Abstract
Diabetes mellitus is a chronic, life-threatening metabolic disorder that occurs worldwide. Despite an increase in the knowledge of the risk factors that are associated with diabetes mellitus, its worldwide prevalence has continued to rise; thus, necessitating more research into its aetiology. Recent researches are beginning to link a dysregulation of the circadian rhythm to impairment of intermediary metabolism; with evidences that circadian rhythm dysfunction might play an important role in the aetiology, course or prognosis of some cases of diabetes mellitus. These evidences thereby suggest possible relationships between the circadian rhythm regulator melatonin, and diabetes mellitus. In this review, we discuss the roles of the circadian rhythm in the regulation of the metabolism of carbohydrates and other macronutrients; with emphasis on the importance of melatonin and the impacts of its deficiency on carbohydrate homeostasis. Also, the possibility of using melatonin and its analogs for the “prophylaxis” or management of diabetes mellitus is also considered.
Keywords: Chronobiology, Dysmetabolism, Insulin, Pancreatic beta cell, Melatonin receptors
Core tip: Diabetes mellitus is a chronic, life-threatening metabolic disorder with a huge disease burden and rising global prevalence that is nearing epidemic proportions. Research has continued to reveal the importance of circadian rhythm and the neurohormone melatonin in the regulation of carbohydrate metabolism. More studies are also revealing the potential roles of melatonin in the pathogenesis, management and modulation of the course of diabetes mellitus; especially type 2 diabetes mellitus. Presently, an array of potential mechanisms exists for melatonin’s roles in diabetes mellitus; however, a complete picture of this is yet to emerge.
INTRODUCTION
Diabetes mellitus is a chronic, life-threatening metabolic disorder with a huge disease burden and rising global prevalence that is nearing epidemic proportions[1]. According to the World Health Organisation (WHO) diabetes factsheet (which was updated in November 2017), in 2014, 8.5% of adults aged 18 years and older had diabetes mellitus; also, diabetes mellitus accounted directly for about 1.6 million deaths in 2015[1]. There have also been projections that diabetes mellitus will be the seventh leading cause of death by 2030[1,2]. As a disorder, diabetes mellitus is associated with increasing morbidity; accounting for a two- to three-fold increase in the risk of cardiovascular and cerebrovascular disease amongst adults[3]. About 2.6% of global blindness has been attributed to diabetes mellitus[4], and it has also been reported to be a leading cause of chronic kidney disease[5].
Presently, management of type 1 diabetes mellitus (T1DM) relies largely on insulin replacement, while that of T2DM is largely dependent on the use of drugs belonging to classes such as biguanides, sulfonylureas, meglitinides, intestinal brush border glucosidase inhibitors and thiazolidinediones. However, cost is a major limitation to the use of drugs (especially in low-income countries); also, there is the risk of side-effects like weight gain, heart failure and gastrointestinal disturbances[6]. The need to drastically reduce the global prevalence of T2DM necessitates a widening of the search for aetiological factors; and over the last two decades, a growing body of evidence has increasingly suggested the role of the biological clock and multiple clock genes in metabolic homeostasis. Data from epidemiological studies have also shown a correlation between circadian dysregulation (due to urbanisation and/or shift-work) and an increase in the prevalence of cardiovascular disease, cancers, inflammatory disorders, obesity, and diabetes mellitus[7-9]. Along this line, both human and rodent studies have demonstrated such relationships. Scheer et al[10] examined the effects of circadian misalignment between the behavioural cycle (feeding/fasting, sleep/wake) and the endogenous circadian rhythm, on metabolic and endocrine predictors of obesity, diabetes, and cardiovascular risk in humans; in their study, they demonstrated that circadian misalignment that occurs acutely (with jet lag) or chronically (with shift-work) was associated with an increased cardiometabolic risk[10]. Genetic polymorphisms involving circadian clock genes and/or circadian locomotor output cycles kaput genes have been linked to the development of metabolic syndrome, obesity, T2DM and hypertension[11-14]. In-vivo or in-vitro rodent studies have also demonstrated a possible link between the disruption of the circadian rhythm[15] or disruption of certain components of the clock genes[16] and the development of hypoinsulinaemia and T2DM.
Evidences demonstrating the importance of chronobiology in intermediary metabolism and the development of diabetes mellitus have also raised questions about the impact that melatonin (a regulator of the circadian rhythm) and its receptors may have on the aetiology, prognosis, prevention and treatment of diabetes mellitus. Certain studies in rodents have reported that melatonin inhibits insulin secretion from beta-cells via its interactions with MT1 and/or MT2 receptors on the beta cell-surface[17]; however, in humans, studies using reverse transcription-polymerase chain reaction demonstrated that human islets expressed mRNAs coding for both melatonin (MT1 and MT2) receptors[18]. Results of single-cell microfluorimetry have also suggested that the expression of MT1 receptor mRNA occurred only on alpha-cells and not on beta-cells[19]. An infusion of exogenous melatonin into dissociated human islet cells and perfused human islets, increased intracellular calcium and glucagon secretion respectively[19]. Genetic mapping and genome-wide association studies have also demonstrated strong associations between the gene for melatonin type 2 receptor (MTNR1B) which is expressed in the pancreatic beta-cells (amongst other tissues), and an increased risk for T2DM[18,20,21]. Again, there have been reports of impaired glucose tolerance following acute melatonin administration[22]. However, a few other studies have also demonstrated that melatonin receptor signalling in β-cell reduced oxidative stress response, militated against proteotoxicity-induced β-cell apoptosis, and restored glucose-stimulated insulin secretion in normal islets exposed to chronic hyperglycaemia or in type 2 diabetes islets[23].
There is a growing body of knowledge associating alterations in circadian rhythms, circadian genes, melatonin and melatonin receptors with derangement of intermediary metabolism and the development of diabetes mellitus. While the implication of this advance in knowledge for the prevention and therapeutic management of diabetes mellitus is evolving, there are strong indications that β-cell melatonin receptor 2 signalling is relevant for the regulation of β-cell survival and function; and by extension, may also be important in T2DM[23]. In this review, we examine relevant literature for the roles of the circadian rhythm in the physiological regulation of carbohydrates, with emphasis on the importance of melatonin in this capacity. The impacts of melatonin deficiency on carbohydrate homeostasis are also discussed. Finally, the possibility of utilising melatonin and its analogues for the “prophylaxis” and treatment of diabetes mellitus are also considered.
Pathogenesis and molecular basis of T2DM
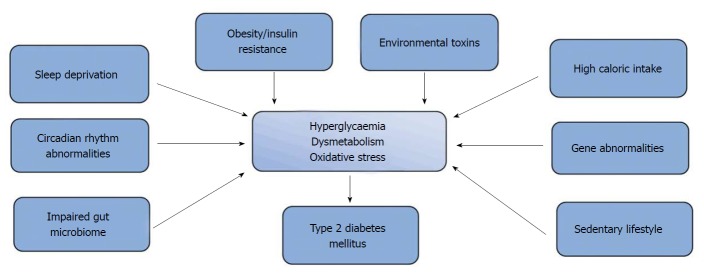
T2DM, which is characterised by impaired insulin secretion (or sensitivity) and hyperglycaemia, has been reported to account for greater than 90% of the total diabetes mellitus case-load[24,25]. It is a polygenic metabolic disorder that results from the interaction of environmental and genetic factors (Figure 1). These factors include obesity[26,27], sedentary lifestyle, high-calorie diet[28] and sleep deprivation[29]. Recently, reports from epidemiological and animal studies have suggested that increased presence of endocrine disruptors like pesticides, dioxins and bisphenol A in the environment may predispose to insulin resistance, alteration of β-cell function and impairment of glucose homeostasis[30]. While reports from genome-wide association studies have demonstrated strong associations between T2DM and over 100 gene variants that are located at four loci[31]; the peroxisome proliferator-activated receptor gamma gene (PPARG), which encodes the nuclear receptor PPAR-γ was the first candidate gene associated with T2DM[32]. Variants of this gene that are expressed in adipose tissue have also been linked to increased transcriptional activity, increased insulin sensitivity and protection against T2DM[22,32]. A number of candidate gene variants including the E23K polymorphisms in KCNJ11 and P12A in PPARG (that have been associated with an increased risk for T2DM)[33] have also been discovered through candidate association studies[34,35]. Studies have also shown that loss-of-function mutations involving KCNJ11 and ABCC8 candidate genes are implicated in hyperinsulinemia in infancy[36].
Figure 1.
Pathophysiology of type 2 diabetes mellitus.
There have been reports that gut microbiota are important in the maintenance of gastrointestinal mucosa permeability, metabolism of dietary polysaccharides (to produce short-chain fatty acids) and the regulation of fat accumulation[37]. These functions make them crucial to the development of obesity and obesity-related diseases[38]. Differences in gut microbiome between lean and obese subjects have also been reported[39]; with suggestions that an important role exists for gut bacteria (and possibly their end-products) in intermediary metabolism. Studies have also demonstrated that alteration in gut microbiota is associated with the development of T2DM and its complications[40]. The importance of gut microbiome to T2DM is affirmed by studies that have shown that transplantation of faecal microbiome from lean donors to subjects with insulin-resistance results in beneficial metabolic changes[41]. Studies in rodents have also demonstrated that modulation of the gut microbiome may also be beneficial in T2DM management[42].
A growing body of evidence suggests an important role for adipose tissue and lipotoxicity in T2DM. Presently, adipose tissue is considered an endocrine organ which influences lipid and glucose metabolism[43,44]. Dysfunctional adipose tissue (characterised by adipocyte hypertrophy, impaired insulin signalling and insulin resistance) results in the release of inflammatory adipokines and large amounts of free fatty acids; causing fat accumulation and lipotoxicity in organs involved in glucose metabolism such as liver, muscle and pancreatic beta cells[45-47]. Reports from a number of human and animal studies have also demonstrated the importance of brown adipose tissue in glucose homeostasis and the regulation of energy expenditure; with the possibility of brown adipose tissue becoming a therapeutic target[48-50].
Another area of extensive research into the pathophysiology of T2DM is the role that oxidative stress plays in the pathogenesis of micro- and macro-vascular diabetic complications[51]. It is believed that oxidative stress (via a common mechanism that involves the production of superoxide, and the inactivation of endothelial nitric oxide synthase and prostacyclin synthase) leads to the development of β-cell dysfunction, insulin resistance, impaired glucose tolerance, and T2DM[52,53]. There have also been suggestions of the involvement of this common mechanism in the development of both microvascular and macrovascular complications associated with T2DM[53,54]. Studies have also shown that T2DM associated increase in oxidative stress occurs as a consequence of hyperglycaemia, hyperinsulinaemia, insulin resistance, and dyslipidaemia[51].
CIRCADIAN RHYTHM
The circadian rhythms can be defined as endogenous rhythms (with behavioural and physiological components) that have a periodicity of about 24 h, and are synchronised through both photic and nonphotic stimuli[55]. These rhythms are known to control important biological processes, including sleep-wake cycle, hormone secretion, body temperature regulation, feeding/energy homeostasis, and cell-cycle regulation[55]. The circadian system is composed of a master clock which is located in the suprachiasmatic nucleus (SCN) of the hypothalamus and a number of peripheral clocks, which together regulate daily variations in many biological processes[56]. The suprachiasmatic nucleus is responsible for generating the circadian rhythms and as such is referred to as the endogenous biological pacemaker[57]. Daily adjustments of the timing of the SCN following exposure to stimuli (zeitgebers) which signals time of day helps to achieve synchrony with the earth’s rotation. A loss of the coordination of these rhythms is known to negatively impact body physiology and behaviours[55].
Anatomically, the SCN is a bilateral structure that contains over 20000 neurons and is a central component of the circadian timing system[56]. It receives input pathways for light and other stimuli that are important in the synchronisation of the pacemaker to the environment; output rhythms are in turn regulated by the pacemaker[55]. Direct (retinohypothalamic) and indirect (retinogeniculate) photic information to the SCN comes from the retina[58]. Retinohypothalamic photic information originates from the ganglion cells of the retina (which contain melanopsin, and are regarded as the primary photoreceptors for the circadian system), nonphotic information comes from the raphe nuclei, while other afferents come from the pons, medulla, basal forebrain and posterior hypothalamus[55]. Arising from the SCN, major efferents project to areas such as the hypothalamus (dorsomedial, subparaventricular zone and the paraventricular nucleus), thalamus, preoptic/retrochiasmatic areas, stria terminalis, lateral septum, and intergeniculate nucleus[55]. Gamma-amino butyric acid is the dominant neurotransmitter that is found in the SCN; however, the SCN core contains vasoactive intestinal polypeptide, gastrin-releasing peptide and bombesin-containing neurons, while somatostatin and neurophysin are predominant within the shell[55].
Circadian timing is affected by several zeitgebers including light, feeding schedules, activity, and the hormone melatonin; of these, light is considered of utmost importance, and the most potent stimulus[55]. Light also modulates pineal gland melatonin secretion through regulation by the SCN, with peak secretion occurring in the middle of the night[57]. Another important marker of internal time (especially during periods of low ambient light) is the circadian rhythm of pineal melatonin. The timing of the endogenous circadian rhythm can be determined by dim light melatonin onset (DLMO) which is regarded as a stable marker of the circadian phase[57]. Melatonin is also associated with the maintenance of sleep propensity rhythm in humans, and as such, it is considered a modulator of internal sleep[59]. There is also evidence suggesting that exogenous melatonin can induce phase shifts in the circadian clock[59].
The genetic control of the circadian rhythms is determined by a core set of clock genes which interact with their own products to form a number of molecular feedback loops, which regulate the circadian rhythm[60]. These genes include three period (Per) homolog genes (Per1; Per2; Per3), two plant cryptochrome gene homologs (Cry1and Cry2), the circadian locomotor output cycles kaput gene (Clock) and the cycle gene (Bmal1)[60]. The interactions of these genes and their products form transcription-translation (molecular) feedback loops that generate the circadian rhythm, and also controls the temporal expression of a number of clock-controlled genes[61].
Circadian rhythm dysregulation and intermediary metabolism
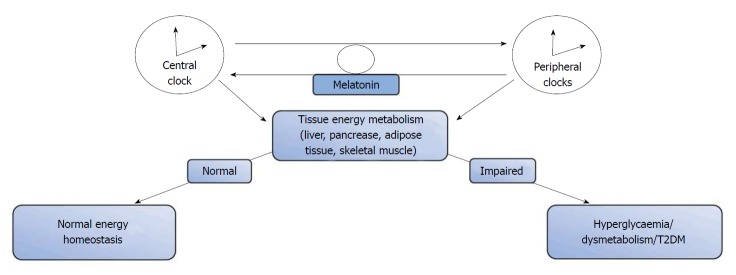
The circadian rhythm is a conserved timing system that modulates behavioural and physiological process to 24-h environmental cycles[55,62]. It is generally accepted that the circadian rhythm depends on zeitgebers or cues for the daily adjustments of its timing; as such, daily cycles of activity/feeding and the biological/molecular rhythm assist in the maintenance of energy homeostasis, linking the circadian clock to metabolic systems[63]. It is known that the molecular clock is present in all metabolic tissues including the liver, intestine, adipose tissue, heart, and retina[62]. This master clock in the SCN works in synchrony with the peripheral clocks, and together, they regulate cellular and physiological functions[64]. Some of these functions which include metabolism and energy homeostasis occur through organs such as the liver, and other peripheral tissues. A part of this task is achieved by regulating the expression and/or activity of certain key metabolic enzymes and transport systems that are involved in the lipogenic and adipogenic pathways[64,65]. However, this relationship is bidirectional, with the metabolic enzymes and transcription activators also interacting with and affecting the clock mechanism. An understanding of this relationship is crucial to appreciating how abnormalities such as mutations in clock genes can disrupt cellular rhythmicity and metabolic homeostasis. Also, clinical studies that focus on shift workers and obese patients further illuminate the link between the circadian clock and energy metabolism[64,65].
There are strong indications that circadian misalignment (or dysfunction) is an emerging risk factor for metabolic diseases[62]. Studies have shown that variations in diet or dietary intake may influence the circadian rhythm of feeding/activity; and this in turn modulates the biological or molecular clock[63]. A number of studies have also associated circadian rhythm disruption and sleep loss/deprivation with obesity[66,67]. Studies in humans who are on night-time shift work also demonstrated that strong associations exist between alterations in circadian rhythm and metabolic parameters such as increased body mass, increased plasma lipid, and glucose levels[68-70]. Karatsoreos et al[71] reported that chronically housing mice in an environment with shortened light/dark cycle resulted in weight gain, alteration of body temperature rhythms, and increased plasma levels of leptin and insulin[71]. Several disorders relating to human psychology and sleep have also been associated with abnormal functioning of the master biological clock. A number of the core hormones that are involved in nutrient metabolism (including insulin, glucagon, adiponectin, corticosterone, leptin and ghrelin) have been shown to undergo circadian oscillation in their levels and activities[72-74]. Studies have also demonstrated that the molecular clock controls mitochondrial posttranslational modification and oxidative metabolism[75]. The molecular clock controls cellular metabolism through its ability to direct the rhythmic synthesis of nicotinamide adenine dinucleotide (NAD+), which is a metabolic cofactor. NAD+ subsequently modulates the activity of the protein deacetylase, sirtuin 1 (SIRT1), which controls cellular metabolism via a feedback loop[76-78]. These nutrient sensors relay information about the cellular nutrient status to the circadian clock, and modulate the activity of clock genes. For example, while the oxidised forms of sodium dehydrogenase (NAD+) redox co-factor inhibits the activity of heterodimers of circadian clock genes like Clock/Bmal1 and Npas2/Bmal1; the reduced forms (NADH) increases their activity[79]. Others, like AMP kinase have also been shown to regulate expression of clock genes[80-83]. Studies in which deletions or mutations in the clock genes result in disruption of the cellular rhythm also provide strong evidence of the cross-talk that occurs between the circadian clock and metabolism.
There are also reports suggesting that key proteins may be involved in the regulation of the core clock mechanism and adipose tissue metabolism; thereby linking the circadian rhythms with lipid metabolism[65]. The role of the circadian clock in the regulation of adipose tissue differentiation has been considered[84]. In-vitro and in-vivo studies have also been used to examine the role of the circadian rhythm in adipocyte physiology. Studies involving cell lines in which clock genes transcription factors like Bmal1 or Rev-Erbα (a nuclear receptor which suppresses Bmal1 expression) were knocked out reported inhibition of adipocyte differentiation[85-87]; while those involving mutations of clock components like Per2[88] or retinoid orphan receptor α[89,90] were associated with an increase in adipogenesis, with these effects mediated by PPARγ[91,92]. Studies in male mice have also demonstrated that the rhythm of expression of the clock genes and adipose PPARγ are decreased by the consumption of high-fat diet[93].
Circadian rhythm and glucose control: Like all other aspects of intermediary metabolism, blood glucose homeostasis is also under circadian regulation; with variations in blood glucose levels occurring with the changes in external synchronisers (activity/feeding and resting/starvation)[94]. During the activity/feeding phase, blood glucose levels are maintained from dietary intake; whereas, during the resting/starvation period, there is a progressive recruitment of glucose from endogenous glucose sources in the liver to maintain blood levels within a relatively narrow margin[94]. The liver also alternates between glycogenolysis and glycogenesis[95,96]. Studies have also shown that daily blood glucose control is also modulated by both the central circadian clock in the SCN as well as by peripheral clocks in the pancreas, liver, muscle and white adipose tissue. This is affirmed by studies in humans, that have observed differences in glycaemic response between meal studies conducted in the morning and those in the evening[97-99]. This alteration in glycaemic control had been attributed to circadian variations in insulin secretion and an increase in hepatic or peripheral insulin resistance[99-101]. Studies using animal models have also shown that insulin secretion follows a rhythmicity that is regulated by peripheral pancreatic β-cell clocks[102]. In humans there have been reports that the set-point for the regulation of the 24-h pulsatile secretion of insulin is higher in obese subjects, T2DM subjects, and their non-diabetic first degree relative[102,103] compared to the general population. Studies using different animal models of circadian clock gene dysfunction (ClockΔ19, Cry1 and Cry2, Bmal1) have also reported evidence of hyperglycaemia, increased insulin sensitivity or impaired insulin secretion[16,104,105].
Gut hormones which are very important in modulating gastric emptying and maintaining glucose homeostasis, like the anorexigenic peptides (glucagon, insulin, glucose inhibitory peptide, glucagon-like peptide-1, amylin, peptide YY) and the orexigenic hormone ghrelin have also been shown to fluctuate with activity/feeding and resting/starvation periods. The variations in their activity pattern are also under circadian control and as such may be altered by circadian disruptors, including altered meal times, dietary compositions and constant light exposure[106,107].
There are evidences supporting the existence of a relationship between gut microbiota and the circadian system; and presently, it is known that intestinal microbiome is regulated by circadian rhythms through the intrinsic circadian clocks[108]. This regulation affects host metabolic function through alteration of microbial community structure as well as their metabolic activities. Up to one-fifth of human gut bacteria exhibit diurnal variations in their activities and abundance; and some species, like Enterobacter aerogenes had been shown to be responsive to the circadian fluctuations in the hormone melatonin[108]. Alterations in the balance of this relationship can lead to changes in the activities and relative composition of gut microbiota. Finally, abnormalities in composition and activities of gut microbiota had been linked to insulin resistance and diabetes mellitus through several mechanisms, such as regulation of adiposity/obesity, regulation of the immune system, modulation of inflammatory processes, and extraction of energy from the diet[109].
Circadian rhythm dysfunction, sleep and T2DM
A number of studies have demonstrated that a dysregulation of the internal circadian clock system or discordance with the external environmental cues has deleterious health consequences, with an associated increase in morbidity and mortality in humans[9]. Increasingly, results from epidemiological[110,111] and animal[8,9,15] studies continue to show associations between circadian rhythm dysfunction (that occur due to sleep loss, shift work or nocturnal lifestyle) and the development of T2DM[8,9,15] (Figure 2). An in-vitro study using rat pancreatic islets revealed that exposure of the islets to continuous light was associated with a disruption of the circadian clock function and reduction in glucose-stimulated insulin secretion, due to a decrease in insulin secretory pulse mass[112]. Also, there have been reports that a disruption of circadian rhythm could induce abnormal insulin release in people at risk of developing T2DM. Gale et al[15] examined the metabolic and physiological changes associated with T2DM following circadian rhythm dysfunction in wild-type, Sprague Dawley and diabetes-prone human islet amyloid polypeptide transgenic rats that were exposed to prolonged episodes of normal light (or experimental disruption in the light-dark cycle), and reported that circadian rhythm disruption accelerated the development of diabetes in diabetes-prone rats, but not in wild-type rats[15]; an effect that has been attributed to pancreatic β-cell loss and dysfunction[15]. Marcheva et al[16] reported that disruption of the clock gene components (Clock and Bmal1) was associated with delays in the phase of oscillation of islet genes that were involved in islet cell growth, glucose metabolism and insulin signalling; resulting in impaired glucose tolerance, reduction in insulin secretion, and alterations in the size and proliferation of pancreatic islets[16]. They also demonstrated that conditional ablation of the pancreatic clock resulted in the development of diabetes mellitus via alteration in β-cell function[16]. Also, there have been suggestions and experimental evidence to show that the mammalian islet clock was responsible for regulating the expression of genes that are involved in sensing glucose levels, insulin secretion, as well as islet cell growth and development[16,113].
Figure 2.
The role of circadian rhythm in the development of type 2 diabetes. T2DM: Type 2 diabetes mellitus.
While we gain new insights into the pathophysiology of T2DM, and continue to understand the roles played by the circadian rhythm[15,16,113]; there is ample scientific evidence to show that a disruption of circadian rhythms alters not only the body weight and adiposity, but it also affects glucose metabolism and glycaemic control. While the magnitude of these effects (as it relates to the development and progression of T2DM) continues to be studied, it is also important to continue to investigate their precise mechanisms, and to determine the relevance of this new knowledge to the therapy and prevention of T2DM.
There appears to be strong relationships between certain sleep parameters and the risk of development of diabetes mellitus. Along this line, numerous evidences from both epidemiological and laboratory studies have continued to reveal and support the fact that poor sleep is strongly associated with the development of glucose- intolerance, insulin resistance, and ultimately T2DM[114].
In a community-based study of adults of both sexes in Xuzhou, China; it was found that after adjustment for a large number of possible aetiological factors, poor sleep-quality and short (≤ 6 h) sleep duration were significantly associated with increased prevalence of diabetes mellitus, when compared with the group of people with good quality of sleep and longer (6-8 h) overnight sleep duration[115]. Again, poor sleep has been known to be associated poor glycaemic control in T2DM patients. In a Japanese study involving 3249 patients with T2DM; an assessment of sleep, using the Pittsburgh Sleep Quality Index (PSQI) showed that (independent of potential confounders) poor subjective sleep quality was associated with less-than-optimal glycaemic control[116].
MELATONIN
Melatonin is a tryptophan-derived indoleamine which is primarily secreted by the pineal gland, with contributions from a number of other tissues including the retina, bone marrow, gastrointestinal tract, skin, ovary and placenta[117,118]. The extra-pineal contribution to melatonin production is small when compared to secretion from the pineal gland; with suggestions that it is only triggered by some specific impulses[119]. Melatonin secretion is regulated by the central circadian clock, as well as by seasonal variations in length of daylight. Production is acutely suppressed by exposure to light, with increased secretion occurring at night in both nocturnal and diurnal species. Plasma concentrations of endogenous melatonin also vary considerably with age[120-122]. Melatonin is a multifunctional molecule that is capable of intracrine, paracrine or autocrine signalling[117]. It can cross all physiological barriers and exert widespread regulatory effects on numerous body tissues. Melatonin is important in the regulation of biologic rhythms[123]; and numerous studies in humans and rodents have reported melatonin’s widespread influence on varied biological and behavioural processes[124-126]. Melatonin plays important roles in neurogenesis, neuroprotection and the maintenance of oxidant/antioxidant balance[127-129]. A few studies have also reported its role in diabetes control[18].
Melatonin’s role in intermediary metabolism
There is increasing scientific evidence to suggest that a derangement of melatonin rhythmicity may have adverse health implications, especially as it relates to its importance in modulating a variety of metabolic functions, as well as its role as a regulator of epigenesis[130]. Studies have demonstrated the presence of high concentrations of extra-pineal melatonin in the gastrointestinal tract (GIT) of a number of mammals[131]. There had also been reports suggesting that extra-pineal melatonin from the GIT contributes significantly to circulating blood melatonin levels (mostly during the day)[131]; although there are evidences to suggest that some of the melatonin in the GIT may be pineal in origin. However; there are reports suggesting that the release of GIT melatonin may be related to the periodicity of food intake rather than being photoperiodic, as occurs with melatonin from the pineal gland[131]. These evidences are stimulating interest in investigating the possible relationship that may exist between melatonin in the GIT and metabolism; especially, since a number of studies in vertebrates had demonstrated exogenous melatonin’s ability to modulate appetite, energy metabolism, anorexigenic hormone/peptide concentration, and body weight[132-134]. Earlier studies evaluating melatonin’s relationship with the GIT and intermediary metabolism reported alterations in the overall food consumption in mice following administration of exogenous melatonin; while a few other studies also demonstrated an increase in tissue and blood melatonin levels with food intake and prolonged food deprivation[131,135]. Studies in zebrafish (Danio rerio) had also demonstrated that melatonin administration induced a decrease in food intake, it also modulated the stimulation of satiety and anorexigenic signals in the liver and intestine[136]. However, a number of studies have suggested that melatonin’s roles in appetite modulation may arise from different mechanisms; with suggestions that its anorexigenic effects could be as a result of its ability to delay gastric emptying[137,138] or via its stimulatory activity on fat mobilisation[139,140]. A number of other studies in fish have also reported that melatonin‘s ability to reduce food consumption may be related to circadian rhythm stimulation (i.e., its ability to promote sleep), and not necessarily due to a direct effect of the hormone[141].
Melatonin, melatonin receptors, glucose metabolism and T2DM
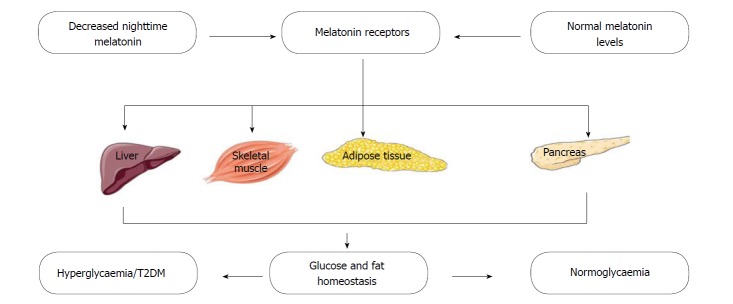
A number of studies have provided evidence that melatonin influences glucose metabolism. In healthy subjects, glucose homeostasis is controlled within a narrow margin via a complex pathway of regulatory mechanisms that involves multiple organs and tissues (Figure 3). Therefore, a disruption of normal glucose balance usually results from a sustained reduction in both pancreatic beta-cell function and insulin secretion[142,143]. In rodents, melatonin has been shown to regulate blood glucose concentration through its ability to bind directly to melatonin receptors on hepatocytes[144] and regulate the uptake of glucose in adipocytes, by modulating the expression of the glucose uptake transporter[145]. Abnormalities of the nocturnal melatonin profile have also been described in diabetic patients, especially in those suffering from diabetic neuropathy[146]. Low melatonin secretion is also independently associated with a higher risk of developing T2DM; an association that further establishes the roles of melatonin in glucose metabolism and insulin sensitivity[147]. Post mortem studies have also indicated an association between diabetes mellitus and decreased melatonin secretion[148]; while some in-vivo and in-vitro studies have demonstrated melatonin’s ability to inhibit the secretion of insulin by pancreatic beta-cells[149]. Presently, a growing body of evidence suggests a relationship between disturbances in melatonin production and impairment of insulin, glucose and lipid metabolism[134,150]; and that of antioxidant capacity[130,151,152]. Results from both in-vivo and in-vitro studies have shown that in patients with metabolic syndrome, night-time melatonin level is related to night-time insulin concentrations[153]. There have also been reports of lower elevations in night-time melatonin levels in diabetic subjects; raising interests in the link between melatonin and hyperglycaemia/diabetes mellitus[154]. Also, melatonin has been reported to stimulate the secretion of glucagon, another hormone that is important in glucose metabolism[155].
Figure 3.
Melatonin, melatonin receptors, glucose and fat homeostasis and type 2 diabetes mellitus. T2DM: Type 2 diabetes mellitus.
Melatonin receptors (MT1 and MT2) have been observed to be present in rodent[156-158] and human[18,19,159] pancreatic islets. The expression of these receptors also varies with the circadian rhythm and feeding status[160]. In humans, several genetic studies have associated MT2 receptor polymorphisms with an increased risk of developing T2DM[150]. Associations between single nucleotide polymorphisms that are situated close to (or within) the gene that encodes MT2 (MTNR1B), and an increased risk of developing T2DM[18,161,162], diminished B-cell function[163,164] and impaired glycaemic control[165-167] have all been reported in cohorts of different regions and ethnicities. Studies have also demonstrated an increase in the expression of MT1 and MT2 receptors in the pancrease of diabetic rats and in subjects with T2DM[168].
Melatonin’s potential roles in prophylaxis or treatment: Experimental and clinical data continue to suggest that both endogenous as well as exogenously-administered melatonin play crucial roles in the improvement of diabetes control. In a rat model of diabetes mellitus, long-term administration of melatonin (1.1 mg/d for 30 wk) attenuated the development of hypertriglyceridaemia, hyperinsulinaemia and hyperleptinaemia[169]. In a study among community-dwelling diabetics, the effect of administration of 2 mg of prolonged-release melatonin (at 9-11 pm for 3 wk) on glucose and lipid metabolism was investigated[148]. This initial administration was followed by an extended period of five months of open-label, prolonged-release melatonin administration to evaluate the effects of prolonged-release melatonin on glycosylated haemoglobin (HbA1c) levels[148]. The results established the safety of prolonged-release melatonin with regards to parameters such as glucose, lipid metabolism, and other routine biochemical indices; also, there were no adverse interactions with routinely-used anti-diabetic drugs, or insulin release[148]. In an earlier study involving twenty-two postmenopausal non-diabetic women, the results suggested that glucose tolerance and insulin sensitivity are reduced following a single oral administration of melatonin 1 mg[170]. However, in diabetic women, use of prolonged-release melatonin (in the short term or long term) did not impair insulin action or glucose tolerance; on the contrary, there was improved glycaemic control upon long-term use[148]. A few other studies have demonstrated that melatonin plus zinc acetate alone, or in combination with metformin improved both fasting and postprandial glycaemic control in T2DM patients[171].
Presently, research continues to unravel the multifaceted effects of melatonin on intermediary metabolism, especially that of glucose; with direct evidences of melatonin’s effects on insulin secretion, pancreatic beta cell activity, hepatic glucose metabolism and insulin sensitivity[172]. Apart from these, melatonin also combats cellular/tissue oxidative stress and inflammation. Therefore, by the modulation of several intracellular signalling pathways and tissue targets, melatonin is emerging to occupy a central role in the understanding of the aetiology and management of diabetes mellitus[173].
Melatonin receptors (MT1 and MT2) have been shown to be present on human pancreatic islets, and the effects of melatonin on insulin secretion are mediated through these receptors[17]. Melatonin is able to affect insulin secretion in two ways, decreasing it by inhibiting cAMP and cGMP pathways, and increasing it by activating the phospholipase C/Inositol triphosphate pathway, which mobilises calcium ions from organelles, consequently increasing insulin secretion. Melatonin also induces production of insulin growth factor and promotes insulin receptor tyrosine phosphorylation; while its supplementation attenuates glucose intolerance and insulin resistance[17].
The use of melatonin in the pharmacotherapy of diabetes mellitus may confer additional benefits over what is obtainable with conventional drugs alone. This is due to its ability to affect several pathways that may be involved in the pathogenesis or progression of the disease. In an experimental model designed to express obese T2DM phenotype, rats with concomitant circadian disruption and diet-induced obesity were treated daily with oral melatonin, metformin, or a combination of the two for 12 wk[174]. It was observed that melatonin alone improved circadian activity/rhythms, attenuated induction of beta-cell failure, and enhanced glucose tolerance. Use of metformin alone only enhanced insulin sensitivity and glucose tolerance. However, combining melatonin with metformin attenuated progression of metabolic dysfunction by improving adiposity, circadian activity, insulin sensitivity, and islet cell failure[174]. The results suggest that attenuation or arrest of circadian dysfunction may be crucial to managing metabolic dysfunction and altering the course of the disease in T2DM. In mice that were given high-fat diet (HFD), oral melatonin at 100 mg/kg per day (for 10 wk) led to a significant reduction in body weight-gain (compared to the HFD controls) and it also reduced hepatic steatosis. Also, there was improved insulin sensitivity and glucose tolerance, with down- regulation of fetuin-A (a hepatokine that is associated with insulin resistance and T2DM) and endoplasmic reticulum stress markers in the liver and serum[175].
One of the ways by which melatonin may be beneficial in the management of T2DM and metabolic syndrome is through its ability to reduce adiposity by modulation of the gut microbiota. In mice that were fed high-fat diet, melatonin treatment significantly reversed gut microbiota dysbiosis, increasing the ratio of the bacteria that are known to be associated with a healthy mucosa while also improving markers of adiposity and inflammation[176].
Some studies have also assessed the impact of melatonin supplementation on the development of microvascular and macrovascular complications of diabetes mellitus and concluded that melatonin has beneficial effects in repairing cardiac injury due to diabetes mellitus[177]. Zhou et al[176] reported that inhibition of the splenic tyrosine kinase (which is activated by hyperglycaemia and contributes significantly to the development of diabetic cardiomyopathy) by melatonin supplementation reversed diabetes-related loss of myocardial function, decreased cardiac fibrosis and preserved the viability of cardiac myocytes[176].
There have been studies that had reported the influence of melatonin on mitochondrial bioenergetics due to its ability to regulate mitochondrial fission/fusion[178,179] and regulate mitophagy/autophagy[180]. In view of the above, Ding et al[181] examined the possible effects of melatonin supplementation on the development of myocardial contractile dysfunction (which has been linked to an increase in mitochondrial fission in subjects with diabetes mellitus), and reported that melatonin attenuated diabetes-induced myocardial dysfunction by decreasing the expression of dynamin-related protein 1, leading to the prevention of mitochondrial fission[181]. Melatonin administration also prevented mitochondrial fragmentation, decreased oxidative stress, and reduced apoptosis of the cardiomyocyte in streptozotocin-induced diabetic mice; however, these were not replicated in the protein deacetylase sirtuin 1 (SIRT1)-/- diabetic mice[181]. Thus, suggesting that melatonin’s cardioprotective effects were exerted through its effects on SIRTI[181].
Melatonin’s antioxidant or oxidative stress-reduction effect is one of the benefits that have increased interests in its possible use in the management of diabetes mellitus and its complications. Studies in rodents have demonstrated that intraperitoneal administration of melatonin (3 mg/kg per day for 4 wk) reduced lipid peroxidation marker (malonyldialdehyde) and increased glutathione levels in the bone tissue of diabetic rats subjected to acute swimming exercise[182]. Mehrzadi et al[183] also examined the effects of melatonin supplementation on the development of diabetes-related retinal injury in rats. Their results showed that while induction of diabetes increased oxidative stress and inflammation, treatment with melatonin for a period of seven weeks attenuated the development of retinal injury; largely through reduction of oxidative stress and inflammation[183]. Studies in human subjects have also demonstrated that melatonin’s cardioprotective effects can be attributed to its ability to reduce oxidative stress and improve cardiometabolic risk[184]. In a randomised, double-blind, placebo-controlled trial, two groups of subjects were administered either melatonin (10 mg) or placebo, once daily for 12 wk[184]. Results of this study showed that (compared to subjects that were administered placebo) melatonin supplementation (in addition to its beneficial effects on glycaemic control, reduction of insulin resistance and improvement of insulin sensitivity) was associated with an increase in the plasma concentration of glutathione, nitric oxide, high density lipoprotein; and a decrease in the levels of malondialdehyde and serum C-reactive protein[184].
A few studies in rodents have also explored the possible use of melatonin as an adjunct to insulin therapy. Oliveira et al[185] reported that 8 weeks of administration of melatonin in drinking water at 0.2 mg/kg body weight (either alone or in combination with insulin (NHP, 1.5 U/100 g/d) improved glycaemic control, increased insulin sensitivity and reduced the expression of hypothalamic genes that are related to reproductive function[185].
CONCLUSION
Research has continued to reveal the importance of circadian rhythm regulation, and the neurohormone melatonin in the regulation of carbohydrate metabolism. More studies are also revealing the potential roles of melatonin in the pathogenesis, management and modulation of the course of diabetes mellitus, especially T2DM; and as shown by these studies, an array of possible mechanisms exists for melatonin’s effects.
However, a complete picture of the role(s) of melatonin in the management of DM is yet to emerge. Also, we are yet to get to the point where melatonin and melatonin receptor agonists may be prescribed as adjuncts or alternatives to already-existing orthodox medications. Finally, we are just beginning to understand how melatonin may be used to prevent or delay the occurrence of diabetes mellitus.
Footnotes
Conflict-of-interest statement: Both authors of this paper declare that there is no conflict of interest related to the content of this manuscript.
Manuscript source: Invited manuscript
Peer-review started: April 16, 2018
First decision: May 24, 2018
Article in press: June 8, 2018
Specialty type: Endocrinology and metabolism
Country of origin: Nigeria
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Das U, Hamasaki H S- Editor: Ji FF L- Editor: A E- Editor: Tan ww
Contributor Information
Adejoke Y Onaolapo, Behavioural Neuroscience/Neurobiology Unit, Department of Anatomy, Ladoke Akintola University of Technology, Ogbomosho 210211, Oyo State, Nigeria.
Olakunle J Onaolapo, Behavioural Neuroscience/Neuropharmacology Unit, Department of Pharmacology, Ladoke Akintola University of Technology, Osogbo 230263, Osun State, Nigeria. olakunleonaolapo@yahoo.co.uk.
References
- 1.World Health Organisation. Diabetes 2017. Available from: http://www.who.int/news-room/fact-sheets/detail/diabetes.
- 2.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 3.Emerging Risk Factors Collaboration, Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio E, Ingelsson E, Lawlor DA, Selvin E, Stampfer M, Stehouwer CD, Lewington S, Pennells L, Thompson A, Sattar N, White IR, Ray KK, Danesh J. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215–2222. doi: 10.1016/S0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bourne RR, Stevens GA, White RA, Smith JL, Flaxman SR, Price H, Jonas JB, Keeffe J, Leasher J, Naidoo K, et al. Causes of vision loss worldwide, 1990-2010: a systematic analysis. Lancet Glob Health. 2013;1:e339–e349. doi: 10.1016/S2214-109X(13)70113-X. [DOI] [PubMed] [Google Scholar]
- 5.United States Renal Data System. 2014 USRDS annual data report: Epidemiology of kidney disease in the United States. Bethesda: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2014. pp. 188–210. [Google Scholar]
- 6.Naveen J, Baskaran V. Antidiabetic plant-derived nutraceuticals: a critical review. Eur J Nutr. 2018;57:1275–1299. doi: 10.1007/s00394-017-1552-6. [DOI] [PubMed] [Google Scholar]
- 7.Knutson KL, Spiegel K, Penev P, Van Cauter E. The metabolic consequences of sleep deprivation. Sleep Med Rev. 2007;11:163–178. doi: 10.1016/j.smrv.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bass J, Takahashi JS. Circadian integration of metabolism and energetics. Science. 2010;330:1349–1354. doi: 10.1126/science.1195027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reddy AB, O’Neill JS. Healthy clocks, healthy body, healthy mind. Trends Cell Biol. 2010;20:36–44. doi: 10.1016/j.tcb.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci USA. 2009;106:4453–4458. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woon PY, Kaisaki PJ, Bragança J, Bihoreau MT, Levy JC, Farrall M, Gauguier D. Aryl hydrocarbon receptor nuclear translocator-like (BMAL1) is associated with susceptibility to hypertension and type 2 diabetes. Proc Natl Acad Sci USA. 2007;104:14412–14417. doi: 10.1073/pnas.0703247104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sookoian S, Gemma C, Gianotti TF, Burgueño A, Castaño G, Pirola CJ. Genetic variants of Clock transcription factor are associated with individual susceptibility to obesity. Am J Clin Nutr. 2008;87:1606–1615. doi: 10.1093/ajcn/87.6.1606. [DOI] [PubMed] [Google Scholar]
- 13.Scott EM, Carter AM, Grant PJ. Association between polymorphisms in the Clock gene, obesity and the metabolic syndrome in man. Int J Obes (Lond) 2008;32:658–662. doi: 10.1038/sj.ijo.0803778. [DOI] [PubMed] [Google Scholar]
- 14.Englund A, Kovanen L, Saarikoski ST, Haukka J, Reunanen A, Aromaa A, Lönnqvist J, Partonen T. NPAS2 and PER2 are linked to risk factors of the metabolic syndrome. J Circadian Rhythms. 2009;7:5. doi: 10.1186/1740-3391-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gale JE, Cox HI, Qian J, Block GD, Colwell CS, Matveyenko AV. Disruption of circadian rhythms accelerates development of diabetes through pancreatic beta-cell loss and dysfunction. J Biol Rhythms. 2011;26:423–433. doi: 10.1177/0748730411416341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marcheva B, Ramsey KM, Buhr ED, Kobayashi Y, Su H, Ko CH, Ivanova G, Omura C, Mo S, Vitaterna MH, et al. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature. 2010;466:627–631. doi: 10.1038/nature09253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharma S, Singh H, Ahmad N, Mishra P, Tiwari A. The role of melatonin in diabetes: therapeutic implications. Arch Endocrinol Metab. 2015;59:391–399. doi: 10.1590/2359-3997000000098. [DOI] [PubMed] [Google Scholar]
- 18.Lyssenko V, Nagorny CL, Erdos MR, Wierup N, Jonsson A, Spégel P, Bugliani M, Saxena R, Fex M, Pulizzi N, et al. Common variant in MTNR1B associated with increased risk of type 2 diabetes and impaired early insulin secretion. Nat Genet. 2009;41:82–88. doi: 10.1038/ng.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramracheya RD, Muller DS, Squires PE, Brereton H, Sugden D, Huang GC, Amiel SA, Jones PM, Persaud SJ. Function and expression of melatonin receptors on human pancreatic islets. J Pineal Res. 2008;44:273–279. doi: 10.1111/j.1600-079X.2007.00523.x. [DOI] [PubMed] [Google Scholar]
- 20.Bonnefond A, Clément N, Fawcett K, Yengo L, Vaillant E, Guillaume JL, Dechaume A, Payne F, Roussel R, Czernichow S, Hercberg S, Hadjadj S, Balkau B, Marre M, Lantieri O, Langenberg C, Bouatia-Naji N; Meta-Analysis of Glucose and Insulin-Related Traits Consortium (MAGIC), Charpentier G, Vaxillaire M, Rocheleau G, Wareham NJ, Sladek R, McCarthy MI, Dina C, Barroso I, Jockers R, Froguel P. Rare MTNR1B variants impairing melatonin receptor 1B function contribute to type 2 diabetes. Nat Genet. 2012;44:297–301. doi: 10.1038/ng.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gaulton KJ, Ferreira T, Lee Y, Raimondo A, Mägi R, Reschen ME, Mahajan A, Locke A, Rayner NW, Robertson N, Scott RA, Prokopenko I, Scott LJ, Green T, Sparso T, Thuillier D, Yengo L, Grallert H, Wahl S, Frånberg M, Strawbridge RJ, Kestler H, Chheda H, Eisele L, Gustafsson S, Steinthorsdottir V, Thorleifsson G, Qi L, Karssen LC, van Leeuwen EM, Willems SM, Li M, Chen H, Fuchsberger C, Kwan P, Ma C, Linderman M, Lu Y, Thomsen SK, Rundle JK, Beer NL, van de Bunt M, Chalisey A, Kang HM, Voight BF, Abecasis GR, Almgren P, Baldassarre D, Balkau B, Benediktsson R, Blüher M, Boeing H, Bonnycastle LL, Bottinger EP, Burtt NP, Carey J, Charpentier G, Chines PS, Cornelis MC, Couper DJ, Crenshaw AT, van Dam RM, Doney AS, Dorkhan M, Edkins S, Eriksson JG, Esko T, Eury E, Fadista J, Flannick J, Fontanillas P, Fox C, Franks PW, Gertow K, Gieger C, Gigante B, Gottesman O, Grant GB, Grarup N, Groves CJ, Hassinen M, Have CT, Herder C, Holmen OL, Hreidarsson AB, Humphries SE, Hunter DJ, Jackson AU, Jonsson A, Jørgensen ME, Jørgensen T, Kao WH, Kerrison ND, Kinnunen L, Klopp N, Kong A, Kovacs P, Kraft P, Kravic J, Langford C, Leander K, Liang L, Lichtner P, Lindgren CM, Lindholm E, Linneberg A, Liu CT, Lobbens S, Luan J, Lyssenko V, Männistö S, McLeod O, Meyer J, Mihailov E, Mirza G, Mühleisen TW, Müller-Nurasyid M, Navarro C, Nöthen MM, Oskolkov NN, Owen KR, Palli D, Pechlivanis S, Peltonen L, Perry JR, Platou CG, Roden M, Ruderfer D, Rybin D, van der Schouw YT, Sennblad B, Sigurðsson G, Stančáková A, Steinbach G, Storm P, Strauch K, Stringham HM, Sun Q, Thorand B, Tikkanen E, Tonjes A, Trakalo J, Tremoli E, Tuomi T, Wennauer R, Wiltshire S, Wood AR, Zeggini E, Dunham I, Birney E, Pasquali L, Ferrer J, Loos RJ, Dupuis J, Florez JC, Boerwinkle E, Pankow JS, van Duijn C, Sijbrands E, Meigs JB, Hu FB, Thorsteinsdottir U, Stefansson K, Lakka TA, Rauramaa R, Stumvoll M, Pedersen NL, Lind L, Keinanen-Kiukaanniemi SM, Korpi-Hyövälti E, Saaristo TE, Saltevo J, Kuusisto J, Laakso M, Metspalu A, Erbel R, Jöcke KH, Moebus S, Ripatti S, Salomaa V, Ingelsson E, Boehm BO, Bergman RN, Collins FS, Mohlke KL, Koistinen H, Tuomilehto J, Hveem K, Njølstad I, Deloukas P, Donnelly PJ, Frayling TM, Hattersley AT, de Faire U, Hamsten A, Illig T, Peters A, Cauchi S, Sladek R, Froguel P, Hansen T, Pedersen O, Morris AD, Palmer CN, Kathiresan S, Melander O, Nilsson PM, Groop LC, Barroso I, Langenberg C, Wareham NJ, O’Callaghan CA, Gloyn AL, Altshuler D, Boehnke M, Teslovich TM, McCarthy MI, Morris AP; DIAbetes Genetics Replication And Meta-analysis (DIAGRAM) Consortium. Genetic fine mapping and genomic annotation defines causal mechanisms at type 2 diabetes susceptibility loci. Nat Genet. 2015;47:1415–1425. doi: 10.1038/ng.3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rubio-Sastre P, Scheer FA, Gómez-Abellán P, Madrid JA, Garaulet M. Acute melatonin administration in humans impairs glucose tolerance in both the morning and evening. Sleep. 2014;37:1715–1719. doi: 10.5665/sleep.4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Costes S, Boss M, Thomas AP, Matveyenko AV. Activation of Melatonin Signaling Promotes β-Cell Survival and Function. Mol Endocrinol. 2015;29:682–692. doi: 10.1210/me.2014-1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prasad RB, Groop L. Genetics of type 2 diabetes-pitfalls and possibilities. Genes (Basel) 2015;6:87–123. doi: 10.3390/genes6010087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang ZQ, Liao YQ, Huang RZ, Chen JP, Sun Hl. Possible role of TCF7L2 in the pathogenesis of type 2 diabetes mellitus. Biotechnol Biotec Eq. 2018 [Google Scholar]
- 26.Muoio DM, Newgard CB. Mechanisms of disease:Molecular and metabolic mechanisms of insulin resistance and beta-cell failure in type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9:193–205. doi: 10.1038/nrm2327. [DOI] [PubMed] [Google Scholar]
- 27.Day C, Bailey CJ. Obesity in the pathogenesis of type 2 diabetes. Br J Diabetes Vasc Dis. 2011;11:55–61. [Google Scholar]
- 28.Kwak SH, Park KS. Recent progress in genetic and epigenetic research on type 2 diabetes. Exp Mol Med. 2016;48:e220. doi: 10.1038/emm.2016.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iyer SR. Sleep and type 2 diabetes mellitus- clinical implications. J Assoc Physicians India. 2012;60:42–47. [PubMed] [Google Scholar]
- 30.Alonso-Magdalena P, Quesada I, Nadal A. Endocrine disruptors in the etiology of type 2 diabetes mellitus. Nat Rev Endocrinol. 2011;7:346–353. doi: 10.1038/nrendo.2011.56. [DOI] [PubMed] [Google Scholar]
- 31.Fuchsberger C, Flannick J, Teslovich TM, Mahajan A, Agarwala V, Gaulton KJ, Ma C, Fontanillas P, Moutsianas L, McCarthy DJ, et al. The genetic architecture of type 2 diabetes. Nature. 2016;536:41–47. doi: 10.1038/nature18642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deeb SS, Fajas L, Nemoto M, Pihlajamäki J, Mykkänen L, Kuusisto J, Laakso M, Fujimoto W, Auwerx J. A Pro12Ala substitution in PPARgamma2 associated with decreased receptor activity, lower body mass index and improved insulin sensitivity. Nat Genet. 1998;20:284–287. doi: 10.1038/3099. [DOI] [PubMed] [Google Scholar]
- 33.Hansen SK, Nielsen EM, Ek J, Andersen G, Glümer C, Carstensen B, Mouritzen P, Drivsholm T, Borch-Johnsen K, Jørgensen T, et al. Analysis of separate and combined effects of common variation in KCNJ11 and PPARG on risk of type 2 diabetes. J Clin Endocrinol Metab. 2005;90:3629–3637. doi: 10.1210/jc.2004-1942. [DOI] [PubMed] [Google Scholar]
- 34.Hani EH, Boutin P, Durand E, Inoue H, Permutt MA, Velho G, Froguel P. Missense mutations in the pancreatic islet beta cell inwardly rectifying K+ channel gene (KIR6.2/BIR): a meta-analysis suggests a role in the polygenic basis of Type II diabetes mellitus in Caucasians. Diabetologia. 1998;41:1511–1515. doi: 10.1007/s001250051098. [DOI] [PubMed] [Google Scholar]
- 35.Gloyn AL, Weedon MN, Owen KR, Turner MJ, Knight BA, Hitman G, Walker M, Levy JC, Sampson M, Halford S, et al. Large-scale association studies of variants in genes encoding the pancreatic beta-cell KATP channel subunits Kir6.2 (KCNJ11) and SUR1 (ABCC8) confirm that the KCNJ11 E23K variant is associated with type 2 diabetes. Diabetes. 2003;52:568–572. doi: 10.2337/diabetes.52.2.568. [DOI] [PubMed] [Google Scholar]
- 36.Gloyn AL, Cummings EA, Edghill EL, Harries LW, Scott R, Costa T, Temple IK, Hattersley AT, Ellard S. Permanent neonatal diabetes due to paternal germline mosaicism for an activating mutation of the KCNJ11 Gene encoding the Kir6.2 subunit of the beta-cell potassium adenosine triphosphate channel. J Clin Endocrinol Metab. 2004;89:3932–3935. doi: 10.1210/jc.2004-0568. [DOI] [PubMed] [Google Scholar]
- 37.Inturri R, Stivala A, Furneri PM, Blandino G. Growth and adhesion to HT-29 cells inhibition of Gram-negatives by Bifidobacterium longum BB536 e Lactobacillus rhamnosus HN001 alone and in combination. Eur Rev Med Pharmacol Sci. 2016;20:4943–4949. [PubMed] [Google Scholar]
- 38.Muñoz-Garach A, Diaz-Perdigones C, Tinahones FJ. Gut microbiota and type 2 diabetes mellitus. Endocrinol Nutr. 2016;63:560–568. doi: 10.1016/j.endonu.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 39.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 40.Zhang Y, Zhang H Microbiota associated with type 2 diabetes and its related complications. Food Science and Human Wellness. 2013;2:167–172. [Google Scholar]
- 41.Vrieze A, Van Nood E, Holleman F, Salojärvi J, Kootte RS, Bartelsman JF, Dallinga-Thie GM, Ackermans MT, Serlie MJ, Oozeer R, et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143:913–6.e7. doi: 10.1053/j.gastro.2012.06.031. [DOI] [PubMed] [Google Scholar]
- 42.Wei X, Tao J, Xiao S, Jiang S, Shang E, Zhu Z, Qian D, Duan J. Xiexin Tang improves the symptom of type 2 diabetic rats by modulation of the gut microbiota. Sci Rep. 2018;8:3685. doi: 10.1038/s41598-018-22094-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89:2548–2556. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 44.Scherer PE. Adipose tissue: from lipid storage compartment to endocrine organ. Diabetes. 2006;55:1537–1545. doi: 10.2337/db06-0263. [DOI] [PubMed] [Google Scholar]
- 45.Cusi K. The role of adipose tissue and lipotoxicity in the pathogenesis of type 2 diabetes. Curr Diab Rep. 2010;10:306–315. doi: 10.1007/s11892-010-0122-6. [DOI] [PubMed] [Google Scholar]
- 46.Ravussin Y, Leibel RL, Ferrante AW Jr. A missing link in body weight homeostasis: the catabolic signal of the overfed state. Cell Metab. 2014;20:565–572. doi: 10.1016/j.cmet.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scherer PE. The Multifaceted Roles of Adipose Tissue-Therapeutic Targets for Diabetes and Beyond: The 2015 Banting Lecture. Diabetes. 2016;65:1452–1461. doi: 10.2337/db16-0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee P, Greenfield JR, Ho KK, Fulham MJ. A critical appraisal of the prevalence and metabolic significance of brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab. 2010;299:E601–E606. doi: 10.1152/ajpendo.00298.2010. [DOI] [PubMed] [Google Scholar]
- 49.Sidossis L, Kajimura S. Brown and beige fat in humans: thermogenic adipocytes that control energy and glucose homeostasis. J Clin Invest. 2015;125:478–486. doi: 10.1172/JCI78362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gastaldelli A, Gaggini M, DeFronzo RA. Role of Adipose Tissue Insulin Resistance in the Natural History of Type 2 Diabetes: Results From the San Antonio Metabolism Study. Diabetes. 2017;66:815–822. doi: 10.2337/db16-1167. [DOI] [PubMed] [Google Scholar]
- 51.Folli F, Corradi D, Fanti P, Davalli A, Paez A, Giaccari A, Perego C, Muscogiuri G. The role of oxidative stress in the pathogenesis of type 2 diabetes mellitus micro- and macrovascular complications: avenues for a mechanistic-based therapeutic approach. Curr Diabetes Rev. 2011;7:313–324. doi: 10.2174/157339911797415585. [DOI] [PubMed] [Google Scholar]
- 52.Ceriello A, Motz E. Is oxidative stress the pathogenic mechanism underlying insulin resistance, diabetes, and cardiovascular disease? The common soil hypothesis revisited. Arterioscler Thromb Vasc Biol. 2004;24:816–823. doi: 10.1161/01.ATV.0000122852.22604.78. [DOI] [PubMed] [Google Scholar]
- 53.Wright E Jr, Scism-Bacon JL, Glass LC. Oxidative stress in type 2 diabetes: the role of fasting and postprandial glycaemia. Int J Clin Pract. 2006;60:308–314. doi: 10.1111/j.1368-5031.2006.00825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 55.Zee PC, Attarian H, Videnovic A. Circadian rhythm abnormalities. Continuum (Minneap Minn) 2013;19:132–147. doi: 10.1212/01.CON.0000427209.21177.aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ramkisoensing A, Meijer JH. Synchronization of Biological Clock Neurons by Light and Peripheral Feedback Systems Promotes Circadian Rhythms and Health. Front Neurol. 2015;6:128. doi: 10.3389/fneur.2015.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Golombek DA, Rosenstein RE. Physiology of circadian entrainment. Physiol Rev. 2010;90:1063–1102. doi: 10.1152/physrev.00009.2009. [DOI] [PubMed] [Google Scholar]
- 58.Dardente H, Cermakian N. Molecular circadian rhythms in central and peripheral clocks in mammals. Chronobiol Int. 2007;24:195–213. doi: 10.1080/07420520701283693. [DOI] [PubMed] [Google Scholar]
- 59.Cajochen C, Kräuchi K, Wirz-Justice A. Role of melatonin in the regulation of human circadian rhythms and sleep. J Neuroendocrinol. 2003;15:432–437. doi: 10.1046/j.1365-2826.2003.00989.x. [DOI] [PubMed] [Google Scholar]
- 60.Takahashi JS, Hong HK, Ko CH, McDearmon EL. The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat Rev Genet. 2008;9:764–775. doi: 10.1038/nrg2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reppert SM, Weaver DR. Molecular analysis of mammalian circadian rhythms. Annu Rev Physiol. 2001;63:647–676. doi: 10.1146/annurev.physiol.63.1.647. [DOI] [PubMed] [Google Scholar]
- 62.Li MD, Li CM, Wang Z. The role of circadian clocks in metabolic disease. Yale J Biol Med. 2012;85:387–401. [PMC free article] [PubMed] [Google Scholar]
- 63.Cribbet MR, Logan RW, Edwards MD, Hanlon E, Bien Peek C, Stubblefield JJ, Vasudevan S, Ritchey F, Frank E. Circadian rhythms and metabolism: from the brain to the gut and back again. Ann N Y Acad Sci. 2016;1385:21–40. doi: 10.1111/nyas.13188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Froy O. Metabolism and circadian rhythms--implications for obesity. Endocr Rev. 2010;31:1–24. doi: 10.1210/er.2009-0014. [DOI] [PubMed] [Google Scholar]
- 65.Froy O, Garaulet M. The circadian clock in white and brown adipose tissue: mechanistic, endocrine and clinical aspects. Endocr Rev. 2018;39:261–273. doi: 10.1210/er.2017-00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Van Cauter E, Spiegel K, Tasali E, Leproult R. Metabolic consequences of sleep and sleep loss. Sleep Med. 2008;9 Suppl 1:S23–S28. doi: 10.1016/S1389-9457(08)70013-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sridhar GR, Sanjana NS. Sleep, circadian dysrhythmia, obesity and diabetes. World J Diabetes. 2016;7:515–522. doi: 10.4239/wjd.v7.i19.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Parkes KR. Shift work and age as interactive predictors of body mass index among offshore workers. Scand J Work Environ Health. 2002;28:64–71. doi: 10.5271/sjweh.648. [DOI] [PubMed] [Google Scholar]
- 69.Karlsson BH, Knutsson AK, Lindahl BO, Alfredsson LS. Metabolic disturbances in male workers with rotating three-shift work. Results of the WOLF study. Int Arch Occup Environ Health. 2003;76:424–430. doi: 10.1007/s00420-003-0440-y. [DOI] [PubMed] [Google Scholar]
- 70.Eckel-Mahan K, Sassone-Corsi P. Metabolism and the circadian clock converge. Physiol Rev. 2013;93:107–135. doi: 10.1152/physrev.00016.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Karatsoreos IN, Bhagat S, Bloss EB, Morrison JH, McEwen BS. Disruption of circadian clocks has ramifications for metabolism, brain, and behavior. Proc Natl Acad Sci USA. 2011;108:1657–1662. doi: 10.1073/pnas.1018375108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.La Fleur SE, Kalsbeek A, Wortel J, Buijs RM. A suprachiasmatic nucleus generated rhythm in basal glucose concentrations. J Neuroendocrinol. 1999;11:643–652. doi: 10.1046/j.1365-2826.1999.00373.x. [DOI] [PubMed] [Google Scholar]
- 73.Ruiter M, La Fleur SE, van Heijningen C, van der Vliet J, Kalsbeek A, Buijs RM. The daily rhythm in plasma glucagon concentrations in the rat is modulated by the biological clock and by feeding behavior. Diabetes. 2003;52:1709–1715. doi: 10.2337/diabetes.52.7.1709. [DOI] [PubMed] [Google Scholar]
- 74.Bodosi B, Gardi J, Hajdu I, Szentirmai E, Obal F Jr, Krueger JM. Rhythms of ghrelin, leptin, and sleep in rats: effects of the normal diurnal cycle, restricted feeding, and sleep deprivation. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1071–R1079. doi: 10.1152/ajpregu.00294.2004. [DOI] [PubMed] [Google Scholar]
- 75.Peek CB, Affinati AH, Ramsey KM, Kuo HY, Yu W, Sena LA, Ilkayeva O, Marcheva B, Kobayashi Y, Omura C, et al. Circadian clock NAD+ cycle drives mitochondrial oxidative metabolism in mice. Science. 2013;342:1243417. doi: 10.1126/science.1243417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nakahata Y, Kaluzova M, Grimaldi B, Sahar S, Hirayama J, Chen D, Guarente LP, Sassone-Corsi P. The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell. 2008;134:329–340. doi: 10.1016/j.cell.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nakahata Y, Sahar S, Astarita G, Kaluzova M, Sassone-Corsi P. Circadian control of the NAD+ salvage pathway by CLOCK-SIRT1. Science. 2009;324:654–657. doi: 10.1126/science.1170803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ramsey KM, Yoshino J, Brace CS, Abrassart D, Kobayashi Y, Marcheva B, Hong HK, Chong JL, Buhr ED, Lee C, et al. Circadian clock feedback cycle through NAMPT-mediated NAD+ biosynthesis. Science. 2009;324:651–654. doi: 10.1126/science.1171641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rutter J, Reick M, Wu LC, McKnight SL. Regulation of clock and NPAS2 DNA binding by the redox state of NAD cofactors. Science. 2001;293:510–514. doi: 10.1126/science.1060698. [DOI] [PubMed] [Google Scholar]
- 80.Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005;1:15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 81.Asher G, Gatfield D, Stratmann M, Reinke H, Dibner C, Kreppel F, Mostoslavsky R, Alt FW, Schibler U. SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell. 2008;134:317–328. doi: 10.1016/j.cell.2008.06.050. [DOI] [PubMed] [Google Scholar]
- 82.Lamia KA, Sachdeva UM, DiTacchio L, Williams EC, Alvarez JG, Egan DF, Vasquez DS, Juguilon H, Panda S, Shaw RJ, et al. AMPK regulates the circadian clock by cryptochrome phosphorylation and degradation. Science. 2009;326:437–440. doi: 10.1126/science.1172156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Huang W, Ramsey KM, Marcheva B, Bass J. Circadian rhythms, sleep, and metabolism. J Clin Invest. 2011;121:2133–2141. doi: 10.1172/JCI46043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kiehn JT, Koch CE, Walter M, Brod A, Oster H. Circadian rhythms and clocks in adipose tissues: Current Insights. ChronoPhysiology and Therapy. 2017;2017:7–17. [Google Scholar]
- 85.Fontaine C, Dubois G, Duguay Y, Helledie T, Vu-Dac N, Gervois P, Soncin F, Mandrup S, Fruchart JC, Fruchart-Najib J, et al. The orphan nuclear receptor Rev-Erbalpha is a peroxisome proliferator-activated receptor (PPAR) gamma target gene and promotes PPARgamma-induced adipocyte differentiation. J Biol Chem. 2003;278:37672–37680. doi: 10.1074/jbc.M304664200. [DOI] [PubMed] [Google Scholar]
- 86.Shimba S, Ishii N, Ohta Y, Ohno T, Watabe Y, Hayashi M, Wada T, Aoyagi T, Tezuka M. Brain and muscle Arnt-like protein-1 (BMAL1), a component of the molecular clock, regulates adipogenesis. Proc Natl Acad Sci USA. 2005;102:12071–12076. doi: 10.1073/pnas.0502383102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang J, Lazar MA. Bifunctional role of Rev-erbalpha in adipocyte differentiation. Mol Cell Biol. 2008;28:2213–2220. doi: 10.1128/MCB.01608-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Grimaldi B, Bellet MM, Katada S, Astarita G, Hirayama J, Amin RH, Granneman JG, Piomelli D, Leff T, Sassone-Corsi P. PER2 controls lipid metabolism by direct regulation of PPARγ. Cell Metab. 2010;12:509–520. doi: 10.1016/j.cmet.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Duez H, Duhem C, Laitinen S, Patole PS, Abdelkarim M, Bois-Joyeux B, Danan JL, Staels B. Inhibition of adipocyte differentiation by RORalpha. FEBS Lett. 2009;583:2031–2036. doi: 10.1016/j.febslet.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 90.Meissburger B, Ukropec J, Roeder E, Beaton N, Geiger M, Teupser D, Civan B, Langhans W, Nawroth PP, Gasperikova D, et al. Adipogenesis and insulin sensitivity in obesity are regulated by retinoid-related orphan receptor gamma. EMBO Mol Med. 2011;3:637–651. doi: 10.1002/emmm.201100172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rosen ED, Hsu CH, Wang X, Sakai S, Freeman MW, Gonzalez FJ, Spiegelman BM. C/EBPalpha induces adipogenesis through PPARgamma: a unified pathway. Genes Dev. 2002;16:22–26. doi: 10.1101/gad.948702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cristancho AG, Lazar MA. Forming functional fat: a growing understanding of adipocyte differentiation. Nat Rev Mol Cell Biol. 2011;12:722–734. doi: 10.1038/nrm3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kohsaka A, Laposky AD, Ramsey KM, Estrada C, Joshu C, Kobayashi Y, Turek FW, Bass J. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab. 2007;6:414–421. doi: 10.1016/j.cmet.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 94.Hutchison AT, Wittert GA, Heilbronn LK. Matching Meals to Body Clocks-Impact on Weight and Glucose Metabolism. Nutrients. 2017;9:pii: E222. doi: 10.3390/nu9030222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Peret J, Macaire I, Chanez M. Schedule of protein ingestion, nitrogen and energy utilization and circadian rhythm of hepatic glycogen, plasma corticosterone and insulin in rats. J Nutr. 1973;103:866–874. doi: 10.1093/jn/103.6.866. [DOI] [PubMed] [Google Scholar]
- 96.Armstrong S. A chronometric approach to the study of feeding behavior. Neurosci Biobehav Rev. 1980;4:27–53. doi: 10.1016/0149-7634(80)90024-x. [DOI] [PubMed] [Google Scholar]
- 97.Bo S, Musso G, Beccuti G, Fadda M, Fedele D, Gambino R, Gentile L, Durazzo M, Ghigo E, Cassader M. Consuming more of daily caloric intake at dinner predisposes to obesity. A 6-year population-based prospective cohort study. PLoS One. 2014;9:e108467. doi: 10.1371/journal.pone.0108467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sonnier T, Rood J, Gimble JM, Peterson CM. Glycemic control is impaired in the evening in prediabetes through multiple diurnal rhythms. J Diabetes Complications. 2014;28:836–843. doi: 10.1016/j.jdiacomp.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 99.Morris CJ, Purvis TE, Mistretta J, Scheer FA. Effects of the Internal Circadian System and Circadian Misalignment on Glucose Tolerance in Chronic Shift Workers. J Clin Endocrinol Metab. 2016;101:1066–1074. doi: 10.1210/jc.2015-3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Morgan LM, Aspostolakou F, Wright J, Gama R. Diurnal variations in peripheral insulin resistance and plasma non-esterified fatty acid concentrations: a possible link? Ann Clin Biochem. 1999;36(Pt 4):447–450. doi: 10.1177/000456329903600407. [DOI] [PubMed] [Google Scholar]
- 101.Van Cauter E, Blackman JD, Roland D, Spire JP, Refetoff S, Polonsky KS. Modulation of glucose regulation and insulin secretion by circadian rhythmicity and sleep. J Clin Invest. 1991;88:934–942. doi: 10.1172/JCI115396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Peschke E, Peschke D. Evidence for a circadian rhythm of insulin release from perifused rat pancreatic islets. Diabetologia. 1998;41:1085–1092. doi: 10.1007/s001250051034. [DOI] [PubMed] [Google Scholar]
- 103.Polonsky KS, Given BD, Van Cauter E. Twenty-four-hour profiles and pulsatile patterns of insulin secretion in normal and obese subjects. J Clin Invest. 1988;81:442–448. doi: 10.1172/JCI113339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, Laposky A, Losee-Olson S, Easton A, Jensen DR, et al. Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 2005;308:1043–1045. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ikeda H, Yong Q, Kurose T, Todo T, Mizunoya W, Fushiki T, Seino Y, Yamada Y. Clock gene defect disrupts light-dependency of autonomic nerve activity. Biochem Biophys Res Commun. 2007;364:457–463. doi: 10.1016/j.bbrc.2007.10.058. [DOI] [PubMed] [Google Scholar]
- 106.Gil-Lozano M, Mingomataj EL, Wu WK, Ridout SA, Brubaker PL. Circadian secretion of the intestinal hormone GLP-1 by the rodent L cell. Diabetes. 2014;63:3674–3685. doi: 10.2337/db13-1501. [DOI] [PubMed] [Google Scholar]
- 107.Gil-Lozano M, Wu WK, Martchenko A, Brubaker PL. High-Fat Diet and Palmitate Alter the Rhythmic Secretion of Glucagon-Like Peptide-1 by the Rodent L-cell. Endocrinology. 2016;157:586–599. doi: 10.1210/en.2015-1732. [DOI] [PubMed] [Google Scholar]
- 108.Voigt RM, Forsyth CB, Green SJ, Engen PA, Keshavarzian A. Circadian Rhythm and the Gut Microbiome. Int Rev Neurobiol. 2016;131:193–205. doi: 10.1016/bs.irn.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 109.Blandino G, Inturri R, Lazzara F, Di Rosa M, Malaguarnera L. Impact of gut microbiota on diabetes mellitus. Diabetes Metab. 2016;42:303–315. doi: 10.1016/j.diabet.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 110.Mikuni E, Ohoshi T, Hayashi K, Miyamura K. Glucose intolerance in an employed population. Tohoku J Exp Med. 1983;141 Suppl:251–256. doi: 10.1620/tjem.141.suppl_251. [DOI] [PubMed] [Google Scholar]
- 111.Kawakami N, Araki S, Takatsuka N, Shimizu H, Ishibashi H. Overtime, psychosocial working conditions, and occurrence of non-insulin dependent diabetes mellitus in Japanese men. J Epidemiol Community Health. 1999;53:359–363. doi: 10.1136/jech.53.6.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Qian J, Block GD, Colwell CS, Matveyenko AV. Consequences of exposure to light at night on the pancreatic islet circadian clock and function in rats. Diabetes. 2013;62:3469–3478. doi: 10.2337/db12-1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kurose T, Hyo T, Yabe D, Seino Y. The role of chronobiology and circadian rhythms in type 2 diabetes mellitus: implications for management of diabetes. Chronophysiology and Therapy. 2014;4:41–49. [Google Scholar]
- 114.Tang Y, Meng L, Li D, Yang M, Zhu Y, Li C, Jiang Z, Yu P, Li Z, Song H, et al. Interaction of sleep quality and sleep duration on glycemic control in patients with type 2 diabetes mellitus. Chin Med J (Engl) 2014;127:3543–3547. [PubMed] [Google Scholar]
- 115.Lou P, Chen P, Zhang L, Zhang P, Yu J, Zhang N, Wu H, Zhao J. Relation of sleep quality and sleep duration to type 2 diabetes: a population-based cross-sectional survey. BMJ Open. 2012;2:pii: e000956. doi: 10.1136/bmjopen-2012-000956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sakamoto R, Yamakawa T, Takahashi K, Suzuki J, Shinoda MM, Sakamaki K, Danno H, Tsuchiya H, Waseda M, Takano T, et al. Association of usual sleep quality and glycemic control in type 2 diabetes in Japanese: A cross sectional study. Sleep and Food Registry in Kanagawa (SOREKA) PLoS One. 2018;13:e0191771. doi: 10.1371/journal.pone.0191771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Onaolapo OJ, Onaolapo AY. Melatonin, adolescence, and the brain: An insight into the period-specific influences of a multifunctional signaling molecule. Birth Defects Res. 2017;109:1659–1671. doi: 10.1002/bdr2.1171. [DOI] [PubMed] [Google Scholar]
- 118.Onaolapo OJ, Onaolapo AY. Melatonin: Medical Uses and Role in Health and Disease. Chapter 4. Melatonin Receptors, Behaviour and Brain Function. In: Correia L and Mayers G (editors). Nova Science Publishers; 2018. pp. 133–158. [Google Scholar]
- 119.Hardeland R, Pandi-Perumal SR, Cardinali DP. Melatonin. Int J Biochem Cell Biol. 2006;38:313–316. doi: 10.1016/j.biocel.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 120.Puig-Domingo M, Webb SM, Serrano J, Peinado MA, Corcoy R, Ruscalleda J, Reiter RJ, de Leiva A. Brief report: melatonin-related hypogonadotropic hypogonadism. N Engl J Med. 1992;327:1356–1359. doi: 10.1056/NEJM199211053271905. [DOI] [PubMed] [Google Scholar]
- 121.Cavallo A. Melatonin and human puberty: current perspectives. J Pineal Res. 1993;15:115–121. doi: 10.1111/j.1600-079x.1993.tb00517.x. [DOI] [PubMed] [Google Scholar]
- 122.Cavallo A, Dolan LM. 6-Hydroxymelatonin sulfate excretion in human puberty. J Pineal Res. 1996;21:225–230. doi: 10.1111/j.1600-079x.1996.tb00290.x. [DOI] [PubMed] [Google Scholar]
- 123.Chan KH, Wong YH. A molecular and chemical perspective in defining melatonin receptor subtype selectivity. Int J Mol Sci. 2013;14:18385–18406. doi: 10.3390/ijms140918385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Onaolapo AY, Adebayo AN, Onaolapo OJ. Exogenous daytime melatonin modulates response of adolescent mice in a repeated unpredictable stress paradigm. Naunyn Schmiedebergs Arch Pharmacol. 2017;390:149–161. doi: 10.1007/s00210-016-1314-7. [DOI] [PubMed] [Google Scholar]
- 125.Onaolapo OJ, Onaolapo AY, Abiola AA, Lillian EA. Central depressant and nootropic effects of daytime melatonin in mice. Ann Neurosci. 2014;21:90–96. doi: 10.5214/ans.0972.7531.210304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Comai S, Gobbi G. Unveiling the role of melatonin MT2 receptors in sleep, anxiety and other neuropsychiatric diseases: a novel target in psychopharmacology. J Psychiatry Neurosci. 2014;39:6–21. doi: 10.1503/jpn.130009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pandi-Perumal SR, Trakht I, Srinivasan V, Spence DW, Maestroni GJ, Zisapel N, Cardinali DP. Physiological effects of melatonin: role of melatonin receptors and signal transduction pathways. Prog Neurobiol. 2008;85:335–353. doi: 10.1016/j.pneurobio.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 128.Tocharus C, Puriboriboon Y, Junmanee T, Tocharus J, Ekthuwapranee K, Govitrapong P. Melatonin enhances adult rat hippocampal progenitor cell proliferation via ERK signaling pathway through melatonin receptor. Neuroscience. 2014;275:314–321. doi: 10.1016/j.neuroscience.2014.06.026. [DOI] [PubMed] [Google Scholar]
- 129.Onaolapo AY, Aina OA, Onaolapo OJ. Melatonin attenuates behavioural deficits and reduces brain oxidative stress in a rodent model of schizophrenia. Biomed Pharmacother. 2017;92:373–383. doi: 10.1016/j.biopha.2017.05.094. [DOI] [PubMed] [Google Scholar]
- 130.Korkmaz A, Reiter RJ, Topal T, Manchester LC, Oter S, Tan DX. Melatonin: an established antioxidant worthy of use in clinical trials. Mol Med. 2009;15:43–50. doi: 10.2119/molmed.2008.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bubenik GA. Gastrointestinal melatonin: localization, function, and clinical relevance. Dig Dis Sci. 2002;47:2336–2348. doi: 10.1023/a:1020107915919. [DOI] [PubMed] [Google Scholar]
- 132.Bermudez FF, Forbes JM, Injidi MH. Involvement of melatonin and thyroid hormones in the control of sleep, food intake and energy metabolism in the domestic fowl. J Physiol. 1983;337:19–27. doi: 10.1113/jphysiol.1983.sp014608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wilson AP, Gaya H. Treatment of endocarditis with teicoplanin: a retrospective analysis of 104 cases. J Antimicrob Chemother. 1996;38:507–521. doi: 10.1093/jac/38.3.507. [DOI] [PubMed] [Google Scholar]
- 134.Wolden-Hanson T, Mitton DR, McCants RL, Yellon SM, Wilkinson CW, Matsumoto AM, Rasmussen DD. Daily melatonin administration to middle-aged male rats suppresses body weight, intraabdominal adiposity, and plasma leptin and insulin independent of food intake and total body fat. Endocrinology. 2000;141:487–497. doi: 10.1210/endo.141.2.7311. [DOI] [PubMed] [Google Scholar]
- 135.Bubenik GA, Pang SF. The role of serotonin and melatonin in gastrointestinal physiology: ontogeny, regulation of food intake, and mutual serotonin-melatonin feedback. J Pineal Res. 1994;16:91–99. doi: 10.1111/j.1600-079x.1994.tb00088.x. [DOI] [PubMed] [Google Scholar]
- 136.Piccinetti CC, Migliarini B, Olivotto I, Simoniello MP, Giorgini E, Carnevali O. Melatonin and peripheral circuitries: insights on appetite and metabolism in Danio rerio. Zebrafish. 2013;10:275–282. doi: 10.1089/zeb.2012.0844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kasimay O, Cakir B, Devseren E, Yegen BC. Exogenous melatonin delays gastric emptying rate in rats: role of CCK2 and 5-HT3 receptors. J Physiol Pharmacol. 2005;56:543–553. [PubMed] [Google Scholar]
- 138.Velarde E, Alonso-Gómez AL, De Pedro N, Azpeleta L, Ortiz L, Delgado MJ. Characterización de la actividad miométrica del intestino de Carassius auratus para el estudio del efecto in vitro de la melatonina en la actividad gastrointestinal. CIVA. 2006:249–258. [Google Scholar]
- 139.Nieminen P, Käkelä R, Mustonen AM, Hyvärinen H, Asikainen J. Exogenous melatonin affects lipids and enzyme activities in mink (Mustela vison) liver. Comp Biochem Physiol C Toxicol Pharmacol. 2001;128:203–211. doi: 10.1016/s1532-0456(00)00190-3. [DOI] [PubMed] [Google Scholar]
- 140.De Pedro N, Martínez-Alvarez RM, Delgado MJ. Melatonin reduces body weight in goldfish (Carassius auratus): effects on metabolic resources and some feeding regulators. J Pineal Res. 2008;45:32–39. doi: 10.1111/j.1600-079X.2007.00553.x. [DOI] [PubMed] [Google Scholar]
- 141.Zhdanova IV. Sleep and its regulation in zebrafish. Rev Neurosci. 2011;22:27–36. doi: 10.1515/RNS.2011.005. [DOI] [PubMed] [Google Scholar]
- 142.Xia Q, Chen ZX, Wang YC, Ma YS, Zhang F, Che W, Fu D, Wang XF. Association between the melatonin receptor 1B gene polymorphism on the risk of type 2 diabetes, impaired glucose regulation: a meta-analysis. PLoS One. 2012;7:e50107. doi: 10.1371/journal.pone.0050107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lardone PJ, Alvarez-Sanchez SN, Guerrero JM, Carrillo-Vico A. Melatonin and glucose metabolism: clinical relevance. Curr Pharm Des. 2014;20:4841–4853. doi: 10.2174/1381612819666131119101032. [DOI] [PubMed] [Google Scholar]
- 144.Poon AM, Choy EH, Pang SF. Modulation of blood glucose by melatonin: a direct action on melatonin receptors in mouse hepatocytes. Biol Signals Recept. 2001;10:367–379. doi: 10.1159/000046904. [DOI] [PubMed] [Google Scholar]
- 145.Lima FB, Machado UF, Bartol I, Seraphim PM, Sumida DH, Moraes SM, Hell NS, Okamoto MM, Saad MJ, Carvalho CR, et al. Pinealectomy causes glucose intolerance and decreases adipose cell responsiveness to insulin in rats. Am J Physiol. 1998;275:E934–E941. doi: 10.1152/ajpendo.1998.275.6.E934. [DOI] [PubMed] [Google Scholar]
- 146.O’Brien IA, Lewin IG, O’Hare JP, Arendt J, Corrall RJ. Abnormal circadian rhythm of melatonin in diabetic autonomic neuropathy. Clin Endocrinol (Oxf) 1986;24:359–364. doi: 10.1111/j.1365-2265.1986.tb01639.x. [DOI] [PubMed] [Google Scholar]
- 147.McMullan CJ, Schernhammer ES, Rimm EB, Hu FB, Forman JP. Melatonin secretion and the incidence of type 2 diabetes. JAMA. 2013;309:1388–1396. doi: 10.1001/jama.2013.2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Garfinkel D, Zorin M, Wainstein J, Matas Z, Laudon M, Zisapel N. Efficacy and safety of prolonged-release melatonin in insomnia patients with diabetes: a randomized, double-blind, crossover study. Diabetes Metab Syndr Obes. 2011;4:307–313. doi: 10.2147/DMSO.S23904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Peschke E, Hofmann K, Pönicke K, Wedekind D, Mühlbauer E. Catecholamines are the key for explaining the biological relevance of insulin-melatonin antagonisms in type 1 and type 2 diabetes. J Pineal Res. 2012;52:389–396. doi: 10.1111/j.1600-079X.2011.00951.x. [DOI] [PubMed] [Google Scholar]
- 150.Espino J, Pariente JA, Rodríguez AB. Role of melatonin on diabetes-related metabolic disorders. World J Diabetes. 2011;2:82–91. doi: 10.4239/wjd.v2.i6.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Nishida S. Metabolic effects of melatonin on oxidative stress and diabetes mellitus. Endocrine. 2005;27:131–136. doi: 10.1385/endo:27:2:131. [DOI] [PubMed] [Google Scholar]
- 152.Peschke E. Melatonin, endocrine pancreas and diabetes. J Pineal Res. 2008;44:26–40. doi: 10.1111/j.1600-079X.2007.00519.x. [DOI] [PubMed] [Google Scholar]
- 153.Robeva R, Kirilov G, Tomova A, Kumanov P. Melatonin-insulin interactions in patients with metabolic syndrome. J Pineal Res. 2008;44:52–56. doi: 10.1111/j.1600-079X.2007.00527.x. [DOI] [PubMed] [Google Scholar]
- 154.Peschke E, Frese T, Chankiewitz E, Peschke D, Preiss U, Schneyer U, Spessert R, Mühlbauer E. Diabetic Goto Kakizaki rats as well as type 2 diabetic patients show a decreased diurnal serum melatonin level and an increased pancreatic melatonin-receptor status. J Pineal Res. 2006;40:135–143. doi: 10.1111/j.1600-079X.2005.00287.x. [DOI] [PubMed] [Google Scholar]
- 155.Bähr I, Mühlbauer E, Schucht H, Peschke E. Melatonin stimulates glucagon secretion in vitro and in vivo. J Pineal Res. 2011;50:336–344. doi: 10.1111/j.1600-079X.2010.00848.x. [DOI] [PubMed] [Google Scholar]
- 156.Poirel VJ, Cailotto C, Streicher D, Pévet P, Masson-Pévet M, Gauer F. MT1 melatonin receptor mRNA tissular localization by PCR amplification. Neuro Endocrinol Lett. 2003;24:33–38. [PubMed] [Google Scholar]
- 157.Stebelová K, Anttila K, Mänttäri S, Saarela S, Zeman M. Immunohistochemical definition of MT(2) receptors and melatonin in the gastrointestinal tissues of rat. Acta Histochem. 2010;112:26–33. doi: 10.1016/j.acthis.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 158.Nagorny CL, Sathanoori R, Voss U, Mulder H, Wierup N. Distribution of melatonin receptors in murine pancreatic islets. J Pineal Res. 2011;50:412–417. doi: 10.1111/j.1600-079X.2011.00859.x. [DOI] [PubMed] [Google Scholar]
- 159.Peschke E, Fauteck JD, Musshoff U, Schmidt F, Beckmann A, Peschke D. Evidence for a melatonin receptor within pancreatic islets of neonate rats: functional, autoradiographic, and molecular investigations. J Pineal Res. 2000;28:156–164. doi: 10.1034/j.1600-079x.2001.280305.x. [DOI] [PubMed] [Google Scholar]
- 160.Soták M, Mrnka L, Pácha J. Heterogeneous expression of melatonin receptor MT1 mRNA in the rat intestine under control and fasting conditions. J Pineal Res. 2006;41:183–188. doi: 10.1111/j.1600-079X.2006.00355.x. [DOI] [PubMed] [Google Scholar]
- 161.Staiger H, Machicao F, Schäfer SA, Kirchhoff K, Kantartzis K, Guthoff M, Silbernagel G, Stefan N, Häring HU, Fritsche A. Polymorphisms within the novel type 2 diabetes risk locus MTNR1B determine beta-cell function. PLoS One. 2008;3:e3962. doi: 10.1371/journal.pone.0003962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Prokopenko I, Langenberg C, Florez JC, Saxena R, Soranzo N, Thorleifsson G, Loos RJ, Manning AK, Jackson AU, Aulchenko Y, et al. Variants in MTNR1B influence fasting glucose levels. Nat Genet. 2009;41:77–81. doi: 10.1038/ng.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Tam CH, Ho JS, Wang Y, Lee HM, Lam VK, Germer S, Martin M, So WY, Ma RC, Chan JC, et al. Common polymorphisms in MTNR1B, G6PC2 and GCK are associated with increased fasting plasma glucose and impaired beta-cell function in Chinese subjects. PLoS One. 2010;5:e11428. doi: 10.1371/journal.pone.0011428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Müssig K, Staiger H, Machicao F, Häring HU, Fritsche A. Genetic variants in MTNR1B affecting insulin secretion. Ann Med. 2010;42:387–393. doi: 10.3109/07853890.2010.502125. [DOI] [PubMed] [Google Scholar]
- 165.Sparsø T, Bonnefond A, Andersson E, Bouatia-Naji N, Holmkvist J, Wegner L, Grarup N, Gjesing AP, Banasik K, Cavalcanti-Proença C, et al. G-allele of intronic rs10830963 in MTNR1B confers increased risk of impaired fasting glycemia and type 2 diabetes through an impaired glucose-stimulated insulin release: studies involving 19,605 Europeans. Diabetes. 2009;58:1450–1456. doi: 10.2337/db08-1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Takeuchi F, Katsuya T, Chakrewarthy S, Yamamoto K, Fujioka A, Serizawa M, Fujisawa T, Nakashima E, Ohnaka K, Ikegami H, et al. Common variants at the GCK, GCKR, G6PC2-ABCB11 and MTNR1B loci are associated with fasting glucose in two Asian populations. Diabetologia. 2010;53:299–308. doi: 10.1007/s00125-009-1595-1. [DOI] [PubMed] [Google Scholar]
- 167.Kan MY, Zhou DZ, Zhang D, Zhang Z, Chen Z, Yang YF, Guo XZ, Xu H, He L, Liu Y. Two susceptible diabetogenic variants near/in MTNR1B are associated with fasting plasma glucose in a Han Chinese cohort. Diabet Med. 2010;27:598–602. doi: 10.1111/j.1464-5491.2010.02975.x. [DOI] [PubMed] [Google Scholar]
- 168.Peschke E, Stumpf I, Bazwinsky I, Litvak L, Dralle H, Mühlbauer E. Melatonin and type 2 diabetes - a possible link? J Pineal Res. 2007;42:350–358. doi: 10.1111/j.1600-079X.2007.00426.x. [DOI] [PubMed] [Google Scholar]
- 169.Nishida S, Segawa T, Murai I, Nakagawa S. Long-term melatonin administration reduces hyperinsulinemia and improves the altered fatty-acid compositions in type 2 diabetic rats via the restoration of Delta-5 desaturase activity. J Pineal Res. 2002;32:26–33. doi: 10.1034/j.1600-079x.2002.10797.x. [DOI] [PubMed] [Google Scholar]
- 170.Cagnacci A, Arangino S, Renzi A, Paoletti AM, Melis GB, Cagnacci P, Volpe A. Influence of melatonin administration on glucose tolerance and insulin sensitivity of postmenopausal women. Clin Endocrinol (Oxf) 2001;54:339–346. doi: 10.1046/j.1365-2265.2001.01232.x. [DOI] [PubMed] [Google Scholar]
- 171.Hussain SA, Khadim HM, Khalaf BH, Ismail SH, Hussein KI, Sahib AS. Effects of melatonin and zinc on glycemic control in type 2 diabetic patients poorly controlled with metformin. Saudi Med J. 2006;27:1483–1488. [PubMed] [Google Scholar]
- 172.Forrestel AC, Miedlich SU, Yurcheshen M, Wittlin SD, Sellix MT. Chronomedicine and type 2 diabetes: shining some light on melatonin. Diabetologia. 2017;60:808–822. doi: 10.1007/s00125-016-4175-1. [DOI] [PubMed] [Google Scholar]
- 173.Wojcik M, Krawczyk M, Wojcik P, Cypryk K, Wozniak LA. Melatonin as a Pleiotropic Molecule with Therapeutic Potential for Type 2 Diabetes and Cancer. Curr Med Chem. 2017;24:3829–3850. doi: 10.2174/0929867324666170718110606. [DOI] [PubMed] [Google Scholar]
- 174.Thomas AP, Hoang J, Vongbunyong K, Nguyen A, Rakshit K, Matveyenko AV. Administration of Melatonin and Metformin Prevents Deleterious Effects of Circadian Disruption and Obesity in Male Rats. Endocrinology. 2016;157:4720–4731. doi: 10.1210/en.2016-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Heo JI, Yoon DW, Yu JH, Kim NH, Yoo HJ, Seo JA, Kim SG, Choi KM, Baik SH, Choi DS, et al. Melatonin improves insulin resistance and hepatic steatosis through attenuation of alpha-2-HS-glycoprotein. J Pineal Res. 2018:e12493. doi: 10.1111/jpi.12493. [DOI] [PubMed] [Google Scholar]
- 176.Zhou H, Yue Y, Wang J, Ma Q, Chen Y. Melatonin therapy for diabetic cardiomyopathy: A mechanism involving Syk-mitochondrial complex I-SERCA pathway. Cell Signal. 2018;47:88–100. doi: 10.1016/j.cellsig.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 177.Xu P, Wang J, Hong F, Wang S, Jin X, Xue T, Jia L, Zhai Y. Melatonin prevents obesity through modulation of gut microbiota in mice. J Pineal Res. 2017:62. doi: 10.1111/jpi.12399. [DOI] [PubMed] [Google Scholar]
- 178.Parameyong A, Govitrapong P, Chetsawang B. Melatonin attenuates the mitochondrial translocation of mitochondrial fission proteins and Bax, cytosolic calcium overload and cell death in methamphetamine-induced toxicity in neuroblastoma SH-SY5Y cells. Mitochondrion. 2015;24:1–8. doi: 10.1016/j.mito.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 179.Chuang JI, Pan IL, Hsieh CY, Huang CY, Chen PC, Shin JW. Melatonin prevents the dynamin-related protein 1-dependent mitochondrial fission and oxidative insult in the cortical neurons after 1-methyl-4-phenylpyridinium treatment. J Pineal Res. 2016;61:230–240. doi: 10.1111/jpi.12343. [DOI] [PubMed] [Google Scholar]
- 180.Coto-Montes A, Boga JA, Rosales-Corral S, Fuentes-Broto L, Tan DX, Reiter RJ. Role of melatonin in the regulation of autophagy and mitophagy: a review. Mol Cell Endocrinol. 2012;361:12–23. doi: 10.1016/j.mce.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 181.Ding M, Feng N, Tang D, Feng J, Li Z, Jia M, Liu Z, Gu X, Wang Y, Fu F, et al. Melatonin prevents Drp1-mediated mitochondrial fission in diabetic hearts through SIRT1-PGC1α pathway. J Pineal Res. 2018:e12491. doi: 10.1111/jpi.12491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Bicer M, Baltaci SB, Patlar S, Mogulkoc R, Baltaci AK. Melatonin has a protective effect against lipid peroxidation in the bone tissue of diabetic rats subjected to acute swimming exercise. Horm Mol Biol Clin Investig. 2018:34. doi: 10.1515/hmbci-2017-0079. [DOI] [PubMed] [Google Scholar]
- 183.Mehrzadi S, Motevalian M, Rezaei Kanavi M, Fatemi I, Ghaznavi H, Shahriari M. Protective effect of melatonin in the diabetic rat retina. Fundam Clin Pharmacol. 2018 doi: 10.1111/fcp.12361. [DOI] [PubMed] [Google Scholar]
- 184.Raygan F, Ostadmohammadi V, Bahmani F, Reiter RJ, Asemi Z. Melatonin administration lowers biomarkers of oxidative stress and cardio-metabolic risk in type 2 diabetic patients with coronary heart disease: A randomized, double-blind, placebo-controlled trial. Clin Nutr. 2017 doi: 10.1016/j.clnu.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 185.Oliveira AC, Andreotti S, Sertie RAL, Campana AB, de Proença ARG, Vasconcelos RP, Oliveira KA, Coelho-de-Souza AN, Donato-Junior J, Lima FB. Combined treatment with melatonin and insulin improves glycemic control, white adipose tissue metabolism and reproductive axis of diabetic male rats. Life Sci. 2018;199:158–166. doi: 10.1016/j.lfs.2018.02.040. [DOI] [PubMed] [Google Scholar]