Abstract
Transcatheter arterial chemoembolization (TACE) is widely accepted as a treatment for patients with hepatocellular carcinoma (HCC) in the intermediate stage according to the Barcelona Clinic Liver Cancer (BCLC) guidelines. Recently, balloon-occluded TACE (B-TACE) was developed in Japan. Despite the lack of a clear definition, B-TACE is generally defined as the infusion of emulsion of chemotherapeutic agents with lipiodol followed by gelatin particles under the occlusion of feeding arteries by a microballoon catheter, which leads to the dense lipiodol emulsion (LE) accumulation in HCC nodules. This phenomenon cannot be explained only by the prevention of proximal migration and leakage of embolization materials; it further involves causing local changes in the hemodynamics of the surrounding occlusion artery and targeted HCC nodules. Balloon-occluded arterial stump pressure plays an important role in the dense LE accumulation in targeted HCC nodules. Although randomized controlled trials comparing the therapeutic effect and the prognosis of B-TACE to those of the other TACE procedures, such as conventional-TACE and drug-eluting beads TACE, are still lacking, B-TACE is thought to be a promising treatment. The purpose of this review is to summarize the mechanism, therapeutic effect, indication, prognosis and complications of B-TACE.
Keywords: Hepatocellular carcinoma, Treatment effect, Transcatheter arterial chemoembolization, Prognosis, Balloon-occluded arterial stump pressure, Dense lipiodol emulsion accumulation, Balloon-occluded transcatheter arterial chemoembolization, Microballoon catheter
Core tip: Balloon-occluded transcatheter arterial chemoembolization (B-TACE) was recently developed in Japan. Despite the lack of a clear definition, B-TACE is generally defined as the infusion of emulsion of chemotherapeutic agents with lipiodol followed by gelatin particles under the occlusion of feeding arteries by a microballoon catheter. Although randomized controlled trials comparing the therapeutic effect and the prognosis of B-TACE to those of the other TACE procedures are still lacking, B-TACE is thought to be a promising treatment. The purpose of this review is to summarize the mechanism, therapeutic effect, indication, prognosis and complications of B-TACE.
INTRODUCTION
Liver cancer ranks sixth as the common malignant neoplasm and second as the cause of death worldwide[1], and hepatocellular carcinoma (HCC) is the most common type of malignant liver tumors[2]. According to the Barcelona Clinic Liver Cancer (BCLC) guidelines, transcatheter arterial chemoembolization (TACE) is an established treatment for patients with HCC in the intermediate stage[3]. The median survival of untreated patients at the intermediate stage, who present with multinodular without vascular invasion or extrahepatic metastasis, performance status 0 and Child-Pugh class A or B, is reported to be 16 mo[4,5], and TACE prolongs their overall survival compared to that of a control group[6,7].
TACE is performed through the injection of single or multiple chemotherapeutic agents after the catheterization of tumor-feeding arteries, followed by the embolization of the same vessels in order to gain a synergistic effect of cytotoxicity and ischemia[3,8]. Conventional-TACE (C-TACE) is defined as the injection of a mixture of anticancer agents with lipiodol followed by embolic materials, such as gelatin particles, calibrated microspheres or polyvinyl alcohol, and drug-eluting beads TACE (DEB-TACE) is defined as the infusion of microspheres onto which chemotherapeutic agents is loaded or adsorbed to achieve a sustained in vivo drug release[8,9].
Recently, balloon-occluded TACE (B-TACE), which was first reported by Irie et al[10], has been developed in Japan. Although it is not mentioned in several guidelines[3,11,12] and its clear definition has not been established, it is generally defined as the infusion of emulsion of chemotherapeutic agents with lipiodol followed by gelatin particles under the occlusion of feeding arteries by a microballoon catheter. The occlusion of the feeding arteries result in the dense lipiodol emulsion (LE) accumulation in targeted nodules[10]. The therapeutic effect of B-TACE was better than that of C-TACE according to several previous reports[13-15], although those were retrospective and small in scale. In addition, randomized controlled trials (RCTs) comparing the therapeutic effect and the prognosis of B-TACE to those of other TACE procedures are still lacking.
Table 1 depicts the key findings of the therapeutic effect and overall survival of B-TACE. With this potential limitation in mind, in the present review, we describe the current understanding of the mechanism, therapeutic effect, indication, overall survival and complications of B-TACE.
Table 1.
Summary of retrospective studies of therapeutic effect and overall survival of balloon-occluded transcatheter arterial chemoembolization for hepatocellular carcinoma
| Ref. | Year | No. of patients | Antitumor agent and embolic materials | Therapeutic effect | Overall survival | Commentary |
| Hatanaka et al[43] | 2018 | 66 | Miriplatin-lipiodol suspension + gelatin particle | CR 53.0%, PR 10.6%, SD 19.7%, PD 16.7%, RR 63.6% | The 1-, 2-, and 3-yr survival rates were 76.8%, 57.3%, and 46.7%, respectively | The number of tumors and α-fetoprotein level were predictive factors for the tumor response and serum albumin and overall response (CR + PR) were predictive factors for prognosis |
| Kawamura et al[40] | 2017 | 30 | Miriplatin-lipiodol suspension + gelatin particle | TE4 51.0%, TE3 8.5%, TE2 19.1%, TE1 21.3%, RR 59.6% | NA | The presence of portal vein visualization, tumor on the subcapsular portion, and successful subsegmental artery embolization were predictive factors for the tumor response |
| Maruyama et al[20] | 2016 | 50 | Epirubicin-lipiodol suspension + gelatin particle | There was no statistically significant difference between the B-TACE group and the C-TACE group | NA | |
| Irie et al[15] | 2016 | 28 | Doxorubicin, mytomysin-lipiodol suspension + gelatin particle | TE4 89.3%, TE3 10.7%, TE2 0%, TE1 0%, RR 100%. The local recurrence rates at 1, 3, and 5 yr were 92.4%, 69.9%, and 69.9%, respectively | The 1-, 3-, and 5-yr survival rates were 96.4%, 60.3% and 31.1%, respectively | B-TACE was an independent factor for improving both the control rate of the primary nodule and the overall survival rates |
| Asayama et al[32] | 2016 | 29 | Miriplatin-lipiodol suspension + gelatin particle | TE4 8.6%, TE3 48.6%, TE2 17.1%, TE1 25.7%, RR 57.1% | NA | |
| Ogawa et al[14] | 2015 | 33 | Miriplatin-lipiodol suspension + gelatin particle | TE4 49.2% | NA | The percentage of TE4 in B-TACE was significantly higher than that in the C-TACE |
| Minami et al[41] | 2015 | 27 | Miriplatin-lipiodol suspension + gelatin particle (epirubicin was used in 3 patients) | Countable HCC (n = 17): TE4 43.8%, TE3 12.5%, TE2 37.5%, TE1 6.3%, RR 56.3%, Uncountable HCC (n = 10): CR 0%, PR 0%, SD 10%, PD 90% | NA | |
| Arai et al[13] | 2014 | 49 | Miriplatin-lipiodol suspension + gelatin particle | TE4 55.1%, TE3 38.8%, TE2 4.1%, TE1 2.0%, RR 93.9% | NA | |
| Ishikawa et al[44] | 2014 | 51 | Miriplatin-lipiodol suspension + gelatin particle | The local recurrence rates at 6, 12 mo were 11.1 %, 26.2%, respectively. The medan recurrence time was 9 mo | NA | The CT value just after B-TACE was a predictive factor for the tumor response |
According to Response Evaluation Criteria in Cancer of the Liver (RECICL), the treatment effect (TE) was defined as follows: TE4, tumor necrosis of 100% or 100% reduction; TE3, tumor necrosis of 50%-100% or 50%-100% reduction in tumor size; TE1, tumor enlargement of > 50% regardless of necrosis; TE2, effect other than TE3 or TE1. B-TACE: Balloon-occluded transcatheter arterial chemoembolization; CR: Complete response; PR: Partial response; SD: Stable disease; PD: Progressive disease; RR: Response rate; NA: Not available; C-TACE: Conventional transcatheter arterial chemoembolization; CT: Computed tomography.
BALLOON-OCCLUDED TACE PROCEDURE
Microballoon catheters
A 3-Fr microballoon catheter was used in the study reported by Irie et al[10]. However, it is sometimes difficult to advance a microballoon catheter into the targeted tumor-feeding arteries selectively[16]. Moreover, a 5- or 6-Fr guiding catheter was required when we used a 3-Fr microballoon catheter for B-TACE, which is more invasive than C-TACE[16]. Microballoon catheters have improved in recent years, and a 1.8-Fr microballoon catheter (Logos, Piolax, Kanagawa, Japan; or Attendant, Terumo Clinical Supply, Tokyo, Japan) is now available, enabling us to insert the catheter more selectively and more peripherally[16]. We are able to coaxially advanced these microballoon catheters through a typical 4-Fr parent catheter[16]. The proximal side consists of two ports in these microballoon catheters: the microballoon lumen and the microguidewire lumen, the diameter of which is 0.017-0.018 inches[16].
B-TACE procedure
Although a clear definition of B-TACE has not been established and several details concerning the procedure vary markedly among institutions, we will attempt to briefly describe the main points of B-TACE.
The right femoral artery is punctured under local anesthesia. A 4- or 5-Fr parent catheter is then placed in the celiac artery (CeA) or superior mesenteric artery (SMA), and angiography is carried out to assess the anatomy of the hepatic artery, tumor stains, the feeding arteries and arteriovenous shunting. In some institutions, cone-beam computed tomography (CBCT) and angiography-assisted computed tomography (CT) is performed to diagnose the HCC and evaluate the tumor-feeding arteries. A microballoon catheter is inserted into the tumor-feeding arteries over the guidewire as selectively as possible. When the selective insertion of the microballoon catheter into the targeted arteries is difficult, such as in patients with tortuous anatomies of the CeA or SMA, using a microballoon catheter as an anchor might be useful for achieving deep cannulation with the parent catheter[17]. The tumor-feeding artery is then occluded, and the emulsion of lipiodol and anticancer drugs is infused under radiographic guidance. The proportion of lipiodol to anticancer drugs was as follow: 70 mg of Miriplatin[18,19], which is most commonly used in B-TACE, was suspended with 3.5 mL of lipiodol (20 mg/mL); 10 mg of epirubicin[20] with 1-2 mL of lipiodol (5-10 mg/mL); 10 mg of doxorubicin hydrochloride and 2 mg of mitomycin C with 5-10 ml of lipiodol (5-10 mg/mL and 1-2 mg/mL, respectively)[10,15]. Digital angiography is sometimes useful for checking for the flow of LE droplets outside the target areas. LE is infused until sufficient filling of the targeted nodule, overflow into the intrahepatic collateral arteries or the presence of portal vein visualization is observed. The fragmented gelatin sponge slurry is then injected into the blood vessels until the distal vessel is embolized. We describe a typical B-TACE procedure in Figures 1-3.
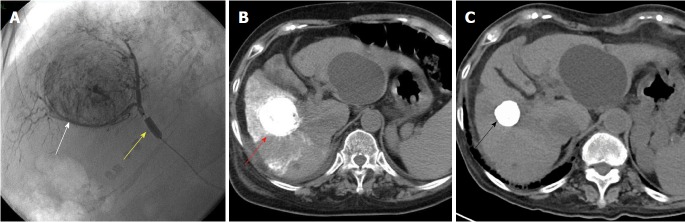
Figure 1.
A 71-year-old female patient with hepatitis-C related hepatocellular carcinoma 45 mm in diameter underwent balloon-occluded transcatheter arterial chemoembolization. A: A 3-Fr microballoon catheter was inserted into a tumor-feeding artery. After achieving occlusion with the microballoon catheter (yellow arrowhead), emulsion of lipiodol and miriplatin was infused until the cancer nodule (white arrowhead) was sufficiently filled. Fragmented gelatin sponge slurry was then injected; B: Just after B-TACE, computed tomography (CT) showed a dense lipiodol emulsion (LE) accumulation in HCC nodules (red arrowhead); C: Four years after B-TACE, CT showed that the volume of the LE accumulation was reduced (black arrowhead) with no local recurrence. HCC: Hepatocellular carcinoma; B-TACE: Balloon-occluded transcatheter arterial chemoembolization.
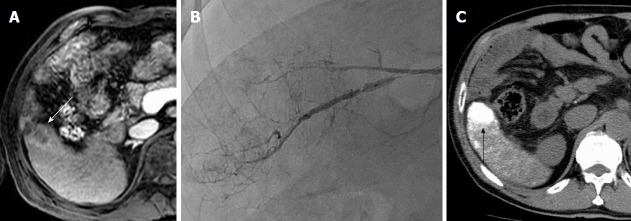
Figure 2.
A 75-year-old man with hepatitis-B related hepatocellular carcinoma. A: Gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid-enhanced magnetic resonance imaging (EOB-MRI) showed that HCC was slightly enhanced in the arterial phase (white arrowhead); B: While angiography did not show the tumor stain obviously, we injected the lipiodol emulsion (LE) and fragmented gelatin sponge slurry from tumor-feeding artery under occlusion with microballoon inflation; C: A favorable LE accumulation (black arrowhead) was seen on computed tomography one day after balloon-occluded transcatheter arterial chemoembolization. HCC: Hepatocellular carcinoma.
Figure 3.
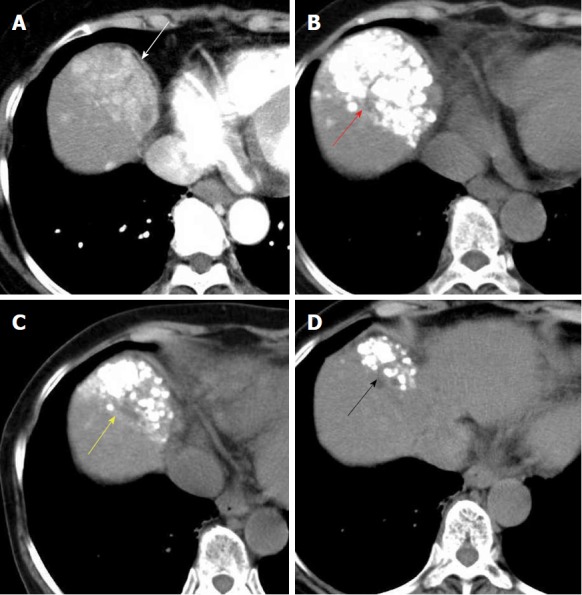
A 74-year-old woman with hepatitis-C related multiple hepatocellular carcinoma. A: Contrast-enhanced computed tomography (CT) showed that multiple HCC nodules were observed, aggregated at segment 8 (white arrowhead); B: Computed tomography showed that the dense lipiodol emulsion (LE) accumulations (red arrowhead) were observed just after balloon-occluded transcatheter arterial chemoembolization (B-TACE) for multiple HCC; C: We carried out additional B-TACE because local recurrence was detected on follow-up CT. Favorable LE accumulations were observed just after additional B-TACE (yellow arrowhead); D: Three month after additional B-TACE, local recurrence was not observed, and the LE accumulations were shrinking (black arrowhead). HCC: Hepatocellular carcinoma.
MECHANISM OF B-TACE
Irie et al[10] reported that they found dense lipiodol emulsion (LE) accumulation in HCC nodules when they used a 3-Fr microballoon catheter in selective TACE with occlusion of feeding arteries. Denser LE accumulation in targeted HCC nodules means more accumulation of anticancer drugs, leading to more therapeutic effect[21,22]. Therefore, revealing this mechanism is important for hepatologists and interventional radiologists in daily clinical practice.
Relationship between the balloon-occluded arterial stump pressure and the dense LE accumulation
The dense LE accumulation caused by the occlusion of feeding arteries cannot be explained only by the prevention of proximal migration and leakage of embolization materials; it also involves causing local changes in the hemodynamics of the surrounding occlusion artery and targeted tumors. Irie et al[10] reported that the pulsatile movement of LE droplets was observed after the inflation of the tip of a balloon catheter, with the peripheral arterial blood flow unable to be stopped. Because stasis of an LE droplet was observed even at the tip of the microballoon catheter, the authors speculated that the distal arterial blood flow did not come from leakage though the space between the balloon and the occluded artery. The intrahepatic collateral arteries, such as the peribiliary plexus[23], interlobar communicating arcade[24] and isolated artery[25], were considered to help maintain the distal arterial blood flow, based on the observation of anastomotic vessels with collateral arteries on digital subtraction angiography (DSA) under occlusion[10]. Consequently, the BOASP is reduced compared to the mean arterial pressure without balloon occlusion and more decreased BOASP allows the injection of LE under higher pressure. He also demonstrated that BOASP is responsible for the dense LE accumulation and the BOASP ≥ 64 mmHg is predictive for the presence of thick collateral arteries and less dense LE accumulation[10].
Matsumoto et al[26] reported that they used a 1.8-Fr microballoon catheter and measured the BOASP at each lobar, segmental and subsegmental arteries before B-TACE treatment. He showed that the BOASP ≥ 64 mmHg was founded at A1, A4, A8, anterior segment artery, right hepatic artery (RHA) and left hepatic artery (LHA) whereas the BOASP ≥ 64 mmHg was never seen at the other segmental and subsegmental arteries and the BOASP was independent of the size and number of tumors[26]. He presumed that this result was affected by the presence of intrahepatic collateral arteries, especially the communicating arcade in the hilum which is a relatively thick anastomosis between the right and left hepatic arteries and originates mainly from A4 and from anterior segment artery or the right hepatic artery[24,26]. BOASP ≥ 64 mmHg at A1 artery could be explained by the studies reported by Miyayama et al[27,28] in which the right branch of the communicating artery in the hilum might have been A1[26]. So, B-TACE treatment in A1, A4, A8, anterior segment artery, RHA and LHA possibly caused less dense LE accumulation in targeted HCC nodules[26]. However, this study is small sample size in measured hepatic arteries and lack of the statistical analysis. A large number of validation studies are needed.
Kakuta et al[29] examined the changes in the stump pressure with and without balloon occlusion as well as after B-TACE treatment. The mean blood pressure at the targeted occluded artery was 97 mmHg before balloon occlusion and decreased to 49.1 mmHg immediately after balloon occlusion, with a statistically significance[29]. Five minutes after balloon occlusion, the mean blood pressure was 50.4 mmHg, and the maintenance of a decreased blood pressure was observed[29]. After the injection of LE and gelatin sponge particles, the mean blood pressure increased to 70.6 mmHg, which was significantly higher than that immediately after balloon occlusion (P < 0.001)[29]. The mean arterial blood pressure without balloon occlusion after B-TACE was not significantly different from that without balloon occlusion before B-TACE (P = 0.9107)[29]. Although no significant differences were observed among the stump pressures at the first-, second- and third-order branches, the proportion of stump pressure < 64 mmHg at third-order branches tended to be higher than that at first- or second-order branches[29]. Advancing a microballoon catheter to a more peripheral artery appeared to cause the stump pressure to decrease more[29]. A further investigation is needed to determine whether or not the occlusion of a more distal artery results in a more decreased BOASP.
Although these previous studies[10,26,29] have provided only preliminary and limited data, the findings indicate that the BOASP plays an important role in the dense LE accumulation in targeted HCC nodules. Further studies regarding whether or not the BOSAP can predict the long-term therapeutic effect should be conducted.
Hemodynamic changes assessed by CBCT and angio-assisted CT
Three reports[30-32] have explored the hemodynamic changes assessed by balloon occlusion using CBCT and angiography-assisted CT. Ishikawa et al[30] reported that the CBCT pixel values of tumor enhancement increased after balloon occlusion in 37 of the 52 nodules, whereas it decreased in the remaining 15 nodules. Yoshimatsu et al[31] showed that the degree of tumor enhancement on selective CT during hepatic angiography (CTHA) increased after balloon occlusion in 3 of the 27 nodules, while it decreased in 11 nodules. Asayama et al[32] also reported that 15 of the 35 nodules showed decreased enhancement or perfusion defects on CTHA with balloon occlusion. While the percentage of decreased tumor enhancement varied markedly among those studies, it remained a negative predictive factor for a dense LE accumulation[30,31] and short-term therapeutic effect[32]. It can be presumed that the blood flow from the occluded artery reduced and the blood flow from the collateral arteries relatively increased[31,32]. In relation to this, a discrepancy was noted between the tumor enhancement on selective CTHA with balloon occlusion and a dense LE accumulation after B-TACE, which was higher than the tumor enhancement grade[31]. Although CBCT[33-35] and CTHA[36,37] are well known to be useful tools for identifying the tumor-feeding arteries, evaluating the embolized area and avoiding nontargeted embolization, the same findings were reported in selective C-TACE[38]. A validation study will be needed to confirm whether or not a decreased tumor enhancement after balloon occlusion can predict the short- and long-term therapeutic effects.
With regard to the analysis of CT findings during arterial portography (CTAP), the reduction in size of the tumorous portal perfusion defects on CTAP with balloon occlusion was observed in 90% (18/20) of nodules. Its mean size significantly decreased from 21.9 to 19.1 mm in diameter (P = 0.0001). No decrease in the tumorous portal perfusion defect area significantly influenced the poor dense LE accumulation in the tumor[31]. The authors speculated that the decrease in the BOASP enabled the pressure gradient from the hepatic artery to the portal vein to also decrease, thus resulting in a relative increase in the blood flow from the portal vein in the surrounding tumor[31]. In other words, no change in the size of the tumorous portal perfusion defect may indicate no decrease in the BOASP[31]. To facilitate our understanding of the mechanism of B-TACE, we summarize the main findings mentioned above and present them in a schematic illustration in Figure 4.
Figure 4.
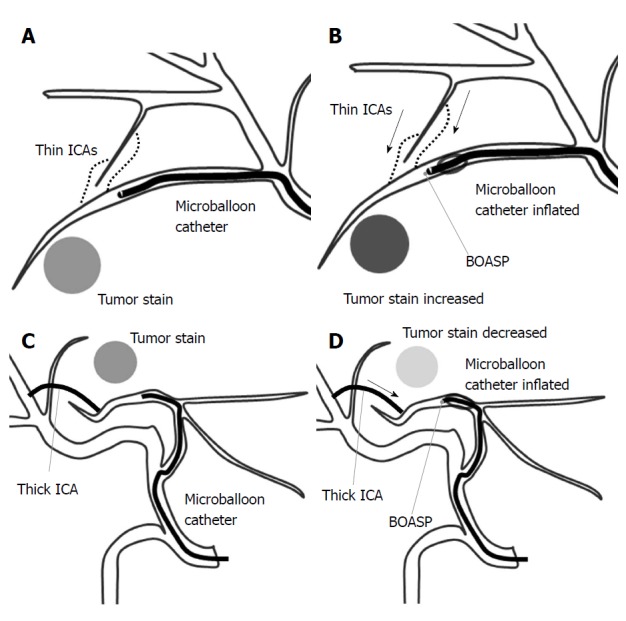
A schematic illustration of the balloon-occluded transcatheter arterial chemoembolization procedure. A: A microballoon catheter is inserted into the tumor-feeding artery and inflated to occlude it. If the distal arterial blood flow cannot be stopped, the intrahepatic collateral arteries (ICAs) may be maintaining the distal arterial blood flow; B: When the ICAs are thin, the balloon-occluded arterial stump pressure (BOASP) is remarkably reduced compared to the arterial pressure without balloon occlusion. In this situation, we might observe increased tumor enhancement on selective computed tomography during hepatic angiography (CTHA) after balloon occlusion. A remarkable reduction in the BOASP is thought to allow us to inject the lipiodol emulsion (LE) under higher pressure, leading to the denser LE accumulation in targeted tumors. The BOASP after the injection of LE and gelatin particles is significantly higher than that before treatment; C and D: When the ICAs are thick, the BOASP is expected to be mildly reduced or unchanged because of the adequate blood flow from a thick ICA. In this situation, we might observe decreased tumor enhancement on CTHA after balloon occlusion. We would probably inject the LE under lower pressure in such a situation, thereby achieving a less-dense LE accumulation.
THERAPEUTIC EFFECT OF B-TACE
Short-term therapeutic effect and local recurrence rate
In many previous studies, the short-term therapeutic effect of B-TACE was evaluated mainly by the Response Evaluation Criteria in Cancer of the Liver (RECICL) proposed by the Liver Cancer Study Group of Japan[39]. The treatment effect (TE) was determined by the radiological findings 1-3 mo after B-TACE treatment and defined as follows: TE4, tumor necrosis of 100% or 100% reduction; TE3, tumor necrosis of 50%-100% or 50%-100% reduction in tumor size; TE1, tumor enlargement of > 50% regardless of necrosis; TE2, effect other than TE3 or TE1[39]. According to previous reports[13,15,32,40,41], the rate of TE4 of targeted HCC nodules treated with B-TACE was 8.6%-89.3%, and that of TE3 was 8.5%-48.6%, with a response rate of 56.3%-100%.
Kawamura et al[40] showed that the presence of portal vein visualization during treatment was associated with an objective response, which is consistent with findings of the study reported by Miyayama et al[42]. However, the evaluation of the therapeutic effect of B-TACE in these studies[13,15,32,40,41] was limited to only targeted lesions and did not include the appearance of new lesions. According to a study[43] evaluated based on the overall response, including target lesions, non-target lesions and the appearance of new lesion, 35 patients (53.0%) experienced complete response (CR), 7 patients (10.6%) partial response (PR), 13 patients (19.7%) stable disease, and 11 patients (16.7%) progressive disease. The rate of a response rate and a disease control rate were 63.6% and 83.3%, respectively. Furthermore, a multivariate analysis showed that a solitary tumor and serum α-fetoprotein level were significantly relevant to the tumor response[43].
Regarding the analysis of the local recurrence rate, Ishikawa et al[44] showed that the median recurrence time and the local recurrence rate at 6 mo and 12 mo were 9 mo and 11.1% and 26.2%. The authors further revealed that only the CT value just after B-TACE was significantly relevant to local recurrence in a multivariate analysis[44]. CBCT was also deemed useful as a substitute for conventional CT to evaluate the degree of dense LE accumulation quantitatively in targeted HCC nodules[45]. Irie et al[15] also showed that the control rates of primary nodule treated by B-TACE at 1, 3 and 5 years were 92.4%, 69.9% and 69.9%, respectively. However, these were retrospective studies, and the number of nodules per patient was small (many patients had solitary nodules). The results should therefore be interpreted with caution when extrapolating to HCC patients with more nodules. A summary of the previous studies is shown in Table 1. A prospective validation study is warranted.
Suitable anticancer agents
The anticancer agents that have been used in B-TACE are as follows: Miriplatin[13,14,31,32,40,41,44], which was the most commonly used; cisplatin[29,31]; epirubicin[20] and a mixture of doxorubicin hydrochloride and mitomycin[10,15]. Miriplatin, which is a lipophilic platinum complex[18,19], has been used in C-TACE[46]. A retrospective study[47] showed that the local recurrence rate was significantly higher after superselective TACE with miriplatin than with epirubucin and mitomycin. This may be attributed to its high viscosity, and warmed miriplatin may reduce its viscosity, possibly overcoming its disadvantages and increasing its therapeutic effect[48,49]. Therefore, B-TACE with warmed miriplatin may exert a better therapeutic effect than that with miriplatin at room temperature. In this connection, arterial vascular damage is less severe in patients treated with miriplatin than in those treated with epirubicin[50]. Although no RCT has compared antitumor agents, miriplatin might be suitable as an anticancer agent in B-TACE. Further studies should be conducted.
Comparing the effectiveness of B-TACE and C-TACE
Reliable data on the comparative efficacy between B-TACE and C-TACE are still lacking, with only four retrospective studies reported. Arai et al[13] reported that the targeted therapeutic effect of B-TACE with miriplatin was better than that of C-TACE, and the mean total dose of miriplatin in B-TACE was higher than that of C-TACE. They presumed that inflating the microballoon catheter decreased the arterial pressure proximal of the tip of the catheter and blocked the backflow of miriplatin, resulting in a higher total dose of miriplatin and a better therapeutic effect on targeted nodules than with C-TACE. Ogawa et al[14] also reported that TE4 was observed in 29 patients (49.2%) treated with B-TACE and in 10 patients (27%) treated with C-TACE, indicating that local efficacy of B-TACE was significantly better than that of C-TACE. Irie et al[15] reported that the short-term treatment effect based on RECICL criteria was significantly better in B-TACE group than that in the C-TACE group, and the tumor control rates of the targeted nodule were significantly improved in the B-TACE group compared to C-TACE group. However, Maruyama et al[20] reported that the local control rate in B-TACE group were not significantly better than in the C-TACE group. The major cause of this was the lack of a significant difference between the two groups in the analysis of the LE ratio. The LE ratio, which was calculated as the ratio of the LE concentration in the targeted tumor to that in the surrounding embolized non-cancerous area, was considered to be an indicator of the selectivity of the LE accumulation in HCC[10]. Alternatively, this finding may have been observed because most patients received B-TACE treatment at the lobar or segmental level, resulting in a less-marked decrease in the BOASP and therefore a less-marked therapeutic effect[26]. More large-scale prospective studies are warranted.
INDICATIONS OF B-TACE
Since no definitive indications of B-TACE have been established to date, we explored potential factors and conditions supporting the performance of B-TACE. Summarizing the findings of the previous studies mentioned above, the following factors and conditions are known to be related to a dense LE accumulation in targeted HCC nodules and the therapeutic effect of B-TACE: a BOASP ≥ 64 mmHg[10]; microballoon occlusion at A1, A4, A8, anterior segment artery, RHA or LHA[26]; decreased tumor enhancement on CBCT and CTHA[30-32]; no decrease in the tumorous portal perfusion defect on CTAP[31]; tumor location on the subcapsular portion[40]; successful subsegmental feeding artery embolization[40]; the presence of portal vein visualization[40]; a high CT value just after B-TACE[44]; solitary tumor[43] and a low serum α-fetoprotein level[43]. Based on studies[13-15] showing that patients with fewer tumor nodules experienced a better therapeutic effect with B-TACE than with C-TACE, B-TACE might be a better indication than C-TACE in patients with fewer tumor nodules. Prospective validation studies are needed to clarify the indications of B-TACE.
The contraindications of B-TACE were thought to be almost the same as those for C-TACE treatment: Child-Pugh class C, no tumor thrombosis in the first branch or main portal vein, extrahepatic metastasis, refractory ascites, hepatic encephalopathy, presence of high-flow arterioportal or arteriovenous shunts, and allergy to contrast medium.
OVERALL SURVIVAL OF B-TACE
Hatanaka et al[43] reported that the survival rates at 1, 3 and 5 years in patients after B-TACE were 76.8%, 57.3% and 46.7%, respectively, and the median survival time was 902 d. They also revealed that a serum albumin level ≥ 3.4 g/dL and an overall response of CR+PR were favorable prognostic factors in a multivariate analysis[43]. Serum albumin is one of the most well-known prognostic factors for patients with HCC and has been adopted and integrated into several staging systems, including the BCLC staging system[3], Cancer of the Liver Italian Program (CLIP) score[51] and Japan Integrated Staging score (JIS score)[52]. According to previous studies of TACE[53,54], the overall response has been reported to be an independent factor affecting for the survival of HCC patients. Although the findings reported by Hatanaka et al[43] were consistent with those of previous studies[53,54], the results were based on a single-arm and single-institution study. Additional validation studies are therefore warranted.
One problem that remains to be solved is whether or not B-TACE improves the overall survival compared to other TACE treatments. Unfortunately, no RCTs have compared B-TACE with other TACE treatments, such as C-TACE and DEB-TACE, and only one historical control study[15] with patients who had solitary or two nodules has been reported. However, while it was small in scale, that study found that B-TACE was a significant factor predicting for the overall survival in a multivariate analysis[15]. A large-scale prospective study is therefore warranted.
COMPLICATIONS OF B-TACE
According to previous reports[13,20,43,44] evaluated by the Common Terminology Criteria for Adverse Effects (CT–CAE), all grades of fever were reported as complications in 44.2%-68% of cases, nausea in 16.3%-28%, abdominal pain in 14%-36.7%, ascites in 12.2%-15.2%, elevated total bilirubin in 34%-62.2%, elevated alanine aminotransferase (ALT) in 78.8%-96%, elevated serum creatine in 8.1%-9.1%, leukocytopenia in 53.1%-59%, thrombocytopenia in 71.3%-87.7%, anorexia in 31.3% and vagovagal reflex in 12%, while a grade 3/4 fever was reported as a complication in 0%-6% of cases, elevated total bilirubin in 0%-6.1%, elevated ALT in 9.1%-18%, leukocytopenia in 0%-12.1% and thrombocytopenia in 7.6%-12.2%. In an analysis comparing the adverse events of B-TACE with those of C-TACE, the rate of increased levels of ALT was significantly higher in the B-TACE group, with no significant differences in the clinical symptoms or other laboratory data between the two groups[13,20].
Hatanaka et al[43] reported no mortalities, but the development of biloma requiring percutaneous transhepatic biliary drainage after B-TACE was observed in 1 patient (1.5%) who had intrahepatic dilation on preoperative CT. Maruyama et al[20] reported that liver abscess was observed in 3 patients (6%), and liver infarction was observed in 1 patient (2%), with bile duct dilation indicated as a significant predictive factor in a multivariate analysis. Careful consideration must be given to perform the B-TACE for the patients with bile duct dilation[43]. Large-scale validation studies are therefore needed to confirm these findings.
CONCLUSION
B-TACE represents a feasible and promising therapy for the treatment of HCC patients, especially those with few nodules. Although no definitive indication of B-TACE in the common clinical practice has yet been established, leaving the decision to perform this procedure up to gastroenterologist or radiologist based on their expertise with or the availability of microballoon catheters, B-TACE may represent an established treatment in HCC patients.
Footnotes
Conflict-of-interest statement: No potential conflicts of interest. No financial support.
Manuscript source: Invited manuscript
Peer-review started: March 27, 2018
First decision: April 11, 2018
Article in press: June 28, 2018
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Guan YS, Lee JI S- Editor: Ji FF L- Editor: A E- Editor: Tan WW
Contributor Information
Takeshi Hatanaka, Department of Gastroenterology, Saiseikai Maebashi Hospital, Gunma 371-0821, Japan.
Hirotaka Arai, Department of Gastroenterology, Maebashi Red Cross Hospital, Gunma 371-0014, Japan.
Satoru Kakizaki, Department of Gastroenterology and Hepatology, Gunma University Graduate School of Medicine, Gunma 371-8511, Japan. kakizaki@gunma-u.ac.jp.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.McGlynn KA, Petrick JL, London WT. Global epidemiology of hepatocellular carcinoma: an emphasis on demographic and regional variability. Clin Liver Dis. 2015;19:223–238. doi: 10.1016/j.cld.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37:429–442. doi: 10.1053/jhep.2003.50047. [DOI] [PubMed] [Google Scholar]
- 5.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–1917. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 6.Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT, Fan ST, Wong J. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164–1171. doi: 10.1053/jhep.2002.33156. [DOI] [PubMed] [Google Scholar]
- 7.Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, Ayuso C, Sala M, Muchart J, Solà R, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734–1739. doi: 10.1016/S0140-6736(02)08649-X. [DOI] [PubMed] [Google Scholar]
- 8.Brown DB, Gould JE, Gervais DA, Goldberg SN, Murthy R, Millward SF, Rilling WS, Geschwind JF, Salem R, Vedantham S, et al. Transcatheter therapy for hepatic malignancy: standardization of terminology and reporting criteria. J Vasc Interv Radiol. 2009;20:S425–S434. doi: 10.1016/j.jvir.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 9.Brown DB, Nikolic B, Covey AM, Nutting CW, Saad WE, Salem R, Sofocleous CT, Sze DY; Society of Interventional Radiology Standards of Practice Committee. Quality improvement guidelines for transhepatic arterial chemoembolization, embolization, and chemotherapeutic infusion for hepatic malignancy. J Vasc Interv Radiol. 2012;23:287–294. doi: 10.1016/j.jvir.2011.11.029. [DOI] [PubMed] [Google Scholar]
- 10.Irie T, Kuramochi M, Takahashi N. Dense accumulation of lipiodol emulsion in hepatocellular carcinoma nodule during selective balloon-occluded transarterial chemoembolization: measurement of balloon-occluded arterial stump pressure. Cardiovasc Intervent Radiol. 2013;36:706–713. doi: 10.1007/s00270-012-0476-z. [DOI] [PubMed] [Google Scholar]
- 11.Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, Zhu AX, Murad MH, Marrero JA. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358–380. doi: 10.1002/hep.29086. [DOI] [PubMed] [Google Scholar]
- 12.Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, Jia J, Tateishi R, Han KH, Chawla YK, Shiina S, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11:317–370. doi: 10.1007/s12072-017-9799-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arai H, Abe T, Takayama H, Toyoda M, Ueno T, Kakizaki S, Sato K. Safety and efficacy of balloon-occluded transcatheter arterial chemoembolization using miriplatin for hepatocellular carcinoma. Hepatol Res. 2015;45:663–666. doi: 10.1111/hepr.12403. [DOI] [PubMed] [Google Scholar]
- 14.Ogawa M, Takayasu K, Hirayama M, Miura T, Shiozawa K, Abe M, Matsumoto N, Nakagawara H, Ohshiro S, Yamamoto T, et al. Efficacy of a microballoon catheter in transarterial chemoembolization of hepatocellular carcinoma using miriplatin, a lipophilic anticancer drug: Short-term results. Hepatol Res. 2016;46:E60–E69. doi: 10.1111/hepr.12527. [DOI] [PubMed] [Google Scholar]
- 15.Irie T, Kuramochi M, Kamoshida T, Takahashi N. Selective balloon-occluded transarterial chemoembolization for patients with one or two hepatocellular carcinoma nodules: Retrospective comparison with conventional super-selective TACE. Hepatol Res. 2016;46:209–214. doi: 10.1111/hepr.12564. [DOI] [PubMed] [Google Scholar]
- 16.Matsumoto T, Endo J, Hashida K, Ichikawa H, Kojima S, Takashimizu S, Watanabe N, Yamagami T, Hasebe T. Balloon-occluded transarterial chemoembolization using a 1.8-French tip coaxial microballoon catheter for hepatocellular carcinoma: technical and safety considerations. Minim Invasive Ther Allied Technol. 2015;24:94–100. doi: 10.3109/13645706.2014.951657. [DOI] [PubMed] [Google Scholar]
- 17.Shibuya K, Tahara H, Takeuchi S, Koyama Y, Tsushima Y. New Method of Parent Catheter Advancement in the Balloon Anchor Technique during Balloon-Occluded Transarterial Chemoembolization for Hepatic Tumors. Case Rep Radiol. 2016;2016:1957129. doi: 10.1155/2016/1957129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kishimoto S, Noguchi T, Yamaoka T, Fukushima S, Takeuchi Y. In vitro release of SM-11355, cis[((1R,2R)-1,2-cyclohexanediamine-N,N’)bis(myristato)] platinum(II) suspended in lipiodol. Biol Pharm Bull. 2000;23:637–640. doi: 10.1248/bpb.23.637. [DOI] [PubMed] [Google Scholar]
- 19.Hanada M, Baba A, Tsutsumishita Y, Noguchi T, Yamaoka T, Chiba N, Nishikaku F. Intra-hepatic arterial administration with miriplatin suspended in an oily lymphographic agent inhibits the growth of tumors implanted in rat livers by inducing platinum-DNA adducts to form and massive apoptosis. Cancer Chemother Pharmacol. 2009;64:473–483. doi: 10.1007/s00280-008-0895-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maruyama M, Yoshizako T, Nakamura T, Nakamura M, Yoshida R, Kitagaki H. Initial Experience with Balloon-Occluded Trans-catheter Arterial Chemoembolization (B-TACE) for Hepatocellular Carcinoma. Cardiovasc Intervent Radiol. 2016;39:359–366. doi: 10.1007/s00270-015-1237-6. [DOI] [PubMed] [Google Scholar]
- 21.Takayasu K, Muramatsu Y, Maeda T, Iwata R, Furukawa H, Muramatsu Y, Moriyama N, Okusaka T, Okada S, Ueno H. Targeted transarterial oily chemoembolization for small foci of hepatocellular carcinoma using a unified helical CT and angiography system: analysis of factors affecting local recurrence and survival rates. AJR Am J Roentgenol. 2001;176:681–688. doi: 10.2214/ajr.176.3.1760681. [DOI] [PubMed] [Google Scholar]
- 22.Hayashi K, Ina H, Tezuka M, Okada Y, Irie T. Local therapeutic results of computed tomography-guided transcatheter arterial chemoembolization for hepatocellular carcinoma: results of 265 tumors in 79 patients. Cardiovasc Intervent Radiol. 2007;30:1144–1155. doi: 10.1007/s00270-007-9169-4. [DOI] [PubMed] [Google Scholar]
- 23.Cho KJ, Lunderquist A. The peribiliary vascular plexus: the microvascular architecture of the bile duct in the rabbit and in clinical cases. Radiology. 1983;147:357–364. doi: 10.1148/radiology.147.2.6836115. [DOI] [PubMed] [Google Scholar]
- 24.Tohma T, Cho A, Okazumi S, Makino H, Shuto K, Mochiduki R, Matsubara K, Gunji H, Ochiai T. Communicating arcade between the right and left hepatic arteries: evaluation with CT and angiography during temporary balloon occlusion of the right or left hepatic artery. Radiology. 2005;237:361–365. doi: 10.1148/radiol.2371040919. [DOI] [PubMed] [Google Scholar]
- 25.Ekataksin W. The isolated artery: an intrahepatic arterial pathway that can bypass the lobular parenchyma in mammalian livers. Hepatology. 2000;31:269–279. doi: 10.1002/hep.510310203. [DOI] [PubMed] [Google Scholar]
- 26.Matsumoto T, Endo J, Hashida K, Mizukami H, Nagata J, Ichikawa H, Kojima S, Takashimizu S, Yamagami T, Watanabe N, et al. Balloon-occluded arterial stump pressure before balloon-occluded transarterial chemoembolization. Minim Invasive Ther Allied Technol. 2016;25:22–28. doi: 10.3109/13645706.2015.1086381. [DOI] [PubMed] [Google Scholar]
- 27.Miyayama S, Matsui O, Taki K, Minami T, Ryu Y, Ito C, Nakamura K, Inoue D, Takamatsu S. Arterial blood supply to the posterior aspect of segment IV of the liver from the caudate branch: demonstration at CT after iodized oil injection. Radiology. 2005;237:1110–1114. doi: 10.1148/radiol.2373041660. [DOI] [PubMed] [Google Scholar]
- 28.Miyayama S, Yamashiro M, Okuda M, Yoshie Y, Nakashima Y, Ikeno H, Orito N, Notsumata K, Watanabe H, Toya D, et al. Main bile duct stricture occurring after transcatheter arterial chemoembolization for hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2010;33:1168–1179. doi: 10.1007/s00270-009-9781-6. [DOI] [PubMed] [Google Scholar]
- 29.Kakuta A, Shibutani K, Ono S, Miura H, Tsushima F, Kakehata S, Basaki K, Fujita H, Seino H, Fujita T, et al. Temporal variations in stump pressure and assessment of images obtained from cone-beam computed tomography during balloon-occluded transarterial chemoembolization. Hepatol Res. 2016;46:468–476. doi: 10.1111/hepr.12579. [DOI] [PubMed] [Google Scholar]
- 30.Ishikawa T, Imai M, Owaki T, Sato H, Nozawa Y, Sano T, Iwanaga A, Seki K, Honma T, Yoshida T, et al. Hemodynamic Changes on Cone-Beam Computed Tomography during Balloon-Occluded Transcatheter Arterial Chemoembolization Using Miriplatin for Hepatocellular Carcinoma: A Preliminary Study. Dig Dis. 2017;35:598–601. doi: 10.1159/000480255. [DOI] [PubMed] [Google Scholar]
- 31.Yoshimatsu R, Yamagami T, Ishikawa M, Kajiwara K, Aikata H, Chayama K, Awai K. Change in Imaging Findings on Angiography-Assisted CT During Balloon-Occluded Transcatheter Arterial Chemoembolization for Hepatocellular Carcinoma. Cardiovasc Intervent Radiol. 2016;39:865–874. doi: 10.1007/s00270-015-1279-9. [DOI] [PubMed] [Google Scholar]
- 32.Asayama Y, Nishie A, Ishigami K, Ushijima Y, Takayama Y, Okamoto D, Fujita N, Morita K, Honda H. Hemodynamic changes under balloon occlusion of hepatic artery: predictor of the short-term therapeutic effect of balloon-occluded transcatheter arterial chemolipiodolization using miriplatin for hepatocellular carcinoma. Springerplus. 2016;5:157. doi: 10.1186/s40064-016-1880-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tognolini A, Louie JD, Hwang GL, Hofmann LV, Sze DY, Kothary N. Utility of C-arm CT in patients with hepatocellular carcinoma undergoing transhepatic arterial chemoembolization. J Vasc Interv Radiol. 2010;21:339–347. doi: 10.1016/j.jvir.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 34.Miyayama S, Yamashiro M, Hattori Y, Orito N, Matsui K, Tsuji K, Yoshida M, Matsui O. Efficacy of cone-beam computed tomography during transcatheter arterial chemoembolization for hepatocellular carcinoma. Jpn J Radiol. 2011;29:371–377. doi: 10.1007/s11604-011-0568-8. [DOI] [PubMed] [Google Scholar]
- 35.Miyayama S, Yamashiro M, Okuda M, Yoshie Y, Sugimori N, Igarashi S, Nakashima Y, Matsui O. Usefulness of cone-beam computed tomography during ultraselective transcatheter arterial chemoembolization for small hepatocellular carcinomas that cannot be demonstrated on angiography. Cardiovasc Intervent Radiol. 2009;32:255–264. doi: 10.1007/s00270-008-9468-4. [DOI] [PubMed] [Google Scholar]
- 36.Takayasu K, Maeda T, Iwata R. Sensitivity of superselective arteriography for small hepatocellular carcinoma compared with proximal arteriography and computed tomography during superselective arteriography. Jpn J Clin Oncol. 2002;32:191–195. doi: 10.1093/jjco/hyf046. [DOI] [PubMed] [Google Scholar]
- 37.Sze DY, Razavi MK, So SK, Jeffrey RB Jr. Impact of multidetector CT hepatic arteriography on the planning of chemoembolization treatment of hepatocellular carcinoma. AJR Am J Roentgenol. 2001;177:1339–1345. doi: 10.2214/ajr.177.6.1771339. [DOI] [PubMed] [Google Scholar]
- 38.Fujita T, Ito K, Tanabe M, Yamatogi S, Sasai H, Matsunaga N. Iodized oil accumulation in hypervascular hepatocellular carcinoma after transcatheter arterial chemoembolization: comparison of imaging findings with CT during hepatic arteriography. J Vasc Interv Radiol. 2008;19:333–341. doi: 10.1016/j.jvir.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 39.Kudo M, Ueshima K, Kubo S, Sakamoto M, Tanaka M, Ikai I, Furuse J, Murakami T, Kadoya M, Kokudo N; Liver Cancer Study Group of Japan. Response Evaluation Criteria in Cancer of the Liver (RECICL) (2015 Revised version) Hepatol Res. 2016;46:3–9. doi: 10.1111/hepr.12542. [DOI] [PubMed] [Google Scholar]
- 40.Kawamura Y, Ikeda K, Fujiyama S, Hosaka T, Kobayashi M, Saitoh S, Sezaki H, Akuta N, Suzuki F, Suzuki Y, et al. Usefulness and limitations of balloon-occluded transcatheter arterial chemoembolization using miriplatin for patients with four or fewer hepatocellular carcinoma nodules. Hepatol Res. 2017;47:338–346. doi: 10.1111/hepr.12754. [DOI] [PubMed] [Google Scholar]
- 41.Minami Y, Minami T, Chishina H, Arizumi T, Takita M, Kitai S, Yada N, Hagiwara S, Tsurusaki M, Yagyu Y, et al. Balloon-Occluded Transcatheter Arterial Chemoembolization for Hepatocellular Carcinoma: A Single-Center Experience. Oncology. 2015;89 Suppl 2:27–32. doi: 10.1159/000440628. [DOI] [PubMed] [Google Scholar]
- 42.Miyayama S, Matsui O, Yamashiro M, Ryu Y, Kaito K, Ozaki K, Takeda T, Yoneda N, Notsumata K, Toya D, et al. Ultraselective transcatheter arterial chemoembolization with a 2-f tip microcatheter for small hepatocellular carcinomas: relationship between local tumor recurrence and visualization of the portal vein with iodized oil. J Vasc Interv Radiol. 2007;18:365–376. doi: 10.1016/j.jvir.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 43.Hatanaka T, Arai H, Shibasaki M, Tojima H, Takizawa D, Toyoda M, Takayama H, Abe T, Sato K, Kakizaki S, et al. Factors predicting overall response and overall survival in hepatocellular carcinoma patients undergoing balloon-occluded transcatheter arterial chemoembolization: A retrospective cohort study. Hepatol Res. 2018;48:165–175. doi: 10.1111/hepr.12912. [DOI] [PubMed] [Google Scholar]
- 44.Ishikawa T, Abe S, Inoue R, Sugano T, Watanabe Y, Iwanaga A, Seki K, Honma T, Nemoto T, Takeda K, et al. Predictive factor of local recurrence after balloon-occluded TACE with miriplatin (MPT) in hepatocellular carcinoma. PLoS One. 2014;9:e103009. doi: 10.1371/journal.pone.0103009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ishikawa T, Abe S, Hoshii A, Yamada Y, Iiduka A, Nemoto T, Takeda K, Yoshida T. Cone-Beam Computed Tomography Correlates with Conventional Helical Computed Tomography in Evaluation of Lipiodol Accumulation in HCC after Chemoembolization. PLoS One. 2016;11:e0145546. doi: 10.1371/journal.pone.0145546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Okusaka T, Okada S, Nakanishi T, Fujiyama S, Kubo Y. Phase II trial of intra-arterial chemotherapy using a novel lipophilic platinum derivative (SM-11355) in patients with hepatocellular carcinoma. Invest New Drugs. 2004;22:169–176. doi: 10.1023/B:DRUG.0000011793.72775.d1. [DOI] [PubMed] [Google Scholar]
- 47.Miyayama S, Yamashiro M, Shibata Y, Hashimoto M, Yoshida M, Tsuji K, Toshima F, Matsui O. Comparison of local control effects of superselective transcatheter arterial chemoembolization using epirubicin plus mitomycin C and miriplatin for hepatocellular carcinoma. Jpn J Radiol. 2012;30:263–270. doi: 10.1007/s11604-011-0043-6. [DOI] [PubMed] [Google Scholar]
- 48.Seko Y, Ikeda K, Kawamura Y, Fukushima T, Hara T, Sezaki H, Hosaka T, Akuta N, Suzuki F, Kobayashi M, et al. Antitumor efficacy of transcatheter arterial chemoembolization with warmed miriplatin in hepatocellular carcinoma. Hepatol Res. 2013;43:942–949. doi: 10.1111/hepr.12041. [DOI] [PubMed] [Google Scholar]
- 49.Kora S, Urakawa H, Mitsufuji T, Osame A, Higashihara H, Yoshimitsu K. Warming effect on miriplatin-lipiodol suspension as a chemotherapeutic agent for transarterial chemoembolization for hepatocellular carcinoma: preliminary clinical experience. Cardiovasc Intervent Radiol. 2013;36:1023–1029. doi: 10.1007/s00270-012-0537-3. [DOI] [PubMed] [Google Scholar]
- 50.Iwazawa J, Hashimoto N, Ohue S, Muramoto O, Mitani T. Chemoembolization-induced arterial damage: Evaluation of three different chemotherapeutic protocols using epirubicin and miriplatin. Hepatol Res. 2014;44:201–208. doi: 10.1111/hepr.12104. [DOI] [PubMed] [Google Scholar]
- 51.The Cancer of the Liver Italian Program (CLIP) investigators. A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients: Hepatology. 1998;28:751–755. doi: 10.1002/hep.510280322. [DOI] [PubMed] [Google Scholar]
- 52.Kudo M, Chung H, Haji S, Osaki Y, Oka H, Seki T, Kasugai H, Sasaki Y, Matsunaga T. Validation of a new prognostic staging system for hepatocellular carcinoma: the JIS score compared with the CLIP score. Hepatology. 2004;40:1396–1405. doi: 10.1002/hep.20486. [DOI] [PubMed] [Google Scholar]
- 53.Gillmore R, Stuart S, Kirkwood A, Hameeduddin A, Woodward N, Burroughs AK, Meyer T. EASL and mRECIST responses are independent prognostic factors for survival in hepatocellular cancer patients treated with transarterial embolization. J Hepatol. 2011;55:1309–1316. doi: 10.1016/j.jhep.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 54.Shim JH, Lee HC, Kim SO, Shin YM, Kim KM, Lim YS, Suh DJ. Which response criteria best help predict survival of patients with hepatocellular carcinoma following chemoembolization? A validation study of old and new models. Radiology. 2012;262:708–718. doi: 10.1148/radiol.11110282. [DOI] [PubMed] [Google Scholar]