Abstract
The scientific evidence supporting pulmonary rehabilitation (PR) for lung cancer patients undergoing cytotoxic chemotherapy is accumulating; however, the feasibility of outpatient‐based PR in these patients has not yet been evaluated in Korea. We conducted an eight‐week outpatient‐based PR feasibility study in a tertiary referral hospital setting. Patients with advanced lung cancer (non‐small cell lung cancer IIIB–IV and small‐cell lung cancer extensive disease) scheduled to undergo first‐line cytotoxic chemotherapy underwent PR consisting of 60‐minute sessions twice a week under the guidance and supervision of a physical therapist, for a total of eight weeks. Feasibility was assessed based on completion of the PR program. In total, 12 patients (median age 68 years) were enrolled; 11 (91.7%) were male with a history of smoking. Among these 12 patients, 9 (75%) completed the eight‐week outpatient‐based PR program. Three patients could not complete the PR program: two were unwilling and one died from complications of lung cancer. This study showed a 75% completion rate of an eight‐week outpatient‐based PR program for advanced lung cancer patients undergoing cytotoxic chemotherapy, which supports its feasibility.
Keywords: Cytotoxic chemotherapy, lung cancer, pulmonary rehabilitation
Introduction
Despite various treatment efforts, lung cancer remains the most common cause of cancer death in the world, primarily as a result of late diagnosis.1 The majority of patients diagnosed with advanced‐stage disease undergo systemic chemotherapy.2 Although new targeted and immunotherapies are in development, cytotoxic chemotherapy remains the preferred treatment for advanced‐stage lung cancer in eligible patients. However, it is essential that patients maintain good performance status to tolerate the rigors of cytotoxic chemotherapy.
Pulmonary rehabilitation (PR) is a multidisciplinary and comprehensive program for patients with chronic respiratory diseases.3 The benefits of PR have been demonstrated in various lung diseases, including chronic obstructive pulmonary disease (COPD)4 and restrictive lung diseases.5, 6 Evidence supporting the use of PR for lung cancer is accumulating.7, 8 In 2013, a Cochrane Review article based on multiple randomized controlled trials concluded that exercise before lung resection in lung cancer patients was helpful for improving postoperative exercise capacity.9 A few studies have reported the feasibility and safety of PR for patients with inoperable lung cancer.10, 11 These studies showed improvement or stability of exercise capacities in patients who completed a PR program. However, in terms of adherence, outpatient‐based PR is considered better than home‐based PR.10 Outpatient‐based PR for patients with advanced lung cancer is a part of multidisciplinary treatment; thus a feasibility study is necessary because of the different medical environments across regions and countries. Accordingly, the aim of the present pilot study was to evaluate the feasibility of an eight‐week outpatient‐based PR program for advanced lung cancer patients undergoing cytotoxic chemotherapy at a tertiary referral hospital in Korea.
Methods
Study design and ethics approval
This single‐center, prospective interventional study was conducted at the Seoul National University Hospital (SNUH, Seoul Korea) from 2014 to 2015. The SNUH institutional review board (H‐1401‐116‐549) approved the study protocol and informed written consent was obtained from all participants.
Subjects
Patients were recruited from among a group of advanced lung cancer (non‐small cell lung cancer [NSCLC] stage IIIB–IV or small cell lung cancer extensive disease [SCLC‐ED]) patients scheduled to undergo first‐line cytotoxic chemotherapy. The inclusion criteria were: age > 20 years and Eastern Cooperative Oncology Group (ECOG) performance status (PS) 0–1. Patients with histories of malignancy within five years, uncontrolled medical disease, or symptomatic bone metastasis to a weight‐bearing site (vertebra and/or femur) were excluded.
Outpatient‐based pulmonary rehabilitation program
Before commencing PR, exercise capacity was evaluated using a six‐minute walk test (6MWT) and a cardiopulmonary test on a cycle ergometer. The outpatient‐based PR program was eight consecutive weeks in duration, with two sessions per week. Guided and supervised 60 minute exercise sessions were conducted with a maximum of three patients per group. The physical therapist measured heart rate and oxygen saturation every 10 minutes to determine the duration of treatment. A typical session was performed in the following order: warm‐up (10 minutes); strengthening exercises (20 minutes); aerobic exercise (20 minutes); and cool‐down (10 minutes) (Fig 1). Strengthening exercises involved dumbbells weighted to 60–80% of the 1 repetition maximum, which was re‐measured every two weeks using the formula described by Epley12 to adjust the intensity of exercise. In aerobic exercise, a fixed cycle was used at an intensity in the range of 65–85% of maximum heart rate, which was based on age, with 13–15 point intensity according to the Borg scale.
Figure 1.
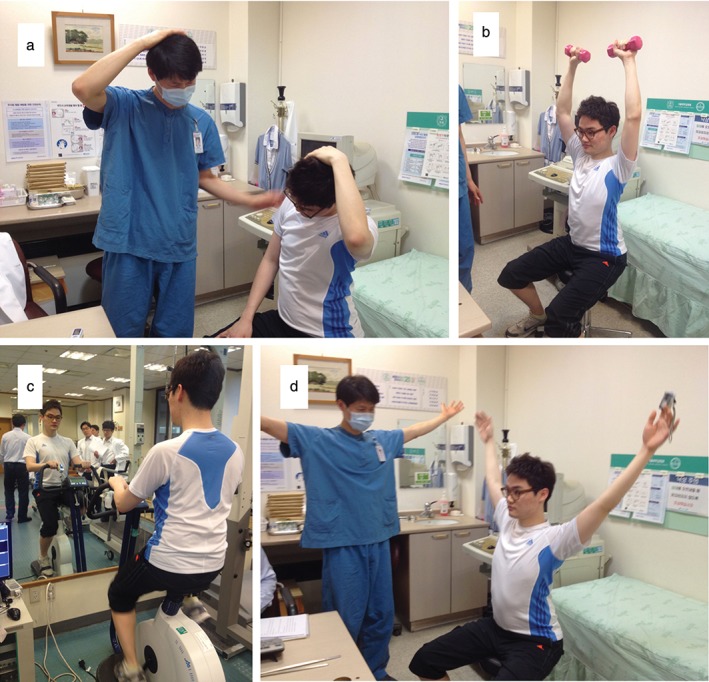
Representative demonstration of pulmonary rehabilitation: (a) warm‐up, (b) strengthening exercise, (c) aerobic exercise, and (d) cool‐down.
Statistical analysis
Statistical analysis was performed using Stata version 13.0 (StataCorp LP, College Station, TX, USA), and representative values were expressed as median ± quartile.
Results
From 2013 to 2014, 14 patients with advanced lung cancer were screened; two were excluded according to the protocol, leaving a total of 12 patients (11 men, 1 woman; median age 68 years). All of the men were smokers and 10 patients were diagnosed with NSCLC (Table 1). The initial ECOG PS results were 41.7% at PS 0 and 58.3% at PS 1. After measurement of exercise capacity, the eight‐week outpatient‐based PR program was started in conjunction with cytotoxic chemotherapy. The median 6MWT distance was 426 m and the maximal oxygen uptake (i.e. VO2 max) was 20.4 mL/kg/minutes (79.0%).
Table 1.
Baseline characteristics of participants
| Variables | Values |
|---|---|
| Age (years), median (IQR) | 68 (61–72) |
| Male (%) | 91.7 |
| Smoking (%) | |
| Never | 8.3 |
| Ever | 91.7 |
| ECOG PS (%) | |
| 0 | 41.7 |
| 1 | 58.3 |
| Pathology (%) | |
| Non‐small cell lung cancer | 83.3 |
| Small cell lung cancer | 16.7 |
| Stage (%) | |
| IIIB | 8.3 |
| IV | 75.0 |
| Extensive disease | 16.7 |
| Six‐minute walk distance (m) | 426 (375–449) |
| Pulmonary function test | |
| FVC (L) | 2.93 (2.85–3.56) |
| FVC pred. (%) | 81.5 (73.5–87.0) |
| FEV1 (L) | 2.24 (1.77–2.50) |
| FEV1 pred. (%) | 82.5 (60.0–86.5) |
| FEV1/FVC | 71.0 (65.5–77.0) |
| CPET | |
| Work (watts) | 60.5 (52.5–77.0) |
| Work pred. (%) | 42.5 (34.5–61.0) |
| VO2 max (mL/kg/minutes) | 20.4 (16.7–23.1) |
| VO2 max pred. (%) | 79.0 (72.5–99.0) |
| AT (% pred. Max VO2) | 42 (33–48) |
Values are presented median (IRQ)
AT, anaerobic threshold; CPET, cardiopulmonary exercise test; ECOG PS, Eastern Cooperative Oncology Group performance status; FVC, forced vital capacity; FEV1, forced expiratory volume in one second; IQR, interquartile range; VO2, maximal oxygen consumption.
Among the 12 patients, 9 successfully completed the program (Fig 2). Figure 3 shows the changes in ECOG PS scores before and after the eight‐week PR program. Two patients refused PR because they were unwilling, and one died from hemoptysis as a result of lung cancer progression.
Figure 2.
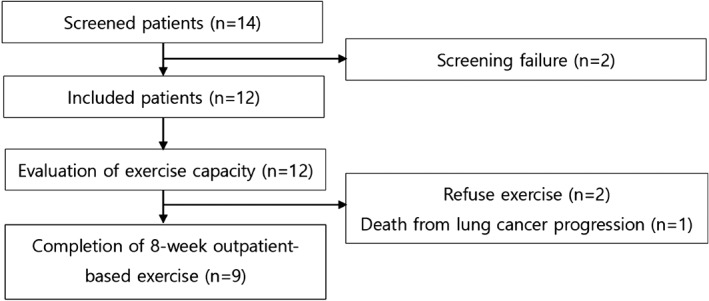
Flowchart of patient selection process.
Figure 3.
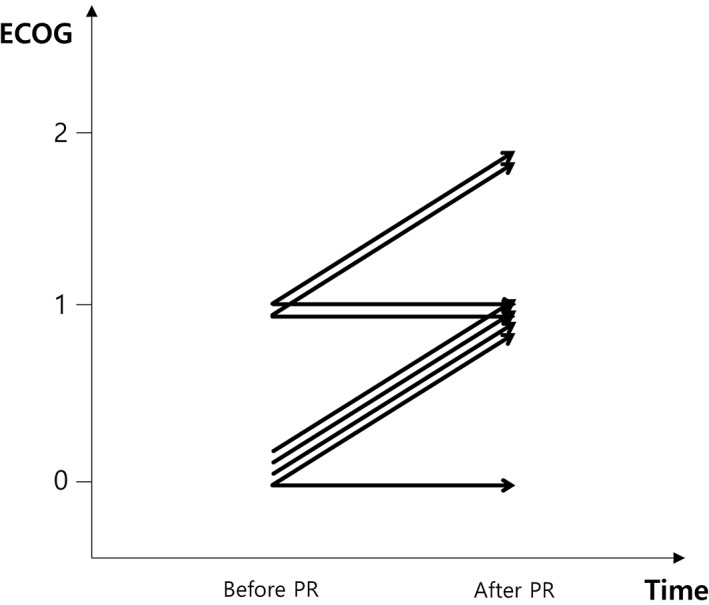
Change in Eastern Cooperative Oncology Group (ECOG) performance status before and after the eight‐week pulmonary rehabilitation (PR) program with cytotoxic chemotherapy. Each arrow represents one patient.
Discussion
The benefit of PR is well established for chronic respiratory disease, and PR should be considered an important part of an integrated patient management program for COPD.13 In lung cancer, exercise capacity measured according to 6MWT was statistically increased after completion of PR in patients who underwent curative lung resection.9 Recently, Olivier et al. reported the feasibility and safety of home‐based PR for patients with advanced lung cancer who were undergoing cytotoxic chemotherapy.11 The results of a randomized controlled trial are anticipated,14, 15 and evidence supporting the use of PR in lung cancer is accumulating.
Lung cancer patients are usually older and have a history of smoking, with various co‐morbidities including COPD and/or ischemic heart disease, that affect treatment. PR may have an influence on physical and psychological aspects. Possible benefits of PR for lung cancer patients undergoing cytotoxic chemotherapy include incremental improvement of exercise capacity and health‐related quality of life,16 and a decrease in cancer‐related fatigue.17 Maintaining a good general condition enables patients to withstand the rigors of additional chemotherapy.
An outpatient‐based PR program is a comprehensive and multidisciplinary treatment, and consumes significant hospital resources; however, accessibility is important to maintain a PR program. Accordingly, the aim of the present pilot study was to evaluate the feasibility of an eight‐week outpatient‐based RP program for lung cancer patients undergoing cytotoxic chemotherapy. We achieved a completion rate of 75% (9/12), which we believe supports the feasibility of such a program. However, there were some limitations to our study. First, the study population was small, given that during the two‐year study period only 14 patients were screened. The most common reasons for refusal were accessibility and unwillingness. It was difficult for patients to participate unless they resided near the hospital. Furthermore, there was considerable resistance to exercise during chemotherapy, which was not expected. To improve accessibility, a well‐structured home‐based PR program may be a viable alternative.18 Second, we could not evaluate treatment efficacy in our study population.
The completion rate of an eight‐week outpatient‐based PR program for advanced lung cancer patients undergoing cytotoxic chemotherapy was 75%, which supports its feasibility.
Disclosure
No authors report any conflict of interest.
Acknowledgment
This study received financial support from The Korean Association of Internal Medicine.
References
- 1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet‐Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65: 87–108. [DOI] [PubMed] [Google Scholar]
- 2. Morgensztern D, Ng SH, Gao F, Govindan R. Trends in stage distribution for patients with non‐small cell lung cancer: A National Cancer Database survey. J Thorac Oncol 2010; 5: 29–33. [DOI] [PubMed] [Google Scholar]
- 3. Nici L, Donner C, Wouters E et al American Thoracic Society/European Respiratory Society statement on pulmonary rehabilitation. Am J Respir Crit Care Med 2006; 173: 1390–413. [DOI] [PubMed] [Google Scholar]
- 4. Ries AL, Bauldoff GS, Carlin BW et al Pulmonary rehabilitation: Joint ACCP/AACVPR evidence‐based clinical practice guidelines. Chest 2007; 131 (5 Suppl): 4S–42S. [DOI] [PubMed] [Google Scholar]
- 5. Varadi RG, Goldstein RS. Pulmonary rehabilitation for restrictive lung diseases. Chest 2010; 137: 247–8. [DOI] [PubMed] [Google Scholar]
- 6. Ho SC, Lin HC, Kuo HP et al Exercise training with negative pressure ventilation improves exercise capacity in patients with severe restrictive lung disease: A prospective controlled study. Respir Res 2013; 14: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bobbio A, Chetta A, Ampollini L et al Preoperative pulmonary rehabilitation in patients undergoing lung resection for non‐small cell lung cancer. Eur J Cardiothorac Surg 2008; 33: 95–8. [DOI] [PubMed] [Google Scholar]
- 8. Bradley A, Marshall A, Stonehewer L et al Pulmonary rehabilitation programme for patients undergoing curative lung cancer surgery. Eur J Cardiothorac Surg 2013; 44: e266–71. [DOI] [PubMed] [Google Scholar]
- 9. Cavalheri V, Tahirah F, Nonoyama M, Jenkins S, Hill K. Exercise training undertaken by people within 12 months of lung resection for non‐small cell lung cancer. Cochrane Database Syst Rev 2013; CD009955. [DOI] [PubMed] [Google Scholar]
- 10. Quist M, Rørth M, Langer S et al Safety and feasibility of a combined exercise intervention for inoperable lung cancer patients undergoing chemotherapy: A pilot study. Lung Cancer 2012; 75: 203–8. [DOI] [PubMed] [Google Scholar]
- 11. Olivier C, Grosbois JM, Cortot AB et al Real‐life feasibility of home‐based pulmonary rehabilitation in chemotherapy‐treated patients with thoracic cancers: A pilot study. BMC Cancer 2018; 18: 178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Epley B. Poundage chart In: Boyd Epley Workout. Body Enterprises, Lincoln, NE: 1985; 86. [Google Scholar]
- 13. Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for Prevention, Diagnosis and Management of Chronic Obstructive Lung Disease 2018. Report. [Cited 3 Apr 2018.] Available from URL: https://goldcopd.org/wp-content/uploads/2017/11/GOLD-2018-v6.0-FINAL-revised-20-Nov_WMS.pdf
- 14. Quist M, Langer SW, Rørth M, Christensen KB, Adamsen L. "EXHALE": Exercise as a strategy for rehabilitation in advanced stage lung cancer patients: A randomized clinical trial comparing the effects of 12 weeks supervised exercise intervention versus usual care for advanced stage lung cancer patients. BMC Cancer 2013; 13: 477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fugazzaro S, Costi S, Mainini C et al PUREAIR protocol: Randomized controlled trial of intensive pulmonary rehabilitation versus standard care in patients undergoing surgical resection for lung cancer. BMC Cancer 2017; 17: 508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mishra SI, Scherer RW, Snyder C, Geigle PM, Berlanstein DR, Topaloglu O. Exercise interventions on health‐related quality of life for people with cancer during active treatment. Cochrane Database Syst Rev 2012; CD008465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cramp F, Byron‐Daniel J. Exercise for the management of cancer‐related fatigue in adults. Cochrane Database Syst Rev 2012; 11: CD006145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Holland AE, Mahal A, Hill CJ et al Home‐based rehabilitation for COPD using minimal resources: A randomised, controlled equivalence trial. Thorax 2017; 72: 57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]


