Abstract
Background
The study was conducted to evaluate the diagnostic performance of magnetic resonance imaging (MRI) for the detection of axillary lymph node metastasis in patients with breast cancer.
Methods
PubMed, Medline, Web of Science, Cochrane Embase, Chinese Biomedical Literature, and China National Knowledge Infrastructure databases were searched for open published studies relevant to the use of MRI for the detection of axillary lymph node metastasis in breast cancer patients. The pooled diagnostic sensitivity, specificity, and the symmetric receiver operating characteristic (SROC) curve was calculated by combining the individual data extracted from 26 included studies.
Results
The pooled diagnostic sensitivity and specificity of MRI to detect axillary lymph node metastasis in patients with breast cancer were 0.77 (95% confidence interval [CI] 0.75–0.80) and 0.90 (95% CI 0.89–0.91), respectively. The pooled positive and negative likelihood ratios were 7.67 (95% CI 5.09–11.53) and 0.23 (95% CI 0.17–0.32), respectively, by random effect method. The area under the SROC curve was 0.93 for MRI to detect axillary lymph node metastasis in breast cancer patients.
Conclusion
With high sensitivity, specificity, and area under the curve, MRI is an effective method to differentiate metastatic axillary lymph node in breast cancer patients, which can provide useful information for surgical procedure selection.
Keywords: Axillary lymph node metastasis, breast cancer, diagnosis, meta‐analysis
Introduction
Statistical studies of cancer have revealed that breast cancer is one of the most common malignant carcinomas diagnosed and the seconding leading cause of cancer related‐death in women.1, 2, 3 Axillary lymph node metastasis is common in breast cancer patients, which also affects the treatment modality and surgical procedure.4 Axillary lymph node status of patients with breast cancer is usually assessed by sentinel lymph node biopsy (SLNB), core needle biopsy (CNB), or fine needle aspiration cytology (FNAC). However, these procedures are mini‐invasive and potentially lead to implantation metastasis. Magnetic resonance imaging (MRI), with high resolution of different tissues, is widely used to diagnose many types of cancers and also affects treatment modality. Previously studies have evaluated its performance for evaluating the axillary lymph node status of patients with breast cancer. However, because of the small sample sizes of previous studies, statistical research is limited. In our present study, we searched open published studies relevant to MRI for the detection of axillary lymph node metastasis in patients with breast cancer and conducted a meta‐analysis to further evaluate diagnostic performance.
Methods
Publication searching
PubMed, Medline, Web of Science, Cochrane Embase, Chinese Biomedical Literature, and China National Knowledge Infrastructure databases were searched for open published studies relevant to the use of MRI to detect axillary lymph node metastasis in patients with breast cancer. The search procedure is demonstrated in Figure 1. The terms “breast cancer” OR “breast carcinoma” OR “breast neoplasm” And “axillary lymph node” And “MRI” OR “magnetic resonance imaging” OR “MR” were used.
Figure 1.
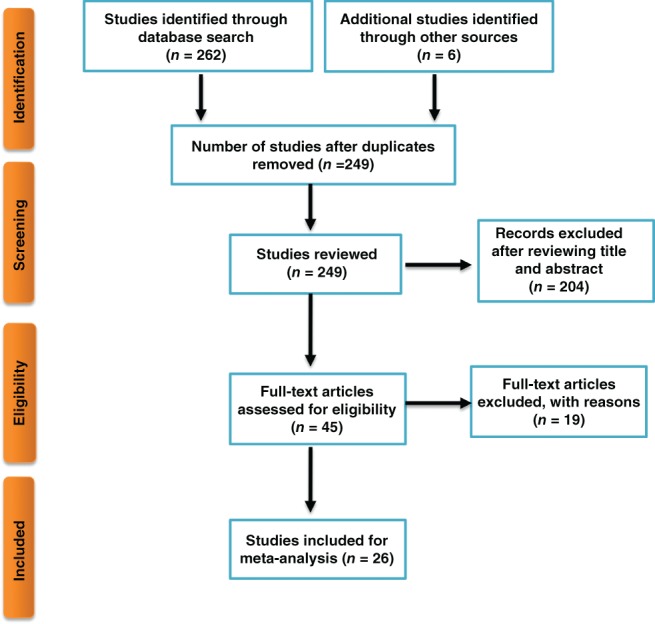
Search procedure.
Study inclusion and exclusion
Two reviewers drew up the inclusion and exclusion criteria and crosschecked the data. The inclusion criteria were: (i) prospective or retrospective diagnostic studies; (ii) studies relevant to the evaluation of MRI to detect axillary lymph node metastasis in patients with breast cancer; (iii) the axillary lymph node metastasis was confirmed by pathological examination; and (iv) the number of true positive (TP), false positive (FP), false negative (FN), and true negative (TN) results could be extracted from each individual study. The exclusion criteria were: (i) duplicate publications or data; (ii) literature review of case reports; (iii) malignant carcinoma other than breast cancer; and (iv) diagnostic data could not be extracted from individual studies.
Initially, 268 relevant studies were identified. Nineteen duplicated publications or data were excluded. After reading the title and abstract, 204 studies were excluded. After review of the full text, a further 19 studies were excluded. Twenty‐six open published studies were finally included in the meta‐analysis (Fig 1). The main features of the included 26 studies are listed in Table 1.
Table 1.
General characteristics of the included publications
| First author | Year | Country | Study type | Reference standard | Lymph node | Age (year) | TP | FP | FN | TN |
|---|---|---|---|---|---|---|---|---|---|---|
| Chen5 | 2014 | China | NR | ALND/CNB | 154 | 21–82 | 70 | 8 | 17 | 59 |
| Meng6 | 2013 | China | NR | SLNB | 35 | 35–61 | 10 | 0 | 1 | 24 |
| Wang7 | 2013 | China | Retrospective | ALND/SLNB | 136 | 21–77 | 55 | 11 | 8 | 62 |
| Xie8 | 2014 | China | Retrospective | ALND/SLNB | 193 | 21–73 | 60 | 9 | 20 | 104 |
| Xu9 | 2004 | China | NR | ALND | 17 | 34–70 | 9 | 2 | 2 | 4 |
| Yin10 | 2013 | China | NR | ALND | 268 | NR | 63 | 52 | 15 | 148 |
| Du11 | 2017 | China | NR | ALND | 229 | NR | 91 | 17 | 11 | 110 |
| Harada12 | 2007 | Japan | Prospective | ALND | 506 | 36–77 | 42 | 73 | 23 | 368 |
| Kimura13 | 2009 | Japan | Prospective | ALND | 10 | 35–79 | 2 | 0 | 0 | 8 |
| Kvistad14 | 2000 | Norway | NR | ALND | 65 | 38–78 | 6 | 1 | 18 | 40 |
| Memarsadeghi15 | 2006 | Australia | Prospective | ALND | 22 | 40–79 | 6 | 23 | 5 | 99 |
| Michel16 | 2002 | Switzerland | Prospective | ALND | 18 | 22–76 | 25 | 9 | 5 | 243 |
| Mumtaz17 | 1997 | UK | NR | ALND | 75 | 29–80 | 21 | 5 | 5 | 64 |
| García Fernández18 | 2011 | Spain | Prospective | ALND | 105 | 52.5 | 14 | 3 | 25 | 63 |
| Hwang19 | 2013 | Korea | Prospective | ALND/SLNB | 349 | 25–79 | 44 | 29 | 48 | 228 |
| Javid20 | 2010 | USA | Prospective | ALND | 47 | NR | 24 | 2 | 4 | 17 |
| Chung21 | 2013 | USA | Prospective | ALND | 110 | NR | 68 | 7 | 0 | 35 |
| Fornasa22 | 2012 | Italy | Prospective | ALND | 43 | NR | 18 | 2 | 1 | 22 |
| Rautiainen23 | 2015 | Finland | Prospective | ALND | 32 | 28–82 | 22 | 0 | 3 | 7 |
| He24 | 2012 | China | Retrospective | ALND | 1242 | NR | 108 | 26 | 34 | 1074 |
| Kamitani25 | 2013 | Japan | Retrospective | ALND | 110 | 25–81 | 24 | 47 | 2 | 37 |
| Kim26 | 2014 | Korea | NR | ALND | 253 | 28–82 | 69 | 26 | 22 | 136 |
| Li27 | 2014 | China | Prospective | SLNB | 121 | 30–58 | 53 | 1 | 3 | 64 |
| Luo28 | 2013 | China | NR | ALND | 78 | 30–63 | 38 | 4 | 7 | 30 |
| Motomura29 | 2011 | Japan | Prospective | SLNB | 102 | 57 | 21 | 7 | 4 | 70 |
| Nakai30 | 2011 | Japan | Prospective | ALND | 216 | 36–77 | 30 | 4 | 6 | 176 |
ALND, axillary lymph node dissection; FN, false negative; FP, false positive; NR, not reported; SLNB, sentinel lymph node biopsy; TN, true negative; TP, true positive.
Data extraction
Two reviewers independently reviewed the data of each included study. General information, such as sample size, year of publication, diagnostic gold standard, and patient age, were extracted. The number of TP, FP, FN, and TN results were carefully extracted from each study and crosschecked.
Statistical methods
All analysis was performed using MetaDiSc 1.4 software (http://www.hrc.es/investigacion/metadisc_en.htm). Statistical heterogeneity across the 26 included studies was assessed by I2 test. The data was pooled by fixed or random effect method according to the heterogeneity. Publication bias was assessed by line regression test. Diagnostic sensitivity and specificity was calculated using the formula: sensitivity = TP/(TP + FN) and specificity = TN/(TN + FP). P < 0.05 was considered statistically significant.
Results
Sensitivity and specificity analysis
The diagnostic sensitivity and specificity of MRI to detect axillary lymph node metastasis in patients with breast cancer were pooled by random effect method because of significant statistical heterogeneity (I2 = 86.6% for sensitivity; I2 = 92.5% for specificity). The pooled sensitivity and specificity were 0.77 (95% confidence interval [CI] 0.75–0.80) (Fig 2) and 0.90 (95% CI 0.89–0.91) (Fig 3), respectively.
Figure 2.
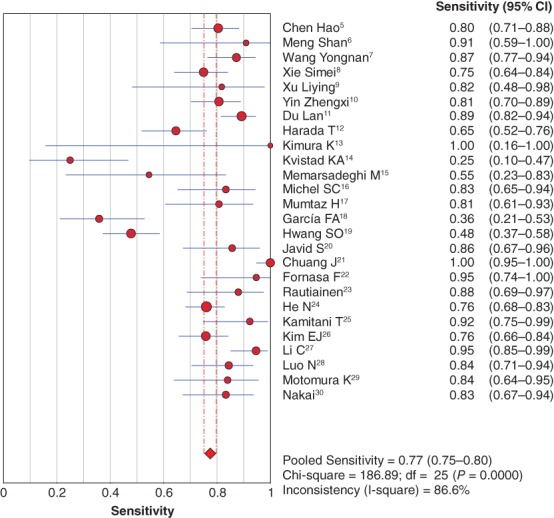
Forest plot of pooled diagnostic sensitivity. CI, confidence interval.
Figure 3.
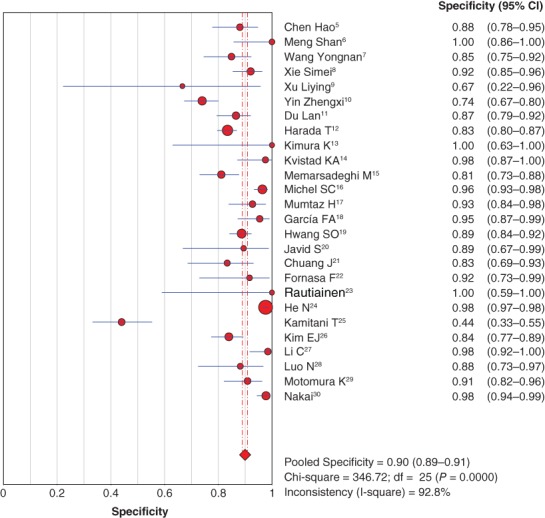
Forest plot of pooled diagnostic specificity. CI, confidence interval.
Positive and negative likelihood ratios
The pooled positive and negative likelihood ratios were 7.67 (95% CI 5.09–11.53) (Fig 4) and 0.23 (95% CI 0.17–0.32), respectively, by random effect method (Fig 5).
Figure 4.
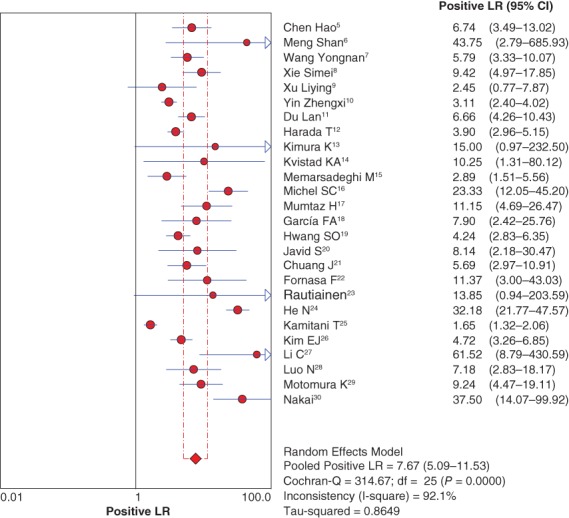
Forest plot of pooled positive likelihood ratio (LR). CI, confidence interval.
Figure 5.
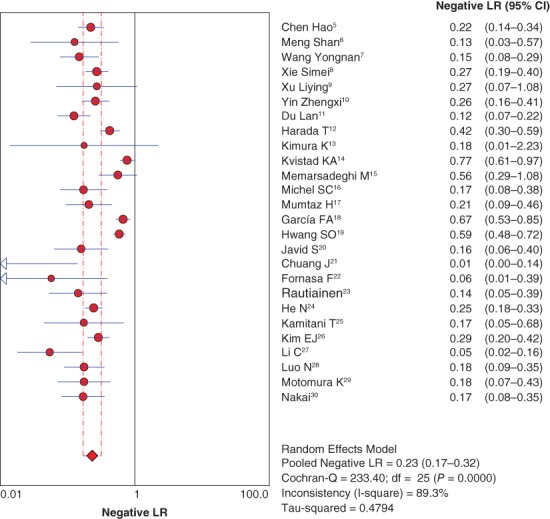
Forest plot of pooled negative likelihood ratio (LR). CI, confidence interval.
Diagnostic odds ratio
Because of significant heterogeneity across the included 26 studies (I2 = 80.9%; P < 0.05), the diagnostic odds ratio (DOR) was pooled by random effect method. The pooled DOR was 36.69, with a 95% confidence interval of 22.09–60.92 (Fig 6).
Figure 6.
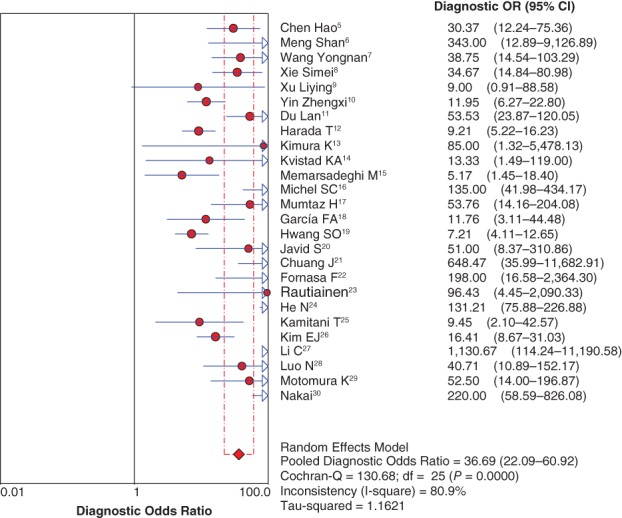
Forest plot of diagnostic odds ratio (OR). CI, confidence interval.
Symmetric receiver operating characteristic curve
The symmetric receiver operating characteristic (SROC) curve was calculated by sensitivity against 1‐specificity using MetaDiSc 1.4 software. The area under the SROC curve (AUC) was 0.93 for MRI for the detection of axillary lymph node metastasis in breast cancer patients (Fig 7).
Figure 7.
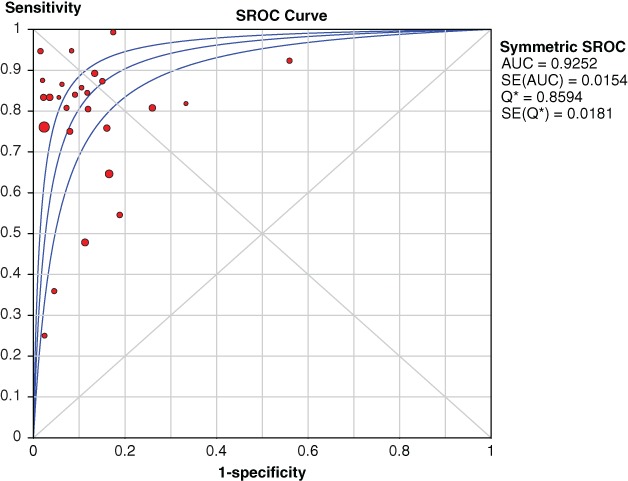
Pooled symmetric receiver operating characteristic (SROC) curves. AUC, area under the curve; SE, standard error.
Evaluation of publication bias
The studies were evaluated by line regression test and no significant publication bias was observed (t = 0.33; P > 0.05) (Fig 8).
Figure 8.
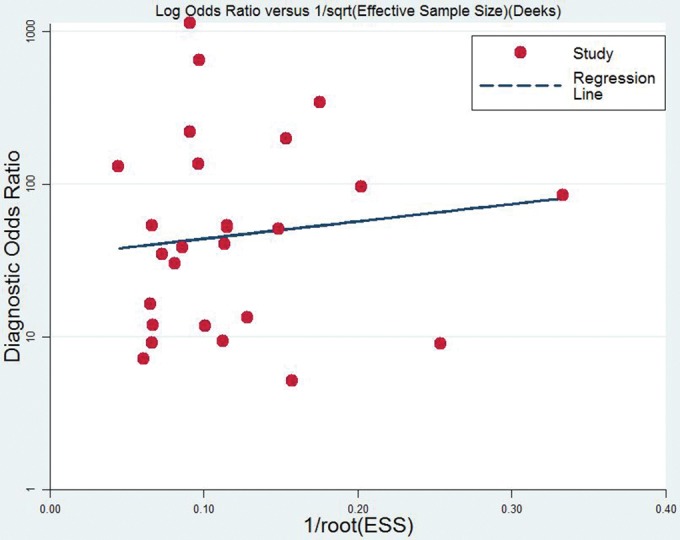
Egger's line regression test to evaluate publication bias.
Discussion
Recent studies have shown that breast cancer has become one of the leading causes of cancer‐related death worldwide. It is estimated that 266 120 new breast cancer patients will be diagnosed in the United States in 2018.31 Breast cancer is the most commonly diagnosed malignant carcinoma in women. Fortunately, the long‐term (five‐year) survival rate of breast cancer in women is approximately 90%.
As described in previous studies, axillary lymph node involvement is one the key factors relevant to prognosis in breast cancer patients. A clear understanding of the status of axillary lymph nodes in breast cancer patients is important not only for prognosis but also to select treatment modality or surgical method. Axillary lymph node dissection (ALND) is the reference standard for evaluating lymph node involvement. However, approximately 40–70% of breast cancer patients have histopathologically negative axillary lymph nodes32 indicating that 40–70% of breast cancer patients undergo unnecessary invasive examinations. Determining axillary lymph node involvement before treatment or dissection is important to reduce such examinations.
The diagnostic performance of MRI to detect axillary lymph node metastasis in patients with breast cancer has been widely discussed. However, the exact diagnostic performance of MRI for discriminating axillary lymph node involvement is not fully understood because of the inconsistent results reported by previous studies.25, 26, 28 These inconsistencies may be the result of: (i) small sample sizes with limited statistical power; (ii) patient inclusion criteria; (iii) MRI examination technology; (iv) types of MRI instruments used; and (v) different reference standards for axillary lymph node involvement.
In our present study, we included 26 studies that evaluated the diagnostic performance of MRI to determine axillary lymph node involvement in breast cancer and pooled the diagnostic sensitivity (0.77, 95% CI 0.75–0.80), specificity (0.90, 95% CI 0.89–0.91), and SROC (0.92) to further assess its value in clinical application. However, there are some limitations to our meta‐analysis. Firstly, we only searched for studies published in English or Chinese, which may have led to publication selection bias. Secondly, significant statistical heterogeneity existed in the sensitivity, specificity, positive and negative likelihood ratios, and ROC effect sizes. Thirdly, not all of the included studies were prospective, reducing the reliability of our results.
Our results show that MRI is an effective method for differentiating metastatic axillary lymph nodes in breast cancer patients because of high sensitivity, specificity, and AUC, and thus can provide useful information for surgical procedure selection.
Disclosure
No authors report any conflict of interest.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017; 67: 7–30. [DOI] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016; 66: 7–30. [DOI] [PubMed] [Google Scholar]
- 3. Li S, Wang X, Yang J et al Clinicopathological features and survival of early stage breast cancer in northwest China: A population‐based retrospective study of 1287 patients. Thorac Cancer 2018; 9: 10–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jessing C, Langhans L, Jensen MB, Talman ML, Tvedskov TF, Kroman N. Axillary lymph node dissection in breast cancer patients after sentinel node biopsy. Acta Oncol 2018; 57: 166–9. [DOI] [PubMed] [Google Scholar]
- 5. Chen H, Sun Y, Yuan F, Hong N. [Diffusion‐weighted imaging in diagnosis of axillary metastatic lymph nodes in patients with breast invasive ductal carcinoma.] Chin J Intervent Imaging Ther 2014; 11: 92–5 (In Chinese.) [Google Scholar]
- 6. Meng S, Li CM, Yang XH, Liu HX, Tang P, Wang J. [Interstitial MR lymphography in diagnosis of axillary lymph node metastasis of breast cancer.] Chin J Med Imag Technol 2013; 29: 1977–80 (In Chinese.) [Google Scholar]
- 7. Wang YN, Zhang AQ, Wan J et al Diagnostic value of MRI in axillary lymph node metastasis of early breast cancer. Int J Surg 2013; 20: 153–6. [Google Scholar]
- 8. Xie SM, Zhang AQ, Zhu CX, Lian ZQ, Zhang Y, Wang Q. [Physical examination and preoperative imaging methods in the prediction of axillary lymph node metastasis in breast cancer.] Chin J Cancer Prev Treat 2014; 21: 1179–83 (In Chinese.) [Google Scholar]
- 9. Xu LY, Xu HB, Yu Q. Clinical evaluation of MRI in the diagnosis of breast cancer. J Clin Radiol 2004; 23: 27–30. [Google Scholar]
- 10. Yin ZX, Shen KW, Li YF, He JR. [The accuracy of preoperatively predicting axillary lymph node status in breast cancer patients by ultrasonography and MRI.] Chin J Gen Surg 2013; 26: 259–62 (In Chinese.) [Google Scholar]
- 11. Du L, Yang JC, Chang Y. [Comparison of diagnostic value of breast MRI and ultrasonography in axillary lymph node metastasis of breast cancer.] Chin Med Herald 2017; 14: 168–71 (In Chinese.) [Google Scholar]
- 12. Harada T, Tanigawa N, Matsuki M, Nohara T, Narabayashi I. Evaluation of lymph node metastases of breast cancer using ultrasmall superparamagnetic iron oxide‐enhanced magnetic resonance imaging. Eur J Radiol 2007; 63: 401–7. [DOI] [PubMed] [Google Scholar]
- 13. Kimura K, Tanigawa N, Matsuki M et al High‐resolution MR lymphography using ultrasmall superparamagnetic iron oxide (USPIO) in the evaluation of axillary lymph nodes in patients with early stage breast cancer: Preliminary results. Breast Cancer 2010; 17: 241–6. [DOI] [PubMed] [Google Scholar]
- 14. Kvistad KA, Rydland J, Smethurst HB, Lundgren S, Fjøsne HE, Haraldseth O. Axillary lymph node metastases in breast cancer: Preoperative detection with dynamic contrast‐enhanced MRI. Eur Radiol 2000; 10: 1464–71. [DOI] [PubMed] [Google Scholar]
- 15. Memarsadeghi M, Riedl CC, Kaneider A et al Axillary lymph node metastases in patients with breast carcinomas: Assessment with nonenhanced versus uspio‐enhanced MR imaging. Radiology 2006; 241: 367–77. [DOI] [PubMed] [Google Scholar]
- 16. Michel SC, Keller TM, Fröhlich JM et al Preoperative breast cancer staging: MR imaging of the axilla with ultrasmall superparamagnetic iron oxide enhancement. Radiology 2002; 225: 527–36. [DOI] [PubMed] [Google Scholar]
- 17. Mumtaz H, Hall‐Craggs MA, Davidson T et al Staging of symptomatic primary breast cancer with MR imaging. AJR Am J Roentgenol 1997; 169: 417–24. [DOI] [PubMed] [Google Scholar]
- 18. García Fernández A, Fraile M, Giménez N et al Use of axillary ultrasound, ultrasound‐fine needle aspiration biopsy and magnetic resonance imaging in the preoperative triage of breast cancer patients considered for sentinel node biopsy. Ultrasound Med Biol 2011; 37: 16–22. [DOI] [PubMed] [Google Scholar]
- 19. Hwang SO, Lee SW, Kim HJ, Kim WW, Park HY, Jung JH. The comparative study of ultrasonography, contrast‐enhanced MRI, and (18)F‐FDG PET/CT for detecting axillary lymph node metastasis in T1 breast cancer. J Breast Cancer 2013; 16: 315–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Javid S, Segara D, Lotfi P, Raza S, Golshan M. Can breast MRI predict axillary lymph node metastasis in women undergoing neoadjuvant chemotherapy. Ann Surg Oncol 2010; 17: 1841–6. [DOI] [PubMed] [Google Scholar]
- 21. Chung J, Youk JH, Kim JA et al Role of diffusion‐weighted MRI: Predicting axillary lymph node metastases in breast cancer. Acta Radiol 2014; 55: 909–16. [DOI] [PubMed] [Google Scholar]
- 22. Fornasa F, Nesoti MV, Bovo C, Bonavina MG. Diffusion‐weighted magnetic resonance imaging in the characterization of axillary lymph nodes in patients with breast cancer. J Magn Reson Imaging 2012; 36: 858–64. [DOI] [PubMed] [Google Scholar]
- 23. Rautiainen S, Könönen M, Sironen R et al Preoperative axillary staging with 3.0‐T breast MRI: Clinical value of diffusion imaging and apparent diffusion coefficient. (Published erratum appears in PLoS One 2015; 10: e0133111). PLoS One 2015; 10: e0122516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. He N, Xie C, Wei W et al A new, preoperative, MRI‐based scoring system for diagnosing malignant axillary lymph nodes in women evaluated for breast cancer. Eur J Radiol 2012; 81: 2602–12. [DOI] [PubMed] [Google Scholar]
- 25. Kamitani T, Hatakenaka M, Yabuuchi H et al Detection of axillary node metastasis using diffusion‐weighted MRI in breast cancer. Clin Imaging 2013; 37: 56–61. [DOI] [PubMed] [Google Scholar]
- 26. Kim EJ, Kim SH, Kang BJ, Choi BG, Song BJ, Choi JJ. Diagnostic value of breast MRI for predicting metastatic axillary lymph nodes in breast cancer patients: Diffusion‐weighted MRI and conventional MRI. Magn Reson Imaging 2014; 32: 1230–6. [DOI] [PubMed] [Google Scholar]
- 27. Li C, Meng S, Yang X, Wang J, Hu J. The value of T2* in differentiating metastatic from benign axillary lymph nodes in patients with breast cancer‐‐a preliminary in vivo study. PLoS One 2014; 9: e84038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Luo N, Su D, Jin G et al Apparent diffusion coefficient ratio between axillary lymph node with primary tumor to detect nodal metastasis in breast cancer patients. J Magn Reson Imaging 2013; 38: 824–8. [DOI] [PubMed] [Google Scholar]
- 29. Motomura K, Ishitobi M, Komoike Y et al SPIO‐enhanced magnetic resonance imaging for the detection of metastases in sentinel nodes localized by computed tomography lymphography in patients with breast cancer. Ann Surg Oncol 2011; 18: 3422–9. [DOI] [PubMed] [Google Scholar]
- 30. Nakai G, Matsuki M, Harada T et al Evaluation of axillary lymph nodes by diffusion‐weighted MRI using ultrasmall superparamagnetic iron oxide in patients with breast cancer: Initial clinical experience. J Magn Reson Imaging 2011; 34: 557–62. [DOI] [PubMed] [Google Scholar]
- 31. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018; 68: 7–30. [DOI] [PubMed] [Google Scholar]
- 32. Kinne DW. Axillary clearance in operable breast cancer: Still a necessity. Recent Results Cancer Res 1998; 152: 161–9. [DOI] [PubMed] [Google Scholar]


