Efficient induction of interferon-stimulated genes (ISGs) prior to infection is known to effectively convert a cell into an antiviral state, blocking viral replication. Additionally, cells can undergo caspase-mediated apoptosis to control viral infection. Here, we identify SMARCA2 and SMARCA4 to be essential for the efficient induction of ISGs but also to be targeted by cellular caspases downstream of the intrinsic apoptotic pathway. We find that C-terminally cleaved SMARCA2 and SMARCA4 accumulate at late stages of infection, when cell damage already had occurred. Cleavage of the C terminus removes domains important for nuclear localization and chromatin binding of SMARCA2 and SMARCA4. Consequently, the cleaved forms are unable to efficiently accumulate in the cell nucleus. Intriguingly, the remaining nuclear C-terminally truncated SMARCA2 still induced ISG expression, although to lower levels. These data suggest that in virus-infected cells caspase-mediated cell death does not completely inactivate the SMARCA2- and SMARCA4-dependent interferon signaling pathway.
KEYWORDS: virus infection, chromatin remodeler, caspase, apoptosis, chromatin remodeling
ABSTRACT
The BAF-chromatin remodeling complex, with its mutually exclusive ATPases SMARCA2 and SMARCA4, is essential for the transcriptional activation of numerous genes, including a subset of interferon-stimulated genes (ISGs). Here, we show that C-terminally truncated forms of both SMARCA2 and SMARCA4 accumulate in cells infected with different RNA or DNA viruses. The levels of truncated SMARCA2 or SMARCA4 strongly correlate with the degree of cell damage and death observed after virus infection. The use of a pan-caspase inhibitor and genetically modified cell lines unable to undergo apoptosis revealed that the truncated forms result from the activity of caspases downstream of the activated intrinsic apoptotic pathway. C-terminally cleaved SMARCA2 and SMARCA4 lack potential nuclear localization signals as well as the bromo- and SnAC domain, with the latter two domains believed to be essential for chromatin association and remodeling. Consistent with this belief, C-terminally truncated SMARCA2 was partially relocated to the cytoplasm. However, the remaining nuclear protein was sufficient to induce ISG expression and inhibit the replication of vesicular stomatitis virus and influenza A virus. This suggests that virus-induced apoptosis does not occur at the expense of an intact interferon-mediated antiviral response pathway.
IMPORTANCE Efficient induction of interferon-stimulated genes (ISGs) prior to infection is known to effectively convert a cell into an antiviral state, blocking viral replication. Additionally, cells can undergo caspase-mediated apoptosis to control viral infection. Here, we identify SMARCA2 and SMARCA4 to be essential for the efficient induction of ISGs but also to be targeted by cellular caspases downstream of the intrinsic apoptotic pathway. We find that C-terminally cleaved SMARCA2 and SMARCA4 accumulate at late stages of infection, when cell damage already had occurred. Cleavage of the C terminus removes domains important for nuclear localization and chromatin binding of SMARCA2 and SMARCA4. Consequently, the cleaved forms are unable to efficiently accumulate in the cell nucleus. Intriguingly, the remaining nuclear C-terminally truncated SMARCA2 still induced ISG expression, although to lower levels. These data suggest that in virus-infected cells caspase-mediated cell death does not completely inactivate the SMARCA2- and SMARCA4-dependent interferon signaling pathway.
INTRODUCTION
Type I and III interferons (IFNs) upregulate hundreds of interferon-stimulated genes (ISGs), thereby establishing an antiviral state of the cell. ISG induction is regulated by specific transcription factors and other cofactors, including the multisubunit chromatin-remodeling BAF complex (also known as SWI/SNF-complex) (1–4). Within its active center, this complex contains either the ATPase subunit SMARCA2 (SWI/SNF-related, matrix-associated, actin-dependent regulator of chromatin, subfamily a, member 2) or SMARCA4 (SWI/SNF-related, matrix-associated, actin-dependent regulator of chromatin, subfamily a, member 4) (1). In order to fulfill its chromatin remodeling function, the BAF complex repositions nucleosomes in an energy-dependent manner, rendering promoter regions accessible for the initiation of transcription (1). To this end, the bromodomain within the C-terminal region of SMARCA2 or SMARCA4 binds to acetylated histones and mediates the correct positioning of the whole BAF complex to DNA (5). This interaction is further supported by the adjacent Snf2-ATP coupling (SnAC) domain (6). Interestingly, SMARCA2 was shown to be particularly important for the full activity of the antiviral MxA protein, as SMARCA2 was responsible for the upregulation of a set of MxA cofactors (3).
Viral replication and dissemination can also be prevented by the induction of programmed cell death, including apoptosis (7, 8). Apoptotic cell death can be divided into an extrinsic death receptor-mediated and intrinsic mitochondrial pathway (9). The latter pathway is characterized by oligomerization of the proapoptotic Bcl-2-family members Bax and Bak within the outer mitochondrial membrane, which eventually leads to its permeabilization (10). Following permeabilization of the outer mitochondrial membrane (MOMP), cytochrome c is released from the mitochondrial intermembrane space into the cytoplasm, where it is bound by apoptotic protease activating factor 1 (Apaf-1), which, together with procaspase-9, initiates the assembly of a multimeric complex called the apoptosome (10). The activated initiator caspase-9 processes the effector caspase-3, -6, and -7 (11, 12), leading to cleavage of protein substrates and cell death (10). During apoptosis, characteristic and drastic morphological cell changes can be observed, including chromosomal DNA fragmentation, cell shrinkage, and membrane blebbing, which lead to the formation of apoptotic bodies that are removed by phagocytes (13).
Although viral replication is controlled by the IFN-mediated induction of ISGs and programmed cell death, very little is known about the interplay of the two antiviral strategies. Here, we provide evidence that both SMARCA2 and SMARCA4, required for efficient induction of ISGs, are targets of active caspases acting downstream of the intrinsic apoptotic pathway. Caspase cleavage occurs at the C terminus, leading to the loss of both the bromodomain and the SnAC domain, but does not completely abrogate chromatin remodeling activity.
RESULTS
Viral infection results in C-terminal truncation of the chromatin-remodeling ATPases SMARCA2 and SMARCA4.
A previous study (14) described potentially cleaved forms of SMARCA2 and SMARCA4 in apoptotic cells. This suggests that these proteins are also caspase substrates during virus-induced apoptosis. Indeed, following infection of HeLa cells with influenza A/seal/Massachusetts/1/1980 (SC35M) at a multiplicity of infection (MOI) of 1 for 24 h, shorter forms of SMARCA2 and SMARCA4 proteins were detected using antibodies specifically recognizing their amino (N) termini (Fig. 1A). Detection of both full-length and shorter forms of SMARCA2 and SMARCA4 was specific, since no signals were observed in SC35M-infected HeLa cells after short interfering RNA (siRNA)-mediated silencing of SMARCA2 or SMARCA4 (Fig. 1B). To investigate whether the accumulation of shorter forms of SMARCA2 or SMARCA4 is a general feature following viral infection, HeLa cells were infected with different RNA and DNA viruses for 24 h at an MOI of 1. Common to all viral infections, we observed SMARCA2 or SMARCA4 with lower molecular weights, albeit to different extents (Fig. 1A). While in most virus-infected cells the full-length SMARCA forms dominated, infection with New Castle disease virus (NDV), La Crosse virus (LACV), and Semliki Forest virus (SFV) led to an almost complete loss of the full-length proteins but persistence of the shorter fragments (Fig. 1A, lanes 18, 20, and 21). Furthermore, transfection of poly(I·C) (1 μg/ml), a double-stranded RNA (dsRNA) analog, likewise resulted in the accumulation of shortened SMARCA2 and SMARCA4 (Fig. 1A, lane 24), whereas stimulation of HeLa cells with IFN-α (1,000 U/ml) had no effect compared to results for uninfected control cells (Fig. 1A, lanes 1 and 2).
FIG 1.
SMARCA2 and SMARCA4 levels in virus-infected cells or after poly(I·C) or staurosporine treatment. (A) HeLa cells were either infected with the indicated viruses (MOI of 1, except for measles [0.5] and A/KAN-1 [0.1]), treated with poly(I·C) (1 μg/ml), IFN-α (1,000 U/ml), or staurosporine (1 μM), or left untreated. After 24 h, cells were lysed and subjected to Western blot analysis using SMARCA2- or SMARCA4-specific antibodies directed against the C (αC-term) or N (αN-term) terminus. Actin served as a loading control. THOV, Thogoto virus; DHOV, Dhori virus; VSV, vesicular stomatitis virus; SeV, Sendai virus; NDV, Newcastle disease virus; RVFV, Rift Valley fever virus; LACV, La Crosse virus; SFV, Semliki Forest virus; HSV-1, herpes simplex virus; MHV68, murine gammaherpesvirus. (B) HeLa cells were transfected with siRNAs targeting either SMARCA2, SMARCA4, or a nontargeting (NT) control siRNA for 72 h and subsequently infected with SC35M. At 24 h postinfection, cells were lysed and subjected to Western blot analysis as described for panel A. (C) HeLa cells were infected with SC35M or NDV, treated with staurosporine as described for panel A or left untreated for the indicated time points, and subjected to Western blot analysis as described for panel A. p.i., postinfection; p.t., posttransfection.
Based on the molecular weight, the shorter forms of SMARCA2 and SMARCA4 could not be assigned to any previously described protein-coding splice variants (15 and UniProtKB accession numbers P51531 [SMCA2_HUMAN] and P51532 [SMCA4_HUMAN]), and we therefore speculated that these shorter forms represent proteolytic cleavage products. To address this, we used antibodies specifically targeting the carboxy (C) terminus of SMARCA2 or SMARCA4 (Fig. 2A depicts antibody binding sites). Indeed, as anticipated for a proteolytic cleavage of the C terminus, these antibodies recognized full-length SMARCA2 or SMARCA4, while the shorter fragments could not be detected (Fig. 1A). Interestingly, in NDV-infected cells, truncated SMARCA2 or SMARCA4 was present already after 8 h, whereas in SC35M-infected cells the shorter fragments appeared after 24 h (Fig. 1C). This markedly correlated with an enhanced cytopathic effect in NDV-infected cells (Fig. 2). Apoptosis triggered by staurosporine, a mitochondrial apoptosis activator, likewise resulted in the appearance of the truncated forms of SMARCA2 and SMARCA4. Together, these data suggest that cell death triggered by either virus infection or other stimuli is accompanied by the accumulation of C-terminally truncated ATPases SMARCA2 and SMARCA4.
FIG 2.
Truncations of SMARCA2 and SMARCA4 result from caspase-mediated cleavage. (A) Domain structure of SMARCA2 and SMARCA4. Antibody binding sites within the N or C terminus of SMARCA2 or SMARCA4 are indicated. The filled arrowhead indicates the caspase cleavage site in SMARCA2. Open arrowheads highlight the predicted nuclear localization signals in SMARCA2, termed NLS1 and NLS2, respectively. QLQ, Gln, Leu, Gln motif; HSA, helicase/SANT-associated domain; BRK, Brahma and Kismet domain; DEXDc, DEAD-like helicase superfamily domain; SNF2_N, SNF2 family N-terminal domain; HELICc, helicase superfamily C-terminal domain; SnAC, Snf2-ATP coupling, chromatin remodeling complex; Bromo, bromodomain. (B) HeLa cells were either pretreated with the pan-caspase inhibitor QVD (10 μM) for 30 min or left untreated (control) and either infected with the indicated viruses (MOI of 1) or treated with IFN-α (1,000 U/ml), poly(I·C) (1 μg/ml), or staurosporine (Stauro; 1 μM) for 24 h while keeping the respective cells under QVD treatment. Samples were subjected to Western blot analysis using SMARCA2- or SMARCA4-specific antibodies directed against the C (αC-term) or N (αN-term) terminus highlighted in panel A. Actin served as a loading control. (C) Images of HeLa cells were obtained 24 h posttreatment as described for panel B. Scale bar, 50 μm.
SMARCA2 and SMARCA4 are cleaved by caspases downstream of the activated intrinsic apoptotic pathway.
As our data suggested that the cleavage of SMARCA2 and SMARCA4 is related to apoptosis, we speculated that caspases were responsible for their cleavage in virus-infected cells. To challenge this hypothesis, HeLa cells were treated with the pan-caspase inhibitor QVD (Q-VD-OPh) (16) prior to infection with SC35M or NDV. As expected, QVD pretreatment prevented SMARCA2 and SMARCA4 cleavage in both SC35M- and NDV-infected cells (Fig. 2B, lanes 10 and 11). Similarly, treatment of HeLa cells with QVD blocked the staurosporine-induced cleavage of SMARCA2 and SMARCA4 (Fig. 2B, lane 12). In all cases QVD treatment markedly reduced the cell death triggered by the different treatments (Fig. 2C). Together these findings suggest that the observed truncations of SMARCA2 and SMARCA4 are a result of caspase-mediated cleavage.
While Bax and Bak are crucial mediators of mitochondrial outer membrane permeabilization (MOMP) and thus control cytochrome c release, Bcl-XL in turn inhibits Bax/Bak function, thereby preventing MOMP (10). To test whether caspases downstream of the intrinsic apoptotic pathway are responsible for SMARCA2 and SMARCA4 cleavage, HeLa Bax/Bak double knockout cells (Bax/Bak dKO) as well as HeLa cells stably overexpressing the antiapoptotic murine Bcl-XL protein (Bcl-XL) were generated (Fig. 3A and B). Cells were NDV infected (MOI of 1), staurosporine treated (1 μM), type I IFN stimulated (1,000 U/ml), or left untreated. In contrast to control cells, cleavage of SMARCA2 and SMARCA4 was completely absent from Bax/Bak dKO or Bcl-XL cells after NDV infection or staurosporine treatment (Fig. 3C, lanes 7, 8, 11, and 12). Furthermore, Bax/Bak dKO or Bcl-XL cells did not show any obvious signs of cytopathic effects after NDV infection or staurosporine treatment compared to control cells (Fig. 3D). In summary, these data indicate that SMARCA2 and SMARCA4 cleavage is mediated by caspases, which are activated during the intrinsic apoptotic pathway.
FIG 3.
Caspases activated downstream of the intrinsic apoptotic pathway are responsible for cleavage of SMARCA2. (A) Bax, Bak, or Bax/Bak CRISPR-Cas9-mediated knockout (KO) HeLa cells were analyzed by Western blot analysis using specific antibodies directed against Bax (αBax) or Bak (αBak). GAPDH served as a loading control. (B) Flow cytometry analysis of intracellularly stained Bcl-XL in HeLa cells (control) and HeLa cells overexpressing Bcl-XL. (C) HeLa cells (control), Bax/Bak knockout cells (dKO), or Bcl-XL cells were stimulated with IFN-α (1,000 U/ml), NDV infected (MOI of 1), or staurosporine treated (1 μM) for 24 h and subjected to Western blot analysis using SMARCA2- or SMARCA4-specific antibodies directed against the N termini (αN-term) or an NDV-specific NP antibody. Actin served as a loading control. (D) Images of HeLa cells were obtained 24 h posttreatment as described for panel C. Scale bar, 50 μm.
Aspartate at position 1318 is the caspase cleavage site of SMARCA2.
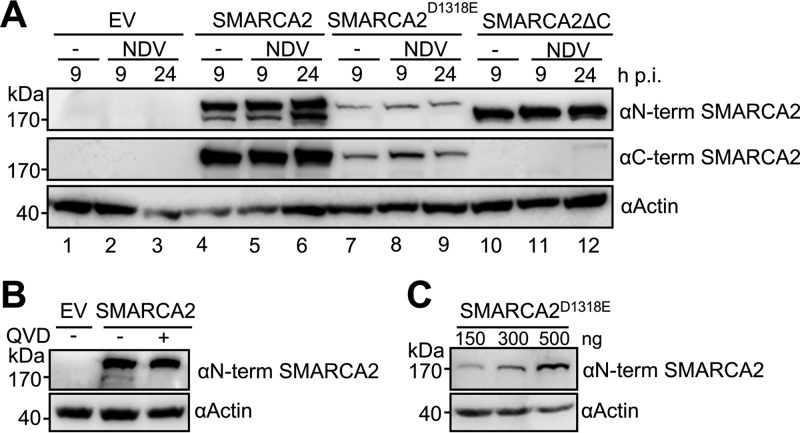
Focusing on SMARCA2, we wanted to identify the possible caspase cleavage site using two prediction tools, CaspDB and Cascleave (17, 18) (Table 1). Considering the high molecular weight of cleaved SMARCA2 and the highest cleavage score obtained with both prediction tools, we assumed that the aspartate at position 1318 (D1318) represents the possible caspase cleavage site (Fig. 2A, closed arrowhead). Using SW-13 cells we transiently overexpressed SMARCA2 or SMARCA2D1318E in which the aspartate at position 1318 was replaced by glutamate (E), thereby preventing caspase cleavage at this site. SW-13 cells lack SMARCA2 and SMARCA4 (19), facilitating the detection of wild-type (wt) and mutant SMARCA2 protein. We additionally cotransfected a C-terminal truncation mutant of SMARCA2 (SMARCA2ΔC), comprising the amino acids 1 to 1318, as a control for cleaved SMARCA2. Following transient expression of the SMARCA2 variants for 24 h, cells were subsequently infected with NDV at an MOI of 1 (or left uninfected) for a further 9 or 24 h (Fig. 4A). To our surprise, cleavage of SMARCA2 could already be observed without virus infection, while NDV infection did not considerably enhance SMARCA2 cleavage (Fig. 4A). Pretreatment with the pan-caspase inhibitor QVD prevented SMARCA2 cleavage (Fig. 4B). As expected, the cleaved form of SMARCA2 and the truncation mutant SMARCA2ΔC were of similar molecular weights, whereas SMARCA2D1318E remained uncleaved after NDV infection (Fig. 4A). Despite the overall lower levels of SMARCA2D1318E compared to those for SMARCA2, transfection of higher concentrations of SMARCA2D1318E expression plasmids did not result in detectable cleavage products (Fig. 4C). These results suggest that the aspartate at position 1318 with the adjacent amino acid motif is the target site of caspase-mediated cleavage of SMARCA2.
TABLE 1.
Predicted caspase cleavage sites of SMARCA2
| Caspase cleavage position | Cleavage scorea |
Cleavage siteb | Predicted molecular wt of resulting protein (in kDa) | |
|---|---|---|---|---|
| CaspDB | Cascleave | |||
| 78 | 0.906 | 0.569 | KPID*GIHD | 8 |
| 618 | 0.811 | 0.763 | SQLD*AWLE | 68 |
| 639 | 0.759 | 0.644 | EESD*SDYE | 70 |
| 673 | 0.884 | 0.730 | SEKD*AKQI | 74 |
| 687 | 0.451 | 0.689 | DVDD*EYSM | 76 |
| 925 | 0.823 | 0.540 | ERVD*LNEE | 104 |
| 990 | 0.994 | 0.779 | LLTD*GSEK | 111 |
| 1097 | 0.891 | 0.665 | LRLD*GTTK | 124 |
| 1318 | 0.987 | 0.910 | DYSD*ALTE | 150 |
| 1571 | 0.671 | 0.553 | SDFD*SDEE | 179 |
Cleavage scores are only listed if the corresponding cleavage site was predicted with CaspDB and Cascleave.
Cleavages sites are indicated by asterisks.
FIG 4.
SMARCA2 is cleaved at position 1318. (A) SW-13 cells were transfected with 300 ng of expression plasmid (pCAGGS) encoding SMARCA2, SMARCA2D1318E, SMARCA2ΔC, or an empty vector control (EV) and at 24 h posttransfection (p.t.) were infected with NDV (MOI of 1) or left uninfected. At the indicated time points cells were harvested and subjected to Western blot analysis using SMARCA2-specific antibodies directed against the N (αN-term) or C (αC-term) terminus. (B) SW-13 cells were transfected as described for panel A and treated with the pan-caspase inhibitor QVD (10 μM) 1 h p.t. or left untreated. Cells were lysed 24 h later and subjected to Western blot analysis using an SMARCA2-specific antibody directed against the N terminus (αN-term). Actin served as a loading control. (C) SW-13 cells were transfected with increasing amounts of expression plasmid (pCAGGS) encoding SMARCA2D1318E, harvested 48 h p.t., and subjected to Western blot analysis as described for panel B.
Truncated SMARCA2 partially relocalizes from the nucleus to the cytoplasm.
Using cNLS Mapper (20), two nuclear localization signals (NLSs) of various lengths were identified in the N- and the C-terminal regions of SMARCA2 (Fig. 2A, open arrowheads), with a very high score for the latter NLS (Table 2). Accordingly, we hypothesized that the truncated form of SMARCA2 would partially localize to the cytoplasm. Indeed, in 40% of all analyzed SW-13 cells, SMARCA2ΔC was confined to the cytoplasm, whereas wt SMARCA2 was predominantly localized to the nucleus (Fig. 5A to C). Thus, the NLS located in the C-terminal domain of SMARCA2 is required for efficient nuclear localization.
TABLE 2.
Predicted NLSs of SMARCA2
| NLS region and position | Sequence | Score |
|---|---|---|
| 1 | ||
| 555–563 | EKKKRRRRK | 5 |
| 555–564 | EKKKRRRRKK | 8 |
| 559–568 | RRRRKKKAEE | 6 |
| 2 | ||
| 1341–1349 | VRLKKRKRR | 6 |
| 1342–1350 | RLKKRKRRR | 10 |
| 1342–1352 | RLKKRKRRRNV | 12.5 |
| 1344–1353 | KKRKRRRNVD | 16 |
FIG 5.
C-terminally truncated SMARCA2 accumulates in the cytoplasm. (A) Confocal microscopy analysis of the intracellular localization of SMARCA2 or SMARCA2ΔC. SW-13 cells were transfected with 500 ng pCAGGS expression plasmid encoding SMARCA2 or SMARCA2ΔC. At 48 h posttransfection cells were fixed, permeabilized, and stained for SMARCA2 using an N terminus-specific antibody (red). Cell nucleus was visualized with DAPI staining (blue). Scale bar, 50 μm. (B) Quantification of the intracellular distribution of SMARCA2 (ctrl.) or SMARCA2ΔC present either in the nucleus (gray) or in both the nucleus and the cytoplasm (white). Shown are the means and standard deviations from three independent experiments. (C, left) Representative images of the intracellular distribution of SMARCA2 or SMARCA2ΔC (red) and DAPI (blue). Scale bar, 10 μm. (Right) Intensity profiles along the white arrows shown in the left panels. The distance covered along the arrow is indicated on the x axis. a.u., arbitrary units.
The C-terminal part of SMARCA2 is not essential for expression of ISGs in SW-13 cells.
The C termini of SMARCA2 and SMARCA4 comprise a bromodomain, which recognizes acetylated histones, thereby positioning their respective ATPase subunits close to DNA (21), an interaction that is further stabilized by the SnAC domain (6). Subsequently, the helicase part of the ATPase subunit repositions or disassembles nucleosomes to render specific promoter regions accessible for transcriptional initiation. Consistent with this, overexpression of SMARCA2 and SMARCA4 in SW-13 cells was shown to boost the expression of a certain subset of ISGs, including the interferon-inducible transmembrane protein 2 (IFITM2) and IFITM3 (2, 4). Additionally, global analyses of SMARCA2 and SMARCA4 activity during viral infection revealed their importance in IFN signaling and also their impact on broader gene regulation (22, 23). To find out whether SMARCA2ΔC lacking both bromo- and SnAC domains still activates ISG expression, we transiently overexpressed either SMARCA2ΔC or SMARCA2 in SW-13 cells for 48 h and compared the mRNA transcriptome to that of SW-13 cells transfected with a vector control. Interestingly, we observed a robust (>2-fold) induction of mainly ISGs but also other mRNA transcripts by both SMARCA2 and SMARCA2ΔC (Fig. 6A; see also Table S1 in the supplemental material). However, this induction was less pronounced in SMARCA2ΔC-transfected cells than in cells transfected with SMARCA2 (Fig. 6B). Interestingly, 10 genes were exclusively induced by SMARCA2 but not SMARCA2ΔC (Fig. 6A and Table S1). Performing quantitative RT-PCR (qRT-PCR), we confirmed the transcriptome data for a selection of factors, previously described to be SMARCA2 or SMARCA4 regulated (2, 4, 22, 23) (Fig. 6C). These results indicate that the C terminus of SMARCA2 is not required for the induction of most ISGs in SW-13 cells.
FIG 6.
Induction of ISGs is less robust in SW-13 cells expressing C-terminally truncated SMARCA2. The overall gene expression profile of SW-13 cells expressing either C-terminally truncated SMARCA2 (SMARCA2ΔC) or full-length SMARCA2 was compared to that of an empty vector control (EV) using RNA sequencing (A and B) and validated by qRT-PCR (C). (A) The Venn diagram illustrates the number of significantly differently expressed (DE) genes for each treatment constellation. Both SMARCA2ΔC (red) and full-length SMARCA2 (blue) share 161 DE genes compared to an empty vector control. From these, only 7 are DE between SMARCA2ΔC and full-length SMARCA2 (green). However, 103 genes are solely differently expressed in full-length SMARCA2-expressing SW-13 cells. (B) Most of the DE genes are upregulated compared to the empty vector control, as indicated by the volcano plot. The log2 fold change is plotted on the x axis and the adjusted P value on the y axis. Each point represents a DE gene of either SMARCA2ΔC (red) or full-length SMARCA2 (blue) that falls within the thresholds of a P value of <0.001 and fold change of >2 (dotted lines). (C) The results of RNA sequencing were validated for 7 ISGs using qRT-PCR. Except for IFITM2, all tested genes presented comparable log2 fold changes in RNA sequencing (gray bars) and qRT-PCR (white bars). (D) Virus replication was determined in SW-13 cells transfected with SMARCA2, SMARCA2ΔC, or empty vector (EV) and infected with either VSV-GFP or SC35M-GFP. All data were normalized to the respective EV control, which was set to 100%. Rel., relative. Three or four independent experiments were performed for SC35M-GFP or VSV-GFP, respectively.
We next determined viral growth of VSV or influenza A virus in SW-13 cells transiently transfected with either SMARCA2ΔC, SMARCA2, or a vector control. Replication of VSV was inhibited equally well by both SMARCA2ΔC and SMARCA2 compared to that of cells transfected with vector control (Fig. 6D). Similarly, SMARCA2ΔC restricted influenza A virus replication, although less robustly than SMARCA2. Together, these data suggest that C-terminal truncation of SMARCA2 only partially abrogates its activity to induce antivirally active ISGs.
DISCUSSION
Several lines of evidence suggest that SMARCA2 and SMARCA4 are proteolytically cleaved by caspases downstream of the activated intrinsic apoptotic pathway. For instance, pretreatment of infected HeLa cells with the pan-caspase inhibitor QVD prevented the appearance of the shorter SMARCA2 and SMARCA4 variants (Fig. 2B). Furthermore, cleavage was not observed in Bax/Bak dKO or Bcl-XL cells, which are unable to undergo mitochondrial apoptosis, indicating that SMARCA2 and SMARCA4 are cleaved by caspases following mitochondrial outer membrane permeabilization (Fig. 3C). Consistent with this finding, the identified caspase cleavage motif within SMARCA2 (DYSD*ALTE) is a bona fide cleavage site recognized by the downstream effector caspase-3 (24–27).
Interestingly, transient expression of SMARCA2 in SW-13 cells, devoid of endogenous SMARCA2 and SMARCA4, was sufficient to initiate cleavage of this protein (Fig. 4A). Since many cancer cell lines downregulate SMARCA2 and SMARCA4 to maintain proliferation and survival (19), the prompt caspase-mediated cleavage might be an intrinsic feature of this cell line to functionally disable these ATPases for sustained proliferation. The suspected need to disable SMARCA2 function in SW-13 cells might also explain why we were not able to express the noncleavable form of SMARCA2 (SMARCA2D1318E) at the same level as the wild-type protein (Fig. 4C).
Caspase-mediated cleavage of SMARCA2 or SMARCA4 at the C terminus leads to the loss of the bromo- and SnAC domains. The bromodomain in particular is believed to be crucial to attach the BAF complex to acetylated histones prior to chromatin remodeling (21). In contrast, SMARCA2 lacking these domains is still able to upregulate a great proportion of ISGs (Fig. 6A). These results are consistent with the observation made by Vangamudi and colleagues (28), who showed that a functional bromodomain of SMARCA2/4 is dispensable for cell proliferation, i.e., gene expression. However, the bromo- and SnAC domains seem to be required for optimal SMARCA2-mediated gene activation (Fig. 6B), suggesting that efficient gene induction relies on binding to acetylated histones. In line with this, a bromodomain deletion mutant of Swi2/Snf2, the SMARCA2/4 homolog in Saccharomyces cerevisiae, exhibited only ∼50% binding and remodeling efficiency on H3- and H4-acetylated nucleosomes compared to wild-type protein (29).
This partial inactivation of the caspase-cleaved chromatin remodeler also might be caused by the loss of the C-terminally localized NLS required for efficient nuclear accumulation of wt SMARCA2 (Fig. 5). Interestingly, a single-nucleotide polymorphism (SNP) in the C-terminal region of SMARCA2 was identified in a Japanese cohort of schizophrenia patients that resulted in a missense polymorphism (30). This SNP led to increased cytoplasmic localization of SMARCA2 of about 30% in human glioblastoma cells. Since the missense mutation was not located within the NLS but downstream of the bromodomain, the underlying mechanism that leads to enhanced cytoplasmic accumulation of SMARCA2 remains unclear.
For the vast majority of cellular proteins cleaved by apoptotic caspases (17), the functional consequence of cleavage is only poorly understood. However, one prominent example is the DNA repair protein PARP, for which it is believed that the smaller 24-kDa fragment, present after cleavage, irreversibly attaches to damaged DNA (31). Acting in a dominant-negative way, it renders the DNA inaccessible for full-length PARP and repair mechanisms, thereby promoting apoptosis (32, 33). Thus, considering their role in chromatin remodeling, it remains to be shown if the absence of the bromo- as well as the SnAC domain of the caspase-cleaved SMARCA2 and SMARCA4 contribute to chromatin condensation, a prerequisite for nuclear breakdown and fragmentation during apoptosis (9, 34).
Together, our data suggest that during virus-induced apoptosis, caspase-mediated cleavage of SMARCA2 and SMARCA4 does not result in the complete inactivation of these chromatin remodelers, which are essential for an intact interferon-mediated antiviral response.
MATERIALS AND METHODS
Cell culture and generation of transgenic and KO HeLa cell lines.
HeLa and SW-13 cells were maintained in Dulbecco's modified Eagle's medium (DMEM) complemented with 10% fetal calf serum (FCS), 2% glutamine, and 1% penicillin-streptomycin at 37°C and 5% CO2.
HeLa cells overexpressing murine Bcl-XL were established by lentiviral transduction with the pEF1α-GWPuro-mBcl-XL construct in which Bcl-XL had been placed under the control of the pEF1a-promoter, followed by puromycin selection (1 μg/ml) 48 h postransduction. Cell lines were established as pools and expression of Bcl-XL was tested by flow cytometry (antibodies were anti-Bcl-XL [2764; Cell Signaling] and DyLight 649-conjugated secondary antibody [705-495-147; Dianova]).
HeLa Bak/Bax dKO cells were generated using CRISPR/Cas9. Guide RNAs (gRNAs) targeting human BAK1 (hBak; guide#1 [internal number], 5′-ACGGCAGCTCGCCATCATCG-3′) and human BAX (hBax; guide#1 [internal number], 5′-CAAGCGCATCGGGGACGAAC-3′) were designed using a web-based server (http://crispr.mit.edu/). gRNAs were cloned into LentiCRISPR v2 (Addgene plasmid 52961 [Feng Zhang]). An empty LentiCRISPR v2 served as a control. For lentivirus production, 293T cells were transfected with corresponding LentiCRISPR v2 vectors and packaging vectors psPAX.2 and psMD2.G (Addgene plasmids 12260 and 12259 [Didier Trono]) using FuGene HD transfection (Promega, USA) by following the manufacturer's instructions. After 48 h, virus-containing supernatant was harvested and filtered. HeLa cells were transduced with lentiviruses containing gRNA against BAK1 and BAX in the presence of 1 μg/ml of Polybrene (TR-1003-G; Millipore). Cells were selected using 1 μg/ml of puromycin 48 h postransduction (ant-pr-1; InvivoGen). Loss of Bax/Bak was confirmed by Western blotting (antibodies were anti-Bak [06536; Millipore], anti-Bax [2772; Cell Signaling], and anti-glyceraldehyde-3-phosphate dehydrogenase [anti-GAPDH; MAB374; Millipore]).
Viral infection.
For infection experiments the following viruses were used. Influenza viruses included A/seal/Massachusetts/1/1980 (SC35M), A/Puerto Rico/8/1934 (PR8), A/WSN/1933 (WSN), A/X-31 (X-31), A/Thailand/1(KAN-1)/2004, B/Yamagata/1/1973, C/Johannesburg/1/(66), and SC35M encoding green fluorescent protein (GFP) (35). Thogoto virus isolate SiAr 126, Dhori virus strain Indian/1313/61, vesicular stomatitis virus (VSV) serotype Indiana encoding GFP (36), mumps virus strain RW, measles virus strain Edmonston, Sendai virus, Newcastle disease virus strain H53, Rift Valley fever virus, La Crosse virus, Semliki Forest virus, herpes simplex virus 1, and murine gamma herpesvirus-68 were kindly provided by Georg Kochs. For viral infection, growth medium was aspirated from cells, washed once with phosphate-buffered saline (PBS) plus 0.2% bovine serum albumin (BSA), and inoculated in DMEM supplemented with 0.2% BSA, 1% penicillin-streptomycin, and 2% glutamine at an MOI of 1 for all viruses listed above, except for measles virus (MOI of 0.5) and influenza A/Thailand/1(KAN-1)/2004 replication-incompetent virus (MOI of 0.1) (see below).
Generation of replication-incompetent influenza A/Thailand/1(KAN-1)/2004 virus.
Replication-incompetent influenza A/Thailand/1(KAN-1)/2004 virus was generated as described earlier (37). Briefly, the DNA transfection system with eight plasmids, established by Hoffman et al. (38), was followed, but instead of pHW2000-PB2, a pPolIPB2(120)Rluc(120) plasmid encoding Renilla luciferase flanked by the noncoding regions and 120 nucleotides of the terminal coding sequence of PB2 from influenza A/WSN/1933 was transfected into 293T cells. For amplification, 293T supernatant was used to infect AX4-PB2 cells, which constitutively express the PB2 protein of influenza A/Puerto Rico/8/34, thereby enabling viral polymerase reassembly and vRNA production in the absence of helper plasmids. AX4-PB2 cells and the pPolIPB2(120)Rluc(120) plasmid were kindly provided by Yoshihiro Kawaoka.
Generation of expression plasmids and transfection.
The open reading frame of wild-type (wt) SMARCA2, cloned from pIND20_SMARCA2 and kindly provided by Bhavatarini Vangamudi, or C-terminally truncated SMARCA2 (SMARCA2ΔC, containing amino acids 1 to 1318) was cloned into the eukaryotic expression plasmid pCAGGS (39). By site-directed mutagenesis the aspartic acid residue (D) at position 1318 within wt SMARCA2 was changed into a glutamic acid (E), resulting in SMARCA2D1318E, thereby destroying the caspase cleavage motif at this site. Transfection of the different pCAGGS expression plasmids into SW-13 cells was performed using Lipofectamine 3000 (Invitrogen) by following the manufacturer's instructions.
Pretreatments.
Transfection of poly(I·C) was performed using Lipofectamine 2000 by following the manufacturer's instructions. QVD (Q-VD-OPh; ApexBio), staurosporine (Sigma-Aldrich), or recombinant human IFN-α (40) treatment was performed by directly adding the respective amount of the reagents to the cell culture medium.
SDS-PAGE and Western blot analysis.
For Western blot analysis, cell supernatant was aspirated and cells directly lysed in 1× Laemmli buffer (41) (30 μl/well for a 24-well plate), briefly sonicated, and boiled at 95°C for 5 min. After separation of the proteins by SDS-PAGE (5% stacking gel, 10% separating gel), proteins were transferred onto a polyvinylidene difluoride (PVDF) membrane using the wet Mini Trans-Blot cell system (Bio-Rad, Munich). Membranes with proteins were blocked in 5% low-fat milk in PBS–0.1% Tween-20 and then incubated with the primary antibodies (anti-actin [1:1,000; Sigma-Aldrich]; anti-NDV-serum, kindly provided by Georg Kochs [1:1,000]; anti-SMARCA2/BRM N terminus [ab15597; 1:1,000; Abcam]; anti-SMARCA2/BRM C terminus [ab12165; 1:10,00; Abcam]; anti-SMARCA4/BRG1 N terminus [EPR3912; ab108318, 1:1,000; Abcam]; and anti-SMARCA4/BRG1 C terminus [ab182790; 1:1,000; Abcam]) in 5% low-fat milk in PBS–0.1% Tween-20 in a reaction tube for 1 h at room temperature under constant rotation. Three washing steps with PBS plus 0.1% Tween-20 (each for 10 min) at room temperature followed before the secondary antibodies (anti-rabbit-HRP; 1:5,000; Jackson Research), resolved in 5% low-fat milk in PBS–0.1% Tween-20, were applied for 45 min at room temperature with constant rotation in a reaction tube. After three more washing steps (PBS plus 0.1% Tween-20, 10 min) at room temperature, visualization of the bands occurred by application of the SuperSignal West Pico chemiluminescent substrate or SuperSignal West Femto maximum sensitivity substrate (Thermo Scientific) and readout with the Odyssey Fc (LI-COR) imaging system.
Immunofluorescence analysis and microscopy.
Immunofluorescence analysis was performed on SMARCA2- and SMARCA2ΔC-transfected SW-13 cells. Cells were plated on coverslips in 24-well plates, and 48 h after transfection cells were fixed in 4% paraformaldehyde for 15 min at room temperature and permeabilized with 0.5% Triton X-100–PBS for 5 min, followed by three wash steps with PBS. Cells were incubated with a specific SMARCA2 antibody detecting the N terminus (ab15597; Abcam) in 5% NGS–PBS at a 1:100 dilution for 1 h at room temperature. After four consecutive washing steps with PBS, the secondary anti-rabbit Cy3-conjugated antibody (Jackson Research) in 5% NGS–PBS was added at a 1:500 dilution and incubated in the dark for 40 min at RT. Subsequently the slides were washed three times with PBS, while the second washing step included a nuclear staining with DAPI (4,6-diamidino-2-phenylindole; 1:10 000 in PBS). The slides were mounted with ProLong Diamond antifade mountant (Life Technologies, Carlsbad, CA, USA) onto a glass slide.
For analysis of SMARCA2-protein distribution, pictures were taken at ×25 magnification as Z stacks off at least 32 vertical slices (x-z plane) with an LSM-I-NLO ZEISS LSM 880 using ZEN Black 2.3 SP1 (Zeiss) software. At least 125 cells per condition and approach were counted and grouped into cells showing nuclear SMARCA2 signals or cells showing both nuclear and cytoplasmic SMARCA2 signal. Additionally, pictures as Z stacks of at least 20 vertical slices (x-z plane) were taken at ×63 magnification, and a maximum intensity projection was calculated for every picture with ZEN Black 2.3 SP1 (Zeiss) software. Protein distribution of SMARCA2 and overlap with that of DAPI as a nuclear marker was analyzed with intensity profiles along the indicated arrows.
Phase contrast images at ×10 magnification of cells present in culture plates were taken with an Axio Observer.Z1 (Zeiss) microscope using AxioVision software.
Prediction of caspase cleavage sites and nuclear localization signals.
In order to predict potential caspase cleavage sites within SMARCA2, the two independent CaspDB and Cascleave online research databases were utilized based on the work of Kumar et al. and Song et al. (17, 18). Cleavage scores (ranging from 0.0 to 0.99) were calculated, with higher scores representing a higher likelihood for cleavage at a particular position (Table 1). Cleavage sites are listed if they are predicted by both CaspDB and Cascleave.
To determine possible NLSs in SMARCA2, cNLS Mapper was used (20). The higher the score, the more likely it is that the protein resides exclusively in the nucleus. According to the provider's platform, the NLSs are grouped in the following categories: scores of 9, 10, and higher depict exclusive nuclear localization, scores of 7 and 8 indicate partial localization to the nucleus, scores of 3, 4, and 5 indicate they are present in both nucleus and cytoplasm, and scores of 1 and 2 indicate they reside in the cytoplasm.
Flow cytometry.
SW-13 cells were transfected with either SMARCA2, SMARCA2ΔC, or a vector control and 48 h later were infected with either VSV-GFP at an MOI of 0.3 or SC35M-GFP at an MOI of 1. Six hours postinfection, GFP-positive cells were quantified by flow cytometry. Briefly, cells were trypsinized, transferred into a fluorescence-activated cell sorting (FACS) tube, washed with PBS–3% FCS, and fixed for 20 min on ice with BD Cytofix/Cytoperm (BD Bioscience) fixation solution. After two washing steps with BD Perm/Wash (BD Bioscience) washing solution, cells were dissolved in the washing solution and analyzed with a FACSCanto flow cytometer (BD Bioscience). Data analysis was performed with FlowJo v10.0.7.
qRT-PCR analysis.
Total RNA was extracted from SW-13 cells transfected with either SMARCA2, SMARCA2ΔC, or a vector control 48 h posttransfection by the Direct-zol RNA MiniPrep kit (Zymo Research). Reverse transcription of 500 ng total RNA was performed using the RevertAid first-strand cDNA synthesis kit (Thermo Scientific) with oligo(dT)18 primers. The RT product was used as the template for quantitative real-time PCR using a SensiFAST SYBR Hi-ROX kit (Bioline) and an ABI7900HT quantitative PCR instrument by denaturation at 95°C for 2 min, followed by 40 cycles at 95°C for 3 s and 60°C for 15 s. Melting curve analyses were performed to verify the amplification specificity. Relative quantification of gene expression was performed according to the relative standard curve method using SDS 2.4 software. Sequences of human primers used were the following: GAPD forward, 5′-TGCACCACCAACTGCTTAGC-3′; GAPD reverse, 5′-GGCATGGACTGTGGTCATGAG-3′; IFITM2 forward, 5′-ATTCTGCTCGTCATCATCCCAG-3′; IFITM2 reverse, 5′-TGATGCAAGACTCGGCTGTG-3′; MX1 forward, 5′-GAAAAATCCAGGCTCGGTGG-3′; MX1 reverse, 5′-TCAATGAGGTCGATGCAGGG-3′; MX2 forward, 5′-CAGAGGCAGCGGAATCGTAA-3′; MX2 reverse, 5′-TGAAGCTCTAGCTCGGTGTTC-3′. We also used actin-γ (Hs_ACTG1_1; Qiagen), IFIT1 (Hs_IFIT1_1; Qiagen), IFNB1 (Hs_IFNB1_1; Qiagen), ISG20 (Hs_ISG20_1; Qiagen), and OAS1 (Hs_OAS1_1; Qiagen).
Transcriptome analysis.
Total RNA was isolated from SW-13 cells (in triplicates) that were transfected with either SMARCA2, SMARCA2ΔC, or an empty vector control using TRIzol reagent (Life Technologies) in combination with an RNeasy minikit (Qiagen, Hilden, Germany) and on-column DNase treatment. For mRNA isolation, the Dynabeads mRNA Direct micro kit (Invitrogen) was used according to the manufacturer's instructions for total RNA inputs of 8 μg. RNA integrity (RIN) and rRNA contamination in total RNA and in mRNA were assessed using an Agilent 2100 Bioanalyzer (Agilent Technologies, Böblingen, Germany) and appropriate chips and kits. Whole-transcriptome libraries were then prepared using the Ion Total RNA-Seq kit v2 (Life Technologies, Carlsbad, CA, USA), and the library quantity was assessed with a KAPA library quantification kit for Ion Torrent (Kapa Biosystems, Wilmington, MA, USA). All libraries were sequenced on an S5XL sequencing system (Life Technologies) using the Ion 540 OT2 and chip kit (Life Technologies).
Transcript abundancies were quantified for all libraries using Salmon software (version 0.9.1) and a human transcript reference (GRCh38.p12; NCBI accession number GCF_000001405.38). Differentially expressed genes were then identified and statistically tested using the DESeq2 package (version 1.18.1) under R (version 3.4.1). Results for genes with an adjusted P value of <0.001 and a minimal fold change of >2 were considered significant.
siRNA-mediated knockdown.
For transient silencing of genes by targeting their corresponding mRNAs, reverse transfection of siRNAs was performed in 24-well cell culture plates. For each approach, 1.5 μl of RNAiMAX (ThermoFisher) was diluted in 125 μl of Opti-MEM-GlutaMAX in an RNase-free reaction tube and incubated for 5 min at room temperature before siRNA, also diluted in 125 μl Opti-MEM-GlutaMAX, was added, briefly vortexed, and incubated for a further 20 min at room temperature. The total volume of 250 μl per well was dispersed, and 8 × 103 cells in the same volume of siRNA transfection medium (DMEM, 20% FCS, 2% glutamine) were seeded on top for 72 h at 37°C, resulting in a final siRNA concentration of 30 nM.
Supplementary Material
ACKNOWLEDGMENTS
We thank Georg Häcker, Christoph Borner, Peter Stäheli, and Sebastian Giese for critical readings of the manuscript and Georg Kochs for providing the viruses mentioned as well as the anti-NDV serum. We also thank Yoshihiro Kawaoka for the AX4-PB2 cells and pPolIPB2(120)Rluc(120), Bhavatarini Vangamudi for the pIND20_SMARCA2 expression plasmid, and Peter Stäheli for recombinant human IFN-α. Furthermore, we thank Jenny Lorke for excellent technical assistance.
This study was supported by funds from the German Research Foundation (DFG; SFB 1160, project 13) to M.S. and by the Excellence Initiative of the German Research Foundation (GSC-4; Spemann Graduate School) to A.H.D. and H.B.
We have no conflicts of interest to declare relating to this study.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JVI.00343-18.
REFERENCES
- 1.Chi T. 2004. A BAF-centred view of the immune system. Nat Rev Immunol 4:965–977. doi: 10.1038/nri1501. [DOI] [PubMed] [Google Scholar]
- 2.Cui K, Tailor P, Liu H, Chen X, Ozato K, Zhao K. 2004. The chromatin-remodeling BAF complex mediates cellular antiviral activities by promoter priming. Mol Cell Biol 24:4476–4486. doi: 10.1128/MCB.24.10.4476-4486.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dornfeld D, Dudek AH, Vausselin T, Gunther SC, Hultquist JF, Giese S, Khokhlova-Cubberley D, Chew YC, Krogan NJ, Garcia-Sastre A, Schwemmle M, Shaw ML. 2018. SMARCA2-regulated host cell factors are required for MxA restriction of influenza A viruses. Sci Rep 8:2092. doi: 10.1038/s41598-018-20458-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu H, Kang H, Liu R, Chen X, Zhao K. 2002. Maximal induction of a subset of interferon target genes requires the chromatin-remodeling activity of the BAF complex. Mol Cell Biol 22:6471–6479. doi: 10.1128/MCB.22.18.6471-6479.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanchez R, Zhou M-M. 2009. The role of human bromodomains in chromatin biology and gene transcription. Curr Opin Drug Discov Dev 12:659–665. [PMC free article] [PubMed] [Google Scholar]
- 6.Sen P, Vivas P, Dechassa ML, Mooney AM, Poirier MG, Bartholomew B. 2013. The SnAC domain of SWI/SNF is a histone anchor required for remodeling. Mol Cell Biol 33:360–370. doi: 10.1128/MCB.00922-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Everett H, McFadden G. 1999. Apoptosis: an innate immune response to virus infection. Trends Microbiol 7:160–165. doi: 10.1016/S0966-842X(99)01487-0. [DOI] [PubMed] [Google Scholar]
- 8.Clem RJ, Fechheimer M, Miller LK. 1991. Prevention of apoptosis by a baculovirus gene during infection of insect cells. Science 254:1388–1390. doi: 10.1126/science.1962198. [DOI] [PubMed] [Google Scholar]
- 9.Elmore S. 2007. Apoptosis: a review of programmed cell death. Toxicol Pathol 35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tait SWG, Green DR. 2010. Mitochondria and cell death: outer membrane permeabilization and beyond. Nat Rev Mol Cell Biol 11:621–632. doi: 10.1038/nrm2952. [DOI] [PubMed] [Google Scholar]
- 11.Bratton SB, Walker G, Srinivasula SM, Sun X-M, Butterworth M, Alnemri ES, Cohen GM. 2001. Recruitment, activation and retention of caspases-9 and -3 by Apaf-1 apoptosome and associated XIAP complexes. EMBO J 20:998–1009. doi: 10.1093/emboj/20.5.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qin H, Srinivasula SM, Wu G, Fernandes-Alnemri T, Alnemri ES, Shi Y. 1999. Structural basis of procaspase-9 recruitment by the apoptotic protease-activating factor 1. Nature 399:549–557. doi: 10.1038/21124. [DOI] [PubMed] [Google Scholar]
- 13.Savill J, Dransfield I, Gregory C, Haslett C. 2002. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol 2:965–975. doi: 10.1038/nri957. [DOI] [PubMed] [Google Scholar]
- 14.Dix MM, Simon GM, Cravatt BF. 2008. Global mapping of the topography and magnitude of proteolytic events in apoptosis. Cell 134:679–691. doi: 10.1016/j.cell.2008.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang M, Sun Y, Ma L, Wang C, Wu JM, Bi A, Liao DJ. 2011. Complex alternative splicing of the smarca2 gene suggests the importance of smarca2-B variants. J Cancer 2:386–400. doi: 10.7150/jca.2.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keoni CL, Brown TL. 2015. Inhibition of apoptosis and efficacy of pan caspase inhibitor, Q-VD-OPh, in models of human disease. J Cell Death 8:1–7. doi: 10.4137/JCD.S23844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumar S, van Raam BJ, Salvesen GS, Cieplak P. 2014. Caspase cleavage sites in the human proteome: CaspDB, a database of predicted substrates. PLoS One 9:e110539. doi: 10.1371/journal.pone.0110539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song J, Tan H, Shen H, Mahmood K, Boyd SE, Webb GI, Akutsu T, Whisstock JC. 2010. Cascleave: towards more accurate prediction of caspase substrate cleavage sites. Bioinformatics 26:752–760. doi: 10.1093/bioinformatics/btq043. [DOI] [PubMed] [Google Scholar]
- 19.Reisman DN, Strobeck MW, Betz BL, Sciariotta J, Funkhouser W Jr, Murchardt C, Yaniv M, Sherman LS, Knudsen ES, Weissman BE. 2002. Concomitant down-regulation of BRM and BRG1 in human tumor cell lines: differential effects on RB-mediated growth arrest vs CD44 expression. Oncogene 21:1196–1207. doi: 10.1038/sj.onc.1205188. [DOI] [PubMed] [Google Scholar]
- 20.Kosugi S, Hasebe M, Tomita M, Yanagawa H. 2009. Systematic identification of cell cycle-dependent yeast nucleocytoplasmic shuttling proteins by prediction of composite motifs. Proc Natl Acad Sci U S A 106:10171–10176. doi: 10.1073/pnas.0900604106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clapier CR, Cairns BR. 2009. The biology of chromatin remodeling complexes. Annu Rev Biochem 78:273–304. doi: 10.1146/annurev.biochem.77.062706.153223. [DOI] [PubMed] [Google Scholar]
- 22.Rialdi A, Campisi L, Zhao N, Lagda AC, Pietzsch C, Ho JSY, Martinez-Gil L, Fenouil R, Chen X, Edwards M, Metreveli G, Jordan S, Peralta Z, Munoz-Fontela C, Bouvier N, Merad M, Jin J, Weirauch M, Heinz S, Benner C, van Bakel H, Basler C, Garcia-Sastre A, Bukreyev A, Marazzi I. 2016. Topoisomerase 1 inhibition suppresses inflammatory genes and protects from death by inflammation. Science 352:aad7993. doi: 10.1126/science.aad7993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramirez-Carrozzi VR, Braas D, Bhatt DM, Cheng CS, Hong C, Doty KR, Black JC, Hoffmann A, Carey M, Smale ST. 2009. A unifying model for the selective regulation of inducible transcription by CpG islands and nucleosome remodeling. Cell 138:114–128. doi: 10.1016/j.cell.2009.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lamkanfi M, Declercq W, Kalai M, Saelens X, Vandenabeele P. 2002. Alice in caspase land. A phylogenetic analysis of caspases from worm to man. Cell Death Differ 9:358–361. [DOI] [PubMed] [Google Scholar]
- 25.Thornberry NA, Rano TA, Peterson EP, Rasper DM, Timkey T, Garcia-Calvo M, Houtzager VM, Nordstrom PA, Roy S, Vaillancourt JP, Chapman KT, Nicholson DW. 1997. A combinatorial approach defines specificities of members of the caspase family and granzyme B. Functional relationships established for key mediators of apoptosis. J Biol Chem 272:17907–17911. [DOI] [PubMed] [Google Scholar]
- 26.Stennicke HR, Renatus M, Meldal M, Salvesen GS. 2000. Internally quenched fluorescent peptide substrates disclose the subsite preferences of human caspases 1, 3, 6, 7 and 8. Biochem J 350(Part 2):563–568. doi: 10.1042/bj3500563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Timmer JC, Enoksson M, Wildfang E, Zhu W, Igarashi Y, Denault JB, Ma Y, Dummitt B, Chang YH, Mast AE, Eroshkin A, Smith JW, Tao WA, Salvesen GS. 2007. Profiling constitutive proteolytic events in vivo. Biochem J 407:41–48. doi: 10.1042/BJ20070775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vangamudi B, Paul TA, Shah PK, Kost-Alimova M, Nottebaum L, Shi X, Zhan Y, Leo E, Mahadeshwar HS, Protopopov A, Futreal A, Tieu TN, Peoples M, Heffernan TP, Marszalek JR, Toniatti C, Petrocchi A, Verhelle D, Owen DR, Draetta GF, Jones P, Palmer WS, Sharma S, Andersen JN. 2015. The SMARCA2/4 ATPase domain surpasses the bromodomain as a drug target in SWI/SNF mutant cancers: insights from cDNA rescue and PFI-3 inhibitor studies. Cancer Res 75:3865–3878. doi: 10.1158/0008-5472.can-14-3798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Awad S, Hassan AH. 2008. The Swi2/Snf2 bromodomain is important for the full binding and remodeling activity of the SWI/SNF complex on H3- and H4-acetylated nucleosomes. Ann N Y Acad Sci 1138:366–375. doi: 10.1196/annals.1414.038. [DOI] [PubMed] [Google Scholar]
- 30.Koga M, Ishiguro H, Yazaki S, Horiuchi Y, Arai M, Niizato K, Iritani S, Itokawa M, Inada T, Iwata N, Ozaki N, Ujike H, Kunugi H, Sasaki T, Takahashi M, Watanabe Y, Someya T, Kakita A, Takahashi H, Nawa H, Muchardt C, Yaniv M, Arinami T. 2009. Involvement of SMARCA2/BRM in the SWI/SNF chromatin-remodeling complex in schizophrenia. Hum Mol Genet 18:2483–2494. doi: 10.1093/hmg/ddp166. [DOI] [PubMed] [Google Scholar]
- 31.Heeres JT, Hergenrother PJ. 2007. Poly(ADP-ribose) makes a date with death. Curr Opin Chem Biol 11:644–653. doi: 10.1016/j.cbpa.2007.08.038. [DOI] [PubMed] [Google Scholar]
- 32.Zhu P, Martinvalet D, Chowdhury D, Zhang D, Schlesinger A, Lieberman J. 2009. The cytotoxic T lymphocyte protease granzyme A cleaves and inactivates poly(adenosine 5′-diphosphate-ribose) polymerase-1. Blood 114:1205–1216. doi: 10.1182/blood-2008-12-195768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith SJ, Long A, Barrow JH, Macarthur DC, Coyle B, Grundy RG, Children's Cancer and Leukaemia Group Biological Studies Committee. 2011. Pediatric high-grade glioma: identification of poly(ADP-ribose) polymerase as a potential therapeutic target. Neuro Oncol 13:1171–1177. doi: 10.1093/neuonc/nor115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tone S, Sugimoto K, Tanda K, Suda T, Uehira K, Kanouchi H, Samejima K, Minatogawa Y, Earnshaw WC. 2007. Three distinct stages of apoptotic nuclear condensation revealed by time-lapse imaging, biochemical and electron microscopy analysis of cell-free apoptosis. Exp Cell Res 313:3635–3644. doi: 10.1016/j.yexcr.2007.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reuther P, Gopfert K, Dudek AH, Heiner M, Herold S, Schwemmle M. 2015. Generation of a variety of stable influenza A reporter viruses by genetic engineering of the NS gene segment. Sci Rep 5:11346. doi: 10.1038/srep11346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stojdl DF, Lichty BD, ten Oever BR, Paterson JM, Power AT, Knowles S, Marius R, Reynard J, Poliquin L, Atkins H, Brown EG, Durbin RK, Durbin JE, Hiscott J, Bell JC. 2003. VSV strains with defects in their ability to shutdown innate immunity are potent systemic anti-cancer agents. Cancer Cell 4:263–275. doi: 10.1016/S1535-6108(03)00241-1. [DOI] [PubMed] [Google Scholar]
- 37.Ozawa M, Victor ST, Taft AS, Yamada S, Li C, Hatta M, Das SC, Takashita E, Kakugawa S, Maher EA, Neumann G, Kawaoka Y. 2011. Replication-incompetent influenza A viruses that stably express a foreign gene. J Gen Virol 92:2879–2888. doi: 10.1099/vir.0.037648-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoffmann E, Neumann G, Kawaoka Y, Hobom G, Webster RG. 2000. A DNA transfection system for generation of influenza A virus from eight plasmids. Proc Natl Acad Sci U S A 97:6108–6113. doi: 10.1073/pnas.100133697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Niwa H, Yamamura K, Miyazaki J. 1991. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 108:193–199. doi: 10.1016/0378-1119(91)90434-D. [DOI] [PubMed] [Google Scholar]
- 40.Horisberger MA, de Staritzky K. 1987. A recombinant human interferon-alpha B/D hybrid with a broad host-range. J Gen Virol 68(Part 3):945–948. doi: 10.1099/0022-1317-68-3-945. [DOI] [PubMed] [Google Scholar]
- 41.Laemmli UK. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.