Abstract
Background
Although metal stents are increasingly being used for endoscopic transmural drainage of pancreatic fluid collection (PFC), the advantages of metal stents in comparison with plastic stents are not clear.
Objective
The aim of this study is to compare the clinical outcomes and adverse events between patients receiving endoscopic transmural drainage of PFCs through metal or plastic stents.
Methods
We performed a systematic literature search to identify all published manuscripts comparing metal and plastic stents for PFC drainage. The primary outcome was clinical success, and the secondary outcomes were technical success, procedure time, overall cost, adverse events, and recurrence.
Results
Seven studies were considered to be appropriate for this meta-analysis. Metal stents showed a higher clinical success rate (odds ratio (OR) 3.39, 95% confidence interval (CI) 2.05–5.60) and a lower overall adverse event rate (OR 0.37, 95% CI 0.21–0.66) than plastic stents. In subgroup analyses, metal stents showed higher clinical success rates than plastic stents both for pseudocyst (OR 5.35, 95% CI 1.35–21.19) and walled-off necrosis (OR 3.37, 95% CI 1.89–5.99).
Conclusions
Metal stents are superior to plastic stents for endoscopic transmural drainage of PFC because they have a higher clinical success rate and lower rate of adverse events.
Keywords: Pancreatic fluid collection, endoscopic ultrasound, metal stent, walled-off necrosis, pseudocyst
Introduction
Pancreatic fluid collection (PFC) develops as a result of acute or chronic pancreatitis, trauma, malignancy, or surgery. Although the majority of PFCs resolve spontaneously, intervention is needed in cases with persistent symptoms. Drainage of symptomatic PFC has been traditionally performed by surgical or percutaneous approaches, but these procedures are accompanied by high rates of morbidity and complications. For these reasons, the emerging technique of endoscopic ultrasound (EUS)-guided transmural drainage has become the mainstay for treatment of PFC.1–3 EUS-guided drainage is preferred over surgical or percutaneous drainage because of comparable success rates, lower morbidity, and better tolerability.4–8 Recent advances in the devices and techniques used for EUS have extended the indicated situations for EUS-guided drainage and improved the therapeutic results.9–11
In its early days, the EUS-guided drainage of PFCs was performed using plastic stents.12–14 However, their small diameter can lead to ineffective drainage for collections with solid debris such as walled-off necrosis (WON).15,16 Furthermore, insertion of multiple plastic stents requires the introduction of multiple guidewires, which is challenging and time-consuming. Recently, specially designed, fully covered, self-expandable metal stents have been used for PFC drainage, and the preliminary data showed promising results.17–20 However, the higher cost of metal stents compared to plastic stents is a concern. Therefore, this more expensive procedure is suitable only if it provides better clinical outcomes.
Recent systematic reviews have not proven that metal stents are superior to plastic stents for PFC drainage in terms of clinical success and adverse events.21,22 However, these reviews only summarized studies using either metal or plastic stents for PFC drainage and did not include direct comparative studies between the two methods. The aim of this meta-analysis is to directly compare the efficacy and safety of metal and plastic stents for PFC drainage.
Materials and methods
Data sources and search strategy
A comprehensive literature search was conducted through 31 January 2017, using the MEDLINE and EMBASE databases without language restrictions. Owing to a lack of prospective studies, retrospective studies were also included in this meta-analysis. We performed the search using combinations of the following terms: (“pancreatic fluid collections” or “pancreatic pseudocyst” or “walled-off necrosis”) and (“endoscopy” or “endoscopic ultrasound”) and “stents.” We also searched for relevant studies in the bibliographies of recently published review articles and editorials.
Study selection and data extraction
Two separate authors (SBY and ISL) independently reviewed all identified articles. Initially, the titles and abstracts of the articles were screened to exclude irrelevant articles. Next, a detailed review of the full manuscripts was conducted to confirm whether the articles met the criteria. The inclusion criteria for the meta-analysis were: (a) studies comparing metal and plastics stents for EUS-guided transmural drainage of PFCs in patients over the age of 18 years and (b) studies reporting the clinical success rate of both methods.
The following data from selected studies were independently extracted by two authors (SBY and ISL) using standardized data extraction forms. The main outcome measure was clinical success rate, which was measured using the definitions set in the individual studies (Table 1). We also collected the technical rate, procedure time, overall cost, incidence of bleeding, stent migration and overall adverse events, and recurrence rate.
Table 1.
Definition of clinical success used in individual studies of the meta-analysis.
| Study authorsref | Definition of clinical success |
|---|---|
| Lee et al. 201423 | Complete resolution or a decrease in size of PFC to ≤ 2 cm in association with complete clinical resolution of symptoms |
| Mukai et al. 201524 | Disappearance of symptoms or inflammation regardless of PFC size |
| Sharaiha et al. 201525 | Complete resolution of PFC at 12-month follow-up |
| Ang et al. 201626 | Complete resolution or a decrease in size of PFC to less than 2 cm |
| Bang et al. 201727 | Resolution of PFC to ≤ 2 cm with clinical resolution of symptoms at eight-week follow-up |
| Bapaye et al. 201728 | Complete resolution of PFC with clinical resolution of symptoms |
| Siddiqui et al. 201729 | Complete resolution of PFC with clinical resolution of symptoms at six months |
PFC: pancreatic fluid collection.
Quality assessment
The risk of bias was assessed by two authors (SBY and ISL) independently using the Jadad scale30 for randomized trials and the Newcastle Ottawa scale31 for other studies. The Jadad scale (range, 0–5) assesses the quality of published clinical trials relevant to random assignment, double blinding, and the flow of patients. The quality of the study is considered to be low when the score is 0–2, and considered to be high when the score is 3–5. The Newcastle Ottawa scale (range, 0–9) measures quality in the three parameters of selection, comparability, and outcome. High-quality studies are scored greater than 7, and moderate-quality studies, between 5 and 7.
Statistical analysis
This meta-analysis was conducted using Comprehensive Meta-Analysis software, version 2.2 (Biostat Inc, Englewood, NJ, USA). The categorical outcome measures of each study, such as clinical success rate or adverse events, were summarized as the odds ratio (OR) and 95% confidence interval (CI). For a conservative approach, the pooled ORs with corresponding 95% CIs were derived using a random-effect model. Forest plots were constructed to visually represent the individual study results and the pooled results. If the pooled data were not suitable for quantitative analysis, numerical and statistical results of each individual study are processed by descriptive method.
We used Cochrane's Q-test and I2 to estimate the heterogeneity of individual studies. I2 values of 20% to 50% suggest moderate heterogeneity, and values >50% suggest high heterogeneity.32 The presence of publication bias was first examined using funnel plots and then confirmed statistically using Egger's test. Subgroup analyses were performed according to type of PFC (i.e. pancreatic pseudocyst or WON). Statistical significance was defined as p < 0.05.
Results
Description of included studies
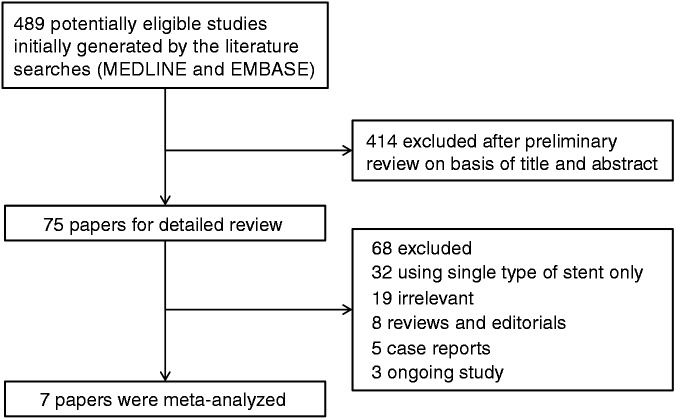
A flow diagram describing the study selection process is shown in Figure 1. A search of the MEDLINE and EMBASE databases identified 489 potentially eligible studies. Of these, 414 studies were excluded after preliminary review of the titles and abstracts. An additional 68 studies were excluded for the following reasons: 32 used only a single type of stent (plastic or metal), 19 were not relevant to our study, eight were reviews or editorials, five were case reports, and three were regarding ongoing research.
Figure 1.
Flow diagram showing the selection process of studies used in the meta-analysis.
Finally, seven papers were considered to be appropriate for this meta-analysis. The main characteristics of the included studies are summarized in Table 2. All were full-length articles published in English. One study is a prospective, randomized study,23 and the remaining six were retrospective studies.24–29 The countries of origin for the studies were: the United States of America (USA) (n = 3),25,27,29 South Korea (n = 1),23 Japan (n = 1),24 Singapore or Thailand (n = 1)26 and India (n = 1).28 The numbers of patients in the plastic- and metal-stent arm groups were 410 and 495, respectively.
Table 2.
Characteristics of studies included in the meta-analysis.
| Study authorsref | Country | No. of study institute | Study design | Study arm |
Age, mean | Male, % | Follow-up period, mean months | Jadad scale | Newcastle Ottawa scale | |
|---|---|---|---|---|---|---|---|---|---|---|
| Plastic | Metal | |||||||||
| Lee et al. 201423 | South Korea | 1 | Prospective, randomized | 25 | 25 | 52.7 | 82.0% | 7 | 3 | |
| Mukai et al. 201524 | Japan | 1 | Retrospective | 27 | 43 | 54.9 | 82.9% | NR | 6 | |
| Sharaiha et al. 201525 | USA | 2 | Retrospective | 118 | 112 | 52.6 | 62.6% | 16a | 7 | |
| Ang et al. 201626 | Singapore and Thailand | 2 | Retrospective | 33 | 16 | 54.0 | 51.0% | NR | 5 | |
| Bang et al. 201727 | USA | 1 | Retrospective | 40 | 20 | 52.2 | 60.0% | 18 | 5 | |
| Bapaye et al. 201728 | India | 2 | Retrospective | 61 | 72 | 42.4 | 87.2% | NR | 6 | |
| Siddiqui et al. 201729 | USA | 2 | Retrospective | 106 | 207 | 52.3 | 76.7% | NR | 7 | |
NR: not reported; USA: United States of America.
Median.
Quality assessment of one randomized trial23 was performed using the Jadad scale. The trial had a Jadad score of 3 and thus was considered to be high quality. The Newcastle Ottawa scale was used for appraising the quality of the other retrospective studies. All six retrospective studies24–29 were scored between 5 and 7, and satisfied the criteria of moderate quality.
Characteristics of PFCs and endoscopic procedures
Characteristics of PFCs and procedure details are summarized in Table 3. Of the seven studies, one involved drainage of a pancreatic pseudocyst only,25 three studies involved drainage of WON only,24,28,29 and three consisted of both pseudocyst and WON.23,26,27 The mean PFC size was 10.2 cm. In the plastic stent group, one or more double pigtail stents were inserted into the PFC, and 7- or 10-Fr diameter catheters were used. In the metal stent group, straight biliary fully covered self-expandable metal stents (SBFCSEMSs) were used in two studies,23,25 lumen-apposing metal stents (LAMSs) were used in three studies,26–28 and both SBFCSEMSs and LAMSs were used in two studies.24,29 Nasocystic drainage and direct endoscopic necrosectomy (DEN) were occasionally performed based on the endoscopist's preference and clinical response.
Table 3.
Characteristics of pancreatic fluid collection and summary of procedure details.
| Study authorsref | PFC type, n | PFC size, mean, cm | Location of PFCs, % (head/body or tail) | Plastic stent type | No. of plastic stents, n (median) | Metal stent diameter and type | Plastic stent in metal stent | Nasocystic drainage | Direct endoscopic necrosectomy |
|---|---|---|---|---|---|---|---|---|---|
| Lee et al. 201423 | NR | 8.7 | 30/70% | 7-Fr DPS | 2–3 (2) | 8 mm, modified SBFCSEMS | No | PS: 24% MS: 32% | No |
| Mukai et al. 201524 | WON | 9.5 | 26/74% | 7-Fr DPS | 1–3 (1) | 10–16 mm, four types of MS | No | PS: 93% MS: 26% | Optional |
| Sharaiha et al. 201525 | Pseudocyst | 9.8 | 13/87% | 10-Fr DPS | 2 | 10 mm, two types of SBFCSEMS | Yes | No | No |
| Ang et al. 201626 | Pseudocyst 31, WON 18 | 10.8 | 6/94% | 10-Fr DPS | 1–2 (1) | 16 mm, LAMS (Nagi™) | No | Optional | PS: 41% MS: 33% |
| Bang et al. 201727 | Pseudocyst 21, WON 39 | 11.3 | 7/93% | 7-Fr DPS | 2 | 15 mm, LAMS (Axios™) | No | PS: 20% MS: 5% | PS: 5% MS: 10% |
| Bapaye et al. 201728 | WON | 10.8 | 11/89% | 7-Fr DPS | 2–5 (4a) | 16 mm, LAMS (Nagi™) | No | Optional | PS: 48% MS: 33% |
| Siddiqui et al. 201729 | WON | 10.2 | 14/86% | 10-Fr DPS | 2 | 10 mm, SBFCSEMS or 10/15 mm, LAMS (Axios™) | Yes (only in SBFCSEMS) | Optional | PS: 4% MS: 19% |
DPS: double-pigtail stent; LAMS: lumen-apposing metal stent; MS: metal stent; NR: not reported; PFC: pancreatic fluid collection; PS: plastic stent; SBFCSEMS: straight biliary fully covered self-expandable metal stent; WON: walled-off necrosis.
Mean.
Outcome measures
Technical and clinical success
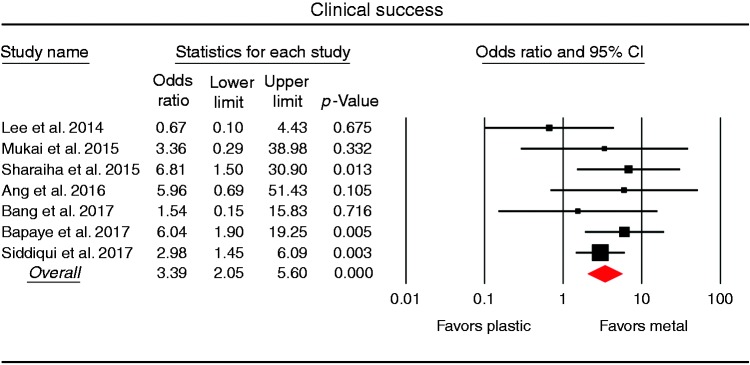
All seven studies compared the technical and clinical success rates of PFC drainage of plastic and metal stents (Table 4). The pooled technical success rate was 97.6% using plastic stents and 99.2% using metal stents, with no statistical difference between metal and plastic stents (OR 2.81, 95% CI 0.69–11.35) and no significant heterogeneity across studies (I2 9.3%; p = 0.294). The pooled analysis of all seven studies demonstrated a significantly higher clinical success rate in the metal stent group (462/491; 94.1%) than in the plastic stent group (342/414; 82.6%), with a pooled OR of 3.39 (95% CI 2.05–5.60) and no significant heterogeneity among the studies (I2 0%; p = 0.489; Figure 2).
Table 4.
Results of procedure for individual studies of the meta-analysis.
| Study authorsref | Technical success |
Clinical success |
Procedure time, median (IQR), minute |
Cost, mean (SD), US dollars |
||||
|---|---|---|---|---|---|---|---|---|
| Plastic | Metal | Plastic | Metal | Plastic | Metal | Plastic | Metal | |
| Lee et al. 201423 | 25/25 (100%) | 25/25 (100%) | 20/22 (90.9%) | 20/23 (87.0%) | 29.5 (23.5–42) | 15.0 (12.5–19.5) | NR | NR |
| Mukai et al. 201524 | 27/27 (100%) | 43/43 (100%) | 25/27 (92.6%) | 42/43 (97.7%) | 42.6 (14.2)a | 28.8 (7.1)a | 5352 (3893) | 6274 (1750) |
| Sharaiha et al. 201525 | 109/118 (92.3%) | 110/112 (98.2%) | 105/118 (89.0%) | 110/112 (98.2%) | NR | NR | NR | NR |
| Ang et al. 201626 | 37/37 (100%) | 12/12 (100%) | 24/37 (64.9%) | 11/12 (91.7%) | NR | NR | 5402 (NR)b | 5804 (NR)b |
| Bang et al. 201727 | 40/40 (100%) | 20/20 (100%) | 37/40 (92.5%) | 19/20 (95.0%) | 25.0 (20–40) | 8.5 (7–10) | 5451 (NR) | 6962 (NR) |
| Bapaye et al. 201728 | 61/61 (100%) | 72/72 (100%) | 45/61 (73.7%) | 68/72 (94.4%) | NR | NR | NR | NR |
| Siddiqui et al. 201729 | 105/106 (99.1%) | 205/207 (99.0%) | 86/106 (81.1%) | 192/207 (92.8%) | NR | NR | NR | NR |
IQR: interquartile range; NR: not reported; SD: standard deviation; US: United States.
Mean.
Singapore dollars.
Figure 2.
Forest plot comparing the clinical success rates between metal and plastic stents for drainage of pancreatic fluid collection. CI: confidence interval.
Procedure time and overall cost
Procedure time for PFC drainage was investigated in three studies and presented as either median with interquartile range (two studies)23,27 or mean with standard deviation (one study).24 In each study, the median or mean procedure time with metal stents was significantly shorter than with plastic stents (p < 0.05 for each). A cost analysis considering the overall treatment costs was conducted in three studies.24,26,27 Costs were expressed in US dollars in two studies24,27 and in Singapore dollars in one study.26 In each study, there was no statistically significant difference in overall cost between plastic and metal stents used in PFC drainage (p > 0.05 for each). A pooled meta-analysis could not be performed on either procedure time or overall costs because of differences among the studies in presentation style or units.
Adverse events and recurrence
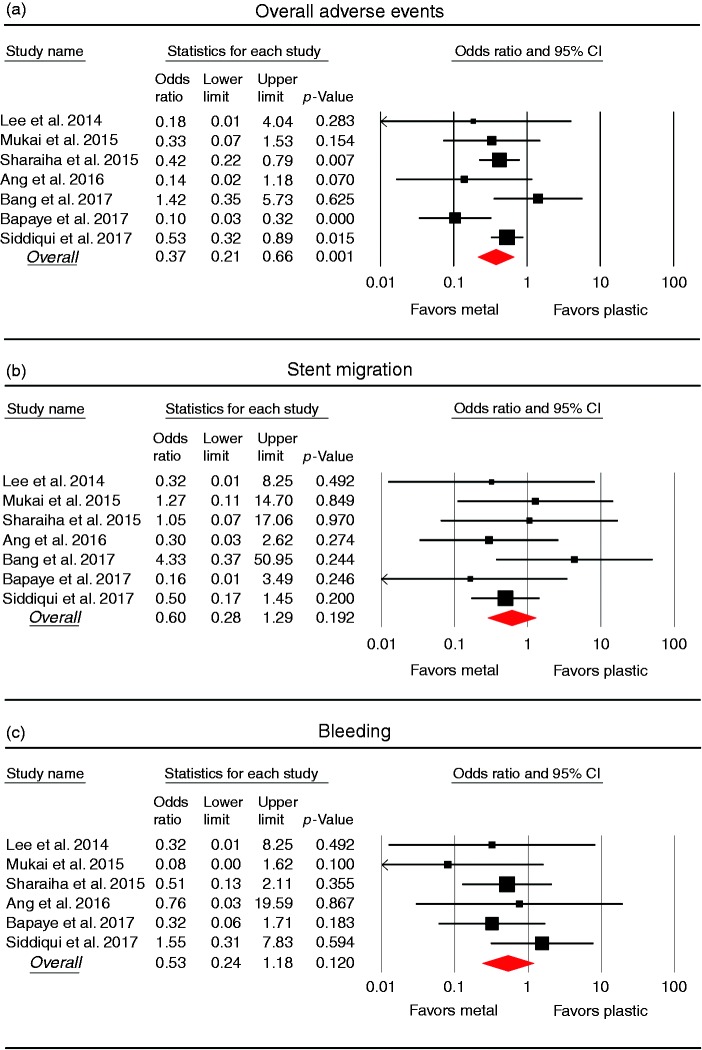
The overall adverse event rate was provided in all seven studies (Table 5, Figure 3). Overall adverse events were significantly lower in the metal stent group (79/495; 16.0%) than in the plastic stent group (123/414; 29.7%), with a pooled OR of 0.37 (95% CI 0.21–0.66) and moderate heterogeneity among the studies (I2 45.1%; p = 0.091). In a comparison of stent migration and bleeding rates, there were no statistically significant differences between plastic and metal stents, with an OR of 0.60 (95% CI 0.28–1.29) for stent migration and 0.53 (95% CI 0.24–1.18) for bleeding. Also, there was no significant heterogeneity in the studies (I2 0%; p = 0.629 for stent migration, and I2 0%; p = 0.489 for bleeding). Recurrence rate was investigated in four studies.23,25,28,29 There was no difference in recurrence between metal and plastic stents (OR 0.65, 95% CI 0.06–6.70), and moderate heterogeneity was observed across the studies (I2 39.8%; p = 0.19).
Table 5.
Adverse events and recurrence for individual studies of the meta-analysis.
| Study authorsref | Overall adverse events |
Stent migration |
Bleeding |
Recurrence |
||||
|---|---|---|---|---|---|---|---|---|
| Plastic | Metal | Plastic | Metal | Plastic | Metal | Plastic | Metal | |
| Lee et al. 201423 | 2/25 (8.0%) | 0/25 (0%) | 1/25 (4.0%) | 0/25 (0%) | 1/25 (4.0%) | 0/25 (0%) | 0/22 (0%) | 1/23 (4.5%) |
| Mukai et al. 201524 | 5/27 (18.5%) | 3/43 (7.0%) | 1/27 (3.7%) | 2/43 (4.7%) | 3/27 (11.1%) | 0/43 (0%) | NR | NR |
| Sharaiha et al. 201525 | 37/118 (31.3%) | 18/112 (16.1%) | 1/118 (0.8%) | 1/112 (0.9%) | 6/118 (5.1%) | 3/112 (2.7%) | 4/118 (3.4%) | 1/112 (0.9%) |
| Ang et al. 201626 | 12/37 (3.3%) | 1/16 (6.3%) | 7/38 (18.4%) | 1/16 (6.3%) | 1/37 (2.7%) | 0/16 (0%) | NR | NR |
| Bang et al. 201727 | 6/40 (15.0%) | 4/20 (20.0%) | 1/40 (2.5%) | 2/20 (10.0%) | NR | NR | NR | NR |
| Bapaye et al. 201728 | 22/61 (36.1%) | 4/72 (2.7%) | 2/61 (3.3%) | 0/72 (0%) | 5/61 (8.2%) | 2/72 (2.7%) | 0/61 (0%) | 0/72 (0%) |
| Siddiqui et al. 201729 | 39/106 (36.8%) | 49/207 (23.7%) | 7/106 (6.6%) | 7/207 (3.4%) | 2/106 (1.9%) | 6/207 (2.9%) | 0/106 (0%) | 0/207 (0%) |
NR: not reported.
Figure 3.
Forest plots comparing adverse event rates between metal and plastic stents for drainage of pancreatic fluid collection. (a) Overall adverse events. (b) Stent migration. (c) Bleeding. CI: confidence interval.
Subgroup analysis
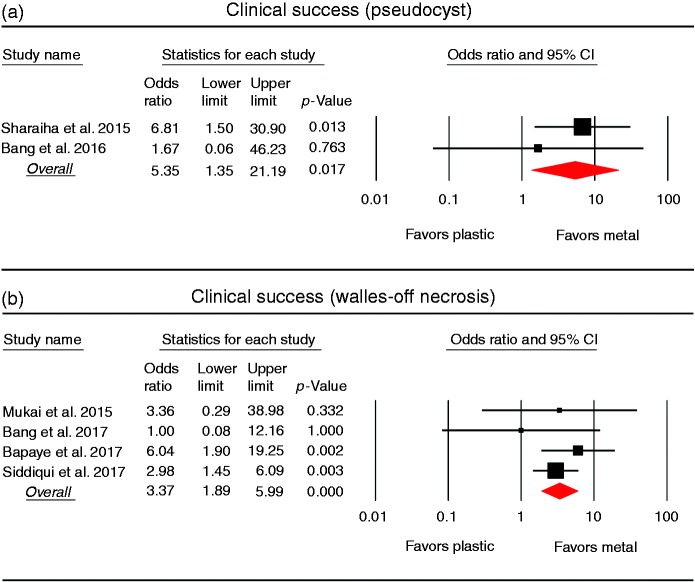
Subgroup analyses were performed to compare the clinical success rates in patients with pancreatic pseudocyst or WON (Figure 4). In the subgroup analysis of pancreatic pseudocyst (two studies, 250 patients), the clinical success rate was higher in the metal stent group (117/119; 98.3%) than in the plastic stent group (119/131; 90.8%). The pooled OR was 5.35 (95% CI 1.35–21.19), and there was no heterogeneity between the studies (I2 0%; p = 0.450). In the subgroup analysis of WON (four studies, 555 patients), the clinical success rate was also significantly higher in the metal stent group (314/335; 93.7%) than in the plastic stent group (180/220; 81.8%). The pooled OR was 3.37 (95% CI 1.89–5.99), and no heterogeneity was found among the studies (I2 0%; p = 0.546).
Figure 4.
Forest plots comparing the clinical success rates between metal and plastic stents for drainage of pancreatic fluid collection. (a) Pseudocyst. (b) Walled-off necrosis. CI: confidence interval.
Publication bias
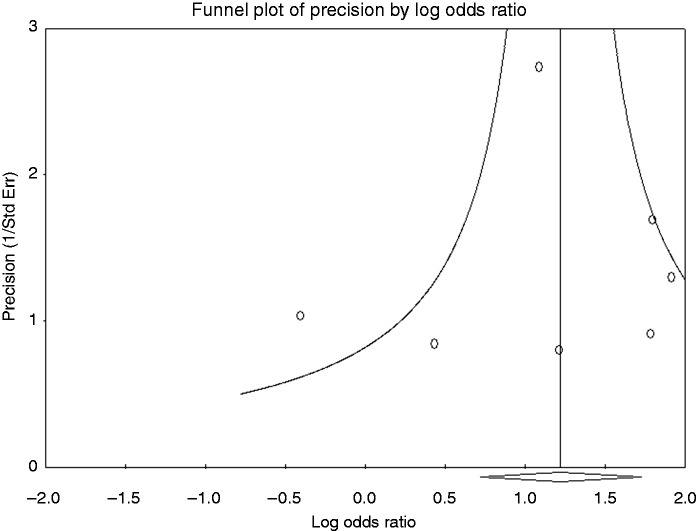
A visual inspection of the funnel plot of the clinical success meta-analysis did not suggest asymmetry (Figure 5). Statistical analysis using Egger's test confirmed that there was no evidence of publication bias in the clinical success rates (p = 0.797). The Egger's test also showed that there was no evidence of publication bias in the meta-analyses of stent migration, bleeding, and overall adverse events (p = 0.999, p = 0.428, and p = 0.407, respectively).
Figure 5.
Funnel plot of meta-analysis on clinical success. The statistical analysis confirmed no evidence of publication bias.
Discussion
Recent studies directly comparing metal and plastic stents for EUS-guided drainage of PFC have shown conflicting results. Some studies have shown superiority of metal stents,25,28,29 but others have not.23,24,26,27 This meta-analysis, which evaluated 905 patients from seven studies, showed that metal stents were superior to plastic stents for PFC drainage both in terms of clinical success and adverse events. There was no significant heterogeneity across the studies and no evidence of publication bias.
All of the individual studies comparing metal and plastic stents were conducted at one or two institutions. Although most of the individual studies have shown that metal stents have a higher clinical success rate than plastic stents, many of them did not show statistical significance. Since the clinical success rate of PFC drainage is more than 80% using either metal or plastic stents, it is difficult for studies conducted at one or two sites to recruit enough patients to prove the superiority of metal stents. Therefore, meta-analysis might be the best analytical method to identify differences between the two stent types.
Our meta-analysis demonstrated that the clinical success of PFC drainage using metal stents (94.1%) was significantly higher compared with plastic stents (82.6%). The increased likelihood of PFC resolution with metal stents could be due to their larger diameter (generally more than 10 mm) compared with plastic stents (7-or 10-Fr.; 2.3 or 3.3 mm). The larger diameter of the metal stents decreases the risk of in-stent occlusion compared with plastic stents. In addition, LAMS, which are designed specifically for drainage of PFC, have allowed endoscopists to perform more aggressive DEN without additional balloon dilation of the opening. Therefore, it is advantageous to use metal stents in cases of PFC that require aggressive debridement due to a large amount of necrotic debris.
The rate of overall adverse events was lower in the metal stent group compared with the plastic stent group. Although the specific reason for this difference is unclear, it can be attributed to the technical difficulty and prolonged procedure time in placing plastic stents. Insertion of multiple plastic stents can be a cumbersome procedure especially for inexperienced endoscopists. The longer procedure time produces a higher probability of adverse events. Additionally, tract dilation prior to DEN also caused more hemorrhages and perforations in the plastic stent group.24 However, an ongoing study has reported several late complications, including bleeding, buried stents, and biliary stricture, in patients with LAMS.33 These possibly conflicting results indicate that, in terms of complications, further observation is needed.
Our study showed that metal stents were superior to plastic stents both for pseudocyst and WON. However, it is unclear whether metal stents should be the first choice for all PFCs including pseudocysts. There were only two individual studies on pseudocysts in our subgroup meta-analysis, making it difficult to draw conclusions.25,27 Unlike WON, the clinical success rate for endoscopic drainage of pseudocysts was excellent ( > 90%) despite the use of plastic stents.15,16 However, for WON, frequent clogging and re-intervention can lead to low clinical success rates and high adverse event rates when using plastic stents. Large-caliber metal stents can be useful in draining PFCs containing necrotic fluid and solid debris. Additionally, wide-diameter metal stents also have the advantage of allowing DEN to be performed more conveniently.
In a recent meta-analysis conducted by Bang et al.,22 no differences were found in the efficacy and safety between metal and plastic stents, where conventional SBFCSEMSs were used in most metal stent cases. On the other hand, dedicated LAMSs were used in five of seven studies included in our meta-analysis. The larger diameter of LAMS allows DEN of WONs easily after stent deployment by passage of endoscopy through stent lumen. In addition, both proximal and distal anchor flanges are designed to prevent stent migration and dislocation. Newly developed LAMSs are expected to be more effective and safer than SBFCSEMSs in PFC drainage, especially in WONs. However, in previous studies comparing the two types of metal stents, LAMS reduced stent migration, but did not improve the treatment success rate.29,34 Further prospective studies are needed to determine which types of metal stents (i.e. LAMS or SBFCSEMS) are better in each type of PFC (i.e. pseudocyst or WON).
There were several limitations to our study. First, only one of the seven studies was a randomized, controlled study; the others were retrospective studies. In addition, all studies were conducted at one or two centers. Although there were no significant differences between the baseline characteristics of the two stent groups in each study, a risk of bias is inevitable in retrospective and single- or dual-center studies. Second, the definitions of clinical success, which was the primary outcome measure in our study, are somewhat different among the individual studies. Meta-analysis has the inherent methodological limitation that it cannot control the variables of the studies involved. Third, there were only two studies comparing the effect of stents on pancreatic pseudocyst: It was not enough to evaluate the superiority of metal stents with only those two studies. Finally, a pooled analysis of cost- and time-effectiveness was not possible.
In conclusion, our study demonstrated that metal stents are superior to plastic stents for endoscopic transmural drainage of PFCs because they have a higher clinical success rate and lower rate of adverse events. Multicenter, prospective, controlled trials are needed to confirm and elaborate on the results of our analysis.
Declaration of conflicting interests
None declared.
Funding
This work was supported by a grant from the National Research Foundation of Korea funded by the Korean Government (NRF-2015R1C1A1A02037568).
Ethics approval
Not applicable.
Informed consent
Not applicable.
References
- 1.Giovannini M, Pesenti C, Rolland AL, et al. Endoscopic ultrasound-guided drainage of pancreatic pseudocysts or pancreatic abscesses using a therapeutic echo endoscope. Endoscopy 2001; 33: 473–477. [DOI] [PubMed] [Google Scholar]
- 2.Baron TH, Harewood GC, Morgan DE, et al. Outcome differences after endoscopic drainage of pancreatic necrosis, acute pancreatic pseudocysts, and chronic pancreatic pseudocysts. Gastrointest Endosc 2002; 56: 7–17. [DOI] [PubMed] [Google Scholar]
- 3.Jacobson BC, Baron TH, Adler DG, et al. ASGE guideline: The role of endoscopy in the diagnosis and the management of cystic lesions and inflammatory fluid collections of the pancreas. Gastrointest Endosc 2005; 61: 363–370. [DOI] [PubMed] [Google Scholar]
- 4.Yusuf TE, Baron TH. Endoscopic transmural drainage of pancreatic pseudocysts: Results of a national and an international survey of ASGE members. Gastrointest Endosc 2006; 63: 223–227. [DOI] [PubMed] [Google Scholar]
- 5.Bakker OJ, van Santvoort HC, van Brunschot S, et al. Endoscopic transgastric vs surgical necrosectomy for infected necrotizing pancreatitis: A randomized trial. JAMA 2012; 307: 1053–1061. [DOI] [PubMed] [Google Scholar]
- 6.Kwon YM, Gerdes H, Schattner MA, et al. Management of peripancreatic fluid collections following partial pancreatectomy: A comparison of percutaneous versus EUS-guided drainage. Surg Endosc 2013; 27: 2422–2427. [DOI] [PubMed] [Google Scholar]
- 7.Varadarajulu S, Bang JY, Sutton BS, et al. Equal efficacy of endoscopic and surgical cystogastrostomy for pancreatic pseudocyst drainage in a randomized trial. Gastroenterology 2013; 145: 583–590. [DOI] [PubMed] [Google Scholar]
- 8.Keane MG, Sze SF, Cieplik N, et al. Endoscopic versus percutaneous drainage of symptomatic pancreatic fluid collections: A 14-year experience from a tertiary hepatobiliary centre. Surg Endosc 2016; 30: 3730–3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gardner TB, Coelho-Prabhu N, Gordon SR, et al. Direct endoscopic necrosectomy for the treatment of walled-off pancreatic necrosis: Results from a multicenter U.S. series. Gastrointest Endosc 2011; 73: 718–726. [DOI] [PubMed] [Google Scholar]
- 10.Varadarajulu S, Phadnis MA, Christein JD, et al. Multiple transluminal gateway technique for EUS-guided drainage of symptomatic walled-off pancreatic necrosis. Gastrointest Endosc 2011; 74: 74–80. [DOI] [PubMed] [Google Scholar]
- 11.Gluck M, Ross A, Irani S, et al. Dual modality drainage for symptomatic walled-off pancreatic necrosis reduces length of hospitalization, radiological procedures, and number of endoscopies compared to standard percutaneous drainage. J Gastrointest Surg 2012; 16: 248–256. [DOI] [PubMed] [Google Scholar]
- 12.Ahn JY, Seo DW, Eum J, et al. Single-step EUS-guided transmural drainage of pancreatic pseudocysts: Analysis of technical feasibility, efficacy, and safety. Gut Liver 2010; 4: 524–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Varadarajulu S, Christein JD, Wilcox CM. Frequency of complications during EUS-guided drainage of pancreatic fluid collections in 148 consecutive patients. J Gastroenterol Hepatol 2011; 26: 1504–1508. [DOI] [PubMed] [Google Scholar]
- 14.Rana SS, Bhasin DK, Rao C, et al. Consequences of long term indwelling transmural stents in patients with walled off pancreatic necrosis & disconnected pancreatic duct syndrome. Pancreatology 2013; 13: 486–490. [DOI] [PubMed] [Google Scholar]
- 15.Varadarajulu S, Bang JY, Phadnis MA, et al. Endoscopic transmural drainage of peripancreatic fluid collections: Outcomes and predictors of treatment success in 211 consecutive patients. J Gastrointest Surg 2011; 15: 2080–2088. [DOI] [PubMed] [Google Scholar]
- 16.Bang JY, Wilcox CM, Trevino JM, et al. Relationship between stent characteristics and treatment outcomes in endoscopic transmural drainage of uncomplicated pancreatic pseudocysts. Surg Endosc 2014; 28: 2877–2883. [DOI] [PubMed] [Google Scholar]
- 17.Itoi T, Binmoeller KF, Shah J, et al. Clinical evaluation of a novel lumen-apposing metal stent for endosonography-guided pancreatic pseudocyst and gallbladder drainage (with videos). Gastrointest Endosc 2012; 75: 870–876. [DOI] [PubMed] [Google Scholar]
- 18.Gornals JB, De la Serna-Higuera C, Sánchez-Yague A, et al. Endosonography-guided drainage of pancreatic fluid collections with a novel lumen-apposing stent. Surg Endosc 2013; 27: 1428–1434. [DOI] [PubMed] [Google Scholar]
- 19.Moon JH, Choi HJ, Kim DC, et al. A newly designed fully covered metal stent for lumen apposition in EUS-guided drainage and access: A feasibility study (with videos). Gastrointest Endosc 2014; 79: 990–995. [DOI] [PubMed] [Google Scholar]
- 20.Huggett MT, Oppong KW, Pereira SP, et al. Endoscopic drainage of walled-off pancreatic necrosis using a novel self-expanding metal stent. Endoscopy 2015; 47: 929–932. [DOI] [PubMed] [Google Scholar]
- 21.Bang JY, Varadarajulu S. Metal versus plastic stent for transmural drainage of pancreatic fluid collections. Clin Endosc 2013; 46: 500–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bang JY, Hawes R, Bartolucci A, et al. Efficacy of metal and plastic stents for transmural drainage of pancreatic fluid collections: A systematic review. Dig Endosc 2015; 27: 486–498. [DOI] [PubMed] [Google Scholar]
- 23.Lee BU, Song TJ, Lee SS, et al. Newly designed, fully covered metal stents for endoscopic ultrasound (EUS)-guided transmural drainage of peripancreatic fluid collections: A prospective randomized study. Endoscopy 2014; 46: 1078–1084. [DOI] [PubMed] [Google Scholar]
- 24.Mukai S, Itoi T, Baron TH, et al. Endoscopic ultrasound-guided placement of plastic vs. biflanged metal stents for therapy of walled-off necrosis: a retrospective single-center series. Endoscopy 2015; 47: 47–55. [DOI] [PubMed] [Google Scholar]
- 25.Sharaiha RZ, DeFilippis EM, Kedia P, et al. Metal versus plastic for pancreatic pseudocyst drainage: Clinical outcomes and success. Gastrointest Endosc 2015; 82: 822–827. [DOI] [PubMed] [Google Scholar]
- 26.Ang TL, Kongkam P, Kwek AB, et al. A two-center comparative study of plastic and lumen-apposing large diameter self-expandable metallic stents in endoscopic ultrasound-guided drainage of pancreatic fluid collections. Endosc Ultrasound 2016; 5: 320–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bang JY, Hasan MK, Navaneethan U, et al. Lumen apposing metal stents (LAMS) for drainage of pancreatic fluid collections: When and for whom?. Dig Endosc 2017; 29: 83–90. [DOI] [PubMed] [Google Scholar]
- 28.Bapaye A, Dubale NA, Sheth KA, et al. Endoscopic ultrasonography-guided transmural drainage of walled-off pancreatic necrosis: Comparison between a specially designed fully covered bi-flanged metal stent and multiple plastic stents. Dig Endosc 2017; 29: 104–110. [DOI] [PubMed] [Google Scholar]
- 29.Siddiqui AA, Kowalski TE, Loren DE, et al. Fully covered self-expanding metal stents versus lumen-apposing fully covered self-expanding metal stent versus plastic stents for endoscopic drainage of pancreatic walled-off necrosis: Clinical outcomes and success. Gastrointest Endosc 2017; 85: 758–765. [DOI] [PubMed] [Google Scholar]
- 30.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary?. Control Clin Trials 1996; 17: 1–12. [DOI] [PubMed] [Google Scholar]
- 31.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010; 25: 603–605. [DOI] [PubMed] [Google Scholar]
- 32.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21: 1539–1558. [DOI] [PubMed] [Google Scholar]
- 33.Bang JY, Hasan M, Navaneethan U, et al. Lumen-apposing metal stents (LAMS) for pancreatic fluid collection (PFC) drainage: May not be business as usual. Gut 2017; 66: 2054–2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vazquez-Sequeiros E, Baron TH, Pérez-Miranda M, et al. Evaluation of the short- and long-term effectiveness and safety of fully covered self-expandable metal stents for drainage of pancreatic fluid collections: Results of a Spanish nationwide registry. Gastrointest Endosc 2016; 84: 450–457. [DOI] [PubMed] [Google Scholar]