Abstract
Background
It is assumed that pregnancy and childbirth increase the risk of developing fecal incontinence (FI).
Objective
We investigated the incidence of FI in groups of nulliparous and parous women.
Methods
Retrospectively, we studied a cross-section of the Dutch female population (N = 680) who completed the Groningen Defecation & Fecal Continence questionnaire. We also analyzed a subgroup of healthy women (n = 572) and a subgroup of women with comorbidities (n = 108).
Results
The prevalence of FI and the Vaizey and Wexner scores did not differ significantly between nulliparous and parous women. Parous women were 1.6 times more likely to experience fecal urgency than nulliparous women (95% CI, 1.0–2.6, p = 0.042). Regression analyses showed that parity, mode of delivery, duration of second stage of labor, obstetrical laceration or episiotomy, and birth weight seem not to be associated with the likelihood of FI.
Conclusions
Pregnancy and childbirth seem not to be associated with the prevalence and severity of FI in the Dutch population. Vacuum and forceps deliveries, however, might result in a higher prevalence of FI. Although the duration of being able to control bowels after urge sensation is comparable between nulliparous and parous women, parous women experience fecal urgency more often.
Keywords: Vaginal delivery, parity, operative delivery, Rome IV criteria, accidental bowel leakage
Key summary
Summary of established knowledge on this subject
– The etiology of fecal incontinence (FI) is multifactorial.
– Pregnancy and childbirth are considered important risk factors for developing post-partum FI.
– Most often, however, FI develops later in life.
What are the significant and/or new findings of this study?
– The prevalence and severity of FI was comparable between nulliparous and parous women.
– The association between FI and pregnancy or childbirth seems to be less significant than was previously thought.
– Vacuum and forceps deliveries, however, might result in a higher prevalence of FI.
– Parous women are more likely to experience fecal urgency than nulliparous women.
Introduction
Pregnancy and childbirth are well-known risk factors for transient postpartum fecal incontinence (FI).1,2 The current theory is that pregnancy and childbirth contribute to the development of FI because of pelvic floor injury due to compression, stretching, or tearing of nerve, muscle, and/or connective tissue.3–5 Such neuromuscular injury may, however, improve during the first year after childbirth, which explains the spontaneous resolution of transient post-partum FI in the majority of women.6,7
Nevertheless, FI often develops later in life.8,9 As a consequence, controversy has arisen about the influence of pregnancy and childbirth on the development of FI in the long term. The current theory is that young women remain fecally continent because they have sufficient spare mechanisms at their disposal to compensate for the neuromuscular damage they sustained during pregnancy and childbirth. Because age influences these spare mechanisms negatively, FI will eventually develop later in life.10 Another possibility, however, is that the influence of pregnancy and childbirth is less significant than the current theory allows.
This hypothesis is supported by the following two findings. First, if pregnancy and childbirth were risk factors for FI, it would seem reasonable to find an increased prevalence of FI in parous women compared to nulliparous women. Several studies, however, found that the prevalence of FI is comparable between parous and nulliparous women.11–13 Additionally, they did not find a higher prevalence of FI with increasing parity.11–13 Second, if pregnancy and childbirth were risk factors for FI, the prevalence of FI would be higher in women than in men. Several studies, however, demonstrated that the prevalence of FI in women is similar to that of men.8,9,14 Possibly, as women grow older causes other than pregnancy, such as comorbidities and surgery in the pelvic area, might be more important risk factors for FI than their childbirth history.9,13,15
Based on these arguments, we hypothesized that pregnancy and childbirth do not increase the risk of FI in the long term. Our aim was therefore to investigate the incidence of FI in groups of nulliparous and parous women.
Materials and methods
Study design
Retrospectively, we analyzed a subgroup of women who were part of a larger database for a previous study.16 For that study, Survey Sampling International (Rotterdam, the Netherlands), created a population-based sample from a database of respondents living in the Netherlands. The database was compiled between September 1 and November 1, 2015. The participants were sent a link that enabled them to fill out the Groningen Defecation & Fecal Continence (DeFeC) questionnaire on their computer (supplementary data). Out of 3081 eligible respondents who started filling out the questionnaire, 1642 (54.2%) filled it out completely. Subsequently, a random selection from among these questionnaires was made by Survey Sampling International to arrive at a representative cohort that was distributed equally regarding sex, region, and age according to the population pyramid of the Netherlands as reported by Statistics Netherlands.17 By doing so, 1259 out of 1642 (76.7%) questionnaires were included in the database. Financial compensation was awarded to the respondents who had fully completed the questionnaire. This study was conducted in compliance with requirements of our local Medical Ethics Review Board.
For the purpose of this study on the association between parity and FI, we excluded all the men (n = 579), which left us with 680 women for our main analyses. For the subanalyses we divided the women into a comorbidity subgroup and a healthy subgroup. The comorbidity subgroup (n = 108) was formed by women who had a history of bowel or pelvic floor surgery (e.g. intestinal resection, perianal fistula operation, hemorrhoid operation) or women who suffered from somatic diseases, such as rectal prolapse, inflammatory bowel diseases, diabetes mellitus, neurological disorders (e.g. cerebrovascular accident, spinal cord injury, multiple sclerosis), or congenital disorders (e.g. anorectal malformation, Hirschsprung’s disease, sacrococcygeal teratoma, or spina bifida) that could have negatively influenced fecal continence. The remaining women, including women with obstetric laceration or episiotomy who had not undergone anal sphincter repair, were included in the healthy subgroup (n = 572).
The women were also divided according to parity, thus we had a nulliparous and a parous subgroup.
Assessment of FI, constipation and urine incontinence
The DeFeC questionnaire is composed of several validated scores for FI, including the Vaizey score18 and Wexner score.19 Additionally, it covers various aspects of associated disorders and causative factors, including constipation, urinary continence, anorectal sensation, diet and medical history.
We defined FI according to the Rome IV criteria for FI, that is recurrent uncontrolled passage of fecal material, including soiling, several times a month for the last six months.20 The criteria were addressed by asking questions number 4.1, 4.2, 4.3 or 4.4 of the DeFeC questionnaire (supplementary data). The severity of FI was assessed by the Vaizey incontinence score18 and the Continence Grading Scale as described by Jorge and Wexner.19 Constipation was also defined according to the Rome IV criteria.20 These criteria consist of the following items: straining, lumpy or hard stools, incomplete evacuation, anorectal blockage, manual maneuvers to facilitate defecation, and reduced stool frequency. In order to meet the criteria for constipation, respondents had to comply with at least two of the aforementioned criteria, and loose stools were rare unless they had used laxatives. Urine incontinence was defined as any involuntary leakage of urine during the past six months.
All medical information was self-reported by the women and, because they filled out the questionnaires anonymously, we could not review their medical records.
Definitions of demographic characteristics
Based on the respondents’ age percentiles, we formed three age groups: the 18- through 38-year-olds, the 39- through 54-year-olds, and the 55- through 80-year-olds. Respondents’ body mass indexes (BMIs) (kg/m2) were classified according to World Health Organization (WHO) guidelines: underweight (<18.5 kg/m2), normal weight (18 to 25 kg/m2), overweight (25 to 30 kg/m2), or obese (>30 kg/m2). Respondents who lived in a village with a maximum of 50,000 inhabitants were classified as living in a rural environment, while respondents who lived in a city of more than 50,000 inhabitants were classified as living in an urban environment. The highest educational level was classified as primary (primary or middle school), secondary (high school or vocational education), or tertiary (university or college).
Statistical analysis
Data were analyzed with SPSS for Windows, version 23.0 (IBM SPSS Statistics, IBM Corporation, Armonk, NY, USA). We reported proportions as prevalence percentages with the corresponding 95% confidence intervals (CIs) and compared the proportions by using the Fisher exact test. We used the Mann-Whitney test to compare continuous variables with categorical variables. We reported median, minimum, and maximum values. We used univariate regression analyses to determine which variables were associated with FI. We defined statistical significance as p ≤ 0.05.
Results
The mean age of the total group of women was 48.7 years (SD 15.3 years). Table 1 shows the demographic characteristics of the total group of women (N = 680), the healthy subgroup (n = 572), and the comorbidity subgroup (n = 108). In the total group and the healthy subgroup, parous women were older (p < 0.001), had a higher BMI (p < 0.001), a lower educational level (p < 0.001), and were employed less often (p < 0.001) than nulliparous women. In the comorbidity group, parous women were also significantly older than nulliparous women (p = 0.045), but no other significant differences were found between nulliparous and parous women.
Table 1.
Demographic characteristics of the total group, healthy subgroup, and comorbidity group.
| Total group (N = 680) |
Healthy subgroup
(n = 572) |
Comorbidity subgroup
(n = 108) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nulliparous n = 291 |
Parous n = 389 |
Nulliparous n = 260 |
p | Parous n = 312 |
Nulliparous n = 31 |
p | Parous n = 77 |
||||||||
| n | % | p | n | % | n | % | n | % | n | % | n | % | |||
| Age (years) | <0.001 | <0.001 | 0.045 | ||||||||||||
| 18–38 | 126 | 43.3 | 60 | 15.4 | 119 | 45.8 | 52 | 16.7 | 7 | 22.6 | 8 | 10.4 | |||
| 39–54 | 100 | 34.4 | 132 | 33.9 | 88 | 33.8 | 112 | 35.9 | 12 | 38.7 | 20 | 26.0 | |||
| 55–80 | 65 | 22.3 | 197 | 50.6 | 53 | 20.4 | 148 | 47.4 | 12 | 38.7 | 49 | 63.6 | |||
| Body mass index (kg/m2) | <0.001 | <0.001 | 0.691 | ||||||||||||
| <18.5 | 11 | 3.8 | 5 | 1.3 | 11 | 4.2 | 5 | 1.6 | 0 | 0.0 | 0 | 0 | |||
| 18.5–25 | 153 | 52.6 | 158 | 40.6 | 143 | 55.0 | 126 | 40.4 | 10 | 32.3 | 32 | 41.6 | |||
| 25–30 | 81 | 27.8 | 126 | 32.4 | 70 | 26.9 | 103 | 33.0 | 11 | 35.5 | 23 | 29.9 | |||
| >30 | 46 | 15.8 | 100 | 25.7 | 36 | 13.8 | 78 | 25.0 | 10 | 32.3 | 22 | 28.6 | |||
| Residence | 0.066 | 0.038 | 0.999 | ||||||||||||
| Rural | 98 | 33.7 | 159 | 40.9 | 86 | 33.1 | 130 | 41.7 | 12 | 38.7 | 29 | 37.7 | |||
| Urban | 193 | 66.3 | 230 | 59.1 | 174 | 66.9 | 182 | 58.3 | 19 | 61.3 | 48 | 62.3 | |||
| Highest educational level | <0.001 | <0.001 | 0.744 | ||||||||||||
| Primary | 49 | 16.8 | 119 | 30.6 | 42 | 16.2 | 96 | 30.8 | 7 | 22.6 | 23 | 29.9 | |||
| Secondary | 102 | 35.1 | 164 | 42.2 | 89 | 34.2 | 135 | 43.3 | 13 | 41.9 | 29 | 37.7 | |||
| Tertiary | 140 | 48.1 | 106 | 27.2 | 129 | 49.6 | 81 | 26.0 | 11 | 35.5 | 25 | 32.5 | |||
| Employed | <0.001 | <0.001 | 0.263 | ||||||||||||
| No | 131 | 45.0 | 238 | 61.2 | 113 | 43.5 | 184 | 59.0 | 18 | 58.1 | 54 | 70.1 | |||
| Yes | 160 | 55.0 | 151 | 38.8 | 147 | 56.5 | 128 | 41.0 | 13 | 41.9 | 23 | 33.3 | |||
Prevalence of FI
Overall, 54 women (7.9%) suffered from FI (Table 2). Compared to the total group, the prevalence of FI was lower in the healthy subgroup (n = 33, 5.8%, p = 0.134) and significantly higher in the comorbidity subgroup (n = 21, 19.4, p < 0.001). Because several demographic characteristics were different between nulliparous and parous women, we also analyzed the prevalence of FI separately for these demographic characteristics (Table 2). Only in the total group did the prevalence of FI increase with increasing BMI (p = 0.018). In all three groups, the prevalence of FI was not influenced by age, educational level, residency, and/or employment status.
Table 2.
Prevalence of fecal incontinence (FI) in relation to demographic characteristics.
| Total group |
Healthy subgroup |
Comorbidity subgroup |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % FI | 95% CI | p | n | % FI | 95% CI | p | n | % FI | 95% CI | p | |
| Overall | 680 | 7.9 | 5.9–10.0 | 572 | 5.8 | 3.9–7.7 | 108 | 19.4 | 11.9–27.0 | |||
| Age (years) | 0.141 | 0.091 | 0.740 | |||||||||
| 18–38 | 186 | 9.1 | 5.0–13.3 | 171 | 7.6 | 3.6–11.6 | 15 | 26.7 | 13.0–52.0 | |||
| 39–54 | 232 | 5.2 | 2.3–8.0 | 200 | 3.0 | 0.6–5.4 | 32 | 18.8 | 4.5–33.0 | |||
| 55–80 | 262 | 9.5 | 6.0–13.1 | 201 | 7.0 | 3.4–10.5 | 61 | 18.0 | 8.1–28.0 | |||
| Body mass index | 0.018 | 0.097 | 0.560 | |||||||||
| <18.5 | 16 | 0.0 | – | 16 | 0.0 | – | 0 | 0.0 | – | |||
| 18.5–25 | 311 | 5.1 | 2.7–7.6 | 269 | 3.7 | 1.4–6.0 | 42 | 14.3 | 3.2–25.3 | |||
| 25–30 | 207 | 9.7 | 5.6–13.7 | 173 | 6.9 | 3.1–10.8 | 34 | 23.5 | 8.5–38.6 | |||
| >30 | 146 | 12.3 | 6.9–17.7 | 114 | 9.6 | 4.1–15.2 | 32 | 21.9 | 6.7–37.0 | |||
| Residence | 0.109 | 0.141 | ||||||||||
| Rural | 257 | 10.1 | 6.4–13.8 | 216 | 6.9 | 3.5–10.4 | 0.360 | 41 | 26.8 | 12.7–41.0 | ||
| Urban | 423 | 6.6 | 4.2–9.0 | 356 | 5.1 | 2.9–7.3 | 67 | 14.9 | 6.2–23.7 | |||
| Highest educational level | 0.087 | 0.258 | ||||||||||
| Primary | 168 | 11.9 | 7.0–16.9 | 138 | 8.7 | 3.9–13.5 | 0.262 | 30 | 26.7 | 9.9–43.5 | ||
| Secondary | 266 | 6.0 | 3.1–8.9 | 224 | 4.9 | 2.1–7.8 | 42 | 11.9 | 1.7–22.1 | |||
| Tertiary | 246 | 7.3 | 4.0–10.6 | 210 | 4.8 | 1.9–7.7 | 36 | 22.2 | 8.0–36.5 | |||
| Employed | 0.118 | 0.440 | ||||||||||
| Yes | 311 | 6.1 | 3.4–8.8 | 275 | 5.1 | 2.5–7.7 | 0.591 | 36 | 13.9 | 2.0–25.8 | ||
| No | 369 | 9.5 | 6.5–12.5 | 297 | 6.4 | 3.6–9.2 | 72 | 22.2 | 12.4–32.1 | |||
CI: confidence interval.
The likelihood of FI following pregnancy and childbirth
We also analyzed the likelihood of FI following pregnancy and childbirth (Table 3). Despite parous women being significantly older and having a significantly higher BMI, the prevalence of FI between nulliparous and parous women in the total group (7.2% versus 8.2%, p = 0.570) and in the healthy subgroup (5.4% versus 6.1%, p = 0.857) was comparable. Also in the comorbidity subgroup the prevalence of FI did not differ significantly between nulliparous and parous women (22.6% versus 17.9%, p = 0.597). Because of the small numbers in the comorbidity subgroup, the more detailed analyses were performed only in the total group and healthy subgroup.
Table 3.
Likelihood of fecal incontinence (FI) following pregnancy and childbirth.
| Total group |
Healthy subgroup |
|||||||
|---|---|---|---|---|---|---|---|---|
| N | % FI | 95% CI | p | n | % FI | 95% CI | p | |
| Overall | 680 | 7.9 | 5.9–10.0 | 572 | 5.8 | 3.9–7.7 | ||
| Pregnancy | 0.570 | 0.857 | ||||||
| Nulliparous | 291 | 7.2 | 4.2–10.2 | 260 | 5.4 | 2.6–8.1 | ||
| Parous | 389 | 8.2 | 5.7–11.3 | 312 | 6.1 | 3.4–8.8 | ||
| Parity | 0.562 | 0.999 | ||||||
| 1 delivery | 88 | 5.7 | 0.7–10.6 | 73 | 5.5 | 0.1–10.8 | ||
| 2 deliveries | 205 | 9.8 | 5.7–13.9 | 163 | 6.1 | 2.4–9.9 | ||
| ≥3 deliveries | 96 | 8.3 | 2.7–14.0 | 76 | 6.6 | 0.9–12.3 | ||
| Mode of delivery | 0.503 | 0.151 | ||||||
| Vaginal | 344 | 8.1 | 5.2–110 | 278 | 5.4 | 2.7–8.1 | ||
| Cesarean section | 25 | 8.0 | –3.4 to 19.4 | 20 | 10.0 | –4.4 to 24.4 | ||
| Both | 20 | 15.0 | –2.1 to 32.1 | 14 | 14.3 | –6.7 to 35.3 | ||
| Longest straining duration | 0.248 | 0.296 | ||||||
| <1 hour | 163 | 6.1 | 2.4–9.9 | 129 | 3.9 | 0.5–7.3 | ||
| 1–2 hours | 100 | 9.0 | 3.3–14.7 | 85 | 5.9 | 0.8–11.0 | ||
| >2 hours | 100 | 12.0 | 5.5–18.5 | 77 | 9.1 | 2.5–15.7 | ||
| Use of instrument | 0.066 | 0.217 | ||||||
| No | 265 | 6.8 | 3.7–9.8 | 217 | 4.6 | 1.8–7.4 | ||
| Yes | 81 | 13.6 | 6.0–21.2 | 65 | 9.2 | 2.0–16.5 | ||
| Obstetrical laceration or episiotomy | 0.845 | 0.999 | ||||||
| No | 128 | 7.8 | 3.1–12.5 | 98 | 6.1 | 1.3–11.0 | ||
| Yes | 235 | 8.9 | 5.3–12.6 | 193 | 5.7 | 2.4–9.0 | ||
CI: confidence interval.
Table 3 shows that parity, mode of delivery, the duration of the second stage of labor, and obstetrical laceration or episiotomy were not associated with the likelihood of FI either. In the total group, however, the prevalence of FI tended to be higher in women who had undergone either a vacuum- or forceps-assisted delivery, in comparison to women who had not needed assistance during vaginal delivery (13.6% versus 6.8%, p = 0.066). Furthermore, in the total group, the median birth weight of the largest newborn was significantly lower in the women with FI compared to women without FI (3200 versus 3500 g, p = 0.028), in contrast to the healthy subgroup (3400 g versus 3500 g, p = 0.803).
Severity of FI, use of medicines, and occurrence of constipation and urine incontinence
When comparing nulliparous and parous women with FI, we found that the Wexner and Vaizey scores did not differ significantly in the total group and the healthy subgroup (Figure 1).
Figure 1.
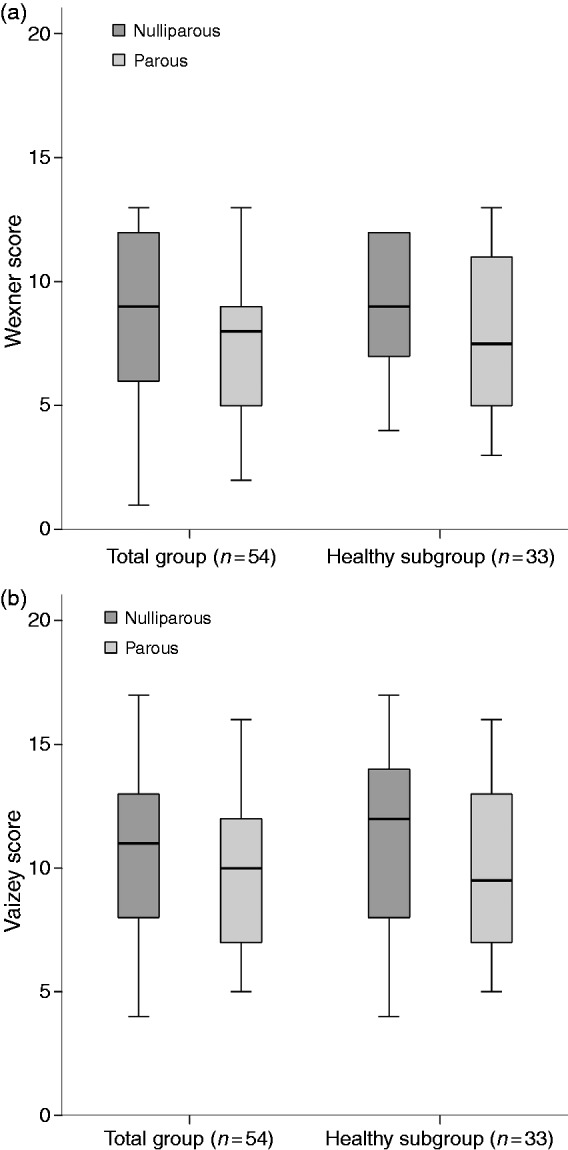
Wexner scores (a) and Vaizey scores (b) in respondents with fecal incontinence (FI) in the total group and the healthy subgroup, for nulliparous and parous women. (a) Wexner scores did not differ significantly between nulliparous and parous women with FI in the total group (p = 0.872) and the healthy subgroup (p = 0.186). (b) Vaizey scores did not differ significantly between nulliparous and parous women with FI in the total group (p = 0.449) and the healthy subgroup (p = 0.321).
Table 4 shows the use of laxatives and anti-diarrhea medicine by women with FI for the total group and the healthy subgroup. In both groups, use of such medicines did not differ significantly between nulliparous and parous women. The prevalence of constipation and urine incontinence did not differ significantly between nulliparous and parous women either.
Table 4.
Use of medicines and prevalence of constipation and urine incontinence in women with FI.
| Total group
(N = 54) |
Healthy subgroup
(n = 33) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Nulliparous
n = 21 |
p | Parous n = 33 |
Nulliparous n = 14 |
p | Parous n = 19 |
|||||
| n | % | n | % | n | % | n | % | |||
| Use of laxatives | 3 | 14.3 | 0.202 | 11 | 33.3 | 3 | 21.4 | 0.698 | 6 | 31.6 |
| Use of anti-diarrhea medicine | 2 | 9.5 | 0.999 | 3 | 9.1 | 2 | 14.3 | 0.172 | 0 | 0.0 |
| Constipation | 10 | 47.6 | 0.392 | 11 | 33.3 | 7 | 50.0 | 0.472 | 6 | 31.6 |
| Urine incontinence | 14 | 66.7 | 0.775 | 20 | 60.6 | 9 | 64.3 | 0.723 | 10 | 52.6 |
Symptoms of FI
The ability to feel urge sensation before defecating, the duration of being able to control bowels after urge sensation was reached, and the ability to differentiate between different types of stool did not differ between nulliparous and parous women (Table 5). Nevertheless, in comparison to nulliparous women, parous women more often indicated that they experienced fecal urgency in the total group (10.3% versus 15.7%, p = 0.053) and in the healthy subgroup (8.5% versus 14.7%, p = 0.027, Table 5).
Table 5.
Symptoms in nulliparous and parous women.
| Total group
(N = 680) |
Healthy subgroup
(n = 572) |
|||||
|---|---|---|---|---|---|---|
| Nulliparous (n = 291) | p | Parous (n = 389) | Nulliparous (n = 260) | p | Parous (n = 312) | |
| n (%) | n (%) | n (%) | n (%) | |||
| Bristol stool chart | 0.610 | 0.928 | ||||
| 1–2 | 44 (15.1) | 50 (12.9) | 34 (13.1) | 39 (12.5) | ||
| 3–5 | 222 (76.3) | 300 (77.1) | 206 (79.2) | 251 (80.4) | ||
| 6–7 | 25 (8.6) | 39 (10.0) | 20 (7.7) | 22 (7.1) | ||
| Defecation frequency | 0.134 | 0.390 | ||||
| <3 times per week | 35 (12.0) | 37 (9.5) | 31 (11.9) | 28 (9.0) | ||
| Once every two days up to twice a day | 234 (80.4) | 334 (85.9) | 213 (81.9) | 269 (86.2) | ||
| >3 times a day | 22 (7.6) | 18 (4.6) | 16 (6.2) | 15 (4.8) | ||
| Feels urge before defecating | 0.682 | 0.466 | ||||
| Yes | 225 (77.3) | 311 (79.9) | 199 (76.5) | 251 (80.4) | ||
| Sometimes | 57 (19.6) | 66 (17.0) | 52 (20.0) | 50 (16.1) | ||
| No | 9 (3.1) | 12 (3.1) | 9 (3.5) | 11 (3.5) | ||
| Duration of being able to control bowels after urge | 0.792 | 0.987 | ||||
| <1 minute | 35 (12.0) | 51 (13.1) | 29 (11.2) | 37 (11.9) | ||
| <5 minutes | 64 (22.0) | 88 (22.6) | 55 (21.2) | 65 (20.8) | ||
| <10 minutes | 62 (21.3) | 71 (18.3) | 55 (21.2) | 63 (20.2) | ||
| Never needs to rush | 130 (44.7) | 179 (46.0) | 121 (46.5) | 147 (47.1) | ||
| Needs to rush to toilet at least monthly to prevent fecal incontinence | 30 (10.3) | 0.053 | 61 (15.7) | 22 (8.5) | 0.027 | 46 (14.7) |
| Can differentiate between stool types | 0.701 | 0.545 | ||||
| Yes | 239 (82.1) | 316 (81.2) | 215 (82.7) | 256 (82.1) | ||
| Difficult | 35 (12.0) | 44 (11.3) | 29 (11.2) | 30 (9.6) | ||
| No | 17 (5.8) | 29 (7.5) | 16 (6.2) | 26 (8.3) | ||
| Abdominal pain at least once a month | 93 (32.0) | 0.058 | 98 (25.2) | 77 (29.6) | 0.184 | 76 (24.4) |
When asked about their quality of life with reference to bowel habits, most nulliparous and parous women in the total group and the healthy subgroup responded with either good or very good (65.3% versus 61.2%, p = 0.416 and 68.5% versus 65.3%, p = 0.528, respectively). In the comorbidity group only 38.7% of the nulliparous women and 44.9% of the parous women qualified their quality of life with reference to bowel habits as either good or very good (p = 0.831).
The likelihood of fecal urgency at least several times a month following pregnancy and childbirth
Because parous women experienced fecal urgency more often in comparison to nulliparous women (Table 5), we analyzed the influence of pregnancy and childbirth on the likelihood of fecal urgency. In the total group, parous women were 1.6 times more likely to experience fecal urgency than nulliparous women (95% CI, 1.0–2.6, p = 0.043). In the healthy subgroup, parous women were 1.8 times more likely to experience fecal urgency than nulliparous women (95% CI, 1.0–3.1, p = 0.028). In neither of the two groups, however, was the presence of fecal urgency associated with other pregnancy-related and/or childbirth-related factors, that, is parity, mode of delivery, longest straining duration, use of instrument, obstetrical laceration or episiotomy, or birth weight of the largest newborn (data not shown).
Discussion
For the total group and the healthy subgroup we demonstrated that in Dutch women pregnancy and childbirth seemed not to be associated with the prevalence and severity of FI. The prevalence of FI in nulliparous and parous women was comparable despite the higher age and BMI of the latter. Furthermore, parity, the mode of delivery, the duration of the second stage of labor, obstetric laceration or episiotomy, and the birth weight of the newborn, seemed to be less associated with the likelihood of FI, than was previously thought. These findings are in accordance with the results of other studies.21–24 Consistent with our finding that the prevalence of FI was comparable between nulliparous and parous women, we also found that both groups of women qualified the quality of their lives with reference to their bowel habits similarly.
After vacuum- or forceps-assisted vaginal delivery, however, we found that the prevalence of FI tended to be higher in the total group. In line with our results, vacuum- or forceps-assisted vaginal delivery is often found to be a risk factor for developing FI,23–25 the reason being that these modes of delivery increase the risk of anal sphincter ruptures.25
The observation that an obstetric laceration was not associated with FI might be considered controversial, because perineal tears Grade 3 or 4 (e.g. involving the external anal sphincter or extending through the external and internal anal sphincters, respectively) are known to be significant risk factors for developing postpartum FI.23,26 Nevertheless, it is also known that after adjusting for bowel disturbances, such as diarrhea or rectal urgency, comorbidities, and age, obstetric laceration is not an important risk factor for developing late-onset FI.27,28 Further studies are necessary to elucidate the influence of obstetric laceration on FI.
In contrast to the current theory that the prevalence of FI increases with age,14,22,29 we found that age was not significantly associated with the prevalence of FI. We have two explanations for this contradictory finding. First, we used a digital survey that could have led to a possible selection bias toward healthy, elderly women who might be more inclined toward using digital devices than their less-healthy peers. This could have led to an underestimation of the prevalence of FI in the elderly women. Secondly, in an earlier study we demonstrated that the function of the anal-external sphincter continence reflex, which is crucial for fecal continence,30 is not influenced by age.31 We therefore hypothesize that the higher prevalence of FI with advancing age is caused by an increase in comorbidities rather than by the aging process itself.
The prevalence of FI was significantly higher in the comorbidity subgroup than in the total group and healthy subgroup. This could be explained by the fact that the comorbidity subgroup consisted of women who were significantly older, had given birth more often, and had a higher BMI compared to the total group of women. Nevertheless, we showed that the prevalence of FI was not associated with age and parity in both groups. BMI was associated with the prevalence of FI, but BMI did not differ significantly between the total group and the comorbidity subgroup. Therefore, the higher prevalence of FI in the comorbidity group had most likely been caused by the comorbidities.
The Wexner and Vaizey scores might seem to be high for the general Dutch population. Apparently, in the general population not only is the prevalence of FI underdiagnosed, but its severity is underestimated. The high prevalence of constipation and urine incontinence we found in the women with FI is supported by earlier studies that demonstrated that FI often coexists with constipation and urine incontinence.16,32,33
Furthermore, in both the total group and the healthy subgroup, we found that parous women experienced fecal urgency at least several times a month to prevent FI almost twice as often as nulliparous women. This could be a sign of urge FI. If, however, these women did not lose their stool before reaching the toilet, they were classified as fecally continent. Regression analyses showed that the likelihood of fecal urgency seems not to be associated with pregnancy and childbirth-related factors, irrespective of having been pregnant or not. Interestingly, the duration of being able to control bowels after urge sensation had been reached did not differ between nulliparous and parous women.
This difference between parous and nulliparous women in the presence of fecal urgency might result from a different interpretation of fecal urgency. Furthermore, parous women might have a different lifestyle compared to nulliparous women, which perhaps leads to their postponing defecation and subsequently experiencing fecal urgency. These findings, however, require elucidation in a follow-up study with a larger study group.
This study was limited by the use of an anonymous survey. As a consequence, we had no access to the women’s medical records and had to rely on their own memory regarding childbirth-related factors, such as the duration of the second stage of labor, the use of vacuum or forceps during delivery, and whether obstetric laceration had occurred or an episiotomy performed. Furthermore, we had no information on the type of episiotomy, the severity of the obstetric laceration, and the age of the women at childbirth. We were also unable to perform objective tests to assess anorectal function and to diagnose underlying causes of FI. Nevertheless, since FI is still an embarrassing topic, the anonymous survey might make women admit their problems more freely and honestly. Finally, the study was limited by the small number of cesarean sections and women with obstetric lacerations. Currently, we are performing a larger follow-up study in which we do have access to medical records, to further elucidate the influence of obstetric laceration on the prevalence of FI in an otherwise healthy population.
Conclusions
In accordance with our hypothesis, we found that the prevalence and severity of FI did not differ between nulliparous and parous women. We conclude therefore that in Dutch women the association between FI and pregnancy or childbirth seems to be less significant than was previously thought. The prevalence of FI, however, tends to be higher after vacuum- and/or forceps-assisted vaginal deliveries. Furthermore, despite the fact that nulliparous and parous women are able to control their bowels equally long after reaching urge sensation, parous women do experience fecal urgency more often.
Supplemental Material
Supplemental Material for Fecal incontinence and parity in the Dutch population: A cross-sectional analysis by Maxime M van Meegdenburg, Monika Trzpis and Paul MA Broens in United European Gastroenterology Journal
Acknowledgments
Author contributions are as follows: Study concept and design: Broens. Acquisition, analysis, and interpretation of the data: van Meegdenburg, Trzpis, and Broens. Drafting of the manuscript: van Meegdenburg. Critical revision of the manuscript for important intellectual content: Trzpis and Broens
Declaration of conflicting interests
None declared.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
This study was conducted in compliance with requirements of our local Medical Ethics Review Board.
Informed consent
Informed consent was not obtained because the data was collected anonymously in the general Dutch population.
References
- 1.Hall W, McCracken K, Osterweil P, et al. Frequency and predictors for postpartum fecal incontinence. Am J Obstet Gynecol 2003; 188: 1205–1207. [DOI] [PubMed] [Google Scholar]
- 2.Guise JM, Morris C, Osterweil P, et al. Incidence of fecal incontinence after childbirth. Obstet Gynecol 2007; 109: 281–288. [DOI] [PubMed] [Google Scholar]
- 3.Snooks SJ, Setchell M, Swash M, et al. Injury to innervation of pelvic floor sphincter musculature in childbirth. Lancet 1984; 2: 546–550. [DOI] [PubMed] [Google Scholar]
- 4.Griffin KM, O’Herlihy C, O’Connell PR, et al. Combined ischemic and neuropathic insult to the anal canal in an animal model of obstetric-related trauma. Dis Colon Rectum 2012; 55: 32–41. [DOI] [PubMed] [Google Scholar]
- 5.Sultan AH, Stanton SL. Occult obstetric trauma and anal incontinence. Eur J Gastroenterol Hepatol 1997; 9: 423–427. [DOI] [PubMed] [Google Scholar]
- 6.Allen RE, Hosker GL, Smith AR, et al. Pelvic floor damage and childbirth: A neurophysiological study. Br J Obstet Gynaecol 1990; 97: 770–779. [DOI] [PubMed] [Google Scholar]
- 7.MacArthur C, Glazener C, Lancashire R, et al. Faecal incontinence and mode of first and subsequent delivery: A six-year longitudinal study. BJOG 2005; 112: 1075–1082. [DOI] [PubMed] [Google Scholar]
- 8.Perry S, Shaw C, McGrother C, et al. Prevalence of faecal incontinence in adults aged 40 years or more living in the community. Gut 2002; 50: 480–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whitehead WE, Borrud L, Goode PS, et al. Fecal incontinence in US adults: Epidemiology and risk factors. Gastroenterology 2009; 137: 512–517. 517.e1–517.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oberwalder M, Dinnewitzer A, Baig MK, et al. The association between late-onset fecal incontinence and obstetric anal sphincter defects. Arch Surg 2004; 139: 429–432. [DOI] [PubMed] [Google Scholar]
- 11.Fritel X, Ringa V, Varnoux N, et al. Mode of delivery and fecal incontinence at midlife: A study of 2,640 women in the Gazel cohort. Obstet Gynecol 2007; 110: 31–38. [DOI] [PubMed] [Google Scholar]
- 12.Varma MG, Brown JS, Creasman JM, et al. Fecal incontinence in females older than aged 40 years: Who is at risk? Dis Colon Rectum 2006; 49: 841–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bharucha AE, Zinsmeister AR, Locke GR, et al. Risk factors for fecal incontinence: A population-based study in women. Am J Gastroenterol 2006; 101: 1305–1312. [DOI] [PubMed] [Google Scholar]
- 14.Ditah I, Devaki P, Luma HN, et al. Prevalence, trends, and risk factors for fecal incontinence in United States adults, 2005–2010. Clin Gastroenterol Hepatol 2014; 12: 636–643. [DOI] [PubMed] [Google Scholar]
- 15.Rey E, Choung RS, Schleck CD, et al. Onset and risk factors for fecal incontinence in a US community. Am J Gastroenterol 2010; 105: 412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meinds RJ, van Meegdenburg MM, Trzpis M, et al. On the prevalence of constipation and fecal incontinence, and their co-occurrence, in the Netherlands. Int J Colorectal Dis 2017; 32: 475–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Central Bureau for Statistics the Netherlands, www.statline.cbs.nl (accessed 23 September 2016).
- 18.Vaizey CJ, Carapeti E, Cahill JA, et al. Prospective comparison of faecal incontinence grading systems. Gut 1999; 44: 77–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jorge JM, Wexner SD. Etiology and management of fecal incontinence. Dis Colon Rectum 1993; 36: 77–97. [DOI] [PubMed] [Google Scholar]
- 20.Drossman DA, Chang L, Chey W, et al. Rome IV: The functional gastrointestinal disorders, Raleigh, NC: Rome Foundation, 2016. [Google Scholar]
- 21.Gyhagen M, Âkervall S, Milsom I. Clustering of pelvic floor disorders 20 years after one vaginal or one cesarean birth. Int Urogynecol J 2015; 26: 1115–1121. [DOI] [PubMed] [Google Scholar]
- 22.Gyhagen M, Bullarbo M, Nielsen TF, et al. Faecal incontinence 20 years after one birth: A comparison between vaginal delivery and caesarean section. Int Urogynecol J 2014; 25: 1411–1418. [DOI] [PubMed] [Google Scholar]
- 23.Volløyhaug I, Mørkved S, Salvesen Ø, et al. Pelvic organ prolapse and incontinence 15–23 years after first delivery: A cross-sectional study. BJOG 2015; 122: 964–971. [DOI] [PubMed] [Google Scholar]
- 24.MacArthur C, Wilson D, Herbison P, et al. Faecal incontinence persisting after childbirth: A 12 year longitudinal study. BJOG 2013; 120: 169–178; discussion 178–179. [DOI] [PubMed] [Google Scholar]
- 25.Rortveit G, Hannestad YS. Association between mode of delivery and pelvic floor dysfunction. Tidsskr Nor Laegeforen 2014; 134: 1848–1852. [DOI] [PubMed] [Google Scholar]
- 26.Borello-France D, Burgio KL, Richter HE, et al. Fecal and urinary incontinence in primiparous women. Obstet Gynecol 2006; 108: 863–872. [DOI] [PubMed] [Google Scholar]
- 27.Bharucha AE, Rao SS. An update on anorectal disorders for gastroenterologists. Gastroenterology 2014; 146: 37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bharucha AE, Zinsmeister AR, Schleck CD, et al. Bowel disturbances are the most important risk factors for late onset fecal incontinence: A population-based case-control study in women. Gastroenterology 2010; 139: 1559–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pretlove SJ, Radley S, Toozs-Hobson PM, et al. Prevalence of anal incontinence according to age and gender: A systematic review and meta-regression analysis. Int Urogynecol J Pelvic Floor Dysfunct 2006; 17: 407–417. [DOI] [PubMed] [Google Scholar]
- 30.Broens PM, Penninckx FM, Ochoa JB. Fecal continence revisited: The anal external sphincter continence reflex. Dis Colon Rectum 2013; 56: 1273–1281. [DOI] [PubMed] [Google Scholar]
- 31.van Meegdenburg MM, Heineman E, Broens PM. Pudendal neuropathy alone results in urge incontinence rather than in complete fecal incontinence. Dis Colon Rectum 2015; 58: 1186–1193. [DOI] [PubMed] [Google Scholar]
- 32.Sze EH, Barker CD, Hobbs G. A cross-sectional survey of the relationship between fecal incontinence and constipation. Int Urogynecol J 2013; 24: 61–65. [DOI] [PubMed] [Google Scholar]
- 33.Linde JM, Nijman RJM, Trzpis M, et al. Urinary incontinence in the Netherlands: Prevalence and associated risk factors in adults. Neurourol Urodyn 2017; 36: 1519–1528. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Fecal incontinence and parity in the Dutch population: A cross-sectional analysis by Maxime M van Meegdenburg, Monika Trzpis and Paul MA Broens in United European Gastroenterology Journal


