Abstract
Background
This study was conducted to investigate the correlation between clinicopathological features and post‐therapeutic response in esophageal squamous cell carcinoma (ESCC) patients. Peripheral blood circulating tumor cells (CTCs) expressing epithelial‐mesenchymal transition markers were identified.
Methods
Peripheral blood samples were collected from 71 patients with newly diagnosed ESCC and 40 healthy volunteers. CTCs were isolated using CanPatrol CTC enrichment technology. RNA‐fluorescent in situ hybridization was used to phenotype the CTCs on the basis of epithelial and/or mesenchymal markers.
Results
The median mesenchymal CTC counts in 71 patients were: 0 in 19 stage I patients, 2 in 31 stage II, and 3 in 21 stage III/IV. The overall diagnostic performance of total CTCs to correctly identify ESCC patients was 0.991. We observed a correlation between increases in tumor size or advanced stage and an increased number of mesenchymal CTCs (P < 0.05). Thirty‐nine patients were administered two cycles of neoadjuvant chemotherapy and their therapeutic response was evaluated: 2 complete response, 20 partial response, 13 stable disease, and 4 progressive disease. After treatment, the positive rate of mesenchymal CTCs was 70.6% in the progressive and stable disease group versus 36.4% in the complete and partial response group (P = 0.05).
Conclusion
The results showed that mesenchymal CTC count is related to ESCC clinical stage and the efficacy of neoadjuvant chemotherapy.
Keywords: Circulating tumor cell, epithelial‐mesenchymal transition, esophageal squamous cell carcinoma
Introduction
Esophageal cancer is the eighth most common cancer in the world, with more than 450 000 new patients diagnosed per year and a five‐year overall survival rate of only 15–25%.1, 2, 3 Currently, surgical resection is the primary treatment for esophageal cancer but incidence and mortality rates remain high, and the remission rate after esophagectomy is relatively low. In addition, radical resection is only recommended for patients who can tolerate surgery and whose tumors can be complete removed.4 Although endoscopy can detect esophageal cancer early, most tumors are diagnosed after regional (30%) or distant metastases (40%) have occurred, thus precluding the opportunity of surgery.5 Therefore, a specific esophageal cancer marker that can predict the metastatic potential of a tumor would greatly help to select the optimum treatment and evaluate treatment efficacy.
In recent years, the role of peripheral blood circulating tumor cells (CTCs) in the metastasis of malignant tumors has gained attention. Studies have found that at the onset of cancer, malignant growth is localized to specific organs; eventually however, almost all tumors metastasize to distant organs via the bloodstream, and this distant metastasis is the leading cause of death of cancer patients. Metastasis begins with epithelial‐mesenchymal transition (EMT), a phenomenon common in normal physiological processes, and critical during tumor invasion and metastasis.6 It is characterized by the gradual evolution of epithelial cells into cells with mesenchymal properties, which enable them to invade the bloodstream and form various CTCs of specific phenotypes.7, 8 The expression of mesenchymal cell surface markers on CTCs, such as vimentin and fibronectin, is linked with the tumor metastasis cascade.9 By producing tumor cells of intermediate phenotypes, EMT is also responsible for primary tumor heterogeneity. Furthermore, the invasiveness resulting from EMT contributes to the production of more CTCs, which eventually extravasate and proliferate in distant tissues and organs, leading to metastasis. During formation of the metastatic lesion, a reverse EMT process can re‐differentiate the tumor cells, thus completing the basic process of tumor metastasis.6, 8 While CTC detection using epithelial markers has become common in recent years, classification of CTCs based on EMT phenotypes still faces many challenges. In this study, CanPatrol CTC enrichment technology was used to separate and classify CTCs into epithelial, hybrid, and mesenchymal based on the expression of epithelial and mesenchymal markers.
At present, the main factors determining the treatment regimen and prognostic evaluation of patients with ESCC are the location of the lesion, the depth of invasion, and the metastatic status at the time of diagnosis. However, there are reports of early‐stage esophageal squamous cell carcinoma (ESCC) patients experiencing recurrence or distant metastasis shortly after treatment, as well as patients at advanced ESCC stage surviving much longer.5, 10 There is a strong possibility that this is a result of the different molecular and biological properties of tumors, including the number of CTCs undergoing EMT. In addition, tumor transition from a localized to a systemic disease is accompanied by CTCs undergoing hematological and metastatic proliferation, which can be detected by different assays.
We assessed the correlation between the clinical stage and local progression of ESCC tumors with CTCs expressing EMT markers. However, it remains unclear whether such heterogeneity exists in CTCs from ESCC patients, and if it does exist, whether it is related to clinical stage, tumor metastasis, and remission of ESCC.
Methods
Patients
Enrollment and clinical staging of tumors
Patients diagnosed with ESCC at the Henan Provincial Cancer Hospital from November 2016 to March 2017 were enrolled in the study. Negative control blood samples were acquired from 40 healthy volunteers. The median age of the ESCC patients was 62.68 ± 7.99 years. Patients were subjected to endoscopic ultrasound (EUS), along‐with chest and abdominal enhanced computed tomography (CT) scans to carry out a preoperative assessment of the clinical stage of ESCC and the status of distant metastasis. Test results were used to make a decision as to whether surgery should be performed. Patients with large tumors, tumor invasion to the adjacent structure, and multiple lymph node or organ metastases were excluded. The clinical staging of the tumors was evaluated according to the seventh edition of the Cancer Staging Manual published by the American Joint Committee on Cancer.
Neoadjuvant chemotherapy
Patients with locally advanced resectable ESCC (cT1‐4aN+M0 or cT3‐4aN0M0) who could not undergo direct surgery were administered two cycles of neoadjuvant chemotherapy consisting of intravenous (IV) drips of 70 mg/m2 paclitaxel on days 1 and 8, and an IV drip of 30 mg/m2 nedaplatin on days 1–3. Each chemotherapy cycle took three weeks, and diagnostic imaging was performed two weeks after the last chemotherapy to re‐evaluate the possibility of surgical treatment. None of the patients had received anti‐tumor therapy prior to this treatment and none had a history of tumors.
Circulating tumor cell (CTC) enrichment and characterization
Isolation and enrichment of CTCs from peripheral blood
The CanPatrol system (SurExam, Guangzhou, China) was used to isolate and enrich CTCs from peripheral blood, as previously described.11 A venous puncture was made to collect a 5 mL blood sample from each subject into an ethylene‐diamine‐tetraacetic acid tube. The erythrocytes were then lysed using a red cell lysis buffer (154 mm NH4Cl, 10 mm KHCO3, and 0.1 mm ethylene‐diamine‐tetraacetic acid in deionized water), and filtered out with an 8 μm diameter pore calibrated membrane (Millipore, Billerica, MA, USA). The filtration system consists of the following components: a filtration tube containing the membrane, a manifold vacuum plate with valve settings (SurExam); an E‐Z 96 Vacuum Manifold (Omega, Norcross, GA, USA); and a mini table vacuum pump (Auto Science, Tianjin, China). Following filtration, the remaining cells were re‐suspended in phosphate buffered saline containing 4% formaldehyde (Sigma, St. Louis, MO, USA) for five minutes, transferred into a filter tube, and re‐filtered. The CTCs were separated and enriched from other blood cells on the basis of size differences between the CTCs and the other cells.12 The pumping valve was set to generate a vacuum pressure of at least 0.08 MPa.
RNA fluorescence in situ hybridization (RNA‐FISH) assay
Circulating tumor cell phenotyping was performed using tri‐colored RNA‐fluorescence in situ hybridization (FISH) assay. The CTC phenotype specific nucleic acid probes, their sequences, and their fluorescent labels are shown in Table 1.
Table 1.
Sequence of RNA probes
| Gene | Sequence(5′ → 3′) |
|---|---|
| EpCAM | TGGTGCTCGTTGATGAGTCAAGCCAGCTTTGAGCAAATGA |
| AAAGCCCATCATTGTTCTGGCTCTCATCGCAGTCAGGATC | |
| TCCTTGTCTGTTCTTCTGACCTCAGAGCAGGTTATTTCAG | |
| CK8 | CGTACCTTGTCTATGAAGGAACTTGGTCTCCAGCATCTTG |
| CCTAAGGTTGTTGATGTAGCCTGAGGAAGTTGATCTCGTC | |
| CAGATGTGTCCGAGATCTGGTGACCTCAGCAATGATGCTG | |
| CK19 | CTGTAGGAAGTCATGGCGAGAAGTCATCTGCAGCCAGACG |
| CTGTTCCGTCTCAAACTTGGTTCTTCTTCAGGTAGGCCAG | |
| CTCAGCGTACTGATTTCCTCGTGAACCAGGCTTCAGCATC | |
| Vimentin | GAGCGAGAGTGGCAGAGGACCTTTGTCGTTGGTTAGCTGG |
| CATATTGCTGACGTACGTCAGAGCGCCCCTAAGTTTTTAA | |
| AAGATTGCAGGGTGTTTTCGGGCCAATAGTGTCTTGGTAG | |
| Twist | ACAATGACATCTAGGTCTCCCTGGTAGAGGAAGTCGATGT |
| CAACTGTTCAGACTTCTATCCCTCTTGAGAATGCATGCAT | |
| TTTCAGTGGCTGATTGGCACTTACCATGGGTCCTCAATAA | |
| CD45 | TCGCAATTCTTATGCGACTCTGTCATGGAGACAGTCATGT |
| GTATTTCCAGCTTCAACTTCCCATCAATATAGCTGGCATT | |
| TTGTGCAGCAATGTATTTCCTACTTGAACCATCAGGCATC |
Tri‐colored RNA‐FISH based on the signal amplification of branching DNA (bDNA) was performed. The sensitivity of this assay depends not on in vitro amplification of the target sequences but rather on the signal amplification of bDNA probes that bind to these sequences after direct binding of capture probes to the target sequences. The bDNA signal amplification probes contain three types of probe sequences: pre‐amplification, amplification, and labeled probes. A specific and contiguous region on the pre‐amplification sequence first hybridizes to the capture probes, followed by the hybridization of the remaining specific regions to multiple bDNA amplification sequences to create a branched structure. Finally, the fluorescent‐labeled probes, whose sequences are complementary to the bDNA amplicons, hybridize to those amplicons to visualize the CTC specific target sequences. This method uses multiple RNA probes simultaneously to capture target cells, in this case CTCs. After the RNA probes hybridize to the target gene, the sensitivity of single copy messenger RNA detection can be multiplied through the fluorescence signal amplification system.11, 13
Statistical analysis
The continuous variables that followed normal distribution were expressed as means and standard deviation, while those that did not fit into normal distribution were expressed as medians and quartiles. Categorical variables were expressed as frequency and percentage. A Mann–Whitney U test was used to analyze the statistical differences between the CTC counts of different N stages and treatment modalities. The correlation between tumor location, clinical staging, and CTC counts was analyzed using a Kruskal–Wallis H test. The correlation between tumor size and CTC counts was determined by Spearman's rank correlation analysis. A Fisher's exact test was used to analyze the correlation between the efficacy of neoadjuvant chemotherapy and the positive rate of mesenchymal CTCs, whereas the changes in mesenchymal CTC counts before and after treatment were analyzed using the Wilcoxon signed‐rank test. Logistic regression analysis was conducted to ascertain whether mesenchymal CTCs were an independent factor influencing the treatment scheme. All P values were examined using two‐sided tests and all statistical analyses were performed using SPSS version 21.0 (IBM Corp., Armonk, NY, USA) and Prism 5.0 (GraphPad Software Inc., San Diego, CA, USA). A P value of < 0.05 was considered statistically significant.
Results
Isolation of CTCs and phenotyping by RNA‐FISH
Circulating tumor cells from 71 ESCC patients were enriched using the CanPatrol system. The CTC counts in different patients ranged from 0 to 104, with a median value of 20. Multiple RNA probes, each targeted against a different CTC‐specific gene and labeled respectively with different fluorescent dyes, were used to subphenotype the CTCs. As shown in Figure 1, epithelial CTCs showed red fluorescence (Fig 1a), while mesenchymal CTCs showed green fluorescence (Fig 1c), corresponding to their specific genes (i.e. EpCAM or CK, and vimentin and Twist), respectively. In addition, a third hybrid population of CTCs expressing both epithelial and mesenchymal specific genes was also observed (Fig 1b).
Figure 1.
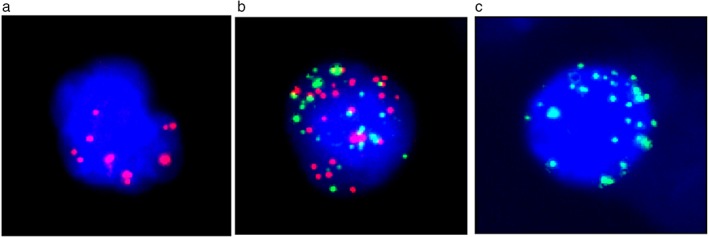
(a) Epithelial circulating tumor cells (CTCs) expressing EpCAM or CKs8, 14, 15 show red fluorescence; (b) hybrid CTCs show both red and green fluorescence; (c) mesenchymal CTCs expressing vimentin and Twist show green fluorescence.
The CTC classification criteria are summarized in Table 2. The positive rates of epithelial, hybrid, and mesenchymal CTCs were 57.7%, 94.4%, and 64.8%, respectively. The total number of CTCs, the number of specific CTCs, and their distribution across various stratifications (such as age, gender, clinical stage etc.) are summarized in Table 3. The median values of epithelial, hybrid, and mesenchymal CTCs were 1 (interquartile range [IQR] 0–3), 16 (IQR 6–33), and 1 (IQR 0–4), respectively, with the hybrid CTCs accounting for the major proportion of total CTCs. In addition, the positive rates of epithelial, hybrid, and mesenchymal CTCs in the blood samples from 40 healthy volunteers in the negative control group were 27.5%, 7.5%, and 0%, respectively. To assess the diagnostic performance of specific CTC phenotypes and their combinations, receiver operating characteristic (ROC) curve analysis was performed. The area under the ROC curve of epithelial, hybrid, and mesenchymal CTCs were 0.668, 0.966, and 0.824, and the optimal cut‐off values were 2, 1, and 0, respectively (Fig 2). The results showed that the area under the curve (AUC) increased to 0.991 with the total CTCs (Table 4).
Table 2.
Classification criteria of CTCs
| Type | Red fluorescence signal point | Green fluorescence signal point | White fluorescence signal point | DAPI |
|---|---|---|---|---|
| Epithelial CTC | + | − | − | + |
| Hybrid CTC | + | + | − | + |
| Mesenchymal CTC | − | + | − | + |
CTC, circulating tumor cell; DAPI, 4′,6‐diamidino‐2‐phenylindole.
Table 3.
Relationship between CTCs and ESCC clinicopathological features
| Total CTC | P | Epithelial CTC | P | Hybrid CTC | P | Mesenchymal CTC | P | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | IQR | M | IQR | M | IQR | M | IQR | ||||||
| Total | 20 | 12–39 | 1 | 0–3 | 16 | 6–33 | 1 | 0–4 | |||||
| Age | < 65 | 16 | 12–32.75 | 0.072 | 0.5 | 0–3 | 0.136 | 14 | 6–27.75 | 0.095 | 1 | 0–4.5 | 0.375 |
| ≥ 65 | 22 | 13–54 | 1 | 0–3 | 17 | 8–42 | 2 | 0–4 | |||||
| Gender | Male | 23 | 12–38.75 | 0.741 | 1 | 0–2.75 | 0.753 | 17 | 6–32.75 | 0.728 | 2 | 0–5 | 0.123 |
| Female | 19 | 12–39 | 1 | 0–3 | 14 | 6–35 | 1 | 0–4 | |||||
| T Staging | Stage I and II | 17.5 | 7.75–41.75 | 0.482 | 1 | 0–4 | 0.289 | 14.5 | 5.75–36.5 | 0.791 | 1 | 0–2 | 0.005† |
| Stage III and IV | 21 | 14–38.5 | 1 | 0–2 | 16 | 8.5–31.5 | 3 | 0.5–6 | |||||
| N Staging | Stage 0 | 20.5 | 10.5–43.25 | 0.799 | 1 | 0–3 | 0.656 | 17 | 6–37.5 | 0.582 | 1 | 0–4.75 | 0.942 |
| Stage I and II | 20 | 12–37 | 1 | 0–3 | 15 | 6–31 | 2 | 0–4 | |||||
| Clinical staging | Stage I | 14 | 7.5–43.25 | 0.524 | 1 | 0–3.75 | 0.557 | 12.5 | 6–37.5 | 0.818 | 0 | 0–1.75 | 0.028‡ |
| Stage II | 21.5 | 11–34.25 | 1 | 0–3.25 | 17 | 5–31.25 | 2 | 0–4.25 | |||||
| Stage III | 22 | 17–39 | 1 | 0–2 | 16 | 10–32 | 3 | 0–7.5 | |||||
| Treatment modality§ | Non‐surgical | 20.5 | 12.78–36 | 0.722 | 0 | 0–1.75 | 0.314 | 16 | 7.25–30.5 | 0.917 | 3.5 | 2–5.75 | 0.011‡ |
| Surgical | 18.5 | 7.75–41.75 | 1 | 0–3 | 15.5 | 5.75–36.5 | 1 | 0–3 | |||||
| Tumor location | Upper segment | 16 | 7–39 | 0.714 | 1 | 0–3 | 0.860 | 15 | 6–29 | 0.844 | 1 | 0–3 | 0.517 |
| Middle segment | 20 | 12–38.25 | 1 | 0–4 | 16.5 | 6–32.25 | 2 | 0–4.25 | |||||
| Lower segment | 38.5 | 3.25–48.5 | 1 | 0–1.25 | 36.5 | 0–42.25 | 2 | 0.75–5.5 | |||||
Correlation is significant at the 0.01 level (two‐tailed).
Correlation is significant at the 0.05 level (two‐tailed).
Cervical esophageal cancer patients were excluded. CTC, circulating tumor cell; ESCC, esophageal squamous cell carcinoma; IQR, interquartile range.
Figure 2.
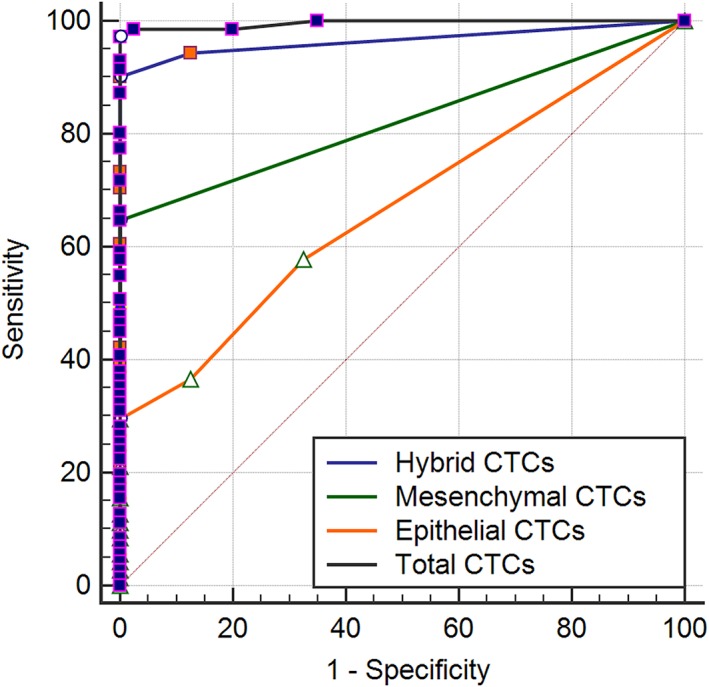
Receiver operating characteristic curves and area under the curves of circulating tumor cells (CTCs).
Table 4.
Area under the ROC curve of CTCs and hypothesis testing
| AUC | 95% LCI | 95% UCI | Youden | P | |
|---|---|---|---|---|---|
| Epithelial CTCs | 0.668 | 0.572 | 0.755 | 0.296 | < 0.01 |
| Hybrid CTCs | 0.966 | 0.913 | 0.991 | 0.901 | < 0.01 |
| Mesenchymal CTCs | 0.824 | 0.740 | 0.890 | 0.648 | < 0.01 |
| Total CTCs | 0.996 | 0.960 | 1.000 | 0.901 | < 0.01 |
CTC, circulating tumor cell; LCI, lower confidence interval; ROC, receiver operating characteristic; UCI, upper confidence interval.
Relationship between CTC subtype counts and clinicopathological features
Of the 71 patients, 19 were in stage I, 31 in stage II, and 21 in stage III or IV. The proportions of the specific CTCs in each patient and different clinical stages are shown in Figure 3a. The mesenchymal CTC counts across the different stages were statistically significant (P = 0.028) (Table 3). As summarized in Table 3, the median values of mesenchymal CTCs through stages I–III/IV were 0 (IQR 0–1.75), 2 (IQR 0–4.25), and 3 (IQR 0–7.5), respectively (Fig 3c). Comparisons of mesenchymal CTCs and clinical stages showed that the number of mesenchymal CTCs in stage III/IV was significantly higher than that in stage I (P = 0.03) (Table 5). In addition, comparison by Kruskal–Wallis H test showed significant differences between the mesenchymal CTC counts of different T stages (Fig 3b). The number of mesenchymal CTCs gradually increased with the T staging, with the maximum differences observed between T1 and T4, and T2 and T4 (P = 0.01) (Table 5). There was also a positive correlation between tumor size and mesenchymal CTC count (P = 0.035) (Table 6). However, there was no statistical difference in the relationship between lymph node metastasis and mesenchymal CTC count (Fig 3e).
Figure 3.
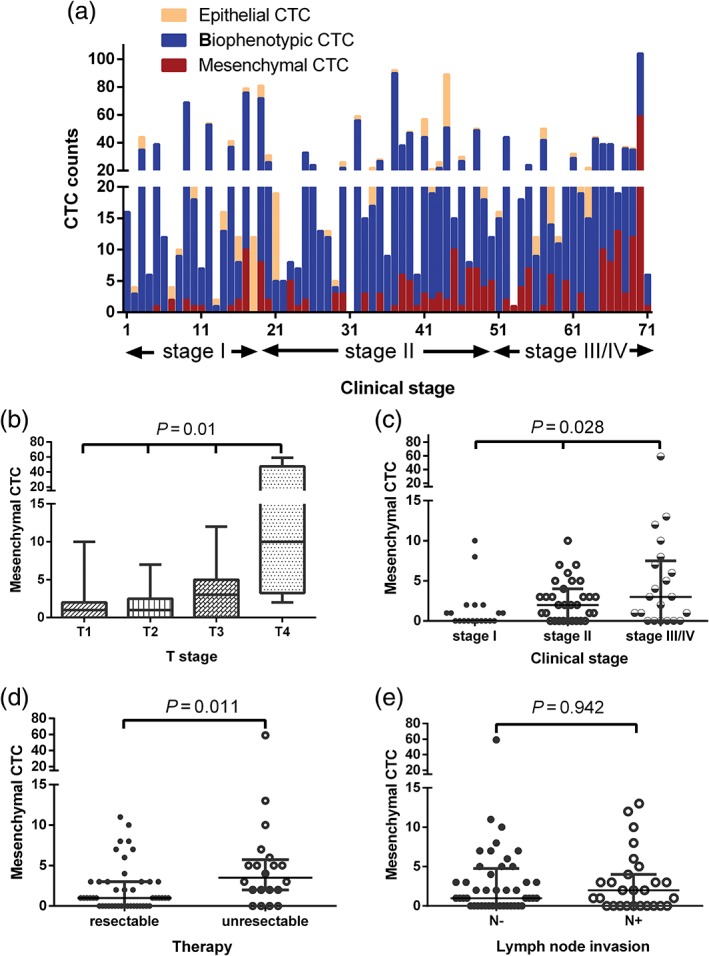
(a) Statistical description of the counts of specific circulating tumor cells (CTCs) during each clinical stage. (b) Kruskal–Wallis H analysis of CTC counts obtained at different T stages. (c) The overall distribution of mesenchymal CTC counts obtained at each clinical stage. (d) The mesenchymal CTC count was higher in non‐surgical esophageal squamous cell carcinoma patients. (e) The relationship between lymph node metastasis and mesenchymal CTC count.
Table 5.
Comparisons of mesenchymal CTCs and clinical staging (Kruskal–Wallis H test)
| Staging | Test statistic | Standard error | Standard test statistic | P |
|---|---|---|---|---|
| Stage I–II | −12.417 | 5.806 | −2.139 | 0.097 |
| Stage I–III/IV | −15.902 | 6.284 | −2.531 | 0.034 |
| T1 ‐ T4 | −31.26 | 11.18 | −2.79 | 0.031 |
| T2 ‐ T4 | −30.97 | 10.97 | −2.82 | 0.029 |
Asymptotic significances (two‐sided tests) are displayed. The significance level is 0.05. CTC, circulating tumor cell.
Table 6.
Correlation between tumor size and CTCs
| Spearman's rho | Total CTC | Epithelial CTC | Hybrid CTC | Mesenchymal CTC |
|---|---|---|---|---|
| Tumor size | ||||
| R | 0.068 | −0.156 | 0.046 | 0.256* |
| P | 0.584 | 0.205 | 0.712 | 0.035 |
*P < 0.05. CTC, circulating tumor cell; P, P value; R, Spearman's correlation coefficient.
Relationship between esophageal squamous cell carcinoma treatment modalities and mesenchymal CTC counts
Among the 71 patients, five had cervical esophageal cancer and were excluded. Forty‐six of the remaining 66 patients met the criteria for surgery, five of whom declined surgery because of coronary heart disease (n = 2), poor pulmonary function (n = 1), or other reasons (n = 2). As shown in Fig 3d, there was a significant difference (P = 0.011) in the number of mesenchymal CTCs between the surgery and non‐surgical groups, with median values of 1 (IQR 0–3) and 3.5 (IQR 2–5.75), respectively. Univariable and multivariable regression analyses were performed on 66 patients to determine the factors affecting treatment efficacy. Six factors were analyzed, four of which – T stage (odds ratio [OR] 8.27, 95% confidence interval [CI] 2.35–29.1), N stage (OR 0.32, 95% CI 0.11–0.96), clinical stage (I vs. III/IV: OR 0.059, 95% CI 0.01–0.54), and mesenchymal CTC counts (OR 0.29, 95% CI 0.98–0.87) – were associated with a positive indication for surgical resection. Multivariable analysis showed that only T staging (OR 11.86) was an independent factor affecting the choice of treatment modality, whereas the other variables were not statistically significant (Table 7).
Table 7.
Univariate and multivariate logistic regression analysis of treatment
| Univariate | Multivariate | ||||||
|---|---|---|---|---|---|---|---|
| Risk factor | OR | 95% CI | P | OR | 95% CI | P | |
| Age (< 65 as reference) | 0.526 | 0.182–1.52 | 0.235 | ||||
| Gender (male as reference) | 0.704 | 0.245–2.021 | 0.514 | ||||
| T stage (T3, T4 as reference) | 8.267 | 2.352–29.06 | 0.001 | 11.862 | 1.510–91.37 | 0.018 | |
| N stage (N0 as reference) | 0.322 | 0.108–0.959 | 0.042 | 0.177 | 0.021–1.455 | 0.107 | |
| Clinical stage (I as reference) | |||||||
| II | 0.118 | 0.014–1.015 | 0.052 | 0.591 | 0.044–8.014 | 0.693 | |
| III/IV | 0.059 | 0.006–0.541 | 0.012 | 3.235 | 0.062–169.1 | 0.561 | |
| Mesenchymal CTC (< 3 as reference) | 0.292 | 0.98–0.87 | 0.027 | 0.456 | 0.124–1.676 | 0.237 | |
CI, confidence interval; CTC, circulating tumor cell; OR, odds ratio.
Evaluation of therapeutic efficacy on the basis of mesenchymal CTC count
Neoadjuvant chemotherapy
The 39 patients unable to undergo direct surgery were administered two cycles of neoadjuvant chemotherapy, after which mesenchymal CTC counts were measured. The treatment response was evaluated on the basis of the Response Evaluation Criteria in Solid Tumors version 1.1, divided into complete response (CR, 2 cases), partial response (PR, 20 cases), stable disease (SD, 13 cases), and progressive disease (PD, 4 cases) (Table 8). The mean assessment interval before and after treatment was 62.22 ± 12.73 days. The positive post‐treatment rates were 36.4% (CR/PR) and 70.6% (SD/PD) (P = 0.05). Three blood samples were collected from each patient before the first round of chemotherapy and every 2–3 weeks after each round. The median time interval between the collections was 30.86 ± 4.39 days. The frequencies of multiple (2–3 times) positive mesenchymal CTC results were 31.8% (CR/PR) and 70.6% (SD/PD) (P = 0.03). There was a clear correlation between mesenchymal CTC count and the efficacy of neoadjuvant therapy in ESCC patients. However, because of the small sample size of this study, the relationship between a change in the mesenchymal CTC count and the efficacy of neoadjuvant therapy before and after treatment could not be evaluated.
Table 8.
Evaluation of therapeutic efficacy by CTC status change
| Positive frequencies of mesenchymal CTC | ||||
|---|---|---|---|---|
| Treatment efficacy | 0–1 time | 2–3 times | Total | CTC positivity after treatment |
| CR/PR | 15 (68.2%) | 7 (31.8%) | 22 | 8 (36.4%) |
| SD/PD | 5 (29.4%) | 12 (70.6%) | 17 | 12 (70.6%) |
| Total | 20 | 19 | 39 | 21 (51.3%) |
| P = 0.03 | P = 0.05 | |||
CR, complete response; CTC, circulating tumor cell; PD, progressive disease; PR, partial response; SD, stable disease.
Surgical treatment
Blood samples were collected from 26 of the 46 patients that underwent surgical treatment without neoadjuvant therapy two weeks after the surgery, and their CTC counts were measured. The postoperative number of mesenchymal CTCs increased significantly compared to the preoperative values (P = 0.037): the median preoperative and postoperative counts were 0 (0–11) and 2.5 (0–18), respectively. However, the total number of CTCs in postoperative patients decreased (Fig 4a). Eighteen of 71 patients underwent four cycles of perioperative chemotherapy following the regimen described above. Blood samples were collected during their last hospital admission for follow‐up and the mean interval from diagnosis to final blood collection was 148.5 (92–210) days. The mesenchymal CTC counts showed a clear downward trend during that interval (Fig 4b); however, this result was not statistically significant (P = 0.11), which might be a result of the small sample size.
Figure 4.
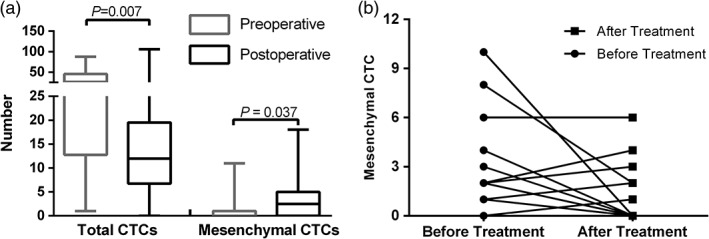
(a) The effect of surgery on total and mesenchymal CTC counts. (b) Changes in mesenchymal CTC status before and after esophageal squamous cell carcinoma treatment.
Discussion
As a result of the advantages of minimal invasiveness and the ease of hematological tests, clinical diagnosis, pathological staging, curative effect assessment, and prognostic evaluation, CTCs enriched from peripheral blood have recently received much attention. Over the past decade, various techniques have been used to detect and isolate CTCs on the basis of phenotypical differences, although none has become the gold standard for CTC testing.16 The capture and detection of CTCs by anti‐EpCAM and CK antibodies is currently the only United States Food and Drug Administration approved CTC testing tool. The biggest drawback of this method is that it can only detect epithelial CTCs and not CTCs that no longer express epithelial markers.17 Because these cells may have already undergone an EMT process and exhibit the characteristics of stem cells as a result, they can escape hematological detection by downregulating their epithelial markers and upregulating their mesenchymal markers, leading to false‐negative results.18 Vimentin and Twist, which are frequently expressed in tumor tissues, can promote tumor metastasis by inducing EMT.19 Vimentin belongs to the family of intermediate filament proteins and is commonly expressed in mesenchymal cells.14 In addition, the expression of vimentin in tumor cells can promote tumor growth and invasion. Previous studies have demonstrated that vimentin is associated with N‐cadherin upregulation.15 Vimentin expression in breast cancer predicts a poor prognosis; Satelli et al. proposed that the CTC load detected by a combination of anti‐vimentin and EpCAM antibodies could be a powerful predictor of the efficacy of breast cancer treatment.20, 21 Twist, a transcriptional factor involved in cell differentiation,22 is also a potential biomarker for EMT.23, 24 A significant number of CTCs expressing Twist have been detected in the blood of breast, stomach, nasopharyngeal, colorectal, and liver cancer patients.12, 25, 26, 27, 28 In addition, detection of the soluble fragment of CK19 (CYFRA 21‐1) in the blood can be used to determine radiotherapy efficacy in ESCC.29 However, the isolation and phenotyping of CTCs from ESCC patients is still in its infancy in China. We were able to detect and phenotype CTCs undergoing the EMT process using the two markers and also detected a small number of epithelial and hybrid CTCs in 40 subjects in the negative control. These results may be a result of vascular endothelial cell damage and shedding during venous puncture; therefore, the separation and phenotyping of CTCs can also effectively reduce false‐positive results.
We used newly developed CanPatrol based CTC enrichment technology11 and a set of fluorescent probes against the epithelial (EpCAM, CK) and mesenchymal (vimentin, Twist) markers to identify the CTC phenotypes. We were able to detect epithelial, mesenchymal, and hybrid CTCs that had partially or completely lost their epithelial features. As with other cancers, ESCC tumors are heterogeneous, as exemplified by the large proportion of hybrid CTCs as well as cells with partial EMT features, which exhibit robust metastatic activity.30 Our results emphasize the importance of the EMT process in tumor metastasis and CTC proliferation. However, the hybrid CTC count was not statistically correlated with ESCC clinical staging and treatment responsiveness (Table 3). A possible explanation could be that hybrid CTCs may not represent a key step in the EMT process and distant metastasis of tumor cells. In addition, as hybrid CTCs also carry some epithelial antigens, it is impossible for hybrid CTCs to completely escape immune surveillance. Previous studies have shown that the anti‐apoptotic ability and tumor‐initiating potential of hybrid CTCs could be higher than that of mesenchymal CTCs, but only when they aggregate into CTC clusters.30 However, CTC clusters were not detected in any of the 71 ESCC patients enrolled in this study. Nevertheless, we did find a correlation between higher mesenchymal CTC count and higher clinical tumor stage (P = 0.028) and larger tumor size (P = 0.035), indicating that the course of disease was longer or the likelihood of local progression was higher with increasing CTC load.
These findings may be useful to assist in choosing the proper course of treatment. Univariable logistical regression showed that the ESCC patients with a mesenchymal CTC count > 3 had a lower probability of tolerating surgery (OR 0.29). Nevertheless, multivariable analysis only identified T staging as an independent risk factor for surgical treatment. The reason for such a discrepancy could be the small sample size, which made the logistic regression model unstable. Further studies with larger cohorts need to be conducted to determine whether mesenchymal CTCs could be a suitable indicator to select treatment options.
Despite the association of mesenchymal CTCs with clinical T staging and tumor size, it is still unclear as to when the tumor starts to undergo the EMT process.31 Although this question is beyond of the scope of this study, we demonstrated that larger tumor size corresponds to a greater ability to undergo EMT and produce mesenchymal CTCs. In fact, the detection of mesenchymal CTCs reflects the EMT process, which has been shown to bestow stem cell‐like characteristics on the tumor and enable the latter to resist radiotherapy and chemotherapy. We evaluated the efficacy of neoadjuvant chemotherapy in ESCC patients and found that patients with multiple positive results of mesenchymal CTCs before and after chemotherapy responded poorly to treatment. Consistent with previous findings, therefore, chemotherapy was well tolerated by the mesenchymal CTCs, most likely by resisting apoptotic signals and escaping immune surveillance.8, 32
Esophageal squamous cell carcinoma is a very aggressive gastrointestinal malignancy and even surgical resection cannot prevent eventual recurrence and distant metastasis. Interestingly, a longitudinal comparison of preoperative and postoperative CTC counts in our cohort showed a decrease in total CTCs (P = 0.007), and an increase in mesenchymal CTCs (P = 0.037) in postoperative patients. These findings are consistent with the results of previous reports of the changes in CTCs before and after surgery;33, 34 however, as these results were all based on epithelial markers, they should be interpreted with caution. The sudden increase in CTCs in the postoperative period is likely a result of the release of a large number of tumor cells in the bloodstream from tumor compression during surgery. However, some epithelial and hybrid CTCs rapidly undergo apoptosis in the peripheral blood, and the diminished tumor burden ceases the constant release of CTCs into the bloodstream, resulting in a decrease in total CTCs. On the other hand, the number of mesenchymal CTCs in the bloodstream eventually increases because EMT endows these cells with increased resistance to apoptotic signals and the ability to evade immune surveillance. In addition, because the patients could not eat for a certain period of time after esophagectomy, their nutritional status and immunity suffered post‐surgery making it difficult to clear the mesenchymal CTC load in the peripheral blood and thus increasing the risk of postoperative recurrence and metastasis. This increased load also strongly indicates that postoperative chemotherapy may reduce the risk of postoperative metastasis in ESCC patients. However, the actual clinical value needs to be validated with further survival analysis. Nevertheless, for the 18 patients who underwent both surgery and perioperative chemotherapy, there was no significant difference in pre‐treatment and post‐treatment mesenchymal CTC counts. Although the CTC count increased in four (20.2%) patients after treatment, imaging tests showed no signs of recurrence or metastasis. The reason for this increase needs to be investigated through follow‐up observation and may be related to the shorter postoperative recovery time at the time of sample collection.
There are several limitations to this study. Because all patient data was obtained from the Department of Thoracic Surgery, there was selection bias in the patients enrolled and the number of late stage ESCC patients included was low. In addition, as vimentin and Twist immunolabeling of postoperative ESCC specimens was not conducted, direct evidence of EMT could not be obtained.
The isolation and quantification of mesenchymal CTCs is significant for evaluating the preoperative state and treatment efficacy of ESCC patients. Compared to previous studies that focused only on CTCs expressing epithelial markers, the information obtained from this study is more valuable for clinical application. We hypothesize that mesenchymal CTCs are a potent marker for prognostic assessment. However, because the application of this technology in ESCC research is still in its infancy, our results need to be validated by studies with a larger sample size.
Disclosure
No authors report any conflict of interest.
Acknowledgment
The authors wish to thank the investigators, surgeons, and nurses who made immense contributions to this study.
References
- 1. Zhang Y. Epidemiology of esophageal cancer. World J Gastroenterol 2013; 19: 5598–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015; 65: 5–29. [DOI] [PubMed] [Google Scholar]
- 3. Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet 2013; 381: 400–12. [DOI] [PubMed] [Google Scholar]
- 4. Wald O, Smaglo B, Mok H, Groth SS. Future directions in esophageal cancer therapy. Ann Cardiothorac Surg 2017; 6: 159–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Okamura S, Fujiwara H, Shiozaki A et al Long‐term survivors of esophageal carcinoma with distant lymph node metastasis. Hepatogastroenterology 2011; 58: 421–5. [PubMed] [Google Scholar]
- 6. Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science 2011; 331: 1559–64. [DOI] [PubMed] [Google Scholar]
- 7. Mego M, Mani SA, Lee BN et al Expression of epithelial‐mesenchymal transition‐inducing transcription factors in primary breast cancer: The effect of neoadjuvant therapy. Int J Cancer 2012; 130: 808–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bonnomet A, Brysse A, Tachsidis A et al Epithelial‐to‐mesenchymal transitions and circulating tumor cells. J Mammary Gland Biol Neoplasia 2010; 15: 261–73. [DOI] [PubMed] [Google Scholar]
- 9. Matsushita D, Uenosono Y, Aeigami T et al Clinical significance of circulating tumor cells in peripheral blood of patients with esophageal squamous cell carcinoma. Ann Surg Oncol 2015; 22: 3674–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lagergren J, Lagergren P. Recent developments in esophageal adenocarcinoma. CA Cancer J Clin 2013; 63: 232–48. [DOI] [PubMed] [Google Scholar]
- 11. Wu S, Liu S, Liu Z et al Classification of circulating tumor cells by epithelial‐mesenchymal transition markers. PLoS One 2015; 10: e0123976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu YK, Hu BS, Li ZL, He X, Li Y, Lu LG. An improved strategy to detect the epithelial‐mesenchymal transition process in circulating tumor cells in hepatocellular carcinoma patients. Hepatol Int 2016; 10: 640–6. [DOI] [PubMed] [Google Scholar]
- 13. Collins ML, Irvine B, Tyner D et al A branched DNA signal amplification assay for quantification of nucleic acid targets below 100 molecules/ml. Nucleic Acids Res 1997; 25: 2979–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Larsson A, Wilhelmsson U, Pekna M, Pekny M. Increased cell proliferation and neurogenesis in the hippocampal dentate gyrus of old GFAP(‐/‐)Vim(‐/‐) mice. Neurochem Res 2004; 29: 2069–73. [DOI] [PubMed] [Google Scholar]
- 15. Zhao Y, Yan Q, Long X, Chen X, Wang Y. Vimentin affects the mobility and invasiveness of prostate cancer cells. Cell Biochem Funct 2008; 26: 571–7. [DOI] [PubMed] [Google Scholar]
- 16. Hardingham JE, Grover P, Winter M, Hewett PJ, Price TJ, Thierry B. Detection and clinical significance of circulating tumor cells in colorectal cancer‐‐20 years of progress. Mol Med 2015; 21(Suppl 1): S25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Esmaeilsabzali H, Beischlag TV, Cox ME, Parameswaran AM, Park EJ. Detection and isolation of circulating tumor cells: principles and methods. Biotechnol Adv 2013; 31: 1063–84. [DOI] [PubMed] [Google Scholar]
- 18. Gorges TM, Tinhofer I, Drosch M et al Circulating tumour cells escape from EpCAM‐based detection due to epithelial‐to‐mesenchymal transition. BMC Cancer 2012; 12: 178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fan F, Samuel S, Evans KW et al Overexpression of snail induces epithelial‐mesenchymal transition and a cancer stem cell‐like phenotype in human colorectal cancer cells. Cancer Med 2012; 1: 5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Satelli A, Brownlee Z, Mitra A, Meng QH, Li S. Circulating tumor cell enumeration with a combination of epithelial cell adhesion molecule‐ and cell‐surface vimentin‐based methods for monitoring breast cancer therapeutic response. Clin Chem 2015; 61: 259–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Domagala W, Lasota J, Bartkowiak J, Weber K, Osborn M. Vimentin is preferentially expressed in human breast carcinomas with low estrogen receptor and high Ki‐67 growth fraction. Am J Pathol 1990; 136: 219–27. [PMC free article] [PubMed] [Google Scholar]
- 22. Yu W, Kamara H, Svoboda KK. The role of twist during palate development. Dev Dyn 2008; 237: 2716–25. [DOI] [PubMed] [Google Scholar]
- 23. Wong IY, Javaid S, Wong EA et al Collective and individual migration following the epithelial‐mesenchymal transition. Nat Mater 2014; 13: 1063–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yang J, Mani SA, Donaher JL et al Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell 2004; 117: 927–39. [DOI] [PubMed] [Google Scholar]
- 25. Strati A, Markou A, Parisi C et al Gene expression profile of circulating tumor cells in breast cancer by RT‐qPCR. BMC Cancer 2011; 11: 422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kallergi G, Papadaki MA, Politaki E, Mavroudis D, Georgoulias V, Agelaki S. Epithelial to mesenchymal transition markers expressed in circulating tumour cells of early and metastatic breast cancer patients. Breast Cancer Res 2011; 13: R59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Si Y, Lan G, Deng Z et al Distribution and clinical significance of circulating tumor cells in nasopharyngeal carcinoma. Jpn J Clin Oncol 2016; 46: 622–30. [DOI] [PubMed] [Google Scholar]
- 28. Zhao R, Cai Z, Li S et al Expression and clinical relevance of epithelial and mesenchymal markers in circulating tumor cells from colorectal cancer. Oncotarget 2016; 8: 9293–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wakatsuki M, Suzuki Y, Nakamoto S et al Clinical usefulness of CYFRA 21‐1 for esophageal squamous cell carcinoma in radiation therapy. J Gastroenterol Hepatol 2007; 22: 715–9. [DOI] [PubMed] [Google Scholar]
- 30. Jolly MK, Boareto M, Huang B et al Implications of the hybrid epithelial/mesenchymal phenotype in metastasis. Front Oncol 2015; 5: 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Aktas B, Tewes M, Fehm T et al Stem cell and epithelial‐mesenchymal transition markers are frequently overexpressed in circulating tumor cells of metastatic breast cancer patients. Breast Cancer Res 2009; 11: R46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bobek V, Matkowski R, Gürlich R et al Cultivation of circulating tumor cells in esophageal cancer. Folia Histochem Cytobiol 2014; 52: 171–7. [DOI] [PubMed] [Google Scholar]
- 33. Lian H, Ding Z, Yuan D, Ma J, Qin J. [Diagnostic value of folate receptor‐positive circulating tumor cell in lung cancer: A pilot study.]. Zhongguo Fei Ai Za Zhi 2016; 19 (12): 813–20. (In Chinese.). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gogenur M, Hillig T, Gogenur I. CytoTrack analysis reveals low presence of circulating tumor cells in the perioperative period in patients with non‐metastatic colorectal cancer. Anticancer Res 2017; 37: 3099–103. [DOI] [PubMed] [Google Scholar]


