Alphaviruses utilize a broad spectrum of cellular factors for efficient formation and function of replication complexes (RCs). Our data demonstrate for the first time that the hypervariable domain (HVD) of chikungunya virus nonstructural protein 3 (nsP3) is intrinsically disordered. It binds at least 3 families of cellular proteins, which play an indispensable role in viral RNA replication. The proteins of each family demonstrate functional redundancy. We provide a detailed map of the binding sites on CHIKV nsP3 HVD and show that mutations in these sites or the replacement of CHIKV HVD by heterologous HVD change cell specificity of viral replication. Such manipulations with alphavirus HVDs open an opportunity for development of new irreversibly attenuated vaccine candidates. To date, the disordered protein fragments have been identified in the nonstructural proteins of many other viruses. They may also interact with a variety of cellular factors that determine critical aspects of virus-host interactions.
KEYWORDS: BIN1, CD2AP, Eilat virus, G3BP1, G3BP2, NAP1L1, viral RNA replication, alphavirus, chikungunya virus, virus-host interactions
ABSTRACT
Alphaviruses are widely distributed in both hemispheres and circulate between mosquitoes and amplifying vertebrate hosts. Geographically separated alphaviruses have adapted to replication in particular organisms. The accumulating data suggest that this adaptation is determined not only by changes in their glycoproteins but also by the amino acid sequence of the hypervariable domain (HVD) of the alphavirus nsP3 protein. We performed a detailed investigation of chikungunya virus (CHIKV) nsP3 HVD interactions with host factors and their roles in viral replication in vertebrate and mosquito cells. The results demonstrate that CHIKV HVD is intrinsically disordered and binds several distinctive cellular proteins. These host factors include two members of the G3BP family and their mosquito homolog Rin, two members of the NAP1 family, and several SH3 domain-containing proteins. Interaction with G3BP proteins or Rin is an absolute requirement for CHIKV replication, although it is insufficient to solely drive it in either vertebrate or mosquito cells. To achieve a detectable level of virus replication, HVD needs to bind members of at least one more protein family in addition to G3BPs. Interaction with NAP1L1 and NAP1L4 plays a more proviral role in vertebrate cells, while binding of SH3 domain-containing proteins to a proline-rich fragment of HVD is more critical for virus replication in the cells of mosquito origin. Modifications of binding sites in CHIKV HVD allow manipulation of the cell specificity of CHIKV replication. Similar changes may be introduced into HVDs of other alphaviruses to alter their replication in particular cells or tissues.
IMPORTANCE Alphaviruses utilize a broad spectrum of cellular factors for efficient formation and function of replication complexes (RCs). Our data demonstrate for the first time that the hypervariable domain (HVD) of chikungunya virus nonstructural protein 3 (nsP3) is intrinsically disordered. It binds at least 3 families of cellular proteins, which play an indispensable role in viral RNA replication. The proteins of each family demonstrate functional redundancy. We provide a detailed map of the binding sites on CHIKV nsP3 HVD and show that mutations in these sites or the replacement of CHIKV HVD by heterologous HVD change cell specificity of viral replication. Such manipulations with alphavirus HVDs open an opportunity for development of new irreversibly attenuated vaccine candidates. To date, the disordered protein fragments have been identified in the nonstructural proteins of many other viruses. They may also interact with a variety of cellular factors that determine critical aspects of virus-host interactions.
INTRODUCTION
The Alphavirus genus in the Togaviridae family contains a wide variety of human and animal pathogens (1). Based on their geographical distribution, they are separated into New World (NW) and the Old World (OW) alphaviruses. In natural circulation, most of the currently known alphaviruses are transmitted by mosquito vectors between vertebrate hosts, in which they induce diseases of different severity (2). The NW alphaviruses, exemplified by Venezuelan (VEEV), eastern (EEEV), and western (WEEV) equine encephalitis viruses, cause a highly debilitating disease. In a wide variety of vertebrate species, including humans, it often results in meningomyeloencephalitis with a frequently lethal outcome (3). Most of the OW alphaviruses are less pathogenic, and their human-associated diseases are characterized by rash, arthritis, and fever (3). Despite a presence on essentially all continents and a significant public health threat, the molecular mechanisms of alphavirus replication and interactions with host cells are insufficiently investigated, and critical aspects of the viral biology remain to be better understood. The importance of the OW alphaviruses was underappreciated for a long time until the recent outbreak of chikungunya fever in both hemispheres with millions of people involved. Chikungunya virus (CHIKV) induces severe polyarthritis characterized by excruciating pain that frequently continues for several years (4–8).
The alphavirus genome is a single-stranded RNA of positive polarity of ∼11.5 kb. It mimics cellular mRNAs in that it has a cap at the 5′ terminus and a poly(A) tail at the 3′ terminus (9). Upon delivery into the cell, the genome is translated into P123 and P1234, the polyprotein precursors of viral nonstructural (ns) proteins (2). The subsequent sequential processing of both ns polyproteins into individual nsPs, nsP1, nsP2, nsP3, and nsP4, differentially regulates the synthesis of the negative-strand RNA intermediates, new viral genomes (G RNA) and subgenomic (SG) RNA (10, 11). The latter RNA is encoded by the 3′ one-third of the genome and translated into viral structural proteins, which ultimately form viral particles (2).
The initially synthesized ns polyproteins are targeted to the plasma membrane (PM). This binding to the internal surface of the PM (12) is mediated by specific alpha-helical peptide and palmitoylated amino acids (aa) of nsP1 (13, 14). After the first cleavage event mediated by nsP2-associated protease activity, the initially formed replication complexes (RCs) contain P123 and nsP4. They are capable of synthesis of the negative-strand RNA on the G RNA template to form the double-stranded RNA (dsRNA) replication intermediates (11, 15). The dsRNA synthesis induces the formation of the membrane spherules, the size of which correlates with the length of the original RNA template (16). The subsequent processing of P123 into nsP1+P23+nsP4 and ultimately into nsP1+nsP2+nsP3+nsP4 transforms spherule-associated RCs into their mature form and makes them active in G and SG RNA synthesis (11, 17, 18). Viral components of RCs, nsPs, exhibit enzymatic activities which are required for viral RNA synthesis. nsP1 and nsP2 facilitate RNA capping, in that nsP2 exhibits RNA triphosphatase activity (19) and nsP1 mediates a cascade of enzymatic reactions which result in the formation of m7GMP, termed Cap(0), at the 5′ termini of viral G and SG RNAs (20, 21). nsP2 also functions as a nucleoside triphosphatase (NTPase) and helicase during replication of viral genomic RNA and transcription of SG RNAs (22–24) and performs all of the steps of P123 and P1234 processing into individual nsPs (18, 25). nsP4 is an RNA-dependent RNA polymerase, the core subunit of viral RC (26–28). Functions of nsP3 in alphavirus replication are not well understood. However, it is absolutely essential for viral RNA replication. nsP3 protein contains three structural domains: two conserved amino-terminal domains that include the macro domain (29) and the Zn-binding alphavirus unique domain (AUD) (30) and the hypervariable carboxy-terminal domain (HVD) (31–33). It is becoming more evident that alphavirus nsP3 proteins, and their HVDs in particular, serve as hubs for assembly of RCs and other large cytoplasmic complexes that are composed of both cellular and viral proteins (34, 35). The initially formed nsP3-containing RCs also mediate recruitment of viral G RNA into the replication process (34).
HVDs of the members of different alphavirus serocomplexes demonstrate essentially no identity at the aa level. These domains were proposed to be disordered and were shown to interact with specific host factors (34, 35). The experimental data suggest that HVDs may play critical roles in the adaptation of alphaviruses to replication in different hosts and mosquito vectors (34). Among other host factors, G3BP proteins (G3BP1 and G3BP2) were found to be the most abundantly binding to HVD of all of the currently investigated OW alphaviruses, such as Sindbis (SINV) and Semliki Forest (SFV) viruses (36–39). G3BP-HVD interactions are particularly important for CHIKV replication, because knockout (KO) of both G3bp genes in murine cells (G3bp dKO cells) makes them no longer capable of supporting CHIKV replication (34).
G3BP-HVD interaction is mediated by a short repeating peptide located in the carboxy-terminal fragment of CHIKV HVD (34, 38). In this study, we demonstrate that despite the absolute requirement of this interaction for CHIKV RC function, G3BP binding alone is insufficient for driving CHIKV RNA replication. Other fragments of nsP3 HVD determine replication of CHIKV in a cell-specific mode. Genetic manipulations with CHIKV HVD differentially affect virus replication in vertebrate and mosquito cells, and this opens an opportunity for designing cell-specific CHIKV mutants.
RESULTS
CHIKV HVD is a completely disordered protein domain.
Recent studies strongly suggested that alphavirus HVDs play critical roles in virus replication by mediating the assembly of protein complexes that facilitate RNA replication and possibly have other activities in virus-host interactions (34). Further understanding of the mechanism of their function implied introduction of wide-range modifications into HVDs and analysis of their effects on binding of specific proteins and on RNA and virus replication. Development of the rationale of mutagenesis and unambiguous interpretation of the data required the detailed knowledge of the HVD structure. This information helps to minimize the effect of the mutations on the overall protein folding and disruption of the potentially critical elements of the secondary structure.
CHIKV HVD is a 199-aa-long C-terminal fragment of nsP3 (Fig. 1A). It is enriched in disorder-promoting amino acids, such as proline, serine, lysine, glutamine, and glutamic acid (Fig. 1A). Computational prediction using IUPred software (40, 41) suggested that CHIKV HVD is likely intrinsically disordered but may have short structured fragments (Fig. 1B). To unambiguously identify the structural state of CHIKV HVD, we analyzed it by nuclear magnetic resonance (NMR) spectroscopy. The 15N-labeled or 15N- and 13C-double-labeled CHIKV HVD was produced in Escherichia coli and purified as a fusion protein with the carboxy-terminal Twin-Strep tag. Figure 1C presents the 1H-15N correlation spectrum of the region, where the cross peaks arising from the one-bond interaction between proton and nitrogen of the amide bond are expected to be observed. It exhibits a reduced chemical shift dispersion, especially in the 1H dimension between 8.5 and 7.5 ppm (Fig. 1C), which is a characteristic of the intrinsically disordered proteins (42). Since the 15N, 13C nuclei are better dispersed even for the intrinsically disordered proteins, the sets of the three-dimensional (3D) experiments involving nitrogen and different types of carbon nuclei were performed (see Materials and Methods). Ultimately, we were able to assign 73% of N, 91% of Cα, 67% of Cβ, and 73% of C′ signals. The detailed analysis of CHIKV HVD structure and full assignment will be published elsewhere.
FIG 1.
CHIKV HVD is an intrinsically disordered domain. (A) Amino acid sequence of CHIKV 181/25 nsP3 HVD. The binding motif for BIN1 is depicted in green. The binding motifs for G3BP1 and G3BP2 are depicted in red. (B) Computational prediction of CHIKV nsP3 disorder probability by IUPred. The known binding motifs for BIN1 and G3BPs are marked by blue lines. (C) 2D best-TROSY spectra of CHIKV nsP3 HVD showing cross peaks corresponding to the one-bond interaction between proton, 1H, and nitrogen, 15N, of the amide bonds. (D) Results of chemical shift analysis of 13C′, 13Cα, and 13Cβ nuclei in CHIKV HVD. The gray bars indicate unassigned carbons.
The deviation of chemical shift for Cα, Cβ, and C′ from the random coil values allows an estimation of the presence of secondary structure elements. The presence of 3 to 4 consecutive values of above 2 ppm is interpreted as the presence of α-helix (positive value) or β-strand (negative value). The majority of carbon signals in CHIKV HVD did not deviate more than 0.7 ppm from the random coil values, and no consecutive signals with a chemical shift more than 2 ppm were detected (Fig. 1D). These experimental data unambiguously demonstrated that CHIKV HVD is a disordered protein domain, and its modifications in the following experiments performed in this study could change only the linear protein-binding motifs but not the protein secondary structure.
Deletion of even one element of the repeat in HVD negatively impacts CHIKV replication.
Our previous studies and those of other groups demonstrated that cellular G3BP1 and G3BP2 proteins interact with the short repeating peptides located at the carboxy termini of the OW alphavirus nsP3s (34, 38). KO of both G3bp genes (dKO) in the NIH 3T3 cells had a strong negative effect on replication of SFV and SINV while rendering CHIKV not viable in this cell line (34). To further evaluate the role of nsP3 HVD-G3BP interaction in virus replication, two recombinant CHIKV 181/25-based mutants were generated (Fig. 2A). The first mutant, CHIKV/Δ1/GFP, had one of the elements of the repeat deleted, and the second, CHIKV/Δ1+2/GFP, had both G3BP-binding sites removed. Since lack of cytopathic effect in cultured cells is not necessarily an indication of the absence of RNA replication, both mutants and the control CHIKV/GFP genomes contained the green fluorescent protein (GFP) gene under the control of an additional SG RNA promoter (Fig. 2A). The analysis of GFP expression was used as another means for indirect assessment of G RNA replication and transcription of SG RNA. The designed mutants were evaluated in terms of their ability to replicate in the cells of vertebrate and mosquito origins.
FIG 2.
Deletions of G3BP-binding peptides in CHIKV nsP3 HVD have deleterious effects on viral replication. (A) Schematic presentation of CHIKV/GFP genome, sequences of the carboxy-terminal fragments of nsP3 HVD in the parental CHIKV/GFP and in the designed deletion mutants, and infectivities of the in vitro-synthesized RNAs in ICA performed on BHK-21 and C7/10 cells. The repeating element is indicated in red. Dots indicate deleted aa in the designed mutants. NV, not viable. The numbers given are the aa numbers in the original full-length CHIKV nsP3. (B and C) BHK-21 and C7/10 cells, respectively, were electroporated with 3 μg of the indicated in vitro-synthesized RNAs, and samples of the media were collected at the indicated time points. Virus titers were determined by plaque assay on BHK-21 cells. The experiments were performed twice using different amounts of in vitro-synthesized RNA and demonstrated similar differences in the rates of infectious virus release. The results of one of the experiments are presented.
Equal amounts of the in vitro-synthesized RNAs of CHIKV/GFP, CHIKV/Δ1/GFP, and CHIKV/Δ1+2/GFP were electroporated into BHK-21 and C7/10 cells. In the infectious center assay (ICA) performed in BHK-21 cells, CHIKV/Δ1/GFP RNA demonstrated essentially the same infectivity as did the parental CHIKV/GFP, but in the repeated experiments, the rates of infectious CHIKV/Δ1/GFP virus release were reproducibly lower (Fig. 2B). CHIKV/Δ1+2/GFP, which lacked both G3BP-binding motifs in nsP3 HVD, failed to replicate in both cell lines with no detectable infectious virus release or cellular GFP expression (Fig. 2B and data not shown). In the subsequent follow-up experiments, we monitored for possible appearance of viable second-site revertants that could develop spreading infection but failed to detect any.
Similarly, in C7/10 cells, the in vitro-synthesized CHIKV/GFP- and CHIKV/Δ1/GFP-specific RNAs exhibited essentially the same infectivity patterns, but at any time posttransfection, titers of CHIKV/Δ1/GFP were between 1 and 2 orders of magnitude lower (Fig. 2A and C). The double deletion mutant CHIKV/Δ1+2/GFP failed to replicate in C7/10 cells. From these experiments, we concluded that deletion of even one G3BP-binding site in CHIKV HVD had a detrimental effect on viral replication rates, and deletion of both of them is lethal for CHIKV growth in both cell types.
Other HVD fragments, besides the G3BP-binding aa repeat, are also critical determinants of CHIKV replication.
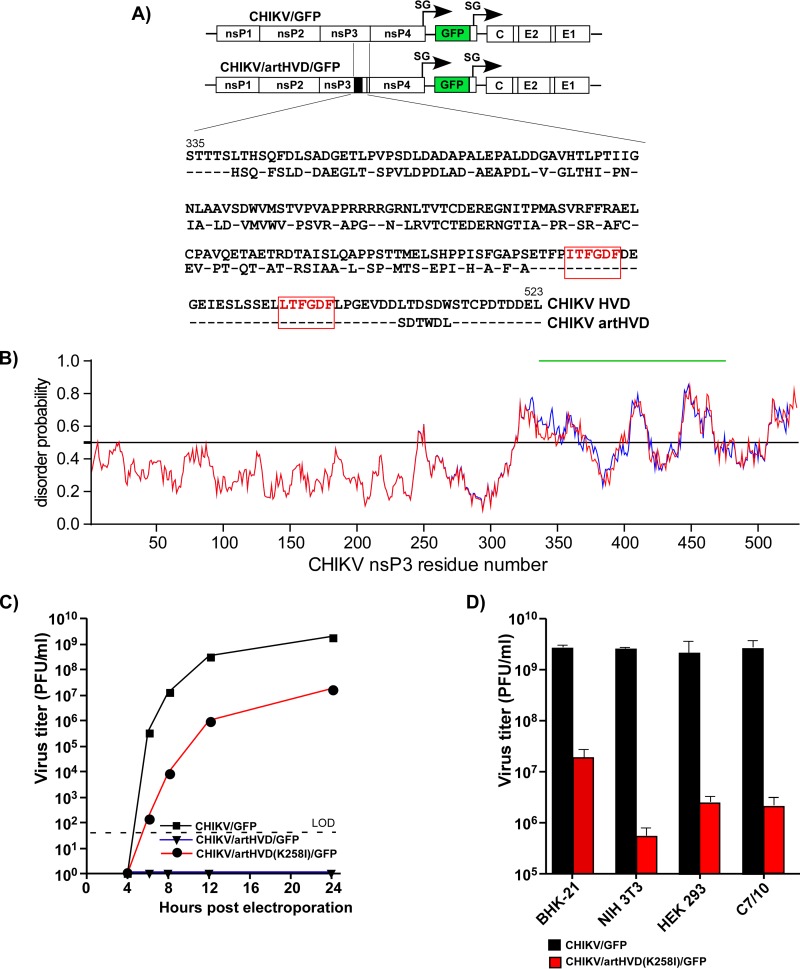
The above experimental data demonstrated that the presence of both HVD-specific, G3BP-binding sites is the prerequisite for efficient CHIKV replication in insect and vertebrate cells. However, this does not necessarily mean that the indicated repeating element is the only critical determinant of the HVD function in viral replication. Therefore, to continue the investigation of the role(s) of CHIKV HVD, we designed a variety of viral mutants that contained strong modifications in the HVD fragments other than the G3BP-binding repeat. The CHIKV/artHVD/GFP mutant encoded an artificial HVD in which the carboxy-terminal, repeat-containing fragment remained intact. The amino acid positions in the rest of the upstream-located HVD fragment were changed to generate essentially random peptide sequence (Fig. 3A). Thus, the newly designed HVD was similar to its natural counterpart in attributes such as length, aa composition, and hydrophobicity profile and, based on the computer predictions, remained intrinsically disordered (Fig. 3B). However, it exhibited a very low level of identity with the sequence of wild-type (wt) CHIKV HVD (Fig. 3A).
FIG 3.
CHIKV nsP3 HVD fragments other than the carboxy-terminal, G3BP-binding repeat determine virus replication. (A) Schematic presentation of the parental CHIKV/GFP genome and its derivative, CHIKV/artHVD/GFP, with artificially designed HVD, and alignment of the wt and mutated HVD sequences. The repeating element is indicated in red. Dashes indicate identical aa. (B) Computational prediction of disorder probability of CHIKV nsP3 with artHVD (shown in blue) and wt CHIKV nsP3 (shown in red) by IUPred. The mutated HVD fragment is indicated by a green line. (C) Replication rates of the indicated viruses, CHIKV/GFP, CHIKV/artHVD/GFP, and its derivative CHIKV/artHVD(K258I)/GFP, which contained an adaptive mutation, K258I, in nsP3. BHK-21 cells were electroporated with 3 μg in vitro-synthesized RNAs. Viral samples were harvested at the indicated time points, and titers were determined by plaque assay on BHK-21 cells. LOD, limit of detection. (D) Titers of CHIKV/GFP and CHIKV/artHVD(K258I)/GFP at 24 h after infection of the indicated cell lines at an MOI of 1 PFU/cell. Titers were determined by plaque assay on BHK-21 cells. The results of one of the reproducible experiments performed in triplicates are presented. Means and standard deviations (SDs) are indicated.
Electroporation of the in vitro-synthesized RNA of the CHIKV/artHVD/GFP variant did not initiate its replication in BHK-21 cells, despite the latter variant containing the intact carboxy-terminal G3BP-binding repeat in its HVD (Fig. 3A and C). This mutant also failed to replicate in mosquito C7/10 cells (data not shown). The above data strongly indicated that (i) the G3BP-binding motifs are not the only indispensable determinants of CHIKV HVD function in RNA replication and (ii) other HVD motifs play an essential role(s) in RC assembly and function similar to the carboxy-terminal G3BP-interacting repeat.
Interestingly, in one of the repeated RNA transfection experiments on BHK-21 cells, we detected selection of a viable second-site revertant of CHIKV/artHVD/GFP. A single adaptive mutation, K258I, was identified in the Zn-binding domain of nsP3. When introduced into the nsP3 sequence of the original CHIKV/artHVD/GFP, it made this variant viable and capable of developing spreading infection in BHK-21 cells (Fig. 3C). However, despite the stimulatory effect of the K258I mutation, viral replication in BHK-21 cells and particularly in other tested cell lines remained low (Fig. 3D), and no further experiments with this second-site revertant were performed in this study.
Fragments of CHIKV HVD differentially determine virus replication in different cell types.
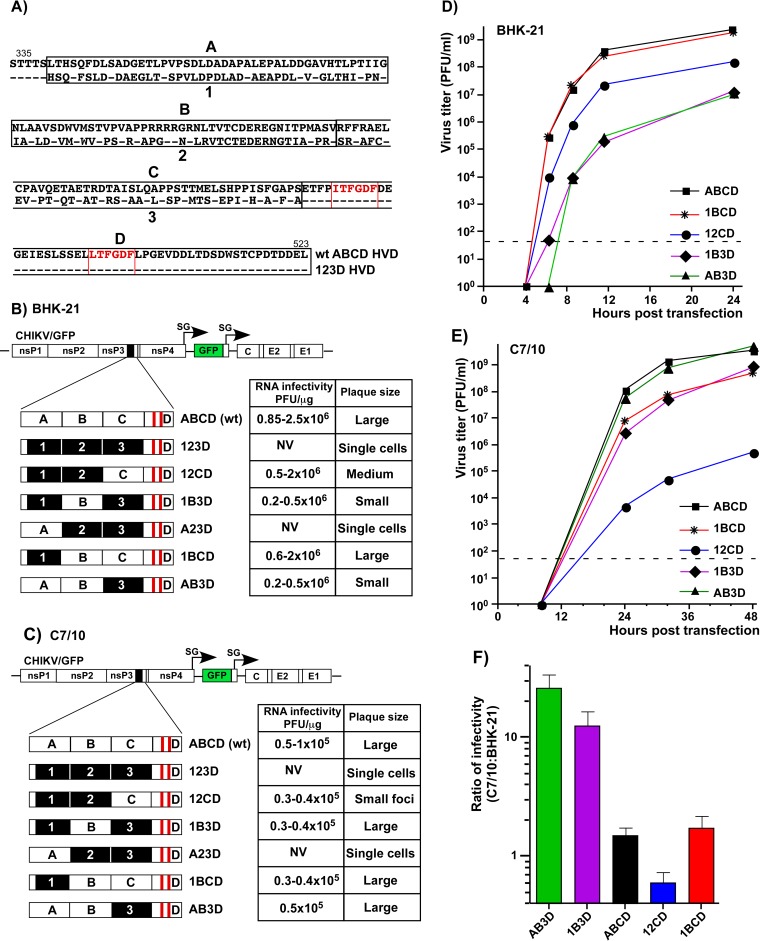
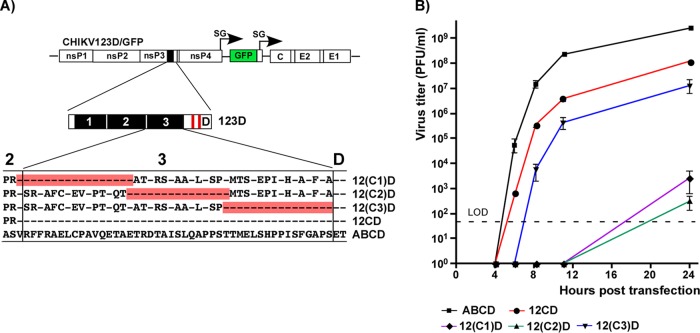
The designed CHIKV/artHVD/GFP mutant was further exploited as a basis for dissecting the functions of natural HVD sequences in CHIKV replication. The fragment of artHVD located between the G3BP-binding sequence and AUD was divided into 3 parts of similar lengths (Fig. 4A). In the cDNA of the CHIKV/artHVD/GFP genome, each of them and their combinations were replaced by the wt counterparts (Fig. 4B and C). For the sake of clarity in presentation of the results, the mutated fragments are labeled 1, 2, and 3, and their wt counterparts are labeled A, B, and C (Fig. 4A, B, and C). The in vitro-synthesized recombinant viral genomes were transfected into BHK-21 and mosquito C7/10 cells to assess the RNA infectivity and viral growth rates. These experiments revealed an interesting interplay of different HVD fragments in determining the level of CHIKV replication in both cell types. The overall dependence of replication rates on the HVD sequence was reproducible in multiple repeating experiments and can be summarized as follows. (i) As described above, the intact G3BP-binding fragment D alone in CHIKV/123D/GFP nsP3 could not drive virus replication in both cell types, and this variant was not viable. (ii) The addition of adjacent, immediately upstream-located fragment C made the CHIKV/12CD/GFP variant viable and capable of replication in BHK-21 cells, albeit not to the wt level (Fig. 4B and D). However, it remained poorly replicating in mosquito C7/10 cells (Fig. 4C and E). In the latter cells, infectivity of CHIKV/12CD/GFP RNA remained essentially at wt level, but at any time postelectroporation, titers of the released virus were 4 to 5 orders of magnitude lower (Fig. 4E). Accordingly, CHIKV/12CD/GFP formed smaller than wt but readily visible plaques in BHK-21 cells and only small foci of GFP-positive cells, but not plaques, in the mosquito cell line (Fig. 4C). (iii) An addition of wt fragment B had a more positive impact on replication of CHIKV/1B3D/GFP variant in C7/10 than BHK-21 cells (Fig. 4C and E). In contrast to CHIKV/123D/GFP, the B fragment-containing CHIKV/1B3D/GFP was viable in BHK-21 cells, but at any time, its titers remained 3 orders of magnitude below those of the virus with natural, wt HVD. (iv) The replacement of the most amino-terminal fragment 1 by its counterpart A (CHIKV/A23D/GFP variant) did not yield noticeable positive effect on infectious virus release and development of spreading infection and remained not viable in both cell types (Fig. 4B and C). (v) However, the same fragment A had detectable positive impact on virus replication in mosquito cells when combined with B and D sequences in CHIKV/AB3D/GFP (Fig. 4E). The latter variant replicated in C7/10 cells to the levels of wt CHIKV/GFP that encoded the entire ABCD-containing HVD (Fig. 4E). (vi) The addition of fragment C to B- and D-containing HVD in CHIKV/1BCD/GFP made the latter variant capable of replication in BHK-21 cells at essentially wt level (Fig. 4D). However, in C7/10 cells, growth rates of CHIKV/1BCD/GFP remained below wt (Fig. 4E) and were similar to those of CHIKV/1B3D/GFP mutant. Thus, the experimental data strongly suggested that fragment C of CHIKV HVD plays a critical role in viral replication in mammalian BHK-21 cells, and fragment B is more important for CHIKV replication in the mosquito C7/10 cell line.
FIG 4.
Fragments of CHIKV nsP3 HVD differentially regulate viral replication. (A) Alignment of amino acid sequences of wt ABCD HVD and mutated 123D HVD counterpart. Fragments A, B, and C in wt HVD and the corresponding mutated fragments 1, 2, and 3 are indicated by black boxes. Red boxes indicate positions of the G3BP-binding sites. Dashes indicate identical aa. (B) Schematic presentation of the original CHIKV/GFP genome and modified HVDs in the genomes of the designed viruses. Equal amounts (3 μg) of the in vitro-synthesized RNAs of the indicated constructs were electroporated into BHK-21 cells, and RNA infectivities and plaque sizes were compared in the ICA. (C) Schematic presentation of HVDs of the same viruses as in panel B, shown for clarity of presentation. Equal amounts of the in vitro-synthesized RNAs of the indicated constructs were electroporated into C7/10 cells, and RNA infectivities and plaque sizes were compared in the ICA performed on C7/10 cells. NV, not viable. (D) Replication rates of the indicated variants were determined in the experiment presented in panel B. Samples were harvested at the indicated time points, and viral titers were determined by plaque assay on BHK-21 cells. The experiments were repeated multiple times using different sets of RNAs with consistent results. (E) Replication rates of the indicated variants were determined in the experiment presented in panel C. At the indicated time points, viral titers were determined by plaque assay on C7/10 cells. The experiments were repeated multiple times using different sets of RNAs with consistent results. The experiments presented in panels D and E show the data of the most representative experiments that used all the indicated variants together. (F) Titers of multiple, randomly selected samples of the indicated viruses harvested from BHK-21 and C7/10 cells were determined in parallel on both BHK-21 and C7/10 cells. The ratios of the determined titers are presented as means and SDs.
Dissection of functional elements in HVD fragments C and B.
Comparing the infectious titers of harvested viral samples on vertebrate and mosquito cell lines provided additional information about the replication step determined by HVD sequences. Stocks of viruses that encoded fragment 3 (mutant) instead of wt fragment C demonstrated 10- to 20-fold-lower infectious titers on BHK-21 than on C7/10 cells (Fig. 4F). This was an indication that in addition to the repeat-containing fragment D, the upstream-located fragment C plays a major role in CHIKV replication in the cell line of vertebrate origin. Lower infectivity of the viruses suggested that the function of C fragment is particularly important at the early step of virus/RNA replication, likely during the formation of viral RCs.
To further dissect the HVD sequences that have a stimulatory effect on CHIKV replication in vertebrate cells, the above-described HVD-specific fragment C was additionally divided into three smaller segments, and each of them was cloned into CHIKV/123D/GFP variant to replace the corresponding mutated counterpart in fragment 3 (Fig. 5A). Equal amounts of the in vitro-synthesized recombinant viral genomes were transfected into BHK-21 cells, and replication rates of the viruses were compared (Fig. 5B). The addition of only-16-aa-long wt peptide, which is adjacent to G3BP-binding-site-containing fragment D, made the designed CHIKV/12(C3)D/GFP variant viable. Thus, the short wt HVD sequence located immediately upstream of G3BP-binding-repeat-containing fragment, but not the other two tested aa sequences, efficiently stimulated virus replication. However, the positive effect of the 16-aa-long peptide was not as strong as that of the entire fragment C (Fig. 5B). The detected stimulatory effect was not specific just to BHK-21 cells. The CHIKV/123(C3)D/GFP variant showed similar replication trends in other tested cell lines, such as HEK 293 and NIH 3T3 cells (data not shown).
FIG 5.
The carboxy terminus of fragment C determines CHIKV replication in vertebrate cells. (A) Schematic presentation of CHIKV/123D/GFP genome and aa sequences of fragment 3 that contained the peptides of mutated HVD restored to wt sequence. The restored fragments are indicated in red, and dashes indicate aa that are identical to those in wt CHIKV nsP3 HVD. (B) BHK-21 cells were electroporated with 3 μg of the in vitro-synthesized RNAs of the indicated constructs. Media were replaced at the indicated time points, and viral titers were determined by plaque assay on BHK-21 cells. The experiments were repeated three times with highly reproducible results. LOD, limit of detection.
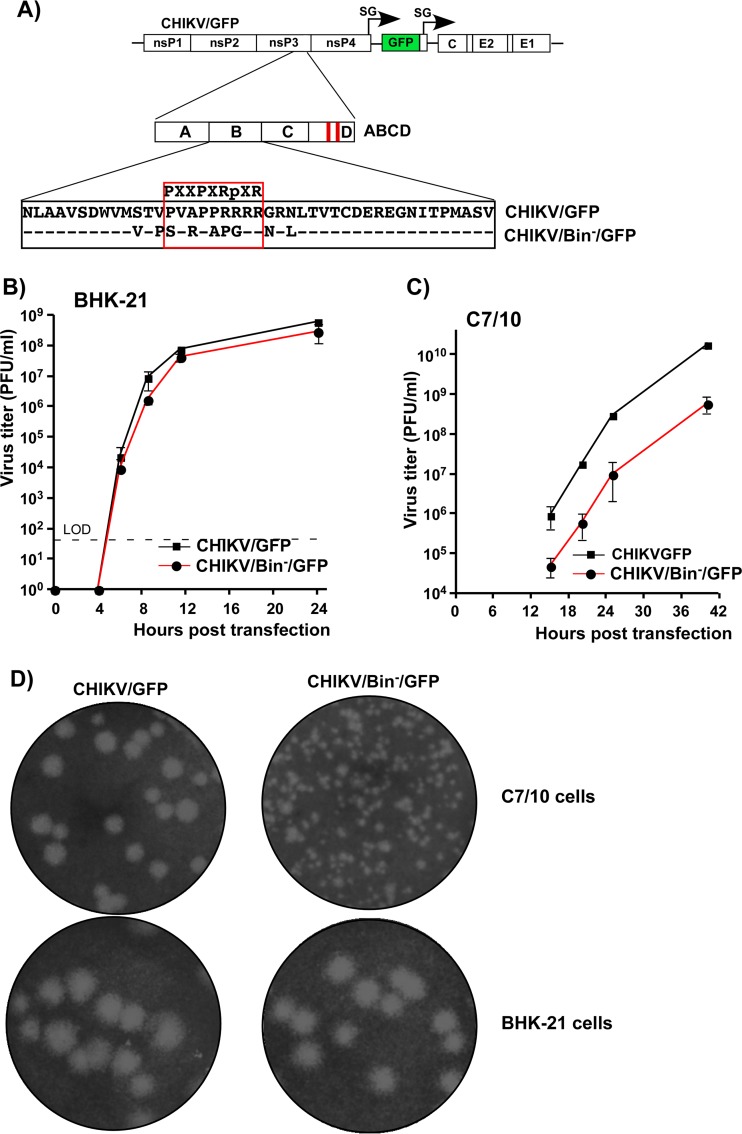
Similarly, CHIKV nsP3 HVD-specific fragment B was additionally investigated in terms of defining the critical element that determines virus replication. The previously published data demonstrated that the HVDs of the studied OW alphaviruses, including CHIKV, contain a proline-rich, SH3-binding motif that can bind with high affinity to BIN1 (amphiphysin II) protein (43). In our study, we introduced a combination of point mutations into this motif of CHIKV HVD (CHIKV/Bin−/GFP) (Fig. 6A) and analyzed their effect on viral replication in BHK-21 and C7/10 cells. Equal amounts of in vitro-synthesized CHIKV/Bin−/GFP and CHIKV/GFP RNAs were electroporated into both cell lines, and viral samples were harvested at different times postelectroporation for assessment of infectious titers. In repeated experiments, these viruses replicated with equal efficiency in BHK-21 cells (Fig. 6B). However, in C7/10 cells, the replication rate of CHIKV/Bin−/GFP was almost 100-fold lower than that of CHIKV/GFP, which encoded wt HVD (Fig. 6C). In addition, the decrease in replication in mosquito cells was further corroborated by a difference in plaque sizes recorded for CHIKV/Bin−/GFP compared to CHIKV/GFP (Fig. 6D). The designed mutant showed smaller plaques in C7/10 and the same-size plaques in BHK-21 cells. Thus, in agreement with the above data on the mapping of the critical aa sequences in CHIKV HVD (Fig. 4), these experiments showed that the previously described BIN1-SH3-binding motif (44) plays an essential role in CHIKV replication in insect cells rather than in the cells of vertebrate origin.
FIG 6.
Proline-rich, SH3-binding motif in CHIKV HVD is more important for viral replication in mosquito than in vertebrate cells. (A) Schematic presentation of CHIKV/GFP genome and its derivative CHIKV/Bin−/GFP with mutated SH3-binding motif. Dashes in the alignment indicate aa that are identical in wt and mutated CHIKV nsP3 HVD. (B and C) BHK-21 and C7/10 cells, respectively, were electroporated with 3 μg of in vitro-synthesized RNAs. At the indicated time points, media were replaced and viral titers were determined by plaque assay on BHK-21 cells. LOD, limit of detection. These experiments were repeated twice using different amounts of RNA and produced consistent results. (D) Size of plaques formed by parental CHIKV/GFP and its CHIKV/Bin−/GFP derivative on BHK-21 and C7/10 cells. For both cell lines, plaques were stained by crystal violet at 3 days postinfection.
Fragments of CHIKV HVD interact with specific cellular proteins.
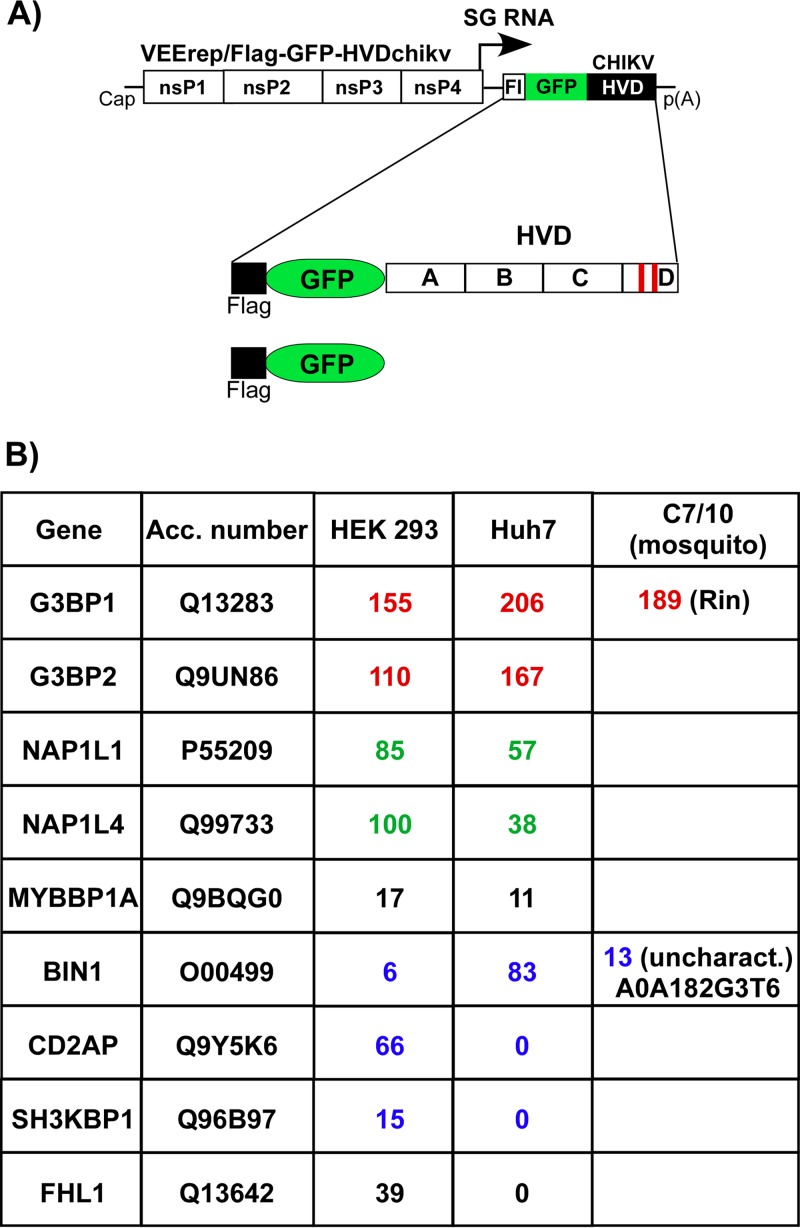
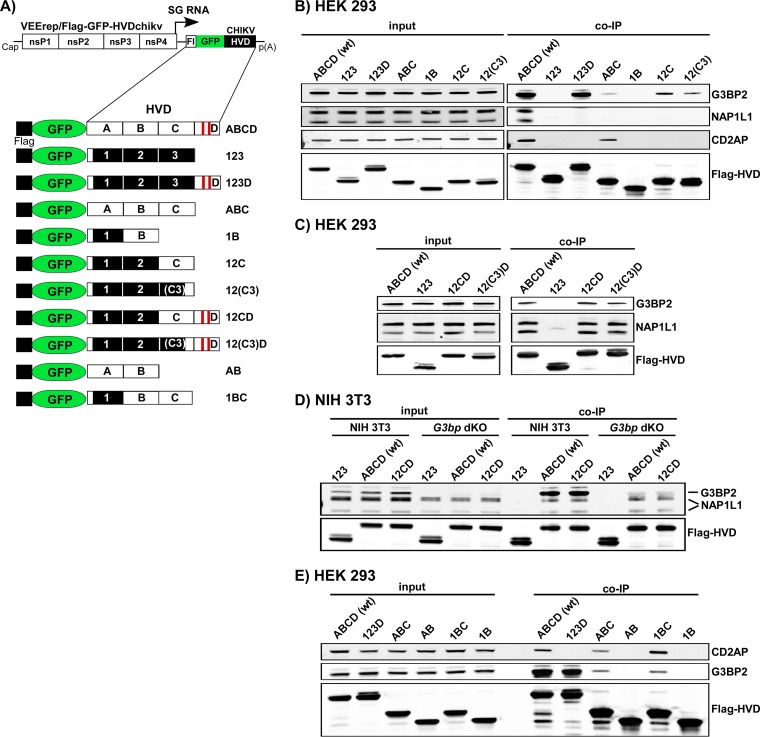
The above data suggested that CHIKV HVD may interact with a variety of host factors, and these interactions determine virus replication in different cell types. To identify the interacting host proteins, we expressed CHIKV nsP3 HVD from VEEV replicon as Flag-GFP-HVD fusions (Fig. 7A). This expression system was applied based on the previous data that VEEV and CHIKV HVDs utilize different host factors for building higher-order complexes involved in virus replication (34). Thus, VEEV nsP3 that is encoded by the replicon itself does not compete with expressed Flag-GFP-HVDchikv fusion for binding factors, particularly at early times postinfection (p.i.). VEEV replicons were packaged into infectious viral particles and used to infect both human and mosquito cells. Complexes of HVD-interacting cellular proteins were isolated at ∼2 h p.i., when expression of Flag-GFP-HVDchikv remained very low and thus physiologically relevant, using magnetic beads with Flag-specific monoclonal antibody (MAb) (see Materials and Methods for details). The coisolated cellular proteins were separated using SDS-PAGE and identified by mass spectrometry. To determine only proteins that interact with HVD with high affinity, we have used a highly stringent cutoff. In agreement with our previously published data (34, 35), Flag-GFP-HVDchikv coprecipitated large amounts of G3BP1 and G3BP2 from any vertebrate cells used and Rin protein from C7/10 cells. In vertebrate cells, CHIKV HVD also coimmunoprecipitated cellular SH3-binding proteins BIN1, CD2AP, and SH3KBP1. In mosquito C7/10 cells, it interacted with A0A182G3T6, the mosquito BIN1 homolog (Fig. 6B). Interestingly, isolation of CD2AP/SH3KBP1 and BIN1 was strongly dependent on the cell type. In human Huh7 and mouse NIH 3T3 cells (35), BIN1, but not CD2AP or SH3KBP1, efficiently interacted with CHIKV HVD. However, in another human cell line, HEK 293 cells, CHIKV HVD preferentially interacted with CD2AP and SH3KBP1, but not with BIN1. The extended list of CHIKV HVD-interacting host proteins in different cell lines is presented in Data Set S1 in the supplemental material.
FIG 7.
Host factors binding to CHIKV HVD in different cell types. (A) Schematic presentation of VEEV replicons that encode Flag-GFP-HVDchikv fusion or Flag-GFP. (B) Indicated cell lines were infected with packaged VEErep/Flag-GFP-HVDchikv replicon or the control replicon expressing only Flag-GFP at an MOI of 20 IU/cell. Cells were harvested at 2 h postinfection, and complexes were immunoprecipitated as described in Materials and Methods. Proteins were separated by SDS-PAGE and identified by mass spectrometry. Total spectra for the most abundant proteins are presented. Groups of functionally similar proteins are indicated by different colors. None of the presented proteins were detected in the control samples generated using Flag-GFP. Acc. number, accession number for UniProt database.
The coimmunoprecipitation (co-IP) samples from both human cell lines also contained high levels of NAP1L1 and NAP1L4 proteins, which we previously found to uniquely interact only with CHIKV HVD and not HVDs of other alphaviruses in mouse NIH 3T3 and hamster BHK-21 cells (34, 35). No NAP1 homolog was found in the co-IP samples derived from mosquito cells. Another coisolated cellular protein was FHL1, but it was present only in the samples isolated from Huh7 cells, not from HEK 293 cells. Interestingly, we have previously found its homolog FHL2 in the co-IP samples from mouse fibroblasts (35).
To confirm the results and to further explore the binding sites, samples from the independent co-IP experiments were analyzed by Western blotting. For this study, we used VEEV replicons expressing various combinations of wt and mutated CHIKV HVD fragments fused with Flag-GFP (Fig. 8A). The specific Abs detected the presence of G3BP in the samples isolated from the cells infected with the constructs encoding the D fragment, VEErep/Flag-GFP-ABCD and VEErep/Flag-GFP-123D (Fig. 8B). Unexpectedly, low levels of G3BP were also found in the co-IP samples from the cells infected with VEErep/Flag-GFP-ABC and VEErep/Flag-GFP-12C that lacked CHIKV HVD fragment D, which contained the canonical G3BP-binding repeat. The low-level binding of G3BP to HVD in the absence of binding repeats indicated the existence of an additional G3BP-binding site(s) in fragment C. Therefore, we then focused on the carboxy-terminal 16-aa-long sequence of fragment C, located immediately upstream of fragment D, which was found crucial in the replication earlier in this study (Fig. 5). We expressed Flag-GFP-12(C3) fusion and analyzed the presence of G3BP in the co-IP sample. The results presented in Fig. 8B demonstrated that the carboxy-terminal element of the C fragment is indeed capable of G3BP binding, despite that its sequence does not contain the canonical G3BP-binding elements (34, 38). However, the presented Western blot analysis suggested that the efficiency of this binding is relatively low.
FIG 8.
Mapping of G3BP-, NAP1-, and CD2AP-binding sites in CHIKV nsP3 HVD. (A) Schematic presentation of VEEV replicon and expressed Flag-GFP-HVD cassettes that contained wt and mutated CHIKV HVD fragments. Fragments with wt aa sequences are indicated by open boxes and labeled A, B, C, and D, and mutated counterparts of A, B, and C are indicated by black boxes and labeled 1, 2, and 3. (B, C, D, and E) Indicated cell lines were infected with packaged replicons encoding indicated fragments of CHIKV HVD. At 2 h postinfection, protein complexes were isolated using magnetic beads with Flag-specific MAbs as described in Materials and Methods. The presence of studied cellular proteins was analyzed by Western blotting using specific Abs and corresponding secondary Abs labeled with different infrared dyes. Membranes were scanned on a Li-Cor imager. The following primary antibodies were used: anti-G3BP2 rabbit polyclonal antibodies (A302-040; Epitomics), anti-CD2AP rabbit polyclonal antibodies (sc-9137; Santa Cruz), anti-NAP1L1 rabbit polyclonal antibodies (14989-1-AP; Proteintech), and anti-Flag mouse monoclonal antibodies (F1804; Sigma).
The members of an NAP1 family, NAP1L1 and NAP1L4, were reproducibly precipitated with CHIKV HVD from vertebrate HEK 293, Huh7, and NIH 3T3 cells (Fig. 7B) (34, 35). Western blot analysis with NAP1L1-specific Abs also definitively detected the latter protein in the co-IP samples generated on the cells infected with VEErep/Flag-GFP-ABCD but not with any other constructs, including VEErep/Flag-GFP-ABC and VEErep/Flag-GFP-123D (Fig. 8B). The most plausible explanation for the lack of NAP1L1 binding to CHIKV HVD deletion mutant constructs was that NAP1L1 binding requires the presence of intact aa sequences of both fragments C and D. Indeed, two isoforms of NAP1L1 were readily detectable in the samples generated from the cells infected with replicons expressing Flag-GFP-12CD and Flag-GFP-12(C3)D fusions (Fig. 8C). This was an indication that NAP1L1 interacts with HVD on the border between C and D fragments (see Fig. 11). Taken together, the data suggested that C3 fragment takes part in interaction with G3BP and/or NAP1 proteins, and thus, a possibility remained that NAP1 proteins bind to CHIKV HVD indirectly through interaction with G3BPs. Therefore, we infected NIH 3T3 G3bp dKO cells, which do not express G3BP1 and G3BP2, with VEErep/Flag-GFP-12CD and VEErep/Flag-GFP-12(C3)D replicons and coimmunoprecipitated HVD-binding proteins (Fig. 8D). NAP1L1 was readily identified in the samples generated from G3bp dKO cells, suggesting that this protein directly interacts with CHIKV HVD.
FIG 11.
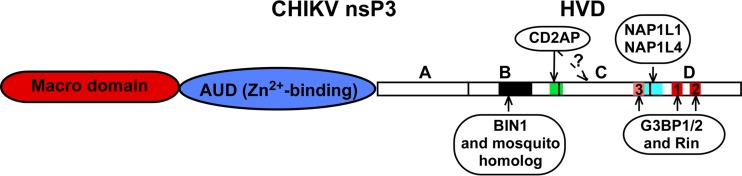
Locations of to-date identified binding sites of cellular proteins in CHIKV nsP3 HVD. CHIKV nsP3 is structurally divided into three domains, which include macro domain, AUD, and HVD. In this study, HVD was further divided into the indicated four fragments, A, B, C, and D. The identified locations of binding sites for cellular proteins are indicated in different colors. A question mark indicates that a possibility remains that CD2AP requires interaction with more than one HVD motif for efficient binding. “3” indicates a position of a noncanonical G3BP-binding site in C3 peptide of CHIKV HVD.
Another vertebrate protein identified by mass spectrometry as interacting with CHIKV HVD (ABCD construct) was CD2AP. Further analysis of its interaction with CHIKV HVD revealed an interesting pattern of its binding to the HVD aa sequences. CD2AP was found only in the co-IP samples from the cells expressing either the full-length ABCD or ABC or BC fragments of HVD (Fig. 8B and E). This was an indication that it likely interacts with the peptide that includes the carboxy-terminal sequence of fragment B and amino terminus of fragment C (see Fig. 11). The most recently published study confirmed the location of this binding site (45).
HVD determines cell specificity of alphavirus replication.
The above data (Fig. 4) suggested that nsP3 HVD interactions with different host factors determine cell specificity of CHIKV replication. In an attempt to additionally examine this possibility, we introduced into this study another alphavirus, Eilat virus (EILV) (46). The latter virus replicates exclusively in mosquito cells. One of the reasons for such cell specificity is that its nsPs facilitate viral RNA replication only in the cells of insect origin. Despite its predisposition to mosquito cell lines, EILV shares genome strategy and nsP identity with other, better-studied alphaviruses, such as SINV, SFV, and VEEV. The sequence alignment of CHIKV and EILV nsP3 HVDs showed that they have essentially no similarity at the aa level with the exception that both domains are enriched in proline-rich motifs that can potentially bind SH3 domain-containing proteins (Fig. 9A). Another important characteristic of EILV relevant to this study is that the carboxy terminus of its nsP3 HVD contains the same canonical repeating aa elements that have been shown to interact with G3BPs in CHIKV HVD (Fig. 9A). In the context of EILV, the latter repeat mediates interaction with the insect-specific homolog of G3BP, Rin (data not shown). In addition, EILV HVD is more than 60 aa shorter than the CHIKV-specific counterpart.
FIG 9.
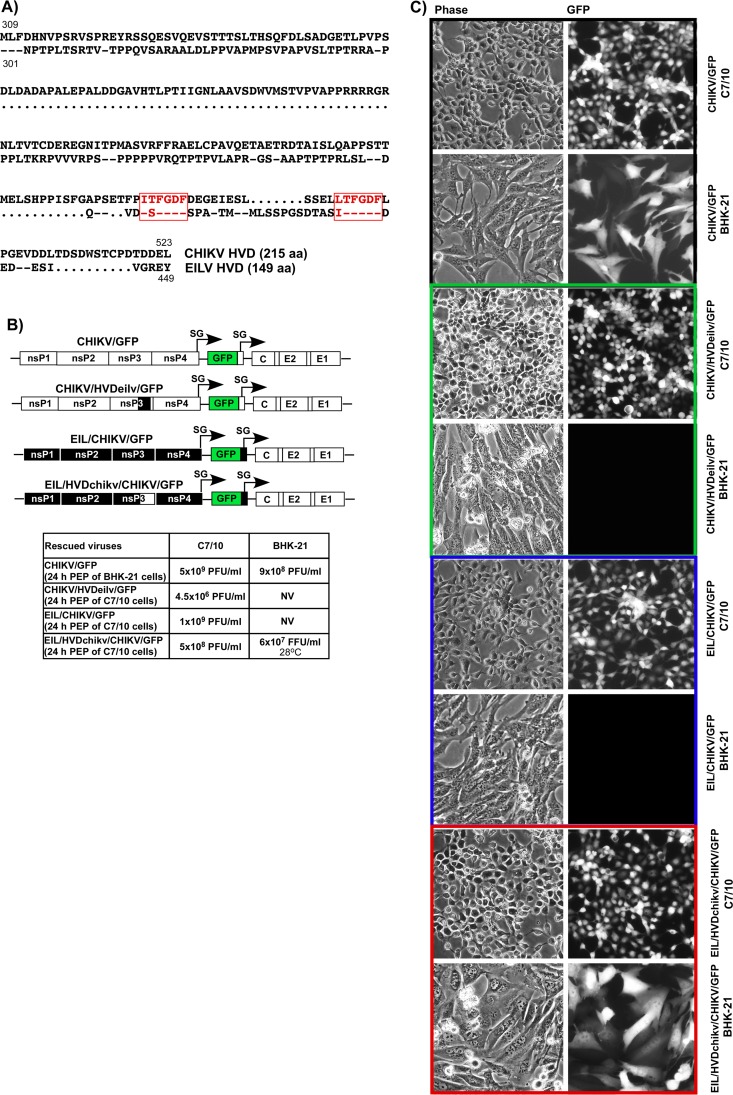
CHIKV and EILV HVDs determine cell specificity of viral replication. (A) Alignment of the aa sequences of CHIKV and EILV HVDs. Dots indicate gaps introduced for better alignment. Dashes indicate identical aa. Red boxes indicate positions of the carboxy-terminal, G3BP/Rin-binding repeats. (B) Schematic presentations of recombinant alphavirus CHIKV and EILV/CHIKV genomes with homologous and heterologous nsP3 HVDs. The white and black boxes indicate CHIKV and EILV genomes, respectively. Titers of rescued viruses after electroporation of 3 μg of the in vitro-synthesized RNAs into indicated cell lines. Titers of the stocks were determined by plaque assay on both BHK-21 and C7/10 cells. NV, not viable. Titers of plaque-forming viruses are shown as PFU/ml, and titers of noncytopathic viruses were measured in GFP-positive focus-forming units (FFU/ml). (C) BHK-21 and C7/10 cells were infected with the indicated viruses, which were rescued by electroporation of permissive cells. Images were taken on a fluorescence microscope.
Based on CHIKV and EILV sequences, we designed a set of recombinant alphavirus genomes and investigated their replication. One of the plasmids contained the genome of the previously designed CHIKV/GFP and encoded wt HVD (Fig. 9B). The second plasmid contained cDNA of the chimeric EIL/CHIKV/GFP (Fig. 9B). Its G RNA encoded the replication machinery of EILV, and the structural genes were derived from CHIKV 181/25, because the natural EILV glycoproteins do not facilitate viral entry into vertebrate cells (47, 48). However, replication of this chimeric virus remains restricted to mosquito-derived cell lines (see below). On the basis of CHIKV/GFP, we designed a CHIKV/HVDeilv/GFP chimeric construct, in which the natural HVD in CHIKV-specific nsPs was replaced by that derived from EILV. Another chimeric viral genome, EIL/HVDchikv/CHIKV/GFP, encoded heterologous CHIKV HVD in EILV ns polyprotein instead of the natural, EILV-specific counterpart.
G RNAs of these 4 constructs were synthesized in vitro and transfected into both BHK-21 and mosquito C7/10 cells. The control CHIKV/GFP RNA efficiently initiated replication in both cell types (Fig. 9B and C), while electroporation of EIL/CHIKV/GFP RNA induced virus replication only in mosquito cells. In repeating experiments, after electroporation of EIL/CHIKV/GFP RNA into BHK-21 cells, no infectious virus was recovered. Within 24 h postelectroporation, very few cells became GFP positive, but no spreading infection was detected during further incubation at either 37°C or 28°C.
In contrast to EIL/CHIKV/GFP, the EIL/HVDchikv/CHIKV/GFP chimera, which encoded CHIKV HVD in EILV nsP3, became viable in both cell types. In BHK-21 cells, it was producing readily detectable levels of GFP and was developing a spreading infection (Fig. 9B and C). Of note, this chimeric virus replicated in BHK-21 cells only at 28°C, a temperature that is used for the cells of mosquito origin, but not at the vertebrate cell-specific 37°C temperature. The most plausible explanation for this temperature-dependent replication is that EILV nsPs are highly adapted to mosquito cell-specific low incubation temperatures. Another distinguishing characteristic of EIL/HVDchikv/GFP/CHIKV was its inability to cause a cytopathic effect (CPE) in BHK-21 cells (data not shown). Even after a 4-day-long incubation of cells under agarose cover at 28°C, we detected only the large foci of GFP-positive cells, but not plaque formation. Most likely, EILV-specific nsP2 does not have nuclear functions, which are exhibited by the OW alphaviruses and are the critical contributor to CPE development and inhibition of the innate immune response (49, 50). The reciprocal replacement of CHIKV HVD by that derived from EILV in CHIKV/HVDeilv/GFP restricted replication of the latter virus to mosquito cells only (Fig. 9B and C). In contrast to parental CHIKV/GFP, the chimeric virus was no longer viable in BHK-21 cells, which are normally highly permissive for CHIKV replication.
These experimental data strongly suggested that HVDs play critical roles in the adaptation of CHIKV and EILV to replication in particular cell types. Despite having very similar G3BP-binding repeating peptides, heterologous HVDs changed cell specificities of replication for both viruses. This was a strong support for the hypothesis that HVD fragments located upstream of the carboxy-terminal G3BP/Rin-binding repeat play an indispensable role in CHIKV replication.
The above data strongly suggested that fragment C of CHIKV HVD, whose presence restores the NAP1-binding aa sequence, plays a critical role in defining virus replication in vertebrate cells. To additionally test this hypothesis, the CHIKV-specific fragment C was cloned upstream of the heterologous EILV-specific fragment, containing the G3BP/Rin1-binding aa repeat, into CHIKV/HVDeilv/GFP (Fig. 10A). The addition of this small fragment made the resulting recombinant CHIKV/HVDeilv(C)/GFP viable in BHK-21 cells. The in vitro-synthesized RNA of this chimeric virus demonstrated the same infectivity as that of CHIKV/GFP. This was an indication that in contrast to parental CHIKV/HVDeilv/GFP, the C-fragment-containing chimera is viable and does not require additional adaptive mutations for initiation of replication. However, at any time postelectroporation, infectious titers of CHIKV/HVDeilv(C)/GFP were 30- to 50-fold below those of CHIKV/GFP. Thus, growth rates of CHIKV/HVDeilv(C)/GFP generally mimicked those of CHIKV, encoding 12CD HVD (Fig. 4D). This was an indication that despite having the C fragment, the chimeric HVD cannot mediate interaction with the complete set of host factors essential for CHIKV replication in vertebrate cells. These results additionally supported our hypothesis that nsP3 HVD is a critical determinant of specificity of CHIKV replication for different cells, with multiple binding host factors being involved.
FIG 10.
Addition of CHIKV HVD fragment C to EILV HVD makes the resulting chimeric CHIKV/HVDeilv(C)/GFP capable of replication in vertebrate cells. (A) Schematic presentation of recombinant CHIKV genomes with wt or heterologous HVDs, infectivity of the in vitro-synthesized RNA in ICA on BHK-21 cells, and titers of viruses harvested 24 h after electroporation of BHK-21 cells. (B) Replication rates of the indicated viruses in BHK-21 cells after electroporation of the in vitro-synthesized RNAs. Viral titers at the indicated time points were determined by plaque assay on BHK-21 cells. (C) GFP expression by replicating viruses in BHK-21 and C7/10 cells. Cells were infected with the indicated viruses at an MOI of 5 PFU/cell. Stocks of CHIKV/GFP and CHIKV/HVDeilv(C)/GFP used for infection were generated on BHK-21 cells, and the stock of CHIKV/HVDeilv/GFP was generated on C7/10 cells. Images were acquired on a fluorescence microscope at 8 h and 16 h after infection of BHK-21 and C7/10 cells, respectively.
DISCUSSION
In recent years, alphavirus nsP3 has emerged as a key player in the assembly and function of viral RCs that serves as a hub for recruitment of numerous host proteins required for viral RNA replication. The amino-terminal macro domain of nsP3 (Fig. 11) exhibits a high level of conservation between alphaviruses and other positive-strand RNA viruses. The macro domain has been demonstrated to bind ADP-ribose, poly(ADP)-ribose, and RNA and has a detectable level of mono(ADP-ribosyl)hydrolase activity (29, 51, 52). The earlier reports have shown that the macro domain can acquire adaptive mutations in response to strong modifications in RNA promoter elements in the alphavirus genome (32, 53). This implied its potential role in viral G RNA replication.
The next conserved Zn-binding domain (Fig. 11), termed AUD, appears to be present only in alphavirus nsP3 and does not have homologs in the nonstructural proteins of other viruses (30). There is no clear information about its function in the literature, except that this and a few other studies have shown that some adaptive mutations in AUD compensated for changes in the downstream-located hypervariable domain, HVD (Fig. 3) (32, 54).
HVD (Fig. 11) exhibits an exceptionally low level of identity between different alphavirus species, and before this study, the definitive information about the CHIKV nsP3 HVD folding was lacking. We carried out detailed characterization of a 199-aa-long CHIKV HVD by NMR spectroscopy at atomic resolution. The results revealed the absence of stable secondary structures in this domain and clearly demonstrated that CHIKV HVD was present in solution in a disordered conformation. Consequently, all of the modifications generated in CHIKV HVD as part of this study could not have affected its secondary structure.
Within recent years, alphavirus HVDs have attracted a lot of attention. The studies of the HVD function in VEEV, EEEV, SFV, SINV, and CHIKV replication strongly suggested that this domain mediates assembly of prereplication complexes at the plasma and endosomal membranes in vertebrate and mosquito cells (32, 34, 35, 38). These evidences implied a critical role of nsP3 HVD in early stages of the alphavirus life cycle. At later times postinfection, it is also involved in the formation of large protein complexes, but their function at this time remains unclear (14, 36, 55). One of the intriguing characteristics of nsP3 HVDs is their ability to interact with distinct sets of cellular proteins, which are specific for virus species and cell types (34–36). Another important feature is a high level of redundancy in the function of these HVD-binding cellular factors in RC assembly and RNA replication. Instead of single host proteins, alphaviruses utilize entire families, such as G3BP or FXR protein families (34–36). Moreover, the interaction of HVDs with particular cellular proteins can be destroyed by either specific mutations in the HVDs or KO of corresponding cellular genes (34, 56). Such changes usually strongly affect replication rates of most alphaviruses but do not make them nonviable. This points to another level of redundancy in that other binding partners can to some extent facilitate readily detectable levels of RC assembly and function. However, the results of this and our previous study (34) demonstrate that compared to other alphaviruses, CHIKV exhibits a unique characteristic. The host factors that belong to different protein families do not have the redundant function(s) in CHIKV RNA replication, and lack of CHIKV HVD interaction with individual cellular proteins or protein families can make virus nonviable. Thus, CHIKV represents a very convenient experimental system for studying functions of different HVD-binding proteins in viral replication.
G3BP proteins of vertebrate cells and their Rin homolog in mosquito cells have been shown to interact with HVDs of the OW alphaviruses and that of EEEV, the NW alphavirus representative (34, 35, 38). For most of the viruses, except EEEV, this interaction is mediated by short aa repeats located at the carboxy termini of their HVDs (38). In the case of CHIKV, G3BP binding is particularly critical. In contrast to the results of the studies performed on SINV and SFV, deletion of even a single element of the G3BP-binding HVD repeat had a strong negative effect on CHIKV replication in both vertebrate and mosquito cells (Fig. 2) (34). Deletion of both elements, in turn, completely abrogated virus replication. This lack of G3BP interaction with HVD-specific repeats may potentially affect virus ability to block the formation of stress granules, which have been proposed to have an antiviral function (57, 58). However, G3bp dKO cells do not support replication of the OW alphaviruses as efficiently as the wt cell lines, although the stress granules cannot be formed (34). Moreover, the NW alphavirus VEEV does not induce redistribution of G3BPs into virus-specific complexes (31, 32). Taken together, these data imply that G3BP-HVD interactions are mainly important for the OW alphavirus RC assembly, and the role of this interaction in stress response to viral infection has to be reevaluated.
Our data have demonstrated that G3BP/Rin binding to HVD is indispensable for CHIKV replication in both vertebrate and mosquito cells. Nevertheless, this interaction alone is insufficient for supporting CHIKV replication. The extensive mutagenesis of CHIKV HVD that eliminated all of the potential binding sites, except those specific to G3BP, made CHIKV/artHVD/GFP virus nonviable (Fig. 3). Similarly, the replacement of natural CHIKV HVD by the EILV-derived counterpart, which also contains a canonical G3BP-binding aa repeats, made the chimeric virus incapable of replication in vertebrate cells (Fig. 9). Sequential restoration of wt sequences in the artificially designed artHVD (Fig. 4) clearly demonstrated that at least one additional binding site for other cellular proteins can restore CHIKV/artHVD/GFP replication, albeit not to the wt level.
Interestingly, further restoration of HVD sites that bind specific host proteins differentially stimulated CHIKV replication in mosquito and vertebrate cells (Fig. 4). The binding site that is important for CHIKV replication in the cells of vertebrate origin was located in the HVD immediately upstream of the G3BP-binding repeats (Fig. 5). Addition of this aa sequence to either carboxy-terminal, repeat-containing CHIKV HVD fragment (Fig. 5 and 8) or to EILV HVD that contained a similar Rin-binding repeat (Fig. 10) restored the ability of CHIKV to replicate in vertebrate cells. This critical HVD-specific peptide was found to interact with cellular NAP1L1 and NAP1L4 proteins. It also contained an additional binding site for G3BP (Fig. 8). However, the latter G3BP-HVD interaction was less efficient than that determined by the downstream-located, canonical repeating peptide, and this G3BP-binding element alone was incapable of driving CHIKV replication (Fig. 2). Therefore, the biological significance of this additional interaction is unlikely but remains a possibility. We have not investigated it further in this study.
Interaction with NAP1L1 is independent of the G3BP binding (Fig. 8), and NAP1L1 appears to be the primary candidate for promoting CHIKV replication in vertebrate cells. Similar to the results of our previous study of G3BP and FXR functions in alphavirus replication (34), here we found that two members of the NAP1 protein family likely facilitate CHIKV replication redundantly. Both NAP1 proteins were reproducibly coimmunoprecipitated with CHIKV HVD, but not with HVDs of other alphaviruses, such as EEEV, VEEV, and SINV (35) (Fig. 8). Therefore, their function in virus replication appears to be specific to CHIKV. Mutations in the NAP1-binding HVD fragment, fragment C, had a stronger effect on CHIKV RNA infectivity and viral replication rates in BHK-21 cells than in mosquito cells (Fig. 4). Importantly, we could not identify a mosquito homolog of NAP1 in immunoprecipitation experiments. This can be a plausible explanation for the poor stimulatory effect of intact fragment C on CHIKV/12CD/GFP replication in C7/10 cells. Based on available data, we hypothesize that similarly to FXRs and G3BPs, NAP1 proteins stimulate the formation of CHIKV replication complexes in vertebrate cells, and this effect might be also determined by the ability of NAP1 proteins to undergo multimerization (59). The exact mechanism of NAP1L1 and NAP1L4 function is now under investigation.
The histone chaperone NAP1 proteins are mostly localized in the cytoplasm. They serve as a carrier of histones during nuclear import, mediate nucleosome assembly and chromatin remodeling, and regulate critical processes in cell biology such as cellular transcription and cell cycle progression (60). Thus, KO or downregulation of the expression by RNA interference (RNAi) of both members of the NAP1 family will likely lead to cell death. To date, there are no published data that such NAP1L1 and NAP1L4 dKO cell lines can be viable. Despite this methodological limitation, the list of NAP1L1 functions is continuously growing. Recent studies have demonstrated interaction of NAP1L1 with the intrinsically disordered, carboxy-terminal fragment of HCV NS5A protein (61). However, the data about the role of this interaction in HCV biology are somewhat contradictory (61, 62).
The second important CHIKV HVD-specific peptide that is critical for viral replication in mosquito, but not in vertebrate BHK-21 or NIH 3T3, cells is represented by the proline-rich motif (Fig. 1A and 6A). This peptide has been previously implicated in interaction with BIN1 protein (44). The latter protein contains both Bin-Amphiphysin-Rus (BAR) and SH3 domains. The SH3 domain has been demonstrated to directly bind to CHIKV and SFV HVDs (43). The BAR domain was proposed to regulate membrane curvature formation during invagination of viral replication complex containing membranous spherules, but this function remains yet to be shown (44). To date, only BIN1, but not BIN2, has been found to interact with CHIKV HVD and HVDs of several OW alphaviruses (34, 35). Our new data suggest that the role of the proline-rich peptide interaction with host factors in viral replication is likely more complicated than was previously expected. In mosquito cells, the latter peptide indeed efficiently interacts with BIN1 homolog (Fig. 7), and the introduced mutations have a negative effect on CHIKV replication (Fig. 6). However, in hamster BHK-21 cells, the effect of the same mutations on virus replication was undetectable (Fig. 6). CHIKV HVD also showed interaction with BIN1 in murine NIH 3T3 and human Huh7 cells, but only very small numbers of BIN1-specific peptides were detected in CHIKV HVD co-IP samples derived from HEK 293 cells (Fig. 7) (35). No BIN1-HVD interaction was also found in human HOS cells (45). Instead, two other SH3 domain-containing proteins, CD2AP and SH3KBP1, coimmunoprecipitated with CHIKV HVD from HEK 293 cells, with CD2AP being the main HVD-interacting partner (Fig. 7 and 8). The amounts of CD2AP and SH3KBP1 in CHIKV HVD co-IP samples from NIH 3T3 cells were relatively small, and they were not coisolated from Huh7 cells (Fig. 7) (35). Thus, it appears that depending on the cell type, CHIKV HVD interacts with either BIN1 or CD2AP/SH3KBP1 proteins. Currently, it is not clear whether the difference in the efficiency of complex formation depends on the relative amount of these proteins in the specific cell lines or/and these proteins compete for binding to HVD. Our data suggest that the CD2AP binding site is located a few aa downstream of the BIN1-interacting peptide at the junction of B and C fragments (Fig. 8 and 11). Recently published data from another group also proposed this location (45). Interestingly, CD2AP and SH3KBP1 have three SH3 domains, and this suggests a possibility that to achieve high binding affinity, the latter proteins may require interaction with more than one HVD-specific proline-rich motif. Thus, a further structural analysis is needed for better understanding cell-specific interaction of BIN1 and CD2AP/SH3KBP1 with CHIKV HVD in different cell types. Using our experimental systems, we previously found that in NIH 3T3 cells, EEEV HVD interacted with four SH3 domain-containing host proteins that included CD2AP, SH3KBP1, and two SH3 and BAR domain-containing proteins, SNX9 and SNX33. However, in these cells, EEEV HVD did not bind BIN1 (35). In contrast to other alphaviruses, VEEV HVD did not detectably bind any BAR domain-containing proteins at all but instead interacted exclusively with CD2AP and SH3KBP1 in NIH 3T3 and BHK-21 cells. Hence, the above data and earlier reports suggest that different alphaviruses have evolved to exploit a wide variety of SH3 domain-containing proteins and appear to use them in a cell-specific mode.
The family of the SH3 domain-containing proteins is represented by ∼300 members (63). They are involved in a range of key processes of cell biology, such as endocytosis, membrane trafficking, and cytoskeletal organization (64). Their common characteristic is interaction with the proline-rich aa sequences, which demonstrate high diversity. The affinity of SH3 domains for the peptide ligand is relatively low, and selectivity is quite poor (63). Therefore, binding of a particular SH3 domain-containing protein is likely determined by both sequences of the binding motif and concentration of the protein relative to other SH3 domain-containing family members. Thus, despite the continuing progress in understanding the molecular mechanism of alphavirus replication, the functional role of cellular SH3 domain-containing proteins in this process remains elusive. Apparently, we are only at the beginning of understanding the complexity of CHIKV HVD interactions with SH3 domain-containing proteins in different cell types and hosts.
Interestingly, similarly to NAP1L1, BIN1 was shown to interact with the disordered fragment of hepatitis C virus (HCV)-specific NS5A protein (62, 65–67). Alteration of this interaction has a negative effect on HCV replication. Thus, NAP1L1 and BIN1 may have similar functions in replication of these two very different groups of positive-strand RNA viruses.
The results of this study demonstrate that CHIKV nsP3 HVD interacts with at least 3 families of cellular proteins. They include two members of the G3BP family, two members of the NAP1 family, and several members of the SH3 domain-containing protein family. The summary of our current knowledge of the location of the binding sites of these cellular factors on CHIKV HVD is presented in Fig. 11. All of these interactions are critically important for virus viability and determine the efficiency of its replication in a cell-specific mode. (i) Binding of G3BPs or mosquito homolog Rin is universally indispensable for viral replication in both vertebrate and mosquito cells. (ii) Interaction with NAP1 proteins with HVD is critical for CHIKV replication in vertebrate cells and is CHIKV specific. (iii) Binding of SH3 domain-containing proteins, which include BIN1, CD2AP, and SH3KBP1, to proline-rich motifs of HVD has a more proviral role in the cells of mosquito origin and plays a less important role during viral growth in vertebrate cells. Identification of different SH3 domain-containing proteins bound to CHIKV HVD in different vertebrate cells suggests that CHIKV and probably other alphaviruses have evolved to redundantly utilize very diverse members of this family for their replication. Manipulations with the set of binding sites in CHIKV HVD change the efficiency of CHIKV replication in different cell types. Similar modifications can likely be introduced into HVDs of other alphaviruses to specifically alter their replication rates.
MATERIALS AND METHODS
Cell cultures.
NIH 3T3, HEK 293, and Vero cells were obtained from the American Type Culture Collection (Manassas, VA). BHK-21 cells were kindly provided by Paul Olivo (Washington University, St. Louis, MO). These cell lines were maintained at 37°C in alpha minimum essential medium (αMEM) supplemented with 10% fetal bovine serum (FBS) and vitamins. The G3bp dKO cell line has been described elsewhere (34). Mosquito C7/10 cells were obtained from Henry Huang (Washington University, St. Louis, MO). They were propagated at 28°C in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% heat-inactivated (HI) FBS and 10% tryptose phosphate broth (TPB).
Plasmid constructs.
The original plasmids containing the infectious cDNAs of the attenuated strain CHIKV 181/25 and Eilat alphavirus (EILV) were kindly provided by Scott Weaver (University of Texas Medical Branch, Galveston, TX) (46, 68). Plasmids containing cDNAs of VEEV replicon encoding Flag-GFP or Flag-GFP fused with different fragments of CHIKV HVD, and plasmids carrying CHIKV and EIL/CHIKV genomes with mutated or heterologous HVDs were designed using standard PCR-based techniques. The detailed schematic presentations of HVDs and their aa sequences are shown in the corresponding figures. All of the introduced modifications were confirmed by sequencing. Plasmids carrying the genomes of helper constructs, which were used for packaging of RNA replicons into infectious viral particles, were described elsewhere (69). Sequences of the plasmids and details of the cloning procedures can be provided upon request.
In vitro RNA transcription and transfection.
Plasmids were purified by ultracentrifugation in CsCl gradients. Then, they were linearized using unique restriction sites located downstream of the poly(A) of viral, replicon, and helper genomes. RNAs were synthesized by SP6 RNA polymerase in the presence of a cap analog (New England BioLabs). Quality of the synthesized RNAs was tested by agarose gel electrophoresis, and aliquots of the transcription reaction mixtures were used for electroporation without additional RNA purification. Electroporations of BHK-21 and C7/10 cells by in vitro-synthesized virus-specific RNAs were performed under previously described conditions (70, 71). VEEV replicon genomes were packaged by coelectroporation of their in vitro-synthesized RNAs and homologous helper RNA. Viruses and packaged replicons were harvested at 20 to 24 h postelectroporation. Viral titers were determined by plaque assay on BHK-21 or C7/10 cells (72). The infectious titers of packaged replicons were determined by infecting BHK-21 cells (5 × 105 cells/well) in 6-well Costar plates with 10-fold dilutions of the samples and counting the number of GFP-positive cells at 6 h postinfection.
Analysis of virus replication.
Because of the capability of alphaviruses for rapid evolution to a more efficiently replicating phenotype, most of the analyses of viral replication were performed using the samples of RNA-electroporated cells. One-tenth of electroporated BHK-21 and C7/10 cells was seeded into 35-mm dishes, and media were replaced at the indicated times posttransfection (see corresponding figure legends for details). Viral titers in the harvested samples were determined by plaque assay on BHK-21 (72) or C7/10 (47) cells (see corresponding figure legends for details).
Infectious center assay (ICA).
Infectivities of viral RNAs were analyzed in parallel with analysis of viral replication. BHK-21 or C7/10 cells were electroporated with the same amounts of in vitro-synthesized genomes of the designed viral mutants and control genome encoding wt nsP3 HVD. Tenfold dilutions of electroporated cells were seeded in 6-well Costar plates containing subconfluent monolayers of naive BHK-21 or C7/10 cells. After 2 h of incubation at 37°C, cells were overlaid with agarose supplemented with MEM and 3% FBS. C7/10 cells were incubated for 2 h at 28°C and then overlaid with DMEM supplemented with 0.6% tragacanth, 5% TPB, and 5% FBS. Plaques were stained with crystal violet after 3 days of incubation at 37°C or 28°C, and RNA infectivity was determined as PFU/μg of electroporated RNA.
Isolation and identification of cellular proteins that interact with alphavirus HVDs.
HEK 293 or other cells in the quantity of 8 × 106 were infected with viral particles containing replicon genomes encoding Flag-GFP fused with different fragments of CHIKV HVD at a multiplicity of infection (MOI) of 20 infectious units (IU)/cell. Cells were harvested at 2 h postinfection (p.i.), when GFP was at the very early stage of expression, and thus, concentrations of expressed fusion proteins remained biologically relevant. Protein complexes were isolated from the postnuclear fraction of the NP-40-lysed cells using magnetic beads loaded with the Flag-specific monoclonal antibody (MAb) (Sigma) as described elsewhere (34). Proteins were separated on 4 to 12% NuPAGE gels (Invitrogen), stained with Coomassie blue, and identified by mass spectrometry as previously described (34). Identified proteins were selected as specifically binding to particular HVDs if, in the repeated experiments, the specific spectra were 20- to 100-fold higher than those detected in the control samples derived from the cells infected with replicons expressing Flag-GFP.
Recombinant CHIKV HVD purification and NMR experiment.
CHIKV HVD-coding sequence (aa 325 to 523, Fig. 1A), containing Twin-Strep tag at the carboxy terminus, was cloned into pE-SUMOpro-3 plasmid (LifeSensors Inc.) encoding the amino-terminal His-SuMO tag. The plasmid was transformed into E. coli Rosetta 2(DE3)pLacI (Novagen). The 15N- and 15N, 13C-labeled proteins were produced by growing cells in M9 minimal medium supplemented with [15N]NH4Cl (2 g/liter) and d-[13C6]glucose (3 g/liter). The recombinant CHIKV HVD was first purified on a HisTrap HP column (GE Healthcare). After cleavage of the SUMO tag, CHIKV HVD was purified on a Strep Tactin Sepharose column (GE Healthcare). Size exclusion chromatography in NMR buffer [25 mM N2HPO4, 50 mM NaCl, 2 mM tris(hydroxypropyl)phosphine] on a Superdex 200 10/30 column (GE Healthcare) was used as a final purification step. The purified protein was concentrated to ∼0.3 to 0.5 mM, and NaN3 was added to 1 mM.
NMR experiments were performed on Bruker Avance III spectrometers operating at 14.1 T equipped with a cryo-enhanced QCI-P probe at a temperature of 298 K. Gradient sensitivity enhanced 3D triple-resonance pulse programs HNCA, HNCO, HN(CO)CACB, H(NCOCA)NNH, and (H)N(COCA)NNH with transverse relaxation-optimized spectroscopy (TROSY) were acquired. In addition, the 2D version of MUSIC (SER, LAVIA, TAVI, and PRO) was applied as well as 15N best-TROSY. All experiments came from the Bruker standard library of pulse programs. The backbone resonances and Cβ were assigned and used in this publication. Full assignment, including side chains, will be published elsewhere. To estimate the secondary structure prosperity of CHIKV HVD, random coil chemical shifts calculated with neighbor correction factor in POTENCI (73) were subtracted from the experimental 13Cα, 13Cβ, and 13C′ chemical shifts. In the figures and in the text, the standard nomenclature for amino acids of the carbon atoms was used, where Cα is the carbon next to the carbonyl group C′ and Cβ is the carbon next to Cα (74).
Supplementary Material
ACKNOWLEDGMENTS
We thank Scott Weaver for providing infectious cDNA clones of CHIKV 181/25 and EILV. We also thank Valeriya Kuznetsova and Arpine Sokratian for technical assistance.
This study was supported by Public Health Service grants AI118867 and AI133159 to E.I.F. and AI095449, AI119627, and AI127744 to I.F.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JVI.00838-18.
REFERENCES
- 1.Griffin DE. 2001. Alphaviruses, p 917–962. In Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE (ed), Fields virology, 4th ed Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 2.Strauss JH, Strauss EG. 1994. The alphaviruses: gene expression, replication, evolution. Microbiol Rev 58:491–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weaver SC, Frolov I. 2005. Togaviruses, p 1010–1024. In Mahy BWJ, ter Meulen V (ed), Virology, vol 2 Hodder Arnold, Salisbury, United Kingdom. [Google Scholar]
- 4.Weaver SC, Lecuit M. 2015. Chikungunya virus infections. N Engl J Med 373:94–95. doi: 10.1056/NEJMc1505501. [DOI] [PubMed] [Google Scholar]
- 5.Weaver SC, Lecuit M. 2015. Chikungunya virus and the global spread of a mosquito-borne disease. N Engl J Med 372:1231–1239. doi: 10.1056/NEJMra1406035. [DOI] [PubMed] [Google Scholar]
- 6.Weaver SC, Forrester NL. 2015. Chikungunya: evolutionary history and recent epidemic spread. Antiviral Res 120:32–39. doi: 10.1016/j.antiviral.2015.04.016. [DOI] [PubMed] [Google Scholar]
- 7.McSweegan E, Weaver SC, Lecuit M, Frieman M, Morrison TE, Hrynkow S. 2015. The Global Virus Network: challenging chikungunya. Antiviral Res 120:147–152. doi: 10.1016/j.antiviral.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsetsarkin KA, Vanlandingham DL, McGee CE, Higgs S. 2007. A single mutation in chikungunya virus affects vector specificity and epidemic potential. PLoS Pathog 3:e201. doi: 10.1371/journal.ppat.0030201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strauss EG, Rice CM, Strauss JH. 1984. Complete nucleotide sequence of the genomic RNA of Sindbis virus. Virology 133:92–110. doi: 10.1016/0042-6822(84)90428-8. [DOI] [PubMed] [Google Scholar]
- 10.Hardy WR, Strauss JH. 1989. Processing the nonstructural proteins of Sindbis virus: nonstructural proteinase is in the C-terminal half of nsP2 and functions both in cis and trans. J Virol 63:4653–4664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lemm JA, Rice CM. 1993. Roles of nonstructural polyproteins and cleavage products in regulating Sindbis virus RNA replication and transcription. J Virol 67:1916–1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laakkonen P, Auvinen P, Kujala P, Kaariainen L. 1998. Alphavirus replicase protein NSP1 induces filopodia and rearrangement of actin filaments. J Virol 72:10265–10269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laakkonen P, Ahola T, Kaariainen L. 1996. The effects of palmitoylation on membrane association of Semliki Forest virus RNA capping enzyme. J Biol Chem 271:28567–28571. doi: 10.1074/jbc.271.45.28567. [DOI] [PubMed] [Google Scholar]
- 14.Frolova EI, Gorchakov R, Pereboeva L, Atasheva S, Frolov I. 2010. Functional Sindbis virus replicative complexes are formed at the plasma membrane. J Virol 84:11679–11695. doi: 10.1128/JVI.01441-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mai J, Sawicki SG, Sawicki DL. 2009. Fate of minus-strand templates and replication complexes produced by a p23-cleavage-defective mutant of Sindbis virus. J Virol 83:8553–8564. doi: 10.1128/JVI.00056-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kallio K, Hellstrom K, Balistreri G, Spuul P, Jokitalo E, Ahola T. 2013. Template RNA length determines the size of replication complex spherules for Semliki Forest virus. J Virol 87:9125–9134. doi: 10.1128/JVI.00660-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lemm JA, Rümenapf T, Strauss EG, Strauss JH, Rice CM. 1994. Polypeptide requirements for assembly of functional Sindbis virus replication complexes: a model for the temporal regulation of minus and plus-strand RNA synthesis. EMBO J 13:2925–2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shirako Y, Strauss JH. 1994. Regulation of Sindbis virus RNA replication: uncleaved P123 and nsP4 function in minus strand RNA synthesis whereas cleaved products from P123 are required for efficient plus strand RNA synthesis. J Virol 185:1874–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vasiljeva L, Merits A, Auvinen P, Kaariainen L. 2000. Identification of a novel function of the alphavirus capping apparatus. RNA 5′-triphosphatase activity of Nsp2. J Biol Chem 275:17281–17287. [DOI] [PubMed] [Google Scholar]
- 20.Ahola T, Kaariainen L. 1995. Reaction in alphavirus mRNA capping: formation of a covalent complex of nonstructural protein nsP1 with 7-methyl-GMP. Proc Natl Acad Sci U S A 92:507–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahola T, Laakkonen P, Vihinen H, Kaariainen L. 1997. Critical residues of Semliki Forest virus RNA capping enzyme involved in methyltransferase and guanylyltransferase-like activities. J Virol 71:392–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rikkonen M, Peranen J, Kaariainen L. 1994. ATPase and GTPase activities associated with Semliki Forest virus nonstructural protein nsP2. J Virol 68:5804–5810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karpe YA, Aher PP, Lole KS. 2011. NTPase and 5′-RNA triphosphatase activities of Chikungunya virus nsP2 protein. PLoS One 6:e22336. doi: 10.1371/journal.pone.0022336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Das PK, Merits A, Lulla A. 2014. Functional cross-talk between distant domains of chikungunya virus non-structural protein 2 is decisive for its RNA-modulating activity. J Biol Chem 289:5635–5653. doi: 10.1074/jbc.M113.503433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rausalu K, Utt A, Quirin T, Varghese FS, Zusinaite E, Das PK, Ahola T, Merits A. 2016. Chikungunya virus infectivity, RNA replication and non-structural polyprotein processing depend on the nsP2 protease's active site cysteine residue. Sci Rep 6:37124. doi: 10.1038/srep37124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tomar S, Hardy RW, Smith JL, Kuhn RJ. 2006. Catalytic core of alphavirus nonstructural protein nsP4 possesses terminal adenylyltransferase activity. J Virol 80:9962–9969. doi: 10.1128/JVI.01067-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rubach JK, Wasik BR, Rupp JC, Kuhn RJ, Hardy RW, Smith JL. 2009. Characterization of purified Sindbis virus nsP4 RNA-dependent RNA polymerase activity in vitro. Virology 384:201–208. doi: 10.1016/j.virol.2008.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Urakova N, Kuznetsova V, Crossman DK, Sokratian A, Guthrie DB, Kolykhalov AA, Lockwood MA, Natchus MG, Crowley MR, Painter GR, Frolova EI, Frolov I. 22 November 2017. Beta-D-N(4)-hydroxycytidine is a potent anti-alphavirus compound that induces high level of mutations in viral genome. J Virol doi: 10.1128/JVI.01965-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Malet H, Coutard B, Jamal S, Dutartre H, Papageorgiou N, Neuvonen M, Ahola T, Forrester N, Gould EA, Lafitte D, Ferron F, Lescar J, Gorbalenya AE, de Lamballerie X, Canard B. 2009. The crystal structures of Chikungunya and Venezuelan equine encephalitis virus nsP3 macro domains define a conserved adenosine binding pocket. J Virol 83:6534–6545. doi: 10.1128/JVI.00189-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shin G, Yost SA, Miller MT, Elrod EJ, Grakoui A, Marcotrigiano J. 2012. Structural and functional insights into alphavirus polyprotein processing and pathogenesis. Proc Natl Acad Sci U S A 109:16534–16539. doi: 10.1073/pnas.1210418109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Foy NJ, Akhrymuk M, Akhrymuk I, Atasheva S, Bopda-Waffo A, Frolov I, Frolova EI. 2013. Hypervariable domains of nsP3 proteins of New World and Old World alphaviruses mediate formation of distinct, virus-specific protein complexes. J Virol 87:1997–2010. doi: 10.1128/JVI.02853-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Foy NJ, Akhrymuk M, Shustov AV, Frolova EI, Frolov I. 2013. Hypervariable domain of nonstructural protein nsP3 of Venezuelan equine encephalitis virus determines cell-specific mode of virus replication. J Virol 87:7569–7584. doi: 10.1128/JVI.00720-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.LaStarza MW, Lemm JA, Rice CM. 1994. Genetic analysis of the nsP3 region of Sindbis virus: evidence for roles in minus-strand and subgenomic RNA synthesis. J Virol 68:5781–5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim DY, Reynaud JM, Rasalouskaya A, Akhrymuk I, Mobley JA, Frolov I, Frolova EI. 2016. New World and Old World alphaviruses have evolved to exploit different components of stress granules, FXR and G3BP proteins, for assembly of viral replication complexes. PLoS Pathog 12:e1005810. doi: 10.1371/journal.ppat.1005810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frolov I, Kim DY, Akhrymuk M, Mobley JA, Frolova EI. 2017. Hypervariable domain of eastern equine encephalitis virus nsP3 redundantly utilizes multiple cellular proteins for replication complex assembly. J Virol 91:e00371-. doi: 10.1128/JVI.00371-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gorchakov R, Garmashova N, Frolova E, Frolov I. 2008. Different types of nsP3-containing protein complexes in Sindbis virus-infected cells. J Virol 82:10088–10101. doi: 10.1128/JVI.01011-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frolova E, Gorchakov R, Garmashova N, Atasheva S, Vergara LA, Frolov I. 2006. Formation of nsP3-specific protein complexes during Sindbis virus replication. J Virol 80:4122–4134. doi: 10.1128/JVI.80.8.4122-4134.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Panas MD, Ahola T, McInerney GM. 2014. The C-terminal repeat domains of nsP3 from the Old World alphaviruses bind directly to G3BP. J Virol 88:5888–5893. doi: 10.1128/JVI.00439-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kristensen O. 2015. Crystal structure of the G3BP2 NTF2-like domain in complex with a canonical FGDF motif peptide. Biochem Biophys Res Commun 467:53–57. doi: 10.1016/j.bbrc.2015.09.123. [DOI] [PubMed] [Google Scholar]
- 40.Dosztanyi Z, Csizmok V, Tompa P, Simon I. 2005. IUPred: web server for the prediction of intrinsically unstructured regions of proteins based on estimated energy content. Bioinformatics 21:3433–3434. doi: 10.1093/bioinformatics/bti541. [DOI] [PubMed] [Google Scholar]
- 41.Dosztanyi Z. 2018. Prediction of protein disorder based on IUPred. Protein Sci 27:331–340. doi: 10.1002/pro.3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Felli IC, Pierattelli R. 2014. Novel methods based on (13)C detection to study intrinsically disordered proteins. J Magn Reson 241:115–125. doi: 10.1016/j.jmr.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 43.Tossavainen H, Aitio O, Hellman M, Saksela K, Permi P. 2016. Structural basis of the high affinity interaction between the alphavirus nonstructural protein-3 (nsP3) and the SH3 domain of amphiphysin-2. J Biol Chem 291:16307–16317. doi: 10.1074/jbc.M116.732412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Neuvonen M, Kazlauskas A, Martikainen M, Hinkkanen A, Ahola T, Saksela K. 2011. SH3 domain-mediated recruitment of host cell amphiphysins by alphavirus nsP3 promotes viral RNA replication. PLoS Pathog 7:e1002383. doi: 10.1371/journal.ppat.1002383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mutso M, Morro AM, Smedberg C, Kasvandik S, Aquilimeba M, Teppor M, Tarve L, Lulla A, Lulla V, Saul S, Thaa B, McInerney GM, Merits A, Varjak M. 2018. Mutation of CD2AP and SH3KBP1 binding motif in alphavirus nsP3 hypervariable domain results in attenuated virus. Viruses 10:E226. doi: 10.3390/v10050226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nasar F, Palacios G, Gorchakov RV, Guzman H, Da Rosa AP, Savji N, Popov VL, Sherman MB, Lipkin WI, Tesh RB, Weaver SC. 2012. Eilat virus, a unique alphavirus with host range restricted to insects by RNA replication. Proc Natl Acad Sci U S A 109:14622–14627. doi: 10.1073/pnas.1204787109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reynaud JM, Kim DY, Atasheva S, Rasalouskaya A, White JP, Diamond MS, Weaver SC, Frolova EI, Frolov I. 2015. IFIT1 differentially interferes with translation and replication of alphavirus genomes and promotes induction of type I interferon. PLoS Pathog 11:e1004863. doi: 10.1371/journal.ppat.1004863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nasar F, Gorchakov RV, Tesh RB, Weaver SC. 2015. Eilat virus host range restriction is present at multiple levels of the virus life cycle. J Virol 89:1404–1418. doi: 10.1128/JVI.01856-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Akhrymuk I, Kulemzin SV, Frolova EI. 2012. Evasion of the innate immune response: the Old World alphavirus nsP2 protein induces rapid degradation of Rpb1, a catalytic subunit of RNA polymerase II. J Virol 86:7180–7191. doi: 10.1128/JVI.00541-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Garmashova N, Gorchakov R, Volkova E, Paessler S, Frolova E, Frolov I. 2007. The Old World and New World alphaviruses use different virus-specific proteins for induction of transcriptional shutoff. J Virol 81:2472–2484. doi: 10.1128/JVI.02073-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Egloff MP, Malet H, Putics A, Heinonen M, Dutartre H, Frangeul A, Gruez A, Campanacci V, Cambillau C, Ziebuhr J, Ahola T, Canard B. 2006. Structural and functional basis for ADP-ribose and poly(ADP-ribose) binding by viral macro domains. J Virol 80:8493–8502. doi: 10.1128/JVI.00713-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McPherson RL, Abraham R, Sreekumar E, Ong SE, Cheng SJ, Baxter VK, Kistemaker HA, Filippov DV, Griffin DE, Leung AK. 2017. ADP-ribosylhydrolase activity of Chikungunya virus macrodomain is critical for virus replication and virulence. Proc Natl Acad Sci U S A 114:1666–1671. doi: 10.1073/pnas.1621485114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Michel G, Petrakova O, Atasheva S, Frolov I. 2007. Adaptation of Venezuelan equine encephalitis virus lacking 51-nt conserved sequence element to replication in mammalian and mosquito cells. Virology 362:475–487. doi: 10.1016/j.virol.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thaa B, Biasiotto R, Eng K, Neuvonen M, Gotte B, Rheinemann L, Mutso M, Utt A, Varghese F, Balistreri G, Merits A, Ahola T, McInerney GM. 2015. Differential phosphatidylinositol-3-kinase-Akt-mTOR activation by Semliki Forest and Chikungunya viruses is dependent on nsP3 and connected to replication complex internalization. J Virol 89:11420–11437. doi: 10.1128/JVI.01579-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fros JJ, Domeradzka NE, Baggen J, Geertsema C, Flipse J, Vlak JM, Pijlman GP. 2012. Chikungunya virus nsP3 blocks stress granule assembly by recruitment of G3BP into cytoplasmic foci. J Virol 86:10873–10879. doi: 10.1128/JVI.01506-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Scholte FE, Tas A, Albulescu IC, Zusinaite E, Merits A, Snijder EJ, van Hemert MJ. 2015. Stress granule components G3BP1 and G3BP2 play a proviral role early in Chikungunya virus replication. J Virol 89:4457–4469. doi: 10.1128/JVI.03612-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Panas MD, Varjak M, Lulla A, Eng KE, Merits A, Karlsson Hedestam GB, McInerney GM. 2012. Sequestration of G3BP coupled with efficient translation inhibits stress granules in Semliki Forest virus infection. Mol Biol Cell 23:4701–4712. doi: 10.1091/mbc.e12-08-0619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McInerney GM, Kedersha NL, Kaufman RJ, Anderson P, Liljestrom P. 2005. Importance of eIF2alpha phosphorylation and stress granule assembly in alphavirus translation regulation. Mol Biol Cell 16:3753–3763. doi: 10.1091/mbc.e05-02-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Park YJ, McBryant SJ, Luger K. 2008. A beta-hairpin comprising the nuclear localization sequence sustains the self-associated states of nucleosome assembly protein 1. J Mol Biol 375:1076–1085. doi: 10.1016/j.jmb.2007.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Park YJ, Luger K. 2006. Structure and function of nucleosome assembly proteins. Biochem Cell Biol 84:549–558. doi: 10.1139/o06-088. [DOI] [PubMed] [Google Scholar]
- 61.Cevik RE, Cesarec M, Da Silva Filipe A, Licastro D, McLauchlan J, Marcello A. 2017. Hepatitis C virus NS5A targets nucleosome assembly protein NAP1L1 to control the innate cellular response. J Virol 91:e00880-. doi: 10.1128/JVI.00880-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Goonawardane N, Gebhardt A, Bartlett C, Pichlmair A, Harris M. 2017. Phosphorylation of serine 225 in hepatitis C virus NS5A regulates protein-protein interactions. J Virol 91:e00805-17. doi: 10.1128/JVI.00805-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mayer BJ. 2001. SH3 domains: complexity in moderation. J Cell Sci 114:1253–1263. [DOI] [PubMed] [Google Scholar]
- 64.Kaneko T, Li L, Li SS. 2008. The SH3 domain—a family of versatile peptide- and protein-recognition module. Front Biosci 13:4938–4952. doi: 10.2741/3053. [DOI] [PubMed] [Google Scholar]
- 65.Aladag A, Hoffmann S, Stoldt M, Bosing C, Willbold D, Schwarten M. 2014. Hepatitis C virus NS5A is able to competitively displace c-Myc from the Bin1 SH3 domain in vitro. J Pept Sci 20:334–340. doi: 10.1002/psc.2618. [DOI] [PubMed] [Google Scholar]
- 66.Schwarten M, Solyom Z, Feuerstein S, Aladag A, Hoffmann S, Willbold D, Brutscher B. 2013. Interaction of nonstructural protein 5A of the hepatitis C virus with Src homology 3 domains using noncanonical binding sites. Biochemistry 52:6160–6168. doi: 10.1021/bi400363v. [DOI] [PubMed] [Google Scholar]
- 67.Nanda SK, Herion D, Liang TJ. 2006. The SH3 binding motif of HCV [corrected] NS5A protein interacts with Bin1 and is important for apoptosis and infectivity. Gastroenterology 130:794–809. doi: 10.1053/j.gastro.2005.12.030. [DOI] [PubMed] [Google Scholar]
- 68.Gorchakov R, Wang E, Leal G, Forrester NL, Plante K, Rossi SL, Partidos CD, Adams AP, Seymour RL, Weger J, Borland EM, Sherman MB, Powers AM, Osorio JE, Weaver SC. 2012. Attenuation of Chikungunya virus vaccine strain 181/clone 25 is determined by two amino acid substitutions in the E2 envelope glycoprotein. J Virol 86:6084–6096. doi: 10.1128/JVI.06449-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Volkova E, Gorchakov R, Frolov I. 2006. The efficient packaging of Venezuelan equine encephalitis virus-specific RNAs into viral particles is determined by nsP1-3 synthesis. Virology 344:315–327. doi: 10.1016/j.virol.2005.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liljeström P, Lusa S, Huylebroeck D, Garoff H. 1991. In vitro mutagenesis of a full-length cDNA clone of Semliki Forest virus: the small 6,000-molecular-weight membrane protein modulates virus release. J Virol 65:4107–4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gorchakov R, Hardy R, Rice CM, Frolov I. 2004. Selection of functional 5′ cis-acting elements promoting efficient Sindbis virus genome replication. J Virol 78:61–75. doi: 10.1128/JVI.78.1.61-75.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lemm JA, Durbin RK, Stollar V, Rice CM. 1990. Mutations which alter the level or structure of nsP4 can affect the efficiency of Sindbis virus replication in a host-dependent manner. J Virol 64:3001–3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nielsen JT, Mulder FAA. 2018. POTENCI: prediction of temperature, neighbor and pH-corrected chemical shifts for intrinsically disordered proteins. J Biomol NMR 70:141–165. doi: 10.1007/s10858-018-0166-5. [DOI] [PubMed] [Google Scholar]
- 74.Markley JL, Bax A, Arata Y, Hilbers CW, Kaptein R, Sykes BD, Wright PE, Wuthrich K. 1998. Recommendations for the presentation of NMR structures of proteins and nucleic acids—IUPAC-IUBMB-IUPAB Inter-Union Task Group on the Standardization of Data Bases of Protein and Nucleic Acid Structures Determined by NMR Spectroscopy. Eur J Biochem 256:1–15. doi: 10.1046/j.1432-1327.1998.2560001.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.