Abstract
Inflammasome‐activated caspase‐1, caspase‐11, caspase‐4, and caspase‐5 cleave GSDMD to unleash its N‐terminal gasdermin‐N domain (GSDMDN term) that perforates the plasma membrane to execute pyroptosis and stimulate inflammation. The mechanism underlying GSDMDN term pore formation is unclear. Mulvihill et al use high‐resolution atomic force microscopy (AFM) to analyze the dynamic pore formation process of GSDMDN term. GSDMDN term protomers are inserted into the lipid membrane to assemble arc‐ or slit‐shaped oligomers that can incorporate additional protomers and grow into large and stable ring‐shaped oligomers to form pores.
Subject Categories: Autophagy & Cell Death, Immunology
One of the most important immune responses to infection and danger signal is pyroptosis. Pyroptosis is a lytic form of programmed cell death that can destruct the pathogen‐residing niche, release cytosolic contents, and induce strong inflammation. Historically, pyroptosis refers to caspase‐1‐mediated monocyte death. Caspase‐1 is activated by the canonical inflammasome complex scaffolded by a pattern recognition receptor that detects a pathogen‐associated molecular pattern or an endogenous danger signal. Murine caspase‐11 and its human orthologs caspase‐4 and caspase‐5, upon activation by direct binding to bacterial lipopolysaccharide (LPS), can also induce pyroptosis. A recent breakthrough in pyroptosis and innate immunity researches is the identification of gasdermin‐D (GSDMD) protein, the key executioner of pyroptotic cell death (Kayagaki et al, 2015; Shi et al, 2015). GSDMD contains an N‐terminal gasdermin‐N and a C‐terminal gasdermin‐C domain linked by a loop. The gasdermin‐N domain has a potent pore‐forming activity that interacts with and is inhibited by the gasdermin‐C domain. Activated caspase‐1 and caspase‐11/4/5 cleave the linking loop in GSDMD, thereby unlocking the autoinhibition on the gasdermin‐N domain. The unleashed gasdermin‐N domain translocates to plasma membrane through binding to membrane phosphoinositides to perforate the membrane, causing cell swelling and ultimately osmotic lysis (Aglietti et al, 2016; Ding et al, 2016; Liu et al, 2016; Sborgi et al, 2016). The GSDMD pore releases cellular contents including the inflammatory cytokines IL‐1β and IL‐18 (Kayagaki et al, 2015; Shi et al, 2015), the other substrates of caspase‐1.
GSDMD belongs to a gasdermin family that contains six members in human and ten in mouse (Shi et al, 2017). All the family members (except for DFNB59) share the two‐domain architecture, and their gasdermin‐N domains are capable of inducing pyroptosis (Shi et al, 2015; Ding et al, 2016). Natural GSDMA3 mutants losing the autoinhibition are constitutively pro‐pyroptotic and cause alopecia with skin inflammation in mice (Shi et al, 2015; Ding et al, 2016). Two recent studies show that GSDME is cleaved and activated by caspase‐3 and its expression confers pyroptotic cell death upon caspase‐3 activation by TNFα or DNA damage (Rogers et al, 2017; Wang et al, 2017). The gasdermin‐N domains of GSDME, GSDMA, and GSDMA3 are also capable of forming pores on liposomes or monolayer membranes in vitro (Shi et al, 2015; Ding et al, 2016). These suggest a common pore‐forming mechanism adopted by the gasdermin family to lyse the cell and execute pyroptosis, which redefines pyroptosis as gasdermin‐mediated programmed necrotic cell death (Shi et al, 2017). However, the mechanism by which the gasdermin‐N domain assembles pores to perforate the lipid membrane is unknown.
To answer this question, Mulvihill et al applied high‐resolution and time‐lapse atomic force microscopy (AFM) and directly imaged the pore‐forming process of GSDMD gasdermin‐N domain (GSDMDNterm) (Mulvihill et al, 2018). Under the force–distance curve‐based AFM, GSDMDNterm, released from caspase‐1 cleavage of full‐length GSDMD, was observed to assemble arc‐, slit‐, and ring‐shaped oligomers that could form transmembrane pores in muscovite mica‐supported membranes made of artificially mixed lipids or E. coli polar lipid extracts (Fig 1). The slit‐ and ring‐shaped oligomers protruded about 3.6 nm from the lipid surface, suggesting a nearly full insertion into the membrane. The ring‐shaped oligomers have an average diameter of 22.6 nm, agreeing with the previous study (Ding et al, 2016). These observations were confirmed in liposome membranes by negative‐stain transmission electron microscopy. Mulvihill et al also checked the effects of lipid composition on GSDMDNterm membrane binding and pore formation. Consistent with the previous studies (Aglietti et al, 2016; Ding et al, 2016; Liu et al, 2016; Sborgi et al, 2016), GSDMDNterm assembled arc‐, slit‐, and ring‐like oligomers in the presence of phosphoinositide (PI(4,5)P2) but not phosphatidylinositol; PI(4,5)P2 did not alter the size of the ring‐shaped oligomers, but greatly facilitated membrane binding of GSDMDNterm with reduced occurrence of arc‐ and slit‐like oligomers and much increased ring‐like oligomers. Notably, cholesterol failed to support GSDMDNterm oligomer formation and even inhibited GSDMDNterm binding to PI(4,5)P2‐containing membrane and oligomer formation. These findings substantiate that GSDMD can only trigger pyroptosis from the inside of mammalian cells as the outer leaflet of plasma membrane contains cholesterol but not phosphoinositides (Ding et al, 2016).
Figure 1. Schematic of the GSDMDNterm pore formation process.
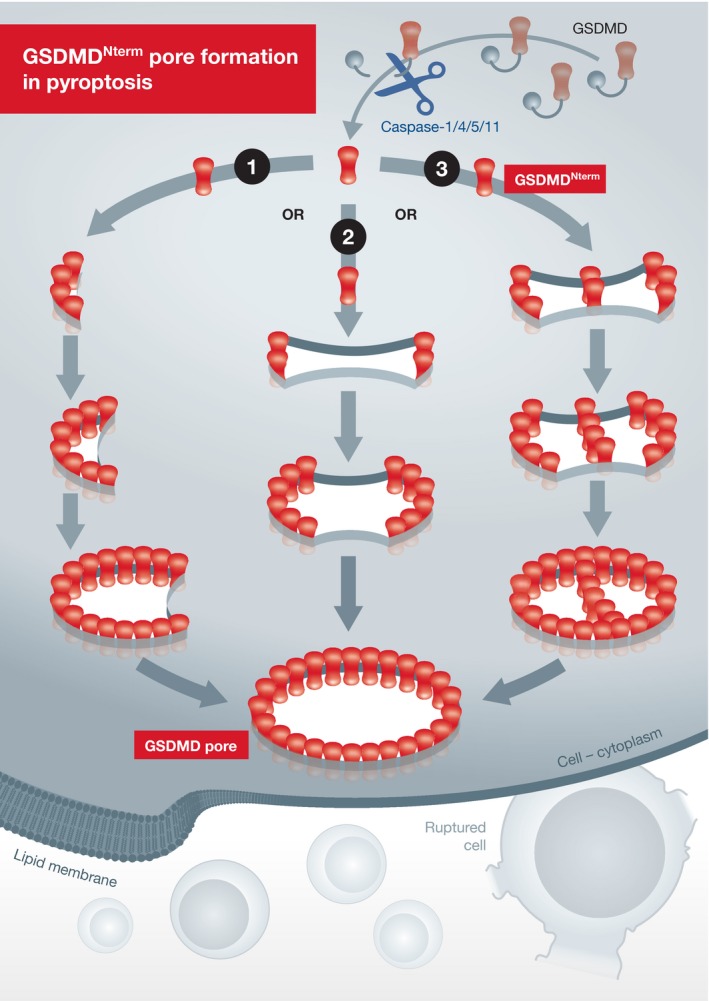
In pyroptosis, site‐specific cleavage of GSDMD by activated caspase‐1 or caspase‐11/4/5 unlocks the autoinhibition on GSDMDNterm, which allows the GSDMDNterm to bind to membrane lipids (specifically phosphoinositides) and translocates to the membrane. The GSDMDNterm protomers are inserted into the lipid membrane and form arc‐ or slit‐shaped transmembrane oligomers. The arc‐ and slit‐shaped oligomers can incorporate additional protomers or oligomers, thereby growing into large and stable ring‐shaped oligomers to assemble complete pores. Remnants GSDMDNterm oligomers are occasionally present in the pores, resulting from the fusion process, and eventually disappear in the final ring‐shaped pores. Formation of the GSDMDNterm pores in the plasma membrane causes cell swelling and ultimately osmotic lysis to execute pyroptotic cell death.
GSDMD can also be cleaved by caspase‐11/4/5. The cleavage occurs at the same site as that by caspase‐1, generating the same GSDMDNterm fragment. Not surprisingly, Mulvihill et al found that caspase‐4/5‐cleaved GSDMD formed similar arc‐, slit‐, and ring‐shaped oligomers and its lipid binding and pore formation properties resembled those of caspase‐1‐cleaved GSDMD. Mulvihill et al further snapshotted GSDMDNterm oligomer formation process by time‐lapse AFM analyses. The arc‐, slit‐, and ring‐shaped transmembrane GSDMDNterm oligomers appeared within 10 min after incubation of GSDMD with caspase‐1. Over time, the arc‐ and slit‐shaped oligomers grew from one or both ends with addition of more monomers or oligomers, and the two ends eventually fused with each other to generate a larger and stable ring‐shaped transmembrane oligomer (Fig 1). The majority of ring‐shaped oligomers assembled from the slit‐like oligomers. These suggest that the arc‐ and slit‐shaped GSDMDNterm oligomers represent intermediate states of transmembrane pore formation. The authors also found that the lipid composition did not evidently affect the dynamic fusion process during the ring‐shaped oligomer formation. Previous studies of other pore‐forming proteins suggest two modes of pore formation, pore growing directly in the membrane (without vertical collapse of pore‐forming proteins) or a mechanism involving prepore‐to‐pore transitions. In the time‐lapse AFM topographs, Mulvihill et al observed no considerable changes of the heights of GSDMDNterm oligomers during formation of the ring‐shaped oligomers. The absence of a vertical collapse event favors the notion that GSDMDNterm pore formation is initiated in lipid membranes and features a continuous growing process without prepore‐to‐pore transitions. Lastly, the authors recorded AFM topographs of the membrane‐bound oligomers at the sub‐nanometer resolution and analyzed the oligomerization of GSDMDNterm pores, which revealed an average of 30 GSDMDNterm monomers (range from 15 to 45) in each ring‐shaped oligomer.
The findings made by Mulvihill et al provide detailed mechanistic insights into transmembrane pore assembly by GSDMDNterm. Given the conserved pore‐forming activity in the entire gasdermin family, the Mulvihill study (Mulvihill et al, 2018), together with the recently determined Cryo‐EM structure of GSDMA3 pores (Ruan et al, 2018), builds an elegant framework for understanding membrane pore formation during other gasdermin‐mediated pyroptosis that are associated with diverse pathophysiological processes.
The EMBO Journal (2018) 37: e100067
See also: https://doi.org/10.15252/embj.201798321 (July 2018)
Contributor Information
Jingjin Ding, Email: jding@moon.ibp.ac.cn.
Feng Shao, Email: shaofeng@nibs.ac.cn.
References
- Aglietti RA, Estevez A, Gupta A, Ramirez MG, Liu PS, Kayagaki N, Ciferri C, Dixit VM, Dueber EC (2016) GsdmD p30 elicited by caspase‐11 during pyroptosis forms pores in membranes. Proc Natl Acad Sci USA 113: 7858–7863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J, Wang K, Liu W, She Y, Sun Q, Shi J, Sun H, Wang DC, Shao F (2016) Pore‐forming activity and structural autoinhibition of the gasdermin family. Nature 535: 111–116 [DOI] [PubMed] [Google Scholar]
- Kayagaki N, Stowe IB, Lee BL, O'Rourke K, Anderson K, Warming S, Cuellar T, Haley B, Roose‐Girma M, Phung QT, Liu PS, Lill JR, Li H, Wu J, Kummerfeld S, Zhang J, Lee WP, Snipas SJ, Salvesen GS, Morris LX et al (2015) Caspase‐11 cleaves gasdermin D for non‐canonical inflammasome signalling. Nature 526: 666–671 [DOI] [PubMed] [Google Scholar]
- Liu X, Zhang Z, Ruan J, Pan Y, Magupalli VG, Wu H, Lieberman J (2016) Inflammasome‐activated gasdermin D causes pyroptosis by forming membrane pores. Nature 535: 153–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulvihill E, Sborgi L, Mari SA, Pfreundschuh M, Hiller S, Müller DJ (2018) Mechanism of membrane pore formation by human gasdermin‐D. EMBO J 37: e98321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers C, Fernandes‐Alnemri T, Mayes L, Alnemri D, Cingolani G, Alnemri ES (2017) Cleavage of DFNA5 by caspase‐3 during apoptosis mediates progression to secondary necrotic/pyroptotic cell death. Nat Commun 8: 14128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan J, Xia S, Liu X, Lieberman J, Wu H (2018) Cryo‐EM structure of the gasdermin A3 membrane pore. Nature 557: 62–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sborgi L, Ruhl S, Mulvihill E, Pipercevic J, Heilig R, Stahlberg H, Farady CJ, Müller DJ, Broz P, Hiller S (2016) GSDMD membrane pore formation constitutes the mechanism of pyroptotic cell death. EMBO J 35: 1766–1778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, Zhuang Y, Cai T, Wang F, Shao F (2015) Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526: 660–665 [DOI] [PubMed] [Google Scholar]
- Shi J, Gao W, Shao F (2017) Pyroptosis: gasdermin‐mediated programmed necrotic cell death. Trends Biochem Sci 42: 245–254 [DOI] [PubMed] [Google Scholar]
- Wang Y, Gao W, Shi X, Ding J, Liu W, He H, Wang K, Shao F (2017) Chemotherapy drugs induce pyroptosis through caspase‐3 cleavage of a gasdermin. Nature 547: 99–103 [DOI] [PubMed] [Google Scholar]


