Influenza viruses cause seasonal epidemics and result in significant human morbidity and mortality. Influenza viruses persist in the human population through generating mutations in the hemagglutinin head domain that prevent antibody recognition. Despite the similar selective pressures on influenza A and B viruses, influenza A virus displays a higher rate and breadth of antigenic variability than influenza B virus. A transposon mutagenesis screen was used to examine if the reduced antigenic variability of influenza B virus was due to inherent differences in mutational tolerance. This study demonstrates that the influenza A virus head domain and the individual antigenic sites targeted by humoral responses are more tolerant to insertions than those of influenza B virus. This finding sheds light on the genetic factors controlling the antigenic evolution of influenza viruses.
KEYWORDS: influenza virus, viral evolution, insertional mutagenesis, transposons
ABSTRACT
Influenza A and B viruses can continuously evade humoral immune responses by developing mutations in the globular head of the hemagglutinin (HA) that prevent antibody binding. However, the influenza B virus HA over time displays less antigenic variation despite being functionally and structurally similar to the influenza A virus HA. To determine if the influenza B virus HA is under constraints that limit its antigenic variation, we performed a transposon screen to compare the mutational tolerance of the currently circulating influenza A virus HAs (H1 and H3 subtypes) and influenza B virus HAs (B/Victoria87 and B/Yamagata88 antigenic lineages). A library of insertional mutants for each HA was generated and deep sequenced after passaging to determine where insertions were tolerated in replicating viruses. The head domains of both viruses tolerated transposon mutagenesis, but the influenza A virus head was more tolerant to insertions than the influenza B virus head domain. Furthermore, all five of the known antigenic sites of the influenza A virus HA were tolerant of 15 nucleotide insertions, while insertions were detected in only two of the four antigenic sites in the influenza B virus head domain. Our analysis demonstrated that the influenza B virus HA is inherently less tolerant of transposon-mediated insertions than the influenza A virus HA. The reduced insertional tolerance of the influenza B virus HA may reveal genetic restrictions resulting in a lower capacity for antigenic evolution.
IMPORTANCE Influenza viruses cause seasonal epidemics and result in significant human morbidity and mortality. Influenza viruses persist in the human population through generating mutations in the hemagglutinin head domain that prevent antibody recognition. Despite the similar selective pressures on influenza A and B viruses, influenza A virus displays a higher rate and breadth of antigenic variability than influenza B virus. A transposon mutagenesis screen was used to examine if the reduced antigenic variability of influenza B virus was due to inherent differences in mutational tolerance. This study demonstrates that the influenza A virus head domain and the individual antigenic sites targeted by humoral responses are more tolerant to insertions than those of influenza B virus. This finding sheds light on the genetic factors controlling the antigenic evolution of influenza viruses.
INTRODUCTION
Influenza A and B viruses (IAV and IBV) are segmented, negative-sense, single-stranded RNA viruses in the family Orthomyxoviridae (1). Each influenza season, IAV and IBV can circulate widely in the human population. Although IAV tends to predominate, IBV represents approximately 25% of the total annual flu burden and can be the major cause of influenza-related disease in some years (2, 3). Despite the importance of influenza viruses to public health, the molecular factors controlling the evolution of these viruses are not well understood.
Influenza viruses continue to cause yearly epidemics due to their ability to rapidly evade preexisting immunity (4). Infection or vaccination elicits primarily strain-specific antibodies to the hemagglutinin (HA) head domain (5). The error-prone replication of influenza viruses (6) allows for the rapid development of mutations in the HA head that prevent antibody binding (7). Despite their phylogenetic and structural relationships (8, 9), the influenza A and B viral HAs display different levels of diversity (10). IAV infects a wide range of hosts, and the HA is divided into 18 known antigenic subtypes (11), with the H1 and H3 subtypes currently circulating in humans (4). IBV is restricted primarily to the human population and confined to one antigenic subtype with two cocirculating antigenic lineages (B/Victoria87 and B/Yamagata88) (12). In addition to the difference in total diversity, the rate of evolution is higher for the human IAV HAs than those of IBV (8, 13–16). It is not understood why IBV remains more conserved than IAV. One possibility is that inherent limitations to amino acid diversity in the IBV HA reduce the evolutionary and antigenic potential of this virus.
We previously used transposon mutagenesis to map the genetic landscape of the H1 IAV HA by generating a library of viruses each with a single 15-nucleotide insertion (17). Deep sequencing the viral library after passaging in tissue culture revealed that the variable head domain of the IAV HA was exceptionally tolerant of insertions compared to the conserved stalk domain. Consequently, our results may reveal that the genetic flexibility in the HA head domain allows it to undergo such varied antigenic changes, while the HA stalk domain remains conserved and can be targeted by broadly protective antibodies (18, 19).
This report describes a transposon mutagenesis study directly comparing the mutagenic potential of the currently circulating human IAV HAs (IAV H1 and H3 subtypes) and the IBV HAs (IBV B/Victoria/87-like and B/Yamagata/88-like lineages). We found the head domain was more tolerant of transposon mutagenesis than the conserved stalk region in both the influenza A and B viral HAs, as observed previously with the H1 HA (17). The results demonstrate that conserved epitopes can be under genetic restrictions that prevent the development of antigenic diversity (17, 20–22). Compared to IAV, IBV tolerates fewer mutations in the head domain and the individual antigenic sites. We hypothesize that this reduced genetic flexibility may be one factor limiting the antigenic variation of the IBV HA.
RESULTS
Development of high-efficiency rescue systems for IAV and IBV.
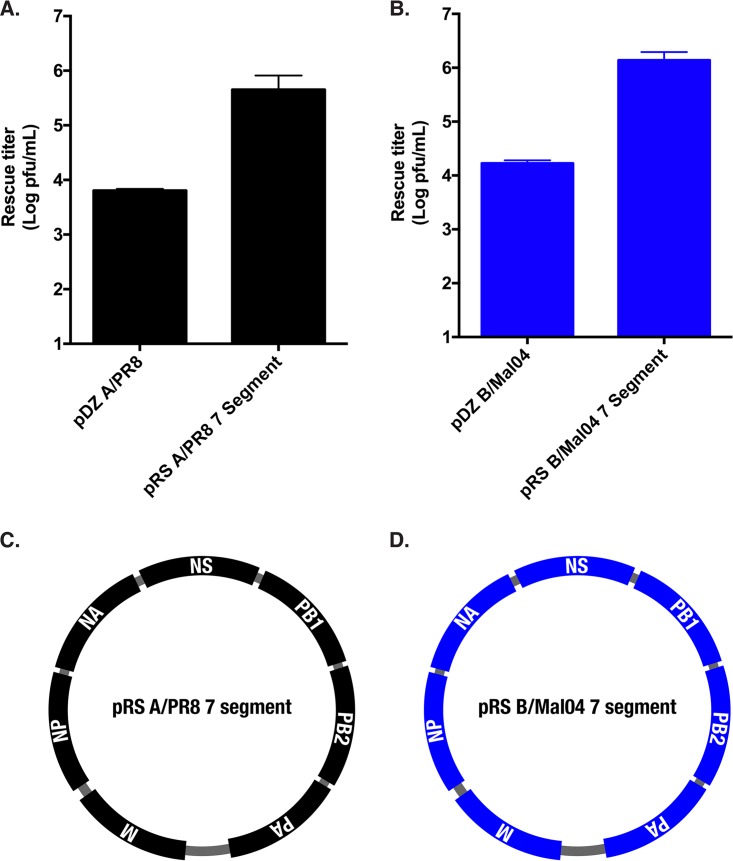
Transposon mutagenesis screens of RNA viruses require the ability to efficiently derive viruses from plasmid templates. Our previous mutagenic screen of the H1N1 A/Puerto Rico/8/1934 (A/PR8) genome was performed with the pDZ rescue system (17, 23). Transfection of plasmids for all eight segments of the A/PR8 backbone produced ∼104 PFU/ml (Fig. 1A). A mouse-adapted version of the B/Victoria/87 lineage (Vic/87) B/Malaysia/2506/2004 (B/Mal04) backbone (24) cloned into the pDZ plasmid produced similar rescue titers (Fig. 1B).
FIG 1.
The pRS 7 segment rescue systems produce high viral titers. (A and B) Rescue titers of WT A/PR8 HA (A) and B/Mal04 HA (B) in the pDZ and pRS segment plasmid backbones were compared. The pDZ WT HA expression vector was transfected into 293T cells with either the seven pDZ plasmids encoding the other viral segments or the pRS 7 segment plasmid. The supernatants were plaqued directly on MDCKs 48 h posttransfection. Data points are the means for three replicates, while the error bars represent the standard deviations. (C and D) Plasmid schematics of the pRS A/PR8 7 segment (C) and pRS B/Mal04 7 segment (D) plasmids. The segments are not to scale, but their order within the plasmid backbone is shown.
To improve the resolution of the screen, a novel high-efficiency rescue system was developed. In past studies, others have demonstrated that reducing the number of required plasmids for rescue could greatly increase virus recovery (25, 26). Therefore, two plasmids, termed pRS A/PR8 7 segment and pRS B/Mal04 7 segment, were generated for the A/PR8 (H1N1) and the B/Mal04 (Vic/87) viruses, respectively (Fig. 1C and D). These plasmids contained all of the segments of the influenza viral genome except the HA. Viral rescue with this system requires only two plasmids: the pRS 7 segment plasmid and an additional plasmid encoding the HA segment. Transfection of the pRS rescue system resulted in an ∼2-log increase in rescue titer (Fig. 1A and B), therefore providing a similarly efficient method to rescue both the influenza A and B viral HAs from plasmid templates in their respective backgrounds.
Characterization of the HA transposon libraries.
To compare the insertional tolerance of the influenza A and B viral HAs, we selected HAs representing the two IAV antigenic subtypes and the two antigenic lineages of IBV circulating in humans: A/Puerto Rico/8/1934 (designated A/PR8; H1N1), A/Hong Kong/1/68 (A/HK68; H3N2), B/Malaysia/2506/2004 (B/Mal04; B/Victoria/87 lineage), and B/Yamagata/16/1988 (B/Ya88; B/Yamagata/88 lineage) (Fig. 2A). DNA clones of these HAs were subjected to transposon mutagenesis as previously described (17) to generate a large library (∼1 × 106 colonies) of HAs, each with a single randomly integrated 15-nucleotide insertion. Transposon integration events result in 5-amino-acid insertions upon translation and cannot lead to the integration of stop codons or induce frameshifts (27). The integration can result in polar or hydrophobic amino acids depending on the reading frame of the insertion.
FIG 2.
Characterization of the HA libraries. (A) Schematic of the A/PR8 (black), A/HK68 (red), B/Mal04 (blue), and B/Ya88 HA (purple) segments utilized in the screen. Color schemes are consistent throughout the figure. (B) Rescue titers of the WT HA and mutagenized HA libraries for the A/PR8, A/HK68, B/Mal04, and B/Ya88 viruses in the pRS 7 segment backbone. The WT HA and the HA library were transfected into 293T cells with the pRS 7 segment plasmid, and the supernatants were plaqued at 48 h posttransfection. The numbers above the graph indicate the fold reduction in rescue titer of the HA library from the WT HA. Data points are the means for four replicates, while the error bars represent the standard deviations. (C) Protocol for rescuing and passaging the HA libraries. The HA library was transfected into 293T cells with the appropriate pRS 7 segment plasmid. This and all following steps were performed at 37°C for A/HAs and 33°C for B/HAs. Two days posttransfection, the supernatants were collected and passaged in 8-day-old embryonated eggs or on MDCK cells. For the egg passage, the viruses were incubated for 2 days for IAV or 3 days for IBV. For the MDCK cell passages, the remaining supernatant was used to infect MDCK cells for 24 h for IAV and 48 h for IBV. Supernatants were collected, and their titers were determined. A second MDCK cell passage was then performed at an MOI of 0.01. RNA was collected from purified virions for the egg passage and from the cells for the MDCK passage 2. (D) Titers of the rescue (P0), MDCK passage 1 (P1), MDCK passage 2 (P2), and egg (Egg) passages. Data points are the means for three replicates, while the error bars represent the standard deviations.
Rescue of the wild-type (WT) IAV A/H1 and A/H3 HAs in the A/PR8 backbone or the WT IBV B/Mal04 and B/Ya88 HAs in the B/Mal04 backbone produced similar titers (Fig. 2B). Viruses were recovered from the IAV HA libraries at a frequency approximately 10-fold less than those from the nontransposed WT HA plasmid (Fig. 2B), possibly revealing that some mutant viruses were not viable. Interestingly, rescue of the IBV HA libraries produced approximately 100-fold fewer viruses than the rescue from those of the nontransposed WT HA plasmid (Fig. 2B). Multiple independent transfections of the IBV HA libraries consistently yielded lower titers than the IAV HA libraries, although the WT HA was recoverable at comparable titers in both IAV and IBV backbones. Therefore, it is unlikely that the reduced recovery of mutants from the IBV HA libraries was due to differences in transfection efficiency and may indicate that fewer mutants in the IBV HA transposon library were viable.
A screen was performed with three independent replicates to directly compare the insertional tolerance of all HA segments. A large pool of transposon mutants was recovered by transfecting 293T cells with the HA library and the appropriate pRS 7 segment plasmid (Fig. 2C). Selection was performed in both Madin-Darby canine kidney (MDCK) cells and embryonated chicken eggs. For cell selection, the recovered mutant library was passaged on the MDCK cell line: the titers of supernatants from the first passage were determined, and then the supernatants were used to infect MDCK cells again at a multiplicity of infection (MOI) of 0.01. Alternatively, passaging in eggs was performed by injecting the undiluted transfection supernatants into 10-day-old embryonated chicken eggs. The viral titers were within approximately 1 log after passaging in MDCK cells and eggs (Fig. 2D).
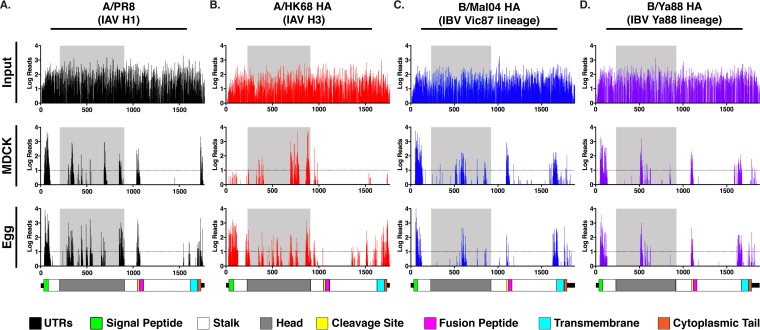
Deep sequencing of the transposon library plasmids revealed that mutational coverage of each HA segment was high (Fig. 3, input), with insertions detected in or after at least 93% of all codons. After selection in MDCK cells (Fig. 3, MDCK) or in eggs (Fig. 3, Egg), insertions were detected only in discrete regions of the HA: the signal peptide, the distal ∼15 amino acids of the ectodomain, the head domain, and the cytoplasmic tail. Insertions were also observed in or near the cleavage site. The fusion peptide and the majority of the stalk domain were relatively resistant to mutations. Different insertional profiles were observed between the MDCK and egg passages in all HA screens. The difference was most striking for the A/HK68 H3 HA, where insertions were more uniformly distributed throughout the HA after egg passaging, while the C terminus of the head domain was highly enriched for insertions after passaging in MDCK cells (Fig. 3B). The serial passaging of the mutant pool in MDCK cells may have reduced the detection of variants with lower fitness that were observed during the single egg passage. However, these results may also highlight different functional restrictions between in ovo and in vitro selections, as has been observed with certain H3 vaccine strains (28, 29).
FIG 3.
Transposon insertions are tolerated in the head domains of the influenza A and B viral HAs. Deep sequencing data for A/PR8 (black) (A), A/HK68 (red) (B), B/Mal04 (blue) (C), and B/Ya88 HA (purple) (D) input libraries (top row), MDCK passage 2 (middle row), and egg passages (bottom row). The number of reads containing transposon insertions is indicated on the y axis, and the numbers along the x axis of the graphs indicate the nucleotide positions in the segment. Dashed lines indicate a threshold of 10 or more reads. A schematic of the HA segments is shown to scale at the bottom to identify the location of insertions: untranslated regions (UTR, black), signal peptide (green), stalk (white), head (gray), cleavage site (yellow), fusion peptide (pink), transmembrane domain (cyan), and cytoplasmic tail (orange). The data for the 15 amino acids at the distal ends of the ectodomain, the cleavage site, and the fusion peptide are shown here but are not included in the analysis in Fig. 7.
Results of the screen are reproducible, and viruses bearing individual insertion sites are recoverable.
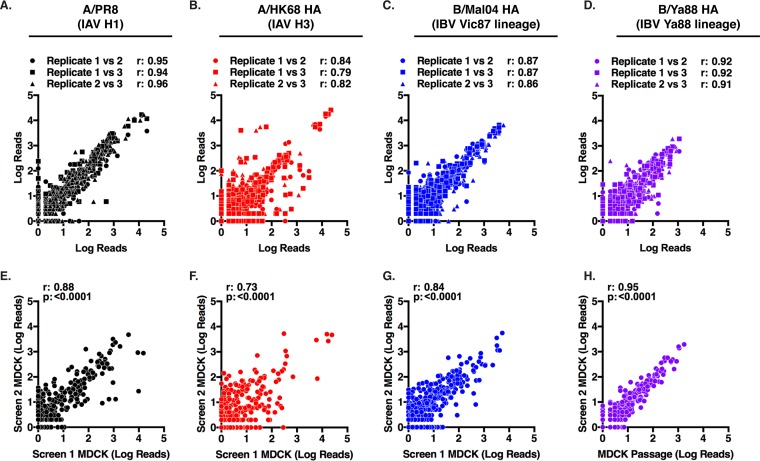
The three independent replicates of each screen exhibited high degrees of reproducibility (Fig. 4A to D). A second independent rescue and selection in MDCK cells was performed for each HA library and found to select for a similar mutant pool (Fig. 4E to H). It is possible that variations in library preparations could have altered the recovered viral population. To test this possibility, a de novo A/PR8 library was generated and screened in MDCK cells. This independent biological replicate produced an insertional profile similar to that of the original library (Fig. 5A). Furthermore, the correlation coefficient of the screens performed with independent libraries (Fig. 5B) was similar to those of replicate screens performed with the same library (Fig. 4E). Insertion sites frequently detected as hits (>10 reads) in both screens were individually cloned and rescued (Fig. 6). All of these mutant viruses were recoverable at titers comparable to those of the WT HA, demonstrating that the detected insertions were viable mutants.
FIG 4.
Consistency of the HA transposon screens. Pairwise analysis of the representation of each insertion mutant between each replicate of a single screen (A to D) and second independent screen performed in MDCK cells (E to H) for A/PR8 (black), A/HK68 (red), B/Mal04 (blue), and B/Ya88 HA (purple). The scatter plot shows the number of reads for a site in one sample plotted against the number of reads for that site in another sample. The r and P values for Pearson's correlation are displayed.
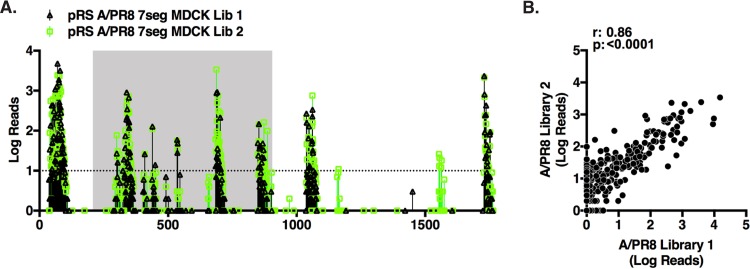
FIG 5.
An independently generated A/PR8 HA library produces results similar to those of the original library. (A) A new A/PR8 HA library (A/PR8 library 2) was generated and rescued as before. Deep-sequencing data from the second MDCK cell passage for the A/PR8 HA library 1 (black, triangles) and library 2 (green, squares) are plotted. The number of reads containing transposon insertions is indicated on the y axis, and the numbers along the x axis of the graph indicate the nucleotide position in the segment. The dashed line indicates a threshold of 10 or more reads. (B) Comparison of the abundance of each mutant after passage in MDCKs between A/PR8 HA library 1 and library 2. The scatter plot shows the number of reads for a site in one sample plotted against the number of reads for that site in another sample. The r and P values for Pearson's correlation are displayed.
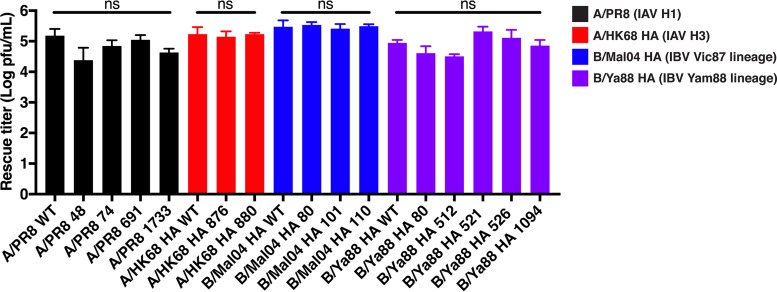
FIG 6.
Mutants bearing individual insertions are recoverable at titers comparable to those of the wild-type virus. Rescue titers of the WT HAs and mutant HAs bearing individual insertions are shown. The HA mutants are numbered by the nucleotide position prior to the insertion. The WT HAs or the mutant HAs were transfected into 293T cells with the appropriate pRS 7 segment plasmid. Supernatants were plaqued at 48 h posttransfection. Data points are the means for four replicates, while the error bars represent the standard deviations. WT HA and mutant HA rescue titers were compared by a one-way analysis of variance (ANOVA) followed by Tukey's multiple-comparison test. NS, not significant.
The IAV head domain is more tolerant to insertions than the IBV HA head.
The overall insertional tolerance of the head and stalk domains was determined for each HA. The head domain of the B/HAs was less tolerant to insertions than the head of the A/HAs (Fig. 7A). The conserved stalk domain was less tolerant to insertions in all the HAs than was the head domain, as was previously observed with the A/PR8 virus (17). The mutational coverage of the input libraries (Fig. 7B) and depth of sequencing were similar among the HAs (Fig. 7C). Therefore, the reduced detection of variants in the IBV HA head domain was likely due to reduced fitness of these mutants and not a lack of mutational or sequencing coverage. These results do not provide a complete map of all the possible insertion sites due to the stochastic nature of this screen and the limitations of deep sequencing. However, under similar conditions, we observe the IAV HAs are more tolerant of transposon insertions than the IBV HAs.
FIG 7.
The head domains of IAV are more tolerant to insertions than those of IBV, and the stalk domains are less tolerant to insertions in all HAs. (A) The number of sites that could tolerate insertions in each region were normalized to the region size to give the relative insertional tolerance of the head and stalk for the A/PR8 (black), A/HK68 (red), B/Mal04 (blue), and B/Ya88 HA (purple) for the MDCK passage 2 data. Color schemes are consistent throughout the figure. Data points are the means for two independent screens in MDCK cells, while the error bars represent the standard errors of the means. The data for the 15 amino acids at the distal ends of the ectodomain, the cleavage site, and the fusion peptide were excluded from this analysis. (B) The proportion of codons mutagenized in each of the viral libraries is plotted. (C) The number of reads aligning to the HA segment are displayed for the MDCK cell and egg passages.
We next looked at the genetic flexibility of the previously defined antigenic sites in both the IAV and IBV head domains (30–32). Insertions were tolerated in all five of the antigenic sites of A/PR8 and the A/HK68 HAs (Fig. 8A and C). In the B/HAs, insertions were tolerated only in the 150-loop and 160-loop antigenic sites, but not in the 120-loop or 190-helix antigenic sites (Fig. 8E and G). Potent neutralizing antibodies tend to bind near the receptor binding pocket (9, 33). Insertions were tolerated near some of the critical receptor binding pocket residues (Fig. 8); however, none of the insertions altered the critical residues required for binding.
FIG 8.
The antigenic sites of the IAV HAs are more tolerant to insertions than those of IBV. Locations of transposon insertions in the A/PR8 (A), A/HK68 (C), B/Mal04 (E), and B/Ya88 (G) HA head domains. (Top) Diagram of the HA segment showing the locations of the head (red), stalk (white), and UTRs (gray). The amino acid positions of the head domain in the HA1 protein are provided in parentheses below the schematic. (Bottom) The primary sequence for the head domain is displayed. All insertional mutants from the MDCK cell and egg passages are denoted on the primary sequence by solid and half-filled triangles, respectively. Locations of antigenic domains within the head domains are indicated by the colored boxes (30–32). The critical residues of the receptor binding pockets are indicated by yellow stars below the primary sequence. Insertions located in the antigenic domains are colored accordingly. Crystal structures of the HAs from lineages A/PR8 (PDB 1RU7) (52) (B), A/HK68 (PDB 4FNK) (53) (D), B/Vic87 lineage (PDB 4FQM) (19) (F), and B/Ya88 (PDB 4M40) (31) (H). The head domain is colored in red, while the stalk domain is colored in gray. Locations of the antigenic domains on the crystal structure are indicated.
DISCUSSION
The host immune system places strong evolutionary pressure on influenza viruses to develop antigenic diversity (1). However, the molecular factors controlling the ability of these viruses to respond to immune selection are not fully understood. Here, we report an insertional tolerance map of the influenza A and B viral HAs to identify regions with the ability to tolerate genetic alterations. The majority of sites in both the influenza A and B viral HAs were intolerant of insertions. In particular, we found that the majority of the stalk domain, the fusion peptide, and the receptor binding site were intolerant to insertions. These highly conserved regions are critical for cell attachment and entry during viral replication. The lack of insertions in these regions reflects the limited sequence variations that these protein domains can tolerate while remaining functional. However, discrete regions were tolerant of insertions, such as the signal peptide, the distal ends of the ectodomain, the head domain, the cleavage site, and the cytoplasmic tail in both the influenza A and B viral HAs.
Our data largely reflect what has been published with screens assaying the effects of single-amino-acid substitutions in the A/H1 HA (21, 34–37). These substitutions result in relatively small changes that provide a finer map of the potential amino acid changes in a specific region. However, substitutions are less likely to be as detrimental to protein function as are the five-amino-acid insertions introduced by transposon mutagenesis. Transposon mutagenesis screens are consequently more likely to identify regions with genetic flexibility and provide insights into the mutational tolerances of a viral region. This study also builds on previous work (17) by providing a mutational tolerance map of the A/H3 HA and both lineages of the B/HAs, adding to the understanding of the evolutionary capabilities of these important pathogens.
Regions identified to be particularly tolerant of insertions in both the influenza A and B viral HAs are commonly known to vary in amino acid composition and length. For example, the signal peptide can display up to 74% amino acid variability between influenza virus subtypes and insertions/deletions in this region are frequently observed (38, 39). The HA head domain undergoes extensive sequence variation to escape antibody responses (1). Naturally occurring insertions of at least 20 amino acids are observed in the HA cleavage site (40) despite having strict sequence requirements identified by substitutional analysis (34). The distal ends of the ectodomain, the regions directly after the signal peptide and before the transmembrane domain, are also known to vary in length (39, 41, 42). These regions do not fall into known stalk epitopes and are unlikely to affect the antigenicity of the HA. In particular, the N terminus of the ectodomain varies by up to 15 amino acids between H1 and H3 isolates (41). Recombinant viruses bearing large insertions on the N terminus of the mature HA1 domain replicate with only minor fitness costs (43). Although more conserved in natural isolates, the cytoplasmic tail is dispensable for replication in vitro (44, 45). Therefore, our screen highlights the known genetic flexibility in these regions in both the IAV and IBV HAs.
Many of the genetically flexible regions were not originally detected in our first transposon screen of the A/PR8 H1N1 HA (17). Due to the improvements in rescue efficiency (Fig. 1), we may have rescued and detected additional viruses in the signal peptide, the distal ends of the ectodomain, the cleavage site, and the cytoplasmic tail that were not recovered in the original A/PR8 H1 screen. These additional mutants can change the competition among viruses in the recovered pool, thereby altering the detection of individual mutations. Additionally, because more viruses were recovered, the passaging was performed at a higher MOI. Increasing the MOI of passages may allow viruses with minor fitness costs to persist in this new screen. In addition to these newly detected sites, the majority of insertion sites detected in the original screen and all of the sites in the head domain were also identified in this screen. Furthermore, the overall finding that the A/PR8 H1 head domain is exceptionally tolerant to insertions compared to the conserved stalk domain is consistent between the two studies.
RNA viruses vary extensively in their ability to undergo antigenic variation in response to immune selection. One possibility is that inherent differences in the mutational capacities among viruses lead to disparate evolutionary capabilities in immunological targets. Here we found that the HA heads of both the influenza A and B viruses were tolerant of insertional mutagenesis. In contrast, we have previously found that the HA of the serologically monotypic measles virus was intolerant to transposon mutations (20). The disparity in mutational tolerances may explain why influenza viruses display extensive antigenic variation, while measles virus remains serologically conserved. Consequently, influenza virus requires yearly revaccination for protection, while the measles virus vaccine first released in 1963 remains effective today (46).
Although the head domains of both the influenza A and B HAs are genetically adaptable, the head domain of IAV HA was found to be more flexible than the IBV HA head. Insertions were detected in all five of the antigenic sites of the IAV HAs; however, only one-half of the antigenic sites in the IBV HAs were tolerant of insertional mutagenesis. The IBV HA 120-loop and 190-helix antigenic regions were identified to be genetically conserved and may be one factor explaining the limited genetic and antigenic variation of IBV (8, 10, 13).
The greater genetic restrictions observed in the IBV head may also explain the unequal ability of IAV and IBV to escape broadly neutralizing head antibodies. Antibodies CR8071 and CR8033 can neutralize both antigenic lineages of IBV by targeting the conserved residues in the base of the head and the receptor binding pocket, respectively (19). These antibodies recognize conserved epitopes that partially overlap the genetically restricted 120-loop and 190-helix antigenic sites (31). Prior attempts to make escape mutants to these antibodies have been difficult or unsuccessful (19). However, IAV does not appear to be limited by such functional restrictions. IAV can easily develop escape mutants to these antibodies binding a conserved receptor pocket epitope (21, 47, 48), indicating that this epitope is inherently tolerant of mutations in IAV. Together, this difference implies that the conservation of these epitopes in the IBV head may be due to its limited tolerance to mutations.
Broadly neutralizing antibodies targeting the HA stalk in IAV and IBV have been described (19, 49, 50). Monoclonal antibodies targeting the stalk can neutralize multiple IAV subtypes and both antigenic lineages of IBV. Because these regions are genetically restricted in both IAV and IBV, these viruses are less likely to develop escape mutations and antigenic variation in the stalk domain than the head domain. Consistent with this framework, a recent paper demonstrated that it is harder for A/H1 viruses to escape stalk antibodies than head antibodies (21). Together, these observations give support to recent efforts to develop a universal influenza vaccine targeting the genetically constrained HA stalk in order to provide broad protection to influenza infection (18).
In summary, this screen provides a map of the insertional tolerance of the A/H1 HA, A/H3 HA, and the HA of both antigenic lineages of IBV. This work demonstrates that the inherent tolerance for mutations in the antigenic regions of influenza viruses is one factor likely contributing to the rapid evolution of these viruses in comparison to other RNA viruses (20, 22). Furthermore, mutagenesis screens can be adapted to other RNA viruses to identify genetically constrained vaccine targets with the potential for long-term efficacy.
MATERIALS AND METHODS
Cells.
Human embryonic kidney cells (293T) were maintained in Dulbecco's modified Eagle's medium (DMEM; Gibco) containing 10% (vol/vol) fetal bovine serum (FBS) and penicillin-streptomycin (PS; Gibco) at 37°C with 5% CO2. Madin-Darby canine kidney (MDCK) cells were grown in minimum essential medium (MEM; Gibco) supplemented with 10% (vol/vol) FBS, l-glutamine (Gibco), sodium bicarbonate (Corning), HEPES (Gibco), and PS at 37°C with 5% CO2.
Development of the pRS 7 segment plasmids.
The A/PR8 and B/Mal04 genomic segments were cloned into the ambisense pDZ vector (23). The genomic segments and the expression elements were PCR amplified using the CloneAmp HiFi PCR Premix (TaKaRa Bio USA, Inc.) from the pDZ plasmids. The PCR primer sets were designed to include an AscI restriction site in the 3′ PCR primer. The pBR322 plasmid backbone was digested with MluI and AscI (NEB) for 16 h at 37°C. The matrix (M) segment insert was then cloned into the pBR322 backbone using the In-Fusion HD kit (TaKaRa Bio USA, Inc.) and transformed into Stbl2 cells (Invitrogen) according to the manufacturers' protocols. Plasmids were purified from subsequent colonies using the QIAprep spin miniprep kit (Qiagen). Plasmids with the correct insert were digested with AscI to linearize the plasmid directly after the insertion. This process was repeated using the In-Fusion HD cloning kit to sequentially add the remaining six non-HA segments into this plasmid in the following order: nucleoprotein (NP), neuraminidase (NA), nonstructural protein (NS), polymerase basic protein 1 (PB1), PB2, and polymerase acidic protein (PA). Each time a new segment was integrated, the AscI site used to linearize the plasmid was ablated and replaced with an AscI at the 3′ end of the newly integrated sequences. The final plasmids, termed pRS A/PR8 7 segment and pRS B/Mal04 7 segment, contained all of the influenza segments of A/PR8 and B/Mal04, respectively, except HA.
Characterization of the HA transposon libraries.
HA transposon libraries were generated as previously described (17). The transposon libraries were independently rescued and passaged in triplicate. For each replicate, two 6-well poly-lysine-coated plates of 70% confluent 293T cells in 1 ml fresh DMEM with 0.35% bovine serum albumin (BSA) (vol/vol), 0.01% FBS (vol/vol), and PS were transfected with 2.8 μg of the appropriate pRS 7 segment plasmid, 1 μg of the plasmid library, and 0.5 μg of the pCAGGS protein expression plasmids for the WT protein, 200 μl Opti-MEM (OM; Gibco), and 20 μl TransIT LT1 (Mirus Bio) per well. All steps, including this one, were performed at 33°C for IBV and 37°C for IAV to account for the growth preferences of the two viruses. Two days posttransfection, the supernatants from the transfections were collected and centrifuged at 4,000 × g for 5 min to clarify the supernatant. A total of 1 μl per ml of tosyl phenylalanyl chloromethyl ketone (TPCK)-treated trypsin was added to the clarified supernatants to activate the viral HA. The supernatant was then used to infect 80% confluent MDCK cells in 15-cm dishes for 2 h. Following the infection, the supernatant was replaced with 20 ml fresh DMEM with 0.35% BSA (vol/vol), 0.01% FBS (vol/vol), and PS with TPCK trypsin. The infection was carried out for 1 day for IAV and 2 days for IBV to account for the difference in growth rates between the two viruses. The supernatants were then collected, their titers were determined, and they were stored at 4°C. A second round of infection was performed as described above, but the viral pools were diluted to an MOI of 0.01 prior to infection. After the infection, the supernatants were collected and 6 ml of TRIzol was used to extract RNA from the cells prior to being frozen at −80°C. After each passage, the viral titer was determined by plaque assays as previously described (51). Selection in eggs was performed by injecting at least seven 10-day-old embryonated chicken eggs with 100 μl of the transfection supernatant. Eggs were then incubated at 37°C for 48 h for IAV or 33°C for 72 h for IBV. Allantoic fluid was harvested from the eggs and clarified by centrifugation at 4,000 × g for 5 min. Virions were purified from the resulting supernatant by centrifugation at 25,000 rpm in a Beckman SW28 rotor for 2 h over a 30% sucrose gradient and lysed in 1 ml TRIzol.
RT-PCR and deep sequencing of HA libraries.
RNA was isolated from the TRIzol lysates using the PureLink TRIzol Plus RNA purification kit (Thermo Fisher Scientific) with on-column PureLink DNase treatment to remove any potential plasmid contamination. The viral RNA was then amplified via reverse transcription (RT)-PCR with the SuperScript III Platinum Taq kit (Thermo Fisher Scientific) with primers specific to the untranslated regions (UTRs) of the HA segment (primer sequences available upon request). The cDNA products were then gel purified with the Qiaex II kit. The three replicates of the original MDCK cell and egg passage cDNAs were pooled in equimolecular ratios. The cDNAs for each of the replicates from the second independent screen in MDCK cells were processed and sequenced as separate samples. The cDNA products and the input library were then sheared by Covaris sonication into 200-bp fragments and prepared for deep sequencing with the NEBNext Ultra II kit and Oligos (New England BioLabs). All the samples were then multiplexed and sequenced on a MiSeq Illumina platform with 150 nucleotide reads, except the B/Ya88 HA library, which was sequenced on a HiSeq with 100 nucleotide reads.
Analysis of the deep-sequencing data.
Reads containing a 10-nucleotide sequence tag (TGCGGCCGCA) found in every insertion site were filtered from the raw reads, trimmed, and mapped to the genome using Bowtie 2. A custom script was then used to count the number of reads with insertions at each genomic location as previously described (17, 20, 22). For the comparison of the insertional tolerances of each HA, we needed to limit the background signal from attenuated viruses or defective particles passaged in our screen. An arbitrary cutoff of 10 reads was used to identify sites that were above background noise. The number of sites in the head and stalk domain (excluding the cleavage site and the fusion peptide) was then determined and divided by the region length. Due to the enrichment of reads in the first and last ∼15 amino acids of the ectodomain, these regions were also excluded from the analysis.
Cloning and rescue of WT HAs and HA mutants bearing individual insertions.
Wild-type clones of A/PR8, A/HK68, B/Mal04, and B/Ya88 HAs were PCR amplified with the CloneAmp HiFi PCR Premix and cloned into a SapI (NEB)-digested pPOLI vector using the In-Fusion HD kit (primers available upon request). HA segments bearing individual inserts were generated by using the CloneAmp HiFi PCR Premix to amplify the wild-type HAs with overlapping primer sets encoding the transposon sequence (primers available upon request). The cDNA products were then cloned into the pPOLI vector using the In-Fusion HD kit. The wild type, the individual mutants, and the HA libraries were rescued, and their titers were determined as described above in quadruplicate to determine the rescue efficiency of those HA preparations. For the experiments with the pDZ rescue system, 0.5 μg of each segment in the pDZ backbone was used. All other rescue parameters were the same.
Data availability.
The original sequence files and the data set can be accessed with the NCBI Gene expression Omnibus (GEO) series record GSE113831. The custom computer script used to analyze the data was originally described by Heaton et al. (17).
ACKNOWLEDGMENTS
We acknowledge Paul E. Leon for his help in generating the B/Ya88 HA library and Mark J. Bailey and Jennifer R. Hamilton for manuscript editing assistance. We thank Maryline Panis, Benjamin R. tenOever, Panchajanya Deshpande, and Milind Mahajan for their help with deep sequencing. We thank David Sachs for developing the custom scripts for analysis of the deep-sequencing data.
This work was supported by the following grants: NIH grant P01AI097092 (P.P.), NIH grant U19 AI109946 (P.P.), and CEIRS grant HHSN272201400008C (P.P.).
REFERENCES
- 1.Shaw ML, Palese P. 2013. Fields virology, p 1151–1185. In Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE (ed), Fields virology, 6th ed Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 2.Heikkinen T, Ikonen N, Ziegler T. 2014. Impact of influenza B lineage-level mismatch between trivalent seasonal influenza vaccines and circulating viruses, 1999-2012. Clin Infect Dis 59:1519–1524. doi: 10.1093/cid/ciu664. [DOI] [PubMed] [Google Scholar]
- 3.Adlhoch C, Snacken R, Melidou A, Ionescu S, Penttinen P, The European Influenza Surveillance Network. 2018. Dominant influenza A(H3N2) and B/Yamagata virus circulation in EU/EEA, 2016/17 and 2017/18 seasons, respectively. Euro Surveill 23(13):18–00146. doi: 10.2807/1560-7917.ES.2018.23.13.18-00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO. 2018. Influenza (seasonal) fact sheet. http://www.who.int/mediacentre/factsheets/fs211/en/ Accessed 13 April 2018.
- 5.Altman MO, Angeletti D, Yewdell JW. 2018. Antibody immunodominance: the key to understanding influenza virus antigenic drift. Viral Immunol doi: 10.1089/vim.2017.0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nobusawa E, Sato K. 2006. Comparison of the mutation rates of human influenza A and B viruses. J Virol 80:3675–3678. doi: 10.1128/JVI.80.7.3675-3678.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith DJ, Lapedes AS, de Jong JC, Bestebroer TM, Rimmelzwaan GF, Osterhaus AD, Fouchier RA. 2004. Mapping the antigenic and genetic evolution of influenza virus. Science 305:371–376. doi: 10.1126/science.1097211. [DOI] [PubMed] [Google Scholar]
- 8.Chen R, Holmes EC. 2008. The evolutionary dynamics of human influenza B virus. J Mol Evol 66:655–663. doi: 10.1007/s00239-008-9119-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Q, Cheng F, Lu M, Tian X, Ma J. 2008. Crystal structure of unliganded influenza B virus hemagglutinin. J Virol 82:3011–3020. doi: 10.1128/JVI.02477-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bedford T, Suchard MA, Lemey P, Dudas G, Gregory V, Hay AJ, McCauley JW, Russell CA, Smith DJ, Rambaut A. 2014. Integrating influenza antigenic dynamics with molecular evolution. Elife 3:e01914. doi: 10.7554/eLife.01914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.CDC. 2017. Types of influenza viruses. https://www.cdc.gov/flu/about/viruses/types.htm Accessed 13 April 2018.
- 12.Langat P, Raghwani J, Dudas G, Bowden TA, Edwards S, Gall A, Bedford T, Rambaut A, Daniels RS, Russell CA, Pybus OG, McCauley J, Kellam P, Watson SJ. 2017. Genome-wide evolutionary dynamics of influenza B viruses on a global scale. PLoS Pathog 13:e1006749. doi: 10.1371/journal.ppat.1006749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vijaykrishna D, Holmes EC, Joseph U, Fourment M, Su YC, Halpin R, Lee RT, Deng YM, Gunalan V, Lin X, Stockwell TB, Fedorova NB, Zhou B, Spirason N, Kuhnert D, Boskova V, Stadler T, Costa AM, Dwyer DE, Huang QS, Jennings LC, Rawlinson W, Sullivan SG, Hurt AC, Maurer-Stroh S, Wentworth DE, Smith GJ, Barr IG. 2015. The contrasting phylodynamics of human influenza B viruses. Elife 4:e05055. doi: 10.7554/eLife.05055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fitch WM, Leiter JM, Li XQ, Palese P. 1991. Positive Darwinian evolution in human influenza A viruses. Proc Natl Acad Sci U S A 88:4270–4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rambaut A, Pybus OG, Nelson MI, Viboud C, Taubenberger JK, Holmes EC. 2008. The genomic and epidemiological dynamics of human influenza A virus. Nature 453:615–619. doi: 10.1038/nature06945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jenkins GM, Rambaut A, Pybus OG, Holmes EC. 2002. Rates of molecular evolution in RNA viruses: a quantitative phylogenetic analysis. J Mol Evol 54:156–165. doi: 10.1007/s00239-001-0064-3. [DOI] [PubMed] [Google Scholar]
- 17.Heaton NS, Sachs D, Chen CJ, Hai R, Palese P. 2013. Genome-wide mutagenesis of influenza virus reveals unique plasticity of the hemagglutinin and NS1 proteins. Proc Natl Acad Sci U S A 110:20248–20253. doi: 10.1073/pnas.1320524110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krammer F, Palese P. 2015. Advances in the development of influenza virus vaccines. Nat Rev Drug Discov 14:167–182. doi: 10.1038/nrd4529. [DOI] [PubMed] [Google Scholar]
- 19.Dreyfus C, Laursen NS, Kwaks T, Zuijdgeest D, Khayat R, Ekiert DC, Lee JH, Metlagel Z, Bujny MV, Jongeneelen M, van der Vlugt R, Lamrani M, Korse HJ, Geelen E, Sahin O, Sieuwerts M, Brakenhoff JP, Vogels R, Li OT, Poon LL, Peiris M, Koudstaal W, Ward AB, Wilson IA, Goudsmit J, Friesen RH. 2012. Highly conserved protective epitopes on influenza B viruses. Science 337:1343–1348. doi: 10.1126/science.1222908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fulton BO, Sachs D, Beaty SM, Won ST, Lee B, Palese P, Heaton NS. 2015. Mutational analysis of measles virus suggests constraints on antigenic variation of the glycoproteins. Cell Rep 11:1331–1338. doi: 10.1016/j.celrep.2015.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doud MB, Lee JM, Bloom JD. 2018. How single mutations affect viral escape from broad and narrow antibodies to H1 influenza hemagglutinin. Nat Commun 9:1386. doi: 10.1038/s41467-018-03665-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fulton BO, Sachs D, Schwarz MC, Palese P, Evans MJ. 2017. Transposon mutagenesis of the Zika virus genome highlights regions essential for RNA replication and restricted for immune evasion. J Virol 91:e00698-. doi: 10.1128/JVI.00698-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quinlivan M, Zamarin D, Garcia-Sastre A, Cullinane A, Chambers T, Palese P. 2005. Attenuation of equine influenza viruses through truncations of the NS1 protein. J Virol 79:8431–8439. doi: 10.1128/JVI.79.13.8431-8439.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wohlbold TJ, Podolsky KA, Chromikova V, Kirkpatrick E, Falconieri V, Meade P, Amanat F, Tan J, ten Oever BR, Tan GS, Subramaniam S, Palese P, Krammer F. 2017. Broadly protective murine monoclonal antibodies against influenza B virus target highly conserved neuraminidase epitopes. Nat Microbiol 2:1415–1424. doi: 10.1038/s41564-017-0011-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neumann G, Fujii K, Kino Y, Kawaoka Y. 2005. An improved reverse genetics system for influenza A virus generation and its implications for vaccine production. Proc Natl Acad Sci U S A 102:16825–16829. doi: 10.1073/pnas.0505587102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang X, Kong W, Ashraf S, Curtiss R III. 2009. A one-plasmid system to generate influenza virus in cultured chicken cells for potential use in influenza vaccine. J Virol 83:9296–9303. doi: 10.1128/JVI.00781-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poussu E, Vihinen M, Paulin L, Savilahti H. 2004. Probing the alpha-complementing domain of E. coli beta-galactosidase with use of an insertional pentapeptide mutagenesis strategy based on Mu in vitro DNA transposition. Proteins 54:681–692. doi: 10.1002/prot.10467. [DOI] [PubMed] [Google Scholar]
- 28.Lu B, Zhou H, Ye D, Kemble G, Jin H. 2005. Improvement of influenza A/Fujian/411/02 (H3N2) virus growth in embryonated chicken eggs by balancing the hemagglutinin and neuraminidase activities, using reverse genetics. J Virol 79:6763–6771. doi: 10.1128/JVI.79.11.6763-6771.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zost SJ, Parkhouse K, Gumina ME, Kim K, Diaz Perez S, Wilson PC, Treanor JJ, Sant AJ, Cobey S, Hensley SE. 2017. Contemporary H3N2 influenza viruses have a glycosylation site that alters binding of antibodies elicited by egg-adapted vaccine strains. Proc Natl Acad Sci U S A 114:12578–12583. doi: 10.1073/pnas.1712377114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caton AJ, Brownlee GG, Yewdell JW, Gerhard W. 1982. The antigenic structure of the influenza virus A/PR/8/34 hemagglutinin (H1 subtype). Cell 31:417–427. doi: 10.1016/0092-8674(82)90135-0. [DOI] [PubMed] [Google Scholar]
- 31.Ni F, Kondrashkina E, Wang Q. 2013. Structural basis for the divergent evolution of influenza B virus hemagglutinin. Virology 446:112–122. doi: 10.1016/j.virol.2013.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Skehel JJ, Stevens DJ, Daniels RS, Douglas AR, Knossow M, Wilson IA, Wiley DC. 1984. A carbohydrate side chain on hemagglutinins of Hong Kong influenza viruses inhibits recognition by a monoclonal antibody. Proc Natl Acad Sci U S A 81:1779–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tzarum N, de Vries RP, Zhu X, Yu W, McBride R, Paulson JC, Wilson IA. 2015. Structure and receptor binding of the hemagglutinin from a human H6N1 influenza virus. Cell Host Microbe 17:369–376. doi: 10.1016/j.chom.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Doud MB, Bloom JD. 2016. Accurate measurement of the effects of all amino-acid mutations on influenza hemagglutinin. Viruses 8:155. doi: 10.3390/v8060155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thyagarajan B, Bloom JD. 2014. The inherent mutational tolerance and antigenic evolvability of influenza hemagglutinin. Elife 3:e03300. doi: 10.7554/eLife.03300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu NC, Young AP, Al-Mawsawi LQ, Olson CA, Feng J, Qi H, Chen SH, Lu IH, Lin CY, Chin RG, Luan HH, Nguyen N, Nelson SF, Li X, Wu TT, Sun R. 2014. High-throughput profiling of influenza A virus hemagglutinin gene at single-nucleotide resolution. Sci Rep 4:4942. doi: 10.1038/srep04942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Du Y, Xin L, Shi Y, Zhang TH, Wu NC, Dai L, Gong D, Brar G, Shu S, Luo J, Reiley W, Tseng YW, Bai H, Wu TT, Wang J, Shu Y, Sun R. 2018. Genome-wide identification of interferon-sensitive mutations enables influenza vaccine design. Science 359:290–296. doi: 10.1126/science.aan8806. [DOI] [PubMed] [Google Scholar]
- 38.Air GM. 1981. Sequence relationships among the hemagglutinin genes of 12 subtypes of influenza A virus. Proc Natl Acad Sci U S A 78:7639–7643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nobusawa E, Aoyama T, Kato H, Suzuki Y, Tateno Y, Nakajima K. 1991. Comparison of complete amino acid sequences and receptor-binding properties among 13 serotypes of hemagglutinins of influenza A viruses. Virology 182:475–485. doi: 10.1016/0042-6822(91)90588-3. [DOI] [PubMed] [Google Scholar]
- 40.Lopez-Martinez I, Balish A, Barrera-Badillo G, Jones J, Nunez-Garcia TE, Jang Y, Aparicio-Antonio R, Azziz-Baumgartner E, Belser JA, Ramirez-Gonzalez JE, Pedersen JC, Ortiz-Alcantara J, Gonzalez-Duran E, Shu B, Emery SL, Poh MK, Reyes-Teran G, Vazquez-Perez JA, Avila-Rios S, Uyeki T, Lindstrom S, Villanueva J, Tokars J, Ruiz-Matus C, Gonzalez-Roldan JF, Schmitt B, Klimov A, Cox N, Kuri-Morales P, Davis CT, Diaz-Quinonez JA. 2013. Highly pathogenic avian influenza A(H7N3) virus in poultry workers, Mexico, 2012. Emerg Infect Dis 19:1531–1534. doi: 10.3201/eid1909.130087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Russell CJ. 2014. Acid-induced membrane fusion by the hemagglutinin protein and its role in influenza virus biology. Curr Top Microbiol Immunol 385:93–116. doi: 10.1007/82_2014_393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tung CS, Goodman JL, Lu H, Macken CA. 2004. Homology model of the structure of influenza B virus HA1. J Gen Virol 85:3249–3259. doi: 10.1099/vir.0.80021-0. [DOI] [PubMed] [Google Scholar]
- 43.Li ZN, Mueller SN, Ye L, Bu Z, Yang C, Ahmed R, Steinhauer DA. 2005. Chimeric influenza virus hemagglutinin proteins containing large domains of the Bacillus anthracis protective antigen: protein characterization, incorporation into infectious influenza viruses, and antigenicity. J Virol 79:10003–10012. doi: 10.1128/JVI.79.15.10003-10012.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Imai M, Watanabe S, Kawaoka Y. 2012. The cytoplasmic tail domain of influenza B virus hemagglutinin is important for its incorporation into virions but is not essential for virus replication in cell culture in the presence of compensatory mutations. J Virol 86:11633–11644. doi: 10.1128/JVI.01479-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jin H, Leser GP, Lamb RA. 1994. The influenza virus hemagglutinin cytoplasmic tail is not essential for virus assembly or infectivity. EMBO J 13:5504–5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moss WJ, Griffin DE. 2012. Measles. Lancet 379:153–164. doi: 10.1016/S0140-6736(10)62352-5. [DOI] [PubMed] [Google Scholar]
- 47.Lee PS, Yoshida R, Ekiert DC, Sakai N, Suzuki Y, Takada A, Wilson IA. 2012. Heterosubtypic antibody recognition of the influenza virus hemagglutinin receptor binding site enhanced by avidity. Proc Natl Acad Sci U S A 109:17040–17045. doi: 10.1073/pnas.1212371109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yoshida R, Igarashi M, Ozaki H, Kishida N, Tomabechi D, Kida H, Ito K, Takada A. 2009. Cross-protective potential of a novel monoclonal antibody directed against antigenic site B of the hemagglutinin of influenza A viruses. PLoS Pathog 5:e1000350. doi: 10.1371/journal.ppat.1000350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ekiert DC, Bhabha G, Elsliger MA, Friesen RH, Jongeneelen M, Throsby M, Goudsmit J, Wilson IA. 2009. Antibody recognition of a highly conserved influenza virus epitope. Science 324:246–251. doi: 10.1126/science.1171491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sui J, Hwang WC, Perez S, Wei G, Aird D, Chen LM, Santelli E, Stec B, Cadwell G, Ali M, Wan H, Murakami A, Yammanuru A, Han T, Cox NJ, Bankston LA, Donis RO, Liddington RC, Marasco WA. 2009. Structural and functional bases for broad-spectrum neutralization of avian and human influenza A viruses. Nat Struct Mol Biol 16:265–273. doi: 10.1038/nsmb.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bouvier NM, Lowen AC, Palese P. 2008. Oseltamivir-resistant influenza A viruses are transmitted efficiently among guinea pigs by direct contact but not by aerosol. J Virol 82:10052–10058. doi: 10.1128/JVI.01226-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gamblin SJ, Haire LF, Russell RJ, Stevens DJ, Xiao B, Ha Y, Vasisht N, Steinhauer DA, Daniels RS, Elliot A, Wiley DC, Skehel JJ. 2004. The structure and receptor binding properties of the 1918 influenza hemagglutinin. Science 303:1838–1842. doi: 10.1126/science.1093155. [DOI] [PubMed] [Google Scholar]
- 53.Ekiert DC, Kashyap AK, Steel J, Rubrum A, Bhabha G, Khayat R, Lee JH, Dillon MA, O'Neil RE, Faynboym AM, Horowitz M, Horowitz L, Ward AB, Palese P, Webby R, Lerner RA, Bhatt RR, Wilson IA. 2012. Cross-neutralization of influenza A viruses mediated by a single antibody loop. Nature 489:526–532. doi: 10.1038/nature11414. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original sequence files and the data set can be accessed with the NCBI Gene expression Omnibus (GEO) series record GSE113831. The custom computer script used to analyze the data was originally described by Heaton et al. (17).