Abstract
Background
Previous studies have elucidated that FOXM1 may predict poor prognosis in patients with multiple solid malignant tumors. In this study we explored the differential expression of FOXM1 in stage IIA esophageal squamous cell carcinoma (ESCC) and investigated its prognostic value.
Methods
Immunohistochemistry (IHC) and Western blot were used to detect FOXM1 expression in ESCC. Correlations between FOXM1 expression and clinicopathological variables, and five‐year lymphatic metastatic recurrence (LMR) and overall survival (OS) of patients were analyzed.
Results
FOXM1 was aberrantly expressed in ESCC. Statistical analysis revealed a close relationship between FOXM1 expression and tumor size (P = 0.024), depth of invasion (P = 0.048), and degree of differentiation (P = 0.043). The five‐year LMR of patients in the FOXM1 overexpression group was significantly increased compared to the low expression group (P = 0.001). The five‐year OS of patients in the FOXM1 overexpression group was significantly reduced compared to the low expression group (P = 0.007). Log‐rank tests demonstrated that large tumor size (P = 0.044), poor differentiation degree (P = 0.005), deep invasion (P = 0.000), and FOXM1 overexpression (P = 0.007) may indicate poor prognosis in stage IIA ESCC. Cox multivariate regression analysis revealed that all of these variables were independent predictors of unfavorable outcome (P < 0.05).
Conclusion
FOXM1 could be a predictor of lymphatic metastatic recurrence in stage IIA ESCC after Ivor Lewis esophagectomy.
Keywords: Esophageal squamous cell carcinoma, FOXM1, lymphatic metastatic recurrence, prognosis
Introduction
Esophageal carcinoma, a common cancer of the digestive tract, is the sixth leading cause of cancer‐related death worldwide.1, 2 In China, the mortality rate of esophageal cancer ranked fourth among all cancers, with standardized mortality rates steadily increasing by an average of 1.06/105 per year from 1991 to 2012. In 2012, the mortality rate was 16.77/105 and the standardized mortality rate was 7.75/105.3 There are two main histopathological types of this disease: esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma. ESCC is the predominant type in China, comprising approximately 90% of all diagnosed esophageal cancer cases in some areas.4, 5 Given improvements at diagnostic level, an increasing number of individuals are now diagnosed at earlier stage; however, prognosis is still not optimistic, with a five‐year overall survival (OS) rate of only 20–30%.5, 6, 7 Lymphatic metastatic recurrence (LMR) is the main reason for treatment failure and poor prognosis.8, 9, 10 Even in stage IIA ESCC, the five‐year survival rate is only 30–50% after complete resection, and many patients may eventually die from LMR.11 To improve the OS of ESCC patients, it is necessary to control locoregional LMR. Several indexes are associated with LMR, Including tumor node metastasis (TNM) staging, but the specificity and sensitivity cannot satisfy our clinical requirements. Therefore, we still need to investigate useful biological markers to predict the risk of LMR for individuals with ESCC to subsequently improve prognosis.
FOXM1 is an important transcriptional factor of the Forkhead box family, characterized by an evolutionarily conserved DNA binding domain called the winged‐helix domain.12, 13 Previous studies have demonstrated that FOXM1 is aberrantly expressed in various malignant tumors, such as prostate and breast adenocarcinoma, cervical and nasopharyngeal carcinoma, and ESCC.14, 15, 16, 17, 18 FOXM1 regulates the cell cycle by influencing the phase transitions from G1 to S and G2 to M and participates in cell proliferation.19, 20, 21 In addition, FOXM1 also participates in the migration and invasion of several tumor cells and promotes cancer metastasis.22, 23, 24 Recently, studies have demonstrated that FOXM1 is overexpressed in ESCC and may predict poor prognosis in patients.25 Nevertheless, the association between FOXM1 expression and LMR still needs to be elucidated.
In the present study we sought to validate the relationships between FOXM1 expression and clinicopathological parameters, and the five‐year LMR and OS rates of stage IIA ESCC patients after Ivor Lewis esophagectomy with two‐field lymphadenectomy. We explored whether FOXM1 can predict the LMR of patients.
Methods
Patients and specimens
Eight pairs of frozen ESCC tissues and corresponding noncancerous esophageal tissues (> 5 cm from the margin of tumor) were collected from Shandong Provincial Hospital Affiliated to Shandong University from June 2016 to 2017. In addition, 178 formalin‐fixed paraffin‐embedded tumor specimens were harvested from patients who underwent Ivor Lewis esophagectomy with two‐field lymphadenectomy from January 2007 to December 2009.
The inclusion criteria were: (i) curative (R0) resection of mid‐thoracic ESCC; (ii) > 12 (13–27) lymph nodes dissected; (iii) no administration of preoperative adjuvant chemotherapy or postoperative treatment; and (iv) all patients were pathologically diagnosed as stage IIA (T2‐3N0M0) after surgery. Patients with hematogenous recurrence were excluded. Detailed clinical data of the patients is presented in Table 1.
Table 1.
Correlation of FOXM1 expression with clinicopathological features of 178 stage IIA ESCC patients
| Parameters | Cases (178) | FOXM1 | P † | Five‐year OS (%) | P ‡ | Five‐year LMR (%) | P ‡ | |
|---|---|---|---|---|---|---|---|---|
| Low expression | Overexpression | |||||||
| Gender | 0.543 | 0.240 | 0.317 | |||||
| Male | 99 | 40 | 59 | 49.4 | 64.6 | |||
| Female | 79 | 36 | 43 | 38.4 | 55.7 | |||
| Age (years) | 0.548 | 0.779 | 0.582 | |||||
| > 50 | 96 | 43 | 53 | 40.6 | 64.6 | |||
| ≤ 50 | 82 | 33 | 49 | 46.3 | 56.1 | |||
| Tumor Size (cm) | 0.024 | 0.044 | 0.020 | |||||
| < 3 | 83 | 43 | 40 | 53.0 | 49.4 | |||
| ≥ 3 | 95 | 33 | 62 | 34.7 | 70.5 | |||
| Depth of invasion | 0.048 | 0.000 | 0.000 | |||||
| T2 | 80 | 41 | 39 | 58.8 | 42.5 | |||
| T3 | 98 | 35 | 63 | 30.6 | 75.5 | |||
| Differentiation degree | 0.043 | 0.005 | 0.014 | |||||
| Low | 67 | 22 | 45 | 31.3 | 70.1 | |||
| Mid‐high | 111 | 54 | 57 | 50.5 | 55.0 | |||
| FOXM1 | 0.007 | 0.001 | ||||||
| Low expression | 76 | 52.6 | 47.4 | |||||
| Overexpression | 102 | 36.3 | 70.6 | |||||
Bold values indicate P < 0.05.
χ2 test.
Log‐rank test.
ESCC, esophageal squamous cell carcinoma; FOXM1, Forkhead box M1; LMR, lymphatic metastatic recurrence.
Approval was obtained from the Research Ethic Committee of Shandong Provincial Hospital Affiliated to Shandong University. Informed consent was obtained from each patient or their relatives.
Immunohistochemistry (IHC) and immunohistochemical score (IHS)
Immunohistochemistry (IHC) was used to examine FOXM1 expression using the streptavidin‐peroxidase method. Anti‐FOXM1 rabbit polyclonal antibodies (Abcam, Cambridge, MA, USA) were diluted at 1:100. The primary antibodies were replaced by phosphate‐buffered saline (PBS) as a negative control. The secondary biotinylated antibody kit was purchased from ZSGB Biotech (Beijing, China).
Two pathologists who were blinded to the clinicopathological data evaluated all sections. The immunohistochemical score (IHS) was measured by combining the quantity score (percentage of positive stained cells in five fields) with the staining intensity score. The quantity score was rated as follows: 0 (< 5%), 1 (5–25%), 2 (26–50%), and 3 (> 50%). The staining intensity was scored as 0 (absent), 1 (weak), 2 (moderate), and 3 (strong). The total score was classified into low expression (0–4) and overexpression (5–9).
Western blot analysis
Protein was extracted from tissue samples, and the concentration was determined using a bicinchoninic acid kit (Thermo Fisher Scientific, Waltham, MA, USA). Equal amounts of protein (40 μg) were separated by 10% sodium dodecyl sulfate‐polyacrylamide gel electrophoresis and transferred onto polyvinylidene fluoride membrane filters (Millipore, Billerica, MA, USA). Briefly, 5% non‐fat dry milk was used to block the non‐specific binding. Membranes were incubated overnight at 4°C with primary antibodies anti‐FOXM1 (1:500) and anti‐glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) (1: 5000; Abcam, Cambridge, MA, USA). After washing, the membranes were incubated with corresponding secondary antibodies (horseradish peroxidase‐conjugated goat anti‐rabbit antibodies, 1: 5000; ZSGB Biotech, Beijing, China) for one hour at room temperature. Finally, the protein levels were quantified by an enhanced chemiluminescence (ECL) detection system (Amersham Imager 600; General Electric, Fairfield, CT, USA).
Follow‐up
All patients underwent a routine examination every three to six months. The examinations mainly consisted of physical examination, B ultrasonography of the abdomen, chest and upper abdomen computed tomography (CT) scan, positron emission tomography (PET), bone scintigraphy, and cerebral CT. If progressive lymph node enlargement was observed in postoperative imaging, biopsy was the first choice to identify whether metastatic lymph node recurrence was involved. When mediastinal lymph node enlargement was identified in CT scans but a biopsy was difficult to obtain, a PET‐CT scan was taken. Follow‐up of this study ended in December 2015; the longest follow‐up period was six years.
Statistical analysis
The χ2 test was used to analyze the relationship between FOXM1 expression and clinicopathological variables. Survival and recurrence curves were calculated using the Kaplan–Meier method. A log‐rank test was used to compare the differences between FOXM1 expression and the survival and recurrence status of patients. Cox regression analysis was used to evaluate independent prognostic factors. A statistically significant difference was defined with a two‐tailed P value < 0.05. All statistical analyses were performed using SPSS version 19.0 (IBM Corp., Armonk, NY, USA).
Results
FOXM1 overexpression in esophageal squamous cell carcinoma (ESCC)
Immunohistochemical assays were used to detect FOXM1 expression in ESCC (Fig 1). FOXM1 expression was mainly observed in the cytoplasm as a yellow or brownish‐yellow stain. In some cases it was also detected in the nuclei of tumor cells. Low or undetected FOXM1 expression was observed in noncancerous tissues. According to IHS standard, we divided all patients into two groups: 102 cases (57.3%) of overexpression, and 76 (42.7%) of low expression. We also validated the differential expression of FOXM1 by Western blot analysis in eight pairs of ESCC and noncancerous tissues. FOXM1 was obviously overexpressed in ESCC compared to noncancerous tissues (P < 0.05), which was consistent with IHC results (Fig 2).
Figure 1.
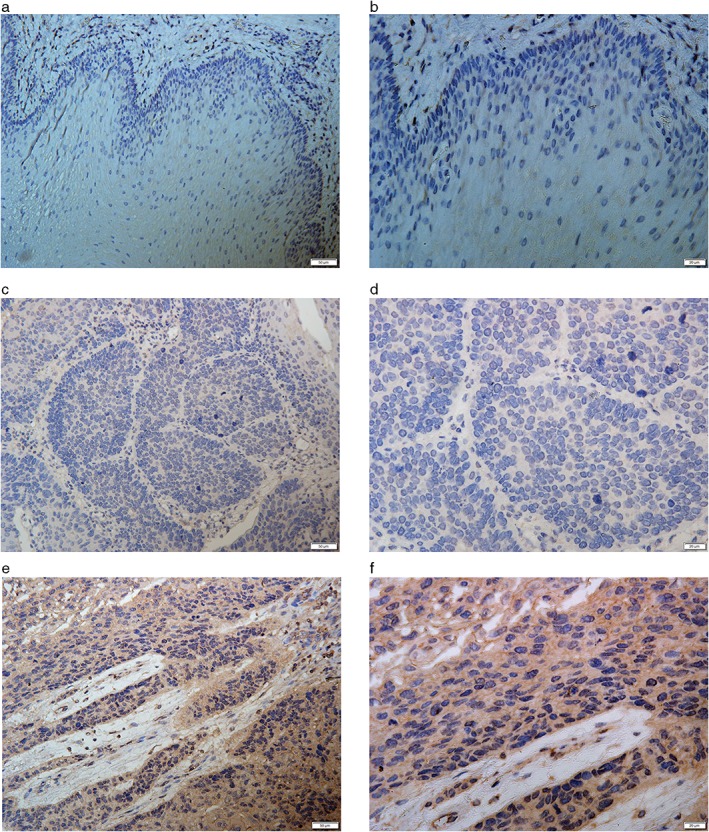
Immunohistochemical staining of FOXM1 in esophageal squamous cell carcinoma (ESCC) and noncancerous tissues. Representative (a,b) negative, (c,d) low, and (e,f) strong positive expression of FOXM1 in ESCC tissue (×200, ×400, respectively).
Figure 2.
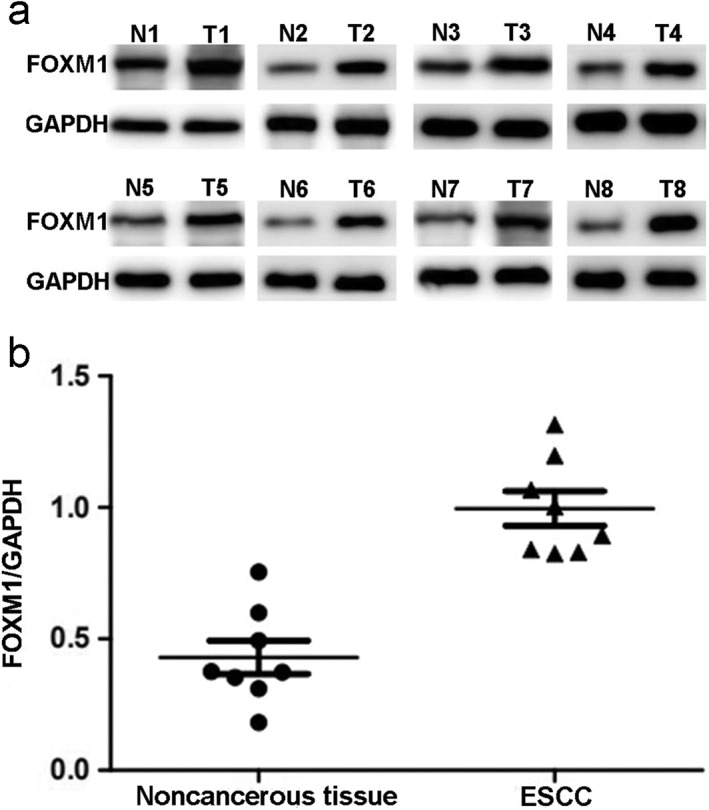
Western blot analysis of FOXM1 protein expression in noncancerous and tumor tissues. (a) Bands of FOXM1 and glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) and (b) quantitative analysis of FOXM1/GAPDH in noncancerous and tumor tissues.
FOXM1 expression and clinicopathological parameters
We used the chi‐square test to detect the relationship between FOXM1 expression and the clinicopathological parameters of patients. FOXM1 expression was significantly associated with tumor size (P = 0.024), tumor differentiation degree (P = 0.043), and depth of invasion (P = 0.048).
FOXM1 expression and prognosis
After thorough follow‐up, 108 cases (60.7%) were diagnosed as LMR within five years. The five‐year OS rate of 178 patients was 41.0%. The median survival time was 49.5 months (9–72). The five‐year LMR and OS rates of patients in the overexpression group were 70.6% and 36.3%, respectively. In the low expression group, the five‐year LMR rate was only 47.4%, and the five‐year OS rate reached 52.6%. Kaplan–Meier analysis revealed that the five‐year OS rate was obviously reduced in patients with FOXM1 overexpression (Fig 3). In contrast, the five‐year LMR rate was correspondingly increased in this group. Univariate analysis showed that tumor size (P = 0.044), depth of invasion (P = 0.000), differentiation degree (P = 0.005), and FOXM1 expression (P = 0.007) were significant prognostic factors. In contrast, gender (P = 0.240) and age (P = 0.779) did not reach statistical significance. To exclude confounding factors, Cox proportional hazards model analysis was performed. Multivariate analysis revealed that tumor size (P = 0.034), differentiation degree (P = 0.024), depth of invasion (P = 0.000), and FOXM1 expression (P = 0.016) were all independent prognostic factors (Table 2). These results indicated that stage IIA ESCC patients with FOXM1 overexpression tend to exhibit a high risk of LMR.
Figure 3.
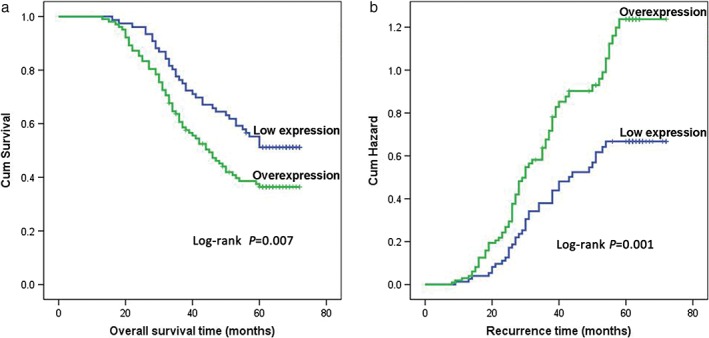
Kaplan–Meier analysis and log‐rank test of FOXM1 for five‐year (a) overall survival and (b) lymphatic metastatic recurrence of 178 stage IIA esophageal squamous cell carcinoma (ESCC) patients.
Table 2.
Multivariate Cox regression analysis of prognostic factors of 178 stage IIA ESCC patients
| Parameters | Five‐year OS | Five‐year LMR | ||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Gender (male vs. female) | 1.271 (0.845–1.912) | 0.250 | 1.246 (0.841–1.846) | 0.273 |
| Age (> 50 vs. ≤ 50 years) | 0.865 (0.578–1.293) | 0.479 | 0.837 (0.567–1.236) | 0.372 |
| Tumor size (≥ 3 cm vs. < 3 cm) | 0.472 (0.236–0.946) | 0.034 | 0.445 (0.233–0.852) | 0.014 |
| T status (T2 vs. T3) | 3.781 (1.847–7.739) | 0.000 | 4.325 (2.200–8.503) | 0.000 |
| Differentiation degree (low vs. mid‐high) | 1.591 (1.064–2.377) | 0.024 | 1.483 (1.003–2.192) | 0.048 |
| FOXM1 (low expression vs. overexpression) | 1.661 (1.100–2.508) | 0.016 | 1.877 (1.252–2.813) | 0.002 |
Bold values indicate P < 0.05. CI, confidence interval; ESCC, esophageal squamous cell carcinoma; HR, hazard ratio; LMR, lymphatic metastatic recurrence; OS, overall survival.
Discussion
It is widely accepted that surgery is the first choice for the treatment of localized ESCC.26, 27 However, the overall survival of ESCC patients remains unsatisfactory. Even in stage IIA ESCC, the five‐year OS rate is only 30–50%. Locoregional LMR is the main recurrence pattern and the most common reason for treatment failure. In contrast to other digestive tract components, the esophagus has its own unique histological structure involving rich lymphatic drainage and anastomosis in the submucosa, which may lead to early lymph node metastasis and postoperative LMR.28, 29, 30 In 1991, Yoshinaka et al. reported that approximately 47% of ESCC patients experienced lymphatic metastasis when the submucosa (T1b) was invaded.31
Evidence indicates that postoperative adjuvant chemotherapy could significantly improve the five‐year survival rate in advanced tumors, but adjuvant chemotherapy has not been demonstrated to be advantageous in early stage ESCC because of significant adverse effects.32, 33, 34 Whether adjuvant therapy should be administered to stage IIA ESCC patients remains controversial. According to the National Comprehensive Cancer Network (NCCN) esophageal cancer guidelines, patients may not accept adjuvant therapy after complete tumor resection.35 In China, patients tend to receive primary surgery if tumors can be completely resected. Ivor Lewis esophagectomy with two‐field lymph node dissection is the main surgical modality for early stage ESCC.27, 36 Thus, pathological stage IIA ESCC patients typically do not receive adjuvant therapy.
However, previous research has demonstrated that some individuals experience postoperative LMR, which is the main cause of death of ESCC patients.37 Therefore, to improve OS, it is crucial to determine the indicators to predict prognosis in stage IIA ESCC patients and administer treatment according to the different prognostic indications. At present, the commonly used TNM staging cannot accurately and sensitively evaluate the prognosis of ESCC patients. Therefore, we argue that it is of great significance to investigate biological markers to predict the potential for recurrence and administer adjuvant therapy to prevent lymphatic metastasis and further improve prognosis.
Upregulation of FOXM1 is related to tumorigenesis and the progression of various solid tumors and might serve as a novel prognostic biomarker.13, 38 Takata et al. reported that ESCC patients with FOXM1 positive expression exhibited poor prognosis.25 Hui et al. reported that low cytoplasmic FOXM1 levels correlated with early stage ESCC, and nuclear FOXM1 expression was observed in young ESCC patients.14 However, reports about the relationship between FOXM1 expression and postoperative LMR in stage IIA ESCC patients are not available. In the present study, we confirmed that FOXM1 is overexpressed in ESCC. In addition, stage IIA ESCC patients with FOXM1 overexpression may exhibit a high risk of LMR and poor prognosis after Ivor Lewis esophagectomy with two‐field lymph node dissection.
The underlying mechanism of FOXM1‐mediated tumor invasion and metastasis remains unclear. Tumor metastasis is a multistep and complex process involving local invasion, intravasation, extravasation, formation of micrometastases, and colonization.39 The epithelial‐mesenchymal transition (EMT) and matrix metalloproteinases (MMPs) exert a profound influence on the processes of tumor invasion and metastasis. During the process of EMT, tumor cells acquire a more peculiar mesenchymal phenotype and gain the ability to metastasize.40, 41 MMP‐2 and MMP‐9 are directly associated with angiogenesis and degradation of basement membrane collagen, leading to metastasis.42 Recently, numerous studies have demonstrated that FOXM1 plays an important role in the activation of EMT and MMPs.23, 24, 43, 44 Wang et al. reported that downregulation of FOXM1 by small interfering RNA could inactivate MMP‐2, MMP‐9, and VEGF, subsequently resulting in the inhibition of cell growth, migration, invasion, angiogenesis, and metastasis of pancreatic cancer.45 They also elucidated that FOXM1 downregulation may attenuate the proliferation and aggressiveness of breast cancer cells by modulating uPA, uPAR, MMP‐2, MMP‐9, and VEGF expression.18 In subsequent studies, we will attempt to elucidate the detailed mechanisms of FOXM1 for regulating ESCC metastasis and detect the associations between FOXM1 expression and EMT markers and MMPs in vitro. In addition, xenograft mouse models can be used to validate the role of FOXM1 in tumor metastasis in vivo.
Some limitations of this study should be noted. First, the number of samples is relatively small. In addition, all participants are Han Chinese with a similar genetic background. Thus, a larger sample size and multicenter randomized studies are needed to confirm the prognostic value of FOXM1.
In conclusion, we identified that FOXM1 is aberrantly expressed in ESCC. Patients with FOXM1 overexpression may exhibit an increased risk of LMR and poor prognosis. FOXM1 may serve as a potential biomarker to predict the LMR in stage IIA ESCC after Ivor Lewis esophagectomy with two‐field lymph node dissection. Some regimes, including postoperative adjuvant therapy and targeted drug treatment, may serve as complementary treatments to control locoregional LMR and subsequently improve the survival of patients with an otherwise poor prognosis.
Disclosure
No authors report any conflict of interest.
Acknowledgments
Shandong Provincial Natural Science Foundation, China (ZR2017MH089) supported the study.
References
- 1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet‐Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65: 87–108. [DOI] [PubMed] [Google Scholar]
- 2. Global Burden of Disease Cancer Collaboration . The global burden of cancer 2013. JAMA Oncol 2015; 1: 505–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tang WR, Fang JY, Wu KS, Shi XJ, Luo JY, Lin K. Epidemiological characteristics and prediction of esophageal cancer mortality in China from 1991 to 2012. Asian Pac J Cancer Prev 2014; 15: 6929–34. [DOI] [PubMed] [Google Scholar]
- 4. Lin Y, Totsuka Y, He Y et al Epidemiology of esophageal cancer in Japan and China. J Epidemiol 2013; 23: 233–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Akhtar J, Wang Z, Yu C, Zhang ZP, Bi MM. STMN‐1 gene: A predictor of survival in stage IIa esophageal squamous cell carcinoma after Ivor‐Lewis esophagectomy? Ann Surg Oncol 2014; 21: 315–21. [DOI] [PubMed] [Google Scholar]
- 6. Sun Z, Ji N, Bi M, Zhang Z, Liu X, Wang Z. Negative expression of PTEN identifies high risk for lymphatic‐related metastasis in human esophageal squamous cell carcinoma. Oncol Rep 2015; 33: 3024–32. [DOI] [PubMed] [Google Scholar]
- 7. Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: Defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol 2006; 24: 2137–50. [DOI] [PubMed] [Google Scholar]
- 8. Shen WB, Gao HM, Zhu SC, Li YM, Li SG, Xu JR. Analysis of the causes of failure after radical surgery in patients with PT3N0M0 thoracic esophageal squamous cell carcinoma and consideration of postoperative radiotherapy. World J Surg Oncol 2017; 15: 192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen G, Wang Z, Liu XY, Liu FY. Recurrence pattern of squamous cell carcinoma in the middle thoracic esophagus after modified Ivor‐Lewis esophagectomy. World J Surg 2007; 31: 1107–14. [DOI] [PubMed] [Google Scholar]
- 10. Liu Q, Cai XW, Wu B, Zhu ZF, Chen HQ, Fu XL. Patterns of failure after radical surgery among patients with thoracic esophageal squamous cell carcinoma: Implications for the clinical target volume design of postoperative radiotherapy. PLoS One 2014; 9: e97225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen H, Wang Z, Yang Z, Shang B, Liu X, Chen G. Prospective study of adjuvant radiotherapy on preventing lymph node metastasis after Ivor‐lewis esophagectomy in esophageal cancer. Ann Surg Oncol 2013; 20: 2721–6. [DOI] [PubMed] [Google Scholar]
- 12. Katoh M, Katoh M. Human FOX gene family (Review). Int J Oncol 2004; 25: 1495–500. [PubMed] [Google Scholar]
- 13. Gartel AL. A new target for proteasome inhibitors: FoxM1. Expert Opin Investig Drugs 2010; 19: 235–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hui MK, Chan KW, Luk JM et al Cytoplasmic Forkhead box M1 (FoxM1) in esophageal squamous cell carcinoma significantly correlates with pathological disease stage. World J Surg 2012; 36: 90–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen Y, Liu Y, Ni H, Ding C, Zhang X, Zhang Z. FoxM1 overexpression promotes cell proliferation and migration and inhibits apoptosis in hypopharyngeal squamous cell carcinoma resulting in poor clinical prognosis. Int J Oncol 2017; 51: 1045–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li XR, Chu HJ, Lv T, Wang L, Kong SF, Dai SZ. miR‐342‐3p suppresses proliferation, migration and invasion by targeting FOXM1 in human cervical cancer. FEBS Lett 2014; 588: 3298–307. [DOI] [PubMed] [Google Scholar]
- 17. Cai Y, Balli D, Ustiyan V et al Foxm1 expression in prostate epithelial cells is essential for prostate carcinogenesis. J Biol Chem 2013; 288: 22527–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ahmad A, Wang Z, Kong D et al FoxM1 down‐regulation leads to inhibition of proliferation, migration and invasion of breast cancer cells through the modulation of extra‐cellular matrix degrading factors. (Retracted in Breast Cancer Res Treat 2016; 158: 607). Breast Cancer Res Treat 2010; 122: 337–46. [DOI] [PubMed] [Google Scholar]
- 19. Wang IC, Chen YJ, Hughes D et al Forkhead box M1 regulates the transcriptional network of genes essential for mitotic progression and genes encoding the SCF (Skp2‐Cks1) ubiquitin ligase. Mol Cell Biol 2005; 25: 10875–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Halasi M, Gartel AL. FOX(M1) news‐‐it is cancer. Mol Cancer Ther 2013; 12: 245–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sullivan C, Liu Y, Shen J et al Novel interactions between FOXM1 and CDC25A regulate the cell cycle. PLoS One 2012; 7: e51277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wei P, Zhang N, Wang Y et al FOXM1 promotes lung adenocarcinoma invasion and metastasis by upregulating SNAIL. Int J Biol Sci 2015; 11: 186–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Meng FD, Wei JC, Qu K et al FoxM1 overexpression promotes epithelial‐mesenchymal transition and metastasis of hepatocellular carcinoma. World J Gastroenterol 2015; 21: 196–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jiang L, Wang P, Chen H. Overexpression of FOXM1 is associated with metastases of nasopharyngeal carcinoma. Ups J Med Sci 2014; 119: 324–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Takata A, Takiguchi S, Okada K et al Clinicopathological and prognostic significance of FOXM1 expression in esophageal squamous cell carcinoma. Anticancer Res 2014; 34: 2427–32. [PubMed] [Google Scholar]
- 26. Mirinezhad SK, Jangjoo AG, Seyednejad F et al Impact of tumor length on survival for patients with resected esophageal cancer. Asian Pac J Cancer Prev 2014; 15: 691–4. [DOI] [PubMed] [Google Scholar]
- 27. Pauthner M, Haist T, Mann M, Lorenz D. Surgical therapy of early carcinoma of the esophagus. Viszeralmedizin 2015; 31: 326–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tachimori Y. Pattern of lymph node metastases of squamous cell esophageal cancer based on the anatomical lymphatic drainage system: Efficacy of lymph node dissection according to tumor location. J Thorac Dis 2017; 9 (Suppl 8): S724–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jia Y, Xiao Z, Jiang W, Chen G, Wang Z. Overexpression of IFITM3 predicts poor prognosis in stage IIA esophageal squamous cell carcinoma after Ivor Lewis esophagectomy. Thorac Cancer 2017; 8: 592–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ji X, Cai J, Chen Y, Chen LQ. Lymphatic spreading and lymphadenectomy for esophageal carcinoma. World J Gastrointest Surg 2016; 8: 90–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yoshinaka H, Shimazu H, Fukumoto T, Baba M. Superficial esophageal carcinoma: A clinicopathological review of 59 cases. Am J Gastroenterol 1991; 86: 1413–8. [PubMed] [Google Scholar]
- 32. Qi Z, Wang YX, Yang Q, Li J, Yao JF, He M. [Survival and the value of adjuvant chemotherapy in esophageal squamous cell carcinoma patients with lymphatic metastasis]. Zhonghua Zhong Liu Za Zhi 2017; 39: 628–33. (In Chinese.). [DOI] [PubMed] [Google Scholar]
- 33. Lyu X, Huang J, Mao Y et al Adjuvant chemotherapy after esophagectomy: Is there a role in the treatment of the lymph node positive thoracic esophageal squamous cell carcinoma? J Surg Oncol 2014; 110: 864–8. [DOI] [PubMed] [Google Scholar]
- 34. Guo X, Mao T, Gu Z, Ji C, Fang W. Clinical study on postoperative recurrence in patients with pN1 esophageal squamous cell carcinoma. Thorac Cancer 2015; 6: 146–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ajani JA, D'Amico TA, Almhanna K et al Esophageal and esophagogastric junction cancers, version 1.2015. J Natl Compr Canc Netw 2015; 13: 194–227. [DOI] [PubMed] [Google Scholar]
- 36. Nozoe T, Kakeji Y, Baba H, Maehara Y. Two‐field lymph‐node dissection may be enough to treat patients with submucosal squamous cell carcinoma of the thoracic esophagus. Dis Esophagus 2005; 18: 226–9. [DOI] [PubMed] [Google Scholar]
- 37. Gandour‐Edwards R, Lara PN Jr, Folkins AK et al Does HER2/neu expression provide prognostic information in patients with advanced urothelial carcinoma? Cancer 2002; 95: 1009–15. [DOI] [PubMed] [Google Scholar]
- 38. Dai J, Yang L, Wang J, Xiao Y, Ruan Q. Prognostic value of FOXM1 in patients with malignant solid tumor: A meta‐analysis and system review. Dis Markers 2015; 2015: 352478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shoji S, Tang XY, Sato H, Usui Y, Uchida T, Terachi T. Metastin has potential as a suitable biomarker and novel effective therapy for cancer metastasis (Review). Oncol Lett 2010; 1: 783–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Said N, Theodorescu D. Permissive role of endothelin receptors in tumor metastasis. Life Sci 2012; 91: 522–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hugo H, Ackland ML, Blick T et al Epithelial‐‐mesenchymal and mesenchymal‐‐epithelial transitions in carcinoma progression. J Cell Physiol 2007; 213: 374–83. [DOI] [PubMed] [Google Scholar]
- 42. Wang Z, Ahmad A, Li Y, Banerjee S, Kong D, Sarkar FH. Forkhead box M1 transcription factor: A novel target for cancer therapy. Cancer Treat Rev 2010; 36: 151–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhang J, Chen XY, Huang KJ et al Expression of FoxM1 and the EMT‐associated protein E‐cadherin in gastric cancer and its clinical significance. Oncol Lett 2016; 12: 2445–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fei BY, He X, Ma J, Zhang M, Chai R. FoxM1 is associated with metastasis in colorectal cancer through induction of the epithelial‐mesenchymal transition. Oncol Lett 2017; 14: 6553–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wang Z, Banerjee S, Kong D, Li Y, Sarkar FH. Down‐regulation of Forkhead box M1 transcription factor leads to the inhibition of invasion and angiogenesis of pancreatic cancer cells. Cancer Res 2007; 67: 8293–300. [DOI] [PubMed] [Google Scholar]


