Abstract
Hepatitis B represents a global health threat because its chronic course and sequelae contribute to a high morbidity and mortality. Hepatitis B virus (HBV) infection can be controlled by vaccines, antiviral treatment, and by interrupting transmission. Rare vaccine escape mutants are serious because they eliminate vaccine protection. Here, we present a 74-year-old vaccinated patient with HBV reactivation 11 years after kidney transplantation. The patient was HBV-positive but HBsAg-negative prior to vaccination 6 years before transplantation. The reactivated virus was HBV genotype F3 with vaccine escape mutations G145R, P120Q, and Q129P. The patient was successfully treated with entecavir. The epidemiological reasons for this subgenotype, which is extremely rare in Western Europe, were unclear. This case illustrates that second-generation vaccines are not always effective in a specific group of patients.
Keywords: Entecavir, Hepatitis B virus, Subgenotype F3, Kidney transplantation, Vaccine escape mutant G145R
Core tip:We report the first documented case of hepatitis B virus (HBV) subgenotype F3 reactivation with vaccine escape mutations in a patient after kidney transplantation. We successfully treated this patient with entecavir. This case illustrates a specific clinical situation in which the current World Health Organization HBV vaccine may be unsuccessful, and third generation vaccines should be considered.
INTRODUCTION
Hepatitis B virus (HBV) is an enveloped virus of the Hepadnaviridae family. HBV is highly hepatotropic and is transmitted parenterally. There are 111.2 million cases of hepatitis[1] and 686000 hepatitis-related deaths reported worldwide each year. Long-term sequelae of hepatitis include liver cirrhosis and liver cell carcinoma, and these are responsible for the majority of hepatitis related deaths[2]. HBV has at least ten different genotypes, each with a specific geographic pattern[3].
There is no cure for chronic HBV infection. Antiviral agents are able to suppress viral replication but have to be taken permanently to prevent reactivation. A plasma-derived vaccine (first generation) was developed in 1982 and was replaced in 1986 by a recombinant monovalent yeast-derived vaccine (second generation). Global prevention strategies include vaccination at birth and during early childhood. However, 5%-10%of immunocompetent vaccines do not induce protective neutralizing antibody production (more so in immunodeficient patients)[4].
Immunization with second-generation vaccines relies on neutralizing antibody production against the “a”determinant, a hydrophilic antigenic domain (residues 100-170) of the HBV surface (S) protein. Specific single mutations, such as G145R, disrupt the structure of this epitope domain so that neutralizing antibodies (anti-HBs) cannot recognize it, thereby eliminating protection[5].
In this study, we report a case of HBV subgenotype F3 reactivation after kidney transplantation. Our findings highlight that current vaccines are not always effective against mutated HBV. Furthermore, current vaccines do not confer protection against HBV subgenotype F3, which is distantly related to those subgenotypes that current vaccines are based upon.
CASE REPORT
A 74-year-old patient was referred to the University Hospital of Bonn with acute hepatitis in March 2015. He initially presented with loss of appetite and acute watery diarrhea for 7 d. He was a frail patient, requiring longterm level III care. Six weeks prior to admission he had slightly elevated alanine aminotransferase (ALT) levels (78 U/L) due to a urinary tract infection.
His medical history included chronic kidney failure due to tuberculous empyema, and the left kidney was resected in 1965. In May 1996, end-stage renal disease was diagnosed and hemodialysis was started. Prior to dialysis, chronic HBV infection had been diagnosed with partial seroconversion (anti-HBc and anti-HBe-positive and anti-HBs-negative). In November 1995, he tested positive for the HBV virus surface antigen (HBsAg). HBsAg declined to borderline levels in July 1996; and in May 1996, only low levels of HBV DNA viremia (9 pg/mL) were detected. Tests for HBs-Ag and HBV DNA have been repeatedly negative since July 1996. Two years later, seroconversion to anti-HBs had still not occurred, and the patient was repeatedly vaccinated with GenHBVax (January 1998, May 1998, and July 2002), which stabilized anti-HBs levels (Figure 1).
Figure 1.
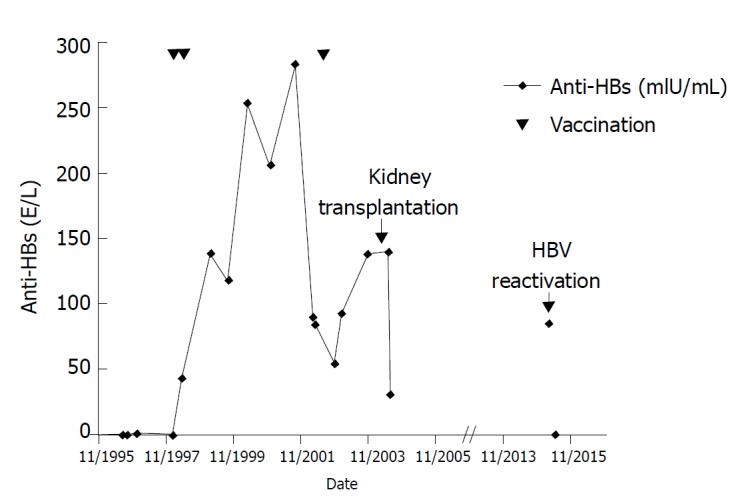
Course of anti-HBs levels since diagnosis of hepatitis B virus infection in 1995. Vaccine was administered in January 1998, May 1998, and July 2002. Kidney transplantation and hepatitis B virus reactivation are indicated.
In June 2004, he underwent cadaveric kidney transplantation and received three immunosuppressive treatments: sirolimus, tacrolimus, and methylprednisolone. This treatment was discontinued several months later because of severe side effects and was converted to 25 mg azathioprine every other day in combination with daily administration of 300 mg allopurinol plus 4 mg prednisolone with close lymphocyte monitoring. Permanent antiviral treatment was not administered. After the immunosuppressant regimen was changed, graft function remained stable with an eGFR (MDRD) grade of IIIb A1.
At admission (March 2015), his liver enzyme levels were massively elevated: ALT, 1462 U/L; aspartate aminotransferase (AST), 1459 U/L; gamma glutamyltransferase 407 U/L; lactate dehydrogenase (LDH), 616 U/L; and bilirubin, 5 mg/dL. Creatinine was elevated to 2.9 mg/dL (eGFR (MDRD) 25 mL/min). Ultrasound and CT scans excluded a tumor, thrombosis, or abscess. Serologic hepatotropic virus evaluation revealed highly replicative HBV infection with 22.5 million IU/mL HBV DNA and positive results for HBsAg (79.11 IU/mL) and anti-HBs (85.2 mIU/mL). Anti-HBe tests were also positive, while anti-HBc IgM tests were negative. Infection with hepatitis C/D viruses, CMV, and HIV was excluded. Markers of past hepatitis A/E virus, HSV, and Epstein Barr virus (EBV) infections were detected. A liver biopsy showed acute hepatitis with multiple disseminated acidophilic single cell necroses, pericentral lipofuscinosis, vacuolar lipid droplets, and minimal periportal fibrosis. The latter was interpreted as chronic toxic damage (Figure 2).
Figure 2.
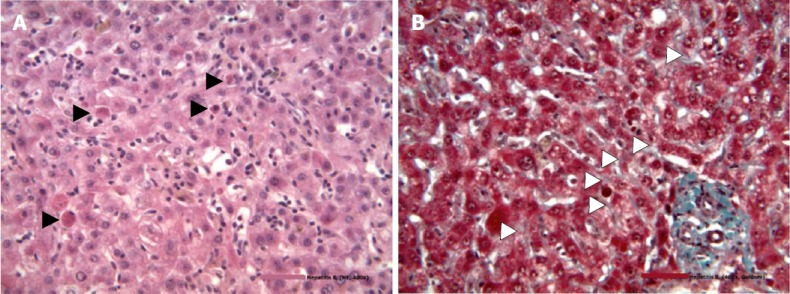
Hematoxylin and eosin stain of a liver specimen from the patient described in this study showing necrosis of hepatocytes (black triangles) without infiltrations and trichrome stain for fibrosis. A: Necrosis of hepatocytes; B: Trichromestain for fibrosis. Periportal fibrosis is indicated by white triangles.
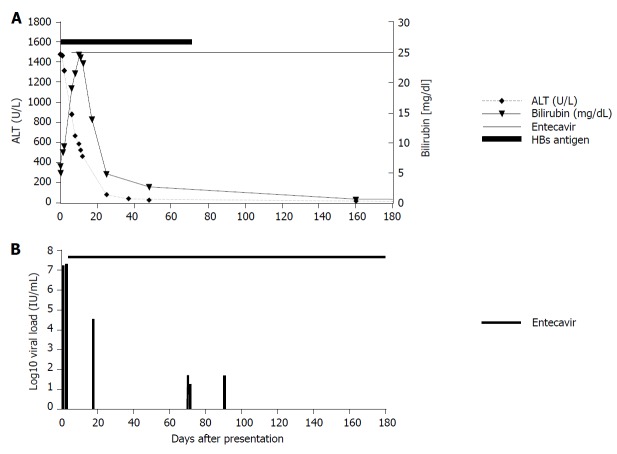
Aminotransaminase levels decreased slightly, but bilirubin peaked at 24 mg/mL 11 d after presentation. Because hepatitis B had reactivated despite high levels of anti-HBs antibodies, we initiated further analyses. Full genome analysis revealed the virus to be subgenotype F3 of genotype F (Figure 3). HBV genotype F is endemic in South America but rare in Europe. There were no reported contacts to South America, Spain, or any other epidemiological links to regions endemic for genotype F. The patient had received blood transfusions following a traffic accident with bilateral hip fracture requiring surgical procedures back in the 1960s, which may represent potential transmission routes. After kidney transplantation and revisions to the hip prosthesis, only minor surgical wound care interventions had been performed before presentation.
Figure 3.
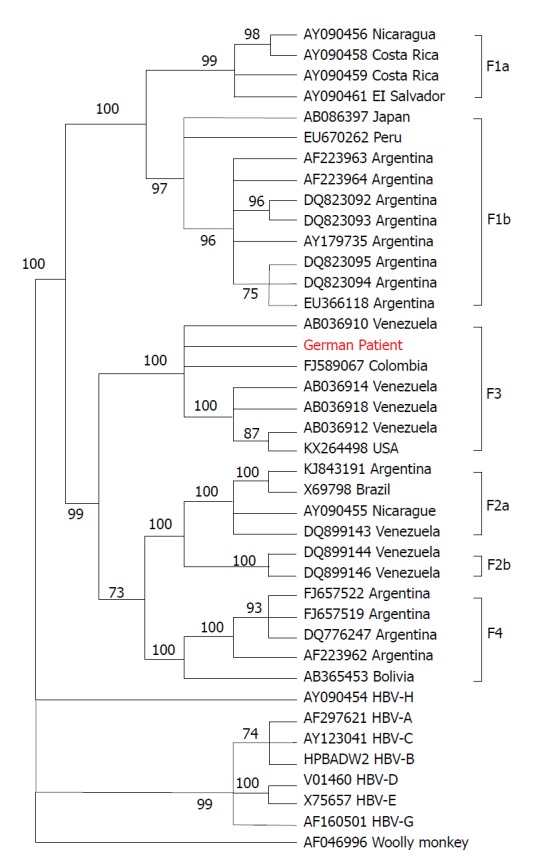
Phylogenetic tree of hepatitis B virus based on complete hepatitis B virus genomes. The tree was constructed using the MEGAv6.0 software package[29], (http//:www.megasoftware.net), with the neighbor-joining method with p-distance and 1000 bootstrap replicates. Bootstrap values of at least 70 are shown at the nodes. Phylogenetic analysis was performed using reference sequences from GenBank, indicated in the tree by their GenBank accession numbers. Country of virus origin is included for all genotype F sequences. Woolly monkey HBV was used as the outgroup. The sequence from the patient described in this study is presented in red. HBV: Hepatitis B virus.
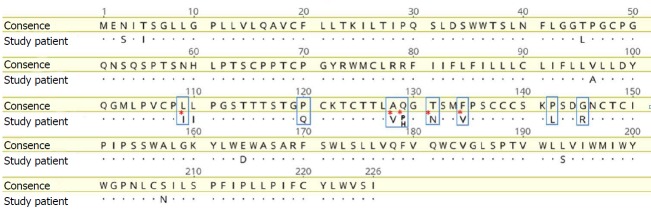
We found eight mutations and amino acid substitutions in the S protein, including the most common vaccine escape mutation G145R (Figure 4). Analysis of the HBV polymerase reverse transcription domain showed no known primary resistance mutations against the nucleos(t)ide analogues lamivudine, telbivudine, entecavir, tenofovir, or adefovir. Sequence analysis showed that wild-type and mutated viruses were present at a ratio of approximately 40:60 for five out of the eight identified mutations, indicating a mixed HBV population. The CD4 cell count was low (121/µL), the B cell count was strongly reduced (2/µL), and immunoglobulin levels were slightly reduced. Therefore, immunosuppressive treatment was reduced to 3 mg prednisolone, 100 mg allopurinol, and 25 mg azathioprine.
Figure 4.
Vaccine escape mutations in the patient’s hepatitis B virus strain. Alignment of the HBs antigen amino acid sequence (SHBs) of the patient´s strain with a HBV genotype F consensus sequence derived from the HIV-grade database (www.hiv-grade.de). Amino acids are named according to the one-letter code. Dots in the patient´s sequence represent amino acids identical to those in the consensus sequence. Positions relevant for drug resistance are indicated by blue boxes. Red asterisks denote positions in the patient´s strain (i.e., 109, 128, 129, 131, and 134) where both the amino acid of the consensus strain and the vaccine escape mutation were observed. HBV: Hepatitis B virus.
Daily treatment with 0.5 mg entecavir was initiated because of reduced kidney function. Viral replication decreased significantly and was negative 5 mo after the diagnosis (Figure 5). The entecavir dosage was later adjusted according to kidney function every fourth day. No complications with liver function were reported following treatment. Immunosuppressive treatment was changed to tacrolimus.
Figure 5.
Course of amino transferases and bilirubin levels during acute hepatitis in March 2015 and HBV viremia levels after diagnosis of hepatitis B virus reactivation. A: Course of amino transferases and bilirubin levels during acute hepatitis in March 2015; B: Course of HBV viremia levels after diagnosis of HBV reactivation. Start of antiviral treatment is indicated. ALT: Alanine aminotransferase.
DISCUSSION
Reactivation of chronic hepatitis B in immunosuppressed patients is serious. In this patient, reactivation occurred approximately 11 years after immunosuppressive therapy was initiated, and no recent causes for reactivation, such as a change in immunosuppressive medication, were reported. HBV reactivation is a well described risk in renal transplant recipients. However, only one report of reactivation due to a vaccine escape mutant (HBV genotype E) in a successfully vaccinated post-transplant patient 4 years after kidney transplantation has been published[6]. Post-renal transplant patients are at risk of de novo HBV infections due to immunosuppression. At least two reported cases of HBV infection have been described in vaccinated kidney transplant patients carrying vaccine escape mutations[7,8]. A de novo infection, albeit unlikely, cannot be excluded completely in our patient.
We identified the immune escape mutation G145R in our patient. This mutation was discovered in 1988[9] and has been described in HBV genotypes A-D worldwide[10]. Reduced antibody binding is sufficient for immunologic breakthrough of HBV escape mutants[5]. We also identified a P120Q mutation in the upstream region of the HBV core gene and two different mutations at positions Q129, Q129P, and H in our patient. Mutations at these positions have been described in immune escape variants or occult infection[10]. The combination of different mutations alters HBV antigenicity even further[11]. All eight mutations found in the case presented here and available previous reports on these specific mutations are provided in the supplemental file. Escape mutants are rare, but they can be selected by vaccination in highly endemic regions[12] and during reactivation of occult HBV.
HBV genotype F is endemic in Central and South America and has four subgenotypes, F1–F4[13]. Prevalence outside South America is very low (up to 7% in Spain[14]and 1.4% in the whole of Europe[15]). Surprisingly, the HBV genotype in our patient was subgenotype F3, subtype adw4q. The phylogenetic tree of this virus was similar to virus strains in Venezuela (Figure 3).
HBV genotype F is the most genetically divergent of the HBV genotypes[16]. It shares a close genetic and geographical association with related orthohepadnaviruses endemic in mammals of Middle and South America, like the woolly monkey, a nonhuman primate, and the Tent-making bat. This suggests an evolutionary link between these viruses in different host species[17]. The distant relationship of HBV genotype F strains with European HBV isolates has specific clinical implications. For example, the current World Health Organization (WHO) vaccine is based on the “a” determinant domain of subgenotype A2 (subtype adw2), which has a different antigen to genotype F (subtype adw4). Therefore, vaccine escape is more likely in genotype F. Moreover, HBV genotype F infections have high viral loads and are typically HBeAg-positive in acute cases. This may facilitate breakthrough when anti-HBs titers are low[18,19]. However, only two cases of vaccine failure in patients infected with HBV subgenotype F have been reported. They were infected with genotype F1b (subtype adw4) after traveling to Northern Argentina[20] and with subgenotype F1 after traveling to Spain[21], respectively. Escape mutants were not detected in either patient, suggesting that the HBV genotype F breakthrough without the help of escape mutations. The patients described in these previous reports were vaccinated before infection. In contrast, our patient was vaccinated after HBV infection. In the 1990s, several clinical pilot trials tested vaccination in HBsAg-positive patients to induce virus-specific cellular immunity and HBsAg loss (“therapeutic vaccination”) using vaccines containing HBsAg and later containing preS2/S proteins[22]. Initial results suggested a small effect on replication, seroconversion, and HBsAg loss, but the concept was later proven unsuccessful, at least with current vaccines[23]. In our case, vaccine escape was an additional negative effect of this vaccination therapy. Escape mutation selection in our patient after second-generation vaccination could be explained by: (1) incomplete neutralization of HBV genotype F by the generated antibodies; and (2) selection pressure from vaccination. To the best of our knowledge, this is the first case describing breakthrough of HBV genotype F due to G145R, P120Q, and Q129P escape mutations in a transplant setting.
The high viral load in our patient presented a substantial risk for virus transmission during blood sampling or laboratory blood work. The risk was aggravated because the virus was HBeAg-negative, which favors the development of severe and fulminant acute hepatitis due to abnormal immunopathology. Fatal outbreaks of HBeAg-negative HBV strains, even with common genotypes, have recently been reported in healthcare settings and nursing homes[24].
This case illustrates the limitations of the conventional HBV vaccine in individuals infected with escape mutant forms of the virus. These limitations include impaired immunogenicity and inability of neutralizing antibodies to recognize the virus. All available second-generation, yeast-derived vaccines are based on the non-glycosylated “a” determinant of the small surface HBV protein (SHBs). Several third-generation vaccines (Hepagene™, Bio-Hep B™, Sci-V-vac™) were developed in the 1990s and contain all three HBV envelope proteins: The SHBs, the medium (MHBs) (preS2-SHBs), and the large (LHBs) (preS1/preS2-SHBs) within subviral particles derived from mammalian cells[25]. The amino terminal of the preS1 domain contains the binding domain for sodium-dependent taurocholate co-transporting peptide (NTCP), which is the high-affinity receptor for HBV cell binding and entry[26]. PreS1-specific antibodies are interesting because the receptor-binding domain of preS1 is relatively conserved across all HBV genotypes. To date, a third-generation vaccine is not globally available; one has been on the market in Israel and in several countries in East Asia and Africa since 2001. The response rate in healthy non-responders is superior to second-generation vaccines[27] as well as in immunosuppressed post-liver transplantation patients who do not respond to second-generation vaccines[28].
In summary, we present a rare case of vaccine es-cape in an immunosuppressed patient infected with the rare HBV subgenotype F3 in Western Europe. This vaccine escape mutant replicated despite high levels of anti-HBs. Fortunately, antiviral agents effectively lowered the viral load and ameliorated liver inflammation. Despite this successful treatment, third-generation vaccines should be introduced as a protective measure to enhance the immunological protection of low-responders and risk groups (e.g., immunosuppressed patients, patients with chronic kidney failure) and possibly induce seroconversion of HBs-Ag to anti-HBs in chronic HBeAg-negative patients with low viral loads. The HBV status of immunosuppressed patients should be carefully monitored in transplant settings to prevent possible reactivation.
ARTICLE HIGHLIGHTS
Case characteristics
A 74-year-old patient with previous hepatitis B infection presented 11 years after kidney transplantation with diarrhea, loss of appetite, and icterus.
Clinical diagnosis
The main clinical finding was acute liver dysfunction.
Differential diagnosis
The differentials of acute liver dysfunction in this patient were acute hepatitis caused by hepatitis B virus (HBV) reactivation or hepatitis D virus (HDV) superinfection or infection with another hepatotropic virus, sepsis, obstruction of bile ducts, cholangitis, tumor, abscess, or thrombosis.
Laboratory diagnosis
Laboratory results revealed acute hepatitis caused by highly replicative HBV subgenotype F3 with immune escape mutations.
Imaging diagnosis
A computed tomography (CT) scan excluded bile duct obstruction, tumor, abscess, and thrombosis.
Pathological diagnosis
Histologic examinations showed acute hepatitis with multiple disseminated acidophilic single cell necroses and pericentral lipofuscinosis, vacuolar lipid droplets, and minimal periportal fibrosis suggesting additional chronic toxic damage.
Treatment
Acute hepatitis B was treated with an antiviral medication, the nucleoside reverse transcriptase inhibitor (NRTI) entecavir (ETV).
Related reports
HBV reactivation has been reported in immunosuppressed patients, patients with de novo infection, and in successfully vaccinated persons (caused by vaccine escape mutations or subgenotype F).
Term explanation
Vaccine or immune escape describes the ability of the HBV to reinfect or reactivate in the presence of neutralizing antibodies that cannot neutralize the virus.
Experiences and lessons
Acute hepatitis caused by hepatitis B infection may occur in a successfully vaccinated patient if the virus escapes antibody neutralization. Escape may be caused by escape mutations that change surface antigens or by a virus genotype that is distantly related to the virus genotype that the second-generation HBV vaccine is based on.
Footnotes
Informed consent statement: The patient gave informed consent for the publication of his medical history. Identifying details were omitted or anonymized.
Conflict-of-interest statement: Spengler U has consulted for AbbVie and has received a royalty from UpToDate for another study. Marsen T has received speakers honoraria from GHD Gesundheits GmbH and Fresenius Medical Care Nephrology for another study. Schlabe S has received sponsoring for educational events from Janssen for another study. All other authors state no conflict of interest.
CARE Checklist (2013) statement: The authors have read the guidelines of the CARE Checklist (2013) and the manuscript adheres to CARE Checklist guidelines.
Manuscript source: Unsolicited manuscript
Peer-review started: April 7, 2018
First decision: April 23, 2018
Article in press: June 8, 2018
Specialty type: Gastroenterology and hepatology
Country of origin: Germany
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Kai K, Kanda T, Saniabadi A S- Editor: Ji FF L- Editor: Filipodia E- Editor: Tan WW
Contributor Information
Stefan Schlabe, German Center of Infectious Diseases Research (DZIF), Partner-Site Cologne-Bonn, Bonn-Cologne35392, Germany; Department of Internal Medicine I, University Hospital of Bonn, Bonn 53127, Germany. Germany.stefan.schlabe@ukbonn.de.
Kathrin van Bremen, German Center of Infectious Diseases Research (DZIF), Partner-Site Cologne-Bonn, Bonn-Cologne35392, Germany; Department of Internal Medicine I, University Hospital of Bonn, Bonn 53127, Germany.
Souhaib Aldabbagh, German Center of Infectious Diseases Research (DZIF), Partner-Site Cologne-Bonn, Bonn-Cologne35392, Germany; Institute of Virology, University Hospital of Bonn, Bonn 53127, Germany.
Dieter Glebe, Institute of Medical Virology, Justus Liebig University Giessen, National Reference Center for Hepatitis B and D Viruses, Biomedical Research Center Seltersberg, Giessen 35392, Germany; German Center of Infectious Diseases Research (DZIF), Partner-Site Giessen, Giessen 35392, Germany.
Corinna M Bremer, Institute of Medical Virology, Justus Liebig University Giessen, National Reference Center for Hepatitis B and D Viruses, Biomedical Research Center Seltersberg, Giessen 35392, Germany; German Center of Infectious Diseases Research (DZIF), Partner-Site Giessen, Giessen 35392, Germany.
Tobias Marsen, Practice of Nephrology and Dialysis, Nephrological Center Cologne-Lindenthal, Cologne 50937, Germany.
Walter Mellin, Practice of Pathology and Cytology, Cologne 50931, Germany.
Veronica Di Cristanziano, Institute of Virology, University Hospital of Cologne, Cologne 50935, Germany.
Anna M Eis-Hübinger, German Center of Infectious Diseases Research (DZIF), Partner-Site Cologne-Bonn, Bonn-Cologne35392, Germany; Institute of Virology, University Hospital of Bonn, Bonn 53127, Germany.
Ulrich Spengler, German Center of Infectious Diseases Research (DZIF), Partner-Site Cologne-Bonn, Bonn-Cologne35392, Germany; Department of Internal Medicine I, University Hospital of Bonn, Bonn 53127, Germany.
References
- 1.GBD 2015 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1545–1602. doi: 10.1016/S0140-6736(16)31678-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Global Burden of Disease Study 2013 Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386:743–800. doi: 10.1016/S0140-6736(15)60692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kramvis A. Genotypes and genetic variability of hepatitis B virus. Intervirology. 2014;57:141–150. doi: 10.1159/000360947. [DOI] [PubMed] [Google Scholar]
- 4.Hepatitis B vaccines. Wkly Epidemiol Rec. 2009;84:405–419. [PubMed] [Google Scholar]
- 5.Waters JA, Kennedy M, Voet P, Hauser P, Petre J, Carman W, Thomas HC. Loss of the common “A” determinant of hepatitis B surface antigen by a vaccine-induced escape mutant. J Clin Invest. 1992;90:2543–2547. doi: 10.1172/JCI116148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Le Pendeven C, Cuzon G, Fontaine H, Zatla F, Schneider V, Amiel C, Khayat R, Nicolas JC, Soussan P. Hepatitis B escape mutant reactivation in a renal transplant patient. J Clin Virol. 2007;40:74–76. doi: 10.1016/j.jcv.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 7.Sayiner AA, Agca H, Sengonul A, Celik A, Akarsu M. A new hepatitis B virus vaccine escape mutation in a renal transplant recipient. J Clin Virol. 2007;38:157–160. doi: 10.1016/j.jcv.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 8.Lu M, Lorentz T. De novo infection in a renal transplant recipient caused by novel mutants of hepatitis B virus despite the presence of protective anti-hepatitis B surface antibody. J Infect Dis. 2003;187:1323–1326. doi: 10.1086/373902. [DOI] [PubMed] [Google Scholar]
- 9.Zanetti AR, Tanzi E, Manzillo G, Maio G, Sbreglia C, Caporaso N, Thomas H, Zuckerman AJ. Hepatitis B variant in Europe. Lancet. 1988;2:1132–1133. doi: 10.1016/s0140-6736(88)90541-7. [DOI] [PubMed] [Google Scholar]
- 10.Coppola N, Onorato L, Minichini C, Di Caprio G, Starace M, Sagnelli C, Sagnelli E. Clinical significance of hepatitis B surface antigen mutants. World J Hepatol. 2015;7:2729–2739. doi: 10.4254/wjh.v7.i27.2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Okamoto H, Tsuda F, Sakugawa H, Sastrosoewignjo RI, Imai M, Miyakawa Y, Mayumi M. Typing hepatitis B virus by homology in nucleotide sequence: comparison of surface antigen subtypes. J Gen Virol. 1988;69:2575–2583. doi: 10.1099/0022-1317-69-10-2575. [DOI] [PubMed] [Google Scholar]
- 12.Hsu HY, Chang MH, Ni YH, Chen HL. Survey of hepatitis B surface variant infection in children 15 years after a nationwide vaccination programme in Taiwan. Gut. 2004;53:1499–1503. doi: 10.1136/gut.2003.034223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alvarado-Mora MV, Pinho JR. Distribution of HBV genotypes in Latin America. Antivir Ther. 2013;18:459–465. doi: 10.3851/IMP2599. [DOI] [PubMed] [Google Scholar]
- 14.Sánchez-Tapias JM, Costa J, Mas A, Bruguera M, Rodés J. Influence of hepatitis B virus genotype on the long-term outcome of chronic hepatitis B in western patients. Gastroenterology. 2002;123:1848–1856. doi: 10.1053/gast.2002.37041. [DOI] [PubMed] [Google Scholar]
- 15.Kurbanov F, Tanaka Y, Mizokami M. Geographical and genetic diversity of the human hepatitis B virus. Hepatol Res. 2010;40:14–30. doi: 10.1111/j.1872-034X.2009.00601.x. [DOI] [PubMed] [Google Scholar]
- 16.Norder H, Hammas B, Lee SD, Bile K, Couroucé AM, Mushahwar IK, Magnius LO. Genetic relatedness of hepatitis B viral strains of diverse geographical origin and natural variations in the primary structure of the surface antigen. J Gen Virol. 1993;74:1341–1348. doi: 10.1099/0022-1317-74-7-1341. [DOI] [PubMed] [Google Scholar]
- 17.Drexler JF, Geipel A, König A, Corman VM, van Riel D, Leijten LM, Bremer CM, Rasche A, Cottontail VM, Maganga GD, et al. Bats carry pathogenic hepadnaviruses antigenically related to hepatitis B virus and capable of infecting human hepatocytes. Proc Natl Acad Sci U S A. 2013;110:16151–16156. doi: 10.1073/pnas.1308049110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pezzano SC, Torres C, Fainboim HA, Bouzas MB, Schroder T, Giuliano SF, Paz S, Alvarez E, Campos RH, Mbayed VA. Hepatitis B virus in Buenos Aires, Argentina: genotypes, virological characteristics and clinical outcomes. Clin Microbiol Infect. 2011;17:223–231. doi: 10.1111/j.1469-0691.2010.03283.x. [DOI] [PubMed] [Google Scholar]
- 19.Stramer SL, Wend U, Candotti D, Foster GA, Hollinger FB, Dodd RY, Allain JP, Gerlich W. Nucleic acid testing to detect HBV infection in blood donors. N Engl J Med. 2011;364:236–247. doi: 10.1056/NEJMoa1007644. [DOI] [PubMed] [Google Scholar]
- 20.O’Halloran JA, De Gascun CF, Dunford L, Carr MJ, Connell J, Howard R, Hall WW, Lambert JS. Hepatitis B virus vaccine failure resulting in chronic hepatitis B infection. J Clin Virol. 2011;52:151–154. doi: 10.1016/j.jcv.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 21.Tacke F, Amini-Bavil-Olyaee S, Heim A, Luedde T, Manns MP, Trautwein C. Acute hepatitis B virus infection by genotype F despite successful vaccination in an immune-competent German patient. J Clin Virol. 2007;38:353–357. doi: 10.1016/j.jcv.2006.12.024. [DOI] [PubMed] [Google Scholar]
- 22.Pol S, Nalpas B, Driss F, Michel ML, Tiollais P, Denis J, Brécho C; Multicenter study group. Efficacy and limitations of a specific immunotherapy in chronic hepatitis B. J Hepatol. 2001;34:917–921. doi: 10.1016/s0168-8278(01)00028-9. [DOI] [PubMed] [Google Scholar]
- 23.Cesar Aguilar J, Y L. Immunotherapy for Chronic Hepatitis B using HBsAg-based Vaccine Formulations: From Preventive Commercial Vaccines to Therapeutic Approach Julio Cesar Aguilar. Euroasian J Hepatogastroenterol. 2014;4:92–97. doi: 10.5005/jp-journals-10018-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seiz PL, Slanina H, Ziebuhr J, Gerlich WH, Glebe D, Schüttler CG. Studies of nosocomial outbreaks of hepatitis B in nursing homes in Germany suggest a major role of hepatitis B e antigen expression in disease severity and progression. Int J Med Microbiol. 2015;305:663–672. doi: 10.1016/j.ijmm.2015.08.016. [DOI] [PubMed] [Google Scholar]
- 25.Shouval D, Ilan Y, Adler R, Deepen R, Panet A, Even-Chen Z, Gorecki M, Gerlich WH. Improved immunogenicity in mice of a mammalian cell-derived recombinant hepatitis B vaccine containing pre-S1 and pre-S2 antigens as compared with conventional yeast-derived vaccines. Vaccine. 1994;12:1453–1459. doi: 10.1016/0264-410x(94)90155-4. [DOI] [PubMed] [Google Scholar]
- 26.Yan H, Zhong G, Xu G, He W, Jing Z, Gao Z, Huang Y, Qi Y, Peng B, Wang H, et al. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. Elife. 2012:3. doi: 10.7554/eLife.00049. [DOI] [PubMed] [Google Scholar]
- 27.Krawczyk A, Ludwig C, Jochum C, Fiedler M, Heinemann FM, Shouval D, Roggendorf M, Roggendorf H, Lindemann M. Induction of a robust T- and B-cell immune response in non- and low-responders to conventional vaccination against hepatitis B by using a third generation PreS/S vaccine. Vaccine. 2014;32:5077–5082. doi: 10.1016/j.vaccine.2014.06.076. [DOI] [PubMed] [Google Scholar]
- 28.Lo CM, Lau GK, Chan SC, Fan ST, Wong J. Efficacy of a pre-S containing vaccine in patients receiving lamivudine prophylaxis after liver transplantation for chronic hepatitis B. Am J Transplant. 2007;7:434–439. doi: 10.1111/j.1600-6143.2006.01636.x. [DOI] [PubMed] [Google Scholar]
- 29.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]