Abstract
Background
Platinum‐based chemotherapy is the standard first‐line treatment for patients with advanced pan‐negative non‐squamous (non‐Sq) non‐small cell lung cancer (NSCLC). However, it is unknown which chemotherapy regimen confers the greatest benefit in such patients. This study explored which chemotherapy regimens were advantageous in non‐Sq NSCLC patients.
Methods
A retrospective study was conducted on 114 patients with advanced non‐Sq NSCLC using platinum‐based chemotherapy in a first‐line setting between January 2013 and December 2015. The study evaluated the most common first‐line regimens including pemetrexed/platinum (PP), paclitaxel/carboplatin, gemcitabine/platinum, and vinorelbine/cisplatin. The primary endpoint was progression‐free survival (PFS), and secondary endpoints were the objective response rate and disease control rate (DCR). Univariate and multivariate logistic analysis was carried out.
Results
Sixty of the 114 patients were administered PP regimens and 54 non‐pemetrexed plus platinum (NPP) regimens. The median PFS was significantly longer in the PP than in the NPP group (7.2 months, 95% confidence interval [CI] 5.3–9.1 vs. 4.9 months, 95% CI 3.2–6.6; P = 0.031). The DCR of the PP regimen was better than that of the NPP regimen (90.0% vs. 74.1%; P = 0.026). Smoking status was an independent predictor of PFS (hazard ratio 2.1, 95% CI 1.4–3.3; P = 0.001) in a final multivariate Cox regression model.
Conclusions
A PP regimen tends to be more beneficial than an NPP regimen for patients with pan‐negative advanced non‐Sq NSCLC. Smoking status may be a valuable predictor for the selection of a chemotherapy regimen in such patients.
Keywords: Chemotherapy, first‐line anticancer treatment, metastatic, non‐small cell lung cancer
Introduction
Lung cancer is the leading cause of cancer‐related mortality in the world. It was estimated that by the end of 2017 in the United States, approximately 222 500 new cases would be diagnosed, with an estimated 155 870 deaths resulting from lung cancer. The overall five‐year survival rate for non‐small cell lung cancer (NSCLC) was only 17.7% from 2006 to 2012.1 However, after an alarming increase, lung cancer has become a great threat in China, with 530 800 new lung cancer cases and 460 300 deaths reported by the Chinese National Cancer Institute in 2013.2 NSCLC accounts for 80–85% of all lung cancer cases. The frequency of adenocarcinoma has dramatically increased in Chinese men, from 28.7% in 2000–2004 to 48.6% in 2009–2012. Adenocarcinoma has also become the most predominant histological subtype of lung cancer in China.3, 4 Recurrence or metastatic disease will inevitably develop in some early‐stage patients after resection, while some patients diagnosed at advanced stage show pleural or pericardial effusion, or distant metastases, the outcomes of which remain very poor. In cases of advanced or metastatic disease, median progression‐free survival (PFS) is 8–10 months and the one‐year survival rate is only 30–40%.5, 6, 7, 8
With the discovery of oncogenic driver mutations and the availability of molecular targeted therapies, the management paradigm for patients with advanced non‐squamous (non‐Sq) NSCLC has dramatically changed in recent years. The Iressa (gefitinib) pan‐Asia study (IPASS) study showed that in a mutation‐positive subgroup, PFS was significantly longer in patients receiving gefitinib than in those treated with carboplatin‐paclitaxel.9
Targeted therapies based on genetic alterations are recommended as first‐line standard treatment for advanced NSCLC if sensitive EGFR gene mutations or ALK gene rearrangements are detected.10, 11, 12, 13, 14 Sensitizing EGFR mutations are found in approximately 10% of Caucasian patients with NSCLC and in up to 50% of Asian patients,15 while the ALK arrangement rate is only about 5–7%.16, 17, 18 In clinical practice, nearly 50% of patients without an EGFR mutation or ALK gene rearrangement require platinum‐doublet chemotherapy. It is unclear, however, which chemotherapy regimens may benefit patients with pan‐negative non‐Sq NSCLC. Therefore, this study aimed to explore which chemotherapy regimen offered greater advantages for patients with advanced pan‐negative non‐Sq NSCLC in clinical practice.
Methods
Patients
We performed a retrospective study of 114 patients with pan‐negative advanced non‐Sq NSCLC (stages IIIB–IV) who received first‐line platinum‐based chemotherapy at the National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College (Beijing, China), between January 2013 and December 2015 (Fig 1). All patients who met the following criteria were registered: aged ≥ 18 years; histologically or cytologically confirmed with unresectable stage IIIB–IV non‐Sq NSCLC or recurrent disease after surgical resection; received platinum doublet chemotherapy as first‐line treatment; and pan‐negative cases: wild‐type EGFR/KRAS confirmed by PCR or the absence of ALK rearrangement confirmed by fluorescence in situ hybridization or Ventana immunohistochemistry, with measurable target lesions documented by computed tomography (CT) images of the chest and abdomen, or magnetic resonance imaging (MRI), defined by Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1, and an Eastern Cooperative Oncology Group performance status (ECOG PS) of ≤ 2. Patients were excluded if they had previous received systemic anticancer treatment for stage IIIB–IV disease or underwent concurrent chemoradiotherapy. Smokers were defined as current or former smokers, while non‐smokers referred to individuals who had smoked < 100 cigarettes in their lifetime. Data was collected from electronic medical records. As an observational study, informed patient consent was not required. The institutional review board approved study.
Figure 1.
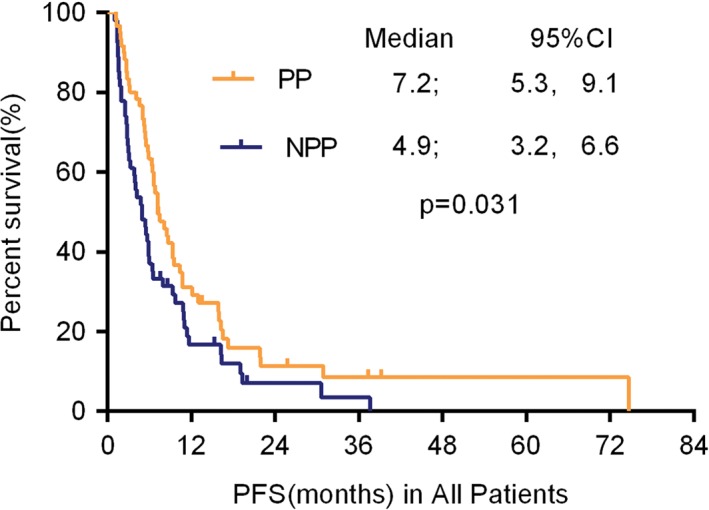
Kaplan–Meier curve for progression‐free survival (PFS) for pemetrexed/platinum (PP) versus non‐pemetrexed/platinum (NPP) regimen in patients with advanced non‐squamous non‐small cell lung cancer without a driver oncogene. The difference was statistically significant (median 7.2 vs. 4.9 months; P = 0.031 by log‐rank test). CI, confidence interval.
Chemotherapy regimens
Patients were stratified into two groups according to treatment regimens: pemetrexed/platinum (PP) and non‐pemetrexed plus platinum (NPP) chemotherapy. The chemotherapy regimens were as follows: (i) pemetrexed 500 mg/m2 on day 1 plus cisplatin 75 mg/m2 divided into three days (day 1–3), with or without antiangiogenic agents (bevacizumab 7.5 mg/kg on day 1 or 15 mL of endostar injected intravenously days 1–14 every 21 days; (ii) gemcitabine 1000 mg/m2 on days 1 and 8 plus cisplatin 75 mg/m2 divided into three days (day 1–3) every 21 days; (iii) paclitaxel 175 mg/m2 on day 1 plus cisplatin 75 mg/m2 divided into three days (day 1–3), with or without antiangiogenic agents (bevacizumab or endostar) every 21 days; and (iv) vinorelbine 25 mg/m2 on days 1 and 8 plus cisplatin 75 mg/m2 divided into three days (day 1–3). Patients that could not tolerate cisplatin were administered carboplatin.
Outcomes
Disease was assessed at baseline and every two cycles after the first dose of study therapy for four or six cycles until radiographic progressive disease (PD) was determined by imaging examination, including a CT scan of the chest and abdomen or MRI of the brain. Scans were then taken at two‐month intervals. Evaluations of the response included: complete response (CR), partial response (PR), stable disease (SD), or PD. Calculations of the objective response rate (ORR) included cases achieving CR and PR, while the disease control rate (DCR) included patients who achieved CR, PR, and SD. The primary endpoint was progression‐free survival (PFS), and secondary endpoints were ORR and DCR. PFS was calculated from the first day of treatment to the date of disease progression. OS was calculated from the date of diagnosis to the date of death from any cause. Patients who were still alive at the final follow‐up (30 November 2016) were regarded as censored, and the duration between the first day of treatment and the final follow‐up was included in the analysis.
Statistical analysis
SPSS version 16.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analyses. Baseline characteristics were presented by applying descriptive statistics. The data were presented as percentages for dichotomous variables and analyzed using a chi‐square test. The primary endpoint of PFS was analyzed using the Kaplan–Meier method; univariate analyses were performed using the log‐rank test; and multivariable analysis using a Cox proportional hazard regression model. All statistical tests were two‐tailed with P < 0.05 considered statistically significant. Variables included age, gender, smoking history, clinical stage, pathological and histological type, tumor differentiation, ECOG PS, and the CYFRA 21‐1 value in blood before treatment. GraphPad Prism 6.0 (GraphPad, La Jolla, CA, USA) was used to present survival curves.
Results
Baseline characteristics
Of the 114 patients, 77 (67.5%) were men and 37 (32.5%) were women; the median age was 65 years (range 39–86); 110 patients (96.5%) had a good PS of 0–1; and 65 (57%) were smokers. Sixty patients were administered a PP chemotherapy and 54 an NPP chemotherapy regimen (a paclitaxel‐containing regimen for 39 patients, a gemcitabine‐containing regimen for 13 patients, and a vinorelbine‐containing regimen for 2 patients). Baseline characteristics were well balanced between the two groups. The characteristics of the 114 patients are displayed in Table 1.
Table 1.
Baseline characteristics of patients with advanced stage IIIB/IV non‐Sq NSCLC
| Characteristics | PP regimen (n = 60) | NPP regimen (n = 54) | P |
|---|---|---|---|
| Age (years) | 0.416 | ||
| ≥ 60 | 29 | 22 | |
| < 60 | 31 | 32 | |
| Gender | 0.311 | ||
| Male | 38 | 39 | |
| Female | 22 | 15 | |
| Smoking history | 0.936 | ||
| Yes | 34 | 31 | |
| No | 26 | 23 | |
| Location of the primary tumor | 0.372 | ||
| Left lung | 25 | 27 | |
| Right lung | 35 | 27 | |
| Clinical stage | 0.452 | ||
| IIIB | 9 | 11 | |
| IV | 51 | 43 | |
| Differentiation | 0.783 | ||
| Low | 49 | 43 | |
| Median–high | 11 | 11 | |
| ECOG | 0.238 | ||
| 0 | 42 | 43 | |
| 1–2 | 18 | 11 | |
| Use of anti‐angiogenic drugs | 0.160 | ||
| Yes | 8 | 3 | |
| No | 52 | 51 | |
| Brain metastasis | 0.953 | ||
| Yes | 8 | 7 | |
| No | 52 | 47 |
ECOG, Eastern Cooperative Oncology Group; non‐Sq, non‐squamous; NPP, non‐pemetrexed/platinum; NSCLC, non‐small cell lung cancer; PP, pemetrexed/platinum.
Efficacy analysis
At least four cycles of treatment were administered to 114 patients. In the PP group, a CR was achieved in one case, PR in 19, and SD in 34. In the NPP group, 14 patients were evaluated with a PR and 26 with SD. No statistical significance was found in the ORR between the groups (33.3% vs. 25.9%; P > 0.05). The DCR of the PP group was higher than in the NPP (90.0% in the PP vs. 74.1% in the NPP; P = 0.026). An efficacy analysis of platinum‐based doublet chemotherapy is presented in Table 2. The median PFS was significantly longer in patients treated with PP than NPP chemotherapy regimens (7.2 months [95% confidence interval, CI 5.3–9.1] vs. 4.9 months [95% CI 3.2–6.6]; P = 0.031), respectively (Fig 1).
Table 2.
Efficacy analysis
| Variable | CR + PR + SD (n = 94) | PD (n = 20) | P |
|---|---|---|---|
| Age (years) | 0.014 | ||
| ≥ 60 | 47 | 4 | |
| < 60 | 47 | 16 | |
| Gender | 0.796 | ||
| Male | 63 | 14 | |
| Female | 31 | 6 | |
| Smoking history | 0.427 | ||
| Yes | 52 | 7 | |
| No | 42 | 13 | |
| Location of the primary tumor | 0.579 | ||
| Left lung | 44 | 12 | |
| Right lung | 50 | 8 | |
| Stage before systemic chemotherapy | 0.742 | ||
| IIIB | 17 | 3 | |
| IV | 77 | 17 | |
| Differentiation | 0.477 | ||
| Low | 77 | 15 | |
| Median–High | 17 | 5 | |
| ECOG | 0.280 | ||
| 0 | 72 | 13 | |
| 1–2 | 22 | 7 | |
| Treatment | 0.026 | ||
| PP regimens | 54 | 6 | |
| NPP regimens | 40 | 14 |
CI, confidence interval; CR, complete response; ECOG, Eastern Cooperative Oncology Group; NPP, non‐pemetrexed/platinum; PD, progressive disease; PP, pemetrexed/platinum; PR, partial response; SD, stable disease.
Univariate and multivariate analyses by Cox regression
Univariate analysis showed that the PFS of patients with pan‐negative advanced non‐Sq NSCLC was significantly associated with the following factors: gender (female vs. male), smoking history (yes vs. no), treatment pattern (PP vs. NPP chemotherapy regimens), and the CYFRA 21‐1 level before treatment of first‐line chemotherapy (abnormal vs. normal) (Table 3). Statistically significant variables in univariate analysis were entered into a Cox proportional hazard regression model.
Table 3.
Univariate survival analyses for PFS
| Variable | B | SE | HR | 95% CI | P |
|---|---|---|---|---|---|
| Age | |||||
| ≥ 60 vs. < 60 | −0.228 | 0.205 | 0.796 | 0.532–1.198 | 0.265 |
| Gender | |||||
| Male vs. female | 0.446 | 0.222 | 1.563 | 1.011–2.415 | 0.044 |
| Smoking history | |||||
| Yes vs. no | 0.763 | 0.215 | 2.144 | 1.405–3.270 | 0.000 |
| Stage IIIB vs. IV | 0.030 | 0.261 | 1.030 | 0.918–1.156 | 0.609 |
| Differentiation | |||||
| Low vs. median–high | −0.025 | 0.262 | 0.923 | 0.584–1.628 | 0.975 |
| ECOG | |||||
| 0 vs. 1–2 points | 0.309 | 0.227 | 1.363 | 0.873–2.127 | 0.173 |
| Treatment | |||||
| PP vs. NPP regimen | −0.429 | 0.201 | 0.651 | 0.439–0.965 | 0.033 |
| CYFRA 21‐1 level | |||||
| Abnormal vs. normal | 0.495 | 0.204 | 1.641 | 1.100–2.447 | 0.016 |
CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; HR, hazard ratio; NPP, non‐pemetrexed/platinum; PD, progressive disease; PFS, progression‐free survival; PP, pemetrexed/platinum; SE, standard error.
Multivariable analyses confirmed that smoking history and the CYFRA 21‐1 level were valuable predictors of PFS of first‐line platinum‐based regimens in patients with pan‐negative non‐Sq NSCLC (Table 4). The PFS rates of non‐smokers and current smokers with pan‐negative non‐Sq NSCLC on PP regimens were significantly different at 16.0 (95% CI 8.5–23.4 months) and 6.3 (95% CI 4.7–8.9) months, respectively (P = 0.001) (Fig 2). In contrast, no statistical significance was observed in the PFS rate of non‐smokers versus current smokers with pan‐negative non‐Sq NSCLC on NPP regimens (5.9 months [95% CI 5.4–6.4] vs. 4.0 months [95% CI 2.3–5.7]; P = 0.312) (Fig 2).
Table 4.
Predictors of PFS analyzed by a Cox regression model
| Variable | B | SE | HR | 95% CI | P |
|---|---|---|---|---|---|
| Smoking (yes vs. no) | 0.761 | 0.247 | 2.140 | 1.319–3.472 | 0.002 |
| CYFRA 21‐1 level (abnormal vs. normal) | 0.482 | 0.211 | 1.620 | 1.071–2.450 | 0.022 |
| Treatment patterns (PP vs. NPP) | −0.333 | 0.212 | 0.717 | 0.473–1.087 | 0.117 |
| Gender (male vs. female) | −0.123 | 0.273 | 0.884 | 0.518–1.509 | 0.651 |
CI, confidence interval; HR, hazard ratio; NPP, non‐pemetrexed/platinum; PD, progressive disease; PFS, progression‐free survival; PP, pemetrexed/platinum; SE, standard error.
Figure 2.
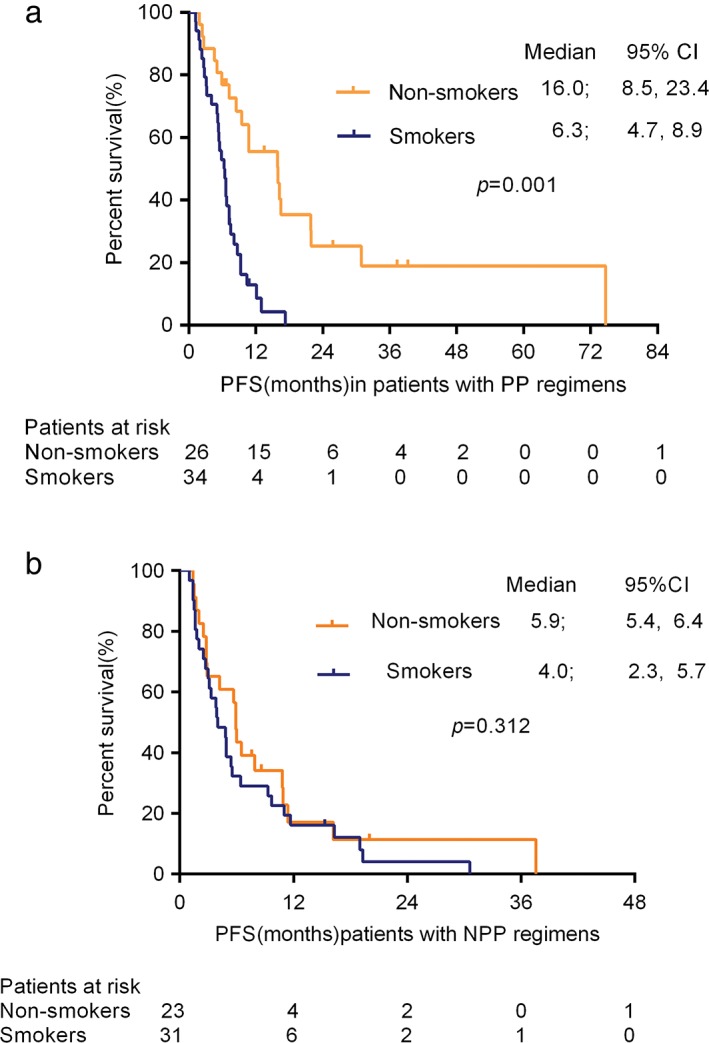
Kaplan–Meier curve for progression‐free survival (PFS) of non‐smokers and current smokers with pan‐negative advanced non‐squamous non‐small cell lung cancer treated with (a) pemetrexed/platinum (PP; median, 16.0 vs. 6.3 months; P = 0.001) and (b) non‐pemetrexed plus platinum (NPP; median 5.9 vs. 4.0 months; P = 0.312) regimens. The difference was statistically significant after the PP regimen, but not after NPP. CI, confidence interval.
Discussion
The most important progress in the field of advanced NSCLC is related to an improved understanding of the biology of NSCLC and the discovery of driver oncogenic mutations. Targeted therapies have been used as first‐line treatment for patients with metastatic non‐Sq NSCLC with a sensitizing EGFR mutation or ALK rearrangement, but were ineffective in a subgroup of patients without driver mutations.12 Platinum‐based chemotherapy is recommended as first‐line treatment in patients with advanced non‐Sq NSCLC, with or without an unknown EGFR mutation/ALK fusion gene arrangement, according to National Comprehensive Cancer Network (NCCN) guidelines.8 However, in a previous study, real world evidence was lacking in a comparison of first‐line treatment patterns routinely used for cases of pan‐negative advanced non‐Sq NSCLC. Furthermore, few reliable predictors regarding the selection of platinum‐based chemotherapy regimens have been identified in patients with advanced NSCLC.
In our study, PP regimens displayed a better DCR than NPP (90.0% vs. 74.1%; P = 0.026) and a statistically improved PFS in patients with pan‐negative advanced non‐Sq NSCLC (median 7.2 vs. 4.9 months; P = 0.031), indicating that a PP chemotherapy regimen tends to be more beneficial. The JMDB study demonstrated that pemetrexed/cisplatin chemotherapy regimens showed a significant survival advantage in patients with adenocarcinomas (12.6 vs. 10.9 months; respectively).8 Patients showed an increased survival benefit after they were stratified by histological type. Unfortunately, the EGFR mutation status of patients was generally unknown; as a noninferiority study, patients were not randomly assigned according to histological types but from a prespecified subset analysis. As such, the JMDB study was neither designed nor powered to answer this question. Thus, we cannot judge whether the benefit of PP chemotherapy regimens in adenocarcinoma cases are related to the presence or absence of an EGFR mutation. Our study analyzed EGFR mutation status and further confirmed the JMDB study results, concluding that PP chemotherapy regimens not only tend to have a survival advantage in adenocarcinoma cases, but might also be beneficial for pan‐negative patients. Although the multivariable Cox proportional hazard model demonstrated that PP regimens are not significantly associated to outcome, this may be a result of the small sample size. In addition, 11 patients were treated with platinum doublet chemotherapy plus anti‐angiogenic drugs (8 in the PP, 3 in the NPP group), and a significant difference in the use of anti‐angiogenic drugs between the two groups was not observed (P = 0.160). The use of anti‐angiogenic agents was slightly lower in both groups in our study, therefore the efficacy advantages of PP regimens are observed statistically. In addition, the IPASS study showed that the ORR using carboplatin–paclitaxel was only 23.5% in an EGFR mutation negative subgroup.9 The ORR of first‐line platinum‐based chemotherapy in our study is similar to that of the EGFR‐negative group in the IPASS study.
Another important finding in the current study was the relationship between smoking and survival. The median survival time for non‐smokers was 16.0 months compared to 6.3 for current or former smokers treated with PP regimens. A statistical significance was not observed in PFS of non‐smokers and current smokers treated with NPP regimens. Smoking status was a valuable predictor of a better response to first‐line platinum‐doublet chemotherapy in advanced pan‐negative NSCLC patients in the clinic. Our data was consistent with that of Igawa et al. in which smoking status was used as a predictor for pemetrexed chemotherapy regimens in wild‐type NSCLC patients.19 A possible explanation for the relationship between smoking status and survival is the expression of thymidylate synthase (TS). A study by Huang et al. demonstrated that TS expression correlated with a history of smoking, and may be the result of oxidative damage to cells caused by smoking.20 A previous study also revealed that TS expression in tumors was significantly higher in smokers than in non‐smokers.21 Furthermore, Giovannetti et al. found that the reduced activity of pemetrexed correlated with high TS expression.22 Huang et al. revealed that DFS and OS in lung adenocarcinoma patients with high TS expression in tissues were significantly shorter compared to patients with low TS expression.20
In summary, our study showed several improvements compared with the IPASS study. First, we analyzed the status of three common driver oncogenes in patients with advanced non‐Sq NSCLC: EGFR and KRAS mutations, and ALK rearrangements. Second, we demonstrated that a simple clinical indicator may help guide patient selection and therapeutic optimization. For patients with advanced pan‐negative non‐Sq NSCLC, our study adds to the accumulating evidence that a PP compared to a NPP regimen may be preferred in patients without a smoking history, and may be used as a simple clinical indicator.
Several limitations of our study must be acknowledged. First, this is a single‐center retrospective study and our sample size may have been inadequate. Second, the study began in 2013, when ROS1 rearrangement and BRAF gene detection were yet not recommended by NCCN guidelines. However, these were presumed to have little influence on the results because of the small proportion of patients included. Third, we did not test the TS expression levels of tumors and thus failed to gather information on the relationship between TS expression and smoking status. A large‐scale prospective study is warranted in future.
Although NCCN guidelines recommend platinum‐based first‐line chemotherapy combined with anti‐angiogenic drugs for patients with pan‐negative NSCLC, for economic reasons, the use of anti‐angiogenic drugs has not been widely accepted in China, thus limiting the therapeutic options for such patients in a clinical setting. With the development of comprehensive molecular profiling of NSCLC, increased driver mutations will continue to be revealed. We look forward to improving the survival benefit via new therapeutic targets and novel targeted drugs in such patients. Meanwhile, immunotherapies are innovative options for pan‐negative NSCLC patients. Researchers continue to pursue reliable predictive biomarkers of immune‐checkpoint inhibitors to deliver more precise diagnosis and customized treatment. New targets, novel targeted drugs, and immunotherapy will become increasingly interesting topics for the treatment of pan‐negative NSCLC patients in the future.
Disclosure
No authors report any conflict of interest.
Contributor Information
Jianming Ying, Email: jmying@hotmail.com.
Yan Wang, Email: wangyanyifu@126.com.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin 2017; 67: 7–30. [DOI] [PubMed] [Google Scholar]
- 2. Chen W, Zheng R, Baade PD et al Cancer statistics in China, 2015. CA Cancer J Clin 2016; 66: 115–32. [DOI] [PubMed] [Google Scholar]
- 3. Zou XN, Lin DM, Wan X. Histological subtypes of lung cancer in Chinese males from 2000 to 2012. Biomed Environ Sci 2014; 27: 3–9. [DOI] [PubMed] [Google Scholar]
- 4. Zhou C. Lung cancer molecular epidemiology in China: Recent trends. Transl Lung Cancer Res 2014; 3: 270–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fossella F, Pereira JR, von Pawel J et al Randomized, multinational, phase III study of docetaxel plus platinum combinations versus vinorelbine plus cisplatin for advanced non‐small‐cell lung cancer: The TAX 326 study group. J Clin Oncol 2003; 21: 3016–24. [DOI] [PubMed] [Google Scholar]
- 6. Smit EF, van Meerbeeck JP, Lianes P et al Three‐arm randomized study of two cisplatin‐based regimens and paclitaxel plus gemcitabine in advanced non‐small‐cell lung cancer: A phase III trial of the European Organization for Research and Treatment of Cancer Lung Cancer Group‐‐EORTC 08975. J Clin Oncol 2003; 21: 3909–17. [DOI] [PubMed] [Google Scholar]
- 7. Ohe Y, Ohashi Y, Kubota K et al Randomized phase III study of cisplatin plus irinotecan versus carboplatin plus paclitaxel, cisplatin plus gemcitabine, and cisplatin plus vinorelbine for advanced non‐small‐cell lung cancer: Four‐Arm Cooperative Study in Japan. Ann Oncol 2007; 18: 317–23. [DOI] [PubMed] [Google Scholar]
- 8. Scagliotti GV, Parikh P, von Pawel J et al Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy‐naive patients with advanced‐stage non‐small‐cell lung cancer. J Clin Oncol 2008; 26: 3543–51. [DOI] [PubMed] [Google Scholar]
- 9. Mok TS, Wu YL, Thongprasert S et al Gefitinib or carboplatin‐paclitaxel in pulmonay adenocarcinoma. N Engl J Med 2009; 361: 947–57. [DOI] [PubMed] [Google Scholar]
- 10. Douillard JY, Ostoros G, Cobo M et al First‐line gefitinib in Caucasian EGFR mutation‐positive NSCLC patients: A phase‐IV, open label, single‐arm study. Br J Cancer 2014; 110: 55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wu YL, Zhou C, Liam CK et al First‐line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation‐positive non‐small‐cell lung cancer: Analyses from the phase III, randomized, open‐label, ENSURE study. Ann Oncol 2015; 26: 1883–9. [DOI] [PubMed] [Google Scholar]
- 12. Rosell R, Carcereny E, Gervais R et al Spanish Lung Cancer Group in collaboration with Groupe Français de Pneumo‐Cancérologie and Associazione Italiana Oncologia Toracica Erlotinib versus standard chemotherapy as first‐line treatment for European patients with advanced EGFR mutation‐positive non‐small‐cell lung cancer (EURTAC): A multicentre, open‐label, randomised phase 3 trial. Lancet Oncol 2012; 13: 239–46. [DOI] [PubMed] [Google Scholar]
- 13. Sequist LV, Yang JC, Yamamoto N et al Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013; 31: 3327–34. [DOI] [PubMed] [Google Scholar]
- 14. Solomon BJ, Mok T, Kim DW et al First‐line crizotinib versus chemotherapy in ALK‐positive lung cancer. N Engl J Med 2014; 371: 2167–77. [DOI] [PubMed] [Google Scholar]
- 15. Hirsch FR, Bunn PA Jr. EGFR testing in lung cancer is ready for prime time. Lancet Oncol 2009; 10: 432–3. [DOI] [PubMed] [Google Scholar]
- 16. Sasaki T, Rodig SJ, Chirieac LR, Jänne PA. The biology and treatment of EML4‐ALK non‐small cell lung cancer. Eur J Cancer 2010; 46: 1773–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Koivunen JP, Mermel C, Zejnullahu K et al EML4‐ALK fusion gene and efficacy of an ALK kinase inhibitor in lung cancer. Clin Cancer Res 2008; 14: 4275–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shaw AT, Yeap BY, Mino‐Kenudson M et al Clinical features and outcome of patients with non‐small‐cell lung cancer who harbor EML4‐ALK. J Clin Oncol 2009; 27: 4247–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Igawa S, Sasaki J, Otani S et al Smoking history as a predictor of pemetrexed monotherapy in patients with non‐squamous small cell lung cancer. Oncology 2016; 91: 41–7. [DOI] [PubMed] [Google Scholar]
- 20. Huang Y, Guo X, Wang H et al Predictive value of thymidylate synthase for the prognosis and survival of lung adenocarinoma patients. Oncol Lett 2015; 9: 252–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Giovannetti E, Mey V, Nannizzi S et al Cellular and pharmacogenetics foundation of synergistic interaction of pemetrexed and gemcitabine in human non‐small cell lung cancer cells. Mol Pharmacol 2005; 68: 110–8. [DOI] [PubMed] [Google Scholar]
- 22. Sun JM, Han J, Ahn JS, Park K, Ahn MJ. Significance of thymidylate synthase and thyroid transcription factor 1 expression in patients with nonsquamous non‐small cell lung cancer treated with pemetrexed‐based chemotherapy. J Thorac Oncol 2011; 6: 1392–9. [DOI] [PubMed] [Google Scholar]


