Abstract
In this study, we evaluated and compared the morphogenetic variability and the degree of recessive homozygosity in patients with manifested ischemic stroke compared to healthy controls. We have evaluated 120 patients with manifested ischemic stroke, of which 64 did not have hypertension and 56 have hypertension. For comparison, we additionally tested 194 healthy individuals without manifested ischemic stroke (controls). For the estimation of the degree of recessive homozygosity, we have performed the homozygously recessive characteristics (HRC) test and tested 19 HRCs. There was a significant difference in the individual variations of 19 HRCs between the controls and patients with manifested ischemic stroke (∑χ2 = 60.162, p < 0.01). The mean values of the tested HRCs significantly differed between the controls and group with manifested ischemic stroke (Controls − 5.71 ± 1.61, Ischemic stroke group − 6.25 ± 1.54, p = 0.012). For the tested individuals with hypertension, the mean values of HRCs did not significantly differ between the controls and those that had manifested ischemic stroke (Controls − 5.28 ± 1.75, Ischemic stroke group − 5.64 ± 1.48, p = 0.435). We found a significant difference in the frequencies of HRCs between those with and without hypertension for controls (p < 0.003) and for those with manifested ischemic stroke (p < 0.001). There are increased degrees of recessive homozygosity along with decreased variability in patients with manifested ischemic stroke compared to controls.
Keywords: manifested ischemic stroke, homozygous recessive characteristics, variability, hypertension
1. Introduction
In the developed world, stroke is considered to be the leading cause of long-term disability and the third most common cause of death [1,2]. The exact role of genetics is still controversial in stroke patients due to the heterogeneous etiopathogenesis [1]. However, numerous monogenic disorders have been described to be associated with the stroke, including those on autosomal, sex and mitochondrial chromosomes as well as polygenic disorders [1,2,3]. These single gene disorders, which have a lower prevalence rate in individuals that are carriers of mutations, are associated with a higher risk for stroke [4]. Thus far, several locations on different chromosomes have been associated with the occurrence of ischemic stroke (1q24.2, 7q36.1, 11p11.2, 13q12.3 and 14q23.1 OMIM 601367) and with hemorrhagic stroke (13q34, 17q23.3 OMIM 614519) [5]. Several studies favor a genetic role in the etiopathogenesis of ischemic stroke, including studies of twins [6] and familial aggregation [7].
The role of hypertension in the pathogenesis of stroke has been previously emphasized by findings in meta-analyses and systematic reviews [8,9]. The mechanism of hypertension is complex and it does not only affect the atherosclerosis processes but is associated with a fibrinoid necrosis of small penetrating arteries of the brain [5].
During the thirty years of developing the homozygous recessive characteristics (HRC) test, the authors of the Belgrade population-genetic school selected 15 to 20 as the most reliable characteristics according to the type of inheritance and variability [10,11,12,13,14,15,16,17,18,19]. By establishing the extremely expressed recessive phenotypes, they actually gained insight into the status of these gene loci and the general homozygosity of individuals and groups. These authors studied the distribution and frequency of a series of highly expressed recessive morphophysiological traits in order to estimate individual and group differences (i.e., comparison between ill and healthy individuals, pupils from special and regular schools and carriers of different blood types). Furthermore, previous population genetics studies showed that the number of tested traits was adequate for comparisons of different subpopulation groups [10,11,12,13,14,15,16,17,18,19].
Thus far, there have only been a few population genetic studies focusing on stroke in Serbia. Thus, we created the hypothesis that increased genetic homozygosity along with decreased variability in individuals with manifested ischemic stroke might be populational genetic parameters that can be used to predict ischemic strokes. The aim of our study was therefore to evaluate and compare the morphogenetic variability and the degree of recessive homozygosity in the patients with manifested ischemic stroke.
2. Material and Methods
2.1. Study Group
The study included 120 patients, who presented with manifested ischemic stroke during the time period of 2014–2017. The diagnosis was established by a board-certified neurologist upon admission of the patient in the hospital. Additionally, 194 healthy individuals without manifested ischemic stroke were assessed (controls). Both individuals from controls and the group with manifested ischemic stroke belonged to the same population (Serbian population) with similar social-economic status and age. Demographic and clinical parameters were taken on examination (gender, age, body mass index (BMI), presence of hypertension and diabetes mellitus, dyslipidemia, family history of hypertension, family history of myocardial infarction (MI), prior percutaneous coronary intervention (PCI) and prior strokes, additionally).
The study was conducted according to the principles of good clinical practice and followed the recommendations of the declaration of Helsinki. The study was approved by the Institutional Review Board of Faculty of Medicine, University of Belgrade.
With regards to the presence of hypertension, the tested individuals were grouped into those with hypertension and those without hypertension. The individuals with any other chronic condition were excluded from the study.
Before inclusion in the study, the patients were informed about study protocol and informed consent was obtained.
To assess the presence of the hypertension, we followed the further recommendations: systolic blood pressure (SBP) ≥ 140 mm Hg or a diastolic blood pressure (DBP) ≥ 90 mm Hg [20].
2.2. Tested Determinants
Homozygously recessive characteristics (HRC) test [10,11,12] was conducted for the estimation of the degree of recessive homozygosity in tested subjects. This test was developed for assessing the proportion of clearly expressed homozygously recessive characteristics, which are considered as qualitative traits, in every individual as the markers of chromosomal homozygosities [12,13,14,15,16,17,18]. The tested HRCs are the markers of genes that are located on different chromosomes [19]. Our study included the estimation of the presence of 19 HRCs in the tested individuals where only the characteristics with extreme appearances were marked as the present trait. In the region of the human head, we tested 13 HRCs: attached ear lobe (OMIM number 128900), continuous frontal hair line (OMIM number 194000), blue eyes (gene location 15q12, 15q13, OMIM number 227220; 5p13 OMIM number 227240; 14q32.1, OMIM number 210750; 9q23 OMIM number 612271), straight hair (1q21.3, OMIM number 139450), soft hair and blond hair (gene location 15q12, 15q13, OMIM number 227220; 14q32.1, OMIM number 210750; 12q21.3 OMIM number 611664; 11q13.3, OMIM number 612267), double hair whorl, opposite hair whorl orientation (OMIM number 139400), an inability to roll, fold and curve the tongue (OMIM number 189300), ear without Darwinian notch, ability to produce a guttural “r” and color blindness (gene location Xq28, OMIM number 303800). In human arms, we tested 6 HRCs: proximal thumb hyperextensibility, index finger longer than the ring finger (OMIM number 136100), left-handedness (gene location 2p12-q22, OMIM number 139900), right thumb over left thumb (hand clasping) (OMIM number 139800), top joint of the thumb >45° and three tendons in the wrist (OMIM) [5].
3. Statistical Analysis
The obtained results were presented as whole numbers with percentages and as continuous variables as mean value ± standard deviation (MV ± SD). For comparisons of the frequencies of HRCs between the controls and patients with manifested ischemic stroke, we used the Chi-squared test (χ2). Mann-Whitney U Test and Students t-test to assess statistical difference between the tested groups of patients. To quantify the strength of the association of the significant predictors and the presence of ischemic stroke, we calculated the odds ratio (OR) with 95% confidence interval (CI). To compare the variability between the studied groups of individuals, we used the variation coefficient (V). The effect size (Cohen’s d) was used to evaluate the correlation between the tested variables. The statistical significance was set at p < 0.05.
4. Results
From 120 patients that were diagnosed with manifested ischemic stroke, 64 (53.33%) did not have hypertension and 56 (46.67%) have hypertension.
There were 194 controls, of which 108 (55.67%) have no hypertension and 86 (44.33%) have hypertension. The demographic and clinical parameters are presented in Table 1.
Table 1.
Demographic and clinical parameters of studied individuals.
| Parameters | Controls N = 194 |
Ischemic Stroke Group N = 120 |
p Values | |
|---|---|---|---|---|
| Gender, N (%) | Males | 82 (42.27%) | 44 (36.67%) | 0.325 * |
| Females | 112 (57.73%) | 76 (63.33%) | ||
| Age, years (MV ± SD) | 67.49 ± 7.12 | 71.32 ± 6.92 | 0.000 ** | |
| BMI (MV ± SD) | 26.34 ± 8.28 | 29.14 ± 7.18 | 0.002 ** | |
| Hypertension, N (%) | 86 (44.33%) | 56 (46.67%) | 0.686 * | |
| Diabetes mellitus, N (%) | 52 (26.80%) | 49 (40.83%) | 0.010 * | |
| Non-insulin dependent, N (%) | 38 (19.59%) | 42 (35.00%) | 0.002 * | |
| Insulin dependent, N (%) | 14 (7.22%) | 7 (5.83%) | 0.634 * | |
| Dyslipidemia, N (%) | 128 (65.98%) | 86 (71.67%) | 0.293 * | |
| Family history of hypertension, N (%) | 104 (53.61%) | 81 (67.50%) | 0.015 * | |
| Family history of MI, N (%) | 78 (40.21%) | 63 (52.50%) | 0.033 * | |
| Prior PCI, N (%) | 84 (43.30%) | 26 (21.67%) | 0.000 * | |
| Prior stroke, N (%) | 0 (0%) | 31 (25.83%) | - | |
* Chi squared test; ** Students t test.
In Table 2, we presented the distribution of HRC frequencies for the controls and patients with manifested ischemic stroke. There were 4 HRCs that significantly differed, of which 3 (blue eyes, inability to transversally tongue roll and right thumb over left thumb) occurred significantly more frequently in patients with manifested ischemic stroke, while 1 (continuous hair line) occurred significantly more frequently in controls. There was a significant difference in the individual variations of 19 HRCs between the controls and patients with manifested ischemic stroke (∑χ2 = 60.162; degree of freedom (df) = 18, p < 0.01) (Table 2).
Table 2.
Frequencies of homozygously recessive characteristics among patients with manifested ischemic stroke and controls.
| Homozygously Recessive Characteristics | Controls N = 194, n (%) |
Ischemic Stroke Group N = 120, n (%) |
χ2 | OR (95% CI) |
|---|---|---|---|---|
| Blond Hair | 52 (26.80) | 29 (24.17) | 0.258 | 0.87 (0.51–1.47) |
| Straight Hair | 121 (62.37) | 88 (73.33) | 1.930 | 1.64 (0.99–2.69) |
| Double Hair Whorl | 19 (9.79) | 12 (10.00) | 0.005 | 1.02 (0.48–2.19) |
| Opposite Hair Whorl Orientation | 40 (20.62) | 29 (24.17) | 0.611 | 1.23 (0.71–2.11) |
| Soft Hair | 92 (47.42) | 73 (60.83) | 3.792 | 1.72 * (1.08–2.73) |
| Continuous Hair Line | 96 (49.48) | 36 (30.00) | 7.669 ** | 0.44 ** (0.27–0.71) |
| Attached Ear Lobe | 31 (15.98) | 14 (11.67) | 1.162 | 0.69 (0.35–1.37) |
| Ear Without Darwinian notch | 18 (9.28) | 17 (14.17) | 2.577 | 1.61 (0.80–3.27) |
| Blue Eyes | 57 (29.38) | 68 (56.67) | 25.349 ** | 3.14 ** (1.95–5.06) |
| Speaking deficiency -guttural “r” | 12 (6.19) | 8 (6.67) | 0.037 | 1.08 (0.43–2.73) |
| Inability to Transversally Tongue Roll | 52 (26.80) | 51 (42.50) | 9.197 ** | 2.02 ** (1.25–3.27) |
| Inability to Longitudinally Tongue Roll | 73 (37.63) | 49 (40.83) | 0.272 | 1.14 (0.72–1.82) |
| Right Thumb over Left Thumb | 97 (50.00) | 78 (65.00) | 4.500 * | 1.86 ** (1.16–2.97) |
| Top Joint of the Thumb >45° | 47 (24.23) | 21 (17.50) | 1.869 | 0.66 (0.37–1.18) |
| Hypermobility of proximal thumb joint | 21 (10.82) | 14 (11.67) | 0.067 | 1.09 (0.53–2.23) |
| Proximal thumb extensibility | 69 (35.57) | 38 (31.67) | 0.428 | 0.84 (0.52–1.36) |
| Three tendons in the wrist | 84 (43.30) | 56 (46.67) | 0.262 | 1.15 (0.73–1.81) |
| Left-handedness | 29 (14.95) | 16 (13.33) | 0.176 | 0.88 (0.45–1.69) |
| Index finger longer than the ring finger | 86 (44.33) | 53 (44.17) | 0.001 | 0.99 (0.63–1.57) |
| ∑χ2 = 60.162 ** | ||||
* p < 0.05; ** p < 0.01.
The significant predictors of tested HRCs in the studied group of ischemic stroke patients were: soft hair despite the fact that it was not significantly more frequent (OR = 1.72), blue eyes (OR = 3.14), inability to transversally tongue the roll (OR = 2.02) and right thumb over left thumb (OR = 1.86). For controls, this was continuous hair line (OR = 0.44). The most significant predictor for the group of patients with ischemic stroke was blue eyes (Table 2).
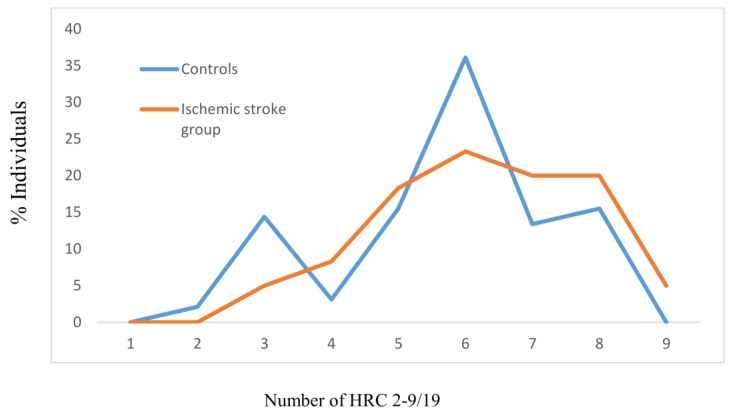
The mean values of the tested HRCs significantly differed between the controls and group with manifested ischemic stroke (MV ± SDControls − 5.71 ± 1.61, MV ± SDIschemic stroke group − 6.25 ± 1.54, z = −2.496, p = 0.012), with the effect size between groups being 34.28% (Figure 1). For the group of tested controls, the most frequent average number of HRC was 6 (36.1%), while for those with manifested ischemic stroke, it was also 6 (23.3%) (Figure 1).
Figure 1.
Frequencies of homozygous recessive characteristics (HRC) in controls and manifested ischemic stroke patients. MV- mean value; SD- standard deviation; z- Mann Whitney U test; V- variability. Controls: N = 194, MV ± SD = 5.71 ± 1.61. Ischemic stroke group: N = 120, MV ± SD = 6.25 ± 1.54 (z = −2.496, p = 0.012; Cohen’s d = 34.28%). VControls = 28.20%, VIschemic stroke group = 24.64%.
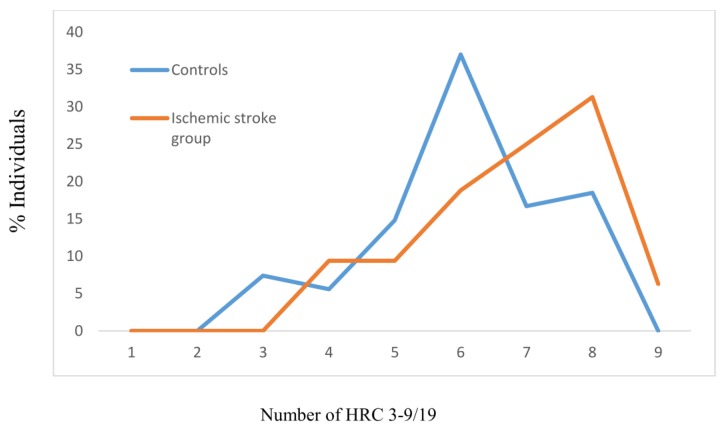
For the group of individuals without hypertension, the mean values of HRCs significantly differed between controls and those with manifested ischemic stroke (MV ± SDControls − 6.02 ± 1.40, MV ± SDIschemic stroke group − 6.78 ± 1.40, z = −3.160, p = 0.002), with the effect size between the groups being 54.29% (Figure 2). The most frequent average number of HRCs for the controls without hypertension was 6 (37%) and for those without hypertension and manifested ischemic stroke, this was 8 (31.3%) (Figure 2).
Figure 2.
Frequencies of homozygous recessive characteristics (HRC) in controls and manifested ischemic stroke patients without hypertension. MV- mean value; SD- standard deviation; z- Mann Whitney U test; V- variability. Controls: N = 108, MV ± SD = 6.02 ± 1.40. Ischemic stroke group: N = 64, MV ± SD = 6.78 ± 1.40 (z = −3.160, p = 0.002; Cohen’s d = 54.29%). VControls = 23.26%, VIschemic stroke group = 20.65%.
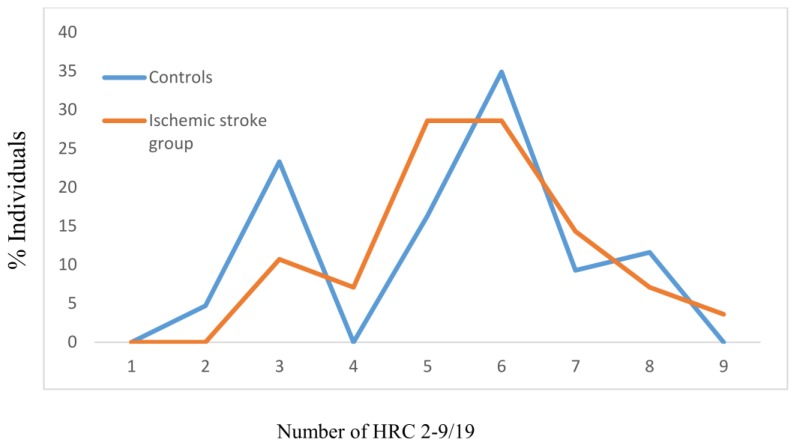
For the tested individuals with hypertension, the mean values of HRCs did not significantly differ between the controls and those with manifested ischemic stroke (MV ± SDControls − 5.28 ± 1.75, MV ± SDIschemic stroke group − 5.64 ± 1.48, z = −0.783, p = 0.435), with the effect size between groups being 22.21% (Figure 3). The most frequent average number of HRCs in the controls with hypertension was 6 (around 35%). For those with manifested ischemic stroke and with hypertension, this number was between 5–6 (slightly below 30%) (Figure 3).
Figure 3.
Frequencies of homozygous recessive characteristics (HRC) in controls and manifested ischemic stroke patients with hypertension. MV- mean value; SD- standard deviation; z- Mann Whitney U test; V- variability. Controls: N = 86, MV ± SD = 5.28 ± 1.75. Ischemic stroke group: N = 56, MV ± SD = 5.64 ± 1.48 (z = −0.783, p = 0.435; Cohen’s d = 22.21%). VControls = 33.14%, VIschemic stroke group = 26.24%.
We found a significant difference in the frequencies of HRCs between those with and without hypertension for controls (p < 0.003) and for those with manifested ischemic stroke (p < 0.001) (Table 3). The effect size between the groups for the controls was 46.70%, while this was higher for those with manifested ischemic stroke (79.14%) (Table 3).
Table 3.
Statistical evaluation of frequencies of homozygous recessive characteristics between groups with regards to the presence of hypertension.
| Groups | With/Without Hypertension | ||
|---|---|---|---|
| Z * | p | Cohen’s d (%) | |
| Controls | −2.943 | <0.003 | 46.70 |
| Ischemic stroke group | 4.037 | <0.001 | 79.14 |
* Mann Whitney U test.
In Table 4, we presented an association between the frequencies of HRCs regarding the presence of ischemic stroke and hypertension. For controls, 3 HRCs (OR = 3.79) were significant predictors for hypertension. For the ischemic stroke group, 5 HRCs (OR = 3.87) and 8 HRCs (OR = 0.17) were significant predictors for the development of hypertension. For those without hypertension, 6 HRCs (OR = 0.39) and 9 HRCs (OR = 2.74) were significant predictors for ischemic stroke.
Table 4.
Association between frequencies of homozygous recessive characteristics regarding the presence of ischemic stroke and hypertension.
| No. of HRCs | OR (95% CI) | |||
|---|---|---|---|---|
| Controls (with/without Hypertension) | Ischemic Stroke Group (with/without Hypertension) | With Hypertension (Ischemic Stroke Group/Controls) | Without Hypertension (Ischemic Stroke Group/Controls) | |
| 2 | - | - | - | - |
| 3 | 3.79 ** (1.58–9.10) | - | 0.40 (0.15–1.06) | - |
| 4 | - | 0.74 (0.20–2.78) | - | 1.76 (0.54–5.70) |
| 5 | 1.12 (0.51–2.44) | 3.87 ** (1.39–10.73) | 2.06 (0.91–4.65) | 0.59 (0.22–1.61) |
| 6 | 0.91 (0.50–1.64) | 1.73 (0.74–4.07) | 0.75 (0.36–1.55) | 0.39 * (0.19–0.82) |
| 7 | 0.51 (0.21–1.24) | 0.50 (0.20–1.28) | 1.63 (0.57–4.62) | 1.67 (0.78–3.56) |
| 8 | 0.58 (0.26–1.31) | 0.17 ** (0.05–0.53) | 0.58 (0.17–1.96) | 2.00 (0.98–4.10) |
| 9 | - | 0.56 (0.10–3.16) | - | 2.74 * (1.14–6.62) |
* p < 0.05; ** p < 0.01.
5. Discussion
Despite the fact that stroke is most frequently considered as a multifactorial disorder where classical patterns of inheritance are hard to demonstrate, there are single gene defects that have been described, which have been predominantly associated with stroke [21]. In the meta-analysis by Hamzi et al., it was stressed that there was an association of some genes with ischemic stroke [22]. Furthermore, another meta-analysis conducted by Casas et al. pointed out that common variants in several genes were associated with an increased risk of stroke, with each having a modest effect [23]. Previously, it was noticed that genes are not only responsible for increased susceptibility to having a stroke but might influence an individual’s response to pharmacological treatment and the ultimate clinical outcome [24]. Moreover, in a multi-ancestry genome-wide study by Malik et al. [25], 32 gene loci were found to be significantly associated with the occurrence of strokes, of which 22 were new discoveries (the location of the gene is on the following chromosomes: 1–7, 9, 10, 12, 13, 15, 17 and 19). This study suggests a wide polygenic determination involved in the expression of the stroke. Therefore, this stresses how heterogeneous and complex the etiology of stroke is. Furthermore, in the era of precision medicine, it was noticed that the polygenic risk core approach is superior to the weighted multi-loci genetic risk score in the assessment of ischemic stroke genetic risks [26].
We have shown that there is a significant difference in the individual variations of tested HRCs between controls and patients with manifested ischemic stroke. Four HRCs were significant predictors in the group with ischemic stroke patients. We noticed that the presence of blue eyes had the highest strength of association with more than 3 times of possibility to be expressed. The presence of a continuous hair line was a significant predictor of stroke in controls, which could have protective effects to the certain degree. These findings suggest that intrinsic changes might exist between these two samples of tested individuals on the population genetic level, assuming that the preferential phenotypes could have certain roles in the development of ischemic stroke. The genes controlling the tested HRCs in this study might have an influence on an individual’s predisposition to the development of ischemic stroke to a certain degree [19].
Furthermore, the findings of this study demonstrated a significantly increased degree of recessive homozygosity in the group of patients with manifested ischemic stroke compared to controls. Despite the fact that the most frequent number of HRCs was 6 in both groups of tested subjects, controls more frequently had this number compared to stroke patients. However, higher numbers of HRCs (indicating the presence of a higher degree of recessive homozygosity) was clearly present in patients with manifested ischemic stroke. Increased recessive homozygosity in the tested group of patients with ischemic stroke might bring the organism into a specific state of genetic-physiological homeostasis, which enables easier expression of this condition [12,19]. Moreover, the increased recessive homozygosity could increase the genetic load, which might influence the processes that lead to a decrease in body immunity, predisposing this organism to developing ischemic stroke [12,27]. Finally, a higher degree of recessive homozygosity in stroke patients might be the result of the pleiotropic effects of genes that are responsible for an individual’s susceptibility to ischemic stroke [12,15].
For the group of tested subjects that did not have hypertension in our study, the patients with manifested ischemic stroke had a significantly higher degree of recessive homozygosity along with decreased variability. Despite the fact that we found no significant differences in the degree of recessive homozygosity between controls and stroke patients with diagnosed hypertension, there is increased recessive homozygosity along with decreased variability in patients with manifested ischemic stroke. These findings might justify the premise that genes determining the evaluated HRCs along with the environmental factors could potentially influence the development and easier expression of manifested ischemic stroke to a certain degree [16]. Our findings are in line with previous reports, which stated that hypertension has a multifactorial origin with both genetic and environmental factors contributing to different degrees [22,23,28]. Therefore, the increased degree of genetic homozygosity in the studied sample of stroke patients with hypertension compared to controls with hypertension implies that preferential phenotypes might exist, which could increase the susceptibility to the easier development of ischemic stroke. Furthermore, the presence of different variations in HRCs between controls and patients with manifested ischemic stroke with and without hypertension might modify the sensitivity of extreme genotype exposures to the risk of being influenced by the processes, which could lead to the onset of ischemic stroke [12].
There was a significant difference in the degree of recessive homozygosity between groups with and without hypertension separately for controls and ischemic stroke patients, with a greater effect size for the group of patients with manifested ischemic stroke. This could provide support for the assumption that there might be a correlation between different combinations of polygenes, which could influence the regulatory processes of resistance to ischemic stroke in stroke patients with hypertension to a certain degree. Furthermore, regarding the presence of hypertension, the different proportion of numbers of HRCs in ischemic stroke group compared to controls was found to be a significant predictor.
The increase in genetic homozygosity is probably correlated with the increase in genetic loads, which may enable easier expression of such condition. Therefore, manifested ischemic stroke patients without hypertension show a higher degree of recessive homozygosity. For the group of patients with hypertension, the association between manifested ischemic stroke and a lower degree of recessive homozygosity also requires the influence of the risk factor.
Furthermore, it should be stressed that the relatively large individual variation in the studied HRCs between controls and group with ischemic stroke, which covers almost all parts of the human body, also provides information about how large this variation in genetic homeostasis can be in human individuals, with a higher chance of individuals with the extreme genotypes having an increased risk of suffering from specific metabolic and developmental malformations. Consequently, the future application of HRC testing can be valuable for predicting these extremely deviant genotypes, which can cause people to be more susceptible to different diseases and conditions.
There are several limitations to this study. The tested HRCs that were evaluated in this study should be improved further as the origin of genetic determination and type of inheritance needs to be better evaluated. Moreover, the pleiotropic effects of the tested HRCs in studied individuals should be also be studied further. Additionally, the pleiotropic causes of risk factors along with tested HRCs should considered in future studies, particularly one using a larger population. This will enable analysis on the population genetic level, which will allow us to obtain a better understanding of the processes that govern the etiopathogenesis of ischemic stroke.
Given the facts above, it can be concluded that the patients with manifested ischemic stroke from our study had an increased degree of recessive homozygosity along with decreased variability compared to controls. The same trend regarding the degree of recessive homozygosity and variability was noticed in the group with hypertension and without hypertension although this occurred to a different degree. The applied methodology potentially might be used in further improved analyses as a sensitive screening tool for early prognosis of susceptibility to ischemic stroke.
Acknowledgments
The study was supported by Ministry of Education, Science and Technological Development of Serbia (175093).
Author Contributions
M.S., S.C., and D.N. was involved in conceptualization, supervision, methodology and writing of the original draft; M.L., and L.N. was involved in resources, formal analysis and writing the original draft. All authors approved the final version of the paper.
Conflicts of Interest
The authors have no conflict of interest.
References
- 1.Francis J., Raghunathan S., Khanna P. The role of genetics in stroke. Postgrad. Med. J. 2007;83:590–595. doi: 10.1136/pgmj.2007.060319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Razvi S.S., Bone I. Single gene disorders causing ischaemic stroke. J. Neurol. 2006;253:685–700. doi: 10.1007/s00415-006-0048-8. [DOI] [PubMed] [Google Scholar]
- 3.Dichgans M. Genetics of ischaemic stroke. Lancet Neurol. 2007;6:149–161. doi: 10.1016/S1474-4422(07)70028-5. [DOI] [PubMed] [Google Scholar]
- 4.Tournier-Lasserve E. New players in the genetics of stroke. N. Engl. J. Med. 2002;347:1711–1712. doi: 10.1056/NEJMcibr022035. [DOI] [PubMed] [Google Scholar]
- 5.Oline Mendelian Inheritance in Man (OMIM) [(accessed on 16 April 2018)]; Available online: http://www.ncbi.nlm.nih.gov.
- 6.Brass L.M., Isaacsohn J.L., Merikangas K.R., Robinette C.D. A study of twins and stroke. Stroke. 1992;23:221–223. doi: 10.1161/01.STR.23.2.221. [DOI] [PubMed] [Google Scholar]
- 7.Brass L.M., Shaker L.A. Family history in patients with transient ischemic attacks. Stroke. 1991;22:837–841. doi: 10.1161/01.STR.22.7.837. [DOI] [PubMed] [Google Scholar]
- 8.Ettehad D., Emdin C.A., Kiran A., Anderson S.G., Callender T., Emberson J., Chalmers J., Rodgers A., Rahimi K. Blood pressure lowering for prevention of cardiovascular disease and death: A systematic review and meta-analysis. Lancet. 2016;387:957–967. doi: 10.1016/S0140-6736(15)01225-8. [DOI] [PubMed] [Google Scholar]
- 9.Katsanos A.H., Filippatou A., Manios E., Deftereos S., Parissis J., Frogoudaki A., Vrettou A.R., Ikonomidis I., Pikilidou M., Kargiotis O., et al. Blood Pressure Reduction and Secondary Stroke Prevention: A Systematic Review and Metaregression Analysis of Randomized Clinical Trials. Hypertension. 2017;69:171–179. doi: 10.1161/HYPERTENSIONAHA.116.08485. [DOI] [PubMed] [Google Scholar]
- 10.Marinkovic D., Ilic M., Spremo B. Studies of human population—Genetic variation. Comparasion of homozygously recessive traits in attendants of special and regular schools in Serbia. Arh. Biol. Nauka. 1990;42:11–12. [Google Scholar]
- 11.Marinković D., Cvjetićanin S. Studes of human population genetic. The frequencies of ABO blood types and homozygously recessive traits among top sportsmen and young intelectuals. Arh. Biol. Nauka. 1991;43:1–2. [Google Scholar]
- 12.Marinkovic D., Cvjeticanin S. Anthropogenetic Homozygosity and Adaptive Variability. HRC-Test in Studies of Human Populations. Serbian Academy of Sciences and Arts; Belgrade, Serbia: 2013. Monographs DCLXXII, Book 8. [Google Scholar]
- 13.Cvjeticanin S., Marinkovic D. Genetic variability in the group of patients with congenital hip dislocation. Genetika. 2005;41:1142–1146. doi: 10.1007/s11177-005-0184-8. [DOI] [PubMed] [Google Scholar]
- 14.Cvjeticanin S., Marinkovic D. Genetic variability and frequencies of ABO blood types among different samples of patients from Serbia. Korean J. Genet. 2005;27:35–40. [Google Scholar]
- 15.Marinkovic D., Cvjeticanin S., Stanojevic M. Population genetic analyses of susceptibility to developing alcohol dependence. Addict. Res. Theory. 2008;16:331–337. doi: 10.1080/16066350801900156. [DOI] [Google Scholar]
- 16.Petricevic B., Cvjeticanin S. Morphogenetic variability and handedness in Montenegro and Serbia. Russ. J. Genet. 2011;43:406–411. doi: 10.1134/S1022795411030100. [DOI] [PubMed] [Google Scholar]
- 17.Branković S., Cvjetićanin S. Anthropogenetic variability in groups of children from regular and special schools from different localities in Serbia. Genetika. 2016;48:743–751. doi: 10.2298/GENSR1602743B. [DOI] [Google Scholar]
- 18.Nikolić D., Cvjeticanin S., Petronic I., Milincic Z., Brdar R., Karan R., Konstantinovic L., Dragin A., Cutovic M. Population genetic analyses of susceptibility to increased body weight. Arch. Med. Sci. 2012;8:998–1002. doi: 10.5114/aoms.2012.32405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cvjeticanin S., Marinkovic D. Morphogenetic variability during selection of elite water polo players. J. Sports Sci. 2009;27:941–947. doi: 10.1080/02640410902960494. [DOI] [PubMed] [Google Scholar]
- 20.Benjamin E.J., Blaha M.J., Chiuve S.E., Cushman M., Das S.R., Deo R., de Ferranti S.D., Floyd J., Fornage M., Gillespie C., et al. Heart disease and stroke statistics—2017 update: A report from the American Heart Association. Circulation. 2017;135:e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hassan A., Markus H.S. Genetics and ischaemic stroke. Pt 9Brain. 2000;123:1784–1812. doi: 10.1093/brain/123.9.1784. [DOI] [PubMed] [Google Scholar]
- 22.Hamzi K., Tazzite A., Nadifi S. Large-scale meta-analysis of genetic studies in ischemic stroke: Five genes involving 152,797 individuals. Indian J. Hum. Genet. 2011;17:212–217. doi: 10.4103/0971-6866.116112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Casas J.P., Hingorani A.D., Bautista L.E., Sharma P. Meta-analysis of Genetic Studies in Ischemic StrokeThirty-two Genes Involving Approximately 18 000 Cases and 58 000 Controls. Arch. Neurol. 2004;61:1652–1661. doi: 10.1001/archneur.61.11.1652. [DOI] [PubMed] [Google Scholar]
- 24.Munshi A., Sharma V. Genetic signatures in the treatment of stroke. Curr. Pharm. Des. 2015;21:343–354. doi: 10.2174/1381612820666140826113502. [DOI] [PubMed] [Google Scholar]
- 25.Malik R., Chauhan G., Traylor M., Sargurupremraj M., Okada Y., Mishra A., Rutten-Jacobs L., Giese A.K., van der Laan S.W., Gretarsdottir S., et al. Multiancestry genome-wide association study of 520,000 subjects identifies 32 loci associated with stroke and stroke subtypes. Nat. Genet. 2018;50:524–537. doi: 10.1038/s41588-018-0058-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hachiya T., Kamatani Y., Takahashi A., Hata J., Furukawa R., Shiwa Y., Yamaji T., Hara M., Tanno K., Ohmomo H., et al. Genetic Predisposition to Ischemic Stroke: A Polygenic Risk Score. Stroke. 2017;48:253–258. doi: 10.1161/STROKEAHA.116.014506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Milašinović S., Cvjetićanin S., Brdar R., Nikolić D. Morphogenetic variability and genetic loads among patients with different expression of developmental hip dysplasia. Genetika. 2017;49:1035–1045. doi: 10.2298/GENSR1703035M. [DOI] [Google Scholar]
- 28.Karan R., Obrenovic-Kircanski B., Cvjeticanin S., Kovacevic-Kostic N., Velinovic M., Milicevic V., Vranes-Stoimirov M., Nikolic D. The Gender Impact on Morphogenetic Variability in Coronary Artery Disease: A Preliminary Study. J. Clin. Med. 2018;7:103. doi: 10.3390/jcm7050103. [DOI] [PMC free article] [PubMed] [Google Scholar]