Abstract
Intradural extramedullary spinal cord metastases in lung cancer are rarely reported, but are a disastrous event because of severe clinical symptoms and poor prognosis. Herein, we report a case of a lung cancer patient with ALK rearrangement who experienced brain, leptomeningeal, and intradural extramedullary spinal cord metastases after developing resistance to crizotinib. After ceritinib therapy, his clinical symptoms improved and magnetic resonance imaging revealed that the intradural extramedullary lesions had reduced.
Keywords: ALK rearrangement, ceritinib, intradural extramedullary spinal cord metastases, leptomeningeal metastases, lung adenocarcinoma
Introduction
Intradural extramedullary spinal cord metastases (IESCM),1 also known as spinal cord pial metastases, are a special clinical manifestation associated with leptomeningeal metastases (LM).2 Tumor infiltration is prominent at the surface of the spinal cord and the nerve roots, with spinal symptoms, such as radiculopathies, myelopathies, or cauda equina syndrome. IESCM in lung cancer is seldom reported. We report a case of ALK gene rearrangement in a lung cancer patient experiencing IESCM and the clinical efficacy of ceritinib therapy as treatment.
Case presentation
A 56‐year‐old Chinese male non‐smoker presented to a respiratory clinic with a one‐month history of enlarged right supraclavicular lymph nodes on 8 July 2014. Right supraclavicular lymph node biopsy and computed tomography (CT)‐guided core needle biopsy of the lung revealed that the tumor histology was lung adenocarcinoma. Immunohistochemistry (IHC) using ALK (D5F3) CDx assay (Ventana Medical Systems, Tuscon, AZ, USA) and ALK fluorescence in situ hybridization (FISH) showed that his tumor was ALK rearrangement positive, while EGFR was wild‐type tested via the amplification refractory mutation system. The patient was diagnosed with lung adenocarcinoma with liver and bone metastases (ALK rearrangement, cT2aN3M1b, stage IV). He received crizotinib therapy and achieved a partial response. Seven months later, his disease progressed and the histological diagnosis of the liver biopsy specimen by IHC was adenocarcinoma with ALK rearrangement. Unfortunately, no ALK resistance mutations were detected by targeted next‐generation sequencing. The patient was administered two cycles of pemetrexed and cisplatin therapy and experienced disease progression. One cycle of docetaxel therapy was subsequently administered, but his disease progressed again (Fig 1a,b).
Figure 1.
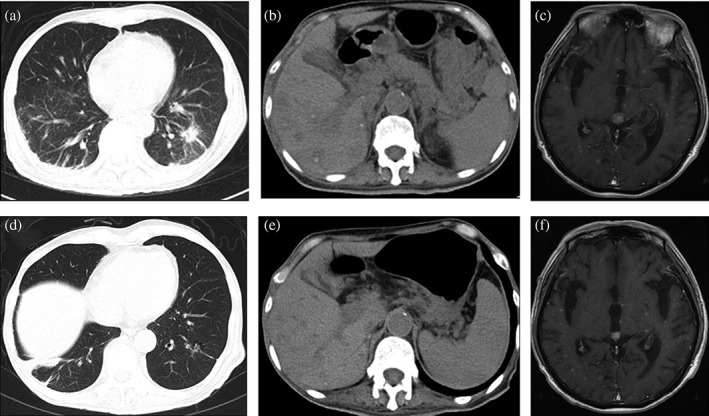
The radiographic response of the patient's primary lung tumor and metastases (a,b,c) before and (d,e,f) after ceritinib treatment. The computed tomography (CT) scan taken before ceritinib treatment showed a solid pulmonary lesion in the (a) left inferior lobe of the lung and (b) multiple liver lesions, and (c) contrast‐enhanced magnetic resonance imaging (MRI) of the head showed multiple brain metastatic lesions. The CT scan and contrast‐enhanced MRI of the head after four weeks of ceritinib treatment showed that (d) the primary lung tumor, (e) multiple liver lesions, and (f) brain metastatic lesions were obviously smaller.
By August 2015, the patient's condition had rapidly deteriorated and he experienced nausea, vomiting, diarrhea, urinary retention, and reduced bilateral lower limb muscle strength with hyperalgesia. His Eastern Cooperative Oncology Group performance status (PS) score was 4. Contrast‐enhanced magnetic resonance imaging (MRI) of the head showed multiple brain metastatic lesions with meningeal enhancement (Fig. 1c), while MRI of the spine (Fig. 2, 3a) showed multiple intradural extramedullary nodules with abnormal and diffuse abnormal enhancement of the pial lining of the spinal cord. He was diagnosed with brain metastases (BM), LM, and IESCM but refused further lumbar puncture to collect cerebrospinal fluid for further pathology because of poor PS. He was administered ceritinib therapy at a dose of 750 mg once daily and his symptoms partially improved, with a PS score of 3. The ceritinib treatment was well tolerated without grade 3–4 toxicity. After four weeks of ceritinib treatment, a partial response (PR) was observed in the patient's extracranial lesions (Fig. 1d,e) and contrast‐enhanced MRI showed improvement in the BM (Fig. 1f) and intradural extramedullary spinal cord lesions (Fig. 3b).
Figure 2.
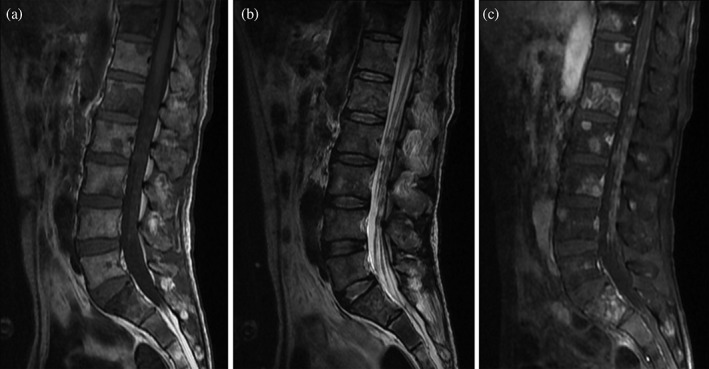
Sagittal sections of magnetic resonance imaging (MRI) of the lumbar spinal cord. T1‐weighted imaging showed (a) multiple hyperintense and T2‐weighted imaging showed (b) multiple hypointense intradural extramedullary nodules. (c) Contrast‐enhanced MRI of the lumbar spine showed multiple intradural extramedullary nodules with abnormal enhancement.
Figure 3.
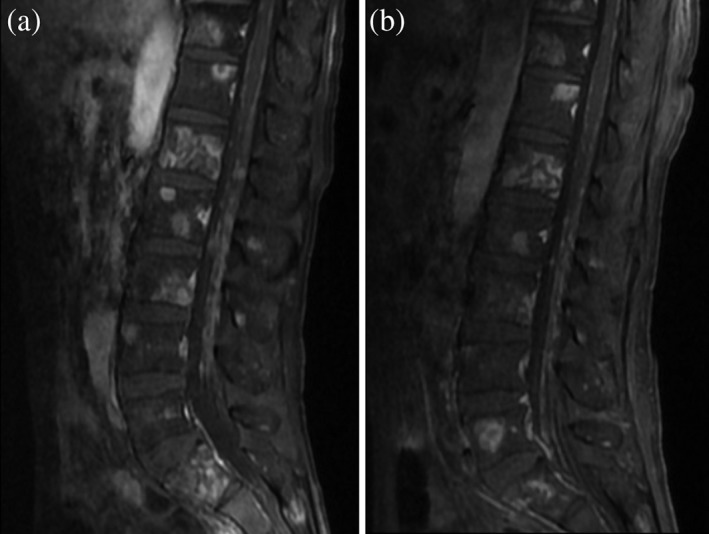
Sagittal sections of contrast‐enhanced magnetic resonance imaging (MRI) of the lumbar spinal cord (a) before and (b) after ceritinib treatment. (a) Multiple intradural extramedullary nodules with abnormal enhancement were observed before ceritinib treatment. (b) The multiple intradural extramedullary nodules improved after ceritinib treatment.
Discussion
Intradural extramedullary spinal cord metastases in lung cancer leads to severe neurological damage and has a grave prognosis. New neurologic signs and symptoms associated with the spinal cord and roots, such as radicular signs including weakness, voiding, and cauda equine problems, and focal or irradiating (radicular) neck and back pain,2, 3 are suggestive of IESCM. In the clinical diagnosis and treatment of lung cancer, oncologists should pay attention to these clinical manifestations of IESCM, as careful neurological assessment is needed to diagnose suspected cases. In such cases, further contrast‐enhanced MRI of the spine, which is the most sensitive and specific way to detect IESCM, should actively be conducted. Doctors can effectively observe the involvement of the vertebral column and canal, subarachnoid space, spinal cord, and spinal nerves, and further identify epidural metastasis, spinal cord metastasis, or IESCM.4, 5 For IESCM, contrast‐enhanced full spinal cord MRI shows multiple intradural extramedullary nodules with abnormal enhancement, and/or spinal pia mater thickening with linear enhancement.6, 7 IESCM can be diagnosed with caution according to neurological symptoms and contrast‐enhanced MRI presentation.
Treating IESCM remains a great challenge and requires multiple disciplinary team discussion. Targeted therapy and/or radiotherapy may be effective for symptom control.8 EGFR‐tyrosine kinase inhibitor (TKI) therapy has demonstrated a survival benefit for EGFR mutant patients with IESCM.9
Molecular targeted agents, such as EGFR and ALK TKIs, can pass through the blood‐brain barrier to a certain extent and thus show treatment effects in some patients, although the cerebrospinal fluid (CSF) concentration could be low. ALK‐TKI treatment for ALK positive non‐small cell lung cancer (NSCLC) patients with LM has been reported.10 Compared to first‐generation ALK inhibitors, second‐generation ALK inhibitors, such as alectinib and ceritinib, have better efficacy in intracranial metastases of ALK positive NSCLC, suggesting better permeability against the blood‐brain barrier. Crizotinib has a very low CSF penetration of 0.26%11 and CSF: half‐maximal inhibitory concentration (IC50) of 0.03, while ceritinib has a CSF penetration of 15%12 and alectinib has a CSF penetration of 63–94% and CSF:IC50 of 1.4,13, 14 which explains the excellent central nervous system efficacy (BM and LM) of second‐generation ALK inhibitors in ALK‐rearranged NSCLC patients pretreated with crizotinib. To date, the proper management of IESCM in ALK‐rearranged lung cancer has not been defined. In our patient with IESCM and ALK rearrangement, second‐generation ALK inhibitors were the best option, and although gene testing of the specimens after a second biopsy did not detect any crizotinib resistance mutations, the clinical outcome was very satisfactory.
In conclusion, we report a case of ALK rearrangement in a lung cancer patient experiencing IESCM after developing resistance to crizotinib, and both his clinical symptoms and intradural extramedullary lesions in contrast‐enhanced MRI improved after ceritinib therapy. Our results suggest that ceritinib treatment should be considered for ALK‐rearranged patients with BM, LM, and even IESCM.
Disclosure
No authors report any conflict of interest.
Acknowledgment
We thank the patient and his family for providing their information for this study.
References
- 1. Kizawa M, Mori N, Hashizume Y, Yoshida M. Pathological examination of spinal lesions in meningeal carcinomatosis. Neuropathology 2008; 28: 295–302. [DOI] [PubMed] [Google Scholar]
- 2. Chamberlain MC. Leptomeningeal metastasis. Semin Neurol 2010; 30: 236–44. [DOI] [PubMed] [Google Scholar]
- 3. Le Rhun E, Weller M, Brandsma D et al EANO–ESMO clinical practice guidelines for diagnosis, treatment and follow‐up of patients with leptomeningeal metastasis from solid tumours. Ann Oncol 2017; 28 (Suppl 4): iv84–99. [DOI] [PubMed] [Google Scholar]
- 4. Loughrey GJ, Collins CD, Todd SM, Brown NM, Johnson RJ. Magnetic resonance imaging in the management of suspected spinal canal disease in patients with known malignancy. Clin Radiol 2000; 55: 849–55. [DOI] [PubMed] [Google Scholar]
- 5. Abdi S, Adams CI, Foweraker KL, O'Connor A. Metastatic spinal cord syndromes: Imaging appearances and treatment planning. Clin Radiol 2005; 60: 637–47. [DOI] [PubMed] [Google Scholar]
- 6. Beall DP, Googe DJ, Emery RL et al Extramedullary intradural spinal tumors: A pictorial review. Curr Probl Diagn Radiol 2007; 36: 185–98. [DOI] [PubMed] [Google Scholar]
- 7. Lim V, Sobel DF, Zyroff J. Spinal cord pial metastases: MR imaging with gadopentetate dimeglumine. AJR Am J Roentgenol 1990; 155: 1077–84. [DOI] [PubMed] [Google Scholar]
- 8. Xu Y, Zhong W, Zhao J, Chen M, Li L, Wang M. [Clinical features of Intradural extramedullary spinal cord metastases in primary lung cancer.]. Chin J Lung Cancer 2016; 19: 539–44 (In Chinese.). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sakai M, Ishikawa S, Ito H et al Carcinomatous meningitis from non‐small‐cell lung cancer responding to gefitinib. Int J Clin Oncol 2006; 11: 243–5. [DOI] [PubMed] [Google Scholar]
- 10. Cheng H, Perez‐Soler R. Leptomeningeal metastases in non‐small‐cell lung cancer. Lancet Oncol 2018; 19: e43–55. [DOI] [PubMed] [Google Scholar]
- 11. Costa DB, Kobayashi S, Pandya SS et al CSF concentration of the anaplastic lymphoma kinase inhibitor crizotinib. J Clin Oncol 2011; 29: e443–5. [DOI] [PubMed] [Google Scholar]
- 12. Zhang I, Zaorsky NG, Palmer JD, Mehra R, Lu B. Targeting brain metastases in ALK‐rearranged non‐small‐cell lung cancer. Lancet Oncol 2015; 16: e510–21. [DOI] [PubMed] [Google Scholar]
- 13. Sakamoto H, Tsukaguchi T, Hiroshima S et al CH5424802, a selective ALK inhibitor capable of blocking the resistant gatekeeper mutant. Cancer Cell 2011; 19: 679–90. [DOI] [PubMed] [Google Scholar]
- 14. Kodama T, Hasegawa M, Takanashi K, Sakurai Y, Kondoh O, Sakamoto H. Antitumor activity of the selective ALK inhibitor alectinib in models of intracranial metastases. Cancer Chemother Pharmacol 2014; 74: 1023–8. [DOI] [PubMed] [Google Scholar]


