Abstract
Introduction
The risk of lymph node metastases (LNM) in submucosal esophageal adenocarcinoma (EAC) patients is subject to debate. These patients might be treated endoscopically if the risk of LNM appears to be low.
Objective
The objective of this article is to evaluate the outcome of patients who underwent an endoscopic resection (ER) and subsequent endoscopic follow-up for a submucosal EAC.
Methods
All patients who underwent ER for submucosal EAC between January 2012 and August 2016 and were subsequently managed with endoscopic follow-up were retrospectively identified. Primary outcome was the number of patients diagnosed with LNM; secondary outcomes included intraluminal recurrences.
Results
Thirty-five patients (median age 68 years) were included: 17 low-risk (submucosal invasion <500 microns, G1–G2, no lymphovascular invasion (LVI)), and 18 high-risk (submucosal invasion >500 microns, and/or G3–G4, and/or LVI, and/or a tumor-positive deep resection margin (R1)) EACs. After a median follow-up of 23 (IQR 15–43) months, in which patients underwent a median of six (IQR 4–8) endoscopies and a median of four (IQR 2–8) endoscopic ultrasound procedures, none of the included patients were diagnosed with LNM. Five (14%) patients developed a local intraluminal recurrence a median of 18 (IQR 11–21) months after baseline ER that were treated endoscopically.
Conclusions
In 35 patients with a submucosal EAC, no LNM were found during a median follow-up of 23 months. Endoscopic therapy may be an alternative for surgery in selected patients with a submucosal EAC.
Keywords: Esophageal adenocarcinoma, lymph node metastases, endoscopic treatment, T1b EAC, early neoplasia
Key summary
Established knowledge on this subject
‐ Mucosal esophageal adenocarcinomas (EACs) can be treated curatively by endoscopic means, since the risk for lymph node metastasis is nihil.
‐ Patients with a submucosal EAC are generally advised to undergo surgery given their presumed risk for lymph node metastases. This risk calculation, however, is based on dated surgical series.
‐ A few recent endoscopic-oriented series suggest that the risk for lymph node metastases might be lower than previously thought.
‐ Submucosal EACs can be subdivided into a low-risk and a high-risk group for lymph node metastases based on the histopathological characteristics of the tumor.
New findings
‐ In 17 patients with a low-risk submucosal EAC, no lymph node metastases were diagnosed during a median follow-up of 22 months.
‐ In 18 patients with a high-risk submucosal EAC, we neither observed lymph node metastases during a median follow-up of 24 months.
‐ Endoscopic therapy in selected submucosal EAC patients may therefore be an alternative to surgery.
Introduction
The optimal treatment of esophageal adenocarcinoma (EAC) with submucosal invasion is subject to debate. Worldwide, most patients with submucosal EAC undergo surgical treatment since it is thought that the risk for lymph node metastases (LNM) in these tumors is high. Guidelines that advise to refer patients for esophagectomy are based on results of surgical series that reported an incidence of LNM of up to 78% in these patients.1–8 Yet, recent studies investigating the risk of LNM in submucosal EAC patients treated endoscopically suggested a much lower incidence of LNM.9–12 Many studies furthermore proposed to subdivide submucosal EACs into two risk groups (low risk vs. high risk), based on histopathological features of the endoscopic resection (ER) specimen. Low-risk submucosal EACs are defined as tumors growing into the superficial submucosa (<500 microns), with good to moderate tumor differentiation, and without evidence of lymphovascular invasion (LVI). The risk of LNM in this subgroup is reported to be so low (<2%) that non-surgical management has become an accepted alternative for many patients.9–12 For high-risk submucosal EACs (i.e. deep submucosal invasion >500 microns, and/or poorly differentiation and/or presence of LVI, and/or a tumor-positive deep resection margin (R1-resection)), the risk for LNM is reported to be higher (16%–37%), and esophagectomy is still considered to be indicated.12–15
The discrepancy between LNM rates reported by surgical and endoscopic series is remarkable. The surgical studies are mainly retrospective series in which the histopathological diagnosis (pT) was not driven by differentiating mucosal from submucosal cancers nor subdividing different types of submucosal cancers. Surgical specimens were generally sectioned at wide intervals and no histopathological review was conducted for the purpose of the study. This makes the data from these surgical series inappropriate for decision making regarding the consequences of histopathological findings of ER specimen.
Evidence for endoscopic follow-up of submucosal EAC patients is scarce (Table 1). Yet, there is a need to treat submucosal EAC patients in a less-invasive way since many patients presenting with this disease have significant comorbidities and an advanced age and are therefore unfit for surgical therapy. The aim of this study was to evaluate the outcome of patients with submucosal EAC who were treated endoscopically, and underwent subsequent endoscopic and endosonographic follow-up.
Table 1.
Overview series on endoscopic treatment and follow-up of submucosal esophageal adenocarcinoma.
| Authors, yearRef | Inclusion period | N | Sm1 (<500 microns) |
Sm2/Sm3 (>500 microns) |
||
|---|---|---|---|---|---|---|
| n | % LNM | n | % LNM | |||
| Manner et al. 201713 | 1996–2010 | 4 | 4 | 0% | ||
| Schölvinck et al. 20169 | 2001–2012 | 33 | 13 LR 10 HR | 0/13 0% 1/10 10% | 10a | 2/10 20% |
| Fotis et al. 201514 | 1994–2013 | 10 | 10 LR | 0/10 0% | ||
| Manner et al. 201512 | 1996–2010 | 43 | 43 37 LR 6 HR | 1/43 2% 1/37 3% 0/6 0% | ||
| Manner et al. 201315 | 1996–2010 | 53a | 53 LR | 1/53 2% | ||
| Alvarez Herrero et al. 201010 | 2000–2008 | 18 | 9 | 0/9 0% | 9 | 0/13 0% |
| Manner et al. 200811 | 1996–2003 | 20a | 20 LR | 0/20 0% | ||
Only patients with R0-resection (for neoplasia) were included in this table.
LR: low risk: superficial submucosal invasion <500 microns, good to moderate tumor differentiation, and no lymphovascular invasion; HR: high risk: deep submucosal invasion >500 microns, and/or poor tumor differentiation, and/or lymphovascular invasion; LNM: lymph node metastases; Sm: submucosal.
Patients and methods
Patients
We retrospectively identified all patients who underwent ER for submucosal EAC between January 2012 and August 2016 in two Dutch tertiary referral centers for the treatment of upper gastrointestinal (GI) neoplasia (Academic Medical Center Amsterdam and the St. Antonius Hospital, Nieuwegein). Patients were included in the study if the ER specimen showed EAC with submucosal invasion, and if the patient underwent endoscopic follow-up after initial endoscopic therapy. Patients were excluded if treated with additional surgical therapy directly after ER, or if metastatic disease was found at baseline.
Endoscopic management
ER procedure
All endoscopies were performed with high-resolution endoscopes with white-light endoscopy and narrow-band imaging (NBI). First, the esophagus was carefully inspected for the presence of visible lesions. In case of Barrett’s esophagus (BE), the extent of the BE was documented according to the Prague C and M classification.16 Visible lesions were classified using the Paris classification.17 The target lesion was then delineated by placing coagulation markers around the lesion. ER was performed by using the multiband mucosectomy (MBM) technique (Duette MBM system, Cook Endoscopy, Limerick, Ireland), ER-cap technique (Olympus, Hamburg, Germany) or by means of endoscopic submucosal dissection (ESD).
Histopathologic evaluation
ER specimens were pinned down on paraffin and fixed in 3.6% buffered formalin for approximately 24 hours. After fixation, the specimens were cut into 2 mm to 3 mm strips. The strips were placed into a cassette (maximum of three strips per cassette) and processed in an automated tissue processor. The paraffin blocks were cut into 4 µm slides and stained with hematoxylin and eosin. For each case a desmin stain was performed to determine the deepest point of invasion and a D2-40 or CD31 when LVI was suspected. All ER specimens were reviewed by a local expert GI pathologist. The following histological features were assessed: depth of tumor infiltration with submucosal invasion measured in microns (invasion of <500 microns was classified as an sm1 EAC; submucosal invasion of ≥500 microns was classified as an sm2–3 EAC), tumor differentiation grade, presence of LVI, radicality of the deep vertical resection margins and of lateral resection margins in case of en bloc resection.
Patients were divided into two risk groups based on the histopathological characteristics of the tumor. Patients were considered as low risk if the EAC was removed radically, if there was superficial (<500 microns: sm1) submucosal infiltration, good to moderate tumor differentiation, and no evidence of LVI. High-risk patients were defined as patients with deep submucosal infiltration (≥500 microns: sm2–3), and/or poor tumor differentiation, and/or presence of LVI, or in case of tumor involvement at the deep vertical resection margin (irradical resection).
Staging
At baseline all patients underwent a staging endoscopic ultrasound (EUS) to assess presence of locoregional lymph nodes and computed tomography (CT) scan of the thorax and abdomen, or a position-emission tomography (PET)/CT scan, to evaluate the presence of distant metastases. Patients with evidence of metastatic disease were excluded. Patients were subsequently discussed in a multidisciplinary team (MDT) meeting, consisting of gastroenterologist, GI surgeon, oncologist, pathologist, radiotherapist, radiologist and nuclear medicine specialist to tailor further treatment. The decision to manage patients endoscopically, and not to perform surgery, was made based on the results of the staging procedures, risk group (low risk or high risk), patient’s characteristics (such as comorbidity and age), and patient’s preference.
Endoscopic therapy and follow-up for patients with a low-risk submucosal EAC was considered standard of care. Patients with a high-risk submucosal EAC were informed about the associated risk for LNM, and extensively consulted about both endoscopic and surgical treatment taking each patient’s age and comorbidity in consideration.
Endoscopic follow-up
If the decision was made to manage the patient endoscopically, he or she entered a follow-up program with gastroscopy every three months and EUS during the first year after baseline ER. After the first year, gastroscopy and EUS were performed every six months to evaluate for the presence of local recurrence or locoregional LNM.
When a visible lesion was detected in the esophagus, it was biopsied or removed with ER. Lymph nodes that appeared suspicious during EUS were punctured using fine-needle aspiration (EUS-FNA).
Any residual Barrett’s epithelium was eradicated using radiofrequency ablation (RFA) or argon plasma coagulation (APC) during subsequent endoscopies, or kept under endoscopic surveillance. In general, low-risk cases underwent ablation therapy at the next follow-up sessions whereas in high-risk cases ablation therapy was postponed for the first 12 months of follow-up. Timing and decision making regarding ablation treatment was further determined by the patients’ general condition and life expectancy and the presence and extent of dysplasia in the residual Barrett’s segment.
Outcome parameters
Primary outcome parameter
Rate of diagnosed LNM in patients with a submucosal EAC.
Secondary outcome parameters
Number of patients diagnosed with a local intraluminal recurrence.
Number of patients diagnosed with distant metastasis.
Ethical considerations and statistical analysis
This study was exempted from official institutional review board approval after assessment of the study design protocol according to our national guidelines (www.ccmo.nl) by the Medical Ethical Committee of the Academic Medical Center Amsterdam.
Data analysis was performed using the SPSS statistical software package (version 23, SPSS Inc, Chicago, IL, USA). The mean (±standard deviation (SD)) was used to describe variables showing a normal distribution and the median (interquartile range (IQR)) for variables with a skewed distribution.
Length of follow-up was calculated from the date of baseline ER to the most recent endoscopy or CT scan. Patients were censored in case of loss to follow-up, or in case of unrelated death.
Results
Patients
Between January 2012 and August 2016, 55 patients underwent diagnostic ER and were found to have a submucosal EAC. Twenty patients were excluded (Figure 1). One patient was excluded because of a Lynch syndrome with multiple gastric carcinomas and a cholangiocarcinoma, and one patient had a simultaneous metastatic sigmoid carcinoma. Another patient was diagnosed with N1 disease with EUS-FNA at baseline and underwent definitive chemoradiation therapy. This patient had a Paris 0-IIa lesion, and the ER specimen showed histopathological characteristics associated with a high risk for LNM. Seventeen patients underwent additional surgical therapy after diagnostic ER. Tumor characteristics of the excluded surgical patients are displayed in Table 2. All these patients were clinically staged as a T1bN0 EAC. However, in one surgical patient with a T1sm1/G3/LVI+ EAC, LNM, two tumor-positive nodes were found in the surgical resection specimen.
Figure 1.
Flow diagram of inclusion and main outcomes.
cT1bN0: clinical T1bN0: CRT: chemoradiotherapy; EAC: esophageal adenocarcinoma; ER: endoscopic resection; IQR: interquartile range; LNM: lymph node metastases.
Table 2.
Tumor characteristics of excluded patients with T1b EAC undergoing surgery.
| Patients | Submucosal invasion depth | Tumor differentiation grade | LVI | Radical resectiona | Risk factors for LNM |
|---|---|---|---|---|---|
| 1 | <500 microns | G2 | Absent | R0 | 0 |
| 2 | <500 microns | G2 | Present | R0 | 1 |
| 3 | ≥500 microns | G2 | Absent | R0 | 1 |
| 4 | <500 microns | G3 | Absent | R0 | 1 |
| 5 | ≥500 microns | G2 | Absent | R0 | 1 |
| 6 | <500 microns | G2 | Present | R0 | 1 |
| 7 | ≥500 microns | G1 | Absent | R0 | 1 |
| 8 | ≥500 microns | G2 | Present | R0 | 2 |
| 9 | ≥500 microns | G2 | Absent | R1 | 2 |
| 10 | <500 microns | G1 | Present | R1 | 2 |
| 11 | ≥500 microns | G2 | Absent | R1 | 2 |
| 12 | ≥500 microns | G3 | Absent | R0 | 2 |
| 13 | <500 microns | G3 | Present | R0 | 2 |
| 14 | ≥500 microns | G3 | Absent | R0 | 2 |
| 15 | <500 microns | G3 | Present | R0 | 2 |
| 16 | ≥500 microns | G3 | Present | R0 | 3 |
| 17 | ≥500 microns | G3 | Present | R0 | 3 |
Deep vertical resection margin.
EAC: esophageal adenocarcinoma; LNM: lymph node metastases; LVI: lymphovascular invasion.
Thirty-five patients with a T1b EAC were included in the final analysis. Median age at the time of ER was 68 (IQR 62–74) years. Of these patients, 30 patients had a BE with a median length of C3M5. Five patients were diagnosed with an adenocarcinoma of the cardia. In 17/35 (49%) patients, the tumor was classified as a low-risk submucosal EAC, and in 18/35 (51%) as a high-risk submucosal EAC. Baseline characteristics are displayed in Tables 3 and 4. In patients with a high-risk submucosal EAC, 10/18 (56%) were considered unfit for surgery owing to comorbidity and 8/18 (44%) patients preferred endoscopic follow-up over surgical treatment after extensive consultation by a surgeon and gastroenterologist. The majority of patients underwent complete staging prior to starting endoscopic follow-up. However, in eight low-risk and one high-risk patients, staging was not complete. In three patients no CT scan or EUS was performed at baseline, and in six patients (including the high-risk patient) no CT scan was performed.
Table 3.
Baseline characteristics.
| Patients | |
|---|---|
| Total, n | 35 |
| Age at ER, years (IQR) | 68 (62–74) |
| Gender, n | |
| Male | 26 (74) |
| Tumor | |
| Location | |
| Barrett | 29 (83) |
| Cardia | 6 (17) |
| Barrett length, cm (IQR) | |
| Circumferential | 3 (1–8) |
| Maximal | 5 (3–9) |
| Paris classification | |
| 0-Is | 10 (29) |
| 0-IIa | 17 (49) |
| 0-IIb | 1 (3) |
| 0-IIa and 0-IIb | 1 (3) |
| 0-IIa and 0-IIc | 4 (11) |
| 0-IIa and 0-Is | 1 (3) |
| 0-IIb and 0-IIc | 1 (3) |
| Size | |
| <2 cm | 19 (54) |
| >2 cm | 16 (46) |
| Endoscopic resection | |
| ER technique | |
| MBM | 17 (49) |
| ER cap | 5 (14) |
| ESD | 13 (37) |
| Resection | |
| En bloc | 18 (51) |
| Histopathological examination ER specimen | |
| Infiltration depth | |
| T1sm1 (<500 microns) | 28 (80) |
| T1sm2–3 (≥500 microns) | 7 (20) |
| Differentiation grade, n | |
| Good (G1) | 4 (11) |
| Moderate (G2) | 23 (66) |
| Poor (G3) | 8 (23) |
| LVI, n | |
| Absent | 28 (80) |
| Present | 7 (20) |
| Radicality of ER (deep vertical margins) | |
| Non-radical | 4 (11) |
| Radical | 31 (89) |
ER: endoscopic resection; ESD: endoscopic submucosal dissection; IQR: interquartile range; LVI: lymphovascular invasion; MBM: multiband mucosectomy.
Table 4.
Tumor characteristics of patients with a high-risk submucosal esophageal adenocarcinoma who underwent endoscopic follow-up.
| Patients | Submucosal invasion depth | Tumor differentiation grade | LVI | Radical resectiona | Risk factors for LNM |
|---|---|---|---|---|---|
| 1 | >500 microns | G2 | Absent | Yes | 1 |
| 2 | >500 microns | G2 | Absent | Yes | 1 |
| 3 | >500 microns | G2 | Absent | Yes | 1 |
| 4 | >500 microns | G2 | Absent | Yes | 1 |
| 5 | >500 microns | G2 | Absent | Yes | 1 |
| 6 | <500 microns | G2 | Present | Yes | 1 |
| 7 | <500 microns | G3 | Absent | Yes | 1 |
| 8 | <500 microns | G3 | Absent | Yes | 1 |
| 9 | <500 microns | G2 | Present | Yes | 1 |
| 10 | <500 microns | G1 | Present | Yes | 1 |
| 11 | >500 microns | G3 | Absent | Yes | 2 |
| 12 | <500 microns | G3 | Present | Yes | 2 |
| 13 | <500 microns | G3 | Present | Yes | 2 |
| 14 | <500 microns | G2 | Present | No | 2 |
| 15 | >500 microns | G3 | Absent | Yes | 2 |
| 16 | <500 microns | G3 | Present | No | 3 |
| 17 | <500 microns | G3 | Absent | No | 2 |
| 18 | <500 microns | G2 | Absent | No | 1 |
Deep vertical resection margin.
LNM: lymph node metastases; LVI: lymphovascular invasion.
Rate of LNM
During a median follow-up of 23 (IQR 15–43) months, none (0%) of the 35 included patients were diagnosed with LNM. The median follow-up was 22 (IQR 15–47) months for the low-risk patients, and a median of 23 (IQR 15–39) months for high-risk patients. During follow-up, a median of six (IQR 4–8) endoscopies and a median of four (IQR 2–8) EUS procedures were performed per patient. In 11/35 (31%) patients, EUS-FNA was performed. In none of these patients were tumor cells diagnosed in the FNA specimen.
In two patients follow-up was discontinued: One patient died 11 months after initial ER of a non-tumor-related cause without evidence of metastatic disease. The other patient refused to undergo additional follow-up endoscopies because of advanced age: Only one EUS was performed three months after therapeutic ER. A CT scan 21 months after ER showed no signs of metastasis.
Esophageal recurrence
In 5/35 (14%) patients, intraluminal recurrences were diagnosed a median of 18 (IQR 11–21) after initial ER (Figure 2). None of the patients showed progression in relation to the initial histopathological tumor characteristics. All patients could be treated successfully by endoscopic means, and during follow-up (median of six (3–27) months) no additional visible lesions were observed. Of interest, none of the patients with an irradical resection (tumor-positive deep vertical resection margin) of the tumor developed local recurrence.
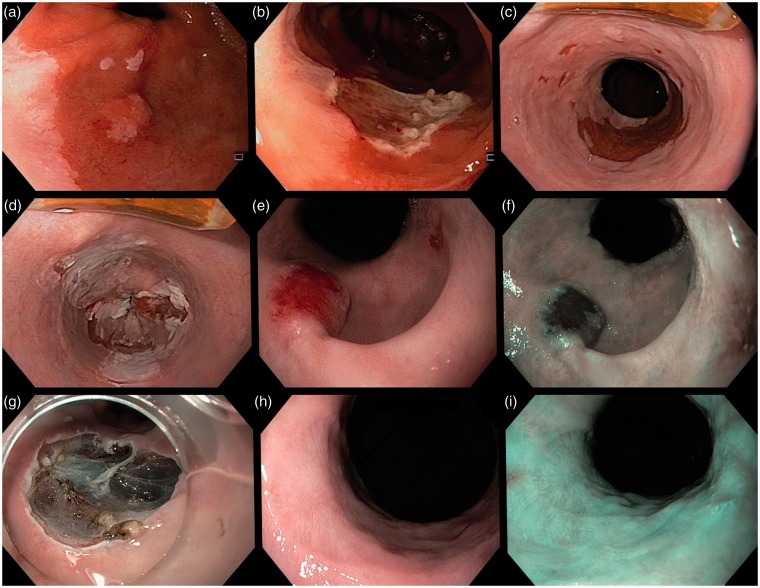
Figure 2.
Endoscopic images of a 59-year-old patient with local recurrence during follow-up after removal of a submucosal esophageal adenocarcinoma. (a) and (b) A C3M4 Barrett’s esophagus with a T1sm1 esophageal adenocarcinoma that was removed with endoscopic resection. (c) and (d) The residual Barrett’s mucosa was removed by radiofrequency ablation. (e) and (f) After 21 months of follow-up, recurrence of a T1sm1 esophageal adenocarcinoma was found. (g) The lesion was removed by endoscopic submucosal dissection. (h) and (i) During the next follow-up endoscopy, the resection scar was seen without signs of residual or recurrent neoplasia.
Further endoscopic treatment of BE after baseline ER
After baseline ER, residual BE was treated in 17/28 (61%) patients by means of ER (n = 1) or RFA (n = 16), complemented with APC for diminutive BE areas. In 2/17 patients therapy is still ongoing: In these patients RFA treatment was initiated >12 months after initial ER. In 3/17 patients treatment was discontinued because of unrelated comorbidity (n = 2) or poor response to RFA (n = 1). At the time treatment was discontinued complete eradication of dysplasia (CE-D) had been achieved, but the whole Barrett’s segment was not eradicated. In the remaining 12 patients, CE-D and CE-intestinal metaplasia was achieved.
Distant metastasis
In none of the patients were distant metastases diagnosed during follow-up.
Discussion
In this cohort study, we evaluated the outcome of 35 patients who were kept under endoscopic and endosonographic follow-up after initial ER for submucosal EAC. In none of the patients were pathological lymph nodes detected during a median follow-up of 23 months.
In our study 49% (17/35) of lesions were classified as low risk. The results for this subgroup are in line with previous studies and add to the evidence that low-risk submucosal EAC have a very low risk of LNM (0%–2% in recent series).9–12 Endoscopic therapy followed by endoscopic follow-up may therefore be preferred over a surgical approach since the LNM rate is lower than the estimated surgical mortality rate of esophagectomy.18
None of the 18 high-risk patients in this study were diagnosed with LNM during a median follow-up of 23 months, which is less than reported in recent endoscopic series (9% to 37%) and surgical series (7%–78%).1–15 Several factors may have contributed to the low LNM rate in our study. First, we have a relatively short follow-up period compared to previous studies, which reported a mean follow-up of 47 and 42 months, respectively.9,13 We cannot exclude the possibility that prolonged follow-up in our 18 patients will increase the LNM rate. However, series have shown that in 75% of cases LNM are diagnosed within the first two years after treatment and follow-up in this study comprises a median of 23 months.6,19 Second, our sample size of 25 patients is relatively small. Yet, taking the previously reported LNM rates into consideration, one would expect that in our study LNM would have been detected in up to 10 of our high-risk patients. Third, during follow-up, only EUS was used to screen for LNM in our patients. We did not include CT scans or abdominal ultrasounds in our follow-up regimen. Yet, EUS is the best available modality for the detection of regional LNM in esophageal cancer and is superior to other imaging modalities (CT or PET-CT), especially when combined with a low threshold for performing EUS-FNA.20 Fourth, we staged patients at baseline with EUS and CT scanning before discussing cases at our MDT and deciding on endoscopic follow-up. This led us to exclude one patient who was found to have a T1bN1 after EUS-FNA at baseline. Last, a fair number of high-risk patients had only superficial submucosal invasion: Eleven of 18 high-risk patients had submucosal invasion of less than 500 microns. Moreover, in 11 (61%) high-risk patients, only one risk factor for the development of LNM was observed (Table 3). Boys et al. reported in a case series that in patients with only one risk factor, LNM were found in 25% (2/8) of patients, compared with 50% (1/2) of patients with three risk factors.21 Therefore, the composition of our cohort may have contributed to the low incidence of LNM found.
Recurrence of luminal disease was seen in 5/35 patients (14%) after a median follow-up of 18 months. All patients were effectively treated endoscopically. It is known that up to 30% of patients will develop metachronous lesions in residual BE after removal of early cancer.22–24 The majority (95%) of intraluminal recurrences can be treated endoscopically, which is underlined by the results of our study.11 After removal of early cancer in BE, it is important to eradicate residual (flat) BE by using ablation therapy, preferably RFA. For low-risk cases, RFA can be performed after baseline staging. For high-risk cases ablation therapy will be postponed for the first 12 months of follow-up. Timing and decision making regarding ablation treatment was further determined by the patients’ general condition and life expectancy and the presence and extent of dysplasia in the residual Barrett’s segment.
We found no distant metastases in our patients. It must be noted that we did not routinely perform CT scans to trace distant metastases. Additional imaging (such as a CT abdomen/thorax) was performed only on indication. However, we believe that the risk of distant metastasis is low in asymptomatic patients without endosonographic signs of local LNM. Some patients with a high-risk submucosal EAC are deemed unfit for surgery or prefer an endoscopic approach over surgical treatment. These patients are offered endoscopic treatment in our center, and a GI surgeon also informs them about the pros and cons of surgical treatment. This study therefore reflects the current clinical practice in which low-risk EAC as well as high-risk EAC were endoscopically treated followed by endoscopic follow-up. This study adds value to the available literature, since it describes a relatively large cohort of submucosal EAC patients treated endoscopically.
Our study has several limitations that need to be addressed. First, we screened only patients with a submucosal EAC who underwent diagnostic ER. We did not look at patients who underwent direct surgical therapy without any endoscopic intervention. This subgroup may have a higher risk for metastatic disease since ER was considered technically not feasible. Subsequently, we included only patients who underwent endoscopic follow-up after initial ER in order to minimize the heterogeneity of the cohort. Of the excluded surgical patients only one patient was diagnosed with LNM and one patient was excluded based on N1-disease diagnosed at baseline. Even if we include these two cases, the total LNM rate is only 4% (2/55). Finally, given the retrospective nature of this study, follow-up visits were not scheduled according to a strict prospective protocol. The vast majority of patients underwent CT scanning and EUS at baseline, and upper endoscopies and EUS every three months in the first year; every six months during the second and third year, and annually thereafter. However, there were gaps in the workup in our study.
Because of these limitations, we have initiated a multicenter prospective cohort study investigating the safety of endoscopic treatment and follow-up of patients with a submucosal EAC.
In conclusion, the absence of LNM in 17 included patients with a low-risk submucosal EAC confirms the results of earlier endoscopic series. Endoscopic therapy for this subgroup seems preferable over prophylactic esophagectomy.
The absence of LNM in 18 submucosal EAC patients with high-risk features suggests that there is at least a subgroup of high-risk patients with a lower risk of LNM than previously thought.
Declaration of conflicting interests
H.T. Künzli, K. Belghazi, S. Meijer, and C.A. Seldenrijk: no conflict of interest.
B. Weusten: Research support for IRB-approved studies from GI Solutions Medtronic, Erbe Medical, C2 Therapeutics, and Boston Scientific. Honorarium-consultancy-speakers fee from C2 Therapeutics.
J.J.G.H.M. Bergman: Research support for IRB-approved studies from GI Solutions Medtronic, Erbe Medical, C2 Therapeutics, Olympus Endoscopy, Fuji Film, Boston Scientific, Ninepoint Medical, Cernostics, Interpase, and Lumen-R. Financial support for training programs from GI Solutions Medtronic and Boston Scientific. Honorarium-consultancy-speakers fee from Boston Scientific, GI Solutions Medtronic, Olympus Endoscopy, Fuji Film, and WATTS-3d.
Ethics approval
This study comprises a retrospective cohort study, for which no official institutional review board (IRB) approval was indicated (after review of protocol and study design by the IRB).
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Informed consent
Informed consent was not required for this study.
References
- 1.Leers JM, DeMeester SR, Oezcelik A, et al. The prevalence of lymph node metastases in patients with T1 esophageal adenocarcinoma a retrospective review of esophagectomy specimens. Ann Surg 2011; 253: 271–278. [DOI] [PubMed] [Google Scholar]
- 2.Stein HJ, Feith M, Bruecher BL, et al. Early esophageal cancer: Pattern of lymphatic spread and prognostic factors for long-term survival after surgical resection. Ann Surg 2005; 242: 566–573. discussion 573–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Griffin SM, Burt AD, Jennings NA. Lymph node metastasis in early esophageal adenocarcinoma. Ann Surg 2011; 254: 736–737. discussion 736–737. [DOI] [PubMed] [Google Scholar]
- 4.Sepesi B, Watson TJ, Zhou D, et al. Are endoscopic therapies appropriate for superficial submucosal esophageal adenocarcinoma? An analysis of esophagectomy specimens. J Am Coll Surg 2010; 210: 418–427. [DOI] [PubMed] [Google Scholar]
- 5.Ancona E, Rampado S, Cassaro M, et al. Prediction of lymph node status in superficial esophageal carcinoma. Ann Surg Oncol 2008; 15: 3278–3288. [DOI] [PubMed] [Google Scholar]
- 6.Westerterp M, Koppert LB, Buskens CJ, et al. Outcome of surgical treatment for early adenocarcinoma of the esophagus or gastro-esophageal junction. Virchows Arch 2005; 446: 497–504. [DOI] [PubMed] [Google Scholar]
- 7.Buskens CJ, Westerterp M, Lagarde SM, et al. Prediction of appropriateness of local endoscopic treatment for high-grade dysplasia and early adenocarcinoma by EUS and histopathologic features. Gastrointest Endosc 2004; 60: 703–710. [DOI] [PubMed] [Google Scholar]
- 8.Bollschweiler E, Baldus SE, Schröder W, et al. High rate of lymph-node metastasis in submucosal esophageal squamous-cell carcinomas and adenocarcinomas. Endoscopy 2006; 38: 149–156. [DOI] [PubMed] [Google Scholar]
- 9.Schölvinck D, Künzli H, Meijer S, et al. Management of patients with T1b esophageal adenocarcinoma: A retrospective cohort study on patient management and risk of metastatic disease. Surg Endosc 2016; 30: 4102–4113. [DOI] [PubMed] [Google Scholar]
- 10.Alvarez Herrero L, Pouw RE, van Vilsteren FG, et al. Risk of lymph node metastasis associated with deeper invasion by early adenocarcinoma of the esophagus and cardia: Study based on endoscopic resection specimens. Endoscopy 2010; 42: 1030–1036. [DOI] [PubMed] [Google Scholar]
- 11.Manner H, May A, Pech O, et al. Early Barrett’s carcinoma with “low-risk” submucosal invasion: Long-term results of endoscopic resection with a curative intent. Am J Gastroenterol 2008; 103: 2589–2597. [DOI] [PubMed] [Google Scholar]
- 12.Manner H, Pech O, Heldmann Y, et al. The frequency of lymph node metastasis in early-stage adenocarcinoma of the esophagus with incipient submucosal invasion (pT1b sm1) depending on histological risk patterns. Surg Endosc 2015; 29: 1888–1896. [DOI] [PubMed] [Google Scholar]
- 13.Manner H, Wetzka J, May A, et al. Early-stage adenocarcinoma of the esophagus with mid to deep submucosal invasion (pT1b sm2–3): The frequency of lymph-node metastasis depends on macroscopic and histological risk patterns. Dis Esophagus 2017; 30: 1–11. [DOI] [PubMed] [Google Scholar]
- 14.Fotis D, Doukas M, Wijnhoven BP, et al. Submucosal invasion and risk of lymph node invasion in early Barrett’s cancer: Potential impact of different classification systems on patient management. United European Gastroenterol J 2015; 3: 505–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manner H, Pech O, Heldmann Y, et al. Efficacy, safety, and long-term results of endoscopic treatment for early stage adenocarcinoma of the esophagus with low-risk sm1 invasion. Clin Gastroenterol Hepatol 2013; 11: 630–635. [DOI] [PubMed] [Google Scholar]
- 16.Sharma P, Dent J, Armstrong D, et al. The development and validation of an endoscopic grading system for Barrett’s esophagus: The Prague C & M criteria. Gastroenterology 2006; 131: 1392–1399. [DOI] [PubMed] [Google Scholar]
- 17.The Paris endoscopic classification of superficial neoplastic lesions: Esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc 2003; 58 (6 Suppl): S3–S43. [DOI] [PubMed]
- 18.Bennett C, Vakil N, Bergman J, et al. Consensus statements for management of Barrett’s dysplasia and early-stage esophageal adenocarcinoma, based on a Delphi process. Gastroenterology 2012; 143: 336–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dresner SM, Lamb PJ, Bennett MK, et al. The pattern of metastatic lymph node dissemination from adenocarcinoma of the esophagogastric junction. Surgery 2001; 129: 103–109. [DOI] [PubMed] [Google Scholar]
- 20.van Vliet EP, Heijenbrok-Kal MH, Hunink MG, et al. Staging investigations for oesophageal cancer: A meta-analysis. Br J Cancer 2008; 98: 547–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boys JA, Worrell SG, Chandrasoma P, et al. Can the risk of lymph node metastases be gauged in endoscopically resected submucosal esophageal adenocarcinomas? A multi-center study. J Gastrointest Surg 2016; 20: 6–12. discussion 12. [DOI] [PubMed] [Google Scholar]
- 22.May A, Gossner L, Pech O, et al. Local endoscopic therapy for intraepithelial high-grade neoplasia and early adenocarcinoma in Barrett’s oesophagus: Acute-phase and intermediate results of a new treatment approach. Eur J Gastroenterol Hepatol 2002; 14: 1085–1091. [DOI] [PubMed] [Google Scholar]
- 23.Ell C, May A, Pech O, et al. Curative endoscopic resection of early esophageal adenocarcinomas (Barrett’s cancer). Gastrointest Endosc 2007; 65: 3–10. [DOI] [PubMed] [Google Scholar]
- 24.Peters FP, Kara MA, Rosmolen WD, et al. Endoscopic treatment of high-grade dysplasia and early stage cancer in Barrett’s esophagus. Gastrointest Endosc 2005; 61: 506–514. [DOI] [PubMed] [Google Scholar]