As CNS invasion by flaviviruses is a rare but life-threatening event, it is critical to understand how brain-resident immune cells elicit protection or injury during disease progression. Microglia have been shown to be important in viral clearance but may also contribute to CNS injury as part of the neuroinflammatory process. By utilizing a microglial depletion model, we can begin to parse out the exact roles of microglia during flaviviral pathogenesis with hopes of understanding specific mechanisms as potential targets for therapeutics.
KEYWORDS: CNS, flavivirus, encephalitis, microglia
ABSTRACT
Flaviviruses account for most arthropod-borne cases of human encephalitis in the world. However, the exact mechanisms of injury to the central nervous system (CNS) during flavivirus infections remain poorly understood. Microglia are the resident immune cells of the CNS and are important for multiple functions, including control of viral pathogenesis. Utilizing a pharmacologic method of microglia depletion (PLX5622 [Plexxikon Inc.], an inhibitor of colony-stimulating factor 1 receptor), we sought to determine the role of microglia in flaviviral pathogenesis. Depletion of microglia resulted in increased mortality and viral titer in the brain following infection with either West Nile virus (WNV) or Japanese encephalitis virus (JEV). Interestingly, microglial depletion did not prevent virus-induced increases in the expression of relevant cytokines and chemokines at the mRNA level. In fact, the expression of several proinflammatory genes was increased in virus-infected, microglia-depleted mice compared to virus-infected, untreated controls. In contrast, and as expected, expression of the macrophage marker triggering receptor expressed on myeloid cells 2 (TREM2) was decreased in virus-infected, PLX5622-treated mice compared to virus-infected controls.
IMPORTANCE As CNS invasion by flaviviruses is a rare but life-threatening event, it is critical to understand how brain-resident immune cells elicit protection or injury during disease progression. Microglia have been shown to be important in viral clearance but may also contribute to CNS injury as part of the neuroinflammatory process. By utilizing a microglial depletion model, we can begin to parse out the exact roles of microglia during flaviviral pathogenesis with hopes of understanding specific mechanisms as potential targets for therapeutics.
INTRODUCTION
West Nile virus (WNV) and Japanese encephalitis virus (JEV) are mosquito-borne members of the Flaviviridae that are, respectively, the leading causes of acute viral meningoencephalitis in the United States and worldwide (1, 2). Following peripheral infection, these viruses can reach the central nervous system (CNS), where they readily infect multiple cell types (including neurons, microglia, and endothelial cells), leading to neurological impairment, sequelae, and death. There are no current effective treatments for flavivirus-induced CNS disease. A vaccine is available for human JEV infections, but wide-scale implementation is difficult; there is also an equine vaccine for WNV. Animal models of WNV infection suggest that intrinsic neuroinflammation, as well as infiltration of the CNS by peripheral immune cells, contributes to effective clearance of WNV from the infected CNS (4). The roles of cellular infiltration and neuroinflammation during JEV disease remain controversial, and proinflammatory molecules have been shown to have both protective and detrimental effects (5–8). Given the importance of the neuroinflammatory response, understanding how these events are regulated may identify potential treatments for flavivirus-induced CNS disease that function by modulating inflammation or innate immune function.
Microglia are the resident innate immune cells of the CNS and have been shown to be vital regulators of neuroinflammatory events in several disease models (9, 10). Mechanisms of immune regulation include clearance of foreign antigen and cellular debris, maintenance of blood-brain barrier (BBB) permeability, and secretion of many factors important in the neuroinflammatory process, such as interferons (IFNs), chemokines, and cytokines (11, 12). Microglia become activated following infection of the CNS with a variety of different viruses (13, 14); however, their exact role in virus-induced CNS disease remains incompletely understood. Studies using ex vivo CNS tissue or mouse models have shown that microglia become morphologically active following WNV infection, phagocytose WNV-infected neurons and antigenic debris, and produce several important cytokines/chemokines involved in WNV-induced neuroinflammatory regulation. The use of minocycline in these studies to inhibit microglial activation improved neuronal survival, suggesting that microglia act to promote disease following WNV infection (15). In contrast, mice with reduced microglial activation have enhanced mortality following WNV infection (16). Microglia have also been implicated in synaptic pruning following WNV infection, resulting in worse disease outcomes (17). Our understanding of the role of microglia in JEV-induced disease is also limited (18, 19). Similar to WNV, JEV induces the expression of cytokines and chemokines in the brains of infected animals, and minocycline is protective against JEV-induced CNS disease (20). One notable difference between WNV and JEV is that WNV does not appear to infect microglia in vivo (21), whereas JEV does.
Many compounds have been shown to inhibit microglial activation (15, 20, 22), although their mechanisms of action remain poorly understood. Colony-stimulating factor 1 (CSF1) is thought to be essential in maintaining homeostasis in microglial populations (23). The CSF1 receptor (CSF1R) kinase inhibitor PLX5622 has been shown to deplete microglia with minimal effects on normal CNS function (24, 25) and has been used to investigate the consequences of microglia depletion in several settings, including recently in a murine coronavirus model (26). Microglial depletion has not yet been studied in the context of flaviviral infection. In this study, we infected mice with WNV or JEV after depletion of microglia with PLX5622 and compared the infection outcome to that seen in infected, control-fed mice. Flavivirus-infected mice treated with PLX5622 had significantly increased mortality compared to infected but untreated controls. Furthermore, WNV and JEV grew to significantly higher titers in the CNS of PLX5622-treated mice, indicating that microglial depletion impeded the ability of the host to control viral replication. Interestingly, the normal virus-induced increased expression of cytokines and chemokines was not blocked in infected PLX5622-treated animals, suggesting that other CNS cells are sufficient to mediate production of these inflammatory factors. Taken together these results indicate that microglia are important in controlling flaviviral infection of the CNS and increase survival in murine models of flavivirus-induced encephalitis.
RESULTS
PLX5622 treatment depletes microglia from the brains of adult Swiss-Webster mice.
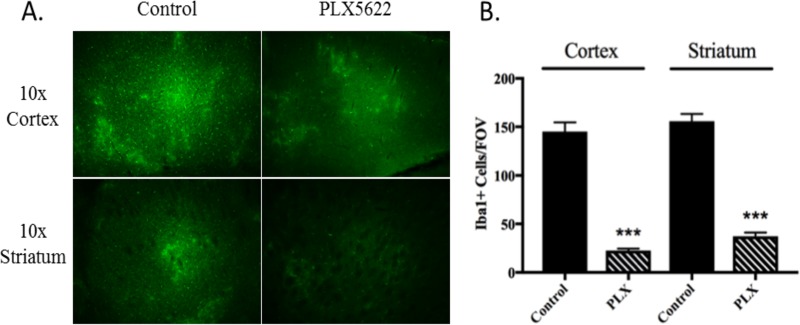
To establish the efficacy of PLX5622 for robust microglia depletion in Swiss-Webster mice, mice were fed with PLX5622 or control chow for 10 days before examining expression of ionized calcium binding adaptor molecule 1 (Iba1), a canonical marker for microglia (27, 28), in brain sections via immunohistochemistry. Iba1+ cells were counted across multiple fields of vision (FOV) in brain sections derived from treated and untreated mice (n = 3) (Fig. 1). Throughout the brains of PLX5622-treated mice, microglia levels were observed to be reduced >80% compared to those in control mice (Fig. 1A and B). These significant reductions were equivalent for both the cortical and striatal regions of the brain, indicating that depletion of microglia was not specific to one site in the CNS. These data establish that in Swiss-Webster mice, PLX5622 treatment can robustly deplete microglia as has been reported for other mouse strains (25, 26). We utilized a PLX5622 pretreatment regimen of 14 days prior to infection for the remainder of the experiments in order to ensure that microglial reduction would be adequate.
FIG 1.
PLX5622 depletes microglia from the CNS of Swiss-Webster mice. (A) Mice were pretreated for 10 days with PLX5622 followed, by staining of brain sections for Iba1+ cells. Microglia were observed to be decreased in both the cortex and striatum at magnifications of both ×10 and ×20. (B) Quantification of FOV counts of Iba1+ cells over various regions of the brain from PLX5622-treated and untreated mice at a magnification of ×20 (n = 3 per group). Errors bars are standard errors of the mean (SEM). ***, P < 0.001.
PLX5622 treatment increases WNV-induced mortality and viral titer in infected Swiss-Webster mice.
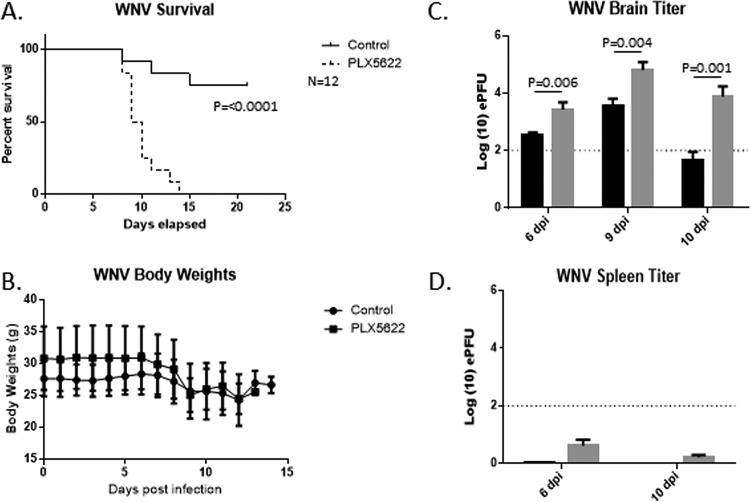
We next sought to determine the effect of PLX5622 treatment on mortality following WNV infection of Swiss-Webster mice. Mice were fed either control or PLX5622 chow for 14 days prior to footpad inoculation with 1000 PFU of WNV (NY99); the mice remained on their respective food types for the duration of the infection time course and were monitored for 21 days postinfection (dpi). Mice treated with PLX5622 had dramatically (P < 0.001) increased mortality following WNV infection (100%) compared to that of control mice (25%) (Fig. 2A).
FIG 2.
PLX5622 increases mortality and viral titer in WNV-infected mice. Mice were untreated or treated with PLX5622 for 14 days. Mice were them infected with WNV and monitored for 21 days. (A) PLX5622 treatment significantly increased mortality following infection with WNV (n = 12 animals per group). (B) Mouse body weight during infection was also monitored, and there was no significant difference between untreated and treated mice. (C) The viral titer in brains was found to be significantly increased in virus-infected, PLX5622-treated mice (gray bars) compared to infected controls (black bars) at 6 dpi (n = 11), 9 dpi (n = 9), and 10 dpi (n = 6). (D) Amounts of viral RNA in spleens were quantified by PCR and compared to a standard curve to determine genomic equivalent PFU. Error bars represent SEM. The dotted line represents our determined limit of accurate detection (102 PFU).
We also measured differences in body weight changes between surviving control and infected mice over the course of disease progression (Fig. 2B). While the average body weight loss for PLX5622-treated mice was slightly increased compared to that for control mice, both sets of mice lost weight at approximately the same rate, indicating similar systemic disease progression for both sets of surviving mice. These findings indicate that microglial depletion by PLX5622 treatment leads to increased mortality following WNV CNS infection.
To assess viral loads in the CNS, whole-brain lysates were collected from treated and untreated infected mice at 6 dpi (11 mice per condition), 9 dpi (9 to 12 mice per condition), and 10 dpi (5 to 9 mice per condition) and assessed quantitatively for WNV RNA (Fig. 2C). The 6-dpi time point was chosen to evaluate early viral CNS entry, 9 dpi was used to determine the WNV titer when mice were becoming clinically ill, and 10 dpi was a readout for when treated mice became moribund. Utilizing a standard curve to determine the genomic equivalent PFU levels for WNV in each brain, significantly increased WNV levels were detected in microglia-depleted PLX5622-treated mice versus untreated controls at all time points, indicating that microglial depletion leads to increased viral titers in the CNS. In contrast, viral loads in the spleens of PLX5622-treated and control-treated WNV-infected mice at 6 and 9 days pi were below the limit of detection for accurate interpretation using this assay, again suggesting that the effects of PLX5662 were not due to off-target, nonmicroglial effects (Fig. 2D).
Microglia are not required for WNV-induced expression of inflammatory genes.
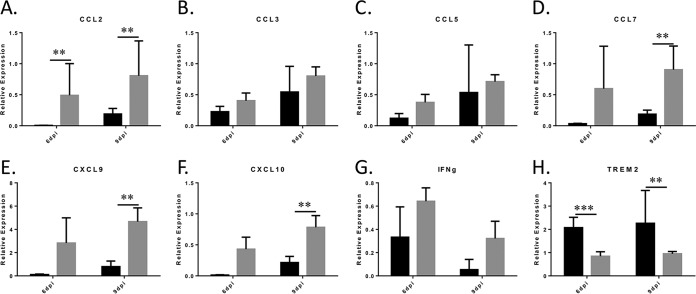
Microglia have important roles in regulating neuroinflammation across disease models (29), but how they modulate cytokine/chemokine levels in vivo during WNV infection is poorly understood. We assessed via reverse transcription-quantitative PCR (RT-qPCR) the genetic expression of several important inflammatory cytokines/chemokines from brain lysates taken from control and PLX5622-treated WNV-infected mice at 6 dpi and 9 dpi (Fig. 3). The genes analyzed included those for cytokines and chemokines with known relevance to WNV infections (CCL2, CCL3, CCL5, CCL7, CXCL9, and CXCL10). We have previously shown that these genes are all significantly upregulated in the brains of WNV-infected mice compared to controls as determined by microarray analysis (30).
FIG 3.
Expression of inflammatory genes is not blocked following WNV infection of PLX5622-treated mice. Relevant microglial and other inflammatory cytokines were analyzed by RT-qPCR with the indicated primers at 6 (n = 6) and 9 (n = 9) dpi. Graphs show the relative expression of genes in control-fed, infected mice (black bars) compared to PLX5622-treated, infected mice (gray bars), with values normalized to β-actin (ΔΔCT) with error bars representing SEM. **, P = 0.01; ***, P < 0.001 (as determined by two-tailed t tests) (GraphPad).
PLX5622 treatment did not decrease virus-induced expression of any of the cytokines/chemokines tested, indicating that microglia are not required for virus-induced expression of these genes. Several of the genes examined were actually elevated in WNV-infected PLX5622 brains compared to WNV-infected controls across the 6-dpi and especially the 9-dpi time points, with significant increases for CCL2 seen at 6 and 9 dpi (Fig. 3A) and for CCL7 (Fig. 3D), CXCL9 (Fig. 3E), and CXCL10 (Fig. 3F) seen at 9 dpi. CCL3 and CCL5, which are frequently associated with WNV infection of the CNS (31), and IFN-γ were also upregulated in PLX5622-treated virus-infected mice compared to control-fed infected mice, although these changes were not significant. TREM2 was significantly decreased at both 6 dpi and 9 dpi (Fig. 3H), reflecting microglial depletion. These data suggest that microglia are not required for increased expression of proinflammatory and immune-modulatory genes following virus infection of the CNS and that their depletion may in fact lead to increased virus-induced expression of these neuroinflammatory factors.
PLX5622 treatment increases mortality of mice infected with JEV.
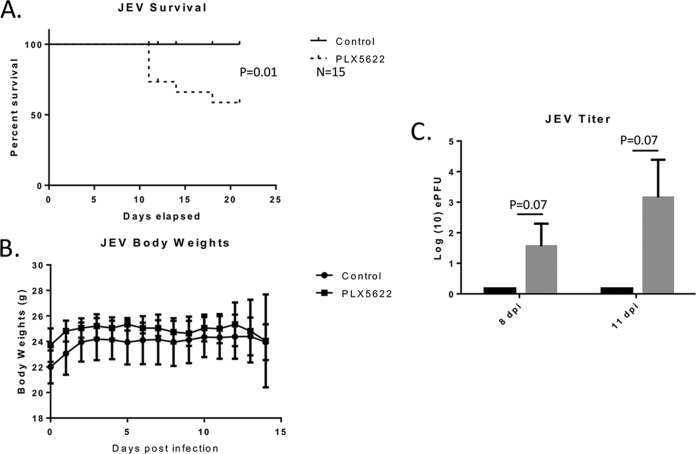
In order to further understand the role that microglial depletion plays during flaviviral infection, we completed an analysis similar to that done for WNV following infection with Japanese encephalitis virus (JEV). Mice were again pretreated for 14 days with PLX5622, followed by footpad infection with 1,000 PFU of JEV (strain p3), and were monitored for 21 days. Microglia-depleted PLX5622-treated animals showed an increase in mortality following JEV infection (P = 0.01) compared to untreated controls, showing an increase in susceptibility similar to what was observed in WNV-infected PLX5662-treated mice (Fig. 4A). There was no significant change in body weights between the PLX5622-treated and untreated groups (Fig. 4B), suggesting a similar profile of systemic infection. JEV-infected PLX5622-treated animals took longer than their WNV-infected counterparts to progress to a moribund state, and collection times for early (8 dpi) and late (11 dpi) time points were adjusted accordingly for comparison to WNV experiments. Only PLX5622-treated mice that showed overt clinical illness had a detectable viral titer in the brain (Fig. 4C) at the 8-dpi or 11-dpi time point, which was consistent with the results of the survival analysis. Although not statistically significant for individual mice with the numbers used, differences between PLX5622 and control mice were statistically different for pooled data at 8 and 11 dpi (P = 0.0075, Fisher's exact test). No virus was detected in the brain at either time point in animals fed the control diet. These data indicate that, similar to the case for WNV-infected animals, PLX5622 also increases mortality and viral load following JEV infection compared to those of control mice.
FIG 4.
PLX5622 increases mortality and viral titer in JEV-infected mice. Mice were untreated or treated with PLX5622 for 14 days. Mice were them infected with JEV (1,000 PFU by footpad injection) and monitored for 21 days. (A) PLX5622 treatment significantly increased mortality following infection with JEV (n = 15 animals per group). (B) Mouse body weight during infection was also monitored and showed no significant difference between untreated and treated mice (n = 5 per group). (C) The viral titer in brains was found to be significantly increased in virus-infected, PLX5622-treated mice compared to infected controls at 8 and 11 dpi (n = 5 per group at each time point). Amounts of viral RNA in brains were quantified by PCR and compared to a standard curve to determine genomic equivalent PFU. Error bars represent SEM.
Microglia are not required for JEV-induced expression of inflammatory genes.
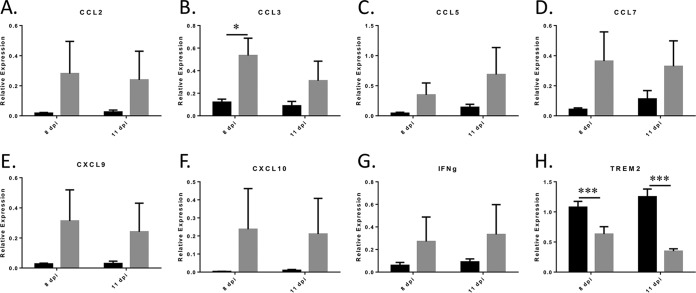
Similar to the case for WNV-infected mice, proinflammatory cytokines and chemokines (CCL2, CCL3, CCL5, CCL7, CXCL9, CXCL10, and IFN-γ) showed changes in expression at the mRNA level following PLX5622 treatment of JEV-infected mice compared to control-fed animals (Fig. 5). However, only the increased expression of CCL3 was found to be statistically significant, presumably since only 40% of the PLX5622-treated, JEV-infected animals showed signs of virus-induced CNS disease. Consistent with microglia depletion following PLX5622 treatment, levels of the microglia-specific TREM2 gene were again decreased in the JEV-infected PLX5622-treated animals compared to control-fed mice (Fig. 5H). Together, these data suggest that microglial depletion by PLX5622 treatment increases both mortality and viral titer during JEV infection and that microglia are not required for the production of chemokines and cytokines in the brains of JEV-infected mice.
FIG 5.
The expression of JEV-induced inflammatory cytokines and chemokines is not blocked following JEV infection of PLX5622-treated mice. PCR analysis of relevant cytokines and chemokines using indicated primers is shown. Graphs show the relative expression of genes in control-fed, infected mice (black bars) compared to PLX5622-treated, infected mice (gray bars), with values normalized to β-actin. Errors bars are SEM. Statistical analyses were conducted as described for Fig. 3. *, P = 0.05; ***, P < 0.001).
DISCUSSION
The aim of this study was to investigate the role of microglia in control of CNS flavivirus infection in murine models. We found that microglial depletion led to increased CNS viral titer and increased mortality in both WNV- and JEV-infected animals. This mirrors a recent report showing that PLX5622 also enhances mortality in mice infected with coronavirus (26). As microglia are the resident innate immune cells of the brain, the resultant increase in mortality and viral load might be expected, at least following WNV infection, where microglia are not targeted for infection; however, our results indicate that this is also true of JEV infections, where microglia are a significant target of the viral infection (12). Interestingly the 100% mortality phenotype in PLX5622-treated mice infected with WNV was not recapitulated by JEV infection. The exact mechanism of this difference is unknown but could reflect differences in the host immune capabilities that are required for viral control, with microglia being more critical in WNV infection than in JEV infection. Alternatively the difference could simply reflect the lower relative lethality of the matched (1,000-PFU) challenge dose in JEV- compared to WNV-infected Swiss-Webster mice. Future work will need to be conducted to determine if increased viral replication in PLX5622-treated, infected mice is due to unchecked viral replication within the neurons (34). Depletion of microglia has been shown to affect the integrity of the blood-brain barrier (BBB), which could also affect viral loads within the CNS; however, earlier studies suggest that PLX5622 does not alter the integrity of the BBB (36). Interestingly, we found no difference in CD3 expression in PLX-treated, infected brains compared to untreated controls (not shown), similar to what was seen during coronavirus infection, suggesting that T-cell involvement seems relatively unaffected following PLX treatment, which also suggests that the BBB remains intact and that the majority of the observed responses are intrinsic to the CNS (26).
This experimental model system has shown utility for investigating the specific inflammatory contributions of microglia to neuroinflammation during viral infection. Proinflammatory cytokines, including CCL2, CCL7, CXCL9, and CXCL10, are broadly upregulated following infection of the brain with WNV and JEV (41, 42). Interestingly, these neuroinflammatory molecules are still upregulated in virus-infected brains following PLX5622 treatment, suggesting that these cytokines/chemokines are released by cells other than microglia. In fact our data suggest that they may be upregulated more in the absence of microglia. Similar conclusions in regard to chemokine/cytokine expression were made following coronavirus infection of PLX5622-treated animals (26). Of these genes, CCL2, which is thought to be potentially of astroglial and neuronal origin (43) and may regulate the pain response and monocyte migration during infection (44, 45), has been implicated in facilitating microglial recruitment during infection (46). CCL2 upregulation may thus be an attempt to facilitate a microglial response even after microglial ablation (47). TREM2 was found to be downregulated in the brain following WNV infection in the presence of PLX5622, consistent with the fact that it is produced predominantly by microglia (48). The disruption in chemokine and cytokine responses in PLX5622-treated mice may contribute to dysfunctional T-cell responses, as has been indicated in previous studies (26).
The measured neuroinflammatory cytokines and chemokines following microglial depletion make it clear that many other cells may be responsible for facilitating neuroinflammatory responses, including astrocytes, neurons, and perivascular macrophages (26). Although neurons are known to be infected by these viruses, their exact role in triggering neuroinflammation remains unclear (8, 34, 42, 49). Astrocytes are also thought to be mediators of various virally induced pathologies but require further investigation as producers of immunoregulatory proteins (50, 51). Additionally innate immune signaling via host pathogen-associated molecular pattern recognition receptors remains poorly understood for the cells of the CNS (52, 53) and warrants future investigation as to how these pathways function in microglia and other cells of the CNS.
These data, taken together, show that PLX5622 treatment leads to microglial depletion, dramatically increased disease severity, and increased titer and mortality following flavivirus infection. We postulate that the animals die faster and at a higher rate due to the increased viral load in the brain and that for some reason chemokine/cytokine production, whether maintained or increased, is not effective at clearing the infection. We speculate that microglia have an additional protective function that is critical in controlling viral infection and may involve their ability to phagocytose infected cells or debris. Alternatively, increased cytokines and chemokines may be inherently detrimental to health rather than supporting recovery.
Future studies using PLX5622-based microglial depletion will help elucidate various roles for microglia following viral infection and give insight on potential mechanisms of CNS injury.
MATERIALS AND METHODS
PLX5622 preparation and administration.
PLX5622 was generously provided by Plexxikon for in vivo experiments. Research Diets prepared the food on the AIN-76A formulation (high sucrose) as previously described (26). Mice were fed for 14 days before infection and monitored following 21 days postinfection.
Immunohistochemistry.
Mice were perfusion fixed with 20 ml of 4% paraformaldehyde (PFA) followed by 20 ml of phosphate-buffered saline (PBS)–EDTA. Brains were processed, immersed in 3% agarose, and then mounted to a Vibratome platform (Leica VT1000S). Slice sections were collected at 100-μm thickness (speed 1 and frequency setting 9), followed by primary anti-Iba1 (1:500; Wako Labs) and secondary (Alexa Fluor 488; Invitrogen) staining, and imaged as described previously (31).
Mice.
Seven- to 10-week-old female Swiss-Webster mice were used for this study (Envigo). In our previous studies we have found no significant difference in lethality between male and female Swiss-Webster mice. Mice were observed daily and body weights determined. All experiments were approved by the Institutional Animal Care and Use Committee (IACUC).
WNV and JEV stocks and inoculation.
Both the NY99 strain of WNV and the p3 strain of JEV were prepared as previously described (54). Briefly, the viruses were propagated in Vero cells following a passage through C6/36 mosquito cells to amplify the virus. Following the observation of cytopathic effect in vitro, the virus was purified through sucrose cushion ultracentrifugation to remove cellular debris and associated growth factors. Viruses were diluted to the indicated inoculums in sterile phosphate-buffered saline and injected in the left rear footpad.
RT-qPCR and determination of viral titers.
Whole-brain homogenates were made using either zirconium beads followed by 2 cycles through a BeadBug homogenizer or a Tenbroeck glass homogenizer. A portion of this homogenate was then mixed with RLT (RNA tissue lysis extraction buffer; Qiagen) and extracted using the Qiagen RNeasy midikit protocol. RNA was quantified on a AlphaSpec NanoDrop instrument, and 1,000 ng was converted to cDNA using the Qiagen iScript kit. RT-qPCR was done on a CFX1000 instrument (Bio-Rad) and analyzed using Bio-Rad software before statistical analysis (two-tailed t test) was finalized using GraphPad. Viral titers were transformed to their respective log10 value; undefined titers were arbitrarily given a value of 1 and thus become 0 on each graph. The PCR primers for equivalent PFU (ePFU) of WNV virus and JEV were as follows: WNV 3′-UTR FOR, 5′-CAGACCACGCTACGGCG-3′; WNV 3′-UTR REV, 5′-CTAGGGCCGCGTGGG-3′; WNV 3′-UTR probe, 5′-FAM/TCTGCGGAG/ZENAGTGCAGTCTGCGAT-3′; JEV NS3 FOR, 5′-AGAGCACCAAGGGAATGAAATAGT-3′; JEV NS3 REV, 5′-AATAGGTTGTAGTTGGGCACTCTG-3′; JEV NS3 probe, 5′-FAM/CCACGCCACZEN/TCGACCCATAGACTG-3′.
ACKNOWLEDGMENTS
This work was supported by the Department of Veteran's Affairs and the National Institutes of Health (R01 NS076512 and R33AI101064) and by the RNA Bioscience Initiative Graduate Scholar's award (S.S.).
K.L.T. is the Louise Baum Endowed Professor and Chair.
REFERENCES
- 1.Davis LE, DeBiasi R, Goade DE, Haaland KY, Harrington JA, Harnar JB, Pergam SA, King MK, DeMasters BK, Tyler KL. 2006. West Nile virus neuroinvasive disease. Ann Neurol 60:286–300. doi: 10.1002/ana.20959. [DOI] [PubMed] [Google Scholar]
- 2.Debiasi RL, Tyler KL. 2006. West Nile virus meningoencephalitis. Nat Clin Pract Neurol 2:264–275. doi: 10.1038/ncpneuro0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reference deleted.
- 4.Shrestha B, Samuel MA, Diamond MS. 2006. CD8+ T cells require perforin to clear West Nile virus from infected neurons. J Virol 80:119–129. doi: 10.1128/JVI.80.1.119-129.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Larena M, Regner M, Lobigs M. 2013. Cytolytic effector pathways and IFN-gamma help protect against Japanese encephalitis. Eur J Immunol 43:1789–1798. doi: 10.1002/eji.201243152. [DOI] [PubMed] [Google Scholar]
- 6.Nazmi A, Dutta K, Das S, Basu A. 2011. Japanese encephalitis virus-infected macrophages induce neuronal death. J Neuroimmune Pharmacol 6:420–433. doi: 10.1007/s11481-011-9271-x. [DOI] [PubMed] [Google Scholar]
- 7.Ding K, Wang H, Xu J, Lu X, Zhang L, Zhu L. 2014. Melatonin reduced microglial activation and alleviated neuroinflammation induced neuron degeneration in experimental traumatic brain injury: possible involvement of mTOR pathway. Neurochem Int 76:23–31. doi: 10.1016/j.neuint.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 8.Nazmi A, Mukhopadhyay R, Dutta K, Basu A. 2012. STING mediates neuronal innate immune response following Japanese encephalitis virus infection. Sci Rep 2:347. doi: 10.1038/srep00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ito D, Tanaka K, Suzuki S, Dembo T, Fukuuchi Y. 2001. Enhanced expression of Iba1, ionized calcium-binding adapter molecule 1, after transient focal cerebral ischemia in rat brain. Stroke 32:1208–1215. doi: 10.1161/01.STR.32.5.1208. [DOI] [PubMed] [Google Scholar]
- 10.Biber K, Owens T, Boddeke E. 2014. What is microglia neurotoxicity (Not)? Glia 62:841–854. doi: 10.1002/glia.22654. [DOI] [PubMed] [Google Scholar]
- 11.Orihuela R, McPherson CA, Harry GJ. 2016. Microglial M1/M2 polarization and metabolic states. Br J Pharmacol 173:649–665. doi: 10.1111/bph.13139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen CJ, Ou YC, Chang CY, Pan HC, Liao SL, Chen SY, Raung SL, Lai CY. 2012. Glutamate released by Japanese encephalitis virus-infected microglia involves TNF-alpha signaling and contributes to neuronal death. Glia 60:487–501. doi: 10.1002/glia.22282. [DOI] [PubMed] [Google Scholar]
- 13.Mori I, Goshima F, Koshizuka T, Imai Y, Kohsaka S, Koide N, Sugiyama T, Yoshida T, Yokochi T, Kimura Y, Nishiyama Y. 2003. Iba1-expressing microglia respond to herpes simplex virus infection in the mouse trigeminal ganglion. Brain Res Mol Brain Res 120:52–56. doi: 10.1016/j.molbrainres.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Mori I, Imai Y, Kohsaka S, Kimura Y. 2000. Upregulated expression of Iba1 molecules in the central nervous system of mice in response to neurovirulent influenza A virus infection. Microbiol Immunol 44:729–735. doi: 10.1111/j.1348-0421.2000.tb02556.x. [DOI] [PubMed] [Google Scholar]
- 15.Quick ED, Seitz S, Clarke P, Tyler KL. 2017. Minocycline has anti-inflammatory effects and reduces cytotoxicity in an ex vivo spinal cord slice culture model of West Nile virus infection. J Virol 91:e00569-17. doi: 10.1128/JVI.00569-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Szretter KJ, Samuel MA, Gilfillan S, Fuchs A, Colonna M, Diamond MS. 2009. The immune adaptor molecule SARM modulates tumor necrosis factor alpha production and microglia activation in the brainstem and restricts West Nile Virus pathogenesis. J Virol 83:9329–9338. doi: 10.1128/JVI.00836-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vasek MJ, Garber C, Dorsey D, Durrant DM, Bollman B, Soung A, Yu J, Perez-Torres C, Frouin A, Wilton DK, Funk K, DeMasters BK, Jiang X, Bowen JR, Mennerick S, Robinson JK, Garbow JR, Tyler KL, Suthar MS, Schmidt RE, Stevens B, Klein RS. 2016. A complement-microglial axis drives synapse loss during virus-induced memory impairment. Nature 534:538–543. doi: 10.1038/nature18283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swarup V, Ghosh J, Duseja R, Ghosh S, Basu A. 2007. Japanese encephalitis virus infection decrease endogenous IL-10 production: correlation with microglial activation and neuronal death. Neurosci Lett 420:144–149. doi: 10.1016/j.neulet.2007.04.071. [DOI] [PubMed] [Google Scholar]
- 19.Hayasaka D, Shirai K, Aoki K, Nagata N, Simantini DS, Kitaura K, Takamatsu Y, Gould E, Suzuki R, Morita K. 2013. TNF-alpha acts as an immunoregulator in the mouse brain by reducing the incidence of severe disease following Japanese encephalitis virus infection. PLoS One 8:e71643. doi: 10.1371/journal.pone.0071643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dutta K, Mishra MK, Nazmi A, Kumawat KL, Basu A. 2010. Minocycline differentially modulates macrophage mediated peripheral immune response following Japanese encephalitis virus infection. Immunobiology 215:884–893. doi: 10.1016/j.imbio.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 21.Salimi H, Cain MD, Klein RS. 2016. Encephalitic arboviruses: emergence, clinical presentation, and neuropathogenesis. Neurotherapeutics 13:514–534. doi: 10.1007/s13311-016-0443-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Waisman A, Ginhoux F, Greter M, Bruttger J. 2015. Homeostasis of microglia in the adult brain: review of novel microglia depletion systems. Trends Immunol 36:625–636. doi: 10.1016/j.it.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 23.De I, Nikodemova M, Steffen MD, Sokn E, Maklakova VI, Watters JJ, Collier LS. 2014. CSF1 overexpression has pleiotropic effects on microglia in vivo. Glia 62:1955–1967. doi: 10.1002/glia.22717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spangenberg EE, Lee RJ, Najafi AR, Rice RA, Elmore MR, Blurton-Jones M, West BL, Green KN. 2016. Eliminating microglia in Alzheimer's mice prevents neuronal loss without modulating amyloid-beta pathology. Brain 139:1265–1281. doi: 10.1093/brain/aww016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spangenberg EE, Green KN. 2017. Inflammation in Alzheimer's disease: lessons learned from microglia-depletion models. Brain Behav Immun 61:1–11. doi: 10.1016/j.bbi.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wheeler DL, Sariol A, Meyerholz DK, Perlman S. 2018. Microglia are required for protection against lethal coronavirus encephalitis in mice. J Clin Invest 128:931–943. doi: 10.1172/JCI97229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Imai Y, Ibata I, Ito D, Ohsawa K, Kohsaka S. 1996. A novel gene iba1 in the major histocompatibility complex class III region encoding an EF hand protein expressed in a monocytic lineage. Biochem Biophys Res Commun 224:855–862. doi: 10.1006/bbrc.1996.1112. [DOI] [PubMed] [Google Scholar]
- 28.Ito D, Imai Y, Ohsawa K, Nakajima K, Fukuuchi Y, Kohsaka S. 1998. Microglia-specific localisation of a novel calcium binding protein, Iba1. Brain Res Mol Brain Res 57:1–9. doi: 10.1016/S0169-328X(98)00040-0. [DOI] [PubMed] [Google Scholar]
- 29.Loane DJ, Kumar A. 2016. Microglia in the TBI brain: the good, the bad, and the dysregulated. Exp Neurol 275:316–327. doi: 10.1016/j.expneurol.2015.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clarke P, Leser JS, Bowen RA, Tyler KL. 2014. Virus-induced transcriptional changes in the brain include the differential expression of genes associated with interferon, apoptosis, interleukin 17 receptor A, and glutamate signaling as well as flavivirus-specific upregulation of tRNA synthetases. mBio 5:e00902-14. doi: 10.1128/mBio.00902-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quick E, Leser J, Clarke P, Tyler K. 2014. Activation of intrinsic immune responses and microglial phagocytosis in an ex vivo spinal cord slice culture model of West Nile virus infection. J Virol 88:13005–13014. doi: 10.1128/JVI.01994-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reference deleted.
- 33.Reference deleted.
- 34.Chakraborty S, Nazmi A, Dutta K, Basu A. 2010. Neurons under viral attack: victims or warriors? Neurochem Int 56:727–735. doi: 10.1016/j.neuint.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reference deleted.
- 36.Elmore MR, Najafi AR, Koike MA, Dagher NN, Spangenberg EE, Rice RA, Kitazawa M, Matusow B, Nguyen H, West BL, Green KN. 2014. Colony-stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain. Neuron 82:380–397. doi: 10.1016/j.neuron.2014.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reference deleted.
- 38.Reference deleted.
- 39.Reference deleted.
- 40.Reference deleted.
- 41.Lannes N, Neuhaus V, Scolari B, Kharoubi-Hess S, Walch M, Summerfield A, Filgueira L. 2017. Interactions of human microglia cells with Japanese encephalitis virus. Virol J 14:8. doi: 10.1186/s12985-016-0675-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klein RS, Lin E, Zhang B, Luster AD, Tollett J, Samuel MA, Engle M, Diamond MS. 2005. Neuronal CXCL10 directs CD8+ T-cell recruitment and control of West Nile virus encephalitis. J Virol 79:11457–11466. doi: 10.1128/JVI.79.17.11457-11466.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pevida M, Gonzalez-Rodriguez S, Lastra A, Garcia-Suarez O, Hidalgo A, Menendez L, Baamonde A. 2014. Involvement of spinal chemokine CCL2 in the hyperalgesia evoked by bone cancer in mice: a role for astroglia and microglia. Cell Mol Neurobiol 34:143–156. doi: 10.1007/s10571-013-9995-7. [DOI] [PubMed] [Google Scholar]
- 44.Piotrowska A, Kwiatkowski K, Rojewska E, Slusarczyk J, Makuch W, Basta-Kaim A, Przewlocka B, Mika J. 2016. Direct and indirect pharmacological modulation of CCL2/CCR2 pathway results in attenuation of neuropathic pain—in vivo and in vitro evidence. J Neuroimmunol 297:9–19. doi: 10.1016/j.jneuroim.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 45.Getts DR, Terry RL, Getts MT, Muller M, Rana S, Shrestha B, Radford J, Van Rooijen N, Campbell IL, King NJ. 2008. Ly6c+ “inflammatory monocytes” are microglial precursors recruited in a pathogenic manner in West Nile virus encephalitis. J Exp Med 205:2319–2337. doi: 10.1084/jem.20080421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li X, Hanson C, Cmarik JL, Ruscetti S. 2009. Neurodegeneration induced by PVC-211 murine leukemia virus is associated with increased levels of vascular endothelial growth factor and macrophage inflammatory protein 1 alpha and is inhibited by blocking activation of microglia. J Virol 83:4912–4922. doi: 10.1128/JVI.02343-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Allen F, Bobanga ID, Rauhe P, Barkauskas D, Teich N, Tong C, Myers J, Huang AY. 2018. CCL3 augments tumor rejection and enhances CD8(+) T cell infiltration through NK and CD103(+) dendritic cell recruitment via IFNgamma. Oncoimmunology 7:e1393598. doi: 10.1080/2162402X.2017.1393598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hsieh CL, Koike M, Spusta SC, Niemi EC, Yenari M, Nakamura MC, Seaman WE. 2009. A role for TREM2 ligands in the phagocytosis of apoptotic neuronal cells by microglia. J Neurochem 109:1144–1156. doi: 10.1111/j.1471-4159.2009.06042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nazmi A, Dutta K, Basu A. 2011. RIG-I mediates innate immune response in mouse neurons following Japanese encephalitis virus infection. PLoS One 6:e21761. doi: 10.1371/journal.pone.0021761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mishra MK, Kumawat KL, Basu A. 2008. Japanese encephalitis virus differentially modulates the induction of multiple pro-inflammatory mediators in human astrocytoma and astroglioma cell-lines. Cell Biol Int 32:1506–1513. doi: 10.1016/j.cellbi.2008.08.020. [DOI] [PubMed] [Google Scholar]
- 51.Das S, Mishra MK, Ghosh J, Basu A. 2008. Japanese encephalitis virus infection induces IL-18 and IL-1beta in microglia and astrocytes: correlation with in vitro cytokine responsiveness of glial cells and subsequent neuronal death. J Neuroimmunol 195:60–72. doi: 10.1016/j.jneuroim.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 52.Jiang R, Ye J, Zhu B, Song Y, Chen H, Cao S. 2014. Roles of TLR3 and RIG-I in mediating the inflammatory response in mouse microglia following Japanese encephalitis virus infection. J Immunol Res 2014:787023. doi: 10.1155/2014/787023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kundu K, Dutta K, Nazmi A, Basu A. 2013. Japanese encephalitis virus infection modulates the expression of suppressors of cytokine signaling (SOCS) in macrophages: implications for the hosts' innate immune response. Cell Immunol 285:100–110. doi: 10.1016/j.cellimm.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 54.Beatman E, Oyer R, Shives KD, Hedman K, Brault AC, Tyler KL, Beckham JD. 2012. West Nile virus growth is independent of autophagy activation. Virology 433:262–272. doi: 10.1016/j.virol.2012.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]