We describe the evolutionary origins and antigenic properties of influenza A viruses isolated from two separate Australian swine populations from 2012 to 2016, showing that these viruses are distinct from each other and from those isolated from swine globally. Whole-genome sequencing of virus isolates revealed a high genotypic diversity that had been generated exclusively through the introduction and establishment of human influenza viruses that circulated in past seasons. We detected six reassortants with gene segments derived from human H1N1/H1N1pdm09 and various human H3N2 viruses that circulated during various periods since 1968. We also found that these swine viruses were not related to swine viruses collected elsewhere, indicating independent circulation. The detection of unique lineages and genotypes in Australia suggests that isolated swine populations that are sufficiently large can sustain influenza virus for extensive periods; we show direct evidence of a sustained transmission for at least 4 years between 2012 and 2016.
KEYWORDS: antigenicity, influenza surveillance, pandemic risk, phylogenetic analysis, reassortment, swine influenza
ABSTRACT
Global swine populations infected with influenza A viruses pose a persistent pandemic risk. With the exception of a few countries, our understanding of the genetic diversity of swine influenza viruses is limited, hampering control measures and pandemic risk assessment. Here we report the genomic characteristics and evolutionary history of influenza A viruses isolated in Australia from 2012 to 2016 from two geographically isolated swine populations in the states of Queensland and Western Australia. Phylogenetic analysis with an expansive human and swine influenza virus data set comprising >40,000 sequences sampled globally revealed evidence of the pervasive introduction and long-term establishment of gene segments derived from several human influenza viruses of past seasons, including the H1N1/1977, H1N1/1995, H3N2/1968, and H3N2/2003, and the H1N1 2009 pandemic (H1N1pdm09) influenza A viruses, and a genotype that contained gene segments derived from the past three pandemics (1968, reemerged 1977, and 2009). Of the six human-derived gene lineages, only one, comprising two viruses isolated in Queensland during 2012, was closely related to swine viruses detected from other regions, indicating a previously undetected circulation of Australian swine lineages for approximately 3 to 44 years. Although the date of introduction of these lineages into Australian swine populations could not be accurately ascertained, we found evidence of sustained transmission of two lineages in swine from 2012 to 2016. The continued detection of human-origin influenza virus lineages in swine over several decades with little or unpredictable antigenic drift indicates that isolated swine populations can act as antigenic archives of human influenza viruses, raising the risk of reemergence in humans when sufficient susceptible populations arise.
IMPORTANCE We describe the evolutionary origins and antigenic properties of influenza A viruses isolated from two separate Australian swine populations from 2012 to 2016, showing that these viruses are distinct from each other and from those isolated from swine globally. Whole-genome sequencing of virus isolates revealed a high genotypic diversity that had been generated exclusively through the introduction and establishment of human influenza viruses that circulated in past seasons. We detected six reassortants with gene segments derived from human H1N1/H1N1pdm09 and various human H3N2 viruses that circulated during various periods since 1968. We also found that these swine viruses were not related to swine viruses collected elsewhere, indicating independent circulation. The detection of unique lineages and genotypes in Australia suggests that isolated swine populations that are sufficiently large can sustain influenza virus for extensive periods; we show direct evidence of a sustained transmission for at least 4 years between 2012 and 2016.
INTRODUCTION
Influenza A viruses (IAV) are enzootic in domestic swine herds and pose a persistent risk to animal and human health (1, 2). The emergence of the 2009 pandemic influenza virus from swine (3, 4) highlighted the capacity of global swine populations to sustain human- and avian-derived IAV genes long term (5), as well as their ability to transmit endemic influenza viruses across continents through the live swine trade (6, 7) and generate novel influenza virus genotypes with pandemic potential through segmental reassortment (6, 8). Human infection with swine influenza A virus (swIAV) has been detected since the 1970s (9), although reporting rates have increased since 2005, with most cases being in the United States, including an epidemic originating from an agricultural fair that was estimated to have infected over 2,000 people during 2011 and 2012 (10).
The vast majority of swIAV circulating in global swine populations have been introduced from humans (11–13). Human-to-swine transmission (reverse zoonosis) of the H1N1 2009 pandemic (H1N1pdm09) influenza virus was recorded at an unprecedented scale around the world (14–16), including in Australia (17). The majority of other human seasonal IAV lineages have been recorded in domestic pigs, including those that originated as pandemics in 1918 (influenza A virus H1N1/1918), 1977 (H1N1/1977), 1968 (H3N2/1968), and 2009 (H1N1pdm09), and several of these established to form stable swIAV lineages (18). Avian IAV have been sporadically isolated from swine (19–21); however, the successful establishment of avian influenza virus genes has been detected only twice, once to form the North American triple reassortant (TR) swine lineage (22) and the second time with the Eurasian avian-like (EA) virus lineage, with both contributing significantly to the global swIAV gene pools. The global migration of swIAV through the live swine trade has enabled the mixing of genetically diverse swIAV across the globe (7). While it is clear that the host barrier for human-to-swine interspecies transmission is low, the ecological conditions required for long-term circulation and reassortment in swine populations globally are not well understood.
Little is known of the patterns of incidence and genetic diversity of swIAV in Australia due to the absence of systematic surveillance. Despite this, Australia was one of the first countries that notified the World Organization for Animal Health (OIE) of spillover H1N1pdm09 virus infections from the human population into farmed pigs approximately 2 months after the first pandemic influenza cases were confirmed in the country in May 2009 (23). Prior to 2009, Australian piggery herds were considered to be free of the major swine influenza virus lineages, such as the classical swine (CS), TR, and EA lineages, an assumption largely based on the negative findings from limited serological surveys using hemagglutination inhibition (HI) assays against human seasonal IAV antigens, A/Hong Kong/1/1968(H3N2) and A/New Jersey/1976(H1N1). Although a small number of additional H1N1pdm09 virus detections have occurred from various investigations of respiratory disease in Australian pigs since 2009 (17), the overall prevalence of H1N1pdm09 or other IAV infections in Australian pig herds is unknown.
In this study, we describe the identification and genetic and antigenic characterization of novel and divergent reassortant H1N2 and H3N2 swine viruses and H1N1pdm09-like viruses from two unrelated commercial piggeries in the Australian states of Western Australia (WA) and Queensland (QLD). We present evidence and the description of an apparent long-term establishment of swIAV containing unique human-origin H3N2 and H1N1 virus genes in Australia. We also report novel reassortant genotypes arising from interactions between the Australian swine influenza and H1N1pdm09 viruses cocirculating in Australian pigs, highlighting the rich genetic and genotypic diversity of swIAV in Australia.
RESULTS
Piggery cases and sampling.
Influenza A virus was detected by PCR in 10 of 15 swine samples collected from July to September 2012 from a large commercial piggery in southwestern Western Australia (WA) that reported respiratory distress associated with a 5-fold increase in herd mortality and subsequently in 4 of 6 swine samples collected during a separate disease investigation from September to November 2016. Haemophilus parasuis, Pasteurella aerogenes, and Streptococcus suis were isolated from necropsy specimens, while immunohistochemical influenza A virus antigen staining was positive in consolidated lung tissue. Influenza A virus was also detected by PCR from 17 of 22 swine samples collected from August to September 2012 from a small piggery in southern Queensland (QLD), which reported respiratory disease that was associated with a 10% weaner mortality, with both Haemophilus parasuis and IAV being isolated from lung tissue.
Influenza A virus subtypes and data set preparation.
We were able to generate the whole genomes for 10 influenza A viruses isolated from 2012 and 2016 in Australia (WA, n = 6; QLD, n = 4) (Table 1), which included one H1N1, seven H1N2, and two H3N2 viruses. Based on the Swine H1 Clade Classification system (24), two viruses from QLD were clade 1A.3.3.2, which is comprised of H1N1pdm09-derived swine H1 viruses, whereas the remaining were part of swine H1 clades 1B.2 (n = 2) and Other-Human (n = 4), which broadly represents several lineages of swIAV derived from human seasonal H1N1 viruses collected globally. Both the H3 subtype viruses collected from WA were derived from human seasonal H3N2 virus. Hence, to comprehensively characterize the evolution of these viruses, we acquired all available genomes of swine influenza and human seasonal influenza viruses collected since 1968, including those from Australia, to assemble data sets for H1-hemagglutinin (HA) (n = 3,420), H3-HA (n = 4,955), N1-neuraminidase (NA) (n = 1,976), N2-NA (n = 5,073), polymerase basic 2 (PB2) (n = 7,854), polymerase basic 1 (PB1) (n = 7,848), polymerase acidic (PA) (n = 8,238), nucleoprotein (NP) (n = 6,514), matrix (MP) (n = 4,822), and nonstructural (NS) (n = 5,230) genes.
TABLE 1.
Swine influenza virus isolated from Australian piggeries, 2012 to 2016
| Strain | Collection date (day/mo/yr) | Subtype | HA cladea |
|---|---|---|---|
| sw/WA/AS12-2111-01/2012 | 20/7/2012 | H1N2 | Other-Human |
| sw/WA/2577766G/2012 | 20/7/2012 | H3N2 | Seasonal H3 |
| sw/WA/2577896X/2012 | 4/8/2012 | H1N2 | Other-Human |
| sw/WA/2577899R/2012 | 4/8/2012 | H1N2 | Other-Human |
| sw/QLD/P12-13921-24/2012 | 6/8/2012 | H1N2 | Other-Human-1B.2b |
| sw/QLD/P12-14238-07/2012 | 24/8/2012 | H1N2 | Other-Human-1B.2 |
| sw/QLD/P12-14238-09/2012 | 24/8/2012 | H1N2 | 1A.3.3.2c |
| sw/QLD/P12-14600-69/2012 | 13/9/2012 | H1N1 | 1A.3.3.2 |
| sw/WA/AS16-2465-03/2016 | 12/9/2016 | H3N2 | Seasonal H3 |
| sw/WA/AS16-3457-09/2016 | 8/11/2016 | H1N2 | Other-Human |
The nomenclature of H1 subtype viruses is based on reference 24.
Previously termed “delta-like.”
Previously termed “npdm.”
Gene origins of Australian swine influenza viruses.
Maximum likelihood phylogenetic analysis of the HA and NA genes revealed four distinct Australian swine HA lineages and four distinct NA lineages (Fig. 1a and b and 2a and b) forming the three influenza virus subtypes H1N1 (n = 1) from QLD, H1N2 (n = 7) from both Australian sites, and H3N2 (n = 2) from WA, with no evidence of mixing of viruses between QLD and WA. Furthermore, the majority of these human-derived Australian swine lineages formed long branches that could be directly traced back to their seed human IAV lineages with no relationship to swine viruses collected elsewhere, suggesting an independent evolution. The exceptions were A/sw/QLD/P12-14600-69/2012(H1N1), which shared all eight gene segments with H1N1pdm09-like viruses circulating in humans and swine, and A/sw/QLD/P12-14238-09/2012(H1N2), which shared seven genes with H1N1pdm09-like viruses circulating in humans and swine.
FIG 1.
(a and b) Evolutionary relationships of the H1-HA (a) and H3-HA (b) genes of Australian swine influenza viruses (2012 to 2016) (red). Swine viruses isolated elsewhere are colored gray, while human lineages are colored black. Nth American, North American. (c to f) The human origins of each of the Australian swine HA gene lineages are shown with a representative set of closely related human or swine viruses: H1N1/pdm09-like (c), H1N1/1995-like (d), H1N1/1977-like (e), and H3N2/1995-like (f). The scale bar represents the number of nucleotide substitutions per site. Branch support values (>70 UFBoot) are shown at the nodes. Blue branches in Fig. 1c reveal the phylogenetic relationships of previously published H1N1pdm09-like viruses from Australian swine.
FIG 2.
(a and b) Evolutionary relationships of the N2-NA (a) and N1-NA (b) genes of Australian swine influenza viruses (2012 to 2016) (red). Swine viruses isolated elsewhere are colored gray, while human lineages are colored black. (c to f) The human origins of each of the Australian swine NA gene lineages are shown with a representative set of closely related human or swine viruses: H3N2/2003-like (c), H3N2/1995-like (d), H3N2/1968-like (e), and H1N1/pdm09-like (f). The scale bar represents the number of nucleotide substitutions per site. Branch support values (>70 UFBoot) are shown at the nodes.
Phylogenetic analysis showed that the H1-HA genes of the four WA viruses collected during 2012 (n = 3) and 2016 (n = 1) were derived from H1N1 viruses that reemerged in humans in 1977 (Fig. 1e), with 87 to 89% nucleotide sequence similarities to H1N1/1977-like viruses (25), whereas the H1-HA genes of two QLD viruses were derived from human seasonal H1N1 viruses circulating during the mid-1990s, with 93 to 95% nucleotide sequence similarities to H1N1/1995-like viruses (Fig. 1d). A further two QLD viruses contained H1N1pdm09-like (clade 1A.3.3.2) HA genes (Fig. 1c) (24). The HA genes of the two H3 viruses collected from WA pigs in 2012 and 2016, respectively, were derived from human seasonal H3N2 viruses circulating during 1995 (Fig. 1f). The respective WA swine H3N2 (swH3N2) virus genes have accumulated 5 to 7% nucleotide sequence divergence from these human H3N2/1995-like viruses. These results highlight that HA segments derived from several previously circulated human seasonal influenza viruses have persisted concurrently in Australian swine populations. The long branch lengths leading to these Australian swine viruses indicate that these lineages had been unsampled for approximately 20 to 40 years since their introduction from humans into swine populations. A linear regression of the root-to-tip distance (data not shown) excluded the possibility of lab-based evolution or erroneous sequencing along these lineages.
The only N1-NA gene detected in this study [A/sw/QLD/P12-14600-69/2012(H1N1)] was H1N1pdm09-like (Fig. 2f). The N2-NA genes of the four WA H1N2 viruses were derived from human H3N2/1968-like pandemic viruses (Fig. 2e), two H3N2 viruses from WA (from 2012 and 2016) were derived from human seasonal H3N2 viruses circulating during the mid-1990s (H3N2/1995-like) (Fig. 2d), and three H1N2 viruses collected from QLD during 2012 were from human seasonal H3N2/2003-like viruses (Fig. 2c). The H3N2/1968-like virus genes in Australian swine represent the independent and continuing evolution of such genes in WA swine for approximately 50 years.
Genotypes of swIAV in Australian swine.
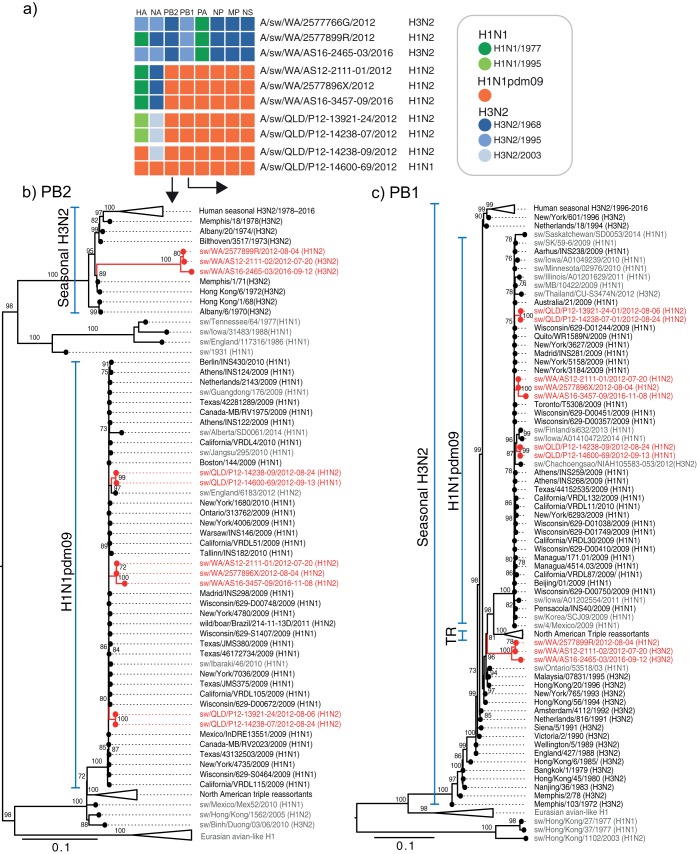
The internal segments (PB2, PB1, PA, NP, MP, and NS) of the Australian swIAV were predominantly derived from H1N1pdm09 viruses, except for three of the swine viruses from WA (the H1N2 and H3N2 viruses from 2012 and one H3N2 virus from 2016) with closely related genotypes that retained four internal human H3N2/1968-like segments (PB2, NP, MP, and NS), a human H3N2/1995-like PB1 segment, and H1N1/1997-like PA segment (Fig. 3). The remaining three H1N2 viruses from WA (A/sw/WA/AS12-2111-01/2012, A/sw/WA/2577896X/2012, and A/sw/WA/AS16-3457-09/2016) contained H1N1pdm09-like internal gene segments with an H1N1/1977-like HA and H3N2/1968-like NA, thereby having segments derived from the past three human influenza pandemics.
FIG 3.
(a) Gene sources of Australian swine influenza viruses. (b and c) Maximum likelihood phylogenetic trees of the PB2 (b) and PB1 (c) data sets are shown to illustrate the internal gene sources of Australian swine viruses. Branch support values (>70 UFBoot) are shown at the nodes. The scale bar represents the number of nucleotide substitutions per site.
While the internal segments of all the QLD swine H1N2 (swH1N2) viruses were also of H1N1pdm09 origin, two related viruses (A/sw/QLD/P12-13921-24/2012 and A/sw/QLD/P12-14238-07/2012) contained human H1N1/1995-like HA and H3N2/2003-like NA, while one virus (A/sw/QLD/P12-14600-69/2012) retained all gene segments of H1N1pdm09 and one virus (A/sw/QLD/P12-14238-09/2012) contained seven H1N1pdm09-like gene segments with H3N2/2003-like NA. A summary of the six different swIAV genotypes (genome constellations) determined by the lineage of origin of each of their gene segments is shown in Fig. 3. Taken together, our results revealed the cocirculation of a rich diversity of human-derived IAV genetic elements in Australian pigs.
The two swine viruses collected during 2016 from WA [A/sw/WA/AS16-2465-03/2016(H3N2) and A/sw/WA/AS16-3457-09/2016(H1N2)] each belonged to one of the two dominant genotypes that were previously detected in 2012 (Fig. 3), indicating sustained transmission of these genotypes in WA swine.
Antigenic relationships of Australian swine influenza viruses.
The antigenic properties of three representative Australian swine viruses detected in 2012, which exhibited long HA branches (H1N1/1977-like, H1N1/1995-like, and H3N2/1995-like viruses; Fig. 1a), were investigated through hemagglutination inhibition (HI) assays using panels of postinfection pig (Table 2) and ferret (Table 3) antisera raised to a set of representative swine and human H1 and H3 viruses. The H3N2/1995-like virus A/sw/WA/2577766G/2012(H3N2) showed varying antigenic cross-reactivity with human seasonal H3N2 viruses isolated during the 1990s, producing the highest heterologous HI titer to antiserum raised to A/Wuhan/359/1995. North American swine viruses that originated from human H3 lineages circulating around the same period also had antigenic cross-reactivity with the WA swH3N2 virus (Table 2). In contrast, ferret antisera to A/Nanchang/933/1995 and A/Sydney/5/1997 did not produce HI activity against A/sw/WA/2577766G/2012 (Table 3). The WA swH3N2 virus was also antigenically and genetically distinct from the zoonotic H3N2v viruses detected in North America (see Fig. 1b for their phylogenetic relationship), although this does not exclude the possibility that the virus possesses a similar risk to human health.
TABLE 2.
Hemagglutination inhibition assay of Australian swine H1N2 and H3N2 influenza viruses using pig antisera raised to human and swine influenza A virus antigens
| Pig antiserumb | HI titer for Australian swIAVa |
||||
|---|---|---|---|---|---|
| H3N2 WA/G/12 | H1N2 WA/X/12 | H1N2 QLD/P12/12 | H3N2 WA/AS16-2/16 | H1N2 WA/AS16-3/16 | |
| Swine H1 | |||||
| sw/Wisconsin/1/196830/1968(H1N1) | <20 | <20 | <20 | <20 | NT |
| sw/Iowa/1973(H1N1) | <20 | <20 | <20 | <20 | NT |
| sw/Illinois/00685/2005(H1N1) | <20 | <20 | <20 | <20 | NT |
| sw/Ohio/511445/2007(H1N1) | <20 | <20 | <20 | <20 | NT |
| sw/Kentucky/02086/2008(H1N1) | <20 | <20 | <20 | <20 | NT |
| sw/Minnesota/02011/2008(H1N2) | <20 | <20 | <20 | <20 | NT |
| Human H1 | |||||
| New Jersey/8/1976(H1N1) | <20 | <20 | <20 | <20 | NT |
| Brazil/11/1978(H1N1) | NT | 80 | 80 | NT | NT |
| Taiwan/1/1986(H1N1) | NT | 40 | 320 | NT | NT |
| Beijing/262/1995(H1N1) | NT | <20 | <20 | NT | NT |
| New Caledonia/20/1999(H1N1) | <20 | <20 | <20 | NT | NT |
| Solomon Islands/3/2006(H1N1) | <20 | <20 | <20 | NT | NT |
| California/04/2009(H1N1pdm09) | <20 | <20 | <20 | 20 | NT |
| Swine H3 | |||||
| sw/Texas/4199-1/1998(H3N2) | 320 | <20 | <20 | 80 | NT |
| sw/Colorado/23619/1999(H3N2) | <20 | <20 | <20 | <20 | NT |
| sw/Nakorn Pathom/2002(H3N2) | <20 | <20 | <20 | <20 | NT |
| sw/Minnesota/01146/2006(H3N2) | 640 | <20 | <20 | 640 | NT |
| sw/New York/A01104005/2011(H3N2) | 40 | <20 | <20 | 40 | NT |
| sw/Iowa/A01480656/2014(H3N2) | <20 | <20 | <20 | 160 | NT |
| Human H3 | |||||
| Port Chalmers/1/1973(H3N2) | <20 | <20 | <20 | NT | NT |
| Bangkok/01/1979(H3N2) | <20 | <20 | <20 | NT | NT |
| Shanghai/11/1987(H3N2) | <20 | <20 | <20 | NT | NT |
| Beijing/32/1992(H3N2) | 40 | <20 | <20 | NT | NT |
| Wuhan/359/1995(H3N2) | 320 | <20 | <20 | NT | NT |
| Sydney/5/1997(H3N2) | 20 | <20 | <20 | NT | NT |
| Moscow/10/1999(H3N2) | <20 | <20 | <20 | NT | NT |
| Fujian/411/2002(H3N2) | <20 | <20 | <20 | NT | NT |
| Brisbane/10/2007(H3N2) | <20 | <20 | <20 | NT | NT |
| Victoria/361/2011(H3N2) | <20 | <20 | <20 | NT | NT |
| Switzerland/9715293/2013(H3N2) | <20 | <20 | <20 | NT | NT |
| Swine Australia | |||||
| sw/WA/2577766G/2012(H3N2) | 1,280 | <20 | <20 | 40 | NT |
| sw/WA/2577896X/2012(H1N2) | <20 | 640 | 20 | 20 | 640 |
| sw/QLD/P12-13921-24/2012(H1N2) | <20 | 40 | 640 | <20 | 160 |
Abbreviations for Australian swine IAV: WA/G/12, A/swine/WA/2577766G/2012(H3N2); WA/X/12, A/swine/WA/2577896X/2012(H1N2); QLD/P12/12, A/swine/QLD/P12-13921-24/2012(H1N2); WA/AS16-2/16, A/swine/WA/AS16-2465-03/2016(H3N2); WA/AS16-3/16, A/swine/WA/AS16-3457-09/2016(H1N2). Positive reactive hemagglutination inhibition titers are shown in bold, with homologous titers being underlined. NT, not tested.
Pig antisera against Australian swine viruses (italics) were raised in this study, whereas the remaining pig antisera and the corresponding virus antigens were kindly donated by the Virus and Prion Research Unit, Agricultural Research Service, USDA, Ames, IA.
TABLE 3.
Hemagglutination inhibition assay of Australian swine H1N2 and H3N2 influenza viruses using ferret antisera raised to human influenza A virus antigens
| Ferret antiserum | HI titer for Australian swIAVa |
||
|---|---|---|---|
| H3N2 WA/G/12 | H1N2 WA/X/12 | H1N2 QLD/P12/12 | |
| Human H1 | |||
| PR/8/1934(H1N1) | <20 | <20 | <20 |
| Texas/36/1991(H1N1) | <20 | <20 | 320 |
| Bayern/7/1995(H1N1) | <20 | <20 | 160 |
| Beijing/262/1995(H1N1) | <20 | <20 | <20 |
| New Caledonia/20/1999(H1N1) | <20 | <20 | <20 |
| Illinois/9/2007(H1N1v) | <20 | <20 | <20 |
| California/7/2009(H1N1pdm09) | <20 | <20 | <20 |
| Human H3 | |||
| Nanchang/933/1995(H3N2) | <20 | <20 | <20 |
| Sydney/5/1997(H3N2) | <20 | <20 | <20 |
| Human H3v, Minnesota/11/2010(H3N2v) | <20 | <20 | <20 |
| Swine Australiab | |||
| sw/WA/2577766G/2012(H3N2) | 1,280 | <20 | <20 |
| sw/WA/2577896X/2012(H1N2) | <20 | 1,280 | <20 |
| sw/QLD/P12-13921-24/2012(H1N2) | <20 | <20 | 640 |
Abbreviations for Australian swine IAV: WA/G/12, A/swine/WA/2577766G/2012(H3N2); WA/X/12, A/swine/WA/2577896X/2012(H1N2); QLD/P12/12, A/swine/QLD/P12-13921-24/2012(H1N2). Positive reactive hemagglutination inhibition titers are shown in bold, with homologous titers being underlined.
Ferret antisera raised against three Australian swine viruses are in italics and were produced in this study.
The H1N2 viruses (A/sw/WA/2577896X/2012 and A/sw/QLD/P12-13921-24/2012) showed the highest heterologous HI titers against pig antisera to past human seasonal H1N1 viruses, A/Brazil/11/1978 and A/Taiwan/1/1986, respectively, but had no antigenic cross-reactivity to any of the other viruses represented in the pig antiserum panel (Table 2). None of the ferret antisera raised to the panel of human seasonal H1 viruses inhibited hemagglutination by the WA swH1N2 virus with H1N1/1977-like HA, while the QLD swH1N2 virus with H1N1/1995-like HA showed antigenic cross-reactivity to A/Texas/36/1991 and A/Bayern/7/1995 (Table 3).
Postinfection pig antisera raised to the three representative Australian swIAV isolates were tested against the related swine viruses isolated from the same WA piggery in 2016, A/sw/WA/AS16-2465-03/2016(H3N2) and A/sw/WA/AS16-3457-09/2016(H1N2) (Table 2). Despite being closely related in HA lineage (H3N2/1995-like) and genotype, the 2016 WA swH3N2 virus was inhibited only by antiserum raised to the 2012 swH3N2 isolate at a titer of 1:40, which was a 5-fold decrease in HI activity relative to the homologous HI titer. This suggested that some antigenic drift had occurred over the 4 years of circulation of this virus in WA swine. In contrast, the genetically related 2012 and 2016 WA swH1N2 viruses that shared the H1N1/1977 HA gene lineage were antigenically stable with no apparent drift over the same period. The 2016 WA swH1N2 virus had an HI titer of 1:640 against pig antiserum raised to A/sw/WA/2577896X/2012, which was equivalent to the homologous titer for this antiserum. We analyzed the amino acid sequences of the mature HA1 protein of the corresponding 2012 and 2016 WA swH3N2 and WA swH1N2. The 2016 WA swH3N2 virus had accumulated nine amino acid substitutions in HA1 compared to the sequence of the 2012 virus, while the 2016 swH1N2 virus had six HA1 amino acid substitutions compared to the sequence of its 2012 counterpart (Fig. 4).
FIG 4.

Comparison of HA1 amino acid substitutions between 2012 and 2016 isolates of Western Australian swine influenza H3N2 (a) and H1N2 (b) viruses and antigenically cross-reactive influenza A virus antigens. Known HA1 antigenic sites are boxed in gray and numbered according to the H3 (47) and H1 (32) numbering systems. Dots denote positions with amino acid residues identical to those of sw/Western Australia/2577766G/2012(H3N2) and sw/Western Australia/2577896X/2012(H1N2).
DISCUSSION
Whole-genome sequencing of influenza viruses collected from two geographically distinct swine populations in Australia revealed the establishment of multiple human influenza H1N1 and H3N2 viruses that have circulated in humans since 1968, filling an important gap in the lack of knowledge of swine influenza virus diversity in Australia (26). Through the phylogenetic analysis of an expansive human and swine influenza virus data set, we have revealed evidence of the long-term establishment of Australian swIAV gene segments derived from several seasonal influenza virus lineages that have previously circulated in humans, including H1N1/1977-like, H1N1/1995-like, H3N2/1968-like, H3N2/2003-like, and H1N1pdm09-like viruses. Each of the lineages, except the H1N1pdm09 segments, contained long branches originating from the respective human viruses, indicating an undetected circulation of these lineages in Australian swine. As our limited sampling only commenced in 2012, the dates of introduction of these lineages into Australian swine populations could not be precisely ascertained; however, we found evidence of the sustained transmission of two swIAV lineages from 2012 to 2016 in a large integrated pig farm in Western Australia. There was no further evidence or reports of sustained ill health in swine at this property over this period. However, the true impacts of influenza on swine in Australia and elsewhere cannot be defined without undertaking a thorough systematic and longitudinal IAV surveillance, together with an analysis of the epidemiological linkage to outbreaks of respiratory syndromes observed in affected pig herds.
It appears that H3N2/1968 pandemic-like virus gene segments could have circulated undetected for up to 44 years in swine populations in Australia, reassorting with other seasonal human influenza virus lineages that were subsequently introduced into pigs and that led to the H1N2 and H3N2 swIAV genotypes detected in 2012 and 2016. H3N2/1968-like viruses [e.g., A/Hong Kong/1/68 (H3N2)] or their descendant gene segments have been observed in pigs since the 1970s, first in Taiwan in 1969 (27) and subsequently in swine across Asia and Europe (18, 28). Our phylogenies indicate that the Australian swine H3N2/1968-like virus genes were independently introduced from human lineages and were not related to swIAV described from other countries.
The H3N2 subtype viruses detected in Western Australian swine appeared to have arisen following the introduction of H3N2/1995-like human seasonal influenza viruses into pigs, which then reassorted to adopt four H3N2/1968-like internal genes (PB2, NP, MP, and NS) and an H1N1/1977-like PA gene. The long tip branches of the H3N2/1995-like HA, NA, and PB1 genes of WA swH3N2 suggest a continual transmission in swine of approximately 20 years. Other independent introductions of human H3N2/1995-like viruses into pigs followed by stable transmission of their gene segments in swine have been reported in East and Southeast Asia and North America (13, 16, 29).
The opportunistic detection of the WA swH3N2 virus from the same pig farm in 2012 and 2016 provided insight into the continuing evolution of this lineage in swine in Australia. There was 2% nucleotide sequence divergence across seven virus gene segments and 1% divergence in the MP gene between the 2012 and 2016 WA swH3N2 viruses. This indicated that there was no particular selective mutation pressure on the virus surface protein genes, likely because of the frequent introduction of naive hosts as part of the pig production system and the absence of influenza vaccination in Australian swine. The WA swH3N2 virus showed antigenic cross-reactivity to A/sw/Minnesota/01146/2006 of the North American swH3N2 lineage and to A/sw/Texas/4199-1/1998 and A/Wuhan/359/1995, which are swine and human viruses that share a human H3N2/1995-like HA genetic lineage. However, there was no HI activity against ferret antiserum to A/Nanchang/933/1995 and similar seasonal H3N2 viruses. More comprehensive HI testing will be needed to better define the antigenic relationships of the WA swH3N2 viruses to the range of human and swine H3 viruses. Despite sharing a direct H3N2 genotype heritage, the A/sw/WA/AS16-2465-03/2016 virus was found to be antigenically distant from A/sw/WA/2577766G/2012. However, compared to the 2012 WA swH3N2 virus, the 2016 virus had accumulated at least five amino acid substitutions at key H3 positions near the HA receptor binding site known to directly affect antigenic clustering, including N145K, K156E, D158N, S189R, and S193N (30, 31).
Interestingly, we isolated three similar swH1N2 viruses from WA forming a genotype containing gene segments exclusively originating from the past three influenza pandemics (1968, 1977, and 2009), with evidence of circulation for at least 4 years in Australia. This genotype clearly emerged from reassortment following anthropological introductions of H1N1pdm09 virus into Australian swine populations (17, 23); however, detection of the circulation of this virus over several years suggests that it is well adapted to transmission in pigs. The A/sw/WA2577899R/2012(H1N2) variant, which carries no H1N1pdm09 genes, could have circulated in swine for more than a decade before the introduction of the 2009 pandemic virus. Similar to the swH3N2 viruses from the same pig farm, the genetically related swH1N2 virus isolated in 2012 and 2016 shared high sequence similarities (98 to 99%) across eight gene segments. However, the 2012 and 2016 WA swH1N2 virus isolates were antigenically similar to each other. The accumulation of six amino acid differences in the HA1 of the 2016 swH1N2 virus did not appear to alter its antigenic properties, despite substitutions in two positions (S162N and K163N; H1 numbering) associated with the H1 Sa antigenic site (32). When tested with the HI antiserum panels, the WA swH1N2 virus showed some antigenic cross-reactivity with A/Brazil/11/1978 but was not antigenically similar to any of the more contemporary (post-1990s) human H1N1 viruses, which concurred with the H1N1/1977-like phylogenetic lineage of its HA.
Both human-to-pig and pig-to-pig transmissions of the H1N1pdm09 virus have left a legacy in generating new swIAV diversity in different parts of the world. A further snapshot of H1N1pdm09-reassorted Australian swIAV variants was provided by the three different H1 genotypes characterized from pigs in QLD, which is an eastern state of the Australian continent and which is therefore expected to contain virus-host dynamics independent of those in Western Australia. First, the QLD sampling confirmed that nonreassorted H1N1pdm09-like viruses continue to be present in Australian swine populations, either from recent human-to-pig spillover infections or through continuous transmission in pigs. The pandemic virus was first detected in pigs in QLD in August 2009 (17). Furthermore, H1N1pdm09 internal genes present in pigs have reassorted with surface protein genes from human H1N1 and H3N2 viruses that appear to have been introduced into Australian swine during the 1995 and 2003 human influenza seasons, respectively, as evidenced by the long HA and NA branches leading to the 2012 QLD swH1N2 viruses. When assessed by HI, the A/sw/QLD/P12-13921-24/2012(H1N2) virus showed antigenic cross-reactivity with both A/Taiwan/1/1986 and A/Texas/36/1991 human H1N1 viruses, which, although they share a relatively close H1 lineage, belong to different antigenic clusters in the human seasonal H1N1 lineage (33, 34). As with the WA swH3N2 viruses, a more comprehensive HI analysis with an expanded panel of both human and swine viruses from different temporal and geographical points will be needed to more clearly map the antigenic relationships of the QLD swH1N2 viruses (35). Reassortant swH1N2 viruses arising from various previously circulating human H1N1 and H3N2 gene lineages have been detected in swine in many other countries (1). However, our phylogenetic analysis showed that the Australian swH1N2 viruses were derived from independent human-to-swine introductions and are not related to those found in swine in other countries.
The unique genome constellations of most of the Australian swIAV lineages show that these viruses have been cocirculating undetected for a number of decades in swine. Australian animals are isolated due to a strict quarantine regimen prohibiting the importation of live swine or fresh pork products, which is in contrast to the wide commercial exchange of live swine in other parts of the world (7). While this may impact the incursion of exotic swine viruses, it has little impact on the human-to-swine transmission of seasonal IAV. The detections of independent swIAV lineages within Australia from a small number of diagnostic investigations suggest that improved surveillance will likely lead to the identification of additional swIAV diversity in Australian swine herds. These viruses, which have been shown to circulate in swine in isolation from nonporcine hosts for extended periods spanning decades, may represent an increased pandemic threat or cause zoonotic human infections due to antigenic variation and the absence of postexposure and postvaccination immunity in the general human population (2, 36). As with swIAV in other parts of the world, more widespread and periodic surveillance is required to gain a better understanding of the diversity of IAV circulating in Australian swine populations in order to better inform influenza risk assessments and global efforts in pandemic influenza preparedness. Our findings suggest that Australian authorities and swine industry bodies should implement periodic active sampling for IAV or enhanced influenza surveillance during any syndromic disease investigation of pigs and piggery workers to determine the prevalence of swIAV and to detect in a timely manner novel IAV genotypes and the presence of porcine reservoirs of past human virus lineages that may pose a risk to both animal and human health.
MATERIALS AND METHODS
Samples and virus isolation.
Swine nasal, tracheal, or pooled lung tissue samples were collected from a commercial piggery in Western Australia (WA) and an unrelated piggery in Queensland (QLD) following independent reports of respiratory distress associated with various degrees of mortality and submitted to our laboratories for diagnostic investigation during 2012. A separate disease investigation was conducted on samples from the same WA farm collected during 2016. Samples were processed for bacteriology and virus propagation in Madin-Darby canine kidney (MDCK) cells and in the allantoic cavities of 9- to 11-day-old specific-pathogen-free embryonated chicken eggs (SPAFAS, Woodend, Australia) according to established protocols (37). Allantoic and supernatant fluids from postinfection chicken eggs or cell cultures showing cytopathic effects were tested for hemagglutinating activity using 0.5% (vol/vol) chicken or turkey red blood cells. Clinical samples as well as egg and MDCK cell isolates were tested in molecular assays, and isolates were tested by serologic analysis.
Serology and antigenic analysis.
The antigenic relationships of the swIAV were investigated by hemagglutination inhibition (HI) assay using panels of ferret and pig antisera raised against representative human and swine IAV, including pig antisera raised against three representative Australian swIAV (see Tables 2 and 3 for the list of viruses and the corresponding antisera). HI assays were performed with either turkey or chicken red blood cells as previously described using standard techniques (32, 37).
Genome sequencing.
Viral RNA was extracted directly from PCR-positive diagnostic samples and infected allantoic fluid or cell culture using a MagMax-96 viral RNA isolation kit (Life Technologies, Scoresby, Australia) with a MagMax Express magnetic particle processor (Life Technologies), according to the manufacturer's instructions. Virus genome segments were amplified by one-step reverse transcription-PCR (RT-PCR) using a SuperScript III one-step RT-PCR system with Platinum Taq DNA polymerase (Life Technologies) and the IAV gene primers with the cycling conditions previously described (38, 39). Specific amplicons were purified using a QIAquick gel extraction kit (Qiagen, Hilden, Germany) and used directly for Sanger sequencing with a BigDye (v3.1) cycle sequencing kit (Life Technologies) and IAV gene-specific primers (40, 41; WHO, 2009 [http://www.who.int/csr/disease/swineflu/guidance/en/]). Sequencing reactions were analyzed on an Applied Biosystems 3130xl genetic analyzer (Life Technologies). Sequence data assembly and editing were performed using Lasergene (v12.0) SeqMan Pro software (DNAStar, Madison, WI). Virus cultures were plaque purified to confirm the viral origin of gene segments from coinfected samples. Randomly selected clones from each sample were selected for full-genome sequencing using both Sanger and Illumina MiSeq (San Diego, SA) next-generation sequencing as previously described (39).
Phylogenetic analysis and genotyping.
The complete swIAV genome coding sequences determined in this study were analyzed with sequences obtained from the Global Initiative on Sharing All Influenza Data (GISAID) database (42). Multiple-sequence alignments of each segment were performed independently using the MUSCLE (v3.5) program (43) followed by manual optimization. The large-scale maximum likelihood (ML) phylogenetic trees shown in Fig. 1a, 2a, and 3b and c were estimated in IQ-TREE (v1.4) (44), using the best-fit nucleotide substitution model. Branch support was estimated using the ultrafast bootstrap (UFBoot) method (45). Swine virus genotypes were determined using the source of the origins of their lineages in each gene segment. Smaller ML phylogenies shown in Fig. 1b to e and 2b to e were estimated in the RAxML (v8.0) program (46), applying the general time-reversible nucleotide substitution model with a gamma distribution of among-site rate variation (GTR+G). Support for individual nodes was estimated with 1,000 rapid bootstrap replicates. Trees were visualized and annotated using the FigTree (v1.4) program (http://tree.bio.ed.ac.uk/software/figtree/).
Ethics statement.
The study was performed in accordance with guidelines of the Australian Animal Health Laboratory (AAHL) Animal Ethics Committee (AEC) (protocol no. 1734), licensed with Agriculture Victoria, Australia, and complies with all relevant requirements of the Prevention of Cruelty to Animals Act (186) and the Regulations and with the Australian code for the care and use of animals for scientific purposes (48). All animal work involving ferrets was approved by the University of Melbourne, Biochemistry & Molecular Biology, Dental Science, Medicine, Microbiology & Immunology, and Surgery, AEC (approval no. 815).
Accession number(s).
The nucleotide sequences generated in this study can be obtained using NCBI GenBank accession numbers MG962421 to MG962468 and MG962477 to MG962508.
ACKNOWLEDGMENTS
We acknowledge the technical support of Amanda Bagnara, Noel Ritson-Bennet, John Bingham, Jeff Butler, Ross Lunt, Som Walker, and Jianning Wang from the CSIRO Australian Animal Health Laboratory (AAHL), Pina Iannello and Rob Shaw from VIDRL, and Bruce Harrower from the Department of Health, QLD. We also thank Amy Vincent of the USDA Agricultural Research Service, Ames, IA, for the kind donation of reference swine antisera and virus antigens used in this study.
The Melbourne WHO Collaborating Centre for Reference and Research on Influenza is supported by the Australian Government Department of Health, and the AAHL Facility is supported by the Department of Agriculture and Water Resources and the National Collaborative Research Infrastructure Strategy (NCRIS). V.D., D.T., and J.M. are supported by contract HHSN272201400006C from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, U.S. Department of Health and Human Services, USA. C.D. is an Australian NHMRC Early Career Research Fellow.
REFERENCES
- 1.Brown IH. 2013. History and epidemiology of swine influenza in Europe. Curr Top Microbiol Immunol 370:133–146. doi: 10.1007/82_2011_194. [DOI] [PubMed] [Google Scholar]
- 2.Kitikoon P, Vincent AL, Gauger PC, Schlink SN, Bayles DO, Gramer MR, Darnell D, Webby RJ, Lager KM, Swenson SL, Klimov A. 2012. Pathogenicity and transmission in pigs of the novel A(H3N2)v influenza virus isolated from humans and characterization of swine H3N2 viruses isolated in 2010-2011. J Virol 86:6804–6814. doi: 10.1128/JVI.00197-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garten RJ, Davis CT, Russell CA, Shu B, Lindstrom S, Balish A, Sessions WM, Xu X, Skepner E, Deyde V, Okomo-Adhiambo M, Gubareva L, Barnes J, Smith CB, Emery SL, Hillman MJ, Rivailler P, Smagala J, de Graaf M, Burke DF, Fouchier RA, Pappas C, Alpuche-Aranda CM, Lopez-Gatell H, Olivera H, Lopez I, Myers CA, Faix D, Blair PJ, Yu C, Keene KM, Dotson PD Jr, Boxrud D, Sambol AR, Abid SH, St George K, Bannerman T, Moore AL, Stringer DJ, Blevins P, Demmler-Harrison GJ, Ginsberg M, Kriner P, Waterman S, Smole S, Guevara HF, Belongia EA, Clark PA, Beatrice ST, Donis R, et al. 2009. Antigenic and genetic characteristics of swine-origin 2009 A(H1N1) influenza viruses circulating in humans. Science 325:197–201. doi: 10.1126/science.1176225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith GJ, Vijaykrishna D, Bahl J, Lycett SJ, Worobey M, Pybus OG, Ma SK, Cheung CL, Raghwani J, Bhatt S, Peiris JS, Guan Y, Rambaut A. 2009. Origins and evolutionary genomics of the 2009 swine-origin H1N1 influenza A epidemic. Nature 459:1122–1125. doi: 10.1038/nature08182. [DOI] [PubMed] [Google Scholar]
- 5.Dunham EJ, Dugan VG, Kaser EK, Perkins SE, Brown IH, Holmes EC, Taubenberger JK. 2009. Different evolutionary trajectories of European avian-like and classical swine H1N1 influenza A viruses. J Virol 83:5485–5494. doi: 10.1128/JVI.02565-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mena I, Nelson MI, Quezada-Monroy F, Dutta J, Cortes-Fernandez R, Lara-Puente JH, Castro-Peralta F, Cunha LF, Trovao NS, Lozano-Dubernard B, Rambaut A, van Bakel H, Garcia-Sastre A. 2016. Origins of the 2009 H1N1 influenza pandemic in swine in Mexico. Elife 5:e16777. doi: 10.7554/eLife.16777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nelson MI, Viboud C, Vincent AL, Culhane MR, Detmer SE, Wentworth DE, Rambaut A, Suchard MA, Holmes EC, Lemey P. 2015. Global migration of influenza A viruses in swine. Nat Commun 6:6696. doi: 10.1038/ncomms7696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vijaykrishna D, Smith GJ, Pybus OG, Zhu H, Bhatt S, Poon LL, Riley S, Bahl J, Ma SK, Cheung CL, Perera RA, Chen H, Shortridge KF, Webby RJ, Webster RG, Guan Y, Peiris JS. 2011. Long-term evolution and transmission dynamics of swine influenza A virus. Nature 473:519–522. doi: 10.1038/nature10004. [DOI] [PubMed] [Google Scholar]
- 9.Smith TF, Burgert EO Jr, Dowdle WR, Noble GR, Campbell RJ, Van Scoy RE. 1976. Isolation of swine influenza virus from autopsy lung tissue of man. N Engl J Med 294:708–710. doi: 10.1056/NEJM197603252941308. [DOI] [PubMed] [Google Scholar]
- 10.Biggerstaff M, Reed C, Epperson S, Jhung MA, Gambhir M, Bresee JS, Jernigan DB, Swerdlow DL, Finelli L. 2013. Estimates of the number of human infections with influenza A(H3N2) variant virus, United States, August 2011-April 2012. Clin Infect Dis 57(Suppl 1):S12–S15. doi: 10.1093/cid/cit273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nelson MI, Stratton J, Killian ML, Janas-Martindale A, Vincent AL. 2015. Continual reintroduction of human pandemic H1N1 influenza A viruses into swine in the United States, 2009 to 2014. J Virol 89:6218–6226. doi: 10.1128/JVI.00459-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nelson MI, Vincent AL. 2015. Reverse zoonosis of influenza to swine: new perspectives on the human-animal interface. Trends Microbiol 23:142–153. doi: 10.1016/j.tim.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nelson MI, Wentworth DE, Culhane MR, Vincent AL, Viboud C, LaPointe MP, Lin X, Holmes EC, Detmer SE. 2014. Introductions and evolution of human-origin seasonal influenza A viruses in multinational swine populations. J Virol 88:10110–10119. doi: 10.1128/JVI.01080-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perera HK, Vijaykrishna D, Premarathna AG, Jayamaha CJ, Wickramasinghe G, Cheung CL, Yeung MF, Poon LL, Perera AK, Barr IG, Guan Y, Peiris M. 2014. Molecular epidemiology of influenza A(H1N1)pdm09 virus among humans and swine, Sri Lanka. Emerg Infect Dis 20:2080–2084. doi: 10.3201/eid2012.140842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vijaykrishna D, Poon LL, Zhu HC, Ma SK, Li OT, Cheung CL, Smith GJ, Peiris JS, Guan Y. 2010. Reassortment of pandemic H1N1/2009 influenza A virus in swine. Science 328:1529. doi: 10.1126/science.1189132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nelson MI, Gramer MR, Vincent AL, Holmes EC. 2012. Global transmission of influenza viruses from humans to swine. J Gen Virol 93:2195–2203. doi: 10.1099/vir.0.044974-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deng YM, Iannello P, Smith I, Watson J, Barr IG, Daniels P, Komadina N, Harrower B, Wong FY. 2012. Transmission of influenza A(H1N1) 2009 pandemic viruses in Australian swine. Influenza Other Respir Viruses 6:e42–e47. doi: 10.1111/j.1750-2659.2012.00337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vincent A, Awada L, Brown I, Chen H, Claes F, Dauphin G, Donis R, Culhane M, Hamilton K, Lewis N, Mumford E, Nguyen T, Parchariyanon S, Pasick J, Pavade G, Pereda A, Peiris M, Saito T, Swenson S, Van Reeth K, Webby R, Wong F, Ciacci-Zanella J. 2014. Review of influenza A virus in swine worldwide: a call for increased surveillance and research. Zoonoses Public Health 61:4–17. doi: 10.1111/zph.12049. [DOI] [PubMed] [Google Scholar]
- 19.Nidom CA, Takano R, Yamada S, Sakai-Tagawa Y, Daulay S, Aswadi D, Suzuki T, Suzuki Y, Shinya K, Iwatsuki-Horimoto K, Muramoto Y, Kawaoka Y. 2010. Influenza A (H5N1) viruses from pigs, Indonesia. Emerg Infect Dis 16:1515–1523. doi: 10.3201/eid1610.100508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peiris JS, Guan Y, Markwell D, Ghose P, Webster RG, Shortridge KF. 2001. Cocirculation of avian H9N2 and contemporary “human” H3N2 influenza A viruses in pigs in southeastern China: potential for genetic reassortment? J Virol 75:9679–9686. doi: 10.1128/JVI.75.20.9679-9686.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karasin AI, Brown IH, Carman S, Olsen CW. 2000. Isolation and characterization of H4N6 avian influenza viruses from pigs with pneumonia in Canada. J Virol 74:9322–9327. doi: 10.1128/JVI.74.19.9322-9327.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou NN, Senne DA, Landgraf JS, Swenson SL, Erickson G, Rossow K, Liu L, Yoon K, Krauss S, Webster RG. 1999. Genetic reassortment of avian, swine, and human influenza A viruses in American pigs. J Virol 73:8851–8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holyoake PK, Kirkland PD, Davis RJ, Arzey KE, Watson J, Lunt RA, Wang J, Wong F, Moloney BJ, Dunn SE. 2011. The first identified case of pandemic H1N1 influenza in pigs in Australia. Aust Vet J 89:427–431. doi: 10.1111/j.1751-0813.2011.00844.x. [DOI] [PubMed] [Google Scholar]
- 24.Anderson TK, Macken CA, Lewis NS, Scheuermann RH, Van Reeth K, Brown IH, Swenson SL, Simon G, Saito T, Berhane Y, Ciacci-Zanella J, Pereda A, Davis CT, Donis RO, Webby RJ, Vincent AL. 2016. A phylogeny-based global nomenclature system and automated annotation tool for H1 hemagglutinin genes from swine influenza A viruses. mSphere 1(6):e00275-. doi: 10.1128/mSphere.00275-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kilbourne ED. 2006. Influenza pandemics of the 20th century. Emerg Infect Dis 12:9–14. doi: 10.3201/eid1201.051254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen J, Fu X, Chen Y, He S, Zheng Y, Cao Z, Yu W, Zhou H, Su S, Zhang G. 2014. Identification of four genotypes of H3N2 swine influenza virus in pigs from southern China. Arch Virol 159:2705–2709. doi: 10.1007/s00705-014-2040-4. [DOI] [PubMed] [Google Scholar]
- 27.Shortridge KF, Webster RG, Butterfield WK, Campbell CH. 1977. Persistence of Hong Kong influenza virus variants in pigs. Science 196:1454–1455. doi: 10.1126/science.867041. [DOI] [PubMed] [Google Scholar]
- 28.Ottis K, Sidoli L, Bachmann PA, Webster RG, Kaplan MM. 1982. Human influenza A viruses in pigs: isolation of a H3N2 strain antigenically related to A/England/42/72 and evidence for continuous circulation of human viruses in the pig population. Arch Virol 73:103–108. doi: 10.1007/BF01314719. [DOI] [PubMed] [Google Scholar]
- 29.Webby RJ, Swenson SL, Krauss SL, Gerrish PJ, Goyal SM, Webster RG. 2000. Evolution of swine H3N2 influenza viruses in the United States. J Virol 74:8243–8251. doi: 10.1128/JVI.74.18.8243-8251.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abente EJ, Santos J, Lewis NS, Gauger PC, Stratton J, Skepner E, Anderson TK, Rajao DS, Perez DR, Vincent AL. 2016. The molecular determinants of antibody recognition and antigenic drift in the H3 hemagglutinin of swine influenza A virus. J Virol 90:8266–8280. doi: 10.1128/JVI.01002-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lewis NS, Anderson TK, Kitikoon P, Skepner E, Burke DF, Vincent AL. 2014. Substitutions near the hemagglutinin receptor-binding site determine the antigenic evolution of influenza A H3N2 viruses in U.S. swine. J Virol 88:4752–4763. doi: 10.1128/JVI.03805-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lorusso A, Vincent AL, Harland ML, Alt D, Bayles DO, Swenson SL, Gramer MR, Russell CA, Smith DJ, Lager KM, Lewis NS. 2011. Genetic and antigenic characterization of H1 influenza viruses from United States swine from 2008. J Gen Virol 92:919–930. doi: 10.1099/vir.0.027557-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nelson MI, Viboud C, Simonsen L, Bennett RT, Griesemer SB, St George K, Taylor J, Spiro DJ, Sengamalay NA, Ghedin E, Taubenberger JK, Holmes EC. 2008. Multiple reassortment events in the evolutionary history of H1N1 influenza A virus since 1918. PLoS Pathog 4:e1000012. doi: 10.1371/journal.ppat.1000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu M, Zhao X, Hua S, Du X, Peng Y, Li X, Lan Y, Wang D, Wu A, Shu Y, Jiang T. 2015. Antigenic patterns and evolution of the human influenza A (H1N1) virus. Sci Rep 5:14171. doi: 10.1038/srep14171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lewis NS, Russell CA, Langat P, Anderson TK, Berger K, Bielejec F, Burke DF, Dudas G, Fonville JM, Fouchier RA, Kellam P, Koel BF, Lemey P, Nguyen T, Nuansrichy B, Peiris JM, Saito T, Simon G, Skepner E, Takemae N, ESNIP3 Consortium, Webby RJ, Van Reeth K, Brookes SM, Larsen L, Watson SJ, Brown IH, Vincent AL. 2016. The global antigenic diversity of swine influenza A viruses. Elife 5:e12217. doi: 10.7554/eLife.12217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shu B, Garten R, Emery S, Balish A, Cooper L, Sessions W, Deyde V, Smith C, Berman L, Klimov A, Lindstrom S, Xu X. 2012. Genetic analysis and antigenic characterization of swine origin influenza viruses isolated from humans in the United States, 1990-2010. Virology 422:151–160. doi: 10.1016/j.virol.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 37.Swenson SL, Foni E, Saito T, Brown I. 2015. Influenza A virus of swine, p 1–14. Manual of diagnostic tests and vaccines for terrestrial animals. World Organization for Animal Health (OIE), Paris, France: http://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/2.08.07_INF_A_SWINE.pdf. [Google Scholar]
- 38.Hoffmann E, Stech J, Guan Y, Webster RG, Perez DR. 2001. Universal primer set for the full-length amplification of all influenza A viruses. Arch Virol 146:2275–2289. doi: 10.1007/s007050170002. [DOI] [PubMed] [Google Scholar]
- 39.Zhou B, Donnelly ME, Scholes DT, St George K, Hatta M, Kawaoka Y, Wentworth DE. 2009. Single-reaction genomic amplification accelerates sequencing and vaccine production for classical and swine origin human influenza A viruses. J Virol 83:10309–10313. doi: 10.1128/JVI.01109-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Choi YK, Goyal SM, Kang SW, Farnham MW, Joo HS. 2002. Detection and subtyping of swine influenza H1N1, H1N2 and H3N2 viruses in clinical samples using two multiplex RT-PCR assays. J Virol Methods 102:53–59. doi: 10.1016/S0166-0934(01)00442-6. [DOI] [PubMed] [Google Scholar]
- 41.Hoper D, Hoffmann B, Beer M. 2009. Simple, sensitive, and swift sequencing of complete H5N1 avian influenza virus genomes. J Clin Microbiol 47:674–679. doi: 10.1128/JCM.01028-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shu Y, McCauley J. 2017. GISAID: Global Initiative on Sharing All Influenza Data—from vision to reality. Euro Surveill 22(13):pii=30494. doi: 10.2807/1560-7917.ES.2017.22.13.30494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Edgar RC. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nguyen LT, Schmidt HA, von Haeseler A, Minh BQ. 2015. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol 32:268–274. doi: 10.1093/molbev/msu300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Minh BQ, Nguyen MA, von Haeseler A. 2013. Ultrafast approximation for phylogenetic bootstrap. Mol Biol Evol 30:1188–1195. doi: 10.1093/molbev/mst024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stamatakis A. 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burke DF, Smith DJ. 2014. A recommended numbering scheme for influenza A HA subtypes. PLoS One 9:e112302. doi: 10.1371/journal.pone.0112302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.National Health and Medical Research Council. 2013. Australian code for the care and use of animals for scientific purposes, 8th ed. National Health and Medical Research Council, Canberra, Australia. [Google Scholar]