Abstract
Knowledge of rare diseases (RD) is often scattered among many data collections and registries of patient cohorts. Therefore, assessing the burden of RD in the general population, developing appropriate policies and planning services for the care of RD patients is difficult. This study aimed at providing a systematic picture of RD occurrence in a population as big as 60 million. Data of diagnoses were certified and collected by a network of 247 specialized centres covering the whole Italian territory. Data received (about 200,000 records) were validated according to formal criteria and, where necessary, corrected by the data sources. Data of age at onset and sex distribution are given for about 400 diseases. Incidence and/or birth prevalence are given for 275 diseases and 47 disease groups, which, altogether, comprise a substantial part of the known rare diseases. Data quality, internal consistency, and external validity of the database have also been assessed and ways to limit the impact of some discrepancies were devised. The information provided by RNMR, cutting across such a wide range of RD, represents a unique coherent basis allowing the prioritization of relevant public health measures and research activities.
Keywords: rare diseases, national registry, data quality, epidemiology, Italy
1. Introduction
Rare diseases (RD) are complex diseases with a very low prevalence (in the EU, a prevalence of less than 5/10,000 in the general population is considered). These conditions are currently estimated to be as many as 6000–8000 and the International Classification of Diseases codes a very limited number of them. Consequently, hospital health records are often not the appropriate sources to get information on RD. Therefore, knowledge on RD is often based on data collections and registries resulting from academic and commercial interests. ORPHANET censused a total of more than 700 registries and databases on RD [1] involving European scientists. They address a variety of aims and differ in their organization, quality and database structure, usually monitoring one disease or a group of related diseases [2]. Moreover, many studies are based on hospital data in regions with a higher prevalence [3]. This situation results in the difficulty of assessing the burden of RD in the general population and developing appropriate priority policies for the care of RD patients and the planning of health and social care services. To overcome these difficulties and support the development of knowledge on RD, the USA NIH-NCATS launched the Global RD Patient Registry and Data Repository—GRDR [4], and the EU Council issued a Recommendation urging Member States to support specific disease information networks, registries, and databases [5]. The EU also supported several initiatives addressing the better exploitation of RD patient data [6,7,8,9,10,11]. All these efforts envisage a system addressed to the registration of many, not to say all, RD and to the improvement of data interoperability. The need for efficient and extensive registries for RDs is also made urgent by international cooperations, such as IRDiRC [12] and the European Reference Networks [13], as well as by the establishment of National Registries [14].
The specificities of RD and the special needs of RD patients were recognized in Italy in 2001 when the National Network of Accredited Centres for the assistance and care of RD patients was established by the Ministerial Decree (MD) 279/2001 [15]. This Decree mandated the notification of new cases to the National Registry of Rare Diseases (RNMR), through Regional Registries (RR). The RNMR central database was placed in the National Institute of Health and has been managed by the National Centre for Rare Diseases since its establishment. This registry can be of interest to the current efforts of promoting data comparability and data retrieval across registries for many reasons. RNMR is organized as a devolved system of 17 regional registries, two registries of Autonomous Provinces, and one interregional registry (Piemonte and Valle d’Aosta), which cover populations spanning from about 10.0 to 0.3 million residents. They are controlled by regional or provincial authorities, which make independent decisions and have their own peculiar needs and resources regarding the delivery of care. Therefore, RNMR may represent a pilot on the feasibility and use of the cooperation among national registries, which will seemingly be developed by mainly following the needs of different countries. Moreover, the set of data collected in the central repository very closely resembles the mandatory Common Data Set proposed by EPIRARE [16], which aimed at building synergies among public health authorities, researchers, patients, and the industry for fulfilling their information needs. To this aim, the EPIRARE Common Data Set was devised to support the production of indicators useful for decision-making in public health and research on RD [6,16,17], as well as to fulfill some patients’ information needs. A recent publication by a regional registry which contributes to RNMR, provided some indicator data relevant to RD and their care [18].
After achieving the full coverage of the national territory in 2011 monitoring, individually, 284 RD and, collectively, many more RD distinguished in 47 groups, it is now possible to use the data contained in RNMR. In this paper, we aim to provide a detailed and systematic picture of the epidemiology of RD in Italy, with particular reference to patients requiring RD dedicated assistance and care. The results have been obtained from 247 accredited hospitals, which cover the whole national territory and serve a population of 60.4 million residents. This population is characterized by a sex ratio of 0.94 (males:females), while the youth (0–14 year) and 65+ people make up 13.9% and 21.4%, respectively. In 2017, non-European foreigners were mostly from Western and Northern Africa (1.70% residents), Eastern and Central-Southern Asia (1.66%), and Latin America (0.58), with all other origins accounting for 0.22%. Since this is the first time that an analysis of RNMR data has been published, special attention has been devoted to assessing and describing the validity of the data collected.
2. Materials and Methods
2.1. Set of Data Communicated to RNMR and Collection Procedures
The data communicated to RNMR by the RR and used for further elaborations were:
Personal identifiers of the patient: name, surname, place and date of birth, sex, and national ID code (represented by the Fiscal Code and by an Encrypted Univocal Patient Code derived from it)
Patient place of residence
Disease diagnosed (defined according to the exemption codes listed in MD 279/2001 [15])
Center making the diagnosis, with geographical location (coded according to the National Health Service Information System [19])
Date of disease onset
Date of diagnosis
Data, which referred to the time point when the RD diagnosis is certified by an Accredited Centre, was communicated immediately to its RR by the Accredited Centre itself. This procedure was formally agreed with the regional authorities in 2007 [20] and ensures that diagnoses are notified based on the most reliable diagnostic protocols and best expert advice. Following this agreement, two slightly different record structures were developed by the centers to better serve their regional health service organization. In some regions, the record reported the centre which ascertained the RD diagnosis for the first time and the Accredited Centre notifying the case, while, in other regions, the record indicated the Accredited Centre entitled to formally certify the diagnosis and notify the case. Correspondingly, the date of diagnosis was reported as the date of the first ascertainment of the diagnosis or as the date on which the diagnosis was formally certified and notified. Piemonte, Valle d’Aosta, and Lombardia communicated both the dates of diagnosis ascertainment and certification, while Sardinia switched from the former to the latter record structure in 2012–2013. The date of diagnosis is a main reference for data selection and elaboration. Therefore, data were kept distinct: records characterized by the diagnosis ascertainment date are indicated as “AD notifications” and the regions using this record structure are referred to as “AD regions”. The records characterized by the diagnosis certification date are indicated as “CD notifications” and the related regions as “CD regions”. The coexistence of two notification practices had an important bearing on the epidemiological data. Indeed, as it will be shown in more detail in the results section, frequency data of a disease in a regional population were usually a mix of data notified by AD and CD regions, due to the migration of patients from the residence region to another region, which could confirm the RD diagnosis and notified the case. Finally, Friuli-Venezia Giulia (FVG) communicated no personal identifiers in compliance with local personal data protection regulations. Procedures of data collection and communication have been described elsewhere [21].
The collected data set and the data flow were established and made mandatory by the supporting MD 279/2001 [15]. Therefore, no patient consent nor ethical approval was required. Data were processed according to the EU and Italian data protection regulations in all participating structures. Data processing procedures were communicated to the National Authority for Personal Data Protection and their approval was obtained.
2.2. Aims, Procedures and Limitations of Data Curation
Data curation aimed at spotting erratic or inconsistent data, at discarding records that referred to cases diagnosed after the end of the reference period (31 December 2014) and at detecting and managing duplicate records. Data communicated by RR were checked upon their reception by the National Centre of Rare Diseases. Regional duplicates (i.e., records sent by the same region, referred to the same national ID code) and records showing data not passing the validation criteria (detailed in Supplementary File 1), were fed back to the experts responsible for pertaining the RR, for them to perform the necessary checks, even with the primary data sources, and to confirm or correct the data. After receiving the data sets resulting from these controls, the residual mistakes and discrepancies were recorded; then, the name, surname, place of birth, and Fiscal Code of the patient were removed by all records and the place of residence of the patient was substituted by her/his region of residence; finally, the regional data sets were merged into a single file. In this file, national duplicates (i.e., records with the same Encrypted Univocal Patient Code) were detected, classified, and managed to obtain the subsets to be used according to the scope of the analyses, as reported in Supplementary File 1 (Table S1. Control of duplicate records and curation of demographic data from Friuli-Venezia Giulia (FVG) could not be carried out. Data curation was accomplished by means of an Excel model.
2.3. Symptoms Onset Age and Distribution between Sexes
These features have been calculated from the data of Subset 1 for diseases with at least four records with valid data. For these analyses, data of individual diseases identified with their specific denomination were used, including those coded with group codes.
2.4. Birth Prevalence and Incidence of Rare Diseases
The data used for the calculation of the incidence rates refer to cases with date of diagnosis ascertainment or certification included in the period from 1 January 2012 to 31 December 2014. The data selected for the assessment of birth prevalence (BP) were limited to cases born between 1 January 2012 and 31 December 2013 and with diagnoses ascertained or certified during the first year of life of the case. In these calculations, the dates of diagnosis ascertainment were only considered in the records from Piemonte, Val d’Aosta, and Lombardia. Records were analysed by codes, so that diseases coded with group codes were not analysed individually.
The calculations of regional incidence and BP rates were carried out as follows. It was assumed that differences in the frequency of each pathology, as resulting from either AD or CD notifications, were mainly due to the different local sensitivities of two notification practices rather than to changes of RD frequencies over time. Therefore, for each pathology and each region, the fraction of AD or CD notifications in their respective reference periods were expressed in percent with respect to the total AD plus CD notifications. The notification practice, which represented at least 90% of total notifications of a region over its reference period, was used to calculate the disease incidence and BP in that region. For those diseases and regions where neither notification system reached 90% total regional notifications, the calculation was not carried out. The assumption was applied considering that the datasets from Piemonte, Valle d’Aosta, and Lombardia, which recorded both dates, showed differences between dates of ascertainment and dates of certification of 4.9 years (95% Confidence Interval: 4.4–5.4 years), 8.5 years (95% CI: 6.1–10.9), and 5.3 years (95% CI: 4.9–5.7), respectively (data not shown).
Mean national incidence and BP rates for the three-year period of 2012–2014 were estimated with the same assumption as above, that the frequency of new cases in the period defined by the diagnosis certification date did not differ significantly from the frequency of new cases in the period defined by the diagnosis ascertainment date. Therefore, AD and CD notifications were merged and their sum was used for national frequency estimates. Regional populations on 31 December 2012, 2013, and 2014 [22] and regional live births in the two-year period of 2012–2013 [23] were used to calculate the denominators for regional and national data.
2.5. Internal Consistency of the Incidence and Birth Prevalence Data and Comparison with Literature Data
The internal consistency of the RNMR database has been checked by comparing the medians of the regional incidence and BP rates obtained from AD and CD notifications. The comparison with literature data was limited to the diseases which were monitored by RNMR and could be traced with certainty to an ORPHA code. In addition, comparisons of BP data were limited to diseases which showed a median onset age lower than six months. Results from the RNMR database were firstly compared with literature data reported by ORPHANET [3,24]. In cases where differences of more than one order of magnitude were observed, a further, dedicated, literature search of incidence and BP estimates in the general population was carried out.
2.6. Statistical Methods
Data were reduced by means of contingency tables using RNMR individual disease and disease group codes for frequency calculations. Specific disease names were used to determine the sex ratio and the age at disease onset. Therefore, these features were determined separately for diseases coded with the same group code. The dispersion of regional notifications was described by means of medians and quartiles to minimize the effects of specific situations of selected regions, such as: unusual low sensitivity or unusually high frequencies due to the notification of cases diagnosed in previous years, which were especially likely in regions which activated a registry or adopted a CD record structure shortly before the observation period. The dispersion of the age at symptoms onset was described by means of median and extreme values due to the skewness of the dispersion of the values. Data of frequency for each disease were reported as the yearly average calculated from the total notifications in the three-year observation period. The use of yearly notifications was not considered due to the enhancement of stochastic effects for most diseases.
3. Results and Discussion
3.1. General Description of the National Database
The National Database was made up of records associated with 195,492 notifications. Its Subset 1, which refers to disease occurrences, was made up of 190,622 records, after excluding 4870 Type 2 duplicate records from the National Database. Subset 1 records regarded 275 individually-coded diseases and 47 disease groups. Records with dates of diagnosis ascertainment and certification in the period 2012–2014 were 26,870 and 36,292, respectively. Both the National Database and its Subset 1 contain 6607 Type 3 duplicate records related to cases with multiple diagnoses (3.5% total records of Subset 1). These duplicates may represent cases with multiple diseases, second opinions, and revised or refined diagnoses. Since it was not possible to distinguish among these different possibilities, no selection was applied at this time. Most of these records referred to group-coded pathologies and the differing diagnoses pertained to the same code. In 1855 records, referring to 925 cases, the diagnoses pointed at diseases belonging to different chapters of the ICD9-CM.
3.2. Data Quality: Missing, Inaccurate or Inconsistent Values
The disease onset date was missing or inaccurate in 26% of records of the National Database. The limited completeness of the onset date prevented the use of this variable to study the incidence of the monitored RD in the whole database, regardless of the notification practice. Due to the importance of this variable, a dedicated analysis was carried out to characterize the incomplete records. The study considered both the notifying region and the disease. Figure S1 shows the distribution of the notified diseases in completeness classes of the onset date per notifying region. Data presented also includes the conventional dates for unknown dates and asymptomatic cases. It can be observed that the completeness of this date can be very different, even in regions notifying comparable and large fractions of the monitored diseases. Completeness (including conventional values) for each disease or disease group code was, on average, 81.9% (SD: 17.5%; n = 317 codes) for AD regions, while it was 54.2 (SD: 23.6; n = 303 codes) for CD regions, with a statistically significant difference among the two groups (p < 0.001). Therefore, it appears that the completeness of this variable depends, to a considerable extent, on the rules for data access and communication associated with CD or AD notification practices. Since it is not expected that these rules introduce a biased selection of the data for a disease, available data were used for the determination of the disease onset age.
The completeness of the variable indicating the centre making the first ascertainment of the diagnosis was only checked in AD records: this indication was missing in 5% of records. The Fiscal Code and other identifiers were missing in all FVG records, which make up about 1.1% of total records, and in an additional 0.2% of records from other regions. Remarks on the other variables affected less than 1% of records. Table S2 (Supplementary File 1) shows detailed remarks regarding the variables of the National Database.
3.3. Data Quality: Selection of the Regions and Reference Period for Frequency Rates Assessment
Table S3 (Supplementary File 1) shows the overall number of RD occurrences per region of residence of the case. The table provides summary evidence that epidemiological analyses of the available data could not rely on a single AD or CD record structure. Consequently, the analyses were carried out for individual regional populations, using the data from the record structure which dominated for each disease.
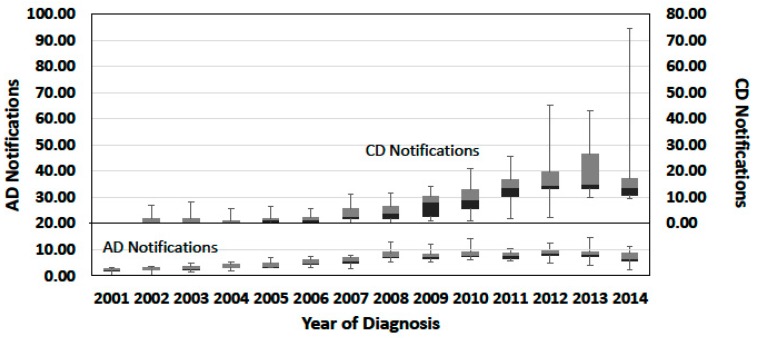
The progress of notifications was studied distinguishing AD from CD notifications, to detect possible differences related to the data collection practice, and the results are summarised in Figure 1. Yearly AD notifications increased from 2001 to 2008 and then remained approximately constant afterwards. The time course of CD notifications showed an increasing trend from 2006 to 2013, when the median of notifications attained the maximum value. The high variance of CD regional notifications is explained by the adoption of the CD record structure by the CD regions at various times till 2011: before 2006, this record structure was only used by Veneto. Moreover, in some regions, the establishment of registries was followed by a peak in the yearly notifications. The variability of the absolute frequencies of regional notifications over time in the periods 2008–2014 and 2012–2014 is presented in Table S4 (Supplementary File 1). Umbria, accounting for about 1.5% of the national population and adopting the CD record structure in 2011, showed by far the highest variability of yearly regional notifications in the period 2012–2014. This period showed the lower variability of both AD and CD notifications with respect to the period 2008–2014. Therefore, it was selected as the reference period for the assessment of RD frequency rates.
Figure 1.
Time course of notifications, by year of diagnosis ascertainment or certification. Dataset: Subset 1; Origin of notifications: Regions using the record structure with the Date of diagnosis Ascertainment (AD) and regions using the record structure with the Date of diagnosis Certification (CD); Selection of records: Records with valid diagnosis ascertainment or certification date; Total records: 115,575 (AD records) and 121,854 (CD records, including those from Lombardia, Piemonte, Sardegna, and Val d’Aosta). Data are expressed as percentage of the total regional notifications (by region of residence) to better compare the progress of AD or CD notifications from regional populations with different dimensions. Absolute numbers of yearly notifications per region of residence are reported in Table S4.
3.4. Symptoms Onset Age and Sex Distribution
Data of age at disease symptoms onset are reported in Table 1 for selected diseases. Table S5 reports data for all 414 diseases in which it was determined. In total, 108 diseases set on in all cases notified before 18 years of age and 15 showed all occurrences setting on after 18. The comparison with literature data could be carried out for almost three hundred diseases using data available in the ORPHANET portal, which mostly referred to the main life stages where the first symptoms set on (Table S5). There was a general agreement between RNMR and literature data, but for about 12% of compared diseases.
Table 1.
Age at symptoms onset of selected rare diseases monitored by RNMR. Dataset: Subset 1; Origin of notifications: all Regions; Record selection: records with diagnosis indicating a specific disease (i.e., excluding disease groups) and at least four records with valid onset date per disease; Total records: 84,859.
| RNMR Code | ORPHA Code | Disease | Records (N) | Median Onset Age (Years) | Life Stages Reported by ORPHANET [24] |
|---|---|---|---|---|---|
| RNG040 | 1791 | Frontofacionasal dysplasia | 7 | −0.28 | Neonatal |
| RN0490 | 3447 | Weaver syndrome | 7 | −0.23 | Neonatal, Antenatal |
| RNG030 | 87 | Apert syndrome | 13 | −0.18 | Antenatal, Neonatal |
| RNG040 | 2108 | Hallermann-Streiff syndrome | 9 | −0.17 | Neonatal, Infancy |
| RP0040 | 1915 | Fetal alcohol syndrome | 56 | −0.14 | Antenatal, Neonatal |
| RNG070 | 313 | Lamellar ichthyosis | 60 | −0.11 | Neonatal |
| RN0400 | 1540 | Jackson-Weiss syndrome | 12 | −0.10 | Neonatal |
| RNG040 | 207 | Crouzon disease | 43 | −0.09 | Infancy, Neonatal |
| RN1150 | 1340 | Cardiofaciocutaneous syndrome | 54 | −0.06 | Antenatal, Neonatal |
| RN0640 | 1114 | Aplasia cutis congenita | 14 | −0.06 | Antenatal, Neonatal |
| RFG010 | 141 | Canavan disease | 6 | −0.06 | Neonatal, infancy (severe form); childhood (mild form) |
| RNG040 | 1452 | Cleidocranial dysostosis | 7 | −0.05 | Neonatal |
| RCG060 | 352 | Galactosemia | 58 | −0.05 | Infancy, Neonatal, Childhood |
| RN0130 | 35,737 | Morning glory syndrome | 10 | −0.05 | Childhood |
| RNG050 | 429 | Hypochondroplasia | 7 | −0.05 | Childhood |
| RP0010 | 290 | Congenital rubella syndrome | 31 | −0.05 | Antenatal, Neonatal |
| RN0390 | 380 | Greig cephalopolysyndactyly syndrome | 15 | −0.04 | Neonatal |
| RDG020 | 326 | Factor V deficiency | 24 | −0.04 | All ages |
| RN0100 | 708 | Peters anomaly | 12 | −0.04 | Infancy, Neonatal |
| RCG040 | 238,583 | Hyperphenylalaninemia | 673 | −0.04 | Neonatal, Infancy |
| RN1040 | 710 | Pfeiffer syndrome | 10 | −0.03 | Antenatal, Neonatal |
| RN1530 | 500 | LEOPARD syndrome | 37 | −0.03 | Childhood |
| RCG040 | 407 | Non-ketotic hyperglycinemia | 6 | −0.03 | Infancy, Neonatal |
| RCG060 | 348 | Fructose-1,6-bisphosphatase deficiency | 4 | −0.03 | All ages |
| RN0510 | 464 | Incontinentia pigmenti | 74 | −0.03 | Neonatal |
| RN1640 | 1466 | COFS syndrome | 6 | −0.03 | Neonatal, Antenatal |
| RN1760 | 912 | Zellweger syndrome | 13 | −0.02 | Neonatal |
| RNG070 | 461 | Recessive X-linked ichthyosis | 60 | −0.02 | Neonatal |
| RP0060 | 415,286 | Bilirubin encephalopathy | 7 | −0.02 | Neonatal |
| RCG050 | 23 | Argininosuccinatelyase deficiency | 12 | −0.02 | Neonatal, All ages |
| RN0910 | 374 | Goldenhar syndrome | 187 | −0.02 | Neonatal, Antenatal |
| RN0600 | 312 | Epidermolytic ichthyosis | 32 | −0.02 | Neonatal |
| RDG020 | 327 | Factor VII deficiency | 98 | −0.02 | All ages |
| RN0990 | 570 | Moebius syndrome | 52 | −0.02 | Neonatal |
| RN0320 | 2368 | Gastroschisis | 57 | −0.02 | Neonatal, Antenatal |
| RDG020 | 328 | Factor X deficiency | 9 | −0.02 | All ages |
| RN0700 | 280 | Wolf-Hirschhorn syndrome | 64 | −0.02 | Neonatal, Antenatal |
| RN1080 | 813 | Silver-Russell syndrome | 85 | −0.02 | Neonatal, Antenatal |
| RN0180 | 1203 | Duodenal atresia | 75 | −0.02 | Antenatal, Neonatal, Infancy, Childhood |
| RN0540 | 1556 | Cutis marmorata telangiectatica congenita | 21 | −0.02 | Neonatal |
| RCG040 | 511 | Maple syrup urine disease | 26 | −0.02 | Infancy, Neonatal, Childhood |
| RNG070 | 634 | Netherton syndrome | 17 | −0.01 | Infancy, Neonatal |
| RN1100 | 808 | Seckel syndrome | 11 | −0.01 | Neonatal, Antenatal |
| RN1310 | 739 | Prader-Willi syndrome | 388 | −0.01 | Neonatal, Antenatal |
| RCG040 | 35 | Propionic acidemia | 4 | −0.01 | Infancy, Neonatal |
| RN0080 | 1764 | Familial dysautonomia | 6 | −0.01 | Neonatal, Antenatal |
| RDG020 | 331 | Factor XIII deficiency | 5 | −0.01 | All ages |
| RN0850 | 138 | CHARGE syndrome | 116 | −0.01 | Neonatal |
| RCG040 | 293,355 | Methylmalonic acidemia | 11 | −0.01 | All ages |
| RN1170 | 744 | Proteus syndrome | 19 | −0.01 | Infancy |
| RN0170 | 1201 | Atresia of small intestine | 77 | −0.01 | Neonatal |
| RN1140 | 107 | Branchio-oto-renal syndrome | 35 | −0.01 | All ages |
| RN1250 | 887 | VACTERL/VATER association | 80 | −0.01 | Neonatal |
| RN1410 | 199 | Cornelia de Lange syndrome | 74 | −0.01 | Neonatal, Antenatal |
| RN0930 | 392 | Holt-Oram syndrome | 23 | −0.01 | Neonatal |
| RDG020 | 98,878 | Hemophilia A | 1209 | −0.01 | Infancy, Neonatal |
| RDG020 | 98,879 | Hemophilia B | 202 | 0.00 | Infancy, Neonatal |
| RN1740 | 899 | Walker-Warburg syndrome | 6 | 0.00 | Infancy, Neonatal |
| RN1010 | 648 | Noonan syndrome | 459 | 0.00 | Neonatal |
| RN0430 | 2911 | Poland syndrome | 299 | 0.00 | Infancy, Neonatal |
| RDG020 | 745 | Protein C deficiency | 233 | 0.00 | Childhood |
| RN0200 | 388 | Hirschsprung disease | 244 | 0.00 | Infancy, Neonatal |
| RN0060 | 2162 | Holoprosencephaly | 46 | 0.00 | Neonatal, Antenatal |
| RN1240 | 857 | Townes-Brocks syndrome | 8 | 0.00 | All ages |
| RN1130 | 1297 | Branchio-oculo-facial syndrome | 7 | 0.00 | Neonatal |
| RNG060 | 1522 | Craniometaphyseal dysplasia | 11 | 0.00 | Childhood |
| RN1210 | 819 | Smith-Magenis syndrome | 45 | 0.00 | Infancy, Neonatal |
| RN0890 | 2053 | Freeman-Sheldon syndrome | 9 | 0.00 | Neonatal |
| RCG050 | 187 | Citrullinemia | 10 | 0.00 | Neonatal, Adult |
| RN0360 | 1465 | Coffin-Siris syndrome | 13 | 0.00 | Neonatal |
| RN0410 | 2311 | Jarcho-Levin syndrome | 11 | 0.00 | Neonatal, Antenatal |
| RN0280 | 950 | Acrodysostosis | 12 | 0.00 | Neonatal, Antenatal |
| RN1660 | 35,125 | Epidermal nevus syndrome | 15 | 0.00 | Childhood, Adolescent, Adult |
| RNG040 | 861 | Treacher-Collins syndrome | 16 | 0.00 | Neonatal |
| RN1450 | 94,068 | Congenital spondyloepiphyseal dysplasia | 14 | 0.00 | Neonatal |
| RNG060 | 289 | Ellis-Van Creveld syndrome | 8 | 0.00 | Neonatal, Antenatal |
| RN1590 | 884 | Pallister-Killian syndrome | 16 | 0.00 | Neonatal, Antenatal |
| RN0670 | 281 | Cri du chat syndrome | 46 | 0.00 | Neonatal |
| RN0790 | 915 | Aarskog-Scott syndrome | 28 | 0.00 | Childhood |
| RN0940 | 2322 | Kabuki make-up syndrome | 112 | 0.00 | Infancy, Neonatal |
| RN0040 | 475 | Joubert syndrome | 96 | 0.00 | Neonatal, Antenatal |
| RN1270 | 904 | Williams syndrome | 316 | 0.00 | Neonatal, Antenatal |
| RCG160 | 567 | Di George syndrome | 359 | 0.00 | Neonatal |
| RN1620 | 783 | Rubinstein-Taybi syndrome | 63 | 0.00 | All ages |
| RN1380 | 110 | Bardet-Biedl syndrome | 51 | 0.00 | Infancy, Neonatal, Antenatal |
| RN0660 | 870 | Down syndrome | 2283 | 0.00 | Antenatal, Neonatal |
| RN0680 | 881 | Turner syndrome | 947 | 0.00 | Infancy, Neonatal, Antenatal, Childhood |
| RN0770 | 3205 | Sturge-Weber syndrome | 134 | 0.00 | Infancy, Neonatal, Childhood, Adolescent |
| RN1330 | 908 | Fragile X syndrome | 288 | 0.00 | Neonatal, Infancy, Childhood |
| RN1510 | 90,308 | Klippel-Trénaunay syndrome | 170 | 0.00 | Infancy, Childhood, Adolescent |
| RN0110 | 77 | Aniridia | 69 | 0.00 | Infancy, Neonatal |
| RFG050 | 83,330 | Werdnig-Hoffman disease | 13 | 0.00 | Infancy, Neonatal |
| RCG040 | 26 | Methylmalonic acidemia with homocystinuria | 16 | 0.00 | All ages |
| RN0860 | 3157 | De Morsier syndrome | 46 | 0.00 | Infancy, Neonatal |
| RN1400 | 191 | Cockayne syndrome | 6 | 0.01 | All ages |
| RN1430 | 220 | Denys-Drash syndrome | 5 | 0.02 | Infancy, Neonatal |
| RN0240 | 2138 | True hermaphroditism | 17 | 0.02 | Infancy, Neonatal |
| RN1190 | 2614 | Nail-patella syndrome | 33 | 0.02 | Neonatal, Infancy, Childhood |
| RN0340 | 974 | Adams-Oliver syndrome | 11 | 0.02 | Neonatal |
| RDG020 | 903 | Von Willebrand disease | 800 | 0.02 | All ages |
| RC0180 | 205 | Crigler-Najjar syndrome | 27 | 0.04 | Neonatal |
| RN1730 | 893 | WAGR syndrome | 8 | 0.07 | Neonatal |
| RN0950 | 98,861 | Primary ciliary dyskinesia, Kartagener type | 345 | 0.08 | Neonatal, infancy |
Note: This table reports pathologies ordered by increasing median age at onset. The number of records is indicated with the only aim of allowing a better assessment of the statistical data presented and cannot be used as an indication of the disease or exemption code frequency.
The sex distribution of diseases unevenly frequent between sexes is presented in Table 2. Eleven diseases occurred with a female:male ratio higher than 9:1. Twelve diseases prevailed in males with a female:male ratio lower than 1:9. Data of sex distribution could be compared with data of 65 diseases reported in the ORPHANET data sheets and further literature as having a predominance in either sex. RNMR results showed a good agreement with literature data, except for three diseases (5% comparisons). Table S6 shows the sex distribution of 441 RD reported to RNMR and the comparison with literature data, where available.
Table 2.
Sex distribution of rare diseases monitored by RNMR with the highest relative frequencies in males or females. Dataset: Subset 1; Origin of notifications: all regions; Record selection: records with diagnosis indicating a specific disease (i.e., excluding disease groups) and at least four records per disease; Total records: 119,762.
| ORPHA Code | Disease | Records (N) | Females (%) | Males (%) | F:M Ratio and Other Literature Information Reported in ORPHANET Data Sheets [24] |
|---|---|---|---|---|---|
| 1791 | Frontofacionasal dysplasia | 7 | 100 | 0 | - |
| 209,981 | Iron refractory iron deficiency anemia | 4 | 100 | 0 | - |
| 881 | Turner syndrome | 1754 | 99 | 1 | Almost exclusively in F |
| 778 | Rett syndrome | 603 | 97 | 3 | Predominant in F |
| 538 | Lymphangioleiomyomatosis | 180 | 97 | 3 | Almost exclusively in F |
| 464 | Incontinentia pigmenti | 95 | 97 | 3 | 20:1 |
| 37,202 | Interstitial cystitis | 1192 | 95 | 5 | 9:1 |
| - | Storage pool deficiency | 11 | 91 | 9 | - |
| 816 | Sjögren-Larsson syndrome | 93 | 90 | 9 | - |
| - | Thrombocytopenic thrombotic purpura-hemolytic-uremic syndrome | 10 | 90 | 10 | - |
| 559 | Marinesco-Sjögren syndrome | 30 | 90 | 10 | - |
| 169,615 | Idiopathic central precocious puberty | 3686 | 89 | 11 | 10:1 |
| 98,973 | Posterior polymorphous corneal dystrophy | 8 | 88 | 13 | - |
| 79,473 | Porphyria variegata | 8 | 88 | 13 | Predominant in F |
| 1297 | Branchio-oculo-facial syndrome | 8 | 88 | 13 | - |
| 3143 | Schmidt syndrome | 72 | 86 | 14 | - |
| 1452 | Cleidocranial dysostosis | 7 | 86 | 14 | - |
| 429 | Hypochondroplasia | 7 | 86 | 14 | - |
| 809 | Mixed connective tissue disease | 1092 | 86 | 14 | 10:1 |
| 3287 | Takayasu arteritis | 346 | 85 | 14 | Predominant in F |
| 2483 | Melkersson-Rosenthal syndrome | 25 | 84 | 16 | - |
| 317 | Erythrokeratodermia variabilis | 6 | 83 | 17 | - |
| 98,958 | Droplet cornea | 17 | 82 | 18 | - |
| 436 | Hypophosphatasia | 11 | 82 | 18 | - |
| - | Secretion deficiency thrombocytopathy | 11 | 82 | 18 | - |
| 125 | Bloom syndrome | 5 | 80 | 20 | - |
| 79,273 | Hereditary coproporphyria | 5 | 80 | 20 | Predominant in F |
| 98,974 | Fuchs endothelial dystrophy | 30 | 80 | 20 | 3–4:1 |
| 581 | Mucopolysaccharidosis type 3 | 5 | 80 | 20 | - |
| 2059 | Fryns syndrome | 5 | 80 | 20 | - |
| 65,684 | Hirayama disease | 5 | 20 | 80 | 1:20 |
| 3447 | Weaver syndrome | 10 | 20 | 80 | - |
| 98,895 | Becker muscular dystrophy | 221 | 19 | 81 | Predominant in M |
| 379 | Chronic granulomatous disease | 87 | 18 | 82 | - |
| 221,117 | Gerstmann syndrome | 6 | 17 | 83 | - |
| 97,360 | Robinow syndrome | 6 | 17 | 83 | - |
| 478 | Kallmann syndrome | 509 | 16 | 84 | 1:5 |
| 101,330 | Porphyria cutanea tarda | 53 | 15 | 85 | Predominant in M |
| - | Opitz syndrome | 20 | 15 | 85 | - |
| 1466 | COFS syndrome | 7 | 14 | 86 | - |
| - | Simpson-Golabi-Behmel syndrome | 7 | 14 | 86 | - |
| 2796 | Pachydermoperiostosis | 15 | 13 | 87 | 1:7 |
| 481 | Kennedy disease | 51 | 12 | 88 | - |
| 98,879 | Hemophilia B | 271 | 11 | 89 | Predominant in M |
| 223 | Nephrogenic diabetes insipidus | 66 | 11 | 89 | - |
| 915 | Aarskog-Scott syndrome | 38 | 8 | 92 | Predominant in M |
| 98,878 | Hemophilia A | 1577 | 7 | 93 | Predominant in M |
| 98,896 | Duchenne muscular dystrophy | 258 | 5 | 95 | Predominant in M |
| 461 | Recessive X-linked ichthyosis | 65 | 3 | 97 | Almost exclusively in M |
| - | Klinefelter syndrome | 2071 | 0 | 100 | - |
| 330 | Factor XII deficiency | 4 | 0 | 100 | - |
| 425 | Familial hypoalphalipoproteinemia | 4 | 0 | 100 | - |
| 510 | Lesch-Nyhan disease | 12 | 0 | 100 | Severe form predominant in M |
| 534 | Lowe syndrome | 5 | 0 | 100 | Almost exclusively in M |
| 649 | Norrie disease | 5 | 0 | 100 | Almost exclusively in M |
| 580 | Mucopolysaccharidosis type 2 | 27 | 0 | 100 | Almost exclusively in M |
| 245 | Nager syndrome | 4 | 0 | 100 | - |
Note: The number of records is indicated with the only aim of allowing a better assessment of the statistical data presented and cannot be used as an indication of the disease or exemption code frequency. Where the percentages in males and females sum to less than 100, the difference represents records with missing sex data.
3.5. Incidence in the General Population and Prevalence in Live Births
Table 3 and Table 4 report, respectively, the rates of diseases (and disease groups) most incident in the general population and most prevalent at birth. National and regional incidence and BP rates of all diseases and disease groups notified to RNMR in Italy are reported in Tables S7 and S8. The collection of data of such a wide range of diseases has been made possible thanks to the sustainability of the registration and, since 2011, full coverage of the population residing in the whole Italian territory. Full population coverage relies on the national network for the diagnosis and care of RD, made up of 247 accredited centres belonging to the public health service [21]. Completeness of case ascertainment relies on mandatory case notification and the possibility for the patients to access RD-specific assistance when their condition is certified by these centres. However, completeness of case ascertainment may depend on the actual need for RD-specific assistance and care. Moreover, the occurrence of diseases and malformations in still births and at termination of pregnancy for foetal anomalies is not recorded. This implies that RNMR data are actually representative of patients requiring care in specialized RD centres.
Table 3.
National Incidence rates of most incident rare diseases and rare disease groups monitored by RNMR in the Italian population. Yearly average in the three-year period 2012–2014.
| ORPHA Code | Disease Name | National Incidence (/Million) |
|---|---|---|
| 156,071 | Keratoconus | 25.53 |
| 803 | Amyotrophic lateral sclerosis | 19.15 |
| 870 | Down syndrome | 7.76 |
| 169,615 | Idiopathic central precocious puberty | 7.33 |
| - | Lichen sclerosus | 5.92 |
| - | Bullous pemphigoid | 4.88 |
| 930 | Idiopathic achalasia | 4.63 |
| 117 | Behçet disease | 4.57 |
| - | Arnold-Chiari malformation | 4.19 |
| 2932 | Chronic inflammatory demyelinating polyneuropathy | 3.77 |
| - | Pemphigus vulgaris | 3.75 |
| - | Klinefelter syndrome | 3.75 |
| 397 | Giant cell arteritis | 3.73 |
| 399 | Huntington disease | 3.38 |
| 37,202 | Interstitial cystitis | 3.19 |
| 881 | Turner syndrome | 2.53 |
| 98,249 | Ehlers-Danlos syndrome | 2.43 |
| 774 | Hereditary hemorrhagic telangiectasia | 2.36 |
| 2331 | Kawasaki disease | 2.08 |
| 732 | Polymyositis | 1.95 |
| 733 | Familial adenomatous polyposis | 1.90 |
| 183 | Churg-Strauss syndrome | 1.88 |
| 221 | Dermatomyositis | 1.87 |
| 809 | Mixed connective tissue disease | 1.80 |
| 558 | Marfan syndrome | 1.79 |
| 900 | Wegener granulomatosis | 1.77 |
| - | Mixed cryoglobulinemia | 1.75 |
| - | Idiopathic torsion dystonia | 1.46 |
| - | Rheumatic endocarditis | 1.36 |
| 761 | Henoch-Schönlein purpura | 1.35 |
| 648 | Noonan syndrome | 1.30 |
| 805 | Tuberous sclerosis | 1.18 |
| 908 | Fragile X syndrome | 1.15 |
| 2911 | Poland syndrome | 1.14 |
| 727 | Microscopic polyangiitis | 1.14 |
| 171 | Primary sclerosing cholangitis | 1.03 |
| 904 | Williams syndrome | 0.98 |
| 2073 | Narcolepsy | 0.97 |
| 478 | Kallmann syndrome | 0.94 |
| 96,346 | Anorectal malformation | 0.93 |
| 388 | Hirschsprung disease | 0.88 |
| 790 | Retinoblastoma | 0.83 |
| 91,378 | Hereditary angioedema | 0.81 |
| 104 | Leber hereditary optic neuropathy | 0.79 |
| 739 | Prader-Willi syndrome | 0.78 |
| 70,590 | Infantile apnea | 0.78 |
| 116 | Beckwith-Wiedemann syndrome | 0.76 |
| 550 | MELAS | 0.73 |
| 49,041 | Retroperitoneal fibrosis | 0.72 |
| 3451 | West syndrome | 0.70 |
| Disease Group Name | ||
| - | Hereditary coagulation defects | 25.16 |
| - | Undifferentiated connective tissue syndromes | 17.75 |
| - | Hereditary retinic dystrophies | 17.60 |
| - | Hereditary anemias | 15.04 |
| - | Neurofibromatoses | 10.08 |
| - | Muscular dystrophies | 8.12 |
| - | Congenital alterations of iron metabolism | 8.04 |
| - | Disturbances of aminoacid transport and metabolism | 7.92 |
| - | Chromosomal duplication/deficiency syndromes | 7.82 |
| - | Hereditary neuropathies | 6.61 |
Table 4.
National BP Rates of most birth-prevalent rare diseases and rare disease groups monitored by RNMR in the Italian population. Rates in live births of the two-year period 2012–2013.
| ORPHA Code | Disease Name | Birth Prevalence (/100,000) |
|---|---|---|
| 870 | Down syndrome | 35.00 |
| - | Esophageal atresia and/or Isolated tracheo-esophageal fistula | 6.67 |
| 96,346 | Anorectal malformation | 6.37 |
| 3451 | West syndrome | 5.69 |
| 388 | Hirschsprung disease | 4.22 |
| 739 | Prader-Willi syndrome | 3.63 |
| - | Biliary atresia | 3.14 |
| 805 | Tuberous sclerosis | 2.84 |
| 116 | Beckwith-Wiedemann syndrome | 2.65 |
| 648 | Noonan syndrome | 2.45 |
| 881 | Turner syndrome | 1.96 |
| 1203 | Duodenal atresia | 1.86 |
| 374 | Goldenhar syndrome | 1.67 |
| 1201 | Atresia of small intestine | 1.67 |
| 290 | Congenital rubella syndrome | 1.47 |
| 904 | Williams syndrome | 1.47 |
| - | Epidermolysis bullosa | 1.37 |
| 2911 | Poland syndrome | 1.27 |
| 2368 | Gastroschisis | 1.18 |
| 138 | CHARGE syndrome | 1.18 |
| - | Lissencephaly | 1.08 |
| 98,861 | Primary ciliary dyskinesia, Kartagener type | 1.08 |
| 464 | Incontinentia pigmenti | 0.78 |
| 887 | VACTERL/VATER association | 0.78 |
| - | Congenital colobomatous optic disc | 0.69 |
| 3205 | Sturge-Weber syndrome | 0.69 |
| - | Alagille syndrome | 0.69 |
| 2162 | Holoprosencephaly | 0.59 |
| 1896 | EEC syndrome | 0.59 |
| 205 | Crigler-Najjar syndrome | 0.59 |
| 77 | Aniridia | 0.49 |
| 70,590 | Infantile apnea | 0.49 |
| 1915 | Fetal alcohol syndrome | 0.39 |
| 199 | Cornelia de Lange syndrome | 0.39 |
| 908 | Fragile X syndrome | 0.39 |
| 90,308 | Klippel-Trénaunay syndrome | 0.39 |
| 912 | Zellweger syndrome | 0.29 |
| 570 | Moebius syndrome | 0.29 |
| 199,642 | Microcephaly | 0.29 |
| 281 | Cri du chat syndrome | 0.29 |
| 783 | Rubinstein-Taybi syndrome | 0.29 |
| 380 | Greig cephalopolysyndactyly syndrome | 0.20 |
| 280 | Wolf-Hirschhorn syndrome | 0.20 |
| 813 | Silver-Russell syndrome | 0.20 |
| 475 | Joubert syndrome | 0.20 |
| 884 | Pallister-Killian syndrome | 0.20 |
| 3157 | De Morsier syndrome | 0.20 |
| 974 | Adams-Oliver syndrome | 0.20 |
| 726 | Alpers syndrome | 0.20 |
| 435 | Ito hypomelanosis | 0.20 |
| Disease Group Name | ||
| - | Disturbances of aminoacid transport and metabolism | 30.10 |
| - | Congenital craniofacial anomalies | 21.57 |
| - | Chromosomal duplication/deficiency syndromes | 8.73 |
| - | Disturbances of carbohydrate transport and metabolism, excluded diabetes mellitus | 5.39 |
| - | Neurofibromatoses | 3.92 |
| - | Congenital chondrodystrophies | 3.53 |
| - | Congenital ichthyoses | 2.94 |
| - | Other congenital anomalies with intellectual disability | 2.84 |
| - | Chromosomal aneuploidy syndromes | 2.55 |
| - | Urea cycle disturbances | 1.18 |
Median regional incidence and BP rates calculated from CD and from AD collection practices are compared in Tables S9 and S10. Incidence data from the two collection practices showed a Pearson correlation coefficient of 0.78, while the Pearson’s correlation coefficient of BP rates was 0.44. The low correlation of BP rates may be related to the limited population monitored and record selection criteria. For both incidence and BP, the rates calculated from the CD collection practice were, on average, higher than those from the AD collection practice. The reason for such a difference may be related to the different time courses shown by CD and AD notifications (Figure 1), which may involve the certification of cases diagnosed before the adoption of the CD practice.
Incidence data for 34 diseases and BP data for 56 diseases could be compared with data retrieved in the ORPHANET compilations (Tables S11 and S12). Diseases with differences of more than one order of magnitude were subject to a dedicated survey of the literature, which showed that information and data justifying our results were published, except for three and four diseases, respectively, for incidence and BP rates.
4. Conclusions
This study determined basic features to assess the reliability and internal consistency of RNMR data. Data of the three-year period 2012–2014 provided information on 275 diseases and 47 disease groups. Moreover, the onset age and sex distribution have been determined for more than 400 individual diseases. This data is mostly representative of patients requiring care in specialized RD centres and is especially suitable to assess the burden of assistance and care by RD-dedicated resources of the public health service.
The external validity of the data collected in the RNMR database was studied by comparing the RD national estimates derived from it with the estimates of frequency in the general population available in the literature. Differences bigger than an order of magnitude were found for 7–8% diseases for which comparisons were possible regarding national incidence and BP. A similar level of agreement with literature data was found regarding the age at disease onset and sex distribution. Therefore, RNMR data is rather consistent with information available in the literature. The remarkable consistency of RNMR data with data of some diseases, which can be traced reliably from hospital records, was also shown in a recent publication [25].
In conclusion, it appears that RNMR results provide basic epidemiological information, which was still lacking for many RD, and overcome the current typical fragmentation of data resulting from the observation of one or a few related diseases in different, selected populations with varied methodologies. In fact, RNMR results compose a unique systematic picture of the occurrence of a wide range of RD in a population as big as about 60 million residents. Although further efforts are still required to achieve the full exploitation of such a complex and extended collection system, this picture represents a novel and sound information basis to improve the assessment of the rare disease burden, and to inform public health policy planning and research prioritization on rare diseases in Italy and beyond.
Acknowledgments
The authors are gratefully indebted with the following experts, who communicated and cured the data used in this publication: Giuliano LOMBARDI, Giandomenico PALKA (Registro Regionale dell’Abruzzo), Antonella ANGIONE, Domenico TRIPALDI (Registro Regionale della Basilicata), Rosalba BARONE (Registro Regionale della Calabria), Generoso ANDRIA, Roberto della Casa, Simona FECAROTTA, Iris SCALA (Registro Regionale della Campania), Maria VIZIOLI, Matteo VOLTA (Registro Regionale dell’Emilia-Romagna), Bruno BEMBI, Laura DEROMA (Registro Regionale del Friuli-Venezia Giulia), Domenico DI LALLO, Esmeralda CASTRONUOVO, Marco PIGNOCCO (Registro Regionale del Lazio), Mirella ROSSI (Registro Regionale della Liguria), Laura BOTTANELLI, Erica DAINA, Sara GAMBA (Registro Regionale della Lombardia), Orazio GABRIELLI (Registro Regionale delle Marche), Maria Lucia DI NUNZIO (Registro Regionale del Molise), Giuseppina ANNICCHIARICO, Maria MALERBA (Registro Regionale della Puglia), Simone BALDOVINO, Dario ROCCATELLO (Registro Interregionale del Piemonte e della Valle d’Aosta), Antonello ANTONELLI, Paolo MOI, Maria Antonietta PALMAS, Rosanna PORCU (Registro Regionale della Sardegna), Lucia BORSELLINO, Salvatore SCONDOTTO (Registro Regionale della Sicilia), Fabrizio BIANCHI, Anna PIERINI, Federica PIERONI (Registro Regionale della Toscana), Paola CASUCCI, Ombretta CHECCONI, Maria Concetta PATISSO (Registro Regionale dell’Umbria), Paola FACCHIN, Monica MAZZUCATO (Registro Regionale del Veneto), Francesco BENEDICENTI, Carla MELANI, Paola ZUECH (Registro della Provincia Autonoma di Bolzano), Annunziata DI PALMA, Annalisa PEDROLLI (Registro della Provincia Autonoma di Trento).
Supplementary Materials
The following are available online at http://www.mdpi.com/1660-4601/15/7/1470/s1: Supplementary File 1: Criteria for data validation; Table S1. Type of national duplicate records and their management; Figure S1. Completeness of disease onset date by notifying region; Table S2. Results of the quality control of data communicated to RNMR; Table S3. Distribution of records by region of residence of the case and validity of diagnosis ascertainment and certification dates; Table S4. Yearly notifications, by region of residence, in different periods of diagnosis ascertainment or confirmation. Table S5: Age at onset of rare diseases reported to RNMR and comparison with literature data. Table S6: Sex distribution of the rare diseases reported to RNMR and comparison with literature data. Table S7: National and regional incidence of rare diseases notified to RNMR during 2012–2014. Table S8: National and regional birth prevalence of rare diseases notified to RNMR (2012–2013). Table S9: Comparison of regional incidence data from AD and CD notifications. Table S10: Comparison of regional birth prevalence data from AD and CD notifications. Table S11: Comparison of national incidence data with literature data. Table S12: Comparison of national birth prevalence data with literature data. References [26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47] are cited in Supplementary Materials.
Author Contributions
Conceptualization, D.T., L.V., A.R., and P.T.; Software, L.F.; Validation, A.R.; Formal Analysis, A.R.; Methodology: L.V.; Resources, D.T.; Data Curation, A.R., P.T.; Writing-Original Draft Preparation, L.V.; Writing-Review & Editing, D.T., L.V., A.R., and P.T.; Visualization, L.V.; Supervision, D.T.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.ORPHANET Rare Disease Registries in EU. ORPHANET Report Series. [(accessed on 15 November 2017)];2017 May; Available online: http://www.orpha.net/orphacom/cahiers/docs/GB/Registries.pdf.
- 2.Taruscio D., Gainotti S., Mollo E., Vittozzi L., Bianchi F., Ensini M., Posada M. The current situation and needs of rare disease registries in Europe. Public Health Genom. 2013;16:288–298. doi: 10.1159/000355934. [DOI] [PubMed] [Google Scholar]
- 3.ORPHANET Prevalence and Incidence of Rare Diseases: Bibliographic Data Prevalence, Incidence or Number of Published Cases Listed by Diseases (in Alphabetical Order). ORPHANET Report Series N.1. [(accessed on 15 July 2017)];2017 Jun; Available online: http://www.orpha.net/orphacom/cahiers/docs/GB/Prevalence_of_rare_diseases_by_alphabetical_list.pdf.
- 4.Rubinstein Y.R., Groft S.C., Bartek R., Brown K., Christensen R.A., Collier E., Farber A., Farmer J., Ferguson J.H., Forrest C.B., et al. Creating a global rare disease patient registry linked to a rare diseases biorepository database: Rare Disease-HUB (RD-HUB) Contemp. Clin. Trials. 2010;31:394–404. doi: 10.1016/j.cct.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.European Union . The Council of the European Union; [(accessed on 23 August 2013)]. Council Recommendation of 8 June 2009 on an Action in the Field of Rare Diseases. Available online: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:C:2009:151:0007:0010:EN:PDF. [Google Scholar]
- 6.European Project for Rare Diseases National Plans Development (EUROPLAN) Selecting Indicators to Evaluate the Achievements of RD Initiatives. [(accessed on 22 November 2017)]; Available online: http://www.europlanproject.eu/_europlanproject/Resources/docs/2008-2011_3.EuroplanIndicators.pdf.
- 7.European Committee of Experts on Rare Diseases (EUCERD) Core Recommendations on Rare Disease Patient Registration and Data Collection. [(accessed on 15 July 2017)];2013 Jun; Available online: http://www.eucerd.eu/wp-content/uploads/2013/06/EUCERD_Recommendations_RDRegistryDataCollection_adopted.pdf.
- 8.EU Patient Registries Initiative (PARENT Joint Action) [(accessed on 3 April 2017)]; Available online: www.patientregistries.eu.
- 9.Building Consensus and Synergies for the Registration of Rare Disease Patients in the European Union (EPIRARE Project) [(accessed on 3 April 2017)]; Available online: www.epirare.eu.
- 10.An Integrated Platform Connecting Databases, Registries, Biobanks and Clinical Bioinformatics for Rare Disease Research (RD-Connect Project) [(accessed on 3 April 2017)]; doi: 10.1007/s11606-014-2908-8. Available online: www.rd-connect.eu. [DOI] [PMC free article] [PubMed]
- 11.Martin S., Kinsner-Ovaskainen A., Lanzoni M., Papadopoulou A. European Platform on Rare Diseases Registration. Oral Presentation at 3rd European Reference Networks Conference (Vilnius, 9 March 2017) [(accessed on 3 April 2017)]; Available online: https://ec.europa.eu/health/sites/health/files/ern/docs/20170309_rt2_05_martin_pres_en.pdf.
- 12.International Rare Disease Research Consortium (IRDiRC) [(accessed on 3 April 2017)]; Available online: http://www.irdirc.org/
- 13.European Union European Reference Networks: Working for Patients with Rare, Low-Prevalence and Complex Diseases. [(accessed on 3 April 2017)]; Available online: https://ec.europa.eu/health/sites/health/files/ern/docs/2017_brochure_en.pdf.
- 14.Taruscio D., Vittozzi L., Choquet R., Heimdal K., Iskrov G., Kodra Y., Landais P., Posada M., Stefanov R., Steinmueller C., et al. National Registries of Rare Diseases in Europe: An Overview of the Current Situation and Experiences. Public Health Genom. 2015;18:20–25. doi: 10.1159/000365897. [DOI] [PubMed] [Google Scholar]
- 15.Ministero della Salute Decreto 18 Maggio 2001, n. 279—Regolamento di Istituzione della Rete Nazionale delle Malattie Rare e di Esenzione dalla Partecipazione al Costo delle Relative Prestazioni Sanitarie, ai Sensi dell’Articolo 5, Comma 1, Lettera b), del Decreto Legislativo 29 Aprile 1998, n. 124. (Gazzetta Ufficiale n. 160 of 12/07/2001 Suppl. Ordinario n.180/L) [(accessed on 3 April 2017)]; Available online: http://www.salute.gov.it/imgs/C_17_normativa_194_allegato.pdf.
- 16.Taruscio D., Mollo E., Gainotti S., Posada M., Bianchi F., Vittozzi L. The EPIRARE proposal of a set of indicators and common data elements for the European platform for rare disease registration. Arch. Public Health. 2014;72:35. doi: 10.1186/2049-3258-72-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fregonese L., Rodwell C., Aymé S. RDTF Report on Health Indicators for Rare Diseases: II—Conceptual Framework for the Use of Health Indicators for the Monitoring of Quality of Care. [(accessed on 15 April 2013)];2011 Sep; Available online: http://nestor.orpha.net/EUCERD/upload/file/RDTFIndicators2011.pdf.
- 18.Mazzucato M., Visonà Dalla Pozza L., Manea S., Minichiello C., Facchin P. A population-based registry as a source of health indicators for rare diseases: The ten-year experience of the Veneto Region’s rare diseases registry. [(accessed on 3 April 2017)];Orphanet J. Rare Dis. 2014 9:37–49. doi: 10.1186/1750-1172-9-37. Available online: http://www.ojrd.com/content/9/1/37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ministero della Salute Elenco delle Strutture di Ricovero Attive al 30 Giugno 2014. [(accessed on 15 June 2015)]; Available online: http://www.salute.gov.it/portale/documentazione/p6_2_8_1_1.jsp?id=13.
- 20.Conferenza Permanente per i Rapporti tra lo Stato, le Regioni e le Province Autonome di Trento e di Bolzano. Accordo, ai Sensi dell’Articolo 4 del Decreto Legislativo 28 Agosto 1997, n.281, tra il Governo, le Regioni e le Province Autonome di Trento e di Bolzano sul Riconoscimento di Centri di Coordinamento Regionali e/o Interregionali, di Presidi Assistenziali Sovraregionali per Patologie a Bassa Prevalenza e sull’Attivazione dei Registri Regionali e/o Interregionali delle Malattie Rare. Rep. 103/CSR del 10 Maggio 2007. [(accessed on 3 April 2017)]; Available online: http://www.regione.lazio.it/malattierare/allegati/ASR-10-5-2007.pdf.
- 21.Taruscio D., Kodra Y., Ferrari G., Vittozzi L., National Rare Diseases Registry Collaborating Group The Italian National Rare Diseases Registry. Blood Transfus. 2014;12(Suppl. 3):606–613. doi: 10.2450/2014.0064-14s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.ISTAT Demographic Tables. [(accessed on 10 May 2016)]; Available online: http://www.istat.it/it/archivio/bilancio+demografico.
- 23.ISTAT Natality Tables. [(accessed on 10 May 2016)]; Available online: http://dati.istat.it/Index.aspx.
- 24.ORPHANET Disease Data Sheets. [(accessed on 3 April 2017)]; Available online: http://www.orpha.net/consor/cgi-bin/Disease_Search_Simple.php?lng=EN.
- 25.Kodra Y., Minelli G., Rocchetti A., Manno V., Carinci A., Conti S., Taruscio D., on behalf of the National Rare Diseases Registry Collaborating Group The Italian National Rare Diseases Registry: A Model of Comparison and Integration with Hospital Discharge Data. J. Public Health. 2017 doi: 10.1093/pubmed/fdx176. [DOI] [PubMed] [Google Scholar]
- 26.Dutly F., Altwegg M. Whipple’s Disease and “Tropheryma whippelii”. Clin. Microbiol. Rev. 2001;14:561–583. doi: 10.1128/CMR.14.3.561-583.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pedrini E., Jennes I., Tremosini M., Milanesi A., Mordenti M., Parra A., Sgariglia F., Zuntini M., Campanacci L., Fabbri N., et al. Genotype-phenotype correlation study in 529 patients with multiple hereditary exostoses: Identification of “protective” and “risk” factors. J. Bone Jt. Surg. Am. 2011;93:2294–2302. doi: 10.2106/JBJS.J.00949. [DOI] [PubMed] [Google Scholar]
- 28.Hausmanowa-Petrusewicz I., Zaremba J., Borkowska J., Szirkowiec W. Chronic proximal spinal muscular atrophy of childhood and adolescence: Sex influence. J. Med. Genet. 1984;21:447–450. doi: 10.1136/jmg.21.6.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.NIH-NCATS Best Vitelliform Macular Dystrophy. [(accessed on 10 November 2017)]; Available online: https://rarediseases.info.nih.gov/diseases/182/best-vitelliform-macular-dystrophy.
- 30.OMIM Entry #162500 Neuropathy, Hereditary, with Liability to Pressure Palsies; HNPP. [(accessed on 5 November 2017)]; Available online: https://www.omim.org/entry/162500#clinicalFeatures.
- 31.Roos D., Thygesen P. Familial recurrent polyneuropathy: A family and a survey. Brain. 1972;95:235–248. doi: 10.1093/brain/95.2.235. [DOI] [PubMed] [Google Scholar]
- 32.Biancalana V., Beldjord C., Taillandier A., Szpiro-Tapia S., Cusin V., Gerson F., Philippe C., Mandel J.-L., French National Working Group on Fragile X Syndrome Five years of molecular diagnosis of fragile X syndrome (1997–2001): A collaborative study reporting 95% of the activity in France. Am. J. Med. Genet. 2004;129A:218–224. doi: 10.1002/ajmg.a.30237. [DOI] [PubMed] [Google Scholar]
- 33.ISTAT Popolazione per Età, Sesso e Stato Civile 2013. [(accessed on 10 November 2017)]; Available online: http://www.tuttitalia.it/statistiche/popolazione-eta-sesso-stato-civile-2013/
- 34.Hazra N., Dregan A., Charlton J., Gulliford M.C., D’Cruz D.P. Incidence and mortality of relapsing polychondritis in the UK: A population-based cohort study. Rheumatology. 2015;54:2181–2187. doi: 10.1093/rheumatology/kev240. [DOI] [PubMed] [Google Scholar]
- 35.Groth K.A., Hove H., Kyhl K., Folkestad L., Gaustadnes M., Vejlstrup N., Stochholm K., Østergaard J.R., Andersen N.H., Gravholt C.H. Prevalence, incidence, and age at diagnosis in Marfan Syndrome. Orphanet J. Rare Dis. 2015;10:153. doi: 10.1186/s13023-015-0369-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kleijer W.J., Laugel V., Berneburg M., Nardo T., Fawcett H., Gratchev A., Jaspers N.G., Sarasin A., Stefanini M., Lehmann A.R. Incidence of DNA repair deficiency disorders in western Europe: Xeroderma pigmentosum, Cockayne syndrome and trichothiodystrophy. DNA Repair. 2008;7:744–750. doi: 10.1016/j.dnarep.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 37.Kubota M., Ohta S., Ando A., Koyama A., Terashima H., Kashii H., Hoshino H., Sugita K., Hayashi M. Nationwide survey of Cockayne syndrome in Japan: Incidence, clinical course and prognosis. Pediatr. Int. 2015;57:339–347. doi: 10.1111/ped.12635. [DOI] [PubMed] [Google Scholar]
- 38.Pascual-Castroviejo I., Lopez-Rodriguez L., de la Cruz Medina M., Salamanca-Maesso C., Roche Herrero C. Hypomelanosis of Ito: Neurological complications in 34 cases. Can. J. Neurol. Sci. 1988;15:124–129. doi: 10.1017/S0317167100027475. [DOI] [PubMed] [Google Scholar]
- 39.EUROCAT Prevalence Tables. [(accessed on 10 November 2017)]; Available online: http://www.eurocat-network.eu/accessprevalencedata/prevalencetables.
- 40.Zenteno-Ruiz J.C., Kofman-Alfaro S., Méndez J.P. 46,XX sex reversal. Arch. Med. Res. 2001;32:559–566. doi: 10.1016/S0188-4409(01)00322-8. [DOI] [PubMed] [Google Scholar]
- 41.Shannon N.L., Maltby E.L., Rigby A.S., Quarrell O.W.J. An epidemiological study of Wolf-Hirschhorn syndrome: Life expectancy and cause of mortality. J. Med. Genet. 2001;38:674–679. doi: 10.1136/jmg.38.10.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Szabó N.Z. Ph.D. Thesis. Department of Paediatrics, Faculty of Medicine, University of Szeged; Szeged, Hungary: 2012. [(accessed on 15 November 2017)]. Epidemiology of Central Nervous System Malformations in South-Eastern Hungary. Available online: http://doktori.bibl.u-szeged.hu/1485/1/SZAB%C3%93_N%C3%93RA_PhD_ertekezes.pdf. [Google Scholar]
- 43.Barisic I., Boban L., Greenlees R., Garne E., Wellesley D., Calzolari E., Addor M.-C., Arriola L., Bergman J.E.H., Braz P., et al. Holt Oram syndrome: A registry-based study in Europe. [(accessed on 10 November 2017)];Orphanet J. Rare Dis. 2014 9:156. doi: 10.1186/s13023-014-0156-y. Available online: https://ojrd.biomedcentral.com/track/pdf/10.1186/s13023-014-0156-y?site=ojrd.biomedcentral.com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Noonan J.A. Noonan syndrome revisited. J. Pediatr. 1999;135:667–668. doi: 10.1016/S0022-3476(99)70082-X. [DOI] [PubMed] [Google Scholar]
- 45.Stoll C., Dott B., Roth M.-P., Alembik Y. Birth prevalence rates of skeletal dysplasias. Clin. Genet. 1989;35:88–92. doi: 10.1111/j.1399-0004.1989.tb02912.x. [DOI] [PubMed] [Google Scholar]
- 46.Holmes L.B. Common Malformations. Oxford University Press; Oxford, UK: 2012. pp. 277–283. [Google Scholar]
- 47.Blyth M., Maloney V., Beal S., Collinson M., Huang S., Crolla J., Temple I.K., Baralle D. Pallister-Killian syndrome: A study of 22 British patients. J. Med. Genet. 2015;52:454–464. doi: 10.1136/jmedgenet-2014-102877. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.