Abstract
Liver resection (LR) is now actively applied to intrahepatic recurrence of liver metastases and hepatocellular carcinoma. Although indications of laparoscopic LR (LLR) have been expanded, there are increased risks of intraoperative complications and conversion in repeat LLR. Controversy still exists for the indication. There are 16 reports of small series to date. These studies generally reported that repeat LLR has better short-term outcomes than open (reduced bleedings, less or similar morbidity and shorter hospital stay) without compromising the long-term outcomes. The fact that complete adhesiolysis can be avoided in repeat LLR is also reported. In the comparison of previous procedures, it is reported that the operation time for repeat LLR was shorter for the patients previously treated with LLR than open. Furthermore, it is speculated that LLR for minor repeat LR of cirrhotic liver can be minimized the deterioration of liver function by LR. However, further experience and evaluation of anatomical resection or resections exposing major vessels as repeat LLR, especially after previous anatomical resection, are needed. There should be a chance to prolong the overall survival of the patients by using LLR as a powerful local therapy which can be applied repeatedly with minimal deterioration of liver function.
Keywords: Hepatocellular carcinoma, Laparoscopic liver resection, Repeat surgery, Metastasis
Core tip: There are 16 reports of repeat laparoscopic liver resection (LLR). They reported that it has better short-term outcomes than open (reduced bleedings, less or similar morbidity and shorter hospital stay). The fact that complete adhesiolysis can be avoided in repeat LLR is also reported. It is speculated that LLR for minor repeat LR of cirrhotic liver can be minimized the deterioration of liver function by LR. Repeated application of LLR as a powerful local therapy, which can be applied repeatedly with minimal deterioration of liver function, could improve the overall survival of the patients.
INTRODUCTION
The neoplastic liver background of hepatocellular carcinoma (HCC) with chronic liver disease (CLD) develops multifocal and metachronous liver tumors repeatedly. Also, metastases of various tumors can occur repeatedly in the liver. Repeat treatments for HCC and metastases, especially of colorectal cancer, are often needed.
Nowadays, liver resection (LR) is often performed to such lesions, if they are resectable without other uncontrollable/distant disease, and the reports for repeat LR has increased[1-4]. Furthermore, indications of laparoscopic LR (LLR) are expanding with the accumulation of experiences and technical/instrumental developments[5-8]. In LLR, surgeons should overcome restricted manipulation, lack of tactile sensation and three-dimensional (3D) vision (which is recently partially resolved by 3D-laparoscope), and disorientation from the lack of an overview of operative field, during liver mobilization, pedicle control and parenchymal transection, which is a trade-off to magnified fine local view[9,10]. Postoperative adhesions with the need for adhesiolysis are known to increase the operation time of subsequent surgeries and the incidence of bowel injury[11,12]. Therefore, increased rates of complications and conversion from laparoscopic to open surgery had been reported in repeat laparoscopic surgery[13]. A previous history of surgery had been among the contraindications for laparoscopic surgery. However, many laparoscopic procedures with previous surgical history, such as cholecystectomy[12,13], appendectomy[14], colectomy[15], and gastrectomy[16], can be performed nowadays with technical and instrumental improvements. On the other, LLR itself remains a demanding procedure and the indications of repeat LLR are under discussion. Adequate dissection of adhesion and mobilization of the involved liver should be performed before repeat LR. Adhesion can disrupt the dissection of hilar area and hepatoduodenal ligament, which is often crucial in LR. The deformity of the liver and surrounding scars and adhesion makes the localization of tumors and the important structures (vessels) difficult. The fact that liver capsule bleeds easily during adhesiolysis and mobilization leads to increase the intraoperative bleeding and create a suboptimal operative field[17]. These changes after previous surgery can increase the risks of intraoperative injury to vascular or biliary structures.
STUDIES OF REPEAT LLR
Only 16 reports of small series were found out under Medline-search with the words “repeat” and “laparoscopic liver resection” and their re-quotations[18-33] (Table 1), although they are gradually increasing. Belli et al[20] reported that LLR with its magnifies view facilitates more meticulous dissection of adhesions strained by the pneumoperitoneum. An additional possible advantage of repeat LLR is reported that complete adhesiolysis can be avoided when the adhesion does not affect the current operative procedure[24,29]. Generally, these studies reported that repeat LLR has better short-term outcomes (similar or longer operation time, reduced bleedings, less blood transfusion, less or similar morbidity and shortened hospital stay) with the comparable long-term outcomes. Each study concluded that repeat LLR is feasible and safe for selected patients, although those studies are the mixtures of the patients with HCC and metastases. The settings of the patients with HCC and metastases are different in LR. The patients with metastases sometimes undergo major LR with the handling of Glissonian pedicles on the soft liver with congestion and/or steatosis. Minor LR on the fibrous hard liver with poor functional reserve and surrounding collateral vessels is often performed for HCC patients. Five studies of repeat LLR, which only include HCC patients[20,24,26,27,31], reported the outcomes for the series of 12, 6, 3, 20 and 8 patients. The conclusions of all studies are that repeat LLR for recurrent HCC in CLD backgrounds is a safe and feasible procedure. It is mentioned that the adhesiolysis was easier and the operation time was shorter in repeat LLR for the patients with previous LLR compared to open LR[20]. Belli et al[20] referred the advantages of LLR for the management during the long history with repeat oncogenesis in cirrhotic patients. Kanazawa et al[27] mentioned that the complication rate and the hospital stay had been decreased in their institute by the introduction of LLR for recurrent HCC patients.
Table 1.
Summary of previous reports of repeat laparoscopic liver resection
| n | Disease | Previous LR (open:lap) | Procedure | Bleeding (mL) | Operating time (min) | Conversion (n) | Postoperative hospital stay (d) | Morbidity | Mortality | Ref. |
| 12 | HCC | 4:8 | LLS (n = 5), Pt (n = 4), Seg (n = 3) | 297 ± 134 272.2 ± 120 | 114.4 ± 11.0 63.9 ± 13.3 | 1 | 7.4 ± 2.5 6.2 ± 3.0 | 26.60% | 0% | [20] |
| 2 | Met | ND | ND | ND | ND | ND | ND | ND | ND | [21] |
| 6 | HCC | 3:3 (Lap RFA, n = 2) | LLS (n = 2), Pt (n = 4) | 283.3 ± 256.3 | 140.8 ± 35.7 | 0 | 5.67 ± 1.63 | 16.7% | 0% | [24] |
| 76 | Met (n = 63), HCC (n = 3), others (n = 10) | 28:44 | LLS (n = 4), Pt, seg (n = 53), above-seg (n = 19) | 300 (0–5000) | 180 (80–570) | 8 | 6 (2–42) | 26% | 0% | [23] |
| 4 | HCC (n = 3), Met (n =1) | 0:4 | LLS (n = 1), Pt (n = 3) | 481.7 ± 449.5 | 312.3 ± 158.4 | 1 | 10.6 ± 7.4 | 23.4% | 0% | [22] |
| 3 | HCC | 0:3 | ND | 281.3 (mean) | 264.6 (mean) | 0 | 8.6 (mean) | 0% | [26] | |
| 17 | ND | ND | ND | ND | ND | ND | ND | ND | ND | [25] |
| 20 | HCC | 15:5 | Pt | 78 (1–1500) | 239 (69–658) | 2 (HALS) | 9 (5–22) | 5% | 0% | [27] |
| 20 | HCC (n = 2), Met (n = 16), others (n = 2) | 0:20 | Minor (n = 14), major (n = 6) | 400 (IQR 150-200 mL) | 285 (IQR 195-360) | 3 | 4 (1-57) | 10% | 0% | [30] |
| 12 | HCC (n = 8), Met (n = 2), others (n = 2) | 8:4 | Pt (n = 9), Subseg (n = 3) | 50 (NC–840) | 301 (104–570) | 0 | 12 (9–30) | 0% | 0% | [29] |
| 11 | HCC | 6:5 | LLS = 2 Subseg = 9 | 100 (50-500) | 200 (131-352) | 0 | 6 (3-17) | 18.2% | 0% | [33] |
| 27 | Met | ND | Major = 25 Minor = 2 | ND (4 patients received transfusion) | 252.5 (180-300) | 1 | 9 (IQR 8-18) | 48.1% | 0% | [32] |
| 8 | HCC | 6:2 | Sec = 2 Seg = 2 Subseg = 4 | 200 (30-5000) | 343 (120-530) | 1 | 3.5 (3-8) | 12.5% | 0% | [31] |
| 20 | HCC (n = 15) Met (n = 5) | 12:8 | Anatomical = 1 Non-anatomical = 19 | 159 +/- 256 | 225 +/- 85 | 1 | 14.2 +/- 5.4 | 0% | 0% | [19] |
| 33 | HCC and combined (n = 18) Met (n = 15) | 21:12 | Anatomical = 11 Non-anatomical = 22 | 30 (NC-1012) | 217 (43-356) | 0 | 6.5 (3-47) | 6.1% | 3% | [18] |
Data are expressed as median (range) or mean ± SD, unless stated otherwise. In the paper from Belli, operation time, bleeding and postoperative hospital stay are described separately for patients whose previous hepatectomy was open (upper) or laparoscopic (lower). LLR: Laparoscopic liver resection; LR: Liver resection; HCC: Hepatocellular carcinoma; LLS: Left lateral sectorectomy; Met: Metastasis; Minor: Resection of 2 segments or less; Major: Resection of 3 segments or more; ND: Not documented; Pt: Partial resection; Sec: Sectionectomy; Seg: Segmentectomy; Subseg: Subsegmentectomy; IQR: Interquartile range; NC: Not countable.
LLR CHARACTERISTICS
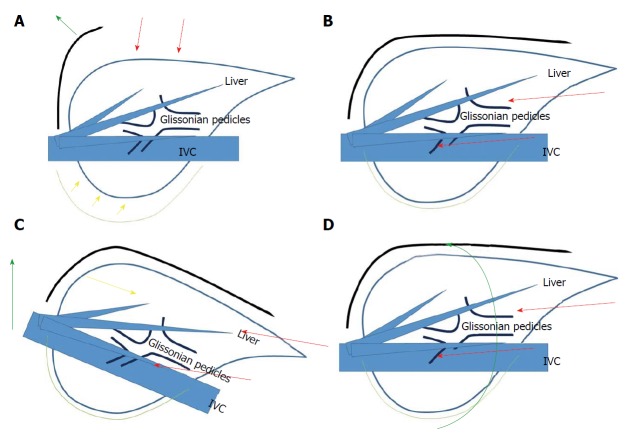
It is previously reported that LLR is especially beneficial for severe CLD patients[34]. LLR with minimal laparotomy and mobilization can minimize the destruction of blood and lymphatic collaterals, as well as the parenchymal injury by compression. It reduces postoperative ascites and liver failure for CLD patients[35]. In LR, resection of the liver inside the subphrenic rib cage is performed. The cage is opened with a big subcostal incision and then the liver is picked up with mobilization in open LR. On the other, laparoscope and forceps intrude into the cage directly from the caudal direction (“Caudal approach”[36-38], Figure 1) and perform LR in the small targeted area without damages to the surrounding area in LLR. LLR also facilitates the usage of postural change and the gravity for handling organs/tumors, since the same surgical view under position changes can be established by the adjustments of laparoscope’s positioning and rotation. That reduces compression on the liver during surgery. Our previous report of the caudal approach posterior sectionectomy in the left lateral position[36] posed the novel concept of “caudal approach” in LLR. Although the supine to semi-lateral positioning had been employed for the other resections, the transection plane of posterior sectionectomy was horizontal and gravity obstructs the exposure of the plane in the supine position. A clear view from the caudal direction and an easy access to postural changes is among the advantages of LLR (Figure 1). We perform parenchymal transection prior to mobilization in the left lateral position for laparoscopic posterior sectionectomy. It facilitates exposure of the cutting plane during the transection in caudal-to-cranial one direction. The transection plane is well-opened between the retroperitoneal-fixed posterior section and the remnant liver falling down to left by gravity. Moreover, the resection of segment(s) 7 should be performed in the deeper and smaller cranial subphrenic space and S6 is an obstacle under the laparoscopic caudal view even in the left lateral position. Semi-prone position with only partial dissection of the retroperitoneum is employed for those resections[39]. Our key aim in LLR is to carry out minimal dissection around the liver with the intrusion and manipulation of laparoscope and forceps to the small target area under postural changes. In the same context, repeat LLR requires smaller (than open) working space between adhesions. Direct approach to the tumor after minimal adhesiolysis for the space where laparoscope and forceps can intrude and do manipulation can be allowed especially in repeat small LLR[23,24,29]. That is why some studies showed that operation time and bleeding amount were similar in primary and repeat LLR[18,29]. The operation time and blood loss are usually much longer and larger in open repeat than open primary LR. Operation time and bleeding amount of repeat partial resection could be reduced under laparoscopic approach.
Figure 1.
Schema of open liver resection (A), laparoscopic liver resection (B), position change in laparoscopic liver resection (tilting the bed for head-up position, C) and position change in laparoscopic liver resection (rotation from supine to semi-prone position, D). Red arrows indicate the directions of the view and manipulation in each approach. A: In the open approach, the subcostal cage containing the liver is opened with a large subcostal incision, and instruments are used to lift the costal arch up. The liver is dissected and mobilized (picked up) from the retroperitoneum; B: In the laparoscopic caudal approach, the laparoscope and forceps are placed into the subcostal cage from caudal direction, and surgery is performed with minimal alteration and destruction of the associated structures; C and D: In the laparoscopic approach, the same surgical view under position changes (tilting the bed and rotation of the patient’s body), acquired by the adjustments of laparoscope’s positioning and rotation, allows for handling large-volume liver/tumor by postural changes.
OUR EXPERIENCES AND FUTURE PERSPECTIVES OF REPEAT LLR
Most reported cases of repeat LLR underwent minor resection of HCC with CLD, as mentioned above. The impact of alterations from the previous surgery on hepatic parenchyma and intrahepatic structure could be smaller in such cases. There were three repeat cases with anatomical resection or resections exposing major vessels (including S8 segmentectomy after 4-times LLR[40]) after previous anatomical resection who developed bile leakage and > 30 d hospital stay, among our 33 repeat and 12 three or more-time repeat LLR cases. Anatomical alterations surrounded by the scars and adhesions on major vessel structures could have big impacts on subsequent anatomical resection or resections exposing major vessels, experiences and evaluations of such setting of repeat LLR are required for the settlement (Table 2).
Table 2.
The summary of present status and future perspectives of repeat laparoscopic liver resection
| Present status |
| There are 16 reports of small series. Controversy still exists in the indication of repeat LLR |
| These studies generally reported that it has better short-term outcomes without compromising the long-term outcomes (similar or longer operation time, reduces bleedings, reduced blood transfusion rate, less or similar morbidity and shorter hospital stay) |
| It facilitates more meticulous dissection of adhesions strained by the pneumoperitoneum using magnified laparoscopic view |
| Complete adhesiolysis can be avoided when the adhesion does not affect the current operative procedure |
| Operation time was shorter and the adhesiolysis was easier for the patients previously treated with LLR than open LR |
| It requires smaller (than open) working space between adhesions (this fact allows for minimal adhesiolysis, and operation time and bleeding amount were similar in primary and repeat LLR, although those from open LR are longer and increased) |
| Future perspectives |
| Further evaluations of anatomical resection or resections exposing major vessels after previous anatomical resection are needed |
| One of the possible advantages for minor repeat LR of CLD liver is that the deterioration of liver function can be minimized |
| It could prolong the overall survival of the HCC patients with CLD as a powerful local therapy which can be applied repeatedly with minimal deterioration of liver function |
LLR: Laparoscopic liver resection; LR: Liver resection; HCC: Hepatocellular carcinoma; CLD: Chronic liver disease.
Footnotes
Conflict-of-interest statement: Morise Z declares no conflicts of interest related to this publication.
Manuscript source: Invited manuscript
Peer-review started: March 27, 2018
First decision: April 13, 2018
Article in press: May 30, 2018
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Aurello P, Bian AZL, Donadon M S- Editor: Ji FF L- Editor: A E- Editor: Tan WW
References
- 1.Petrowsky H, Gonen M, Jarnagin W, Lorenz M, DeMatteo R, Heinrich S, Encke A, Blumgart L, Fong Y. Second liver resections are safe and effective treatment for recurrent hepatic metastases from colorectal cancer: a bi-institutional analysis. Ann Surg. 2002;235:863–871. doi: 10.1097/00000658-200206000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wanebo HJ, Chu QD, Avradopoulos KA, Vezeridis MP. Current perspectives on repeat hepatic resection for colorectal carcinoma: a review. Surgery. 1996;119:361–371. doi: 10.1016/s0039-6060(96)80133-4. [DOI] [PubMed] [Google Scholar]
- 3.Morise Z, Sugioka A, Fujita J, Hoshimoto S, Kato T, Hasumi A, Suda T, Negi H, Hattori Y, Sato H, et al. Does repeated surgery improve the prognosis of colorectal liver metastases? J Gastrointest Surg. 2006;10:6–11. doi: 10.1016/j.gassur.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 4.Itamoto T, Nakahara H, Amano H, Kohashi T, Ohdan H, Tashiro H, Asahara T. Repeat hepatectomy for recurrent hepatocellular carcinoma. Surgery. 2007;141:589–597. doi: 10.1016/j.surg.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen KT, Gamblin TC, Geller DA. World review of laparoscopic liver resection-2,804 patients. Ann Surg. 2009;250:831–841. doi: 10.1097/SLA.0b013e3181b0c4df. [DOI] [PubMed] [Google Scholar]
- 6.Buell JF, Thomas MT, Rudich S, Marvin M, Nagubandi R, Ravindra KV, Brock G, McMasters KM. Experience with more than 500 minimally invasive hepatic procedures. Ann Surg. 2008;248:475–486. doi: 10.1097/SLA.0b013e318185e647. [DOI] [PubMed] [Google Scholar]
- 7.Wakabayashi G, Cherqui D, Geller DA, Buell JF, Kaneko H, Han HS, Asbun H, O Rourke N, Tanabe M, Koffron AJ, et al. Recommendations for laparoscopic liver resection: a report from the second international consensus conference held in Morioka. Ann Surg. 2015;261:619–629. doi: 10.1097/SLA.0000000000001184. [DOI] [PubMed] [Google Scholar]
- 8.Morise Z, Wakabayashi G. First quarter century of laparoscopic liver resection. World J Gastroenterol. 2017;23:3581–3588. doi: 10.3748/wjg.v23.i20.3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buell JF, Thomas MJ, Doty TC, Gersin KS, Merchen TD, Gupta M, Rudich SM, Woodle ES. An initial experience and evolution of laparoscopic hepatic resectional surgery. Surgery. 2004;136:804–811. doi: 10.1016/j.surg.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 10.Vibert E, Perniceni T, Levard H, Denet C, Shahri NK, Gayet B. Laparoscopic liver resection. Br J Surg. 2006;93:67–72. doi: 10.1002/bjs.5150. [DOI] [PubMed] [Google Scholar]
- 11.Beck DE, Ferguson MA, Opelka FG, Fleshman JW, Gervaz P, Wexner SD. Effect of previous surgery on abdominal opening time. Dis Colon Rectum. 2000;43:1749–1753. doi: 10.1007/BF02236862. [DOI] [PubMed] [Google Scholar]
- 12.Karayiannakis AJ, Polychronidis A, Perente S, Botaitis S, Simopoulos C. Laparoscopic cholecystectomy in patients with previous upper or lower abdominal surgery. Surg Endosc. 2004;18:97–101. doi: 10.1007/s00464-003-9001-4. [DOI] [PubMed] [Google Scholar]
- 13.Wiebke EA, Pruitt AL, Howard TJ, Jacobson LE, Broadie TA, Goulet RJ Jr, Canal DF. Conversion of laparoscopic to open cholecystectomy. An analysis of risk factors. Surg Endosc. 1996;10:742–745. doi: 10.1007/BF00193048. [DOI] [PubMed] [Google Scholar]
- 14.Wu JM, Lin HF, Chen KH, Tseng LM, Tsai MS, Huang SH. Impact of previous abdominal surgery on laparoscopic appendectomy for acute appendicitis. Surg Endosc. 2007;21:570–573. doi: 10.1007/s00464-006-9027-5. [DOI] [PubMed] [Google Scholar]
- 15.Law WL, Lee YM, Chu KW. Previous abdominal operations do not affect the outcomes of laparoscopic colorectal surgery. Surg Endosc. 2005;19:326–330. doi: 10.1007/s00464-004-8114-8. [DOI] [PubMed] [Google Scholar]
- 16.Nunobe S, Hiki N, Fukunaga T, Tokunaga M, Ohyama S, Seto Y, Yamaguchi T. Previous laparotomy is not a contraindication to laparoscopy-assisted gastrectomy for early gastric cancer. World J Surg. 2008;32:1466–1472. doi: 10.1007/s00268-008-9542-8. [DOI] [PubMed] [Google Scholar]
- 17.Szomstein S, Lo Menzo E, Simpfendorfer C, Zundel N, Rosenthal RJ. Laparoscopic lysis of adhesions. World J Surg. 2006;30:535–540. doi: 10.1007/s00268-005-7778-0. [DOI] [PubMed] [Google Scholar]
- 18.Ome Y, Hashida K, Yokota M, Nagahisa Y, Yamaguchi K, Okabe M, Kawamoto K. The feasibility and efficacy of pure laparoscopic repeat hepatectomy. Surg Endosc. 2018 doi: 10.1007/s00464-018-6066-7. [DOI] [PubMed] [Google Scholar]
- 19.Noda T, Eguchi H, Wada H, Iwagami Y, Yamada D, Asaoka T, Gotoh K, Kawamoto K, Takeda Y, Tanemura M, et al. Short-term surgical outcomes of minimally invasive repeat hepatectomy for recurrent liver cancer. Surg Endosc. 2018;32:46–52. doi: 10.1007/s00464-017-5632-8. [DOI] [PubMed] [Google Scholar]
- 20.Belli G, Cioffi L, Fantini C, D’Agostino A, Russo G, Limongelli P, Belli A. Laparoscopic redo surgery for recurrent hepatocellular carcinoma in cirrhotic patients: feasibility, safety, and results. Surg Endosc. 2009;23:1807–1811. doi: 10.1007/s00464-009-0344-3. [DOI] [PubMed] [Google Scholar]
- 21.Nguyen KT, Laurent A, Dagher I, Geller DA, Steel J, Thomas MT, Marvin M, Ravindra KV, Mejia A, Lainas P, et al. Minimally invasive liver resection for metastatic colorectal cancer: a multi-institutional, international report of safety, feasibility, and early outcomes. Ann Surg. 2009;250:842–848. doi: 10.1097/SLA.0b013e3181bc789c. [DOI] [PubMed] [Google Scholar]
- 22.Ahn KS, Han HS, Yoon YS, Cho JY, Kim JH. Laparoscopic liver resection in patients with a history of upper abdominal surgery. World J Surg. 2011;35:1333–1339. doi: 10.1007/s00268-011-1073-z. [DOI] [PubMed] [Google Scholar]
- 23.Shafaee Z, Kazaryan AM, Marvin MR, Cannon R, Buell JF, Edwin B, Gayet B. Is laparoscopic repeat hepatectomy feasible? A tri-institutional analysis. J Am Coll Surg. 2011;212:171–179. doi: 10.1016/j.jamcollsurg.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 24.Hu M, Zhao G, Xu D, Liu R. Laparoscopic repeat resection of recurrent hepatocellular carcinoma. World J Surg. 2011;35:648–655. doi: 10.1007/s00268-010-0919-0. [DOI] [PubMed] [Google Scholar]
- 25.Cannon RM, Brock GN, Marvin MR, Buell JF. Laparoscopic liver resection: an examination of our first 300 patients. J Am Coll Surg. 2011;213:501–507. doi: 10.1016/j.jamcollsurg.2011.04.032. [DOI] [PubMed] [Google Scholar]
- 26.Tsuchiya M, Otsuka Y, Maeda T, Ishii J, Tamura A, Kaneko H. Efficacy of laparoscopic surgery for recurrent hepatocellular carcinoma. Hepatogastroenterology. 2012;59:1333–1337. doi: 10.5754/hge12302. [DOI] [PubMed] [Google Scholar]
- 27.Kanazawa A, Tsukamoto T, Shimizu S, Kodai S, Yamamoto S, Yamazoe S, Ohira G, Nakajima T. Laparoscopic liver resection for treating recurrent hepatocellular carcinoma. J Hepatobiliary Pancreat Sci. 2013;20:512–517. doi: 10.1007/s00534-012-0592-9. [DOI] [PubMed] [Google Scholar]
- 28.Montalti R, Berardi G, Laurent S, Sebastiani S, Ferdinande L, Libbrecht LJ, Smeets P, Brescia A, Rogiers X, de Hemptinne B, et al. Laparoscopic liver resection compared to open approach in patients with colorectal liver metastases improves further resectability: Oncological outcomes of a case-control matched-pairs analysis. Eur J Surg Oncol. 2014;40:536–544. doi: 10.1016/j.ejso.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 29.Isetani M, Morise Z, Kawabe N, Tomishige H, Nagata H, Kawase J, Arakawa S. Pure laparoscopic hepatectomy as repeat surgery and repeat hepatectomy. World J Gastroenterol. 2015;21:961–968. doi: 10.3748/wjg.v21.i3.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shelat VG, Serin K, Samim M, Besselink MG, Al Saati H, Gioia PD, Pearce NW, Abu Hilal M. Outcomes of repeat laparoscopic liver resection compared to the primary resection. World J Surg. 2014;38:3175–3180. doi: 10.1007/s00268-014-2728-3. [DOI] [PubMed] [Google Scholar]
- 31.Goh BKP, Teo JY, Chan CY, Lee SY, Cheow PC, Chung AYF. Laparoscopic repeat liver resection for recurrent hepatocellular carcinoma. ANZ J Surg. 2017;87:E143–E146. doi: 10.1111/ans.13628. [DOI] [PubMed] [Google Scholar]
- 32.Hallet J, Sa Cunha A, Cherqui D, Gayet B, Goéré D, Bachellier P, Laurent A, Fuks D, Navarro F, Pessaux P; French Colorectal Liver Metastases Working Group, Association Française de Chirurgie. Laparoscopic Compared to Open Repeat Hepatectomy for Colorectal Liver Metastases: a Multi-institutional Propensity-Matched Analysis of Short- and Long-Term Outcomes. World J Surg. 2017;41:3189–3198. doi: 10.1007/s00268-017-4119-z. [DOI] [PubMed] [Google Scholar]
- 33.Chan AC, Poon RT, Chok KS, Cheung TT, Chan SC, Lo CM. Feasibility of laparoscopic re-resection for patients with recurrent hepatocellular carcinoma. World J Surg. 2014;38:1141–1146. doi: 10.1007/s00268-013-2380-3. [DOI] [PubMed] [Google Scholar]
- 34.Morise Z, Sugioka A, Kawabe N, Umemoto S, Nagata H, Ohshima H, Kawase J, Arakawa S, Yoshida R. Pure laparoscopic hepatectomy for hepatocellular carcinoma patients with severe liver cirrhosis. Asian J Endosc Surg. 2011;4:143–146. doi: 10.1111/j.1758-5910.2011.00081.x. [DOI] [PubMed] [Google Scholar]
- 35.Morise Z, Ciria R, Cherqui D, Chen KH, Belli G, Wakabayashi G. Can we expand the indications for laparoscopic liver resection? A systematic review and meta-analysis of laparoscopic liver resection for patients with hepatocellular carcinoma and chronic liver disease. J Hepatobiliary Pancreat Sci. 2015;22:342–352. doi: 10.1002/jhbp.215. [DOI] [PubMed] [Google Scholar]
- 36.Tomishige H, Morise Z, Kawabe N, Nagata H, Ohshima H, Kawase J, Arakawa S, Yoshida R, Isetani M. Caudal approach to pure laparoscopic posterior sectionectomy under the laparoscopy-specific view. World J Gastrointest Surg. 2013;5:173–177. doi: 10.4240/wjgs.v5.i6.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soubrane O, Schwarz L, Cauchy F, Perotto LO, Brustia R, Bernard D, Scatton O. A Conceptual Technique for Laparoscopic Right Hepatectomy Based on Facts and Oncologic Principles: The Caudal Approach. Ann Surg. 2015;261:1226–1231. doi: 10.1097/SLA.0000000000000737. [DOI] [PubMed] [Google Scholar]
- 38.Wakabayashi G, Cherqui D, Geller DA, Han HS, Kaneko H, Buell JF. Laparoscopic hepatectomy is theoretically better than open hepatectomy: preparing for the 2nd International Consensus Conference on Laparoscopic Liver Resection. J Hepatobiliary Pancreat Sci. 2014;21:723–731. doi: 10.1002/jhbp.139. [DOI] [PubMed] [Google Scholar]
- 39.Morise Z. Laparoscopic liver resection for posterosuperior tumors using caudal approach and postural changes: A new technical approach. World J Gastroenterol. 2016;22:10267–10274. doi: 10.3748/wjg.v22.i47.10267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morise Z, Isetani M, Kawabe N, Tomishige H, Nagata H, Arakawa S, Ikeda M, Kamio K. Case report of the fourth laparoscopic liver resection and review of repeat laparoscopic resection for recurrent hepatocellular carcinoma in cirrhotic liver. Hepatoma Res. 2016;2:253–258. [Google Scholar]