Abstract
Background
Although patients with EGFR‐mutant non‐small‐cell lung cancer (NSCLC) benefit from treatment with EGFR‐tyrosine kinase inhibitors (TKIs), outcomes are limited by the eventual development of acquired resistance. We conducted a retrospective study to evaluate the efficacy and feasibility of EGFR‐TKI therapy beyond focal progression, associated with microwave ablation.
Methods
Patients with metastatic EGFR‐mutant NSCLC treated with EGFR‐TKIs at our institutions from May 2012 to December 2017 were identified. Patients with single lesion progression, treated with MWA, and continually administered EGFR‐TKI therapy until further progression, were included in the study. Initial response to target therapy, median progression‐free survival (PFS1), and first progression site were recorded. The median time to progression after local therapy (PFS2) was also assessed. Overall survival was calculated from the initiation of EGFR‐TKIs to the date of final follow‐up or death.
Results
Fifteen out of 205 patients (10%) satisfied the inclusion criteria. Local therapy was well tolerated, and complete ablation was performed in 11 (73.3%) patients. The median PFS1 was 9.5 months (range 6–41), and the median PFS2 was 8 months (range 3–24). The corresponding 6 and 12 month PFS rates were 73.3% and 26.7%, respectively. Median overall survival was 23 months (range 15–64).
Conclusion
The longer disease control observed in our patients suggests that continuation of EGFR‐TKI beyond focal progression associated to microwave ablation is an efficacious therapeutic strategy.
Keywords: Acquired resistance, continuation of EGFR‐TKI, local treatment, MWA, NCSLC
Introduction
EGFR‐tyrosine kinase inhibitors (TKIs), such as gefitinib, erlotinib, or afatinib, are the standard first‐line therapy for patients with advanced and metastatic non‐small cell lung cancer (NSCLC) harboring sensitive EGFR mutations.1 Compared with standard chemotherapy, EGFR‐TKI treatment has shown a significant improvement in progression‐free survival (PFS), objective response rates (ORR), and quality of life in multiple prospective phase III studies.2, 3, 4, 5
However, patients who initially respond to treatment often ultimately develop acquired resistance to EGFR‐TKIs, with median PFS of 9–13 months.2, 3, 4, 5 Despite clinical evidence of progression on EGFR‐TKI therapy, growth can often be indolent and asymptomatic, and may not necessitate an immediate switch in therapy. Continued EGFR inhibition appears to provide continued clinical benefit, particularly when the disease is controllable with local therapy options, such as radiotherapy, surgery, or both.6, 7, 8, 9, 10
Microwave ablation (MWA), as a local therapy strategy, has been used as an alternative treatment for patients with advanced and medically inoperable stage I NSCLC.11, 12 This study describes our experience of using local MWA while continuing the same targeted therapy to treat EGFR‐mutant (MT) metastatic or recurrent NSCLC patients with disease progression confined to a single site.
Methods
Patients and eligibility
We conducted a retrospective analysis of NSCLC patients in our institutions between May 2012 and December 2017 who developed focal disease progression in a single lesion during therapy with an EGFR‐TKI and were then continuously treated with the EGFR‐TKI combined with locoregional MWA to the site of progression until further progression was observed. The inclusion criteria were: (i) histologically confirmed EGFR‐MT recurrent or metastatic stage IIIB/IV NSCLC; (ii) a tumor harboring an EGFR mutation (examined either through direct sequencing or allele‐specific PCR assays) known to be associated with objective clinical benefit (partial response [PR] or stable disease [SD] longer than 6 months) from treatment with an EGFR‐TKI (such as gefitinib, erlotinib, afatinib); (iii) focal disease progression in a single lesion while on continuous treatment with an EGFR‐TKI; and (iv) willing to provide written informed consent. The internal review board approved this retrospective study.
Treatment methods
All patients enrolled were orally administered 150 mg erlotinib and 250 mg of gefitinib or 40 mg of afatinib daily. Patients underwent routine chest and abdominal computed tomography (CT) scans or positron emission tomography scans every one to two months to assess the local response according to Response Evaluation Criteria in Solid Tumors (RECIST).13 Additional procedures including CT, magnetic resonance imaging, and bone scintigraphy were applied to evaluate metastatic sites. Patients continued oral EGFR‐TKI during MWA intervals until disease progression, death, or the appearance of intolerable toxicity. If their oncologist and interventional radiologist deemed it safe, patients underwent a biopsy at the site of their progressive disease before MWA to elucidate mechanisms of acquired drug resistance.
Microwave ablation
For MWA, we used a commercially available system (ECO‐2450B MWA, ECO Microwave Institute, Nanjing, China) and a 14‐gauge cooled‐shaft antenna (FORSEA, Vision Microwave Electronic Institute, Nanjing, China). The output power was generally set at 50–70 W. If the tumor could not be covered by one ablation session according to the size, location, and geometry, multiple sequential ablations were performed to achieve complete necrosis. Following treatment, CT scanning was again performed to evaluate the immediate necrotic conditions after ablation and to examine whether there were any complications, such as bleeding or pneumothorax.
Response evaluation
Primary technical success was defined as a complete lack of enhancement in the ablation zone on initial follow‐up contrast CT. A thin (< 5 mm), symmetric rim of peripheral enhancement at the ablation zone was considered to indicate benign peritumoral enhancement. Irregular nodular enhancement (> 15 HU) at the ablation site was considered to indicate recurrent or residual disease. The response to EGFR‐TKIs was assessed according to RECIST version 1.1.
Statistical analysis
Progression‐free survival was determined according to Kaplan–Meier method. First PFS (PFS1) was measured from the time of initiation of targeted therapy to first progression of disease. Second PFS (PFS2) was measured from the date of focal progression until further progression of disease (defined by RECIST) or death from any cause. Overall survival (OS) was calculated from the date of initiation of the EGFR‐TKI to the date of death. OS was censored at the date of the last visit for patients whose deaths could not be confirmed. SPSS version 16.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis.
Results
Patient characteristics
Initially, 205 lung cancer patients treated with MWA at our institutions between May 2012 and December 2017 were identified, but only 15 patients satisfied the inclusion criteria. Patient characteristics are shown in Table 1.
Table 1.
Clinical characteristics of patients with EGFR‐mutant NSCLC according to first progression site
| N = 21 | No | % |
|---|---|---|
| Gender | ||
| Male | 7 | 46.7 |
| Female | 8 | 53.3 |
| Age (years) | ||
| Median | 54.2 | |
| Range | 29–81 | |
| Histology | ||
| Adenocarcinoma | 14 | 93.3 |
| Squamous cell carcinoma | 1 | 6.7 |
| ECOG PS | ||
| 0 | 2 | 13.3 |
| 1 | 10 | 66.7 |
| 2 | 3 | 20.0 |
| Smoking history | ||
| Never | 11 | 73.3 |
| Former | 4 | 16.7 |
| EGFR mutation type | ||
| 19 del | 9 | 60.0 |
| 21 L858R | 5 | 33.3 |
| 18 G719X | 1 | 6.7 |
| Best response to TKI | ||
| CR | 2 | 13.3 |
| PR | 8 | 53.4 |
| SD | 5 | 33.3 |
| Line of EGFR‐TKI | ||
| First‐line | 9 | 60 |
| Second/third‐line | 6 | 40 |
| Site of RECIST PD | ||
| Lung | 10 | 66.7 |
| Liver | 4 | 16.6 |
| Adrenal | 1 | 6.7 |
CR, complete response; ECOG PS, Eastern Cooperative Oncology Group performance status; NSCLC, non‐small cell lung cancer; PD, progressive disease; PR, partial response; SD, stable disease; TKI, tyrosine kinase inhibitor.
The median age of the included patients was 53 years (range 29–81); eight (53%) patients were female; 11 were never smokers; and 33% (5/15) had received at least one chemotherapy regimen before commencing EGFR‐TKI treatment. All patients had an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0–2 before MWA was performed. Fourteen patients had adenocarcinomas, and one patient had squamous cell carcinoma. All patients harbored EGFR‐sensitive mutations (9 exon 19 deletions, 5 exon 21 L858R mutations, and 1 exon 18 G719X).
Seven patients were treated with erlotinib 150 mg/day (two patients in the CTONG 0901 clinical trial), six with gefitinib 250 mg/day, and two with afatinib 40 mg/day (both in the LUX‐LUNG 6 clinical trial). Patient characteristics and treatment history are listed in Table 1. Ten patients underwent repeat tumor tissue biopsies to elucidate the mechanisms of acquired drug resistance to EGFR‐TKI before MWA. Six (60%) patients acquired the exon 20 EGFR mutation T790M, two (20%) developed MET amplification, and one developed small cell histologic transformation. The other biopsy did not reveal any new mutations.
Response to therapy, survival, and toxicity
At the first response assessment, two patients (13%) had achieved a complete response (CR) to treatment, eight (53%) a PR, and five (34%) had SD.
The cutoff date for follow‐up was December 2017, and the median follow‐up duration was 17 months from the initial TKI therapy to physician assessment of progressive disease (PD) (range 9–64 months). At the time of the data cutoff, seven patients (46.7%) exhibited physician assessed PD and four (16.7%) had died.
Ten patients (67%) first experienced progression in the lung, four (28%) in the liver, and one (7%) in the left adrenal gland during PFS1. The median time from PFS1 to the start of MWA was 2.8 weeks. The median PFS 1, measured from the start of frontline TKI therapy until focal disease progression, was 9.5 months (range 6–41) (Fig 1).
Figure 1.
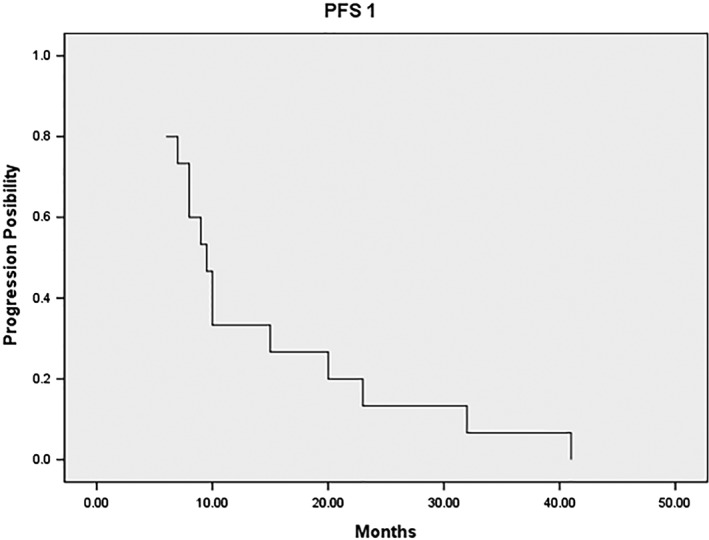
Median progression‐free survival 1 (PFS1), measured from the initiation of frontline tyrosine kinase inhibitor therapy until focal disease progression, was 9.5 months (range 6–41).
All 15 patients were treated with MWA to the single site of focal PD. Fifteen patients underwent 15 MWA sessions corresponding to 20 antennas for 15 progressed sites. The mean size of the metastatic sites was 3.3 cm (range 1.5–6.8 cm). Complete ablation was observed in 11 (73.3%) patients, and incomplete ablation in 4 (26.7%). Pain was the most common complication, occurring in 33.3% (5/15). Postoperative pneumothorax occurred in two cases (7.1%) but did not require chest tube drainage. Most MWA procedures were well tolerated. No patients died during the procedure or within 30 days after MWA.
The median PFS2, measured from the date of diagnosis of focal progression until further progression (defined by RECIST) or death from any cause, was 8 months (range 3–24). The 6 and 12 month PFS2 rates were 73.3% and 26.7%, respectively (Fig 2). The median OS was 23 months (range 15–64).
Figure 2.
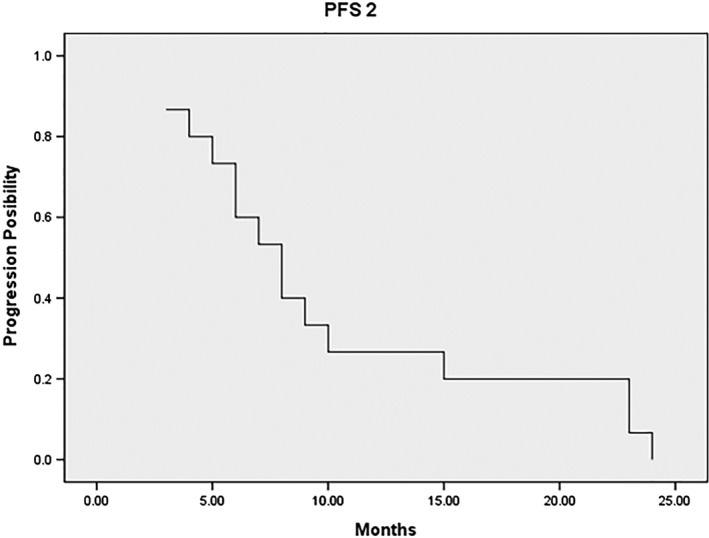
Median progression‐free survival 2 (PFS2), measured from the date of diagnosis of focal progression until further progression or death from any cause, was 8 months (range 3–24).
The most common adverse event caused by TKIs was grade 1 or 2 rash. No grade III or IV toxicities were reported. The toxicities observed during the first EGFR‐TKI treatment continued but did not worsen during the study period.
Discussion
EGFR‐TKIs currently represent the cornerstone of therapy for NSCLC. Premature discontinuation of EGFR‐TKI therapy may result in rapid progression or disease flare, with reintroduction of TKI therapy leading to decreased tumor growth.14, 15 Many patients diagnosed with EGFR‐MT cancers can safely continue the original therapy with their original EGFR inhibitor beyond the first signs of radiographic progression. A retrospective analysis of 41 of patients treated with first‐line erlotinib showed that 21 (50%) were able to delay a change in systemic therapy for > 3 months after RECIST progression and 21% could delay a treatment switch for more than 12 months.16 The prospective clinical trial ASPIRATION showed that patients could safely receive treatment with erlotinib for a median of 3.1 months after initial progression and again, patients who responded well to first‐line erlotinib were most likely to benefit from post‐progression therapy.17 This study confirmed the prospective feasibility of continuing erlotinib therapy in selected patients following RECIST PD without undue toxic effects.
In some cases, when patients with EGFR‐MT NSCLC progress on EGFR‐TKIs, but the progression only occurs in a limited number of sites (oligoprogressive disease), it may be reasonable to consider locoregional therapy to the sites of resistant disease and continue the original EGFR‐TKI. This approach is supported by several retrospective analyses. In a cohort of 27 patients with central nervous system (CNS) and/or extra‐CNS oligoprogressive disease, Weickhardt et al. reported that continuing EGFR‐TKI therapy after disease progression in association with local ablative therapy (surgery and radiotherapy) postponed the initiation of second‐line chemotherapy by 3 months in 19 patients and 12 months in 8.7 Moreover, Yu et al. reported that patients with EGFR‐MT lung cancers with acquired resistance to EGFR‐TKI therapy can be treated with either radiation, surgery, or radiofrequency ablation to the progressive sites and continue on the original targeted therapy for a median of 10 months.8
Radiation therapy of isolated CNS progression,6 a single RECIST progressive lesion,18 or PD of skeletal regions10 in patients with EGFR‐MT NSCLC treated with EGFR‐TKIs and continued systemic administration of the TKI have been reported, and 80 days to 10.9 months additional disease control achieved.
Microwave ablation has been reported as a local therapy for patients with continued EGFR‐TKI in advanced NSCLC that developed extra‐CNS oligoprogressive disease during EGFR‐TKI treatment. In a retrospective study, Ni et al. compared the outcomes in two groups of patients who had progressed on EGFR‐TKI treatment. Thirty‐nine patients received MWA as local therapy for the progression sites and continued on the same TKIs (MWA group), while 26 patients switched to cytotoxic chemotherapy after progression.19 The results show that continuing EGFR‐TKI therapy with MWA beyond disease progression significantly prolonged OS (MWA group median OS 27.7, cytotoxic chemotherapy group median OS 20.0 months). Similar to Ni et al., we found that maintaining the EGFR pathway blockade, despite focal disease progression, is a fundamental strategy; we observed a PFS2 of 8 months with 6 and 12 month PFS rates of 73.3% and 26.7%, respectively.
It is plausible to consider that a TKI‐resistant clone may develop in the progression sites of disease, which are only a small fraction of the total alleles, while the remainder of the cancer burden remains sensitive to EGFR‐TKI therapy.20 Patients with EGFR‐MT disease who progress often experience a disease flare when the EGFR‐TKI is discontinued,15 and re‐challenge of these patients with the same EGFR‐TKI after only a short time off therapy can lead to a second response.21, 22 This theory could partly explain the effectiveness of local ablative therapy while continuing the same targeted therapy after acquired resistance.
The two most frequent mechanisms of resistance to EGFR‐TKIs are the T790M mutation in exon 20 of the EGFR gene and MET amplification.23 Re‐biopsy after development of acquired resistance and genomic analysis of progression sites should be included as routine because they may provide useful information for tailoring subsequent treatment strategies.
In conclusion, MWA with continuous administration of EGFR‐TKIs after the determination of PD in a single progression site might represent an effective treatment option. Further prospective clinical trials are required in patients who develop local progression to confirm whether the treatment used in the present study is beneficial.
Disclosure
No authors report any conflict of interest.
Contributor Information
Xiaoming Chen, Email: cxmdj@sina.com.
Weijun Fan, Email: fanweijun1964@163.com.
References
- 1. Maemondo M, Inoue A, Kobayashi K et al Gefitinib or chemotherapy for non‐small‐cell lung cancer with mutated EGFR. N Engl J Med 2010; 362: 2380–8. [DOI] [PubMed] [Google Scholar]
- 2. Mok TS, Wu YL, Thongprasert S et al Gefitinib or carboplatin‐paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009; 361: 947–57. [DOI] [PubMed] [Google Scholar]
- 3. Rosell R, Carcereny E, Gervais R et al Erlotinib versus standard chemotherapy as first‐line treatment for European patients with advanced EGFR mutation‐positive non‐small‐cell lung cancer (EURTAC): A multicentre, open‐label, randomised phase 3 trial. Lancet Oncol 2012; 13: 239–46. [DOI] [PubMed] [Google Scholar]
- 4. Mitsudomi T, Morita S, Yatabe Y et al Gefitinib versus cisplatin plus docetaxel in patients with non‐small‐cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): An open label, randomised phase 3 trial. Lancet Oncol 2010; 1: 121–8. [DOI] [PubMed] [Google Scholar]
- 5. Zhou C, Wu YL, Chen G et al Erlotinib versus chemotherapy as first‐line treatment for patients with advanced EGFR mutation‐positive non‐small‐cell lung cancer (OPTIMAL, CTONG‐0802): A multicentre, open‐label, randomised, phase 3 study. Lancet Oncol 2011; 12: 735–42. [DOI] [PubMed] [Google Scholar]
- 6. Shukuya T, Takahashi T, Naito T et al Continuous EGFR‐TKI administration following radiotherapy for non‐small cell lung cancer patients with isolated CNS failure. Lung Cancer 2011; 74: 457–61. [DOI] [PubMed] [Google Scholar]
- 7. Weickhardt AJ, Scheier B, Burke JM et al Local ablative therapy of oligoprogressive disease prolongs disease control by tyrosine kinase inhibitors in oncogene‐addicted non‐small‐cell lung cancer. J Thorac Oncol 2012; 7: 1807–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yu HA, Sima CS, Huang J et al Local therapy with continued EGFR tyrosine kinase inhibitor therapy as a treatment strategy in EGFR‐mutant advanced lung cancers that have developed acquired resistance to EGFR tyrosine kinase inhibitors. J Thorac Oncol 2013; 8: 346–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen Q, Quan Q, Ding L et al Continuation of epidermal growth factor receptor tyrosine kinase inhibitor treatment prolongs disease control in non‐small‐cell lung cancers with acquired resistance to EGFR tyrosine kinase inhibitors. Oncotarget 2015; 6: 24904–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hong SH, Kim YS, Lee JE et al Clinical characteristics and continued epidermal growth factor receptor tyrosine kinase inhibitor administration in EGFR‐mutated non‐small cell lung cancer with skeletal metastasis. Cancer Res Treat 2013; 48: 1110–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wei Z, Ye X, Yang X et al Microwave ablation in combination with chemotherapy for the treatment of advanced non‐small cell lung cancer. Cardiovasc Intervent Radiol 2015; 38: 135–42. [DOI] [PubMed] [Google Scholar]
- 12. Yang X, Ye X, Zheng A et al Percutaneous microwave ablation of stage I medically inoperable non‐small cell lung cancer: Clinical evaluation of 47 cases. J Surg Oncol 2013; 110: 758–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Therasse P, Arbuck SG, Eisenhauer EA et al New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000; 92: 205–16. [DOI] [PubMed] [Google Scholar]
- 14. Chmielecki J, Foo J, Oxnard GR et al Optimization of dosing for EGFR‐mutant non‐small cell lung cancer with evolutionary cancer modeling. Sci Transl Med 2011; 3: 90ra59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chaft JE, Oxnard GR, Sima CS, Kris MG, Miller VA, Riely GJ. Disease flare after tyrosine kinase inhibitor discontinuation in patients with EGFR‐mutant lung cancer and acquired resistance to erlotinib or gefitinib: Implications for clinical trial design. Clin Cancer Res 2011; 17: 6298–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lo PC, Dahlberg SE, Nishino M et al Delay of treatment change after objective progression on first‐line erlotinib in epidermal growth factor receptor‐mutant lung cancer. Cancer 2015; 121: 2570–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Park K, Yu CJ, Kim SW et al First‐line erlotinib therapy until and beyond Response Evaluation Criteria in Solid Tumors progression in Asian patients with epidermal growth factor receptor mutation‐positive non‐small‐cell lung cancer: The ASPIRATION study. JAMA Oncol 2016; 2: 305–12. [DOI] [PubMed] [Google Scholar]
- 18. Conforti F, Catania C, Toffalorio F et al EGFR tyrosine kinase inhibitors beyond focal progression obtain a prolonged disease control in patients with advanced adenocarcinoma of the lung. Lung Cancer 2013; 81: 440–4. [DOI] [PubMed] [Google Scholar]
- 19. Ni Y, Bi J, Ye X et al Local microwave ablation with continued EGFR tyrosine kinase inhibitor as a treatment strategy in advanced non‐small cell lung cancers that developed extra‐central nervous system oligoprogressive disease during EGFR tyrosine kinase inhibitor treatment: A pilot study. Medicine (Baltimore) 2016; 95 (25): e3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Suda K, Mizuuchi H, Maehara Y, Mitsudomi T. Acquired resistance mechanisms to tyrosine kinase inhibitors in lung cancer with activating epidermal growth factor receptor mutation‐‐diversity, ductility, and destiny. Cancer Metastasis Rev 2012; 31: 807–14. [DOI] [PubMed] [Google Scholar]
- 21. Oxnard GR, Janjigian YY, Arcila ME et al Maintained sensitivity to EGFR tyrosine kinase inhibitors in EGFR‐mutant lung cancer recurring after adjuvant erlotinib or gefitinib. Clin Cancer Res 2011; 17: 6322–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tomizawa Y, Fujita Y, Tamura A et al Effect of gefitinib re‐challenge to initial gefitinib responder with non‐small cell lung cancer followed by chemotherapy. Lung Cancer 2010; 68: 269–72. [DOI] [PubMed] [Google Scholar]
- 23. Yu HA, Arcila ME, Rekhtman N et al Analysis of tumor specimens at the time of acquired resistance to EGFR‐TKI therapy in 155 patients with EGFR‐mutant lung cancers. Clin Cancer Res 2013; 19: 2240–7. [DOI] [PMC free article] [PubMed] [Google Scholar]


