There is an urgent need for a vaccine against widespread herpes simplex virus infections. The present study demonstrates that immunization of HLA transgenic rabbits with a peptide/CXCL10 prime/pull vaccine triggered mobilization of HSV-specific CD8+ T cells locally into the cornea and TG, the sites of acute and latent herpesvirus infections, respectively. Mobilization of antiviral CD8+ T cells into the cornea and TG of rabbits that received the prime/pull vaccine was associated with protection against ocular herpesvirus infection and disease following an ocular HSV-1 challenge. These results highlight the importance of the prime/pull vaccine strategy to bolster the number and function of protective CD8+ T cells within infected tissues.
KEYWORDS: HSV-1, ocular, herpes, HLA transgenic rabbit, CD8+ T cell, prime/pull vaccine, CXCL10, herpes simplex, prime/pull
ABSTRACT
Herpes simplex virus 1 (HSV-1) is a prevalent human pathogen that infects the cornea, causing potentially blinding herpetic disease. A clinical herpes vaccine is still lacking. In the present study, a novel prime/pull vaccine was tested in a human leukocyte antigen (HLA) transgenic rabbit model of ocular herpes (HLA Tg rabbits). Three peptide epitopes were selected, from the HSV-1 membrane glycoprotein C (UL44400–408), the DNA replication binding helicase (UL9196–204), and the tegument protein (UL25572–580), all preferentially recognized by CD8+ T cells from “naturally protected” HSV-1-seropositive healthy asymptomatic (ASYMP) individuals (who never had recurrent corneal herpetic disease). HLA Tg rabbits were immunized with a mixture of these three ASYMP CD8+ T cell peptide epitopes (UL44400–408, UL9196–204, and UL25572–580), which were delivered subcutaneously with CpG2007 adjuvant (prime). Fifteen days later, half of the rabbits received a topical ocular treatment with a recombinant neurotropic adeno-associated virus type 8 (AAV8) vector expressing the T cell-attracting CXCL10 chemokine (pull). The frequency and function of HSV-specific CD8+ T cells induced by the prime/pull vaccine were assessed in the peripheral blood, cornea, and trigeminal ganglion (TG). Compared to the cells generated in response to peptide immunization alone, the peptide/CXCL10 prime/pull vaccine generated frequent polyfunctional gamma interferon-positive (IFN-γ+) CD107+ CD8+ T cells that infiltrated both the cornea and TG. CD8+ T cell mobilization into the cornea and TG of prime/pull-vaccinated rabbits was associated with a significant reduction in corneal herpesvirus infection and disease following an ocular HSV-1 (strain McKrae) challenge. These findings draw attention to the novel prime/pull vaccine strategy for mobilizing antiviral CD8+ T cells into tissues to protect against herpesvirus infection and disease.
IMPORTANCE There is an urgent need for a vaccine against widespread herpes simplex virus infections. The present study demonstrates that immunization of HLA transgenic rabbits with a peptide/CXCL10 prime/pull vaccine triggered mobilization of HSV-specific CD8+ T cells locally into the cornea and TG, the sites of acute and latent herpesvirus infections, respectively. Mobilization of antiviral CD8+ T cells into the cornea and TG of rabbits that received the prime/pull vaccine was associated with protection against ocular herpesvirus infection and disease following an ocular HSV-1 challenge. These results highlight the importance of the prime/pull vaccine strategy to bolster the number and function of protective CD8+ T cells within infected tissues.
INTRODUCTION
Herpes simplex virus 1 (HSV-1) infection remains one of the most prevalent viral infections, with a staggering 3.72 billion affected individuals worldwide (1, 2). HSV-1, a member of the neurotropic alphaherpesvirus family (3–9), causes a spectrum of clinical ocular manifestations ranging from blepharitis, conjunctivitis, and neovascularization to disciform stromal edema and blinding herpetic stromal keratitis (HSK) (10, 11). In the United States alone, over 450,000 people have a history of potentially blinding ocular herpetic disease requiring medical attention, antiviral drug treatments, or, in severe cases, corneal transplantation (12–14). Current antiviral drug therapies (e.g., acyclovir and derivatives) do not eliminate the virus but reduce herpetic symptoms, but by only ∼45% (15, 16). The development of an effective vaccine would present an unparalleled alternative to antiviral drugs. It would be a powerful and cost-effective means to reduce corneal herpetic infection and disease (reviewed in reference 3).
Indirect observations in humans (17–19) indicate that trigeminal ganglion (TG)- and cornea-resident CD8+ T cells play a critical role in preventing HSV-1 reactivation from latently infected sensory neurons of TG and subsequent corneal herpetic infection and disease (20, 21). The most recent herpes vaccine clinical trials, which used recombinant glycoproteins B and D (gB and gD), failed to protect humans from herpetic disease despite inducing strong HSV-specific neutralizing antibodies (22, 23). These failures emphasize the need to induce strong T cell responses (in addition to humoral responses) for protection against ocular herpes. To protect against infectious pathogens, T cells must be able to traffic from lymph nodes and then the circulation into the cornea and TG, the sites of acute and latent infections, respectively.
Homing of HSV-specific CD8+ T cells to preferred inflamed anatomical locations, such as the cornea and TG, is thought to be regulated by chemokines/chemokine receptors and is affected by the vaccine strategy (24–28). Evidence is growing to support the hypothesis that HSV epitope-specific CD8+ T cells do possess qualities that direct them to the cornea and TG (1, 20, 29). However, the TG appears to be an immunologically restricted compartment that is not “open” to the homing of CD8+ T cells induced by a parenteral vaccine from the circulation into the infected TG (30). This is likely due to low levels of T cell-attracting chemokines, i.e., CXCL9, CXCL10, CXCL11, and CCL5, in noninfected TG. This low level of T cell-attracting chemokines does not allow migration of sufficient numbers of antiviral CD8+ T cells from the circulation into the TG. In the present study, we hypothesized that a prime/pull vaccine strategy that “primes” sufficient numbers of functional antiviral CD8+ T cells in the periphery and “pulls” them locally into the cornea and TG would likely result in significant reductions of corneal herpetic infection and disease. To test this hypothesis, we used the recently developed humanized HLA transgenic rabbits (HLA Tg rabbits), the most reliable small animal model of ocular herpesvirus infection and disease (21, 31–35).
We report that immunization of HLA Tg rabbits with a mixture of three immunodominant HSV-1 epitopes (prime), selected as being recognized preferentially by CD8+ T cells from “naturally protected” HSV-1-seropositive asymptomatic (ASYMP) individuals, followed by a topical ocular application of a recombinant neurotropic adeno-associated virus type 8 (AAV8) vector expressing the CXCL10 chemokine (pull) (i) induced potent and polyfunctional HSV-specific gamma interferon (IFN-γ)-producing CD107+ CD8+ T cells in peripheral blood and (ii) increased the size of CD8+ T cell infiltrates locally in the cornea and TG. This mobilization of antiviral CD8+ T cells was associated with significant reductions in both ocular herpesvirus infection and the severity of corneal herpetic disease following ocular HSV-1 challenge. These preclinical findings for the HLA-A*02:01 Tg rabbit, a reliable animal model of ocular herpesvirus infection and disease, strongly suggest that an HSV-1 human epitope peptide/CXCL10-based prime/pull vaccine displays promising features of safety, immunogenicity, and protective efficacy that should be considered in the development of a clinical ocular herpes vaccine.
RESULTS
Frequent IFN-γ+ CD107a/b+ CD8+ T cells from HLA-A*02:01-positive asymptomatic individuals preferentially target the HSV-1 UL44400–408, UL9196–204, and UL25572–580 epitopes.
We first searched the deduced amino acid sequences of the HSV-1 UL44, UL9, and UL25 proteins for potential HLA-A*02:01 binding regions. Nine potential antigenic HLA-A*02:01-restricted regions (i.e., CD8+ T cell epitopes) in three proteins (Table 1) were identified by use of the previously described BIMAS and SYFPEITHI algorithms (7). Potential cleavage sites for the human proteasome were identified using NetChop and MHC Pathway (7). Three of nine epitope peptides exhibited high affinities for soluble HLA-A*02:01, with 50% inhibitory concentrations (IC50) ranging from 13 to 16 nM (Table 1, last column). These three potential epitopes, one from each of the three proteins, were retained for antigenicity studies in humans and immunogenicity and protective efficacy studies in HLA Tg rabbits. We then compared the frequencies of CD8+ T cells specific to several potential epitopes selected from the HSV-1 UL44 membrane protein, the UL9 DNA replication origin binding helicase, and the UL25 tegument protein (Table 1) in HLA-A*02:01+ asymptomatic (ASYMP) versus symptomatic (SYMP) individuals.
TABLE 1.
Potential HLA-A*02:01 epitopes selected from the HSV-1 UL44, UL9, and UL25 proteinsa
| Antigen | Peptide | Sequence | Mol wt | HLA-A*0201 IC50 (nM) |
|---|---|---|---|---|
| UL44 | UL447–15 | GLAVVLWSL | 957.17 | 43 |
| UL4414–22 | SLLWLGAGV | 915.09 | 54 | |
| UL44400–408 | FLGDDPSPA | 917.98 | 13 | |
| UL9 | UL9172–180 | VLDEVMSTL | 1,006.19 | 45 |
| UL9196–204 | ALMLRLLRI | 1,098.47 | 16 | |
| UL9583–591 | FIYLALLEA | 1,052.29 | 50 | |
| UL25 | UL2535–43 | FWMSPVFNL | 1,140.37 | 96 |
| UL25572–580 | FIPQYLSAV | 1,037.23 | 15 | |
| UL25266–274 | RLPRYLACL | 1,104.4 | 76 |
The sequences of the HSV-1 UL44, UL9, and UL25 proteins were submitted for screening of potential HLA-A*02:01 epitopes by use of several computer algorithms, as described in Materials and Methods. Nine peptides were selected on the basis of the HLA-A*0201 binding motif sequence from HSV-1 strain 17. Sequences in bold are the immunodominant epitopes used to immunize HLA transgenic rabbits.
The characteristics of the SYMP and ASYMP study populations used in the present study, with respect to gender, age, HLA-A*02:01 frequency distribution, HSV-1/HSV-2 seropositivity, and status of ocular herpetic disease, are presented in Table 2 and detailed in Materials and Methods. Since HSV-1 is the main cause of ocular herpes, only individuals who were HSV-1 seropositive and HSV-2 seronegative were enrolled in the present study. The low frequencies of peripheral blood mononuclear cell (PBMC)-derived HSV-specific CD8+ T cells complicate direct ex vivo tetramer detection of CD8+ T cells by use of a typical number of PBMCs (i.e., 1 × 106 cells), and a prior expansion of CD8+ T cells following HSV-1 or peptide in vitro stimulation would hamper a reliable determination of the frequencies of HSV-1 UL44, UL9, and UL25 epitope-specific CD8+ T cells. Therefore, we opted to determine the frequencies of HSV-1 epitope-specific CD8+ T cells ex vivo by using a large number of PBMCs (∼10 × 106) per tetramer/CD8 monoclonal antibody (MAb) panel.
TABLE 2.
Characteristics of individuals enrolled in this study
| Subject-level characteristic | Value for all subjects (n = 39) |
|---|---|
| Gender (no. [%]) | |
| Female | 15 (51) |
| Male | 14 (49) |
| Race (no. [%]) | |
| Caucasian | 19 (66) |
| Non-Caucasian | 10 (34) |
| Age (median [range]) (yr) | 39 (21–67) |
| HSV status (no. [%]) | |
| HSV-1 seropositive | 29 (100) |
| HSV-2 seropositive | 0 (0) |
| HSV-1 and -2 seropositive | 0 (0) |
| HSV seronegative | 10 (100) |
| HLA status (no. [%]) | |
| HLA-A*02:01 positive | 24 (83) |
| HLA-A*02:01 negative | 5 (17) |
| Herpes disease statusa (no. [%]) | |
| Asymptomatic (ASYMP) | 19 (66) |
| Symptomatic (SYMP) | 10 (34) |
Definitions of symptomatic and asymptomatic individuals are detailed in Materials and Methods.
As shown in Fig. 1A (representative data) and B (averages of frequencies), high frequencies of CD8+ T cells against the UL44400–408, UL9196–204, and UL25572–580 epitopes were detected in ASYMP individuals. Interestingly, the average frequencies of CD8+ T cells specific to the UL9196–204 epitope were consistently and significantly higher in ASYMP individuals (Fig. 1A and B, black circles) than in SYMP individuals (Fig. 1A and B, white circles) (P = 0.005). The average frequencies of CD8+ T cells specific to the UL44400–408 and UL25572–580 epitopes were at similar levels in both SYMP and ASYMP individuals. The average frequencies of CD8+ T cells specific to the UL44400–408, UL9196–204, and UL25572–580 immunodominant epitopes in HLA-A*02:01-seronegative controls (SeroNeg) did not yield any significant percentages, confirming the HSV antigen specificity of CD8+ T cells (Fig. 1A and B, white squares). We next focused on determining the function of CD8+ T cells specific to the immunodominant UL44400–408, UL9196–204, and UL25572–580 epitopes.
FIG 1.
Frequent IFN-γ+ CD107+ CD8+ T cells specific to the HSV-1 UL44400–408, UL9196–204, and UL25572–580 epitopes detected HSV-seropositive ASYMP HLA-A*0201+ individuals. PBMCs (∼10 × 106) derived from HSV-seropositive (SeroPos) asymptomatic (ASYMP) and symptomatic (SYMP) individuals and from HSV-seronegative (SeroNeg) individuals were stimulated in vitro with 10 μM (each) immunodominant CD8+ T cell peptide epitopes (UL44400–408, UL9196–204, and UL25572–580) (Table 1). (A) Representative FACS contour plots of UL44400–408, UL9196–204, and UL25572–580 tetramer-specific CD8+ T cells detected in one HSV-seropositive ASYMP individual (top row), one HSV-seropositive SYMP individual (middle row), and one SeroNeg individual (bottom row). (B) Average frequencies of CD8+ T cells specific to the UL44400–408, UL9196–204, and UL25572–580 epitopes detected in 10 HLA-A*0201+ ASYMP (closed circles), eight HLA-A*0201+ SYMP (open circles), and five HSV-seronegative (open squares) individuals. The nominal P values indicate statistical significance detected between ASYMP and SYMP individuals and between SeroPos ASYMP and SeroNeg individuals. A general linear model was used, and we compared the least-squares means by the Dunnett procedure for multiple comparisons. (C) Frequencies of proliferative CFSElow CD8+ T cells, detected by a CFSE dilution method following in vitro stimulation with the UL44400–408, UL9196–204, and UL25572–580 peptides. The top row shows contour plots of individual tetramer-specific CFSElow CD8+ T cells in HLA-A*0201+ ASYMP individuals. The bottom row shows contour plots of tetramer-specific CFSElow CD8+ T cells in HLA-A*0201− ASYMP individuals. (D) Frequencies of HSV-1 UL44400–408, UL9196–204, and UL25572–580 epitope-specific IFN-γ+ CD8+ T cells (top two rows) and CD107+ CD8+ T cells (bottom two rows) in HLA-A*0201+ and HLA-A*0201− ASYMP individuals. The results are representative of two independent experiments.
We compared the CD8+ T cell proliferative responses to the UL44400–408, UL9196–204, and UL25572–580 immunodominant epitopes from HLA-A*02:01+ ASYMP individuals versus HLA-A*02:01− ASYMP individuals (controls). PBMCs were labeled with carboxyfluorescein succinimidyl ester (CFSE) and then restimulated in vitro for 6 days with each of the HSV-1 UL44400–408, UL9196–204, and UL25572–580 peptides. As shown in Fig. 1C (upper panels), significant percentages of the CFSElow CD8+ T cells were detected for one ASYMP HLA-A*02:01+ individual when cells were stimulated with the UL44400–408, UL9196–204, and UL25572–580 peptides. The UL44400–408 and UL25572–580 peptides induced the most proliferation of CD8+ T cells (22.9% and 31.6% of dividing CFSElow CD8+ T cells, respectively). The UL9196–204 peptide induced a modest yet significant amount of dividing CFSElow CD8+ T cells (13.0% of dividing CFSElow CD8+ T cells). In contrast, none of the remaining six low-affinity peptides from the HSV-1 UL44, UL9, and UL25 proteins induced significant proliferation of CD8+ T cells in ASYMP HLA-A*02:01+ individuals (Table 2). No significant percentages of dividing CFSElow CD8+ T cells were induced by any of the UL44, UL9, and UL25 peptides in HLA-A*02:01− individuals, confirming the HLA restriction of the T cell responses (data not shown).
Because a lack of proliferative response may not always reflect a lack of T cell response (36, 37), we studied IFN-γ and CD107a/b expression by HSV-1 epitope-specific CD8+ T cells. PBMCs from HLA-A*02:01+ and HLA-A*02:01− ASYMP individuals were stimulated in vitro for 72 h with each of the UL44, UL9, and UL25 peptides (Table 1). We then used intracellular fluorescence-activated cell sorter (FACS) staining to measure the frequencies of IFN-γ+ CD8+ T and CD107a/b+ CD8+ T cells, as described in Materials and Methods.
Figure 1D (top two rows) shows representative contour plots of percentages of IFN-γ+ CD8+ T cells detected in one HLA-A*02:01+ and one HLA-A*02:01− ASYMP individual following stimulation with equimolar amounts of the HSV-1 UL44400–408, UL9196–204, and UL25572–580 peptides. The highest percentage of IFN-γ+ CD8+ T cells detected from HLA-A*02:01+ ASYMP individuals was directed against the UL44400–408 peptide (6.5%). The UL9196–204 and UL25572–580 peptides induced up to 4.7% IFN-γ+ CD8+ T cells from one HLA-A*02:01+ ASYMP individual. In parallel experiments, no significant percentage of IFN-γ+ CD8+ T cells was detected in the PBMCs of the HLA-A*02:01− ASYMP individuals when cells were stimulated with equimolar amounts of the UL44400–408, UL9196–204, and UL25572–580 peptides, confirming the HLA restriction of the T cell responses. These results indicate that the HSV-1 peptides UL44400–408, UL9196–204, and UL25572–580 contain at least one immunodominant IFN-γ-producing CD8+ T cell epitope.
We then examined the ability of the UL44400–408, UL9196–204, and UL25572–580 peptides to induce cytotoxic CD8+ T cells from HLA-A*02:01+ and HLA-A*02:01− ASYMP individuals. Figure 1D (lower two rows) shows representative contour plots of the percentages of CD107a/b+ CD8+ T cells detected in one HLA-A*02:01+ and one HLA-A*02:01− HSV-1-seropositive individual following stimulation with equimolar amounts of the HSV-1 UL44400–408, UL9196–204, and UL25572–580 peptides. The highest percentages of CD107a/b+ CD8+ T cells detected in HLA-A*02:01+ ASYMP individuals were directed mainly against the UL44400–408 and UL9196–204 peptides (6.7% of CD107a/b+ CD8+ T cells for both peptides). The UL25572–580 peptide induced up to 4.8% CD107a/b+ CD8+ T cells from one HLA-A*02:01+ ASYMP individual. In parallel experiments, no significant percentage of CD107a/b+ CD8+ T cells was detected in HLA-A*02:01− ASYMP controls when cells were stimulated with equimolar amounts of the UL44400–408, UL9196–204, and UL25572–580 peptides, confirming the HLA specificity of the CD8+ T cell responses.
Altogether, these results demonstrate that the HSV-1 UL44400–408, UL9196–204, and UL25572–580 epitopes are targeted by frequent IFN-γ-producing CD107a/b+ CD8+ T cells from HLA-A*02:01-positive ASYMP individuals. In contrast, low frequencies of IFN-γ-producing CD107+ CD8+ T cells were detected from HLA-A*02:01-negative ASYMP controls in response to equimolar amounts of each of the three peptides. These results confirm the immunodominance of the UL44400–408, UL9196–204, and UL25572–580 epitopes.
Since the UL44400–408, UL9196–204, and UL25572–580 peptides were preferentially recognized by polyfunctional CD8+ T cells from naturally protected HSV-seropositive ASYMP individuals who never had any recurrent ocular herpetic disease, we next set out to test whether a mixture of these three peptides can be used as a vaccine to protect against ocular herpes. In the remainder of this study, the safety, immunogenicity, and protective efficacy of the UL44400–408, UL9196–204, and UL25572–580 peptides were evaluated in the HLA-A*02:01-positive transgenic rabbit model (HLA Tg rabbit), a reliable preclinical animal model of ocular herpesvirus infection and disease.
Selection of HLA-A*02:01 transgenic rabbits for preclinical evaluation of a UL44400–408, UL9196–204, and UL25572–580 peptide-based vaccine against ocular herpes.
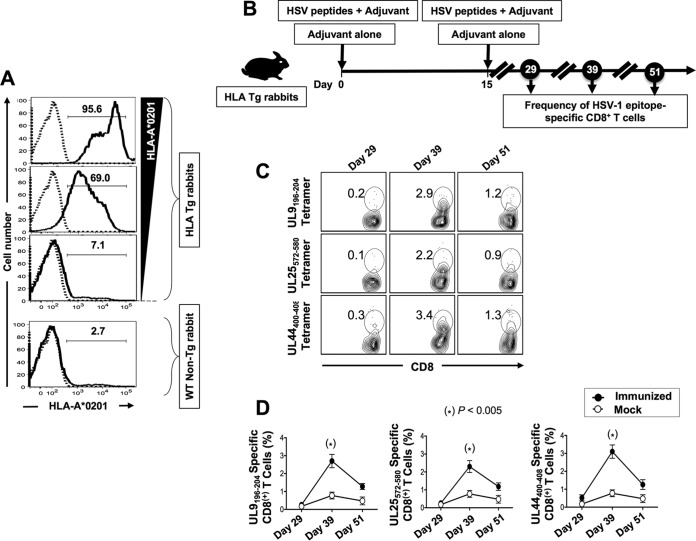
HLA-A*02:01-positive transgenic rabbit breeders were selected based on their high expression of HLA-A*02:01 molecules, since the expression of the rabbits' own major histocompatibility complex (MHC) class I molecules might interfere with the human HLA-A*02:01-restricted responses (Fig. 2A). The high expression of HLA-A*02:01 molecules in the selected HLA Tg rabbits should result in rabbit CD8+ T cells using the human HLA-A*02:01 molecules at both the thymus selection and peripheral effector levels (35). All selected HLA Tg rabbits had similarly high expression of HLA-A*02:01 molecules in over 95% of PBMCs, as assessed by FACS (Fig. 2A, upper panel). HLA transgenic rabbits with low to moderate expression of HLA-A*02:01 molecules (i.e., 7% to 69%) were excluded from the study. Wild-type (non-HLA transgenic) rabbits were used as controls of HLA specificity. As expected, no HLA-A*02:01 expression was detected in nontransgenic wild-type rabbits (Fig. 2A, lower panel). The specificity of anti-HLA-A*02:01 MAb was confirmed using an isotype IgG control. Based on their high expression of HLA-A*02:01 molecules, a group of 30 HLA Tg rabbits were enrolled for preclinical evaluation of a UL44400–408, UL9196–204, and UL25572–580 peptide-based vaccine, as shown in Fig. 2B.
FIG 2.
Frequencies of UL9196–204, UL25572–580, and UL44400–408 tetramer-specific CD8+ T cells induced in HLA Tg rabbits following peptide immunization. (A) Detection of HLA-A*02:01 molecules in PBMCs from HLA Tg rabbits. PBMCs from either HLA Tg rabbits or wild-type nontransgenic rabbits were stained first with the BB7.2 MAb and then with a PE-conjugated anti-mouse secondary IgG antibody and analyzed by flow cytometry (see Materials and Methods). (B) Illustration of UL44, UL9, and UL25 peptide immunization regimen and immunological characterization. HLA Tg rabbits were immunized twice, on days 0 and 15, with a mixture of the UL9196–204, UL25572–580, and UL44400–408 peptides in CpG (ODN2007) adjuvant, or they received adjuvant alone (mock group). (C) HLA Tg rabbits were bled on days 29, 39, and 51 after initial immunization (i.e., on days 14, 24, and 36 after the second immunization), and the frequencies of UL9196–204, UL25572–580, and UL44400–408 epitope-specific CD8+ T cells were detected using the corresponding HLA-A*02:01-peptides/tetramers. Shown are representative contour plots of the frequencies of tetramer+ CD8+ T cells specific to the UL9196–204, UL25572–580, and UL44400–408 epitopes detected in PBMCs from immunized rabbits. The number at the top of each box represents the percentage of epitope-specific CD8+ T cells. (D) Average frequencies of tetramer-specific CD8+ T cells detected in all animals on days 29, 39, and 51 after initial immunization. Solid circles represent peptide-immunized rabbits. Open circles represent mock-immunized rabbits. The results are representative of two independent experiments.
Frequent HSV-specific CD8+ T cells are induced in HLA Tg rabbits following immunization with a mixture of the UL44400–408, UL9196–204, and UL25572–580 peptides.
To gain insight into the immunogenicity of the HSV-1 UL44400–408, UL9196–204, and UL25572–580 peptide-based vaccine, HLA Tg rabbits (n = 20) were immunized twice, at 2-week intervals, with a mixture of the HSV-1 UL44400–408, UL9196–204, and UL25572–580 peptides, as illustrated in Fig. 2B. Because parallel induction of CD4+ T helper cells is crucial for the priming and maintenance of HSV-specific CD8+ T cell responses (7, 38), the mixture of the three CD8+ T cell epitopes was delivered together with the PADRE peptide, a promiscuous HSV-1 CD4+ T helper epitope that binds to most MHC class II molecules, including rabbit MHC class II molecules (7, 35).
The frequencies of HSV-1 epitope-specific CD8+ T cells were determined for PBMCs from each animal on days 29, 39, and 51 after the first immunization (i.e., on days 14, 24, and 36 after the second immunization), using HLA-A*02:01/epitope-specific tetramers. As shown in Fig. 2C and D, each of the three peptides (UL9196–204, UL25572–580, and UL44400–408) generated high frequencies of epitope-specific CD8+ T cells in the majority of immunized HLA Tg rabbits. The maximum frequencies of epitope-specific CD8+ T cells were attained on day 39 after the first immunization.
Similar to those in HLA-A*02:01-positive individuals, the highest frequencies of CD8+ T cells detected in the HLA-A*02:01-positive transgenic rabbits were against the UL44400–408 and UL9196–204 epitopes (3.4% and 2.9%, respectively). The average frequency of CD8+ T cells specific to the remaining epitope, UL25572–580, was at a moderate level in HLA-A*02:01-positive transgenic rabbits (2.2%). The average frequencies of CD8+ T cells specific to the UL44400–408, UL9196–204, and UL25572–580 immunodominant epitopes in nontransgenic control rabbits did not yield any significant percentages (data not shown), confirming the HLA restriction of CD8+ T cells induced in HLA Tg rabbits following immunization with the mixture of the three HSV human epitope peptides.
A prime/pull vaccine based on immunization with a mixture of UL44400–408, UL9196–204, and UL25572–580 peptides followed by CXCL10 treatment bolsters the function of HSV epitope-specific CD8+ T cells in HLA transgenic rabbits.
We next determined whether priming with a mixture of the HSV-1 UL44400–408, UL9196–204, and UL25572–580 peptides followed by topical ocular treatment with CXCL10 would (i) boost the function of HSV-1 epitope-specific CD8+ T cells and (ii) pull more CD8+ T cells into the cornea and TG, the sites of acute and latent HSV-1 infection, respectively, in both humans and rabbits.
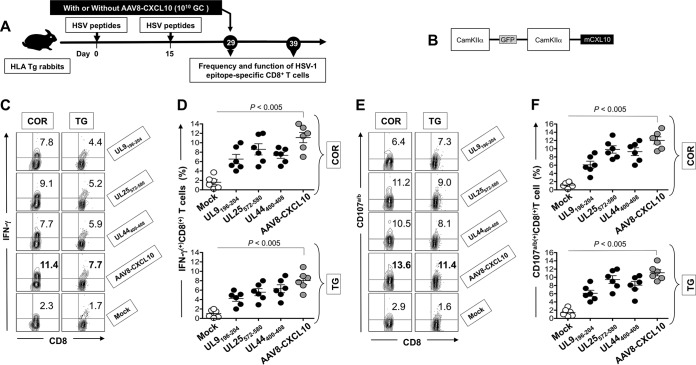
HLA Tg rabbits (n = 12) were immunized twice, at 2-week intervals, with the mixture of UL44400–408, UL9196–204, and UL25572–580 peptides, as shown in Fig. 3A. Using the optimal CamKIIα promoter, we constructed a neurotropic recombinant nonreplicating adeno-associated virus type 8 vector (rAAV8) expressing the T cell-attracting CXCL10 chemokine (rAAV8-CamKIIα-GFP-CamKIIα-CXCL10) (Fig. 3B). On day 29, half of the rabbits (n = 6) were left untreated and the other half (n = 6) received a topical ocular treatment with 1010 genome copies (GC) of the rAAV8-CamKIIα-GFP-CamKIIα-CXCL10 vector. An additional group of HLA Tg rabbits was mock vaccinated and received an empty AAV8 vector as a control (mock group). Ten days following CXCL10 treatment, representative FACS plots (Fig. 3C and E) and average frequencies (Fig. 3D and F) of HSV-1 UL44400–408, UL9196–204, and UL25572–580 epitope-specific functional CD8+ T cells were determined for the cornea (COR) and TG (the sites of acute and latent HSV-1 infection).
FIG 3.
A prime/pull immunization regimen bolsters the function of HSV epitope-specific CD8+ T cells in HLA Tg rabbits. (A) Illustration of the prime/pull immunization regimen and immunology studies in HLA Tg rabbits. HLA Tg rabbits (n = 12) were primed twice, on day 0 and day 15, with a mixture of the UL9196–204, UL25572–580, and UL44400–408 peptides in CpG (ODN2007) adjuvant. On day 29 (14 days after the final peptide immunization), half of the rabbits (n = 6) were left untreated and the other half (n = 6) received a topical ocular treatment with 1010 GC/eye of the AAV8 vector expressing CXCL10. (B) Schematic of the AAV8 vector construct expressing the CXCL10 chemokine and GFP, both under the control of the neurotropic CamKIIα promoter. The frequencies of CD8+ T cells that expressed IFN-γ (C and D) and CD107a/b (E and F) were determined by FACS analysis of cells from the cornea and TG of immunized AAV8-CXCL10-treated and untreated HLA Tg rabbits 10 days after treatment, as described in Materials and Methods. (C) Representative contour plots of IFN-γ+ CD8+ T cells specific to the UL9196–204, UL25572–580, and UL44400–408 epitopes detected in the cornea (COR) (left panels) and TG (right panels) for immunized/untreated, immunized/rAAV8-CXCL10-treated, and mock-immunized rabbits. (D) Average frequencies of IFN-γ+ CD8+ T cells specific to the UL9196–204, UL25572–580, and UL44400–408 epitopes detected in the cornea (top) and TG (bottom) for six immunized/untreated, six immunized/rAAV8-CXCL10-treated, and six mock-immunized rabbits. (E) Representative contour plots of CD107a/b+ CD8+ T cells specific to the UL9196–204, UL25572–580, and UL44400–408 epitopes detected in the cornea (left panels) and TG (right panels) for immunized/untreated, immunized/rAAV8-CXCL10-treated, and mock-immunized rabbits. (F) Average frequencies of CD107a/b+ CD8+ T cells specific to the UL9196–204, UL25572–580, and UL44400–408 epitopes detected in the cornea (top) and TG (bottom) for six immunized/untreated, six immunized/rAAV8-CXCL10-treated, and six mock-immunized rabbits. The results are representative of two independent experiments.
Significantly higher frequencies of (i) HSV-specific IFN-γ+ CD8+ T cells (Fig. 3C and D) and (ii) HSV-specific CD107a/b+ CD8+ T cells (Fig. 3E and F) were detected in the cornea and TG for HLA Tg rabbits that were vaccinated and treated with CXCL10 than for HLA Tg rabbits that received the peptide vaccine alone (P < 0.05), indicating that treatment with the CXCL10 chemokine contributed to increased function of cornea- and TG-resident HSV-specific CD8+ T cells. No corneal pathology was associated with the chemokine-encoding, nonreplicating rAAV8 vector.
These results demonstrate that topical ocular treatment with the CXCL10 chemokine bolsters the functional HSV-specific CD8+ T cells in the cornea and TG of HLA-A*02:01 Tg rabbits.
A human epitope peptide/CXCL10-based prime/pull vaccine induces strong protection against ocular herpesvirus infection and disease.
We next determined whether priming with the mixture of the HSV-1 UL44400–408, UL9196–204, and UL25572–580 peptides followed by topical ocular treatment with the rAAV8-CamKIIα-GFP-CamKIIα-CXCL10 vector would provide better protective immunity which would reduce ocular herpesvirus infection and disease following HSV-1 challenge.
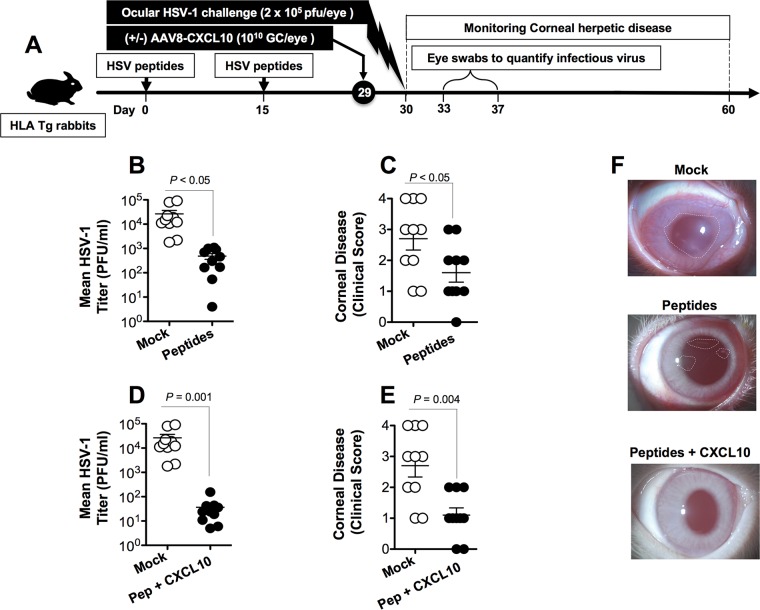
A group of HLA-A*02:01 Tg rabbits (n = 20) received subcutaneous immunizations with the mixture of three HSV-1 peptides (UL9196–204, UL25572–580, and UL44400–408) on day 0 and day 15. On day 29, half of the rabbits (n = 10) were left untreated and the other half (n = 10) received a topical ocular treatment with 1010 GC of the rAAV8-CamKIIα-GFP-CamKIIα-CXCL10 vector. An additional group of control HLA Tg rabbits (n = 10) was mock vaccinated and received an equivalent amount of empty AAV8 vector (mock group). Two days following CXCL10 treatment, animals from all three groups received an ocular HSV-1 challenge (2 × 105 PFU; strain McKrae) without scarification (Fig. 4A). The rabbits were then assessed for up to 30 days postchallenge for ocular herpetic disease and ocular viral replication.
FIG 4.
Protective immunity against ocular herpesvirus replication and corneal disease induced by a peptide/rAAV8-CXCL10 prime/pull vaccine in HLA transgenic rabbits. (A) Illustration of the prime/pull vaccine regimen and virology and disease studies. Two groups of age-matched HLA-A*02:01 Tg rabbits (n = 20 each) were immunized subcutaneously twice (on day 0 and day 15) with a pool of three CD8+ peptide epitopes (UL9196–204, UL25572–580, and UL44400–408). On day 29, half of the immunized animals (n = 10) received a topical application of 1 × 1010 GC/eye of AAV8 expressing the CXCL10 chemokine. The other half was left untreated. As a negative control, a third group of HLA Tg rabbits (n = 10) received the adjuvant alone (mock). Two weeks after the final immunization, HLA Tg rabbits were challenged ocularly (both eyes, without scarification) with 2 × 105 PFU of HSV-1 (strain McKrae) and examined for signs of ocular disease and for virus titer. (B and D) Virus titers were determined from eye swabs on day 7 postinfection. (C and E) Ocular herpetic disease was scored according to a standard scale from 0 to 4 for 30 days postchallenge, as described in Materials and Methods. (F) Representative images showing ocular disease detected in rabbits that received the mock vaccine, the peptide vaccine, or the peptide/rAAV8-CXCL10 prime/pull vaccine. The results are representative of two independent experiments.
As shown in Fig. 4B and D, there was a significant decrease of viral titers detected on day 7 postinfection (the peak of viral replication) in the eye swabs of peptide-vaccinated rabbits compared to those for mock-vaccinated rabbits (P < 0.05). Furthermore, the pathological clinical scores observed for the peptide-vaccinated rabbits were significantly lower than those recorded for the mock-vaccinated rabbits (P < 0.01 for all) (Fig. 4C, E, and F). Moreover, the mixture of the UL9196–204, UL25572–580, and UL44400–408 peptides combined with CXCL10 treatment showed a trend toward providing better protection than the mixture of the UL9196–204, UL25572–580, and UL44400–408 peptides alone (i.e., without CXCL10 treatment) (compare Fig. 4B and C to Fig. 4D and E).
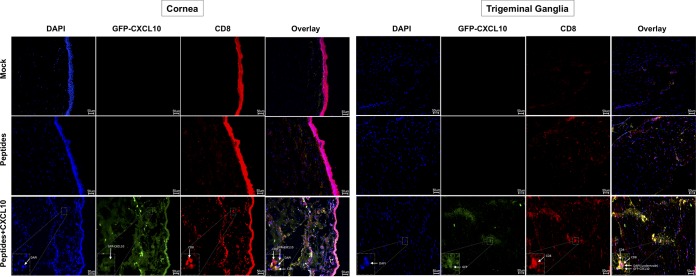
Furthermore, the topical ocular treatment with the CXCL10 chemokine appeared to bolster the CD8+ T cell infiltrates in both the cornea and TG of protected HLA-A*02:01 Tg rabbits. Green fluorescent protein (GFP) appeared to be coexpressed with CD8+ T cell infiltrates in both the cornea (Fig. 5, bottom left panels) and TG (Fig. 5, bottom right panels) of CXCL10-treated protected rabbits. The accumulation of CD8+ T cell infiltrates in the cornea and TG suggests migration of protective CD8+ T cells from the circulation into the cornea and TG, where the neurotropic CXCL10 chemokine-encoding AAV8 vector is expressed.
FIG 5.
Peptide priming followed by topical ocular application of a neurotropic AAV8 vector expressing CXCL10 increases the amounts of CD8+ T cell infiltrates in the cornea and TG. Two groups of age-matched HLA-A*02:01 rabbits (n = 12 each) were immunized subcutaneously twice (on day 0 and day 15) with a pool of three CD8+ peptide epitopes (UL9196–204, UL25572–580, and UL44400–408). On day 29, half of the immunized animals (n = 6) received a topical application of 1 × 1010 GC/eye of neurotropic rAAV8 expressing mouse CXCL10 together with GFP, both under the control of the neurotropic CamKII-α promoter. The other half was left untreated. As a negative control, a third group of HLA transgenic rabbits (n = 6) received adjuvant alone (mock). Two weeks after the final immunization, HLA Tg rabbits were challenged ocularly (both eyes, without scarification) with 2 × 105 PFU of HSV-1 (strain McKrae). Rabbit cornea (A) and TG (B) sections were harvested from all groups of rabbits and costained using DAPI, GFP, and a MAb specific to rabbit CD8+ T cells. The images shown are confocal microscopy images (magnification, ×20). Blue, DAPI (DNA stain); green, GFP-AAV8; yellow, CD8+ cells. The data are representative of two independent experiments.
Altogether, these results (i) indicate that immunization with a mixture of the UL9196–204, UL25572–580, and UL44400–408 peptides combined with CXCL10 treatment contained the ocular HSV-1 load, decreased ocular herpetic disease, and protected against lethal ocular herpes in the HLA Tg rabbit model of ocular herpes; (ii) suggest that bolstering the number and function of HSV-specific CD8+ T cells that infiltrate the cornea and TG through a prime/pull vaccine improved protection against ocular herpesvirus infection and disease; and (iii) support HLA-A*02:01 Tg rabbits as a useful animal model for investigating the underlying mechanisms by which CD8+ T cells specific to human HSV-1 CD8+ T cell epitopes mediate control of ocular herpesvirus infection and disease.
DISCUSSION
Naturally protected HSV-1-seropositive healthy ASYMP individuals (who have never had recurrent corneal, oral, or genital herpetic disease) develop CD8+ T cells that appear to preferentially target a set of human ASYMP epitopes (6, 39–47). These ASYMP CD8+ T cells are phenotypically and functionally distinct and appear to be associated with less frequent and reduced severity of ocular herpetic disease (6, 20, 21, 34, 41, 42, 44–47). The present study extends these correlations by demonstrating that in vivo immunization with these human ASYMP CD8+ T cell epitopes induced protective CD8+ T cells associated with herpetic corneal disease in the humanized HLA Tg rabbit model of ocular herpes. We characterized the T cell immunogenicity and protective efficacy of three human ASYMP CD8+ T cell epitopes that were identified from three HSV-1 antigens, namely, the HSV-1 membrane protein (UL44), the ATP-dependent helicase protein (UL9), and the tegument protein (UL25) (1), as being recognized preferentially by CD8+ T cells from ASYMP individuals. We showed that immunization with a mixture of these three HSV-1 ASYMP peptides (UL44400–408, UL9196–204, and UL25572–580) elicited polyfunctional CD8+ T cell responses in HLA Tg rabbits and was associated with protection from ocular herpesvirus infection and disease following an ocular challenge. Moreover, we demonstrated that immunized HLA Tg rabbits that received a topical ocular application of a neurotropic AAV8 vector expressing the CXCL10 chemokine developed frequent and functional HSV-specific CD107+ CD8+ cytotoxic T cells in the TG and cornea. These potent tissue-resident CD8+ T cells protected HLA Tg rabbits from virus replication in the eye and corneal herpetic disease (keratitis) following an ocular challenge with HSV-1 (strain McKrae). The results of this preclinical trial (i) support implementation of a prime/pull ASYMP peptide vaccine strategy to strengthen the protective efficacy of tissue-resident CD8+ T cells against ocular herpes and (ii) indicate that ASYMP CD8+ T cell epitopes selected from the HSV-1 protein antigens UL44, UL9, and UL25 are logical candidates to be included in the next generation of ocular herpes prime/pull peptide vaccines.
Complications from HSV-1 infection range from mild symptoms, such as cold sores and genital lesions, to more serious complications, such as permanent brain damage from encephalitis in adults and neonates and blinding corneal inflammation (3, 48). Ocular HSV-1 infection is the leading cause of virus-induced corneal blindness in industrialized countries. Changes in sexual behavior among young adults have been associated with a recent increase in genital HSV-1 infection, resulting from oral-genital rather than genital-genital contact. Approximately 450,000 adults in the United States have a history of recurrent herpetic ocular disease (symptomatic [SYMP] individuals), with approximately 20,000 individuals per year experiencing recurrent and potentially blinding ocular herpetic lesions (3, 4, 6–8). Seropositive SYMP and ASYMP individuals are different with regard to CD8+ T cell epitope specificity and the magnitude and phenotype of HSV-specific CD8+ T cells (3–8, 36, 39, 47, 49). Thus, a vaccine that converts the presumably nonprotective profile of CD8+ T cells seen in SYMP patients into the protective profile seen in ASYMP individuals will likely lead to a decrease in ocular herpes. In the present study, we demonstrated that immunization with a mixture of peptide vaccines exclusively bearing human epitopes from the HSV-1 proteins UL44, UL9, and UL25 that are mainly recognized by CD8+ T cells from HSV-1-seropositive healthy ASYMP individuals (1) reduced the amount of infectious virus in tears and lessened the occurrence of ocular herpes following ocular challenge in prophylactically immunized HLA Tg rabbits. This peptide vaccine excludes SYMP epitopes that are recognized mostly by CD8+ T cells from SYMP individuals with a history of numerous episodes of recurrent ocular herpes disease. It remains to be determined whether this ASYMP epitope vaccine given therapeutically to latently infected HLA Tg rabbits will (i) significantly decrease virus reactivation from TG (virus shedding in tears) and/or recurrent ocular disease and (ii) increase the numbers and functions of local HSV-1 UL44, UL9, and UL25 epitope-specific CD8+ T cells over the existing immune response induced by the primary infection. Preclinical studies in HLA Tg rabbits to assess the protective efficacy of these ASYMP epitope vaccines in a therapeutic setting will be the subject of future reports.
Traditional vaccine formulations using native or recombinant proteins are generally ineffective in the induction of CD8+ T cell responses (50). Clinical trials of HSV vaccines using selected HSV proteins mixed with adjuvant have shown limited efficacy in seronegative women but not in men (reviewed in reference 3). This limitation results from the basic biology of antigen processing and presentation of epitopes to CD8+ T cells, necessitating the endogenous synthesis and presentation of HLA class I molecules. In contrast, a mixture of three HSV-1 ASYMP peptides, UL44400–408, UL9196–204, and UL25572–580, induced strong CD8+ T cell responses, as these peptides present the optimal 9-mer length and the preferred peptide binding motif (xLxxxxxxV/L) for binding with high affinity to HLA-A*02:01 molecules expressed by the HLA Tg rabbits. Moreover, there are several other advantages of a vaccine comprised of multiple peptide epitopes, as follows. (i) Multiple epitopes selected from the viral envelope, tegument, capsid, and regulatory proteins can be included, as demonstrated by the UL44400–408, UL9196–204, and UL25572–580 multiple-epitope vaccine in this study. Immunization with the promiscuous CD4+ T helper epitope (PADRE peptide) together with the mixture of CD8+ T cell peptides generated significant PADRE peptide-specific CD4+ T cell responses in HLA Tg rabbits compared to those after immunization with the mixture of CD8+ T cell peptides alone (not shown), indicating that CD4+ T cells fulfill their role as T helpers by providing help for the priming and establishment of CD8+ T cell memory. The multiple-epitope vaccine approach is likely to produce more efficacious protection than that with any one of the three epitopes used individually. (ii) Through identification of protective human ASYMP CD8+ T cell epitopes from HSV-1 proteins, herpes vaccine formulations can be simplified, enabling molecular-level vaccine characterization and improved safety profiles. However, a potential concern of a multipeptide vaccine is the possibility of epitope competition among the epitope peptides for specific HLA-A*02:01 molecules. This may impair the induction of a full spectrum of T cell responses against all of the desired ASYMP epitopes. One approach to overcoming this possibility is to administer individual peptides at different injection sites. To the best of our knowledge, the immunogenicity and protective efficacy of asymptomatic epitopes from the HSV-1 UL44, UL9, and UL25 proteins have not previously been described for rabbits. Thus, the results of the present investigation have revealed, for the first time, the immunogenicity and protective efficacy of several new CD8+ T cell epitopes from many HSV-1 proteins, expanding the panel and diversity of HSV-1 epitopes. Our results highlight the potential of peptide mixture-based vaccines for activating potent functional CD8+ T cell responses against ocular herpes compared to those after immunization with a single immunodominant peptide (51–55).
Homing of HSV-specific CD8+ T cells to preferred inflamed anatomical locations, such as the cornea and TG, is thought to be regulated by chemokines/chemokine receptors, among other factors, and is affected by the vaccine strategy (24–28). In this study, we demonstrated that immunization of HLA Tg rabbits with a mixture of ASYMP peptides followed by a topical ocular application of a neurotropic AAV8 vector expressing the T cell-attracting CXCL10 chemokine was effective at boosting the number and function of HSV-specific CD8+ T cells in corneal and TG tissues and that this correlated with protection against ocular herpes challenge. Several studies, including ours, have shown that HSV-specific CD8+ T cells may exhaust their functional activity by “seeing” more viral antigen in HSV-1-infected TG (51, 56–58). In the present study, more functional (less exhausted) CD8+ T cells were detected in the TG of HSV-1-infected HLA Tg rabbits following the prime/pull vaccine and viral challenge than in those of mock-vaccinated and infected control HLA rabbits. Thus, it seems likely that the prime/pull vaccine contributes, by a still-to-be-determined mechanism, to curbing CD8+ T cell exhaustion in HSV-1-infected TG. Our vaccine strategy was adapted from the recently reported prime/pull genital herpes vaccine strategy from the study of Shin and Iwasaki, which used a mouse model of genital herpes (59). The polyfunctionality of induced CD8+ T cells may play a critical role in protection against ocular herpesvirus infection and disease, since the quality of CD8+ T cell responses, rather than their quantity (i.e., the frequency of CD8+ T cells and the magnitude of CD8+ T cell responses), correlated better with protection. These findings are in agreement with our recent reports in showing that the CXCL10/CXCR3-dependent mobilization of polyfunctional HSV-specific CD8+ TEM and CD8+ TRM cells within infected mouse tissues allows efficient protection against recurrent herpesvirus infection and disease (20, 29). As a control, treatment with AAV8-CXCL10 alone, without prior priming with peptides, did not lead to significant mobilization of CD8+ T cells compared to that with the prime/pull vaccine, indicating the importance of the “prime” and “pull” steps. An immediate practical application of these preclinical findings in HLA Tg rabbits is the development of an ocular herpes prime/pull peptide vaccine strategy that will include T cell-attracting chemokines, such as the CXCL10 chemokine, to “pull” more antiviral CD8+ T cells from the circulation and boost their number and function within infected tissues, such as the cornea and TG. Moreover, a prime/pull immunotherapeutic vaccine is expected to stop or reduce attempts of HSV-1 reactivation from latency, with the potential to produce a sustained reduction of blinding recurrent herpetic disease.
Since the HLA Tg rabbit model is the only small animal model that provides spontaneous reactivation of HSV-1 and recurrent corneal disease (in contrast to mice), a human prime/pull therapeutic strategy is currently being explored in our laboratory, using the humanized HLA Tg rabbit models of recurrent ocular herpes. In latently infected HLA Tg rabbits, CD8+ T cells are first primed with human immunodominant CD8+ T cell epitopes selected from HSV-1 proteins (prime) and then treated with an AAV8 vector expressing T cell-attracting chemokines (CXCL9, CXCL10, and CXCL11) (pull) to increase the number of functional antiviral CD8+ T cells in latently infected TG, which will reduce virus growth at the point of reactivation and protect against recurrent ocular herpesvirus infection and disease. This study first induced HSV-specific activated CD8+ T cells in the periphery (prime) and then pulled them into the trigeminal ganglia following ocular administration of CXCL10 chemokine. Overall, the preclinical results for HLA Tg rabbits presented in this report suggest an opportunity to develop a T cell-based ocular herpes prime/pull vaccine approach based on peptide immunization that would induce functional HSV-specific CD8+ T cells in the periphery combined with topical ocular delivery of T cell-attracting chemokines, such as CXCL9, CXCL10, and CXCL11, that would mobilize protective CD8+ TEM and TRM cells into infected TG and corneal tissues.
The human population is genetically diverse in terms of HLA haplotypes. Thus, a question of practical importance is the translation of the current immunological findings in a single HLA Tg rabbit strain to the development of an epitope-based vaccine for a genetically heterogeneous human population. We selected the HLA-A*02:01 haplotype in this study because (i) >51% of humans, regardless of ethnicity and race, have this haplotype (4); and (ii) we have HLA-A*02:01 Tg rabbits available for our in vivo studies. Previous comparisons of HLA frequencies among cohorts of genital herpes patients showed associations of HLA-B27 and -Cw2 with symptomatic disease (60). In contrast, we have not detected any correlations between any particular HLA class I or class II haplotype and resistance or susceptibility to ocular herpes disease (unpublished data). Thus, we do not think that the difference in ocular herpetic disease is due to a particular HLA haplotype. Although the high degree of HLA polymorphism is often mentioned as a major hindrance to the use of epitope-based vaccines, this can be overcome by the inclusion of multiple supertype-restricted epitopes, recognized in the context of diverse related HLA alleles, and by designing mixtures of peptide-based vaccines with higher epitope densities. The multiple-epitope vaccine can be designed to target various HLA class I haplotypes, besides HLA-A*02:01 (the most common human MHC class I haplotype), allowing even more coverage in vaccine clinical trials (reviewed in reference 4). Thus, broad population coverage can be established, provided that epitopes corresponding to multiple HLA supertype families are incorporated into the vaccine. A combination of nine HLA supertypes can provide >99% coverage of the entire repertoire of HLA molecules (61, 62). The multiepitope-based herpes vaccine could also include several T cell epitopes present not only in one herpesvirus glycoprotein, such as gD, but also in several different proteins chosen to represent the HLA supertypes known to provide recognition in a large proportion of the global population. Such a multiepitope T cell-based vaccine would be broadly effective in the vast majority of individuals, regardless of their HLA haplotype.
Both rabbit and mouse ocular herpes models have been successful for the study of ocular HSV-1 infection and immunity, and each model has resulted in new information and discoveries related to human HSV-1 ocular disease (reviewed in references 63 and 64). Mice have been the animal model of choice for most immunologists over the years, and results from mice have yielded remarkable insights into the role of CD8+ T cells in protection against primary herpesvirus infection (7, 39, 65–69). Unfortunately, spontaneous reactivation of HSV-1 in mice is extremely rare, so the relevance of these findings to in vivo HSV-1 spontaneous reactivation cannot be determined (70). The rabbit ocular herpes model has been especially important for investigating viral reactivation and recurrent ocular disease (63, 64, 71). Unlike those of mouse eyes, but similar to those of human eyes, the surfaces of rabbit eyes are relatively immunologically isolated from systemic immune responses (63, 72, 73). Using the humanized HLA transgenic rabbit model of ocular HSV-1, which mounts “human-like” CD8+ T cell immune responses (HLA Tg rabbits), we found that immunization of HLA Tg rabbits with three human CD8+ T cell epitopes induced strong CD8+ T cell-dependent protective immunity against ocular herpesvirus infection and disease. From a practical standpoint, rabbit corneas are significantly larger than mouse corneas and offer plentiful amounts of tissues for phenotypic and functional characterization of HSV-specific T cells in individual tissues (21, 32, 63, 72, 73). To overcome the hurdle of rabbits not mounting T cell responses specific to human HLA-restricted human epitopes, we introduced the humanized HLA Tg rabbit model of ocular herpes whereby the rabbits express human leukocyte antigen (HLA class I) molecules (35, 74). Ocularly infected HLA Tg rabbits mounted HLA-A*02:01-restricted CD8+ T cell responses to UL44, UL9, and UL25 epitopes similar to those from HLA-A*02:01-positive, HSV-seropositive humans. Since expression of the rabbits' own MHC class I molecules might interfere with the human HLA-A*02:01-restricted responses (9) and to ensure that all rabbits had a high level of expression of HLA-A*02:01 molecules in over 90% of their CD8+ T cells, only HLA Tg rabbits with these characteristics were used in this investigation. These results confirm our previous report that all gD epitopes recognized by CD8+ T cells from HSV-1-infected HLA Tg rabbits are recognized by CD8+ T cells from HLA-A*02:01-positive, HSV-seropositive humans (35). Thus, the HLA Tg rabbit is a useful model for preclinical testing of candidate vaccines bearing human T cell epitopes. The HLA Tg rabbit model allows for testing of the hypothesis that a vaccine that induces appropriate human T cell responses to HSV-1 can decrease HSV-induced ocular disease. These findings should guide the development of a safe and effective T cell-based herpes vaccine.
Systematic detailed comparisons of epitope specificities of CD8+ T cells in HSV-1-infected TG and corneal herpetic lesions or circulating in the blood or lymph may shed light on which epitopes are important at different stages of ocular herpesvirus infection/reactivation. Distinct epitopes have been identified at different anatomical sites, such as the cornea, the TG, or the blood (75). Differences in tissue locations may occur between T cells that recognize different HSV antigens (75). Studies in mice suggest that frequent CD8+ T cells specific to epitopes in ribonucleotide reductase (UL39 and UL40), ICP8 (UL29), and gB (UL27) mostly reside at the TG relative to the spleen, whereas CD8+ T cells specific to epitopes from late antigens have lower frequencies at the TG (30, 76, 77). As CD8+ T cells are known to maintain viral latency at the TG, it is likely that they do this by targeting HSV epitopes expressed from early or immediate early proteins that are expressed early during reactivation. Whether HSV-specific CD8+ T cells that localize to infected human TG also target HSV epitopes expressed from early or immediate early proteins remains to be determined. The induction of homing of appropriate T cells to inflamed corneal and TG tissues by use of specific adjuvants or delivery platforms capable of inducing tissue-resident memory T cells is crucial.
In conclusion, four principal findings were determined by the preclinical results obtained with HSV-1-infected HLA Tg rabbits in this study. First, a human herpes vaccine that exclusively contains a mixture of human ASYMP CD8+ T cell epitopes derived from the HSV-1 UL44, UL9, and UL25 proteins provided protection in HLA-A*02:01 Tg rabbits against ocular herpesvirus infection and disease. Second, frequent polyfunctional HSV-1 ASYMP epitope-specific CD8+ T cells were induced by mixtures of ASYMP human epitopes from the UL44, UL9, and UL25 proteins and correlated with protection against ocular herpesvirus infection and disease in HLA Tg rabbits following HSV-1 ocular challenge. Third, the results demonstrate that the CXCL10 chemokine axis is of paramount importance in mobilizing protective CD8+ T cells to the TG and corneal tissues associated with clearance of ocular herpesvirus infection and disease. Fourth, the study validates the HLA-A*02:01 Tg rabbit model of ocular herpes for preclinical testing of future herpes vaccine candidates bearing human ASYMP CD8+ T cell epitopes against ocular herpes. Overall, the preclinical results determined for HLA Tg rabbits draw attention to the prime/pull vaccine strategy as an alternative to currently used protein-based vaccines to strengthen the protective efficacy of tissue-resident CD8+ T cells against ocular herpesvirus infection and disease.
MATERIALS AND METHODS
Human study population.
During the last 15 years (i.e., January 2003 to January 2018), we screened 875 individuals for HSV-1 and HSV-2 seropositivity. Five hundred seventy-four of these individuals were white, 301 were nonwhite (African, Asian, Hispanic, and other), 446 were female, and 429 were male. In this sample, a cohort of 306 immunocompetent individuals, ranging from 21 to 67 years old (median, 39 years), were seropositive for HSV-1 and seronegative for HSV-2. All patients were negative for HIV and hepatitis B virus (HBV), with no history of immunodeficiency. Seven hundred ninety-two patients were HSV-1, HSV-2, or HSV-1/HSV-2 seropositive, among which 698 patients were healthy and defined as asymptomatic (ASYMP). These patients had never had any herpes disease (ocular, genital, or dermal), based on self-reporting and clinical examination. Even a single episode of any herpetic disease would exclude the individual from this group. The remaining 94 patients were defined as HSV-seropositive symptomatic (SYMP) individuals who suffered from frequent and severe recurrent genital, ocular, and/or orofacial lesions. Signs of recurrent disease in SYMP patients were defined as herpetic lid lesions, herpetic conjunctivitis, dendritic or geographic keratitis, stromal keratitis, and iritis consistent with HSK, with one or more episodes per year for the past 2 years. However, at the time of blood collection, SYMP patients had no recurrent disease (other than corneal scarring) and had had no recurrences during the past 30 days. They had no ocular disease other than HSK, had no history of recurrent genital herpes, and were HSV-1 seropositive and HSV-2 seronegative. Because the spectrum of recurrent ocular herpetic diseases is wide, our emphasis was mainly on the number of recurrent episodes, not the severity of the recurrent disease. No attempt was made to assign specific T cell epitopes to the severity of recurrent lesions. Patients were also excluded if they (i) had an active ocular (or elsewhere) herpetic lesion, or had had one within the past 30 days, (ii) were seropositive for HSV-2, (iii) were pregnant or breastfeeding, or (iv) were on acyclovir or another related antiviral drug or any immunosuppressive drugs at the time of blood draw. From this large cohort of SYMP and ASYMP individuals, 29 patients were enrolled in this study (Table 2). The SYMP and ASYMP groups were matched for age, gender, serological status, and race. We also collected and tested blood samples from 10 healthy control individuals who were seronegative for both HSV-1 and HSV-2 and had no history of ocular herpes, genital lesions, or orofacial herpes disease. All clinical investigations in this study were conducted according to the principles of the Declaration of Helsinki. All subjects were enrolled at the University of California, Irvine (UC Irvine), under approved Institutional Review Board-approved protocols (IRB2003-3111 and IRB2009-6963). Written informed consent was received from all participants prior to inclusion in the study.
HLA-A*02:01 transgenic rabbits.
A colony of HLA Tg rabbits, maintained at UC Irvine, was used for all experiments. HLA Tg rabbits were derived from New Zealand White rabbits (78). HLA Tg rabbits retain their endogenous rabbit MHC locus and express human HLA-A*02:01 under the control of its normal promoter (78). Prior to this study, the expression of HLA-A*02:01 molecules on the PBMCs of each HLA Tg rabbit was confirmed by FACS analysis. Briefly, rabbit PBMCs were stained with 2 μl of anti-HLA-A2 MAb (clone BB7.2; BD Biosciences, USA) at 4°C for 30 min. The cells were washed and analyzed by use of an LSR II flow cytometer (Becton Dickinson, Mountain View, CA). The acquired data were analyzed with FlowJo software (TreeStar, Ashland, OR). All rabbits used in these studies had similarly high levels of HLA-A*02:01 expression (>90%). This eliminated any potential bias due to the variability of HLA-A*02:01 molecule levels in different animals. New Zealand White rabbits (non-Tg control rabbits) purchased from Western Oregon Rabbit Co. were used as controls. All rabbits were housed and treated in accordance with Association for Research in Vision and Ophthalmology (ARVO), Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC), and National Institutes of Health (NIH) guidelines.
HLA-A*02:01 typing.
HLA-A2 typing of humans and HLA Tg rabbits was performed using a commercial sequence-specific primer (SSP) kit (SSPR1-A2; One Lambda, Canoga Park, CA) following the manufacturer's instructions. Genomic DNAs extracted from PBMCs of HSV-seropositive SYMP and ASYMP individuals were analyzed on a Tecan DNA workstation, using a 96-well plate with a 2-μl volume per well, as previously described (6). The yield and purity of each DNA sample were tested using a UV spectrophotometer. The integrity of DNA samples was ascertained by electrophoresis on agarose gels. Each DNA sample was then subjected to multiple small-volume PCRs using primers specific to areas of the genome surrounding the single point mutations associated with each allele. Only primers that matched the specific sequence of a particular allele would amplify a product. The PCR products were subsequently electrophoresed in a 2.5% agarose gel with ethidium bromide buffer, and the pattern of amplicon generation was analyzed using HLA Fusion software (One Lambda, Inc., Canoga Park, CA). Additionally, the HLA-A2 status was confirmed by staining PBMCs with an anti-HLA-A2 MAb, BB7.2 (BD Biosciences, USA), at 4°C for 30 min. The cells were washed, acquired on a BD LSR II flow cytometer, and analyzed using FlowJo software, version 9.9.4 (TreeStar, Ashland, OR).
Human PBMC isolation.
Individuals (negative for HIV and HBV and with or without any HSV infection history) were recruited at the UC Irvine Institute for Clinical and Translational Science (ICTS). Between 40 and 100 ml of blood was drawn into yellow-top Vacutainer tubes (Becton Dickinson, USA). The serum was isolated and stored at −80°C for detection of anti-HSV-1 and -HSV-2 antibodies, as we previously described (39). PBMCs were isolated by gradient centrifugation using leukocyte separation medium (Cellgro, USA). The cells were washed in phosphate-buffered saline (PBS) and resuspended in complete culture medium consisting of RPMI 1640 and 10% fetal bovine serum (FBS; Bio-Products, Woodland, CA) supplemented with 1× penicillin–streptomycin–l-glutamine, 1× sodium pyruvate, 1× nonessential amino acids, and 50 μM 2-mercaptoethanol (Life Technologies, Rockville, MD). Freshly isolated PBMCs were also cryopreserved in 90% fetal calf serum (FCS) and 10% dimethyl sulfoxide (DMSO) in liquid nitrogen for future testing.
Human T cell flow cytometry assays.
The following anti-human antibodies were used: anti-CD3 (clone SK7)–phycoerythrin (PE)–Cy7, anti-CD8 (clone SK1)–allophycocyanin (APC)–Cy7, anti-IFN-γ (clone B27)–Alexa Fluor 647 (BD Biosciences), anti-CD107a (clone H4A3)–fluorescein isothiocyanate (FITC), and anti-CD107b (clone H4B4)–FITC (BioLegend). For surface staining, MAbs against cell markers were added to a total of 1 × 106 cells in 1× PBS containing 1% FBS and 0.1% sodium azide (FACS buffer) for 45 min at 4°C. After washing with FACS buffer, cells were permeabilized for 20 min on ice by use of a Cytofix/Cytoperm kit (BD Biosciences) and then washed twice with Perm/Wash buffer (BD Bioscience). Intracellular cytokine MAbs were then added to the cells and incubated for 45 min on ice in the dark. Cells were washed again with Perm/Wash and FACS buffers and fixed in PBS containing 2% paraformaldehyde (Sigma-Aldrich, St. Louis, MO). For each sample, 100,000 total events were acquired on a BD LSR II flow cytometer. For the gating strategy, peripheral blood mononuclear cells were stained for surface and intracellular markers. Ab capture beads (BD Biosciences) were used as individual compensation tubes for each fluorophore in the experiment. To define positive and negative populations, we employed fluorescence-negative controls for each fluorophore used in this study when initially developing staining protocols. In addition, we further optimized gating by examining known negative cell populations for background expression. The gating strategy was similar to that used in our previous work (7). Briefly, we gated single cells, lymphocytes, CD3+ cells, and CD8+ cells before finally gating human epitope-specific CD8+ T cells by use of HSV-specific tetramers. Data analysis was performed using FlowJo, version 9.9.4 (TreeStar, Ashland, OR). Statistical analyses were done using GraphPad Prism, version 5 (GraphPad, La Jolla, CA).
The intracellular assay to detect IFN-γ and CD107a/b in response to in vitro peptide stimulations was performed as described previously (35, 39), with a few modifications. On the day of the assay, 1 × 106 PBMCs were stimulated in vitro with peptide pools (10 μg/ml/peptide) at 37°C for an additional 6 h in a 96-well plate with BD Golgi Stop (BD Biosciences) and 10 μl each of anti-CD107a–FITC and anti-CD107b–FITC. Phytohemagglutinin (PHA) (5 μg/ml; Sigma) and no peptide were used as positive and negative controls, respectively. At the end of the incubation period, the cells were transferred to a 96-well round-bottomed plate, washed twice with FACS buffer, and then stained with PE-conjugated anti-human CD8 for 45 min at 4°C. Intracellular staining for the detection of IFN-γ and CD107a/b was performed as outlined above. The cells were washed again and fixed, and then 50,000 total events were acquired on a BD LSR II flow cytometer and data analysis was performed using FlowJo, version 9.9.4 (TreeStar, Ashland, OR).
Peptide synthesis.
HLA-A*02:01 binding peptides (9-mers) from the HSV-1 UL44, UL9, and UL25 proteins with high estimated half-times of dissociation (t1/2) were synthesized by 21st Century Biochemicals (Marlboro, MA). All peptides were purified to a purity of 95% to 98% as determined by reversed-phase high-performance liquid chromatography (HPLC) (Vydac C18 column) and mass spectroscopy (Voyager matrix-assisted laser desorption ionization–time of flight [MALDI-TOF] system). Stock solutions (1 mg/ml) were made in PBS. All peptides were aliquoted and stored at −20°C until assayed.
Design and construction of AAV8 vector expressing CXCL10 chemokine.
under the control of neurotropic CamKIIα promoters. Human adeno-associated virus subtype 8 (AAV8), first described in the 1990s by Mishra and Rose (79) and by Johnston et al. (80), was used in this study. The open reading frame (ORF) for the chemokine CXCL10 was cloned into the SalI/EcoRI sites of a pAAV-CamKIIα-MCS-CamKIIα-EGFP vector to determine the best vector for targeted delivery of chemokines to neuronal cells of rabbit TG. This pAAV vector was cotransfected with a helper plasmid into HEK293 cells to produce the recombinant AAV. Two days after transfection, cell pellets were harvested, and viruses were released through three freeze-thaw cycles. AAVs were purified by CsCl gradient ultracentrifugation followed by desalting. Viral titers (GC per milliliters) were determined by real-time PCR.
Prime/pull vaccination and ocular herpes challenge.
HLA-A*02:01 Tg rabbits (8 to 10 weeks old) with similarly high expression of HLA-A*02:01 molecules (>90%) were used as described above. Groups of age-matched HLA-A*02:01 rabbits were immunized twice subcutaneously (2 weeks apart) with a mixture of three CD8+ peptide epitopes (each at 100 μM) delivered with the CD4+ T helper epitope (PADRE) emulsified with CpG (ODN2007) in a total volume of 200 μl of saline. As a negative control, a group of HLA Tg rabbits was injected with adjuvant alone. On day 29, half of the rabbits were left untreated, and the other half received a topical ocular treatment with 1010 viral genome copies (GC) of the rAAV8-CamKIIα-GFP-CamKIIα-CXCL10 vector. An additional group of control HLA Tg rabbits were mock vaccinated and received an empty AAV8 vector (mock group). Two days following CXCL10 treatment, animals from all three groups received an ocular HSV-1 challenge (2 × 105 PFU of the McKrae strain; both eyes) without scarification.
Herpes simplex virus production.
HSV-1 strain McKrae was used in this study. The virus was triple plaque purified and prepared as previously described (81–83).
Rabbit corneal disease clinical scores.
Rabbits were examined for ocular disease (i.e., corneal keratitis) for 30 days after the challenge (2 × 105 PFU/eye). The extent of ocular disease was determined by an investigator for whom identifications were masked, using fluorescein staining and slit lamp examination before challenge and on days 1, 4, 7, 10, 14, and 21 thereafter. A standard scale from 0 to 4 was used, as follows: 0, no disease; 1, 25% staining; 2, 50% staining; 3, 75% staining; and 4, 100% staining.
Detection of rabbit ocular infectious virus.
Tears were collected from both eyes by use of a Dacron swab (type 1; Spectrum Laboratories, Los Angeles, CA) on days 3, 5, and 7 postchallenge. Individual swabs were transferred to 2-ml sterile cryogenic vials containing 1 ml culture medium and stored at −80°C until use. The HSV-1 titers in tear samples were determined by standard plaque assays on RS cells as previously described (84).
Rabbit peripheral blood mononuclear cell isolation.
Twenty milliliters of blood was drawn from rabbit ear veins into yellow-top Vacutainer tubes (Becton Dickinson, USA). Sera were isolated by centrifugation for 10 min at 800 × g. PBMCs were isolated by gradient centrifugation using leukocyte separation medium (Cellgro, USA). The cells were washed in PBS and resuspended in complete culture medium consisting of RPMI 1640 medium and 10% FBS (Bio-Products, Woodland, CA, USA) supplemented with 1× penicillin–l-glutamine–streptomycin, 1× sodium pyruvate, 1× nonessential amino acids, and 50 μM 2-mercaptoethanol (Life Technologies, Rockville, MD, USA). Aliquots of freshly isolated PBMCs were also cryopreserved in 90% FBS and 10% DMSO in liquid nitrogen for future testing.
Flow cytometry assays of rabbit T cells.
PBMCs were analyzed by flow cytometry. The following antibodies were used: mouse anti-rabbit CD8 (clone MCA1576F; Serotec), mouse anti-human CD107a (clone H4A3)–FITC (BioLegend), mouse anti-human CD107b (clone H4B4)–FITC (BioLegend), and rat anti-IFN-γ (clone XMG1.2; BD Biosciences). For surface staining, MAbs against various cell markers were added to a total of 1 × 106 cells in phosphate-buffered saline containing 1% FBS and 0.1% sodium azide (FACS buffer) and left for 45 min at 4°C. For intracellular staining, MAbs were added to the cells and incubated for 45 min on ice and in the dark. Cells were washed again with Perm/Wash and FACS buffers and fixed in PBS containing 2% paraformaldehyde (Sigma-Aldrich, St. Louis, MO). For the measurement of CD107a/b and IFN-γ, cells were first stimulated in vitro with individual peptides. Briefly, 1 × 106 cells were transferred to a 96-well flat-bottomed plate and stimulated with peptides (10 μg/ml in 200 μl complete culture medium) in the presence of BD GolgiStop (10 μg/ml) for 6 h at 37°C. PHA (5 μg/ml) (Sigma-Aldrich, St. Louis, MO) and no peptide were used as positive and negative controls, respectively. At the end of the incubation period, the cells were transferred to a 96-well round-bottomed plate and washed once with FACS buffer. Surface and intracellular staining was done as described above. For the gating strategy, peripheral blood mononuclear cells were stained for surface and intracellular markers. To define positive and negative populations, we employed fluorescence-negative controls for each fluorophore used in this study. Briefly, we gated single cells, lymphocytes, CD3+ cells, and CD8+ cells before finally gating human epitope-specific CD8+ T cells by use of HSV-specific tetramers. A total of 50,000 events were acquired by an LSR II flow cytometer (Becton Dickinson, Mountain View, CA), followed by analysis using FlowJo software (TreeStar, Ashland, OR).
Rabbit CD8+ T cell tetramer assays.
For tetramer-specific CD8+ T cell responses, PBMCs were analyzed for the frequency of CD8+ T cells specific to each of the three immunizing CD8+ T cell epitopes by using the corresponding HLA-A2-peptides/tetramers, provided by the NIH tetramer facility (7, 36, 39). A human beta-2-microglobulin was incorporated into the tetramers, as no rabbit beta-2-microglobulins are currently available. Briefly, the cells were first incubated with 1 μg/ml of each of the three PE-labeled HLA-A2-peptides/tetramers at 37°C for 30 to 45 min. The cells were washed twice and then stained with 1 μg/ml of FITC-conjugated mouse anti-rabbit CD8 MAb (clone MCA1576F; Serotec). After two additional washes, cells were fixed with 2% formaldehyde in FACS buffer. A total of 50,000 events were acquired by an LSR II flow cytometer, followed by analysis using FlowJo software. The absolute numbers of individual peptide-specific CD8+ T cells were calculated using the following formula: (number of events in CD8+/tetramer+ cells) × (number of events in gated lymphocytes)/(total number of events acquired).
Rabbit immunohistochemistry.
Rabbit TG were cut into 8-μm-thick sections by use of a cryostat. Sections were washed with 1× PBS, permeabilized using 0.05% Triton X-100 in 1× PBS for 15 min, and blocked using 10% FBS in 1× PBS for 1 h. Sections were stained using a mouse anti-rabbit CD8 antibody (1:200) (clone MCA1576F; Serotec) overnight at 4°C. After secondary fluorescence staining, sections were washed with 1× PBS and mounted after DAPI (4′,6-diamidino-2-phenylindole) staining (1:10,000 dilution). Immunofluorescence colocalization of CD8+ cells with AAV8-GFP was examined using a Keyence BZ-X700 fluorescence microscope at a magnification of ×40 and imaged using a z-stack.
Statistical analyses.
Data for each assay were compared by analysis of variance (ANOVA) and Student's t test, using GraphPad Prism, version 5 (GraphPad, La Jolla, CA). Differences between the groups were identified by ANOVA and multiple-comparison procedures, as we previously described (36). Data are expressed as means ± standard deviations (SD). Results were considered statistically significant if the P value was <0.05.
ACKNOWLEDGMENTS
This work was supported by Public Health Service Research R01 grants EY026103, EY019896, and EY024618 from the National Eye Institute (NEI), by R21 grant AI110902 and R41 grant AI138764-01 from the National Institute of Allergy and Infectious Diseases (NIAID) (to L.B.), and in part by The Discovery Center for Eye Research (DCER) and a Research to Prevent Blindness (RPB) grant.
This work is dedicated to the memory of the late Steven “Steve” L. Wechsler (1948–2016), whose numerous pioneering works on herpesvirus infection and immunity laid the foundation for this line of research.
We thank Elmostafa Bahraoui from the Université Paul Sabatier Toulouse (Toulouse, France) for critical readings of the study and Dale Long from the NIH Tetramer Facility (Emory University, Atlanta, GA) for providing the tetramers used in this study.
We declare that no conflicts of interest exist.
REFERENCES
- 1.Khan AA, Srivastava R, Chentoufi AA, Kritzer E, Chilukuri S, Garg S, Yu DC, Vahed H, Huang L, Syed SA, Furness JN, Tran TT, Anthony NB, McLaren CE, Sidney J, Sette A, Noelle RJ, BenMohamed L. 2017. Bolstering the number and function of HSV-1-specific CD8+ effector memory T cells and tissue-resident memory T cells in latently infected trigeminal ganglia reduces recurrent ocular herpes infection and disease. J Immunol 199:186–203. doi: 10.4049/jimmunol.1700145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Velzen M, Jing L, Osterhaus AD, Sette A, Koelle DM, Verjans GM. 2013. Local CD4 and CD8 T-cell reactivity to HSV-1 antigens documents broad viral protein expression and immune competence in latently infected human trigeminal ganglia. PLoS Pathog 9:e1003547. doi: 10.1371/journal.ppat.1003547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuo T, Wang C, Badakhshan T, Chilukuri S, BenMohamed L. 2014. The challenges and opportunities for the development of a T-cell epitope-based herpes simplex vaccine. Vaccine 32:6733–6745. doi: 10.1016/j.vaccine.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Samandary S, Kridane-Miledi H, Sandoval JS, Choudhury Z, Langa-Vives F, Spencer D, Chentoufi AA, Lemonnier FA, BenMohamed L. 2014. Associations of HLA-A, HLA-B and HLA-C alleles frequency with prevalence of herpes simplex virus infections and diseases across global populations: implication for the development of an universal CD8+ T-cell epitope-based vaccine. Hum Immunol 75:715–729. doi: 10.1016/j.humimm.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chentoufi AA, Dervillez X, Rubbo PA, Kuo T, Zhang X, Nagot N, Tuaillon E, Van De Perre P, Nesburn AB, BenMohamed L. 2012. Current trends in negative immuno-synergy between two sexually transmitted infectious viruses: HIV-1 and HSV-1/2. Curr Trends Immunol 13:51–68. [PMC free article] [PubMed] [Google Scholar]
- 6.Dervillez X, Qureshi H, Chentoufi AA, Khan AA, Kritzer E, Yu DC, Diaz OR, Gottimukkala C, Kalantari M, Villacres MC, Scarfone VM, McKinney DM, Sidney J, Sette A, Nesburn AB, Wechsler SL, BenMohamed L. 2013. Asymptomatic HLA-A*02:01-restricted epitopes from herpes simplex virus glycoprotein B preferentially recall polyfunctional CD8+ T cells from seropositive asymptomatic individuals and protect HLA transgenic mice against ocular herpes. J Immunol 191:5124–5138. doi: 10.4049/jimmunol.1301415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chentoufi AA, Zhang X, Lamberth K, Dasgupta G, Bettahi I, Nguyen A, Wu M, Zhu X, Mohebbi A, Buus S, Wechsler SL, Nesburn AB, BenMohamed L. 2008. HLA-A*0201-restricted CD8+ cytotoxic T lymphocyte epitopes identified from herpes simplex virus glycoprotein D. J Immunol 180:426–437. doi: 10.4049/jimmunol.180.1.426. [DOI] [PubMed] [Google Scholar]
- 8.Zhang X, Dervillez X, Chentoufi AA, Badakhshan T, Bettahi I, BenMohamed L. 2012. Targeting the genital tract mucosa with a lipopeptide/recombinant adenovirus prime/boost vaccine induces potent and long-lasting CD8+ T cell immunity against herpes: importance of MyD88. J Immunol 189:4496–4509. doi: 10.4049/jimmunol.1201121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farooq AV, Shukla D. 2012. Herpes simplex epithelial and stromal keratitis: an epidemiologic update. Surv Ophthalmol 57:448–462. doi: 10.1016/j.survophthal.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liesegang TJ. 2001. Herpes simplex virus epidemiology and ocular importance. Cornea 20:1–13. doi: 10.1097/00003226-200101000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Banerjee K, Biswas PS, Rouse BT. 2005. Elucidating the protective and pathologic T cell species in the virus-induced corneal immunoinflammatory condition herpetic stromal keratitis. J Leukoc Biol 77:24–32. doi: 10.1189/jlb.0904486. [DOI] [PubMed] [Google Scholar]
- 12.Dana MR, Qian Y, Hamrah P. 2000. Twenty-five-year panorama of corneal immunology: emerging concepts in the immunopathogenesis of microbial keratitis, peripheral ulcerative keratitis, and corneal transplant rejection. Cornea 19:625–643. doi: 10.1097/00003226-200009000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Thomas J, Rouse BT. 1997. Immunopathogenesis of herpetic ocular disease. Immunol Res 16:375–386. doi: 10.1007/BF02786400. [DOI] [PubMed] [Google Scholar]
- 14.Kumaraguru U, Davis I, Rouse BT. 1999. Chemokines and ocular pathology caused by corneal infection with herpes simplex virus. J Neurovirol 5:42–47. doi: 10.3109/13550289909029744. [DOI] [PubMed] [Google Scholar]
- 15.Farooq AV, Valyi-Nagy T, Shukla D. 2010. Mediators and mechanisms of herpes simplex virus entry into ocular cells. Curr Eye Res 35:445–450. doi: 10.3109/02713681003734841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.HEDS. 1998. Acyclovir for the prevention of recurrent herpes simplex virus eye disease. Herpetic Eye Disease Study Group. N Engl J Med 339:300–306. [DOI] [PubMed] [Google Scholar]
- 17.Koelle DM, Gonzalez JC, Johnson AS. 2005. Homing in on the cellular immune response to HSV-2 in humans. Am J Reprod Immunol 53:172–181. doi: 10.1111/j.1600-0897.2005.00262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Posavad CM, Huang ML, Barcy S, Koelle DM, Corey L. 2000. Long term persistence of herpes simplex virus-specific CD8+ CTL in persons with frequently recurring genital herpes. J Immunol 165:1146–1152. doi: 10.4049/jimmunol.165.2.1146. [DOI] [PubMed] [Google Scholar]
- 19.Jing L, Laing KJ, Dong L, Russell RM, Barlow RS, Haas JG, Ramchandani MS, Johnston C, Buus S, Redwood AJ, White KD, Mallal SA, Phillips EJ, Posavad CM, Wald A, Koelle DM. 2016. Extensive CD4 and CD8 T cell cross-reactivity between alphaherpesviruses. J Immunol 196:2205–2218. doi: 10.4049/jimmunol.1502366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Srivastava R, Khan AA, Garg S, Syed SA, Furness JN, Vahed H, Pham T, Yu HT, Nesburn AB, BenMohamed L. 2017. Human asymptomatic epitopes identified from the herpes simplex virus tegument protein VP13/14 (UL47) preferentially recall polyfunctional effector memory CD44high CD62Llow CD8+ TEM cells and protect humanized HLA-A*02:01 transgenic mice against ocular herpesvirus infection. J Virol 91:e01793-16. doi: 10.1128/JVI.01793-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khan AA, Srivastava R, Chentoufi AA, Geertsema R, Thai NT, Dasgupta G, Osorio N, Kalantari M, Nesburn AB, Wechsler SL, BenMohamed L. 2015. Therapeutic immunization with a mixture of herpes simplex virus 1 glycoprotein D-derived “asymptomatic” human CD8+ T-cell epitopes decreases spontaneous ocular shedding in latently infected HLA transgenic rabbits: association with low frequency of local PD-1+ TIM-3+ CD8+ exhausted T cells. J Virol 89:6619–6632. doi: 10.1128/JVI.00788-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stanberry LR. 2004. Clinical trials of prophylactic and therapeutic herpes simplex virus vaccines. Herpes 11(Suppl 3):161A–169A. [PubMed] [Google Scholar]
- 23.Belshe PB, Leone PA, Bernstein DI, Wald A, Levin MJ, Stapleton JT, Gorfinkel I, Morrow RLA, Ewell MG, Stokes-Riner A, Dubin G, Heineman TC, Schulte JM, Deal CD. 2012. Efficacy results of a trial of a herpes simplex vaccine. N Engl J Med 366:34–43. doi: 10.1056/NEJMoa1103151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iijima N, Iwasaki A. 2014. T cell memory. A local macrophage chemokine network sustains protective tissue-resident memory CD4 T cells. Science 346:93–98. doi: 10.1126/science.1257530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee AY, Eri R, Lyons AB, Grimm MC, Korner H. 2013. CC chemokine ligand 20 and its cognate receptor CCR6 in mucosal T cell immunology and inflammatory bowel disease: odd couple or axis of evil? Front Immunol 4:194. doi: 10.3389/fimmu.2013.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Srivastava R, Hernandez-Ruiz M, Khan AA, Fouladi MA, Kim GJ, Ly VT, Yamada T, Lam C, Sarain SAB, Boldbaatar U, Zlotnik A, Bahraoui E, BenMohamed L. 2018. CXCL17 chemokine-dependent mobilization of CXCR8(+)CD8(+) effector memory and tissue-resident memory T cells in the vaginal mucosa is associated with protection against genital herpes. J Immunol 200:2915–2926. doi: 10.4049/jimmunol.1701474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen Y, Chauhan SK, Lee HS, Saban DR, Dana R. 2014. Chronic dry eye disease is principally mediated by effector memory Th17 cells. Mucosal Immunol 7:38–45. doi: 10.1038/mi.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnston C, Zhu J, Jing L, Laing KJ, McClurkan CM, Klock A, Diem K, Jin L, Stanaway J, Tronstein E, Kwok WW, Huang ML, Selke S, Fong Y, Magaret A, Koelle DM, Wald A, Corey L. 2014. Virologic and immunologic evidence of multifocal genital herpes simplex virus 2 infection. J Virol 88:4921–4931. doi: 10.1128/JVI.03285-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Srivastava R, Khan AA, Chilukuri S, Syed SA, Tran TT, Furness J, Bahraoui E, BenMohamed L. 2017. CXCL10/CXCR3-dependent mobilization of herpes simplex virus-specific CD8+ TEM and CD8+ TRM cells within infected tissues allows efficient protection against recurrent herpesvirus infection and disease. J Virol 91:e00278-17. doi: 10.1128/JVI.00278-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Himmelein S, St Leger AJ, Knickelbein JE, Rowe A, Freeman ML, Hendricks RL. 2011. Circulating herpes simplex type 1 (HSV-1)-specific CD8+ T cells do not access HSV-1 latently infected trigeminal ganglia. Herpesviridae 2:5. doi: 10.1186/2042-4280-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Srivastava R, Dervillez X, Khan AA, Chentoufi AA, Chilukuri S, Shukr N, Fazli Y, Ong NN, Afifi RE, Osorio N, Geertsema R, Nesburn AB, Wechsler SL, BenMohamed L. 2016. The herpes simplex virus latency-associated transcript gene is associated with a broader repertoire of virus-specific exhausted CD8+ T cells retained within the trigeminal ganglia of latently infected HLA transgenic rabbits. J Virol 90:3913–3928. doi: 10.1128/JVI.02450-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perng GC, Osorio N, Jiang X, Geertsema R, Hsiang C, Brown D, BenMohamed L, Wechsler SL. 2016. Large amounts of reactivated virus in tears precedes recurrent herpes stromal keratitis in stressed rabbits latently infected with herpes simplex virus. Curr Eye Res 41:284–291. doi: 10.3109/02713683.2015.1020172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jester JV, Morishige N, BenMohamed L, Brown DJ, Osorio N, Hsiang C, Perng GC, Jones C, Wechsler SL. 2016. Confocal microscopic analysis of a rabbit eye model of high-incidence recurrent herpes stromal keratitis. Cornea 35:81–88. doi: 10.1097/ICO.0000000000000666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Srivastava R, Khan AA, Huang J, Nesburn AB, Wechsler SL, BenMohamed L. 2015. A herpes simplex virus type 1 human asymptomatic CD8+ T-cell epitopes-based vaccine protects against ocular herpes in a “humanized” HLA transgenic rabbit model. Invest Ophthalmol Vis Sci 56:4013–4028. doi: 10.1167/iovs.15-17074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chentoufi AA, Dasgupta G, Christensen ND, Hu J, Choudhury ZS, Azeem A, Jester JV, Nesburn AB, Wechsler SL, BenMohamed L. 2010. A novel HLA (HLA-A*0201) transgenic rabbit model for preclinical evaluation of human CD8+ T cell epitope-based vaccines against ocular herpes. J Immunol 184:2561–2571. doi: 10.4049/jimmunol.0902322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang X, Chentoufi AA, Dasgupta G, Nesburn AB, Wu M, Zhu X, Carpenter D, Wechsler SL, You S, BenMohamed L. 2009. A genital tract peptide epitope vaccine targeting TLR-2 efficiently induces local and systemic CD8+ T cells and protects against herpes simplex virus type 2 challenge. Mucosal Immunol 2:129–143. doi: 10.1038/mi.2008.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nesburn AB, Bettahi I, Zhang X, Zhu X, Chamberlain W, Afifi RE, Wechsler SL, BenMohamed L. 2006. Topical/mucosal delivery of sub-unit vaccines that stimulate the ocular mucosal immune system. Ocul Surf 4:178–187. doi: 10.1016/S1542-0124(12)70164-7. [DOI] [PubMed] [Google Scholar]
- 38.Zhang X, Issagholian A, Berg EA, Fishman JB, Nesburn AB, BenMohamed L. 2005. Th-cytotoxic T-lymphocyte chimeric epitopes extended by Nepsilon-palmitoyl lysines induce herpes simplex virus type 1-specific effector CD8+ Tc1 responses and protect against ocular infection. J Virol 79:15289–15301. doi: 10.1128/JVI.79.24.15289-15301.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chentoufi AA, Binder NR, Berka N, Durand G, Nguyen A, Bettahi I, Maillere B, BenMohamed L. 2008. Asymptomatic human CD4+ cytotoxic T-cell epitopes identified from herpes simplex virus glycoprotein B. J Virol 82:11792–11802. doi: 10.1128/JVI.00692-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chentoufi AA, BenMohamed L. 2010. Future viral vectors for the delivery of asymptomatic herpes epitope-based immunotherapeutic vaccines. Future Virol 5:525–528. doi: 10.2217/fvl.10.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chentoufi AA, Kritzer E, Yu DM, Nesburn AB, BenMohamed L. 2012. Towards a rational design of an asymptomatic clinical herpes vaccine: the old, the new, and the unknown. Clin Dev Immunol 2012:187585. doi: 10.1155/2012/187585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dasgupta G, Chentoufi AA, Kalantari M, Falatoonzadeh P, Chun S, Lim CH, Felgner PL, Davies DH, BenMohamed L. 2012. Immunodominant “asymptomatic” herpes simplex virus 1 and 2 protein antigens identified by probing whole-ORFome microarrays with serum antibodies from seropositive asymptomatic versus symptomatic individuals. J Virol 86:4358–4369. doi: 10.1128/JVI.07107-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dasgupta G, Nesburn AB, Wechsler SL, BenMohamed L. 2010. Developing an asymptomatic mucosal herpes vaccine: the present and the future. Future Microbiol 5:1–4. doi: 10.2217/fmb.09.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dervillez X, Gottimukkala C, Kabbara KW, Nguyen C, Badakhshan T, Kim SM, Nesburn AB, Wechsler SL, Benmohamed L. 2012. Future of an “asymptomatic” T-cell epitope-based therapeutic herpes simplex vaccine. Future Virol 7:371–378. doi: 10.2217/fvl.12.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khan AA, Srivastava R, Lopes PP, Wang C, Pham TT, Cochrane J, Thai NT, Gutierrez L, Benmohamed L. 2014. Asymptomatic memory CD8+ T cells: from development and regulation to consideration for human vaccines and immunotherapeutics. Hum Vaccin Immunother 10:945–963. doi: 10.4161/hv.27762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Khan AA, Srivastava R, Spencer D, Garg S, Fremgen D, Vahed H, Lopes PP, Pham TT, Hewett C, Kuang J, Ong N, Huang L, Scarfone VM, Nesburn AB, Wechsler SL, BenMohamed L. 2015. Phenotypic and functional characterization of herpes simplex virus glycoprotein B epitope-specific effector and memory CD8+ T cells from symptomatic and asymptomatic individuals with ocular herpes. J Virol 89:3776–3792. doi: 10.1128/JVI.03419-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Srivastava R, Khan AA, Spencer D, Vahed H, Lopes PP, Thai NT, Wang C, Pham TT, Huang J, Scarfone VM, Nesburn AB, Wechsler SL, BenMohamed L. 2015. HLA-A02:01-restricted epitopes identified from the herpes simplex virus tegument protein VP11/12 preferentially recall polyfunctional effector memory CD8+ T cells from seropositive asymptomatic individuals and protect humanized HLA-A*02:01 transgenic mice against ocular herpes. J Immunol 194:2232–2248. doi: 10.4049/jimmunol.1402606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chentoufi AA, BenMohamed L, Van De Perre P, Ashkar AA. 2012. Immunity to ocular and genital herpes simplex viruses infections. Clin Dev Immunol 2012:732546. doi: 10.1155/2012/732546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chentoufi AA, Dasgupta G, Nesburn AB, Bettahi I, Binder NR, Choudhury ZS, Chamberlain WD, Wechsler SL, BenMohamed L. 2010. Nasolacrimal duct closure modulates ocular mucosal and systemic CD4+ T-cell responses induced following topical ocular or intranasal immunization. Clin Vaccine Immunol 17:342–353. doi: 10.1128/CVI.00347-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.BenMohamed L, Wechsler SL, Nesburn AB. 2002. Lipopeptide vaccines—yesterday, today, and tomorrow. Lancet Infect Dis 2:425–431. doi: 10.1016/S1473-3099(02)00318-3. [DOI] [PubMed] [Google Scholar]
- 51.Chentoufi AA, Kritzer E, Tran MV, Dasgupta G, Lim CH, Yu DC, Afifi RE, Jiang X, Carpenter D, Osorio N, Hsiang C, Nesburn AB, Wechsler SL, BenMohamed L. 2011. The herpes simplex virus 1 latency-associated transcript promotes functional exhaustion of virus-specific CD8+ T cells in latently infected trigeminal ganglia: a novel immune evasion mechanism. J Virol 85:9127–9138. doi: 10.1128/JVI.00587-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Channappanavar R, Twardy BS, Suvas S. 2012. Blocking of PDL-1 interaction enhances primary and secondary CD8 T cell response to herpes simplex virus-1 infection. PLoS One 7:e39757. doi: 10.1371/journal.pone.0039757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. 2010. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med 207:2187–2194. doi: 10.1084/jem.20100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jin HT, Anderson AC, Tan WG, West EE, Ha SJ, Araki K, Freeman GJ, Kuchroo VK, Ahmed R. 2010. Cooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infection. Proc Natl Acad Sci U S A 107:14733–14738. doi: 10.1073/pnas.1009731107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fourcade J, Sun Z, Benallaoua M, Guillaume P, Luescher IF, Sander C, Kirkwood JM, Kuchroo V, Zarour HM. 2010. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J Exp Med 207:2175–2186. doi: 10.1084/jem.20100637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Allen SJ, Hamrah P, Gate D, Mott KR, Mantopoulos D, Zheng L, Town T, Jones C, von Andrian UH, Freeman GJ, Sharpe AH, BenMohamed L, Ahmed R, Wechsler SL, Ghiasi H. 2011. The role of LAT in increased CD8+ T cell exhaustion in trigeminal ganglia of mice latently infected with herpes simplex virus 1. J Virol 85:4184–4197. doi: 10.1128/JVI.02290-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mott KR, Bresee CJ, Allen SJ, BenMohamed L, Wechsler SL, Ghiasi H. 2009. Level of herpes simplex virus type 1 latency correlates with severity of corneal scarring and exhaustion of CD8+ T cells in trigeminal ganglia of latently infected mice. J Virol 83:2246–2254. doi: 10.1128/JVI.02234-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Frank GM, Lepisto AJ, Freeman ML, Sheridan BS, Cherpes TL, Hendricks RL. 2010. Early CD4(+) T cell help prevents partial CD8(+) T cell exhaustion and promotes maintenance of herpes simplex virus 1 latency. J Immunol 184:277–286. doi: 10.4049/jimmunol.0902373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shin H, Iwasaki A. 2012. A vaccine strategy that protects against genital herpes by establishing local memory T cells. Nature 491:463–467. doi: 10.1038/nature11522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lekstrom-Himes JA, Hohman P, Warren T, Wald A, Nam JM, Simonis T, Corey L, Straus SE. 1999. Association of major histocompatibility complex determinants with the development of symptomatic and asymptomatic genital herpes simplex virus type 2 infections. J Infect Dis 179:1077–1085. doi: 10.1086/314729. [DOI] [PubMed] [Google Scholar]
- 61.Sette A, Livingston B, McKinney D, Appella E, Fikes J, Sidney J, Newman M, Chesnut R. 2001. The development of multi-epitope vaccines: epitope identification, vaccine design and clinical evaluation. Biologicals 29:271–276. doi: 10.1006/biol.2001.0297. [DOI] [PubMed] [Google Scholar]
- 62.Sette A, Newman M, Livingston B, McKinney D, Sidney J, Ishioka G, Tangri S, Alexander J, Fikes J, Chesnut R. 2002. Optimizing vaccine design for cellular processing, MHC binding and TCR recognition. Tissue Antigens 59:443–451. doi: 10.1034/j.1399-0039.2002.590601.x. [DOI] [PubMed] [Google Scholar]
- 63.Webre JM, Hill JM, Nolan NM, Clement C, McFerrin HE, Bhattacharjee PS, Hsia V, Neumann DM, Foster TP, Lukiw WJ, Thompson HW. 2012. Rabbit and mouse models of HSV-1 latency, reactivation, and recurrent eye diseases. J Biomed Biotechnol 2012:612316. doi: 10.1155/2012/612316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kollias CM, Huneke RB, Wigdahl B, Jennings SR. 2015. Animal models of herpes simplex virus immunity and pathogenesis. J Neurovirol 21:8–23. doi: 10.1007/s13365-014-0302-2. [DOI] [PubMed] [Google Scholar]
- 65.Wuest T, Farber J, Luster A, Carr DJ. 2006. CD4+ T cell migration into the cornea is reduced in CXCL9 deficient but not CXCL10 deficient mice following herpes simplex virus type 1 infection. Cell Immunol 243:83–89. doi: 10.1016/j.cellimm.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Conrady CD, Zheng M, Stone DU, Carr DJ. 2012. CD8+ T cells suppress viral replication in the cornea but contribute to VEGF-C-induced lymphatic vessel genesis. J Immunol 189:425–432. doi: 10.4049/jimmunol.1200063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dasgupta G, Chentoufi AA, Nesburn AB, Wechsler SL, BenMohamed L. 2009. New concepts in herpes simplex virus vaccine development: notes from the battlefield. Expert Rev Vaccines 8:1023–1035. doi: 10.1586/erv.09.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Knickelbein JE, Khanna KM, Yee MB, Baty CJ, Kinchington PR, Hendricks RL. 2008. Noncytotoxic lytic granule-mediated CD8+ T cell inhibition of HSV-1 reactivation from neuronal latency. Science 322:268–271. doi: 10.1126/science.1164164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.BenMohamed L, Osorio N, Srivastava R, Khan AA, Simpson JS, Wechsler SL. 2015. Decreased reactivation of a herpes simplex virus type 1 (HSV-1) latency associated transcript (LAT) mutant using the in vivo mouse UV-B model of induced reactivation. J Neurovirol 21:508–517. doi: 10.1007/s13365-015-0348-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Feldman LT, Ellison AR, Voytek CC, Yang L, Krause P, Margolis TP. 2002. Spontaneous molecular reactivation of herpes simplex virus type 1 latency in mice. Proc Natl Acad Sci U S A 99:978–983. doi: 10.1073/pnas.022301899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Esteves PJ, Abrantes J, Baldauf HM, BenMohamed L, Chen Y, Christensen N, González-Gallego J, Giacan L, Hu J, Kaplan G, Keppler OT, Knight KL, Kong XP, Lanning DK, Le Pendu L, de Matos AL, Liu J, Liu S, Lopes AM, Lu S, Lukehar S, Manabe YC, Neves F, McFadden G, Pan R, Peng X, de Sousa-Pereira P, Pinheiro A, Rahman M, Ruvoën-Clouet N, Subbian S, Tuñón MJ, van der Loo W, Vaine M, Via LE, Wang S, Mage R. 2018. The wide utility of rabbits as models of human diseases. Exp Mol Med 50:66. doi: 10.1038/s12276-018-0094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hill JM, Nolan NM, McFerrin HE, Clement C, Foster TP, Halford WP, Kousoulas KG, Lukiw WJ, Thompson HW, Stern EM, Bhattacharjee PS. 2012. HSV-1 latent rabbits shed viral DNA into their saliva. Virol J 9:221. doi: 10.1186/1743-422X-9-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nesburn AB, Bettahi I, Dasgupta G, Chentoufi AA, Zhang X, You S, Morishige N, Wahlert AJ, Brown DJ, Jester JV, Wechsler SL, BenMohamed L. 2007. Functional Foxp3+ CD4+ CD25(bright+) “natural” regulatory T cells are abundant in rabbit conjunctiva and suppress virus-specific CD4+ and CD8+ effector T cells during ocular herpes infection. J Virol 81:7647–7661. doi: 10.1128/JVI.00294-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dasgupta G, BenMohamed L. 2011. Of mice and not humans: how reliable are animal models for evaluation of herpes CD8(+)-T cell-epitopes-based immunotherapeutic vaccine candidates? Vaccine 29:5824–5836. doi: 10.1016/j.vaccine.2011.06.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jing L, Haas J, Chong TM, Bruckner JJ, Dann GC, Dong L, Marshak JO, McClurkan CL, Yamamoto TN, Bailer SM, Laing KJ, Wald A, Verjans GM, Koelle DM. 2012. Cross-presentation and genome-wide screening reveal candidate T cells antigens for a herpes simplex virus type 1 vaccine. J Clin Invest 122:654–673. doi: 10.1172/JCI60556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.St Leger AJ, Hendricks RL. 2011. CD8+ T cells patrol HSV-1-infected trigeminal ganglia and prevent viral reactivation. J Neurovirol 17:528–534. doi: 10.1007/s13365-011-0062-1. [DOI] [PubMed] [Google Scholar]
- 77.St Leger AJ, Peters B, Sidney J, Sette A, Hendricks RL. 2011. Defining the herpes simplex virus-specific CD8+ T cell repertoire in C57BL/6 mice. J Immunol 186:3927–3933. doi: 10.4049/jimmunol.1003735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hu J, Peng X, Schell TD, Budgeon LR, Cladel NM, Christensen ND. 2006. An HLA-A2.1-transgenic rabbit model to study immunity to papillomavirus infection. J Immunol 177:8037–8045. doi: 10.4049/jimmunol.177.11.8037. [DOI] [PubMed] [Google Scholar]
- 79.Mishra L, Rose JA. 1990. Adeno-associated virus DNA replication is induced by genes that are essential for HSV-1 DNA synthesis. Virology 179:632–639. doi: 10.1016/0042-6822(90)90130-J. [DOI] [PubMed] [Google Scholar]
- 80.Johnston KM, Jacoby D, Pechan PA, Fraefel C, Borghesani P, Schuback D, Dunn RJ, Smith FI, Breakefield XO. 1997. HSV/AAV hybrid amplicon vectors extend transgene expression in human glioma cells. Hum Gene Ther 8:359–370. doi: 10.1089/hum.1997.8.3-359. [DOI] [PubMed] [Google Scholar]
- 81.Nesburn AB, Burke RL, Ghiasi H, Slanina SM, Wechsler SL. 1998. A therapeutic vaccine that reduces recurrent herpes simplex virus type 1 corneal disease. Invest Ophthalmol Vis Sci 39:1163–1170. [PubMed] [Google Scholar]
- 82.Nesburn AB, Burke RL, Ghiasi H, Slanina SM, Wechsler SL. 1998. Therapeutic periocular vaccination with a subunit vaccine induces higher levels of herpes simplex virus-specific tear secretory immunoglobulin A than systemic vaccination and provides protection against recurrent spontaneous ocular shedding of virus in latently infected rabbits. Virology 252:200–209. doi: 10.1006/viro.1998.9454. [DOI] [PubMed] [Google Scholar]
- 83.Nesburn AB, Slanina S, Burke RL, Ghiasi H, Bahri S, Wechsler SL. 1998. Local periocular vaccination protects against eye disease more effectively than systemic vaccination following primary ocular herpes simplex virus infection in rabbits. J Virol 72:7715–7721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nesburn AB, Ramos TV, Zhu X, Asgarzadeh H, Nguyen V, BenMohamed L. 2005. Local and systemic B cell and Th1 responses induced following ocular mucosal delivery of multiple epitopes of herpes simplex virus type 1 glycoprotein D together with cytosine-phosphate-guanine adjuvant. Vaccine 23:873–883. doi: 10.1016/j.vaccine.2004.08.019. [DOI] [PubMed] [Google Scholar]