Abstract
Study Design
In vitro cadaveric study.
Purpose
To compare biomechanical performance, trial and implant insertion, and disc distraction during implant placement, when two interbody devices, an in situ continuously expandable spacer (CES) and a traditional static spacer (SS), were used for transforaminal lumbar interbody fusion.
Overview of Literature
Severe degenerative disc diseases necessitate surgical management via large spacers to increase the disc space for implants. Next-generation interbody devices that expand in situ minimize insertion forces, optimize fit between vertebral endplates, and limit nerve root retraction. However, the literature lacks characterization of insertion forces as well as details on other parameters of expandable and static spacers.
Methods
Ten cadaveric segments (L5–S1) were divided into two groups (n=5) and implanted with either CES or SS. Each specimen experienced unconstrained pure moment of ±6 Nm in flexion–extension, lateral bending, and axial rotation to assess the contribution of CES and SS implants in biomechanical performance. Radiographic analysis was performed during trial and implant insertion to measure distraction during spacer insertion at the posterior, central, and anterior disc regions. Pressure sensors measured the force of trial and implant insertion.
Results
Biomechanical analysis showed no significant differences between CES and SS in all planes of motion. A total 2.6±0.9 strikes were required for expandable spacer trials insertion and 2.6±0.5 strikes for CES insertion. A total of 8.4±3.8 strikes were required to insert SS trials and 4.2±1.6 strikes for SS insertion. The total force per surgery was 330 N for CES and 635 N for SS. Fluoroscopic analysis revealed a significant reduction in distraction during implant insertion at the posterior and anterior disc regions (CES, 0.58 and 0.14 mm; SS, 1.04 and 0.78 mm, respectively).
Conclusions
Results from the three study arms reveal the potential use of expandable spacers. During implant insertion, CESs provided similar stability, required less insertion force, and significantly reduced over-distraction of the annulus compared with SS.
Keywords: Intervertebral disc degeneration, Lumbar region, Range of motion, Equipment design, Annulus fibrosus
Introduction
Degenerative disc disease (DDD) is characterized by narrowing of the intervertebral space and consequent subluxation and eventual arthrosis of facet joints. These mechanical alterations may cause discogenic or facetinduced pain. Treatment of patients with DDD requires re-expansion of the disc space (indirect decompression), attainment of immediate stabilization of the segment, maintenance of sagittal alignment after removal of the symptomatic disc, and use of an autogenous bone graft to ensure gradual intervertebral fusion [1]. The use of interbody devices is widespread, and their material composition and design have evolved along with biomechanical and clinical advances.
Early attempts at posterior interbody fusion resulted in blood loss, inconsistent fusion rates, graft extrusion due to use of bone chips, and donor site morbidity [2-4]. In 1988, Bagby [5] devised the first artificial interbody device, which used a twin threaded-cage support to neutralize the compressive forces in the involved segment, while providing immediate three-dimensional stability, essential for the incorporation of the fragile cancellous bone graft contained within. However, as Folman et al. [1] noted, the subsequent use of large spacers required the sacrifice of posterior stabilizing structures and iatrogenic over-distraction of the annulus during implant insertion that was not maintained after final positioning, thus compromising stability in extension and axial rotation (AR) [6]. Supplementary pedicle screw fixation [7,8] or restoration of posterior elements [9] was recommended. Moreover, undue retraction of the dural sac and roots posed immediate risk of inadvertent dural laceration with potential neurological deficit [8,10-12] or delayed epidural fibrosis [13].
Next-generation interbody spacers, which are designed to expand vertically within the disc space, enable controlled restoration of disc height while minimizing dissection of posterior elements and nerve root retraction that would otherwise be required for full-sized static spacers (SS) [1,14]. Furthermore, expandable interbody devices may reduce iatrogenic over-distraction that occurs during implant insertion and positively correlates with adjacent segment disease [15]. Bhatia et al. [16] highlighted the requirement for posterior instrumentation in addition to Staxx XD (Spine Wave Inc., Shelton, CT, USA), an expandable polyether ether ketone spacer that utilizes “stackable inserts” to increase the disc height in quantized 1-mm increments. More recently, Kim et al. [17] showed promising clinical outcomes, restoration of intervertebral disc height, and improved fusion rates using an uninterrupted, continuously expandable interbody device in minimally invasive surgery for transforaminal lumbar interbody fusion (TLIF) procedures. To our knowledge, no study has yet compared the stability provided by a continuous expandable cage inserted via the posterolateral approach with that provided by a traditional SS. Furthermore, the literature currently lacks characterization of insertion forces and details about other clinical parameters of expandable and static spacers.
This study compared two different interbody devices, an in situ continuously expandable spacer (CES) and a traditional SS, used in conjunction with TLIF. The former is an uninterrupted, continuously expandable device that, in its smallest, fully contracted form, is inserted at a height of 8 mm and may be expanded within the disc space to a height of 12 mm (Fig. 1). The latter is a traditional static oblique device that is inserted at its final height following sequential dilation and sizing of the disc space before insertion. We first conducted biomechanical comparisons of spacers in the presence of posterior instrumentation, while simultaneously measuring the impact required by the surgeon to insert both trials and implants. Finally, we analyzed radiographic evidence of annular distraction upon insertion of the final implant. In its reduced configuration, the device was capable of vertical expansion once installed in the intervertebral space. We hypothesized that the vertically expandable spacer provides a fixation equivalent to that of its static predecessors, while requiring less impaction force.
Fig. 1.
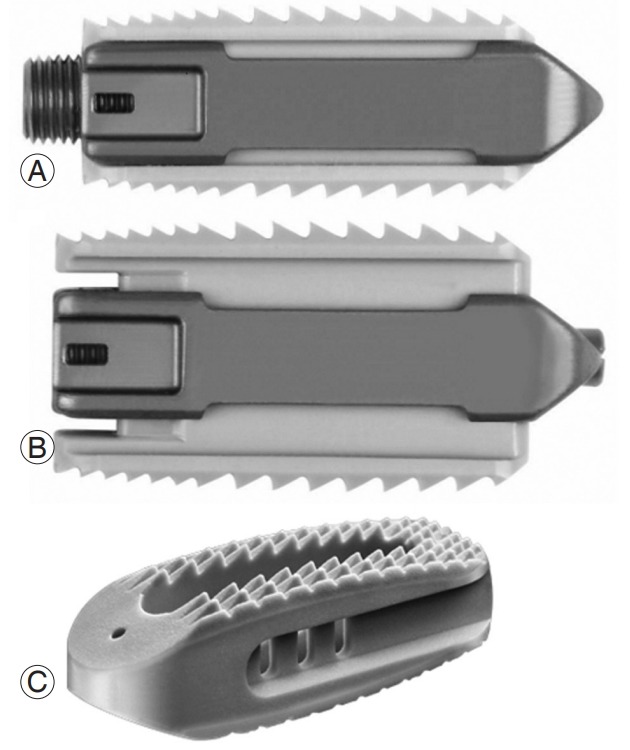
Continuously expandable spacer in minimized (A) and expanded (B) forms and static spacer (C).
Materials and Methods
1. Specimen preparation
Ten (L5–S1) fresh-frozen human cadaveric spines were randomly assigned into two equal groups (SS group: age, 60.6±10.6 years, two males and three females; CES group: age, 61.8±4.3 years, four males and one female). Radiographs in both anteroposterior and lateral views were obtained for each spine to ensure the absence of fracture, deformity, and metastatic disease. Spines were dissected by careful denuding of the paravertebral musculature, and the disruption of spinal ligaments, joints, and discs was avoided. Segments were potted at L5 and sacrum using a 1:1 mixture of Bondo auto filler (Bondo MarHyde Corp., Atlanta, GA, USA) and fiberglass resin (Home-Solution All Purpose, Bondo MarHyde Corp.). Specimens were stored in double plastic bags at −20°C.
2. Testing protocol
1) Biomechanics
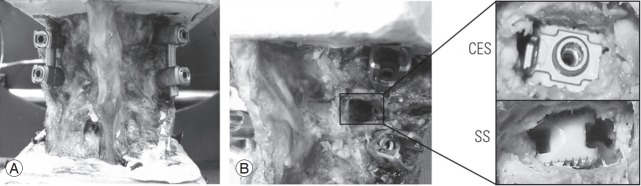
Each specimen was thawed overnight and affixed to a custom-built six-degrees-of-freedom (6-DOF) testing apparatus that was used to simulate physiological loads. The cranial portion of the specimen (L5) was affixed via rectangular metal tubing to a 6-DOF motor gimbal assembly that applied a pure, unconstrained rotational moment independently around the X-, Y-, and Z-axes corresponding to flexion–extension (FE), AR, and lateral bending (LB). The test platform included linear-bearing guide rails (X- and Z-axes), and a pneumatic-controlled linear actuator (Y-axis) allowed unconstrained translation. An unconstrained pure moment of ±6 Nm was applied for all loading modes and surgical constructs at a motor rate of 1°/sec in FE and LB and 0.5°/sec in AR [18]. The testing sequence was as follows: intact (or unperturbed spine); injured state (facetectomy and discectomy via the TLIF approach); bilateral pedicle screws (BPS) and rods (REVERE; Globus Medical Inc., Audubon, PA, USA); and TLIF interbody spacer with BPS (BPS+spacer). The tested interbody devices included a CES (CALIBER, Globus Medical Inc.) and a traditional SS (SUSTAIN, Globus Medical Inc.), which were inserted by a trained surgeon and laboratory personnel. Interbody spacers used throughout the study had a footprint of 10×26 mm and lordosis of 7°, and the heights were determined by proper trialing of the disc space. Both experimental devices are approved by the Food and Drug Administration and are currently available in market. Selected constructs are shown in Fig. 2. A total of three load–unload cycles were performed for each motion, and data obtained during the final cycle were used for analysis.
Fig. 2.
Biomechanical testing images of selected constructs. (A) Bilateral pedicle screws with rods. (B) TLIF facetectomy and discectomy: expandable TLIF interbody spacer (CES) and static interbody spacer (SS). TLIF, transforaminal lumbar interbody fusion; CES, continuously expandable spacer; SS, traditional static spacer.
Plexiglas markers, each with three infrared light-emitting diodes, were rigidly secured to L5 and S1 vertebral bodies to track motion, and the motion analysis system (Optotrak Certus; Northern Digital Inc., Waterloo, ON, Canada) was placed approximately 6 feet in front of the specimens. Markers denoting a rigid body were aligned approximately along the sagittal curvature of the spine. Optotrak Certus software superimposed the coordinate systems of two adjacent vertebral bodies to inferentially determine relative Eulerian rotations in each of the three planes, with an accuracy of 0.1 mm and resolution of 0.01 mm [19]. Range of motion (ROM) was reported across L5–S1 and was normalized per specimen to the intact condition.
2) Impact testing
The force required to insert trials and implants was determined while specimens were instrumented for biomechanical testing. A digital sensor measuring force and pressure (Fig. 3A) was firmly attached to a slap hammer that provided a flat surface and guided the direction of impact (Fig. 3B). The slap hammer was then connected to the trial/implant holder and mallet impulses were administered linearly through the trial or implant (Fig. 3C). Slap hammers are typically used for the removal of trial or interbody spacers, requiring the handle to move away from the specimen. However, preliminary testing determined that moving the handle toward the specimen produced a force necessary to push a trial/implant into the disc space following a thorough discectomy. The design of the slap hammer and the secured interface between the hammer and trial/implant holder consistently produced a force perpendicular to the digital sensor, which was not the case when using a traditional Cottle mallet during preliminary testing. Pressure was measured using pressure mapping software (I-Scan Pressure Measurement System; Tekscan Inc., Boston, MA, USA) through digital pressure film (Model 6900) and was calibrated linearly at 0 and 75 N. The trial or implant was positioned in such a way that it was plano-parallel to the outermost portion of the discectomy window. One practitioner recorded the number of mallet impulses necessary to reach full insertion. The force of each impulse was collected by the pressure film during insertion. The average force per mallet impact as well as the total force (number of impacts×force/impact) were recorded. Both the CES trial and implant were inserted at their smallest, fully contracted height to simulate clinical use and in accordance with the manufacturer’s instructions. Following trialing, the CES implant was expanded in situ until limited by tactile feel or torque limit (2.0 Nm). Trialing of SS started from the smallest size (8 mm) and was completed sequentially via tactile feel.
Fig. 3.
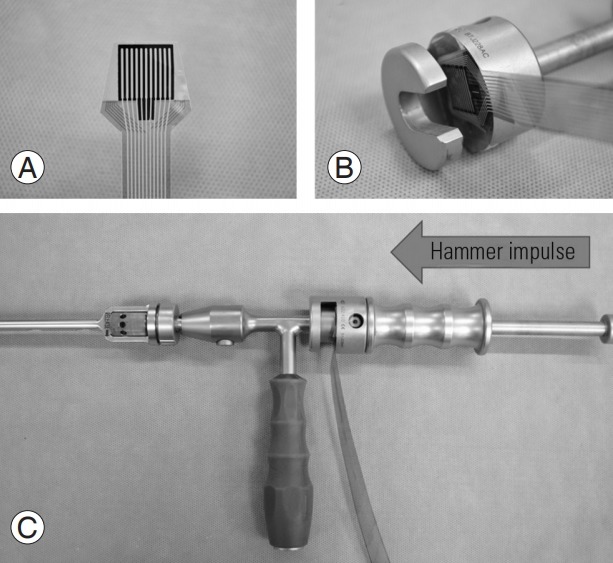
Representative photographs of impulse test set-up including (A) digital pressure film, (B) film placed at the interface of the slap hammer handle to guide the direction of the mallet impulse linearly through the trial and implant, and (C) complete set-up of slap hammer, digital pressure film, and implant/trial holder.
3) Fluoroscopic imaging
Expansion of the intervertebral disc annulus during implant insertion was fluoroscopically measured. A continuous video was recorded during implant insertion, and the frame representing the largest posterior disc height expansion was isolated. A standard of known length was used to calculate the disc space before and after insertion, and the change in distance was calculated for anterior, central, and posterior portions of the disc space (Fig. 4). These changes in disc height were tabulated using ImageJ software (National Institutes of Health, Bethesda, MD, USA) from visible landmarks such as the notable edges or intersection points on an X-ray.
Fig. 4.
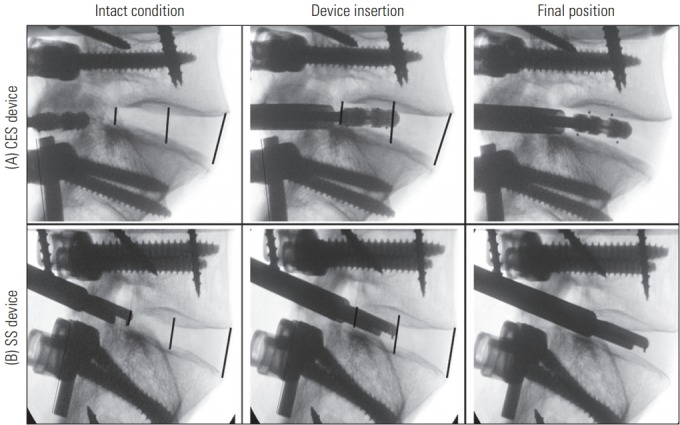
Representative radiographic analysis of disc height upon insertion of expandable spacer (A) and SS (B). Black bars indicate anterior, central, and posterior disc height measurements. CES, continuously expandable spacer; SS, traditional static spacer.
3. Statistical analysis
Statistical analysis was performed using IBM SPSS ver. 22.0 software (IBM Corp., Armonk, NY, USA). Biomechanical data were normalized to the average mean of intact ROM. One-way analysis of variance with repeated measures and Bonferroni post hoc analysis were performed to assess the differences in ROM between constructs within both spacer groups; t-tests were used to compare differences in stability between static and expandable spacers. Impaction data were assessed by independent t-tests for comparison of average strikes per procedure, force per strike, and total force for insertion of both static and expandable interbody trials and spacers. Fluoroscopic data were assessed by independent t-tests for comparison of distraction required for the insertion of the static and expandable spacers at anterior, central, and posterior regions of the disc space. Statistical significance was set at p≤0.05 for all tests.
Results
1. Biomechanical testing
A summary of the biomechanical results is shown in Table 1 and Fig. 5. All data are reported as percentage of mean intact motion. The results of the independent t tests found no statistically significant differences between BPS (CES versus SS) and BPS+spacer (CES versus SS) constructs in FE (p=0.803 and p=0.877, respectively), LB (p=0.532 and p=0.527, respectively), and AR (p=0.842 and p=0.816, respectively). For the BPS+spacer construct relative to intact motion, ROM was 39%, 52%, and 60%, in FE, LB, and AR, respectively, in the CES group (p=0.007, p=0.007, and p=0.302, respectively), and 41%, 41%, and 62%, respectively, in the SS group (p=0.019, p=0.43, and p=0.671, respectively). In the CES group, both BPS and BPS+spacer significantly reduced motion relative to the injured condition, in FE (124%), LB (127%), and AR (160%) (BPS: p=0.003, p=0.005, and p=0.011; BPS+spacer: p=0.006, p=0.005, and p=0.024, respectively; p≤0.05). Although BPS and BPS+spacer (SS group) reduced motion relative to injured, only FE showed statistical significance (p=0.034, p=0.034).
Table 1.
Mean raw ROM for FE, LB, and AR at L5–S1 segments
| ROM | Intact | Injured | BPS | BPS+spacer |
|---|---|---|---|---|
| Continuously expandable spacer (°) | ||||
| FE | 9.14±1.6 | 11.4±1.4 | 3.0±0.4 | 3.8±0.7 |
| LB | 7.0±0.9 | 8.9±1.4 | 2.6±0.9 | 2.9±1.0 |
| AR | 3.5±1.1 | 5.6±0.7 | 1.8±0.6 | 4.2±0.6 |
| Traditional static spacer (°) | ||||
| FE | 11.0±4.8 | 11.9±1.4 | 4.0±2.9 | 4.3±2.8 |
| LB | 8.1±3.7 | 9.6±4.8 | 3.9±2.6 | 4.2±2.8 |
| AR | 3.5±1.8 | 5.1±2.5 | 1.8±0.5 | 2.1±0.7 |
Values are presented as mean±standard deviation.
ROM, range of motion; FE, flexion–extension; LB, lateral bending; AR, axial rotation; BPS, bilateral pedicle screw.
Fig. 5.
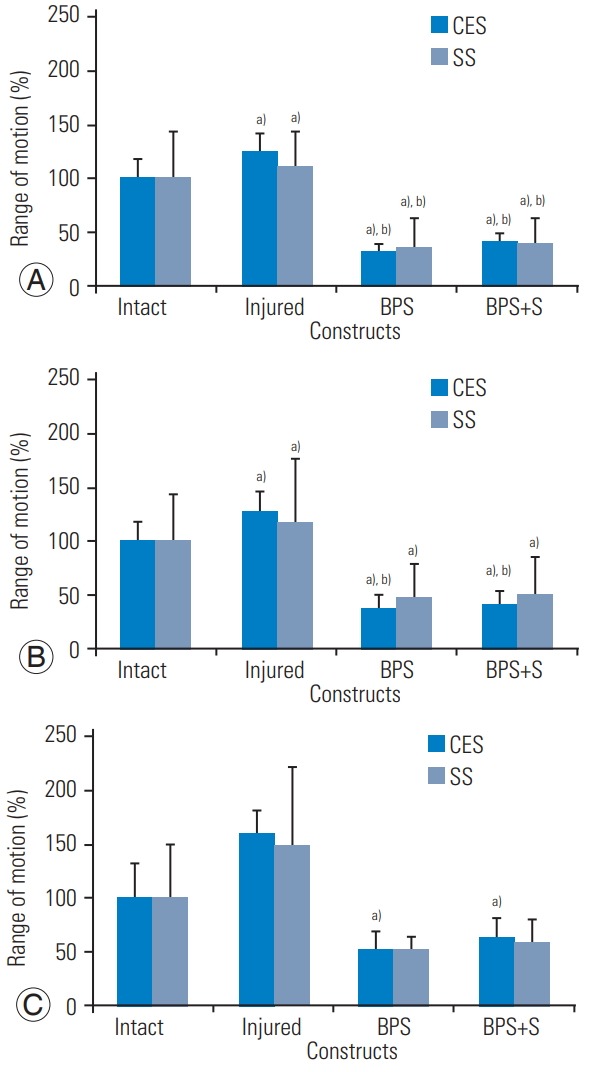
Biomechanical range of motion in flexion–extension (A), lateral bending (B), and axial rotation (C). No significant differences were found between operative constructs (BPS and BPS+spacer) in the CES and SS Groups (p≥0.05). BPS, bilateral pedicle screws; CES, continuously expandable spacer; SS, traditional static spacer. a)vs. intact (p≤0.05). b)vs. injured (p≤0.05).
2. Impact testing
The results of impact testing are presented in Figs. 6 and 7. The average disc height (post-discectomy) was 8.6 mm. The average size of the expandable spacers that could be inserted was 10.3 mm (at final expansion), and the average size of the SS was 9.2 mm. The expandable interbody trial is always inserted at the smallest size (8 mm), and the static trial is inserted at sequentially larger sizes (8 to 10 mm). Insertion of expandable spacer trials required 2.6±0.9 strikes, while insertion of expandable spacers required 2.6±0.5 strikes. A total of 8.4±3.8 strikes were required to insert SS trials and 4.2±1.6 strikes for SS. This amounted to 3.8±2.1 strikes for SS and an average of 2.2 trial sizes used per implant trial. Insertion of static trial required significantly more strikes than the expandable trial (p=0.011); however, this relationship was not significant during insertion of the implant (p=0.73) (Fig. 6).
Fig. 6.
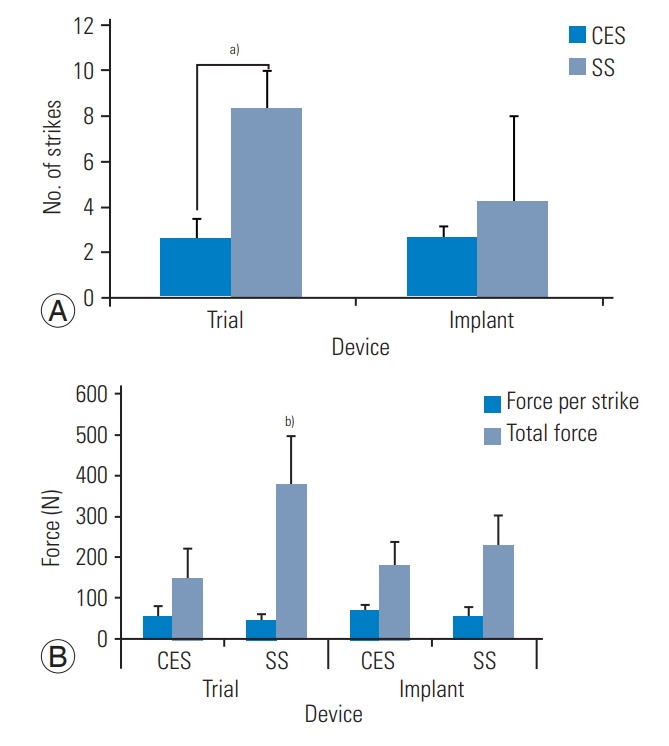
Impact testing results. (A) Average strikes per procedure: number of mallet strikes (top) needed to insert CALIBER and static trials and implant. (B) Implation force: work of impaction (bottom) shows average strike force and total force of surgery. CES, continuously expandable spacer; SS, traditional static spacer. a)p≤0.05. b)vs. trial (p≤0.05).
Fig. 7.
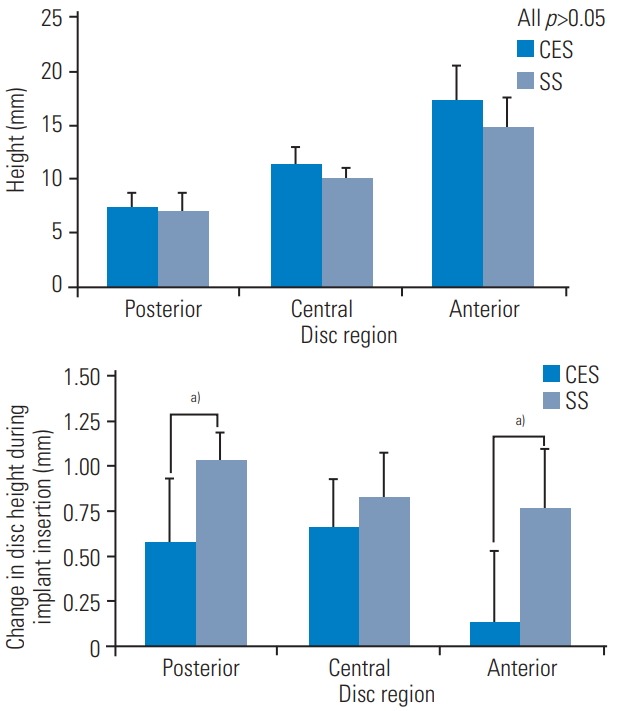
Average disc heights and distraction of the disc during the implant insertion phase. (A) Intact disc height. (B) Ancillary distraction due to implant insertion. During insertion, interbody spacers extend (or distract) the disc at L5–S1. The graph shows maximum distraction during that process, which can be calculated radiographically according to Fig. 4. CES, continuously expandable spacer; SS, traditional static spacer. a)p≤0.05.
The average force per strike required to insert spacers and trials was relatively constant. The total required force per surgery (number of strikes×force/strike) was 150.9±71.5 N to insert expandable spacer trials and 378.3±123.0 N to insert expandable spacers. The total required force per surgery was 378.3±123.3 N to insert SS trials and 228.5±78.5 N to insert SS. The total force required for the insertion of the SS trial was significantly higher than that of the expandable trial (150.9 versus 378.3 N, p=0.007) due to the significant number of strikes required for insertion of the static trial (2.6 versus 8.4 strikes, p=0.011). Additionally, the total force was greater for the static implant than the expandable implant, in part because the total force is related to the number of strikes (180.4 versus 228.5, p=0.298).
3. Fluoroscopic imaging
Intact posterior, central, and anterior disc heights between CES and SS groups (posterior, 7.4 versus 6.9 mm, p=0.674; central, 11.3 versus 10.0 mm, p=0.204; and anterior, 17.3 versus 14.8 mm, p=0.234, respectively) are shown in Fig. 7. Radiographic analysis (Fig. 4) shows that disc extension (iatrogenic over-distraction during implant insertion) occurred anteriorly, centrally, and posteriorly during the implant insertion phase. A clear trend between the CES and SS groups showed less extension of the disc space in the CES group. During insertion, distraction of the disc in the anterior, central, and posterior portions was 0.58, 0.65, and 0.14 mm, respectively, for the CES group, and 1.04, 0.83, and 0.78 mm, respectively, for the SS group. Statistical differences between the two groups were evident in the anterior (p=0.029) and posterior disc spaces (p=0.024), but not in the central disc space (p=0.315).
Discussion
This study compared two different interbody devices used in conjunction with a TLIF approach. The first was a continuously expandable device that, in its smallest, fully contracted form, is inserted at a height of 8 mm and may be expanded within the disc space to a height of 12 mm. The other was a traditional static oblique interbody device that is inserted at its final height on the basis of sequential dilation and sizing of the disc space before insertion. Three factors were evaluated representing clinical and functional parameters. We compared biomechanical performance, insertion of trials and implants, and iatrogenic over-distraction during implant insertion, when two different interbody devices, an in situ CES and a traditional SS, were used in conjunction with the TLIF approach. Results from the present study confirm the hypothesis that expandable interbody technology diminishes impaction forces and reduces iatrogenic over-distraction during implant insertion, while providing biomechanical stability similar to their static interbody counterparts.
Treatment of patients with DDD requires re-expansion of the disc space (indirect decompression), attainment of immediate stabilization of the segment, maintenance of sagittal alignment after removal of the symptomatic disc, and use of autogenous bone graft to ensure gradual intervertebral fusion [1]. The use of interbody spacers in spinal fusion is recommended for the following reasons: they provide structural support, increase foraminal height (indirect decompression), act as a space filler after the removal of potentially painful herniations, and increase anterior column load sharing.
The benefits of a minimally invasive TLIF, or of an interbody device that can be inserted at minimal size, are numerous. These methods may prevent excessive nerve root retraction, the destruction of paravertebral muscle, and neurophysiological damage to surrounding tissues. Several studies have shown deleterious effects of nerve root retraction and scar tissue formation near nerve roots [20-22]. Many studies have focused on electromyographic measurements, evaluating nerve roots by electron microscopy and comparing magnetic resonance imaging intensities after muscle retraction. Nagayama et al. [22] showed that retraction of the nerve root produced visible demyelination of the nerve root (as observed using transmission microscopy) that was even more severe than that resulting from puncture of the root. Indirect deterioration of nerve roots occurs via hypoxia of veins and the vascular supply surrounding the nerve root, both of which are often compressed near degenerated discs [21].
The results of our study show that regardless of spacer design, BPS+spacer greatly reduced motion compared with both intact and injured conditions. To date, there has been no kinematic evaluation of vertically expandable TLIF spacer designs. However, our results using a static PEEK TLIF spacer in combination with bilateral pedicle fixation are consistent with previous cadaveric biomechanical studies [23-26]. Cho et al. [23] found that BPS with static, nonarticulating TLIF spacer reduced intact motion to 25%, 31%, and 60% in FE, LB, and AR, respectively, compared with 41%, 41%, and 62%, respectively, in the present study. Nevertheless, there were no functional differences in biomechanical performance between the expandable and static spacers when supplemented with posterior rods, in any loading direction. These findings are not surprising given that both devices provide adequate height restoration, enter the disc at similar trajectories, present similar footprints, and are used in the presence of posterior instrumentation. Mild differences between CES and SS groups are more attributable to the differences in the innate behavior of the spine than to the performance of the devices.
Some differences in injury biomechanics were evident after spacer insertion, particularly in AR. The injury was tested after spacer impaction; therefore, a dislodged endplate or movement of disc material created the disparity observed in the injury. Theoretically, using an expandable spacer may lead to preservation of more disc material and endplate around the implant compared with using a SS.
Differences between interbody devices were also investigated in terms of two factors that may lead to denervation and damage to surrounding tissue during insertion: force of impact and expansion of the disc space during insertion. The latter is an underestimated yet hugely important factor that influences adjacent segment disease. Kaito et al. [15] found that over-distraction of the disc space during posterior lumbar interbody fusion was positively correlated with adjacent segment disease during a follow-up of only 3 years. Expandable devices, inserted in minimized form, may be less likely to cause over-distraction as the surgeon is not likely to rely on impaction and is likely to radiographically monitor expansion.
Our results show that distraction of the annulus upon insertion varies for the two devices. As observed radiographically, iatrogenic distraction during insertion is less pronounced with an expandable spacer than with a traditional spacer. This was statistically confirmed at both posterior and anterior aspects of the disc. A reduced danger of lacerating nerve roots (particularly L5) and a decreased likelihood of stretching ligament and annular components upon insertion of expandable devices may have pronounced effects on adjacent level disease.
The results of impact testing revealed a trend toward a greater effort (mallet strikes) required to insert traditional trials and spacers than the expandable versions. The total force required per surgery was greater for the static implant. One may conclude that it takes less overall effort to insert CES than a traditional TLIF spacer. The particular scenario largely depends on disc size (post-discectomy) and whether the practitioner sequentially trials up to the final height or directly skips smaller trials. In all but one specimen, every sequential increase in trial size required greater effort for insertion than the previous size (e.g., more mallet hits were required to insert a 9-mm than an 8-mm trial).
The aforementioned benefits of a vertically expandable posterior spacer do not negatively affect fusion rate and sagittal balance [27,28]. A retrospective analysis by Yee et al. [28] (n=89 patients) observed similar lumbar lordosis results after 1 year between static and expandable TLIF spacers (2° versus 5°, respectively). Additionally, Barrett-Tuck et al. [27] observed significantly improved pain scores following 12 months with solid fusion noted in 92.3% (n=12) of patients.
Although the present study successfully characterized a novel expandable device aimed at reducing segmental motion, effort of insertion, and iatrogenic over-distraction during implant insertion, it is not without limitations. Similar to other human cadaveric studies, the lack of availability and the high cost of specimens resulted in a small sample size. Using a larger sample size may reduce the likelihood of type I and II errors. Additionally, captured motion data only reflects the immediate postoperative condition and do not account for patient factors such as bone healing and biomechanical features of the final fusion mass. Finally, anatomic differences between cadaveric specimens introduced variability; however, this was minimized by normalizing ROM data to the average intact condition. Similarly, specimens were randomly grouped to account for bone mineral density.
Conclusions
In this study, researchers compared CES with a traditional TLIF spacer. The results showed biomechanical equivalence in the two groups. Clinically, the impaction force required to insert CES trials and implants was less than that for traditional SS, and less ancillary distraction was noted on annulus material. These promising results show that biomechanics can be maintained through minimally invasive techniques in a TLIF model.
Acknowledgments
The authors would like to thank Kelly Baker, PhD, and Dolores Matthews, MEd, ELS, for contributions in editing this manuscript.
Both transforaminal interbody spacers (CALIBER and SUSTAIN; Globus Medical Inc., Audubon, PA, USA) and pedicle screws (REVERE, Globus Medical Inc.) examined in this study have been cleared by the Food and Drug Administration for the indications discussed.
Footnotes
The authors would like to disclose that the study was performed at Globus Medical Inc., using its 6-DOF motion simulator. Surgeon JT has no financial link to Globus Medical Inc. and was not provided financial benefit for his involvement in the study. Cadaveric specimens and related materials were provided by Globus Medical Inc., at which JAH, BSB, MM, and SK are employees.
References
- 1.Folman Y, Shabat S, Gepstein R. B-twin expandable spinal spacer for posterior lumbar interbody stabilization: mechanical testing. J Surg Orthop Adv. 2006;15:203–8. [PubMed] [Google Scholar]
- 2.Briggs H, Keats S. Laminectomy and foraminotomy with chip fusion; operative treatment for the relief of low-back pain and sciatic pain associated with spondylolisthesis. J Bone Joint Surg Am. 1947;29:328–34. [PubMed] [Google Scholar]
- 3.Cloward RB. The treatment of ruptured lumbar intervertebral discs by vertebral body fusion: I. indications, operative technique, after care. J Neurosurg. 1953;10:154–68. doi: 10.3171/jns.1953.10.2.0154. [DOI] [PubMed] [Google Scholar]
- 4.Lin PM. Posterior lumbar interbody fusion technique: complications and pitfalls. Clin Orthop Relat Res. 1985;((193)):90–102. [PubMed] [Google Scholar]
- 5.Bagby GW. Arthrodesis by the distraction-compression method using a stainless steel implant. Orthopedics. 1988;11:931–4. doi: 10.3928/0147-7447-19880601-13. [DOI] [PubMed] [Google Scholar]
- 6.Abumi K, Panjabi MM, Kramer KM, Duranceau J, Oxland T, Crisco JJ. Biomechanical evaluation of lumbar spinal stability after graded facetectomies. Spine (Phila Pa 1976) 1990;15:1142–7. doi: 10.1097/00007632-199011010-00011. [DOI] [PubMed] [Google Scholar]
- 7.Brantigan JW, Steffee AD, Lewis ML, Quinn LM, Persenaire JM. Lumbar interbody fusion using the Brantigan I/F cage for posterior lumbar interbody fusion and the variable pedicle screw placement system: two-year results from a Food and Drug Administration investigational device exemption clinical trial. Spine (Phila Pa 1976) 2000;25:1437–46. doi: 10.1097/00007632-200006010-00017. [DOI] [PubMed] [Google Scholar]
- 8.Freeman BJ, Licina P, Mehdian SH. Posterior lumbar interbody fusion combined with instrumented postero-lateral fusion: 5-year results in 60 patients. Eur Spine J. 2000;9:42–6. doi: 10.1007/s005860050007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jun BY. Posterior lumbar interbody fusion with restoration of lamina and facet fusion. Spine (Phila Pa 1976) 2000;25:917–22. doi: 10.1097/00007632-200004150-00004. [DOI] [PubMed] [Google Scholar]
- 10.Agazzi S, Reverdin A, May D. Posterior lumbar interbody fusion with cages: an independent review of 71 cases. J Neurosurg. 1999;91(2 Suppl):186–92. doi: 10.3171/spi.1999.91.2.0186. [DOI] [PubMed] [Google Scholar]
- 11.Elias WJ, Simmons NE, Kaptain GJ, Chadduck JB, Whitehill R. Complications of posterior lumbar interbody fusion when using a titanium threaded cage device. J Neurosurg. 2000;93(1 Suppl):45–52. doi: 10.3171/spi.2000.93.1.0045. [DOI] [PubMed] [Google Scholar]
- 12.Ray CD. Threaded titanium cages for lumbar interbody fusions. Spine (Phila Pa 1976) 1997;22:667–79. doi: 10.1097/00007632-199703150-00019. [DOI] [PubMed] [Google Scholar]
- 13.Wetzel FT, LaRocca H. The failed posterior lumbar interbody fusion. Spine (Phila Pa 1976) 1991;16:839–45. doi: 10.1097/00007632-199107000-00027. [DOI] [PubMed] [Google Scholar]
- 14.Cole CD, McCall TD, Schmidt MH, Dailey AT. Comparison of low back fusion techniques: transforaminal lumbar interbody fusion (TLIF) or posterior lumbar interbody fusion (PLIF) approaches. Curr Rev Musculoskelet Med. 2009;2:118–26. doi: 10.1007/s12178-009-9053-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaito T, Hosono N, Mukai Y, Makino T, Fuji T, Yonenobu K. Induction of early degeneration of the adjacent segment after posterior lumbar interbody fusion by excessive distraction of lumbar disc space. J Neurosurg Spine. 2010;12:671–9. doi: 10.3171/2009.12.SPINE08823. [DOI] [PubMed] [Google Scholar]
- 16.Bhatia NN, Lee KH, Bui CN, Luna M, Wahba GM, Lee TQ. Biomechanical evaluation of an expandable cage in single-segment posterior lumbar interbody fusion. Spine (Phila Pa 1976) 2012;37:E79–85. doi: 10.1097/BRS.0b013e3182226ba6. [DOI] [PubMed] [Google Scholar]
- 17.Kim CW, Doerr TM, Luna IY, et al. Minimally invasive transforaminal lumbar interbody fusion using expandable technology: a clinical and radiographic analysis of 50 patients. World Neurosurg. 2016;90:228–35. doi: 10.1016/j.wneu.2016.02.075. [DOI] [PubMed] [Google Scholar]
- 18.Goel VK, Panjabi MM, Patwardhan AG, Dooris AP, Serhan H, American Society for Testing and Materials Test protocols for evaluation of spinal implants. J Bone Joint Surg Am. 2006;88 Suppl 2:103–9. doi: 10.2106/JBJS.E.01363. [DOI] [PubMed] [Google Scholar]
- 19.Schmidt J, Berg DR, Ploeg HL, Ploeg L. Precision, repeatability and accuracy of Optotrak optical motion tracking systems. Int J Exp Comput Biomech. 2009;1:114–27. [Google Scholar]
- 20.Blom S, Lemperg R. Electromyographic analysis of the lumbar musculature in patients operated on for lumbar rhizopathy. J Neurosurg. 1967;26:25–30. doi: 10.3171/jns.1967.26.1part1.0025. [DOI] [PubMed] [Google Scholar]
- 21.Jayson MI. The role of vascular damage and fibrosis in the pathogenesis of nerve root damage. Clin Orthop Relat Res. 1992;(279):40–8. [PubMed] [Google Scholar]
- 22.Nagayama R, Nakamura H, Yamano Y, et al. An experimental study of the effects of nerve root retraction on the posterior ramus. Spine (Phila Pa 1976) 2000;25:418–24. doi: 10.1097/00007632-200002150-00005. [DOI] [PubMed] [Google Scholar]
- 23.Cho W, Wu C, Mehbod AA, Transfeldt EE. Comparison of cage designs for transforaminal lumbar interbody fusion: a biomechanical study. Clin Biomech (Bristol, Avon) 2008;23:979–85. doi: 10.1016/j.clinbiomech.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 24.Harris BM, Hilibrand AS, Savas PE, et al. Transforaminal lumbar interbody fusion: the effect of various instrumentation techniques on the flexibility of the lumbar spine. Spine (Phila Pa 1976) 2004;29:E65–70. doi: 10.1097/01.brs.0000113034.74567.86. [DOI] [PubMed] [Google Scholar]
- 25.Kettler A, Niemeyer T, Issler L, et al. In vitro fixator rod loading after transforaminal compared to anterior lumbar interbody fusion. Clin Biomech (Bristol, Avon) 2006;21:435–42. doi: 10.1016/j.clinbiomech.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 26.Cannestra AF, Peterson MD, Parker SR, Roush TF, Bundy JV, Turner AW. MIS expandable interbody spacers: a literature review and biomechanical comparison of an expandable MIS TLIF with conventional TLIF and ALIF. Spine (Phila Pa 1976) 2016;41 Suppl 8:S44–9. doi: 10.1097/BRS.0000000000001465. [DOI] [PubMed] [Google Scholar]
- 27.Barrett-Tuck R, Del Monaco D, Block JE. One and two level posterior lumbar interbody fusion (PLIF) using an expandable, stand-alone, interbody fusion device: a VariLift(®) case series. J Spine Surg. 2017;3:9–15. doi: 10.21037/jss.2017.02.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yee TJ, Joseph JR, Terman SW, Park P. Expandable vs static cages in transforaminal lumbar interbody fusion: radiographic comparison of segmental and lumbar sagittal angles. Neurosurgery. 2017;81:69–74. doi: 10.1093/neuros/nyw177. [DOI] [PubMed] [Google Scholar]