. SSEP was acquired with continuous averaging of up to 500 sweeps and was repeatedly compared to the baseline responses obtained soon after the patient was positioned. An amplitude decrease of 50% from that at baseline was considered a significant SSEP change [11]. As per the definitions by Kim et al. [12], a true-positive case is defined by a TcMEP alert with a persistent amplitude decrease at the operation’s closure followed by postoperative observation of a new motor deficit; a false-positive case is defined by an alert with a persistent amplitude decrease at closure with no new postoperative deficits; a true-negative case is characterized by the absence of any intraoperative TcMEP alert and new postoperative deficits; and a false-negative case is the absence of an alert in a patient with a new postoperative motor deficit. If decreased TcMEP normalizes after the surgical procedures and the patient exhibits no new motor deficit, the case is considered a rescue case. For statistical analyses, rescue cases were excluded from the analysis of accuracy because we believed that the temporary decrease in amplitude did not accurately indicate real motor deficits without a wake-up test. The relationship between TcMEP monitoring and postoperative motor deficits was analyzed. Furthermore, we assessed the differences between nerve root injury cases and true-negative cases.
5. Statistical analyses
Categorical variables were analyzed using chi-square test or Fisher’s exact test, as appropriate, and are expressed as absolute numbers and percentages. We used the Shapiro-Wilk tests to assess the normality of the continuous variables. Normally distributed continuous variables were analyzed using unpaired t-tests and are expressed as means±standard deviations. Age and intraoperative TcMEP amplitudes did not follow a normal distribution; they were analyzed using the Mann-Whitney U-test and are expressed as medians and interquartile ranges (IQRs). Nerve root injury or unilateral TcMEP waveform deterioration after correction procedures are critically affected by several factors, including the preoperative Cobb angle, combined usage of 3-column osteotomies, number of levels fuzed, length of surgery, and EBL [13-15]. The predictors of neurological events were examined using multivariate logistic regression analyses. Statistical analyzes were conducted using the IBM SPSS ver. 23.0 (IBM Corp., Armonk, NY, USA). All p-values <0.05 were considered statistically significant.
Results
IONM revealed 17 true-positive, one false-negative, 17 false-positive, 248 true-negative, and 12 rescue cases (Fig. 1). Therefore, the sensitivity was 94%, and the specificity was 94%. We identified six true-positive cases with neurological events related to nerve root injury and one false-negative case (case 7) with a motor deficit of the left tibialis anterior muscle soon after operation (Table 2). Intraoperative TcMEPs and SSEP amplitude changes in seven patients with nerve root injury are shown in Table 2. The median alarm period’s TcMEP amplitude of the six true-positive cases with nerve root injury was 18% (IQR, 8%–23%) of baseline, and the median final TcMEP amplitude of those was 11% (IQR, 8%–12%) of baseline. The alarm point for SSEP monitoring was not reached by any patient. Most cases of paralysis, excluding case 3, exhibited motor function improvement within 3 months. Furthermore, a patient with a false-negative experienced motor function improvement within 1 month.
Fig. 1.
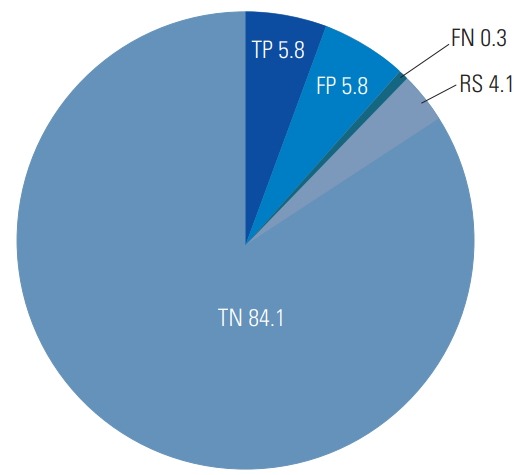
Results of transcranial motor evoked potential monitoring (unit: %). TP, true-positive; FP, false-positive; FN, false-negative; TN, true-negative; RS, rescue cases.
Table 2.
Monitoring data of patients with nerve root injury
| Case | Muscle group involved | Transcranial motor evoked potential amplitudes (%) |
Result of monitoring | Short-latency somatosensory evoked potential (mV) |
Procedure prior to alarm | ||
|---|---|---|---|---|---|---|---|
| Alarm period | Final | Control | Final | ||||
| 1 | R. TA | 15 | 7 | True-positive | - | - | Correction of kyphosis |
| 2 | R. quadriceps | 24 | 29 | True-positive | - | - | Rod rotation |
| 3 | L. TA | 23 | 10 | True-positive | 1.12 | 1.15 | Screw insertion |
| 4 | L. TA | 21 | 11 | True-positive | 0.81 | 0.54 | Rod rotation |
| 5 | R. quadriceps | 6 | 12 | True-positive | 0.31 | 0.43 | Correction of kyphosis |
| 6 | L. quadriceps | 6 | 3 | True-positive | 0.82 | 0.65 | Rod rotation |
| 7 | L. TA | - | 74 | False-negative | 1.68 | 1.07 | Unknown |
R, right; L, left; TA, tibialis anterior.
The baseline characteristics, ODI scores, and surgical factors in nerve root injury cases and true-negative cases are shown in Table 3. The baseline characteristics, ODI scores, and surgical factors of the true-negative cases and the nerve root injury cases did not differ significantly. Preoperative and early postoperative spinopelvic parameter measurements are presented in Table 4. Compared with the true-negatives cases, the nerve root injury cases were associated with significantly lower preoperative SS values (p=0.023), higher preoperative PT values (p=0.007), and larger degrees of change in PT (p=0.001); however, there was no significant difference in the Cobb angle or any postoperative parameter. The receiver operating characteristic (ROC) curve analysis indicated that the best cutoff value for the degree of change in PT for detecting nerve root injury was 17.5°, with sensitivity and specificity of 83.3% and 76.4%, respectively. The area under the ROC curve was 0.84 (95% confidence interval [CI], 0.768–0.905; p=0.005). With respect to the risk factors among the radiographic parameters, after controlling for the relevant confounding variables, including age, sex, height, body weight, preoperative Cobb angle, and preoperative LL, a change in PT of >17.5° (odds ratio, 17.5; 95% CI, 1.994–153.560; p=0.010) (Table 5) was independently associated with intraoperative nerve root injury. Fig. 2 shows the preoperative and postoperative whole-spine standing radiographs for a typical true-positive case with nerve root injury.
Table 3.
Retrospective comparison of baseline characteristics, ODI scores, and surgical factors between cases with nerve root injury and truenegative cases
| Variable | Cases with nerve root injury (n=7) | True-negative cases (n=248) | p-value |
|---|---|---|---|
| Age (yr) | 70 (67–71) | 68 (50–74) | 0.655 |
| Male sex | 1 (14.3) | 45 (18.1) | 0.631 |
| Height (cm) | 146.7±7.2 | 150.9±10.0 | 0.266 |
| Body weight (kg) | 52.1±6.7 | 50.9±10.7 | 0.765 |
| Body mass index (kg/m2) | 24.3±3.1 | 22.4±3.7 | 0.188 |
| American Society of Anesthesiologists classification score | 2.1±0.7 | 1.8±0.5 | 0.106 |
| ODI scores | |||
| Preoperatively | 49.2±16.8 | 41.0±21.2 | 0.391 |
| 6 Months postoperatively | 38.8±14.0 | 32.0±19.7 | 0.441 |
| 1 Year postoperatively | 39.4±15.8 | 28.7±20.2 | 0.240 |
| Surgical factors | |||
| Length of surgery (min) | 404±56 | 360±93 | 0.214 |
| Estimated blood loss (mL) | 1,956±862 | 1,380±1,050 | 0.153 |
| No. of levels fused | 8.3±1.1 | 9.7±2.6 | 0.154 |
| 3-Column osteotomies | 2 (28.6) | 94 (37.9) | 0.471 |
| Multi-stage operation | 2 (28.6) | 59 (23.8) | 0.530 |
| Lumbar lateral interbody fusion | 2 (28.6) | 46 (18.5) | 0.391 |
Values are presented as mean (range), number (%), or mean±standard deviation.
ODI, Oswestry Disability Index.
Table 4.
Retrospective comparison of radiographic parameters between cases with nerve root injury and true-negative cases
| Variable | Cases with nerve root injury (n=7) | True-negative cases (n=248) | p-value |
|---|---|---|---|
| Preoperative parameters | |||
| SVA (mm) | 108.6±48.7 | 98.5±88.6 | 0.766 |
| TK (°) | 29.4±29.4 | 25.9±20.5 | 0.657 |
| LL (°) | 3.9±17.2 | 19.3±26.4 | 0.125 |
| PT (°) | 38.0±5.7 | 29.8±13.4 | 0.007 |
| PI (°) | 47.6±6.7 | 51.2±11.8 | 0.415 |
| SS (°) | 9.6±8.0 | 21.4±13.6 | 0.023 |
| PI–LL (°) | 43.7±15.4 | 31.9±26.8 | 0.249 |
| Cobb angle (°) | 25.3±25.3 | 33.6±23.8 | 0.367 |
| C7-CSVL (mm) | 25.1±43.0 | 29.1±31.9 | 0.748 |
| Postoperative parameters (early postoperatively) | |||
| SVA (mm) | 46.8±40.9 | 40.9±50.5 | 0.778 |
| TK (°) | 32.8±9.0 | 33.5±12.2 | 0.889 |
| LL (°) | 44.2±13.7 | 42.6±12.8 | 0.762 |
| PT (°) | 14.8±5.2 | 20.2±9.1 | 0.155 |
| PI (°) | 48.7±9.5 | 51.1±10.9 | 0.589 |
| SS (°) | 33.8±8.1 | 30.9±9.1 | 0.431 |
| PI–LL (°) | 4.5±7.8 | 8.6±13.8 | 0.474 |
| Cobb angle (°) | 10.1±16.1 | 10.6±10.5 | 0.906 |
| C7-CSVL (mm) | 27.5±24.7 | 18.1±18.2 | 0.748 |
| Amount of change (postoperative–preoperative value) | |||
| SVA (mm) | 71.8±58.2 | 57.9±84.5 | 0.689 |
| LL (°) | 40.3±19.2 | 23.0±25.4 | 0.099 |
| PT (°) | 21.5±5.0 | 9.4±12.1 | 0.001 |
| Cobb angle (°) | 15.1±13.5 | 23.6±17.6 | 0.209 |
Values are presented as mean±standard deviation.
SVA, sagittal vertical axis; TK, thoracic kyphosis; LL, lumbar lordosis; PT, pelvic tilt; PI, pelvic incidence; SS, sacral slope; C7-CSVL, C7 central sacral vertical line.
Table 5.
Multivariate logistic regression analysis of adjusted risk factors related to intraoperative nerve root injury
| Risk factor among radiographic parameters | Odds ratio (95% confidence interval) | p-value |
|---|---|---|
| Amount of change in pelvic tilt >17.5° | 17.5 (1.994–153.560) | 0.010 |
Fig. 2.
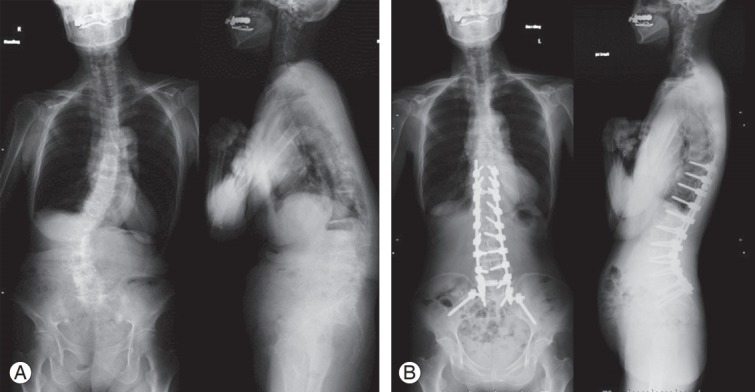
(A) Preoperative whole spine standing radiographs in case 6 (a true-positive case). Preoperative LL was 7°, SS was 13°, PT was 37°, PI–LL was 43°, SVA was 113 mm, and Cobb angle was 46°. (B) Postoperative whole spine standing radiographs in case 6 (a true-positive case). This case had alarm after rod rotation. Postoperative LL was 52°, SS was 31°, PT was 13°, PI–LL was -8°, SVA was -10 mm, and Cobb angle was 9°. LL, lumbar lordosis; SS, sacral slope; PT, pelvic tilt; PI, pelvic incidence; SVA, sagittal vertical axis.
Discussion
With respect to the detection of nerve root injuries using TcMEP monitoring, there is conflicting evidence on the effect of segmental innervation on individual muscles. Neurological injury is an important and significant complication of posteriorly based osteotomies for correcting fixed sagittal plane deformities [5,16]. In spite of radiologically successful decompression and fusion, a neurological complication can cause severe negative effects. In our study, although cases without intraoperative amplitude recovery had moderate or severe postoperative paralysis, most cases of paralysis, excluding a case of permanent paralysis, exhibited motor function improvement within 3 months. It is important to detect intraoperative nerve root injury using TcMEP monitoring to ensure better clinical outcomes.
TcMEP-based nerve root monitoring has certain limitations. Tsutsui et al. [3] reported that TcMEPs may not accurately reflect segmental injury because they did not always alter when the insult was restricted to a limited area in a single spinal nerve root in a cat model. Hence, TcMEP monitoring is believed to be inadequate for detecting isolated nerve root injury. Multiple and variable nerve innervations to several lower extremity muscles, root injury resulting in partial muscle weakness, and disagreement about what constitutes a meaningful amplitude change are among the underlying reasons cited in the literature [17-19]. In our study, we experienced a false-negative case, wherein the patient’s left tibialis anterior muscle grade had deteriorated by 2 grades. However, she experienced motor function improvement within 1 month. This case exemplifies certain limitations of nerve root monitoring.
However, Valone et al. [20] reported that TcMEP monitoring could detect evolving nerve root injuries in a porcine model; therefore, it could serve as a valuable intraoperative tool to prevent or limit nerve root injury. Lieberman et al. [5] reported that TcMEP monitoring may highlight the need for intraoperative corrections, including widened decompressions and limited deformity correction to prevent further, severe neural damage. Langeloo et al. [10] reported that TcMEP monitoring from multiple myotomes was helpful in spinal deformity surgery. Similar to the protocol followed in their study, we placed electrodes on multiple muscles of each lower extremity, including eight muscles that were partly innervated by the L2–5 and S1 nerve roots. These nerves are at the maximum risk of injury in our hospital because most pedicle subtraction osteotomies are performed at L4, and posterior column osteotomies often included L4, L5, and S1. To summarize, there were reports of nerve root deficits associated with intraoperative TcMEP changes [5,10]; however, there were nerve root deficits without remarkable TcMEP changes [3,6]. Our results showed that the 6 cases, excluding a false-negative case, exhibited intraoperative loss of TcMEP amplitudes, and neurological deficits related to nerve root injury appeared soon after operation. Thus, multi-channel TcMEP monitoring may be useful for detecting nerve root injury during ASD surgery.
The present study showed no significant difference with respect to the baseline characteristics and surgical factors, including the amount of bleeding, length of surgery, number of levels fuzed, and presence of 3-column osteotomies, between the patients with and without the neurological complications related to nerve root injuries. Similarly, Kim et al. [15] reported that the risk of neurologic complications did not increase with the use of 3-column osteotomies or decompression. With regard to the radiographic parameters in our series, the preoperative PT was significantly higher in cases with nerve root injury; however, the postoperative PT was not significantly increased. Nerve root injury has previously been shown to occur significantly more frequently in cases with high SVA values or high deformity angulation ratios (i.e., the Cobb angle divided by the number of vertebral bodies) [15,21]; however, our study showed no such differences. Cases with high preoperative PT probably need larger correction amounts for better alignment. In multivariate logistic analyses, a change in PT of >17.5° had an odds ratio of 17.5. The results indicate that an intraoperative change in PT is a significant risk factor for intraoperative nerve root injury. It is noteworthy that TcMEPs are useful for detecting early nerve root injury caused by multiple factors, including iatrogenic compression, traction, and nerve reserve capacity.
This study has certain limitations. First, the data were retrospectively reviewed; this limits our ability to deduce causal relationships and determine the intraoperative interventions that were performed when significant TcMEP changes were identified (e.g., decompression or decreased correction). We believe that the relationship between Tc-MEP changes and neurologic complications is probably attributable to these intraoperative adjustments. Second, we did not evaluate other, more traditional forms of IONM, such as EMG monitoring. EMG monitoring has a high incidence of false-positives and poor positive predictive value for nerve root injuries [22-24]. However, Sutter et al. [1] reported outcomes for 409 patients who underwent lumbar surgeries with multimodal neurophysiologic monitoring, including EMG monitoring. They reported 90% sensitivity and 99.7% specificity for detecting nerve root injuries. Future prospective studies should examine EMG monitoring. Finally, we defined our alarm point as a TcMEP amplitude <30% of that at baseline; however, this cut off may not be optimal. In sum, we plan to follow up this study with a well-designed prospective study to determine suitable TcMEP monitoring alarm criteria, identify the common interventions, and identify the interventions that prove most effective for preventing nerve root injuries.
Conclusions
Multiple-channel TcMEP monitoring may be useful for detecting nerve root injuries during ASD surgeries. A change in PT of >17.5° is a significant risk factor for intraoperative nerve root injury.
Acknowledgments
We thank the following medical engineers of our hospital who performed the intraoperative monitoring: Suzuki S, Ichikawa M, Takayanagi T, Nakamura M, Takiyama H, and Kamimoto S.
Footnotes
No potential conflict of interest relevant to this article was reported.
Author Contributions
Yoshida G and Kobayashi S were responsible for the study’s conception and design. Ushirozako H acquired, analyzed, and interpreted data; drafted the article; and approved the final version on behalf of all authors. All authors critically revised the article and reviewed the submitted version. Matsuyama Y supervised the study.
References
- 1.Sutter MA, Eggspuehler A, Grob D, Porchet F, Jeszenszky D, Dvorak J. Multimodal intraoperative monitoring (MIOM) during 409 lumbosacral surgical procedures in 409 patients. Eur Spine J. 2007;16 Suppl 2:S221–8. doi: 10.1007/s00586-007-0432-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fehlings MG, Brodke DS, Norvell DC, Dettori JR. The evidence for intraoperative neurophysiological monitoring in spine surgery: does it make a difference? Spine (Phila Pa 1976) 2010;35(9 Suppl):S37–46. doi: 10.1097/BRS.0b013e3181d8338e. [DOI] [PubMed] [Google Scholar]
- 3.Tsutsui S, Tamaki T, Yamada H, Iwasaki H, Takami M. Relationships between the changes in compound muscle action potentials and selective injuries to the spinal cord and spinal nerve roots. Clin Neurophysiol. 2003;114:1431–6. doi: 10.1016/s1388-2457(03)00090-7. [DOI] [PubMed] [Google Scholar]
- 4.Kobayashi S, Matsuyama Y, Shinomiya K, et al. A new alarm point of transcranial electrical stimulation motor evoked potentials for intraoperative spinal cord monitoring: a prospective multicenter study from the Spinal Cord Monitoring Working Group of the Japanese Society for Spine Surgery and Related Research. J Neurosurg Spine. 2014;20:102–7. doi: 10.3171/2013.10.SPINE12944. [DOI] [PubMed] [Google Scholar]
- 5.Lieberman JA, Lyon R, Feiner J, Hu SS, Berven SH. The efficacy of motor evoked potentials in fixed sagittal imbalance deformity correction surgery. Spine (Phila Pa 1976) 2008;33:E414–24. doi: 10.1097/BRS.0b013e318175c292. [DOI] [PubMed] [Google Scholar]
- 6.Pateder DB, Kostuik JP. Lumbar nerve root palsy after adult spinal deformity surgery. Spine (Phila Pa 1976) 2005;30:1632–6. doi: 10.1097/01.brs.0000170292.87470.92. [DOI] [PubMed] [Google Scholar]
- 7.Potter BK, Freedman BA, Verwiebe EG, Hall JM, Polly DW, Jr, Kuklo TR. Transforaminal lumbar interbody fusion: clinical and radiographic results and complications in 100 consecutive patients. J Spinal Disord Tech. 2005;18:337–46. doi: 10.1097/01.bsd.0000166642.69189.45. [DOI] [PubMed] [Google Scholar]
- 8.Tsai RY, Yang RS, Nuwer MR, Kanim LE, Delamarter RB, Dawson EG. Intraoperative dermatomal evoked potential monitoring fails to predict outcome from lumbar decompression surgery. Spine (Phila Pa 1976) 1997;22:1970–5. doi: 10.1097/00007632-199709010-00005. [DOI] [PubMed] [Google Scholar]
- 9.Winter RB. Neurologic safety in spinal deformity surgery. Spine (Phila Pa 1976) 1997;22:1527–33. doi: 10.1097/00007632-199707010-00022. [DOI] [PubMed] [Google Scholar]
- 10.Langeloo DD, Lelivelt A, Louis Journee H, Slappendel R, de Kleuver M. Transcranial electrical motorevoked potential monitoring during surgery for spinal deformity: a study of 145 patients. Spine (Phila Pa 1976) 2003;28:1043–50. doi: 10.1097/01.BRS.0000061995.75709.78. [DOI] [PubMed] [Google Scholar]
- 11.Gunnarsson T, Krassioukov AV, Sarjeant R, Fehlings MG. Real-time continuous intraoperative electromyographic and somatosensory evoked potential recordings in spinal surgery: correlation of clinical and electrophysiologic findings in a prospective, consecutive series of 213 cases. Spine (Phila Pa 1976) 2004;29:677–84. doi: 10.1097/01.brs.0000115144.30607.e9. [DOI] [PubMed] [Google Scholar]
- 12.Kim DH, Zaremski J, Kwon B, et al. Risk factors for false positive transcranial motor evoked potential monitoring alerts during surgical treatment of cervical myelopathy. Spine (Phila Pa 1976) 2007;32:3041–6. doi: 10.1097/BRS.0b013e31815d0072. [DOI] [PubMed] [Google Scholar]
- 13.Boachie-Adjei O, Yagi M, Nemani VM, et al. Incidence and risk factors for major surgical complications in patients with complex spinal deformity: a report from an SRS GOP site. Spine Deform. 2015;3:57–64. doi: 10.1016/j.jspd.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 14.Kobayashi K, Imagama S, Ito Z, et al. Transcranial motor evoked potential waveform changes in corrective fusion for adolescent idiopathic scoliosis. J Neurosurg Pediatr. 2017;19:108–15. doi: 10.3171/2016.6.PEDS16141. [DOI] [PubMed] [Google Scholar]
- 15.Kim HJ, Iyer S, Zebala LP, et al. Perioperative neurologic complications in adult spinal deformity surgery: incidence and risk factors in 564 patients. Spine (Phila Pa 1976) 2017;42:420–7. doi: 10.1097/BRS.0000000000001774. [DOI] [PubMed] [Google Scholar]
- 16.Berven SH, Deviren V, Smith JA, Emami A, Hu SS, Bradford DS. Management of fixed sagittal plane deformity: results of the transpedicular wedge resection osteotomy. Spine (Phila Pa 1976) 2001;26:2036–43. doi: 10.1097/00007632-200109150-00020. [DOI] [PubMed] [Google Scholar]
- 17.Byrne CA, Lyons GM, Donnelly AE, O’Keeffe DT, Hermens H, Nene A. Rectus femoris surface myoelectric signal cross-talk during static contractions. J Electromyogr Kinesiol. 2005;15:564–75. doi: 10.1016/j.jelekin.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 18.Gundanna M, Eskenazi M, Bendo J, Spivak J, Moskovich R. Somatosensory evoked potential monitoring of lumbar pedicle screw placement for in situ posterior spinal fusion. Spine J. 2003;3:370–6. doi: 10.1016/s1529-9430(03)00144-x. [DOI] [PubMed] [Google Scholar]
- 19.Magit DP, Hilibrand AS, Kirk J, et al. Questionnaire study of neuromonitoring availability and usage for spine surgery. J Spinal Disord Tech. 2007;20:282–9. doi: 10.1097/01.bsd.0000211286.98895.ea. [DOI] [PubMed] [Google Scholar]
- 20.Valone F, 3rd, Lyon R, Lieberman J, Burch S. Efficacy of transcranial motor evoked potentials, mechanically elicited electromyography, and evoked electromyography to assess nerve root function during sustained compression in a porcine model. Spine (Phila Pa 1976) 2014;39:E989–93. doi: 10.1097/BRS.0000000000000442. [DOI] [PubMed] [Google Scholar]
- 21.Wang XB, Lenke LG, Thuet E, Blanke K, Koester LA, Roth M. Deformity angular ratio describes the severity of spinal deformity and predicts the risk of neurologic deficit in posterior vertebral column resection surgery. Spine (Phila Pa 1976) 2016;41:1447–55. doi: 10.1097/BRS.0000000000001547. [DOI] [PubMed] [Google Scholar]
- 22.Weiss DS. Spinal cord and nerve root monitoring during surgical treatment of lumbar stenosis. Clin Orthop Relat Res. 2001;(384):82–100. doi: 10.1097/00003086-200103000-00011. [DOI] [PubMed] [Google Scholar]
- 23.Bose B, Wierzbowski LR, Sestokas AK. Neurophysiologic monitoring of spinal nerve root function during instrumented posterior lumbar spine surgery. Spine (Phila Pa 1976) 2002;27:1444–50. doi: 10.1097/00007632-200207010-00014. [DOI] [PubMed] [Google Scholar]
- 24.Beatty RM, McGuire P, Moroney JM, Holladay FP. Continuous intraoperative electromyographic recording during spinal surgery. J Neurosurg. 1995;82:401–5. doi: 10.3171/jns.1995.82.3.0401. [DOI] [PubMed] [Google Scholar]


