In cancer cells, certain promoters become aberrantly methylated, contributing to the phenotype of the tumor. KSHV infection seems to modify cellular CpG methylation, but only a few methylated promoters have been identified in KSHV-infected cells. Here, we investigated the CpG methylation of the human genome in KSHV-associated primary effusion lymphoma (PEL) and KSHV-infected cells. We have identified many hyper- and hypomethylated gene promoters and correlated their methylation with cellular gene expression. These differentially methylated cellular promoters can distinguish KSHV-positive cells from uninfected cells and may serve as the foundation for the use of these differentially methylated regions as potential biomarkers for KSHV-associated malignancies. Drugs that reverse these cancerous methylation patterns have the potential to inhibit tumor growth. Here, we show that treating PEL cells with a demethylating drug (5-aza-2′-deoxycytidine) led to inhibition of cell growth, raising the possibility of testing this drug for the treatment of PEL.
KEYWORDS: DNA methylation, Kaposi's sarcoma-associated herpesvirus, human herpesviruses, primary effusion lymphoma, transcriptional repression
ABSTRACT
Kaposi's sarcoma-associated herpesvirus (KSHV, HHV-8) is a gammaherpesvirus associated with several human malignancies. DNA methylation at CpG dinucleotides is an epigenetic mark dysregulated in many cancer types and in KSHV-infected cells. Several previous studies have analyzed in detail the CpG methylation of the KSHV episomal genomes, but little is known about the impact of KSHV on the human genome. Our knowledge of cellular CpG methylation in the context of KSHV infection is currently limited to four hypermethylated human gene promoters. Therefore, we undertook a comprehensive CpG methylation analysis of the human methylome in KSHV-infected cells and KSHV-associated primary effusion lymphoma (PEL). We performed Infinium HumanMethylation450K and MethylationEpic BeadChip arrays and identified panels of hyper- and hypomethylated cellular promoters in KSHV-infected cells. We combined our genome-wide methylation analysis with high-throughput RNA sequencing (RNA-seq) to add functional outcomes to the virally induced methylation changes. We were able to correlate many downregulated genes with promoter hypermethylation and upregulated genes with hypomethylation. In addition, we show that treating the cells with a demethylating agent leads to reexpression of these downregulated genes, indicating that, indeed, DNA methylation plays a role in the repression of these human genes. Comparison between de novo infection and PEL suggests that the virus induces initial hypermethylation followed by a slow increase in genome-wide hypomethylation. This study extends our understanding of the relationship between epigenetic changes induced by KSHV infection and tumorigenesis.
IMPORTANCE In cancer cells, certain promoters become aberrantly methylated, contributing to the phenotype of the tumor. KSHV infection seems to modify cellular CpG methylation, but only a few methylated promoters have been identified in KSHV-infected cells. Here, we investigated the CpG methylation of the human genome in KSHV-associated primary effusion lymphoma (PEL) and KSHV-infected cells. We have identified many hyper- and hypomethylated gene promoters and correlated their methylation with cellular gene expression. These differentially methylated cellular promoters can distinguish KSHV-positive cells from uninfected cells and may serve as the foundation for the use of these differentially methylated regions as potential biomarkers for KSHV-associated malignancies. Drugs that reverse these cancerous methylation patterns have the potential to inhibit tumor growth. Here, we show that treating PEL cells with a demethylating drug (5-aza-2′-deoxycytidine) led to inhibition of cell growth, raising the possibility of testing this drug for the treatment of PEL.
INTRODUCTION
Kaposi's sarcoma-associated herpesvirus (KSHV, HHV-8) is the causative agent of all forms of Kaposi's sarcoma (KS) and is tightly associated with primary effusion lymphoma (PEL) and multicentric Castleman's disease (1, 2). Like other herpesviruses, KSHV has two transcriptional programs, latency and lytic/productive phases. Following infection, the linear double-stranded DNA (dsDNA) viral genome enters the nucleus and closes on itself to become a closed circular genome that remains in the infected nucleus as an episome (3). The latency program enables the virus to remain in the infected host for life, and latency is the major phase in herpesvirus-associated malignancies (although lytic proteins have a role in the pathogenesis) (4, 5). KSHV-infected cells present a unique pattern of cellular gene expression (6–9).
DNA methylation at CpG dinucleotides is an epigenetic mark that has been studied extensively in the context of cancer. Methylation of the cytosine residue in the CpG dinucleotide is carried out by the DNA methyltransferases DNMT1, DNMT3a, and DNMT3b (10). DNMT1 is primarily responsible for reestablishing the methylation pattern during DNA replication and, hence, is considered the maintenance methyltransferase, while DNMT3a and DNMT3b can methylate the CpG cytosine residue in a background of unmethylated DNA and function as de novo methyltransferases. Many promoters contain CpG islands, and these islands are protected from methylation in normal tissues (11). In cancer cells, some of these CpG islands become aberrantly hypermethylated, and this is usually correlated with transcription repression (12). On the other hand, global hypomethylation has been described in cancer cells (13). Whole-genome bisulfite sequencing revealed a notable loss of methylation stability in colon cancer, which involved CpG islands, CpG island shores, and large (up to several megabases) blocks of hypomethylation (14).
DNA methylation is regulated by KSHV on several levels. The latency-associated nuclear antigen (LANA/ORF73) encoded by KSHV leads to CpG methylation by interacting with the cellular de novo DNA methyltransferase, DNMT3a, and recruiting DNMT3a to certain cellular promoters that become methylated and repressed (15). The KSHV-encoded microRNA, miR-K12-4-5p, targets Rbl2, the negative regulator of DNMTs, leading to increased levels of DNMT3a and, to a lesser extent, DNMT1 and DNMT3b (16). Expression of miR-K12-4-5p leads to CpG methylation of the KSHV episomal genome and the cellular α-globin-2. An additional mechanism by which KSHV might modify the human methylome is via the Polycomb complex that creates the histone mark histone H3 trimethylated on Lys27 (H3K27me3) and can direct cellular CpG methylation via its interaction with DNMTs (17, 18). KSHV infection leads to upregulation of the Polycomb catalytic subunit, EZH2, by the latent proteins vFLIP and LANA (19). In addition, LANA has the ability to recruit the Polycomb complex to chromatin through its interaction with EZH2 (20).
A recent study on RNA N6-methyladenosine (m6A) and N6,2′-O-dimethyladenosine (m6Am) modifications revealed that KSHV modulates viral and cellular RNA methylation (21). The pattern of CpG DNA methylation throughout the KSHV episomal genome has been investigated in detail (18, 22, 23). In contrast, our knowledge of cellular CpG DNA methylation in the context of KSHV infection is very limited. Transcription repression via CpG DNA hypermethylation of p16INK4a (CDKN2A) (24), the transforming growth factor β (TGF-β) type II receptor (TbetaRII, TGFBR2) (25), and PDZ-LIM domain-containing protein 2 (PDLIM2) (26) promoters has been detected in KSHV-infected primary effusion lymphoma (PEL) cell lines. Hypermethylation of H-cadherin (CDH13) promoter has been reported in LANA-expressing endothelial cells and PEL (15). In summary, while the importance of CpG methylation in disease and cancer is well established and while KSHV infection seems to modify cellular CpG methylation, only a few methylated promoters have been analyzed in KSHV-infected cells.
In this study, we performed a comprehensive CpG methylation analysis to reveal the human methylome in KSHV-infected cells and generated panels of hyper- and hypomethylated cellular promoters. Integration of our genome-wide methylation analysis with the matched high-throughput RNA sequencing (RNA-seq) data added functional outcomes for these virally induced methylation changes. Reversal of these methylation patterns by treating the cells with a demethylating agent led to reexpression of these downregulated genes and inhibition of PEL cell growth.
RESULTS
Mapping the human methylome in KSHV-associated primary effusion lymphoma.
DNA methylation microarrays are a robust and affordable tool in assessing epigenetic changes in cancer cell lines and tissues at the whole human genome level (27–29). The Infinium HumanMethylation450K BeadChip (here, 450K BeadChip) arrays measure the methylation status of over 450,000 CpG sites at single-base resolution and without the requirement for a methylated DNA capture step, which bypasses the challenges associated with capture-dependent coverage bias and allows free access to most genomic target sites (27). It includes CpGs from 21,231 genes covering 96% of CpG islands and 99% of the annotated RefSeq genes, with multiple probes per gene.
We started our analysis with the KSHV-associated primary effusion lymphoma (PEL) cell lines, BC3 and BCBL1, and the KSHV-negative lymphoma cell line BJAB, a well-accepted control in the field (15, 25). The 450K BeadChip methylation results are β values between 0 (unmethylated) and 1 (fully methylated) for each CpG analyzed. We performed two biological replicates for each cell line, and the scatterplot for β values of each biological replicate demonstrated Pearson correlation coefficient (r) values of 0.9957 in BJAB, 0.9953 in BC3, and 0.9878 in BCBL1 cells, ensuring the reliability of this assay for further analysis (see Fig. S1A to C in the supplemental material). The principal-component analysis (PCA) of the global methylation clearly differentiates PEL cell lines from BJAB cells (Fig. 1A); such grouping of the PEL cell lines indicates that their methylation pattern is distinct from that of the KSHV-negative lymphoma. The threshold we used to determine differentially methylated probes, Δβ ≥ 0.25 or Δβ ≤ −0.25, as previously described (30), identified too many probes to generate a heat map. Therefore, we used the threshold Δβ ≥ 0.5 or Δβ ≤ −0.5 to generate the heat map for the most differentially methylated probes (n = 61,148) between PEL and BJAB cells (Fig. 1B), and many of the differences in methylation appear common between BC3 and BCBL1 cells where most changes are hypomethylation. Analysis of all CpGs that passed the data normalization (n = 421,499) in these three cell lines with a threshold difference of Δβ ≥ 0.25 or Δβ ≤ −0.25 (30) revealed 6.2% hypermethylation and 30.2% hypomethylation in BC3 cells and 6% hypermethylation and 27.5% hypomethylation in BCBL1 cells relative to levels in BJAB cells (Fig. 1C). While some methylation changes were unique to a specific cell line, since we were interested in KSHV and not an individual cell line, all further analysis was performed on common methylation changes shared by both BC3 and BCBL1 cells, which, in any case, presented the vast majority.
FIG 1.
Global cellular CpG DNA methylation in PEL. (A) Principal-component analysis for all 450K BeadChip raw β values (presented between 0 and 1) of the CpG methylation variability in BJAB versus BC3 and BCBL1 cells. (B) Heat map of the most differentially methylated CpG sites (Δβ ≥ 0.5 and Δβ ≤ −0.5) comparing PEL and BJAB samples. (C) Pie charts presenting the percentage of hyper- and hypomethylated CpGs in the two different PEL cell lines relative to levels in BJAB cells (hyper- and hypomethylation thresholds were set as Δβ ≥ 0.25 and Δβ ≤ −0.25, respectively).
The 450K BeadChip methylation results were uploaded on the UCSC Genome Browser (https://genome.ucsc.edu) to visualize the results genome wide (31). We first analyzed the previously reported four hypermethylated promoters; both TGFBR2 and PDLIM2 are heavily hypermethylated in both BC3 and BCBL1 cells compared to levels in BJAB cells, p16INK4a (CDKN2A) is covered by a single probe that is hypermethylated in BC3 cells, and CDH13, an epithelium-specific gene not expressed in B cells is hypermethylated in all three B cell lines, as expected (Fig. S2). Examples of three hypermethylated gene promoters identified in our analysis, DUSP10, EHD3, and CD40, are presented in Fig. 2A to C. These three promoters contain CpG islands (Fig. 2A to C, green boxes) with several probes that are heavily hypermethylated in both BCBL1 and BC3 cells compared to levels in BJAB cells. To validate our 450K BeadChip methylation results, we decided to use a different method that we have previously used (23) for DNA CpG methylation analysis, i.e., preferential binding to the methylated DNA binding domain of MBD2 (methyl-CpG binding domain protein 2). DNA was incubated with methylated DNA binding beads (MBD2 beads; MethylMiner DNA Enrichment kit), and DNA from the unbound (unmethylated) fraction, from a wash with 450 mM NaCl (partially methylated/low CpG content), and from elution with 2,000 mM NaCl (methylated) was amplified by quantitative PCR (qPCR) with specific primers for the methylated genomic region. For all three cellular promoters, the binding of DNA from PEL cell lines to the methylated DNA binding beads was higher than the DNA level from BJAB cells (Fig. 2D to F). The methylated DNA binding assay indicates that all three genes were significantly hypermethylated in both BC3 and BCBL1 cells compared to levels in BJAB cells, giving us confidence in our genome-wide methylation analysis.
FIG 2.
Hypermethylated genes are downregulated in PEL. (A to C) Genome browser view of our 450K BeadChip methylation analysis. Each column presents a single CpG analyzed, where column height is relative to the β value (between 0 and 1). The three gene promoters presented (DUSP10, EHD3, and CD40) are aligned with the probe location on the gene exon/intron map (UCSC Genome Browser) (31). Gray arrows indicate the direction of transcription, green boxes represent CpG islands, and brown columns represent the human bisulfate sequencing methylation hub for B cells (72). (D to F) CpG methylation analysis using a methylated DNA enrichment assay. DNA from the unbound (unmethylated) fraction, from the wash with 450 mM NaCl (partially methylated), and from elution with 2,000 mM NaCl (methylated) was subjected to qPCR with primers that amplify the promoter region of the three genes presented in panels A to C. (G to I) Relative mRNA expression levels of DUSP10, EHD3, and CD40, as indicated, were detected by RT-qPCR. Results are presented relative to levels of β-actin. All experiments were performed in triplicate, and data are presented as means + standard deviations. One-tailed t tests were performed (*, P ≤ 0.05; **, P ≤ 0.01). (J) Protein expression levels for CD40 and DUSP10 were detected by Western blot analysis with the respective antibodies. Equal amounts of total protein were loaded and validated by β-actin antibody.
Hypermethylated genes in PEL cells are repressed.
CpG methylation within the promoter region is associated with repression of gene expression (32, 33). To check the expression levels of these three hypermethylated genes, we performed reverse transcription-quantitative PCR (RT-qPCR). All three genes were dramatically repressed in both PEL cell lines compared to levels in BJAB cells (Fig. 2G to I). For CD40 and DUSP10, we also validated gene downregulation at the protein level (Fig. 2J). Such dramatic repression is usually associated with DNA methylation epigenetic regulation (34). To examine the contribution of DNA methylation to transcription repression of the hypermethylated gene promoters, cells were treated with the demethylating agent 5-aza-2′-deoxycytidine (5-AZA-dC; decitabine). All three genes were reactivated in both BC3 (Fig. S3A) and BCBL1 (Fig. 3A) cells following 5-AZA-dC treatment. The induction of CD40 was quite mild, suggesting strong involvement of repressed chromatin in this promoter; to test this hypothesis, we treated the cells with a drug that increases open chromatin. It is well established that demethylating agents work synergistically with histone deacetylase inhibitors such as trichostatin A (TSA) to reactivate silenced CpG-methylated genes (35). As expected, on hypermethylated promoters TSA alone had only a marginal effect on gene reexpression, while a combination of both drugs resulted in robust gene reexpression (Fig. 3B and S3B). For example, in BC3 cells TSA alone activated CD40 promoter by 1.4-fold, and 100 nM 5-AZA-dC activated the CD40 promoter by 1.6-fold while a combination of 5-AZA-dC and TSA resulted in 44-fold induction. In BCBL1 cells TSA alone induced CD40 promoter by 9-fold, and 100 nM 5-AZA-dC induced the CD40 promoter by 3-fold while a combination of the two resulted in 271-fold induction. This synergistic effect between 5-AZA-dC and TSA characterizes many promoters repressed by DNA CpG methylation. By performing methylation DNA binding capture, we confirmed that, following 5-AZA-dC treatment, these cellular promoters harbor reduced CpG methylation (Fig. 3C and S3C). Our results are consistent with the literature that small changes in the methylation following 5-AZA-dC treatment result in significant gene reexpression (36). One possible explanation for this phenomenon is that demethylation does not spread evenly in the cell population, and cells that undergo stronger demethylation contribute more to gene reexpression. It is worth noting that more robust demethylation was obtained with a higher dose (500 nM) of 5-AZA-dC (data not shown). These results further support the role of DNA methylation in the repression of these human genes in PEL cells and the functional importance of our methylation analysis for cellular gene expression.
FIG 3.
Hypermethylated genes can be reexpressed by 5-AZA-dC in PEL. (A) BCBL1 cells were treated with 20 and 100 nM concentrations of the demethylating agent 5-aza-2′-deoxycytidine (5-AZA-dC) or vehicle (DMSO) daily for 4 days, followed by four additional days of incubation without drug (total of 8 days). (B) The experiment as described for panel A was performed, but on day 7 cells were treated with 300 nM trichostatin A (TSA) or vehicle (DMSO) for 24 h. All experiments were performed in triplicate, and data represented as means + standard deviations. One-tailed t tests were performed (*, P ≤ 0.05; **, P ≤ 0.01). (C) Methylated DNA enrichment with MBD2 beads was performed to determine promoter methylation following 5-AZA-dC treatment. DNA from the unbound (unmethylated) fraction, from the washes with 450 and 600 mM NaCl (partially methylated), and from elution with 2,000 mM NaCl (methylated) was subjected to qPCR with primers that amplify the promoter region.
Hypomethylated promoters in PEL cells are associated with expressed genes.
The promoters of many tissue-specific genes do not contain CpG islands but have a few CpGs whose methylation tightly regulates their expression (37). We selected three hypomethylated promoters identified in our screen: MUC13, LGALS1, and PCDHB5. The 450K BeadChip methylation results detected hypomethylation in the regulatory regions of these genes (Fig. 4A to C). MBD2 possesses high affinity for CpG islands and reduced affinity for low-content CpG regions (38, 39), such as hypomethylated promoters that lack CpG islands, which may explain the relatively low binding in all three cell lines. Still, we were able to detect lower binding of the MBD2 beads for DNA from PEL cells than from BJAB cells (Fig. 4D to F), supporting hypomethylation in PEL. Next, we determined the expression levels of these hypomethylated genes by RT-qPCR and found high expression levels in PEL cells compared to those in BJAB cells (Fig. 4G to I). For LGALS1 and MUC13, we also validated gene upregulation at the protein level (Fig. 4J). To examine the contribution of DNA methylation to the expression of these genes, we treated the BJAB cells that possess hypermethylation and transcription repression relative to PEL cell levels with a demethylating agent. MUC13 and LGALS1 were reactivated in BJAB cells following 5-AZA-dC treatment (Fig. S4A), and all three genes showed robust synergistic reactivation following combined treatment with 5-AZA-dC and TSA (Fig. S4B). We did not observe reduced methylation of these cellular promoters in BJAB cells following 5-AZA-dC treatment using methylation DNA binding capture (Fig. S4C), probably due to the fact that our binding assay was not sensitive enough to detect minor methylation changes in non-CpG island regions. Nevertheless, it is worth noting that minor demethylation was detected at a higher dose (500 nM) of 5-AZA-dC (data not shown). Collectively, these results support the role of DNA hypomethylation in the expression of these human genes in KSHV-positive cells. In conclusion, we have identified three promoters that become hypomethylated and expressed in PEL cells.
FIG 4.
Hypomethylated genes are upregulated in PEL. (A to C) Genome browser view of our 450K BeadChip methylation analysis, as described in the legend of Fig. 2A, but for the three hypomethylated promoters (MUC13, LGALS1, and PCDHB5). (D to F) CpG methylation analysis using a methylated DNA enrichment assay (MethylMiner), as described for Fig. 2D to F. (G to I) Relative mRNA expression levels of MUC13, LGALS1, and PCDHB5, as indicated, were detected by RT-qPCR. Results are presented relative to the level of β-actin. All experiments were performed in triplicate, and data are presented as means + standard deviations. One-tailed t tests were performed (*,P ≤ 0.05; **, P ≤ 0.01). (J) Protein expression levels for LGALS1 and MUC13 were detected by Western blot analysis with the respective antibodies. Equal amounts of total protein were loaded and validated by the level of β-actin antibody.
Global analysis of gene expression reveals a correlation between methylation and gene expression in PEL cells.
To determine the functional importance of the differentially methylated genes in our 450K BeadChip results, we performed RNA-seq on RNA isolated from the same cell lines. Using the RNA-seq data, we generated two lists of differentially expressed genes (over 2-fold difference and a false discovery rate [FDR] of ≤1%); 1,650 genes were upregulated and 1,525 were downregulated in PEL (both BC3 and BCBL1) cells compared to levels in BJAB cells (Table S1). To get better insight into the connection between CpG methylation and gene expression, we generated panels of hyper- and hypomethylated promoters in PEL cells with the criterion of Δβ ≥ 0.25 or Δβ ≤ −0.25 in at least two probes per promoter (including the TSS200 region, 5′ untranslated region [UTR], and first exon) and commonality between the two PEL cell lines tested. Our panels include 656 hyper- and 3,447 hypomethylated promoters in PEL cells compared to levels in BJAB cells (Table S2). Out of the 656 hypermethylated promoters, 215 genes were downregulated (32.8%). Out of the 3,447 hypomethylated promoters, 531 genes were upregulated (15.4%) (Fig. 5A and Table S3). To further investigate the correlation between promoter methylation and gene expression, we generated lists of up- and downregulated genes whose promoters were differentially methylated. Within this group, 91.5% of the hypermethylated promoters were downregulated genes, and 84.8% of the hypomethylated promoters were upregulated genes (Fig. 5B). This analysis supports a correlation specifically for hypermethylation with suppression of gene expression and highlights the importance of DNA CpG methylation in the regulation of cellular genes in PEL.
FIG 5.

PEL CpG methylation correlates with gene expression. (A) Venn diagrams present the commonality between differentially methylated genes from the 450K BeadChip analysis and differentially expressed genes identified by RNA-seq in PEL cells relative to levels in BJAB cells. (B) Percentage of up- and downregulated genes which are differentially methylated and associated with either hyper or hypomethylated gene promoters.
Interestingly, when we analyzed our PEL hypermethylated promoter panel on the platform GREAT (Genomic Regions Enrichment of Annotations Tool [http://great.stanford.edu/public/html/index.php]) for gene expression signatures in published data (Molecular Signatures Database [MSigDB] perturbation) (40), the first hit was a paper on PEL gene expression analysis (6), highlighting the tight connection between our methylation signature and gene expression specific to KSHV-associated lymphoma. In that study, the authors identified several unique downregulated genes in PEL: CD19, CD20, CD79a, and CD79b. In our methylation analysis, we have found hypermethylation of CD19, CD79a, and CD79b in both BC3 and BCBL1 cells, in agreement with their predicted expression.
Limited KSHV lytic induction by 5-AZA-dC.
Lytic induction of PEL cells following treatment with a high dose of 5-azacytidine (5 μM) has been previously reported (22). Therefore, we followed the expression of the KSHV lytic gene K8.1 in cells treated with low doses of 5-AZA-dC (20 nM, 100 nM, and 500 nM). We also followed the expression levels of another late gene, ORF25, and the immediate early gene, ORF50 (Fig. S5A and B). Our results indicate only minor lytic induction with 20 nM, while dose-dependent lytic induction was observed following treatment with 100 nM and 500 nM (Fig. 6A). The induction was more profound in BCBL1 than BC3 cells, a phenomenon that we have observed for cellular genes as well (Fig. 3A and S3A). One possible explanation for the higher expression of the late lytic genes K8.1 and ORF25 than of the immediate early gene ORF50 (RTA) is the fact that RNA isolation was done 4 days following the end of 5-AZA-dC treatment and, thus, toward the late phase of lytic induction. The relatively small upregulation of K8.1, ORF25, and ORF50 compared to that of cellular genes following 5-AZA-dC treatment, as determined by RT-qPCR, prompted us to perform immunofluorescence assays (IFA) to follow viral and cellular protein expression during 5-AZA-dC treatment (Fig. 6B and S6). KSHV-encoded K8α lytic protein expression could be detected only sporadically. In sharp contrast, the cellular protein DUSP10 was profoundly induced by the 100 nM treatment, with over 90% of the cells expressing DUSP10. This set of experiments suggests that while 5-AZA-dC treatment leads to cellular gene reexpression in the vast majority of the treated cells, under these conditions only a small minority turn on the KSHV lytic cycle. It is important to note that our study focuses mainly on the cellular genes, so we chose these 5-AZA-dC treatment conditions (20 nM and 100 nM) to investigate cellular gene reexpression where minimal KSHV lytic induction takes place. Therefore, we can conclude that cellular gene reexpression is the result of 5-AZA-dC treatment and not indirectly attributable to lytic virus induction.
FIG 6.
5-AZA-dC treatment leads to inhibition of cell growth with only limited KSHV lytic induction. (A) BC3 and BCBL1 cells were treated with 5-AZA-dC as described in the legend of Fig. 3 (with the addition of a treatment with 500 nM). RNA was isolated, and RT-qPCR was performed with primers for the KSHV lytic gene K8.1 to evaluate lytic induction. Results are presented as fold change relative to the level in control untreated cells. (B) BCBL1 cells from the experiment shown in panel A (day 8) were fixed to a slide and immunostained with anti-K8α (red), anti-DUSP10 (green) antibodies, and DAPI (blue) to evaluate KSHV lytic induction and cellular DUSP10 reexpression, respectively. Scale bar, 10 μm. (C) Growth curves showing total cell count of BC3 and BCBL1 cells under daily treatment with 20 nM, 100 nM, and 500 nM concentrations of the demethylating agent 5-AZA-dC or vehicle (DMSO) for 4 days. Cells were counted every 24 h via hemocytometer. (D) Charts present cell viability at day 8 for the experiment shown in panel C (4-day treatment followed by 4 additional days of incubation without drug). All experiments were performed in triplicate, and data are presented as means + standard deviations. One-tailed t tests were performed (*, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001).
Low doses of 5-AZA-dC inhibit PEL cell growth.
Treatment of cancer cells with clinically relevant low (nanomolar) doses of 5-aza-cytidine (5-AZA) and 5-aza-2′-deoxycytidine (5-AZA-dC) can produce sustained changes in gene expression and in critical signaling pathways involved in tumorigenesis and, specifically, can inhibit the growth of transformed cells without inducing immediate cytotoxic effects such as DNA damage and apoptosis (41, 42). Several clinical trials have indicated that demethylating agents impair cancer proliferation and cause cancer regression in some patients (43, 44). To test whether treatment with low doses of a demethylating agent can inhibit PEL cell growth, we treated both BC3 and BCBL1 cell lines with three different concentrations of 5-AZA-dC (20 nM, 100 nM, and 500 nM). Treatment was administered daily for four consecutive days, and every 24 h total cell count was measured. Our results revealed significant inhibition of cell growth at 96 h of treatment in both the BC3 and BCBL1 cell lines for all three doses (Fig. 6C). Next, cell viability of all treated cells after recovery from the drug treatment was determined. Cells were counted after four additional days of incubation without drug (total of 8 days), and the ratio between number of live cells and total number of cells was evaluated. Our results revealed that cell viability was dramatically reduced in both the BC3 and BCBL1 cell lines with the 500 nM treatment (Fig. 6D). Treatment with 100 nM produced mild cell death, whereas the 20 nM treatment was not associated with significant cell death. These findings suggest that 5-AZA-dC treatment with lower doses may be beneficial in inhibiting proliferation of KSHV tumors without causing the serious cytotoxic side effects associated with higher doses (42). Additionally, cell viability was evaluated for TSA treatment (Fig. S5C and D), and only marginal cell death was detected under the tested conditions.
Mapping the human methylome during de novo infection.
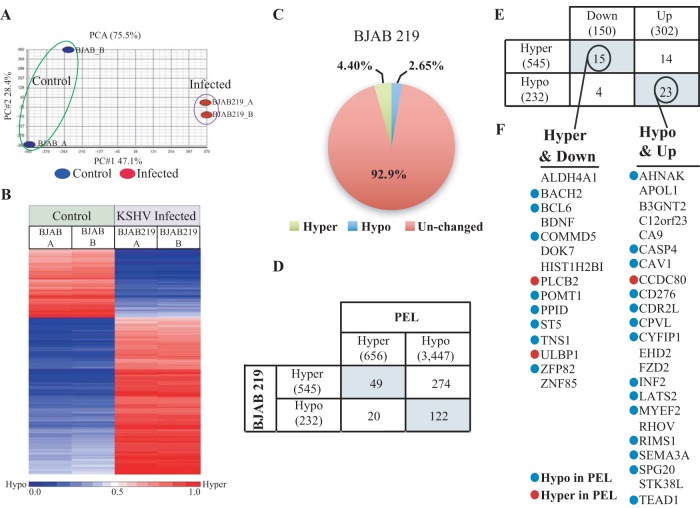
While our global methylation analysis in PEL and BJAB cells revealed many differentially methylated genes and may explain the unique pattern of gene expression in this lymphoma, this analysis still lacks a direct connection between KSHV infection and cellular DNA methylation as we were comparing different cell lines. In order to directly link DNA methylation to KSHV infection, we performed de novo infection of BJAB cells with the recombinant KSHV, rKSHV.219 (45). Following infection of BJAB cells, two flasks were maintained in parallel: one with the infected cells (BJAB219) and a second with uninfected cells as controls. The BJAB219 cells were latently infected with KSHV based on high LANA (latent) and low ORF50 and K8.1 (lytic) gene expression (Fig. S7). DNA was isolated and subjected to bisulfite treatment and hybridized to the MethylationEpic BeadChip (here, Epic BeadChip). The scatterplot for β values of each biological replicate demonstrated a Pearson correlation coefficient, r, of 0.9976 in BJAB and 0.9977 in BJAB219 cells, ensuring the reliability of this assay for further analysis (Fig. S8). A principal-component analysis (PCA) of the global methylation clearly differentiates infected cells (BJAB219) from the parental BJAB cells (Fig. 7A). The most differentially methylated probes between BJAB219 and BJAB (2,534; Δβ ≥ 0.5 or Δβ ≤ −0.5) are presented in the heat map (Fig. 7B). In order to be able to compare results between the BJAB219 and PEL cells, the probes used for the analysis were only probes that were analyzed in PEL and passed the data normalization (n = 391,952). Differentially methylated probes with a threshold difference of Δβ ≥ 0.25 or Δβ ≤ −0.25 (30) revealed 4.4% hypermethylation and 2.65% hypomethylation in BJAB cells infected with KSHV (Fig. 7C). Next, we identified differentially methylated cellular promoters in BJAB219 cells; using the same criteria as in PEL cells (Δβ ≥ 0.25 or Δβ ≤ −0.25 in at least two probes per promoter), we identified 545 hyper- and 232 hypomethylated promoters in BJAB219 cells (Table S2). The genes transcribed by these hypermethylated promoters were analyzed on STRING (https://string-db.org) (46), and many negative regulators of cell proliferation, cellular process, and RNA polymerase II were detected. On the other hand, genes transcribed by the hypomethylated promoters do not include negative regulators but do include positive regulators of neuron and synaptic development, cellular signaling, and morphogenesis. Within the top 10 hits, 7 involve neurogenesis and synaptic transmission, indicating that hypomethylation is associated with loss of cell identity leading to expression of neuronal genes in a B cell. Interestingly, loss of cell identity (hypomethylation of olfactory receptors) characterized our results on hypomethylation in PEL cells.
FIG 7.
Global cellular CpG DNA methylation in BJAB cells de novo infected with KSHV. (A) Principal-component analysis of raw β values of the CpG methylation variability in BJAB versus BJAB219 cells. (B) Heat map of most differentially methylated CpG sites (Δβ ≥ 0.5 and Δβ ≤ −0.5) in BJAB and BJAB219 cells. (C) Pie chart presenting the percentage of hyper- and hypomethylated CpGs in BJAB219 cells relative to levels in BJAB cells (hyper- and hypomethylation thresholds were set as Δβ ≥ 0.25 and Δβ ≤ −0.25, respectively). (D) Table presenting the common differentially methylated genes in both PEL and BJAB219 cells relative to levels in the control. (E) Table presenting the common differentially methylated and expressed genes in BJAB219 cells. (F) Lists of the hypermethylated and downregulated or hypomethylated and upregulated genes.
RNA-seq analysis in BJAB219 cells identified 150 down- and 302 upregulated genes (Table S1). Next, we intersected the down- and upregulated genes with hyper- and hypomethylated gene promoters and identified 15 genes that were both hypermethylated and downregulated and 23 genes that were both hypomethylated and upregulated in BJAB219 cells (Fig. 7E and F). A search for common regulatory elements shared by the promoters of these 15 hypermethylated and repressed genes using the GREAT platform identified ZEB1 (TCF-8), a transcription repressor known to impose promoter hypermethylation (47). ZEB1 is an important epithelial-mesenchymal transition (EMT) factor that is induced by WNT signaling. ZEB1 levels were shown to be upregulated following KSHV infection of primary human dermal microvascular endothelial cells (DMVEC) and of the endothelial-like cell line EAHY926 (EAHY) (48), suggesting its relevance in the repression and hypermethylation of cellular genes in BJAB219 cells. ZEB1 might be important for hypermethylation in PEL cells as well since we were able to locate ZEB1 binding sites next to many differentially hypermethylated probes in BCBL1 and BC3 cells.
A search for common differentially methylated cellular promoters between BJAB219 and PEL cells identified 49 hyper- and 122 hypomethylated promoters (Fig. 7D). While the majority of hypomethylated promoters in BJAB219 cells remain hypomethylated in PEL cells (n = 122), it seems that many hypermethylated promoters in BJAB219 cells become hypomethylated in PEL cells (n = 274). We speculate that the vast hypomethylation in PEL contributes to this observation. The levels of hypermethylation in de novo infection (4.4%) are close to the level in PEL cells (6.1%), while hypomethylation levels are very distinct, with only 2.65% in de novo infection in contrast to 28.8% in PEL (Fig. 8A and B). This suggests that the kinetics of hyper- and hypomethylation during KSHV infection are very distinct. It seems that relatively early during KSHV infection, the virus imposes hypermethylation on the cellular genome and that hypomethylation slowly accumulates over time or as a result of cellular transformation (Fig. 8C, model).
FIG 8.

Suggested model. (A and B) Charts presenting the percentage of hyper- and hypomethylated CpGs in BJAB219, BC3, and BCBL1 cells relative to the level in the control (from the global methylation analysis). (C) Schematic presentation of the suggested model. Initial hypermethylation is followed by a slow increase in hypomethylation that is the dominant form in PEL.
DISCUSSION
Here, we present the first global cellular CpG methylation analysis in KSHV-infected cells. Our methylation analysis in PEL cells revealed a distinct methylation pattern compared to that in BJAB cells, with around 6% (6.2% in BC3 and 6% in BCBL1 cells) hypermethylation and up to 30% (30.2% in BC3 and 27.5% in BCBL1 cells) hypomethylation relative to levels in BJAB cells. Our ability to detect methylation of known KSHV-methylated promoters and validate the methylation status of three representative hyper- and hypomethylated promoters via a different method (binding to the methylated DNA binding domain of MBD2) gave us confidence in our global methylation analysis. The three hypermethylated promoters we analyzed, DUSP10, EHD3, and CD40, were significantly repressed in PEL cells, as determined by RT-qPCR. To test the involvement of CpG methylation in gene repression, we treated the cells with the demethylating agent 5-AZA-dC and found that all three genes were reactivated following 5-AZA-dC treatment. This observation supports the role of DNA methylation in the transcription repression of these genes in KSHV-infected cells. The three hypomethylated promoters we analyzed, MUC13, LGALS1, and PCDHB5, are upregulated in PEL cells. Here, the contribution of DNA methylation to their expression was tested by treating the control BJAB cells (where the promoter is methylated) with 5-AZA-dC. Interestingly, LGALS1 (galectin-1) is released by a variety of tumors, where it contributes to malignant transformation and metastasis (49). Previous studies detected upregulation of LGALS1 in KSHV-infected cells (8), and its suppression prevented angiogenesis and tumorigenesis in a KS mouse model (50). Our study extends LGALS1 upregulation to the PEL cell lines, BC3 and BCBL1, suggesting its relevance also in PEL. Out of these six gene promoters EHD3 (51), MUC13 (52), and LGALS1 (53) have been shown previously to be regulated by promoter CpG methylation, in agreement with our observations.
To add functional outcomes to our methylation panels, we performed RNA-seq on RNA from the same cell lines and identified 1,650 upregulated and 1,525 downregulated genes in PEL cells (both BC3 and BCBL1) compared with levels in BJAB cells. Out of the 656 hypermethylated promoters in PEL cells, 215 genes were downregulated (33%), and out of the 3,447 hypomethylated promoters, 531 genes were upregulated (15%). In other words, we have identified 215 genes that are both downregulated and hypermethylated and 531 genes that are both upregulated and hypomethylated in PEL cells. Our observation that many hypermethylated promoters were not correlated with gene repression is in agreement with previous studies indicating that many promoters that become hypermethylated in cancer cells are already repressed in normal (control) cells (54, 55).
Sequences around hypermethylated regulatory regions (sequences ±100 bp of hypermethylated probes within the promoter region) in PEL analyzed using GREAT identified genes in the pathway of B cell activation, inflammation, and immune response. Modulation of B cell differentiation is consistent with the fact that KSHV infects germinal center B cells and induces their proliferation and maturation by the virus-encoded K1 and K15 genes, leading to B cell activation in a unique pathway that bypasses B cell antigen receptor (BCR) activation (56). Concurrently, KSHV inhibits BCR intracellular transport to the cell surface by K1 (57) and blocks BCR-induced Ca+2 influx by K15 (58). Hypermethylated regulatory regions in BJAB219 analyzed using GREAT identified genes in the regulation of cholesterol transport and gap junction. Recently, it was shown that the cholesterol pathway is repressed in KSHV-infected cells (59). GREAT analysis for consensus binding sites at the promoters of hypermethylated and repressed genes in BJAB219 cells detected enrichment for ZEB1 (TCF-8) binding sites. Interestingly, ZEB1 binding sites were enriched at hypermethylated promoters in PEL cells as well. Depending on the cellular context, ZEB1 can activate transcription or repress transcription via establishment of DNA hypermethylation (47). The fact that ZEB1 is induced by WNT signaling, a cellular pathway activated by KSHV-encoded LANA (60), and the observation that ZEB1 levels were upregulated following KSHV infection (48) support the possible involvement of ZEB1 in KSHV-induced methylation.
Another complex we have found using GREAT to be enriched at hypermethylated regulatory elements in both PEL and BJAB219 cells is the Polycomb repressive complex and the histone mark it leaves on the chromatin, histone H3 trimethylated on Lys27 (H3K27me3). The H3K27me3 chromatin mark is established on unmethylated CpG island genes early in development and then maintained in differentiated cell types by the presence of an EZH2-containing Polycomb complex. In cancer cells, as opposed to normal cells, the presence of this complex recruits DNA methyltransferases, leading to de novo methylation (17, 61). Several studies linked Polycomb to KSHV infection, including upregulation of the Polycomb catalytic subunit EZH2 by vFLIP and LANA (19) and recruitment of EZH2 to the viral episomal genome (18, 20, 62). It would be interesting to follow this complex on the cellular genome as well.
Sequences around hypomethylated regulatory regions in PEL analyzed using GREAT identified genes involved in olfactory receptor activity and a list of genomic imprinted genes (where CpG methylation marks the parental origin of the allele). Sequences around hypomethylated regulatory regions in BJAB219 cells analyzed using GREAT identified genes involved in neuron and synaptic development. Collectively, it seems that hypomethylation induced by de novo infection (BJAB219) as well as chronic infection (PEL) leads to loss of cell identity, hypomethylation of olfactory and neuronal genes that are not expressed in normal B cells, and loss of methylation at imprinted genes.
Since the PEL cells with methylated promoters harbor a latent virus that might be activated into the lytic cycle under different stress conditions, including DNA demethylating agents, we followed KSHV lytic induction under the same 5-AZA-dC treatments. Lytic induction of PEL cells following treatment with a high dose of 5-azacytidine (5 μM) has been previously reported (22). Our results indicate that lytic induction was observed following treatment with 100 nM and 500 nM, but no significant lytic induction was observed with 20 nM. We also performed immunofluorescence assays (IFA) to follow the percentage of cells that express viral (K8α lytic protein) and cellular (DUSP10) proteins following 5-AZA-dC treatment. The cellular protein DUSP10 was profoundly induced by the 100 nM treatment, such that over 90% of the cells expressed DUSP10. In sharp contrast, KSHV-encoded K8α lytic protein expression could be detected only sporadically. These experiments suggest that while 5-AZA-dC treatment leads to cellular gene reexpression in the vast majority of the treated cells, under these conditions only a small minority turn on the KSHV lytic cycle.
The vast number of cellular genes regulated via DNA methylation in PEL raises the possibility that their suppression might contribute to PEL cell growth as malignant cells. Consequently, we tested the effects of a demethylating drug on cell growth and viability. Treatment with low doses of 5-AZA-dC resulted in efficient growth inhibition of PEL cells. This growth inhibition was observed even with the lowest dose of 20 nM, a dose where no lytic viral gene expression could be detected. Previously, the combination of 5-AZA-dC with 1,25-dihydroxyvitamin D3 [1,25(OH)2D3], the biologically active form of vitamin D3, on BCBL1 and BC1 cell growth was investigated (26). In that study, 5-AZA-dC (50 nM) alone only partially inhibited cell growth, while a combination with 1,25(OH)2D3 led to complete cell growth inhibition. One possible explanation for our ability to observe significant cell growth inhibition already with 20 nM 5-AZA-dC is that, in the previous study, cells were treated only once at the beginning of the experiment, whereas we added fresh medium containing 5-AZA-dC daily for 4 days. Regardless, both studies support the notion that treatment of PEL cells with 5-AZA-dC leads to inhibition of cell growth. Similar to treatment of other cancers, 5-AZA-dC also inhibited BJAB cell growth (data not shown). 5-AZA-dC has been approved by the FDA for the treatment of myelodysplastic syndromes (MDS) (63), and several clinical trials have investigated its efficiency on several cancer types (64, 65). Therefore, our study supports the possibility of testing 5-AZA-dC for the treatment of PEL.
Global methylation analysis in de novo-infected BJAB cells revealed 4.4% hypermethylation and 2.65% hypomethylation, while the hypermethylation in de novo-infected cells was close to the level in PEL cells (4.4% versus 6%, respectively). The hypomethylation seems very different, with only 2.65% in de novo-infected cells and up to 30% in PEL cells. This suggests that, following KSHV infection, the virus imposes hypermethylation on the cellular genome, and hypomethylation slowly accumulates until it reaches the levels in PEL. Our finding that many hypermethylated promoters in BJAB219 cells become hypomethylated in PEL cells (n = 274; 50.2%), but not vice versa (n = 20; 8.6%), supports this model.
Epstein-Barr virus (EBV), along with KSHV, belongs to the human gammaherpesviruses and is associated with several human malignancies including nasopharyngeal carcinoma (NPC) and Burkitt lymphoma. Within a list of 150 hypermethylated and repressed genes in nasopharyngeal carcinoma (66), we found 9 hypermethylated in PEL cells and 5 hypermethylated in BJAB219 cells. Within a list of genes nearest to the top 100 differentially methylated regions (DMR) in EBV-positive Burkitt lymphoma (67), 33 were differentially methylated in PEL and 7 were differentially methylated in BJAB219 cells, suggesting that in addition to many unique gene targets, some common targets are differentially methylated during the process of transformation by these closely related viruses.
We realize that the transcriptional analysis results are somewhat underpowered, with three different cell lines and two biological replicates for each. The main limitation here was the complexity and the high cost of transcriptional profiling. The de novo infection was performed on the BJAB cell line. On one hand, the BJAB line is the control for the PEL cells and therefore very relevant to the first part of the study; on the other hand, these cells are established tumor cells, and it will be interesting in the future to follow the global cellular CpG methylation following KSHV infection on primary cells.
To conclude, our study is the first attempt to document global CpG methylation in KSHV-infected cells and a KSHV-associated malignancy. We have identified many epigenetically modified gene promoters, and while the role of these genes in KSHV pathogenesis is a subject for future investigation, these differentially methylated cellular promoters can distinguish KSHV-positive cells from uninfected cells and may provide the foundation for the development of biomarkers for KSHV-associated malignancies.
MATERIALS AND METHODS
Cell culture.
BC3, BCBL1, and BJAB cells (kindly provided by Richard F. Ambinder) were cultured in RPMI 1640 medium supplemented with 20% fetal bovine serum (FBS), 2 mM l-glutamine, penicillin-streptomycin (100 U/ml and 100 μg/ml, respectively), and 1 mM sodium-pyruvate in 5% CO2 at 37°C. The Vero-rKSHV.219 cell line was kindly provided by Jeffrey Vieira and cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% FBS, penicillin-streptomycin (100 U/ml and 100 μg/ml, respectively), 2 mM l-glutamine, and 2 μg/ml puromycin.
KSHV de novo infection.
Vero-rKSHV.219 cells (45) were induced with 1.25 mM sodium butyrate and 20 ng/ml 12-O-tetradecanoylphorbol-13-acetate (TPA) and after 48 h were cocultured with BJAB cells (1:1 cell ratio) in RPMI 1640 medium for an additional 48 h. Medium containing the BJAB infected cells was collected and transferred to a new 25-cm2 flask for an additional 48 h prior to addition of 0.8 μg/ml puromycin for permanent selection.
Drug treatment.
Cells were treated with 20 nM, 100 nM, and 500 nM concentrations of the demethylating agent 5-aza-2′-deoxycytidine (decitabine; 5-AZA-dC) (Sigma) or vehicle (dimethyl sulfoxide [DMSO]; Santa Cruz Biotechnology) daily for 4 days (fresh RPMI 1640 medium containing 5-AZA-dC was added every 24 h), followed by three additional days of incubation without drug (total of 7 days). On day 7 cells were treated with 300 nM trichostatin A (TSA) (Sigma), a histone deacetylase (HDAC) inhibitor, or vehicle (DMSO) for 24 h.
DNA isolation and Illumina 450K BeadChip analysis.
Genomic DNA (gDNA) was isolated from cells using a DNeasy blood and tissue kit (Qiagen). Next, gDNA samples underwent bisulfite conversion (catalog number D5001; Zymo Research) and then were hybridized to the HumanMethylation450K BeadChip (Illumina) or MethylationEpic BeadChip (Illumina) according to the manufacturer's protocol. The 450K BeadChip array was performed in a single-base extension reaction, stained, and imaged on an Illumina HiScan. The raw data were exported from GenomeStudio and normalized using the ChAMP R pipeline (68) that has the ability to run a series of programs in which the output of one program is used as an input for the next one. The different programs decrease biases from known technical issues, such as adjustment for type I and type II probes, background correction, and batch effects between chips. Statistical analysis was performed using the limma program within the ChAMP pipeline and JMP Genomics software. The methylation analysis was performed at the Genomics Center of the Biomedical Core Facility, the Faculty of Medicine, Technion.
DNA methylation data analysis.
Methylation rates of selected CpG sites were calculated (using GenomeStudio methylation module software) as methylation β values ranging from 0 (completely unmethylated) to 1 (completely methylated). Probes with a detection P value of over 0.05 or blank β value were excluded from further analyses. Differences in β values (Δβ) between KSHV-infected cells and control samples were determined as Δβ of ≥0.25 and Δβ of ≤−0.25 and named as hyper- and hypomethylated, respectively (30). Partek Genomics Suite software was used to create Venn diagrams, heat maps, PCAs, and scatterplots.
Methylated DNA enrichment.
Genomic DNA was isolated using a DNeasy blood and tissue kit (Qiagen) and sheared by sonication to fragments of ∼500 bp. The sheared DNA (1 μg) was added to 10 μl of MBD bead slurry (MethylMiner DNA enrichment kit; Invitrogen, Carlsbad, CA) and incubated on a rotating mixer for 1 h, as described previously (23). The DNA fragments were eluted into distinct subpopulations based on the degree of methylation: noncaptured fraction (NC; representing unmethylated DNA fragments), a fraction eluting with 450 mM NaCl (representing partially methylated DNA fragments), and a fraction eluting with 2,000 mM NaCl (representing methylated DNA fragments). The fractions were then ethanol precipitated and resuspended in H2O. Enrichment of the different fractions was measured by real-time quantitative PCR (qPCR) with Power SYBR green PCR master mix (Applied Biosystems) and analyzed on a CFX96 Touch real-time PCR detection system (Bio-Rad). The primers used were the following: MUC13 (5′-TTCCCTTCCTGGCTACCTTT-3′ and 5′-TTGCCTTTCTGTGGTTTGAC-3′), PCDHB5 (5′-GGGTGGATTGTGTACGGAGT-3′ and 5′-CCTCCCACAAAAGCAACAAT-3′), LGALS1 (5′-GCCCTATCCTGACTTGCAATTG-3′ and 5′-CGCTCCCACCCTTTTAACTG-3′), CD40 (5′-GATAGGTGGACCGCGATTG-3′ and 5′-GGGGCAAAAACAACTCACAG-3′), DUSP10 (5′-GGTTGGGCCAATAAGAATCC-3′ and 5′-TTAACACCGAGGACCCAGAG-3′), and EHD3 (5′-GGAATTGACCTCAGCAACTGAAG-3′ and 5′-CGGCTGCGAGGACAGAGT-3′). All the reactions were run in triplicate. To determine promoter methylation following 5-AZA-dC treatment, cells were treated with 100 nM 5-AZA-dC daily for 4 days, and DNA was isolated and subjected to methylated DNA enrichment. Since it is expected that 5-AZA-dC will lead to both partial and complete demethylation, we included two elution steps for partially methylated DNA (450 mM and 600 mM NaCl).
RNA isolation, RT-qPCR, and gene expression.
Total RNA was isolated using a NucleoSpin RNA kit (Macherey-Nagel), and reverse transcription was preformed using a high-capacity cDNA reverse transcription kit (Applied Biosystems) and hexanucleotide random primers. The qPCR was performed using Fast SYBR green master mix (Applied Biosystems), and results were analyzed with a CFX96 Touch real-time PCR detection system (Bio-Rad). β-Actin was used as an internal control (reference gene) for mRNA expression. The primers used were as follows: β-actin (5′-TGCCATCCTAAAAGCCACCCCACTTCTCTC-3′ and 5′-AAGCAATGCTATCACCTCCCCTGTGTGGAC-3′), MUC13 (5′-CTGGCACCGCTTTATTGGA-3′ and 5′-ATTATCTGCACAGGGATCATCTTG-3′), PCDHB5 (5′-GCAGAAATTCGCCTGAAAAGG-3′ and 5′-CCCACCATCTGTGGCTACAA-3′), LGALS1 (5′-AACCTGGAGAGTGCCTTCGA-3′ and 5′-GCTGTCTTTGCCCAGGTTCA-3′), CD40 (5′-TCTCACCTCGCTATGGTTCGT-3′ and 5′-GCATGCAGTGGGTGGTTCT-3′), DUSP10 (5′-AAGAGGAGCGCCAGATGGT-3′ and 5′-GGCTGAAAACTGGCAATTCAA-3′), EHD3 (5′-CGACAACAAGCCCATGGTT-3′ and 5′-GCTCAGGCCCAATCCTCAT-3′), K8.1 (5′-AAAGCGTCCAGGCCACCACAGA-3′ and 5′-GGCAGAAAATGGCACACGGTTAC-3′), LANA (5′-GAAGTTGTAGGAAACGAAACAGGT-3′ and 5′-ACACTGTGGGACTTCCAGGTATAG-3′), ORF50 (5′-GCAGCCACAAAAATGGCGCAAGATG-3′ and 5′-GGAGCACACACTGGTAGAGTTGG-3′), and ORF 25 (5′-ACAGTTTATGGCACGCATAGTG-3′ and 5′-GGTTCTCTGAATCTCGTCGTGT-3′). All the reactions were run in triplicate.
RNA sequencing and analysis.
Total RNA was isolated as described above. rRNA was removed by an NEBNext rRNA depletion kit (E6310RNA; NEB), and then RNA was subjected to cDNA synthesis and library preparation with an NEBNext Ultra Directional RNA Library Prep kit for Illumina (E7420S; NEB) at the next-generation genomic center in the Azrieli Faculty of Medicine, Bar-Ilan University. The cDNA libraries were sequenced on an Illumina HiSeq2000 for a 50-base single read. Raw reads were aligned to the human genome 19 (hg19) build with TopHat (a splice junction mapper), and more than 86% reads were uniquely aligned to the reference genome (69). Mapped reads were used to estimate gene and transcript expression using the Cufflinks tool (70). Differential gene expression analysis with a false discovery rate (FDR) of ≤1% and fold change (FC) of ≥2 was performed using the Cuffdiff program of the Cufflinks package with default settings (71). To be more conservative and eliminate infinite fold differences in cases where there was a nil denominator, 0.01 was the default value when the read value was undetected.
Immunofluorescence assay.
Cells were seeded on poly-d-lysine (Sigma)-treated slides and incubated for 2 h and then washed with phosphate-buffered saline (PBS) and fixed with 4% paraformaldehyde for 10 min, permeabilized with 0.25% Triton X-100 in PBS for 10 min, and blocked with 1% bovine serum albumin (BSA) (with 22.52 mg/ml glycine) in PBS with Tween 20 (PBST). Slides were then incubated with rabbit anti-DUSP10 (PA5-30425; Thermo Fisher Scientific) and mouse anti-K8α (SAB5300152; Sigma) primary antibodies in 1% BSA in PBST, followed by incubation with anti-mouse Alexa 594 (ab150116; Abcam) and anti-rabbit Alexa 488 (ab150077; Abcam) secondary antibodies. LANA expression in BJAB219 cells was detected with mouse anti-LANA (NCL-HHV8-LNA; Novocastra) followed by anti-mouse Alexa 633 (SAB4600333; Sigma) secondary antibody. Alexa 633 emits fluorescence in the far-red spectrum and therefore can be distinguished from a red fluorescent protein (RFP) lytic marker. Cell nuclei were stained with 4′,6′-diamidino-2-phenylindole (DAPI; blue). Fluorescence was detected by an Imager.M2 (Zeiss) upright fluorescence microscope.
Western blot analysis.
Cells were lysed in 0.5 ml of radioimmunoprecipitation assay (RIPA) buffer (50 mM Tris [pH 7.4], 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS plus protease inhibitor cocktail [Thermo Fisher]), sonicated, and cleared by centrifugation. The antibodies used for Western blot analysis included rabbit anti-CD40 (ab224639; Abcam), rabbit anti-DUSP10 (PA5-30425; Thermo Fisher Scientific), rabbit anti-galectin-1/LGALS1 (ab154351; Abcam), rabbit anti-MUC13 (ab65109; Abcam), and mouse anti-β-actin (a1978; Sigma) antibodies.
Cell count and viability.
Cells were treated daily with 20 nM, 100 nM, and 500 nM concentrations of the demethylating agent 5-AZA-dC or vehicle (DMSO) for 4 days, during which cells were counted every 24 h via a hemocytometer to evaluate total cell count. Cell viability was determined at day 8 of the experiment (4 days of treatment followed by 4 additional days of incubation without drug) via a hemocytometer as the ratio between the number of live cells and the total number of cells counted by differential staining with trypan blue. To evaluate TSA cytotoxicity, cell viability was measured after 24 h of treatment with 300 nM TSA or vehicle (DMSO).
Data availability.
Global methylation analysis obtained from the HumanMethylation450K BeadChip and MethylationEpic BeadChip (Illumina) analyses and RNA-seq data are available at http://biodb.md.biu.ac.il/biu/shamay_lab_data.html.
Supplementary Material
ACKNOWLEDGMENTS
We thank Liat Linde and Nili Avidan for help in the analysis of the 450K and Epic BeadChip results, Jeffrey Vieira for providing the Vero-rKSHV.219 cells, Richard F. Ambinder and S. Diane Hayward for reagents, and S. Diane Hayward for critically reading the manuscript.
We are grateful for the support of the Elias, Genevieve and Georgianna Atol Charitable Trust to the Daniella Lee Casper Laboratory in Viral Oncology. This work was supported by grants from the Israel Science Foundation (https://www.isf.org.il) to M.S. (1134/16) and a Research Career Development Award from the Israel Cancer Research Fund (https://www.icrfonline.org/) to M.S. (01282).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JVI.00008-18.
REFERENCES
- 1.Henke-Gendo C, Schulz TF. 2004. Transmission and disease association of Kaposi's sarcoma-associated herpesvirus: recent developments. Curr Opin Infect Dis 17:53–57. doi: 10.1097/00001432-200402000-00011. [DOI] [PubMed] [Google Scholar]
- 2.Chang Y, Cesarman E, Pessin MS, Lee F, Culpepper J, Knowles DM, Moore PS. 1994. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 266:1865–1869. doi: 10.1126/science.7997879. [DOI] [PubMed] [Google Scholar]
- 3.Renne R, Lagunoff M, Zhong W, Ganem D. 1996. The size and conformation of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) DNA in infected cells and virions. J Virol 70:8151–8154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang J, Zhu L, Lu X, Feldman ER, Keyes LR, Wang Y, Fan H, Feng H, Xia Z, Sun J, Jiang T, Gao SJ, Tibbetts SA, Feng P. 2015. Recombinant murine gamma herpesvirus 68 carrying KSHV G protein-coupled receptor induces angiogenic lesions in mice. PLoS Pathog 11:e1005001. doi: 10.1371/journal.ppat.1005001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ganem D. 2006. KSHV infection and the pathogenesis of Kaposi's sarcoma. Annu Rev Pathol 1:273–296. doi: 10.1146/annurev.pathol.1.110304.100133. [DOI] [PubMed] [Google Scholar]
- 6.Klein U, Gloghini A, Gaidano G, Chadburn A, Cesarman E, Dalla-Favera R, Carbone A. 2003. Gene expression profile analysis of AIDS-related primary effusion lymphoma (PEL) suggests a plasmablastic derivation and identifies PEL-specific transcripts. Blood 101:4115–4121. doi: 10.1182/blood-2002-10-3090. [DOI] [PubMed] [Google Scholar]
- 7.Wang HW, Trotter MW, Lagos D, Bourboulia D, Henderson S, Makinen T, Elliman S, Flanagan AM, Alitalo K, Boshoff C. 2004. Kaposi sarcoma herpesvirus-induced cellular reprogramming contributes to the lymphatic endothelial gene expression in Kaposi sarcoma. Nat Genet 36:687–693. doi: 10.1038/ng1384. [DOI] [PubMed] [Google Scholar]
- 8.Viollet C, Davis DA, Tekeste SS, Reczko M, Ziegelbauer JM, Pezzella F, Ragoussis J, Yarchoan R. 2017. RNA sequencing reveals that Kaposi sarcoma-associated herpesvirus infection mimics hypoxia gene expression signature. PLoS Pathog 13:e1006143. doi: 10.1371/journal.ppat.1006143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poole LJ, Yu Y, Kim PS, Zheng QZ, Pevsner J, Hayward GS. 2002. Altered patterns of cellular gene expression in dermal microvascular endothelial cells infected with Kaposi's sarcoma-associated herpesvirus. J Virol 76:3395–3420. doi: 10.1128/JVI.76.7.3395-3420.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bestor TH. 2000. The DNA methyltransferases of mammals. Hum Mol Genet 9:2395–2402. doi: 10.1093/hmg/9.16.2395. [DOI] [PubMed] [Google Scholar]
- 11.Antequera F, Boyes J, Bird A. 1990. High levels of de novo methylation and altered chromatin structure at CpG islands in cell lines. Cell 62:503–514. doi: 10.1016/0092-8674(90)90015-7. [DOI] [PubMed] [Google Scholar]
- 12.Baylin SB. 2005. DNA methylation and gene silencing in cancer. Nat Clin Pract Oncol 2(Suppl 1):S4–S11. doi: 10.1038/ncponc0354. [DOI] [PubMed] [Google Scholar]
- 13.Goelz SE, Vogelstein B, Hamilton SR, Feinberg AP. 1985. Hypomethylation of DNA from benign and malignant human colon neoplasms. Science 228:187–190. doi: 10.1126/science.2579435. [DOI] [PubMed] [Google Scholar]
- 14.Hansen KD, Timp W, Bravo HC, Sabunciyan S, Langmead B, McDonald OG, Wen B, Wu H, Liu Y, Diep D, Briem E, Zhang K, Irizarry RA, Feinberg AP. 2011. Increased methylation variation in epigenetic domains across cancer types. Nat Genet 43:768–775. doi: 10.1038/ng.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shamay M, Krithivas A, Zhang J, Hayward SD. 2006. Recruitment of the de novo DNA methyltransferase Dnmt3a by Kaposi's sarcoma-associated herpesvirus LANA. Proc Natl Acad Sci U S A 103:14554–14559. doi: 10.1073/pnas.0604469103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu F, Stedman W, Yousef M, Renne R, Lieberman PM. 2010. Epigenetic regulation of Kaposi's Sarcoma-associated herpesvirus latency by virus-encoded microRNAs that target Rta and the cellular Rbl2-DNMT pathway. J Virol 84:2697–2706. doi: 10.1128/JVI.01997-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schlesinger Y, Straussman R, Keshet I, Farkash S, Hecht M, Zimmerman J, Eden E, Yakhini Z, Ben-Shushan E, Reubinoff BE, Bergman Y, Simon I, Cedar H. 2007. Polycomb-mediated methylation on Lys27 of histone H3 pre-marks genes for de novo methylation in cancer. Nat Genet 39:232–236. doi: 10.1038/ng1950. [DOI] [PubMed] [Google Scholar]
- 18.Günther T, Grundhoff A. 2010. The epigenetic landscape of latent Kaposi sarcoma-associated herpesvirus genomes. PLoS Pathog 6:e1000935. doi: 10.1371/journal.ppat.1000935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He M, Zhang W, Bakken T, Schutten M, Toth Z, Jung JU, Gill P, Cannon M, Gao S-J. 2012. Cancer angiogenesis induced by Kaposi sarcoma-associated herpesvirus is mediated by EZH2. Cancer Res 72:3582–3592. doi: 10.1158/0008-5472.CAN-11-2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Toth Z, Papp B, Brulois K, Choi YJ, Gao SJ, Jung JU. 2016. LANA-mediated recruitment of host polycomb repressive complexes onto the KSHV genome during de novo infection. PLoS Pathog 12:e1005878. doi: 10.1371/journal.ppat.1005878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tan B, Liu H, Zhang S, da Silva SR, Zhang L, Meng J, Cui X, Yuan H, Sorel O, Zhang SW, Huang Y, Gao SJ. 2018. Viral and cellular N6-methyladenosine and N6,2′-O-dimethyladenosine epitranscriptomes in the KSHV life cycle. Nat Microbiol 3:108–120. doi: 10.1038/s41564-017-0056-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen J, Ueda K, Sakakibara S, Okuno T, Parravicini C, Corbellino M, Yamanishi K. 2001. Activation of latent Kaposi's sarcoma-associated herpesvirus by demethylation of the promoter of the lytic transactivator. Proc Natl Acad Sci U S A 98:4119–4124. doi: 10.1073/pnas.051004198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shamay M, Hand N, Lemas MV, Koon HB, Krown SE, Wrangle J, Desai P, Ramos JC, Ambinder RF. 2012. CpG methylation as a tool to characterize cell-free Kaposi sarcoma herpesvirus DNA. J Infect Dis 205:1095–1099. doi: 10.1093/infdis/jis032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Platt G, Carbone A, Mittnacht S. 2002. p16INK4a loss and sensitivity in KSHV associated primary effusion lymphoma. Oncogene 21:1823–1831. doi: 10.1038/sj.onc.1205360. [DOI] [PubMed] [Google Scholar]
- 25.Di Bartolo DL, Cannon M, Liu Y-F, Renne R, Chadburn A, Boshoff C, Cesarman E. 2008. KSHV LANA inhibits TGF-β signaling through epigenetic silencing of the TGF-β type II receptor. Blood 111:4731–4740. doi: 10.1182/blood-2007-09-110544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun F, Xiao Y, Qu Z. 2015. Oncovirus Kaposi sarcoma herpesvirus (KSHV) represses tumor suppressor PDLIM2 to persistently activate nuclear factor kappaB (NF-κB) and STAT3 transcription factors for tumorigenesis and tumor maintenance. J Biol Chem 290:7362–7368. doi: 10.1074/jbc.C115.637918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bibikova M, Barnes B, Tsan C, Ho V, Klotzle B, Le JM, Delano D, Zhang L, Schroth GP, Gunderson KL, Fan JB, Shen R. 2011. High density DNA methylation array with single CpG site resolution. Genomics 98:288–295. doi: 10.1016/j.ygeno.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 28.Sandoval J, Heyn H, Moran S, Serra-Musach J, Pujana MA, Bibikova M, Esteller M. 2011. Validation of a DNA methylation microarray for 450,000 CpG sites in the human genome. Epigenetics 6:692–702. doi: 10.4161/epi.6.6.16196. [DOI] [PubMed] [Google Scholar]
- 29.Shen J, Wang S, Zhang YJ, Wu HC, Kibriya MG, Jasmine F, Ahsan H, Wu DP, Siegel AB, Remotti H, Santella RM. 2013. Exploring genome-wide DNA methylation profiles altered in hepatocellular carcinoma using Infinium HumanMethylation 450 BeadChips. Epigenetics 8:34–43. doi: 10.4161/epi.23062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kulis M, Merkel A, Heath S, Queiros AC, Schuyler RP, Castellano G, Beekman R, Raineri E, Esteve A, Clot G, Verdaguer-Dot N, Duran-Ferrer M, Russinol N, Vilarrasa-Blasi R, Ecker S, Pancaldi V, Rico D, Agueda L, Blanc J, Richardson D, Clarke L, Datta A, Pascual M, Agirre X, Prosper F, Alignani D, Paiva B, Caron G, Fest T, Muench MO, Fomin ME, Lee ST, Wiemels JL, Valencia A, Gut M, Flicek P, Stunnenberg HG, Siebert R, Kuppers R, Gut IG, Campo E, Martin-Subero JI. 2015. Whole-genome fingerprint of the DNA methylome during human B cell differentiation. Nat Genet 47:746–756. doi: 10.1038/ng.3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kent WJ, Sugnet CW, Furey TS, Roskin KM, Pringle TH, Zahler AM, Haussler D. 2002. The human genome browser at UCSC. Genome Res 12:996–1006. doi: 10.1101/gr.229102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keshet I, Lieman-Hurwitz J, Cedar H. 1986. DNA methylation affects the formation of active chromatin. Cell 44:535–543. doi: 10.1016/0092-8674(86)90263-1. [DOI] [PubMed] [Google Scholar]
- 33.Cedar H. 1988. DNA methylation and gene activity. Cell 53:3–4. doi: 10.1016/0092-8674(88)90479-5. [DOI] [PubMed] [Google Scholar]
- 34.Costello JF, Berger MS, Huang HS, Cavenee WK. 1996. Silencing of p16/CDKN2 expression in human gliomas by methylation and chromatin condensation. Cancer Res 56:2405–2410. [PubMed] [Google Scholar]
- 35.Cameron EE, Bachman KE, Myohanen S, Herman JG, Baylin SB. 1999. Synergy of demethylation and histone deacetylase inhibition in the re-expression of genes silenced in cancer. Nat Genet 21:103–107. doi: 10.1038/5047. [DOI] [PubMed] [Google Scholar]
- 36.Kirkin AF, Dzhandzhugazyan KN, Guldberg P, Fang JJ, Andersen RS, Dahl C, Mortensen J, Lundby T, Wagner A, Law I, Broholm H, Madsen L, Lundell-Ek C, Gjerstorff MF, Ditzel HJ, Jensen MR, Fischer W. 2018. Adoptive cancer immunotherapy using DNA-demethylated T helper cells as antigen-presenting cells. Nat Commun 9:785. doi: 10.1038/s41467-018-03217-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Han H, Cortez CC, Yang X, Nichols PW, Jones PA, Liang G. 2011. DNA methylation directly silences genes with non-CpG island promoters and establishes a nucleosome occupied promoter. Hum Mol Genet 20:4299–4310. doi: 10.1093/hmg/ddr356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Menafra R, Brinkman AB, Matarese F, Franci G, Bartels SJ, Nguyen L, Shimbo T, Wade PA, Hubner NC, Stunnenberg HG. 2014. Genome-wide binding of MBD2 reveals strong preference for highly methylated loci. PLoS One 9:e99603. doi: 10.1371/journal.pone.0099603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fraga MF, Ballestar E, Montoya G, Taysavang P, Wade PA, Esteller M. 2003. The affinity of different MBD proteins for a specific methylated locus depends on their intrinsic binding properties. Nucleic Acids Res 31:1765–1774. doi: 10.1093/nar/gkg249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. 2005. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A 102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsai HC, Li H, Van Neste L, Cai Y, Robert C, Rassool FV, Shin JJ, Harbom KM, Beaty R, Pappou E, Harris J, Yen RW, Ahuja N, Brock MV, Stearns V, Feller-Kopman D, Yarmus LB, Lin YC, Welm AL, Issa JP, Minn I, Matsui W, Jang YY, Sharkis SJ, Baylin SB, Zahnow CA. 2012. Transient low doses of DNA-demethylating agents exert durable antitumor effects on hematological and epithelial tumor cells. Cancer Cell 21:430–446. doi: 10.1016/j.ccr.2011.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Palii SS, Van Emburgh BO, Sankpal UT, Brown KD, Robertson KD. 2008. DNA methylation inhibitor 5-Aza-2′-deoxycytidine induces reversible genome-wide DNA damage that is distinctly influenced by DNA methyltransferases 1 and 3B. Mol Cell Biol 28:752–771. doi: 10.1128/MCB.01799-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schnekenburger M, Grandjenette C, Ghelfi J, Karius T, Foliguet B, Dicato M, Diederich M. 2011. Sustained exposure to the DNA demethylating agent, 2′-deoxy-5-azacytidine, leads to apoptotic cell death in chronic myeloid leukemia by promoting differentiation, senescence, and autophagy. Biochem Pharmacol 81:364–378. doi: 10.1016/j.bcp.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 44.Belinsky SA, Grimes MJ, Picchi MA, Mitchell HD, Stidley CA, Tesfaigzi Y, Channell MM, Liu Y, Casero RA Jr, Baylin SB, Reed MD, Tellez CS, March TH. 2011. Combination therapy with vidaza and entinostat suppresses tumor growth and reprograms the epigenome in an orthotopic lung cancer model. Cancer Res 71:454–462. doi: 10.1158/0008-5472.CAN-10-3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vieira J, O'Hearn PM. 2004. Use of the red fluorescent protein as a marker of Kaposi's sarcoma-associated herpesvirus lytic gene expression. Virology 325:225–240. doi: 10.1016/j.virol.2004.03.049. [DOI] [PubMed] [Google Scholar]
- 46.Szklarczyk D, Franceschini A, Wyder S, Forslund K, Heller D, Huerta-Cepas J, Simonovic M, Roth A, Santos A, Tsafou KP, Kuhn M, Bork P, Jensen LJ, von Mering C. 2015. STRING v10: protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res 43:D447–D452. doi: 10.1093/nar/gku1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang J, Zhou C, Jiang H, Liang L, Shi W, Zhang Q, Sun P, Xiang R, Wang Y, Yang S. 2017. ZEB1 induces ER-alpha promoter hypermethylation and confers antiestrogen resistance in breast cancer. Cell Death Dis 8:e2732. doi: 10.1038/cddis.2017.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gasperini P, Espigol-Frigole G, McCormick PJ, Salvucci O, Maric D, Uldrick TS, Polizzotto MN, Yarchoan R, Tosato G. 2012. Kaposi sarcoma herpesvirus promotes endothelial-to-mesenchymal transition through Notch-dependent signaling. Cancer Res 72:1157–1169. doi: 10.1158/0008-5472.CAN-11-3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paz A, Haklai R, Elad-Sfadia G, Ballan E, Kloog Y. 2001. Galectin-1 binds oncogenic H-Ras to mediate Ras membrane anchorage and cell transformation. Oncogene 20:7486–7493. doi: 10.1038/sj.onc.1204950. [DOI] [PubMed] [Google Scholar]
- 50.Croci DO, Salatino M, Rubinstein N, Cerliani JP, Cavallin LE, Leung HJ, Ouyang J, Ilarregui JM, Toscano MA, Domaica CI, Croci MC, Shipp MA, Mesri EA, Albini A, Rabinovich GA. 2012. Disrupting galectin-1 interactions with N-glycans suppresses hypoxia-driven angiogenesis and tumorigenesis in Kaposi's sarcoma. J Exp Med 209:1985–2000. doi: 10.1084/jem.20111665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Desmond JC, Raynaud S, Tung E, Hofmann WK, Haferlach T, Koeffler HP. 2007. Discovery of epigenetically silenced genes in acute myeloid leukemias. Leukemia 21:1026–1034. doi: 10.1038/sj.leu.2404611. [DOI] [PubMed] [Google Scholar]
- 52.Sung HY, Park AK, Ju W, Ahn J-H. 2014. Overexpression of mucin 13 due to promoter methylation promotes aggressive behavior in ovarian cancer cells. Yonsei Med J 55:1206–1213. doi: 10.3349/ymj.2014.55.5.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Satelli A, Rao US. 2011. Galectin-1 is silenced by promoter hypermethylation and its re-expression induces apoptosis in human colorectal cancer cells. Cancer Lett 301:38–46. doi: 10.1016/j.canlet.2010.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moarii M, Boeva V, Vert JP, Reyal F. 2015. Changes in correlation between promoter methylation and gene expression in cancer. BMC Genomics 16:873. doi: 10.1186/s12864-015-1994-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sproul D, Nestor C, Culley J, Dickson JH, Dixon JM, Harrison DJ, Meehan RR, Sims AH, Ramsahoye BH. 2011. Transcriptionally repressed genes become aberrantly methylated and distinguish tumors of different lineages in breast cancer. Proc Natl Acad Sci U S A 108:4364–4369. doi: 10.1073/pnas.1013224108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Steinbruck L, Gustems M, Medele S, Schulz TF, Lutter D, Hammerschmidt W. 2015. K1 and K15 of Kaposi's sarcoma-associated herpesvirus are partial functional homologues of latent membrane protein 2A of Epstein-Barr Virus. J Virol 89:7248–7261. doi: 10.1128/JVI.00839-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee BS, Alvarez X, Ishido S, Lackner AA, Jung JU. 2000. Inhibition of intracellular transport of B cell antigen receptor complexes by Kaposi's sarcoma-associated herpesvirus K1. J Exp Med 192:11–21. doi: 10.1084/jem.192.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pietrek M, Brinkmann MM, Glowacka I, Enlund A, Havemeier A, Dittrich-Breiholz O, Kracht M, Lewitzky M, Saksela K, Feller SM, Schulz TF. 2010. Role of the Kaposi's sarcoma-associated herpesvirus K15 SH3 binding site in inflammatory signaling and B-cell activation. J Virol 84:8231–8240. doi: 10.1128/JVI.01696-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Serquina AKP, Kambach DM, Sarker O, Ziegelbauer JM. 2017. Viral microRNAs repress the cholesterol pathway, and 25-hydroxycholesterol inhibits infection. mBio 8:e00576-. doi: 10.1128/mBio.00576-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fujimuro M, Wu FY, ApRhys C, Kajumbula H, Young DB, Hayward GS, Hayward SD. 2003. A novel viral mechanism for dysregulation of beta-catenin in Kaposi's sarcoma-associated herpesvirus latency. Nat Med 9:300–306. doi: 10.1038/nm829. [DOI] [PubMed] [Google Scholar]
- 61.Vire E, Brenner C, Deplus R, Blanchon L, Fraga M, Didelot C, Morey L, Van Eynde A, Bernard D, Vanderwinden JM, Bollen M, Esteller M, Di Croce L, de Launoit Y, Fuks F. 2006. The Polycomb group protein EZH2 directly controls DNA methylation. Nature 439:871–874. doi: 10.1038/nature04431. [DOI] [PubMed] [Google Scholar]
- 62.Toth Z, Maglinte DT, Lee SH, Lee HR, Wong LY, Brulois KF, Lee S, Buckley JD, Laird PW, Marquez VE, Jung JU. 2010. Epigenetic analysis of KSHV latent and lytic genomes. PLoS Pathog 6:e1001013. doi: 10.1371/journal.ppat.1001013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kantarjian H, Issa JP, Rosenfeld CS, Bennett JM, Albitar M, DiPersio J, Klimek V, Slack J, de Castro C, Ravandi F, Helmer R III, Shen L, Nimer SD, Leavitt R, Raza A, Saba H. 2006. Decitabine improves patient outcomes in myelodysplastic syndromes: results of a phase III randomized study. Cancer 106:1794–1803. doi: 10.1002/cncr.21792. [DOI] [PubMed] [Google Scholar]
- 64.How J, Minden MD, Brian L, Chen EX, Brandwein J, Schuh AC, Schimmer AD, Gupta V, Webster S, Degelder T, Haines P, Stayner LA, McGill S, Wang L, Piekarz R, Wong T, Siu LL, Espinoza-Delgado I, Holleran JL, Egorin MJ, Yee KW. 2015. A phase I trial of two sequence-specific schedules of decitabine and vorinostat in patients with acute myeloid leukemia. Leuk Lymphoma 56:2793–2802. doi: 10.3109/10428194.2015.1018248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pera B, Tang T, Marullo R, Yang SN, Ahn H, Patel J, Elstrom R, Ruan J, Furman R, Leonard J, Cerchietti L, Martin P. 2016. Combinatorial epigenetic therapy in diffuse large B cell lymphoma pre-clinical models and patients. Clin Epigenetics 8:79. doi: 10.1186/s13148-016-0245-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhao W, Mo Y, Wang S, Midorikawa K, Ma N, Hiraku Y, Oikawa S, Huang G, Zhang Z, Murata M, Takeuchi K. 2017. Quantitation of DNA methylation in Epstein-Barr virus-associated nasopharyngeal carcinoma by bisulfite amplicon sequencing. BMC Cancer 17:489. doi: 10.1186/s12885-017-3482-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hernandez-Vargas H, Gruffat H, Cros MP, Diederichs A, Sirand C, Vargas-Ayala RC, Jay A, Durand G, Le Calvez-Kelm F, Herceg Z, Manet E, Wild CP, Tommasino M, Accardi R. 2017. Viral driven epigenetic events alter the expression of cancer-related genes in Epstein-Barr-virus naturally infected Burkitt lymphoma cell lines. Sci Rep 7:5852. doi: 10.1038/s41598-017-05713-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Morris TJ, Butcher LM, Feber A, Teschendorff AE, Chakravarthy AR, Wojdacz TK, Beck S. 2014. ChAMP: 450k chip analysis methylation pipeline. Bioinformatics 30:428–430. doi: 10.1093/bioinformatics/btt684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim D, Pertea G, Trapnell C, Pimentel H, Kelley R, Salzberg SL. 2013. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol 14:R36. doi: 10.1186/gb-2013-14-4-r36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, van Baren MJ, Salzberg SL, Wold BJ, Pachter L. 2010. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol 28:511–515. doi: 10.1038/nbt.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley DR, Pimentel H, Salzberg SL, Rinn JL, Pachter L. 2012. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat Protoc 7:562–578. doi: 10.1038/nprot.2012.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hodges E, Molaro A, Dos Santos CO, Thekkat P, Song Q, Uren PJ, Park J, Butler J, Rafii S, McCombie WR, Smith AD, Hannon GJ. 2011. Directional DNA methylation changes and complex intermediate states accompany lineage specificity in the adult hematopoietic compartment. Mol Cell 44:17–28. doi: 10.1016/j.molcel.2011.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Global methylation analysis obtained from the HumanMethylation450K BeadChip and MethylationEpic BeadChip (Illumina) analyses and RNA-seq data are available at http://biodb.md.biu.ac.il/biu/shamay_lab_data.html.