The ocular epithelial cells currently used to study HCMV infections in vitro have historical significance based upon their role in retinitis, an HCMV disease most often seen in AIDS patients. However, with the successful implementation of highly active antiretroviral therapy (HAART) regimens, the incidence of HCMV retinitis has rapidly declined, and therefore, the relevance of studying ocular epithelial cell HCMV infection has decreased as well. Our introduction here of oral epithelial cells provides two alternative in vitro models for the study of HCMV infection that complement and extend the physiologic relevance of the ocular system currently in use.
KEYWORDS: HCMV, cytomegalovirus, herpes, keratinocyte, lytic, oral, pentamer, periodontal
ABSTRACT
Human cytomegalovirus (HCMV) productive replication in vitro is most often studied in fibroblasts. In vivo, fibroblasts amplify viral titers, but transmission and pathogenesis require the infection of other cell types, most notably epithelial cells. In vitro, the study of HCMV infection of epithelial cells has been almost exclusively restricted to ocular epithelial cells. Here we present oral epithelial cells with relevance for viral interhost transmission as an in vitro model system to study HCMV infection. We discovered that HCMV productively replicates in normal oral keratinocytes (NOKs) and telomerase-immortalized gingival cells (hGETs). Our work introduces oral epithelial cells for the study of HCMV productive infection, drug screening, and vaccine development.
IMPORTANCE The ocular epithelial cells currently used to study HCMV infections in vitro have historical significance based upon their role in retinitis, an HCMV disease most often seen in AIDS patients. However, with the successful implementation of highly active antiretroviral therapy (HAART) regimens, the incidence of HCMV retinitis has rapidly declined, and therefore, the relevance of studying ocular epithelial cell HCMV infection has decreased as well. Our introduction here of oral epithelial cells provides two alternative in vitro models for the study of HCMV infection that complement and extend the physiologic relevance of the ocular system currently in use.
INTRODUCTION
Human cytomegalovirus (HCMV) is a betaherpesvirus that infects the majority of the population worldwide. In healthy individuals, infection is generally subclinical, but the virus causes severe disease in individuals with compromised, suppressed, or incompletely developed immune systems (1, 2). For example, HCMV causes retinitis and blindness in AIDS patients, organ dysfunction or failure in transplant recipients, and birth defects, including microcephaly and hearing loss, in neonates. Furthermore, research continues into possible roles for HCMV in human cancers such as glioblastoma (3–5). No vaccine exists, but antiviral drugs are available, including the recently approved drug letermovir. The drugs are useful against productive (lytic) infection but select for resistant viruses (6, 7). Furthermore, the approved antivirals do not target the latent reservoirs that permit the virus to infect its host for their lifetime and that reactivate to allow spread within and between hosts.
HCMV infects an array of cell types, including myeloid-lineage cells and endothelial, fibroblast, smooth muscle, glial, and epithelial cells (8). It is speculated, and in many cases known, that infection of a specific cell type has a unique biological or clinical role (9). For example, latency is established in the myeloid and perhaps endothelial cell lineages, and fibroblasts are sites of viral amplification. Furthermore, smooth muscle and glial cell infection may play roles in chronic pathologies associated with HCMV infection (atherosclerosis and cancer, respectively), and epithelial cell infection contributes to both organ disease and viral dissemination through urine, breast milk, and saliva. Therefore, studying HCMV infection in different and relevant cell types can reveal novel insights about replication, persistence, and pathogenesis and point to unique opportunities for novel antiviral interventions.
At the height of the AIDS epidemic, HCMV was a common opportunistic infection in patient cohorts. Retinitis caused by HCMV occurred in 25% to 42% of AIDS patients (10), often leading to blindness. Within the eye, the retinal pigmented epithelium (RPE) was defined as the primary site of infection (11). The severity of the clinical syndrome and the identification of the major relevant cell type sparked interest in studying in vitro HCMV infections in RPE cells (12–14), which were found to be productive rather than latent (see below for further discussion). Importantly, RPE cells were instrumental in determining that the viral pentameric glycoprotein complex (15, 16) was essential for infection of epithelial cells (17). The development and widespread use of highly active antiretroviral therapy (HAART) regimens not only stabilized the AIDS epidemic in developed countries but also dramatically reduced the incidence of retinitis caused by HCMV by ∼80% (10). Therefore, while epithelial cells still remain an import in vivo target of HCMV and thus a priority for in vitro study, both the biological and clinical relevance of studying RPE cells has substantially decreased.
Surprisingly, HCMV infection in other types of epithelial cells has received far less study. Sporadic reports of in vitro HCMV infections of epithelial cells from the cervix (13), cochlea (18), kidney (19), mammary gland (20), and thyroid (21) have appeared. These infections were found to be productive. There is a significant body of work examining murine cytomegalovirus (MCMV) replication in the salivary gland (22). However, despite the high concentration of infectious HCMV found in saliva and the identification of HCMV in oral epithelial cells in vivo (23–26), cultured human oral epithelial cell populations have not been used in vitro to study HCMV infections. More work has examined infection by the gammaherpesviruses Epstein-Barr virus (EBV) and Kaposi's sarcoma-associated herpesvirus (KSHV) in oral epithelial cells from stratified squamous epithelia (see below for a description of oral epithelial cell differentiation). For EBV and KSHV, undifferentiated cells supported latent infection, whereas differentiated cells supported productive infection (27–29). Interestingly, this mimics HCMV infection of myeloid cells, where the virus establishes latency within undifferentiated cells but replicates productively in fully differentiated cells.
We examined HCMV infection of two undifferentiated oral epithelial cell cultures, telomerase-immortalized normal oral keratinocytes (NOKs) and telomerase-immortalized gingival cells (hGETs), to define the mode of infection. We determined that these oral epithelial cells support productive HCMV infection. This work establishes NOKs and hGETs as new models for the study of HCMV productive infection, dissemination, and antiviral sensitivity.
RESULTS
HCMV replicates productively in RPE cells.
The accepted and appropriate definition for productive infection is the release of infectious progeny virus. Latency is defined as the maintenance of the viral genome over time without generating infectious progeny with the capacity for future reactivation to a productive infection. These criteria take time to be realized, and therefore, molecular events that happen quickly after infection are often used as surrogates to predict whether an infection will be productive or latent. For example, productive infections are often characterized by the rapid and high-level accumulation of the viral immediate early 1 (IE1) protein (30). IE1 (UL123) transcription is initiated by the action of the tegument-delivered pp71 protein in the nucleus (31). Therefore, productive infection is inferred when tegument-delivered pp71 transits to the nucleus and when the IE1 protein accumulates. Latent infections are often characterized by the absence of IE1 protein accumulation. As IE1 drives the productive cycle and is a target for immune-mediated cell killing, keeping IE1 protein levels low or absent would appear to be a reasonable strategy to establish a latent infection in vitro, and even more so in vivo. IE1 protein accumulation during latency is prevented because the gene that encodes it (UL123) is transcriptionally silenced, in part, because tegument-delivered pp71, the protein that activates its transcription, is sequestered in the cytoplasm (32). While IE1 (UL123) is transcriptionally silenced during latency, other viral loci are not, including the long noncoding RNA (lncRNA) B2.7 (33). B2.7 is also expressed during productive infections. Therefore, latency is inferred when B2.7 (or another latency-associated transcript) accumulates but IE1 (UL123) RNAs fail to accumulate to productive-phase levels and when tegument-delivered pp71 is cytoplasmic.
HCMV requires the viral pentameric protein complex in its envelope to enter RPE cells (17). To test if the pentamer is also required for viral entry into oral epithelial cells, we used a matched pair (13, 17) of pentamer-negative (AD169) and pentamer-positive (ADrUL131) viral strains. To distinguish between productive and latent infections, we monitored tegument-delivered pp71 subcellular localization, B2.7 and IE1 (UL123) mRNA levels, and IE1 protein accumulation.
In tissues in vivo or in organotypic raft cultures in vitro, oral epithelial cells form a stratified squamous epithelium consisting of a basal layer of dividing, undifferentiated cells and multiple suprabasal layers of nondividing, differentiated cells (34). These cells express specific keratin proteins and therefore are classified as keratinocytes. They also differentially express the involucrin protein. Involucrin marks the differentiated cells within the upper (suprabasal) levels of a stratified squamous epithelium but is not found in undifferentiated (basal) cells (35). Prior to rafting, oral epithelial cells can be maintained in vitro as monolayers in their undifferentiated state by subconfluent culture in serum-free medium. As monolayers, they can be differentiated either through treatment with fetal bovine serum (FBS) and calcium or by the addition of methylcellulose to the growth medium (25, 36, 37). Our NOKs and hGETs maintained as monolayers in serum-free media did not express involucrin, but involucrin expression (differentiation) could be induced either by FBS and calcium or by methylcellulose (Fig. 1). Involucrin expression is restricted to keratinocytes and stratified squamous epithelia and therefore is not detected (Fig. 1) in RPEs or normal human dermal fibroblasts (NHDFs), a common model for productive HCMV infection.
FIG 1.

Calcium- or methylcellulose-dependent differentiation of monolayer NOKs or hGETs. NOKs or hGETs were left untreated and undifferentiated (U) or differentiated with calcium (Ca) or methylcellulose (MC). Protein lysates were analyzed by Western blotting with the indicated antibodies. Untreated RPE and HF cell lysates served as controls. Results are representative of data from three independent experiments.
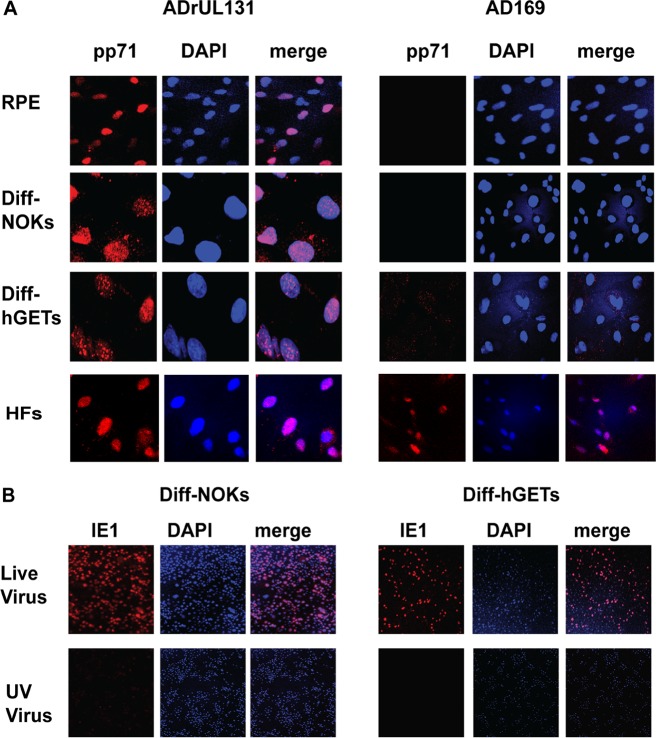
As expected based on previous reports of productive HCMV replication, upon infection of RPE cells with ADrUL131, tegument-delivered pp71 entered the nucleus (Fig. 2), both B2.7 and IE1 (UL123) RNAs were expressed (Fig. 3), the IE1 protein accumulated in the nucleus (Fig. 4), and the late proteins UL44 and pp28 also accumulated (Fig. 5A). For all these assays (Fig. 2 to 5), human fibroblasts (HFs) served as a positive control for productive infection. Importantly, infectious progeny were released from RPE cells (Fig. 6A), defining the infection as productive. The parent virus, AD169, does not express the pentamer and, as previously described (13, 17), was unable to enter RPE cells, a conclusion based on an inability to detect intracellular pp71 (Fig. 2) or IE1 (Fig. 4) proteins, B2.7 or IE1 (UL123) RNAs (Fig. 3), or infectious progeny virions (Fig. 6A).
FIG 2.
Pentamer-competent HCMV delivers tegument-incorporated pp71 to the nucleus of differentiated NOKs or hGETs. (A) The indicated cells grown on coverslips were infected at a multiplicity of infection (MOI) of 3 with UV-inactivated pentamer-competent (AdrUL131) or UV-inactivated pentamer-deficient (AD169) HCMV for 24 h. The intracellular localization of tegument-delivered pp71 was determined by indirect immunofluorescence microscopy. (B) Differentiated NOKs or hGETs grown on coverslips were infected with either live virus or the UV-inactivated virus preparations used for panel A. IE1 expression was visualized by indirect immunofluorescence. Results are representative of data from three independent experiments. DAPI, 4′,6-diamidino-2-phenylindole.
FIG 3.
Pentamer-competent HCMV transcribes the viral IE1 (UL123) and B2.7 genes in NOKs or hGETs. (A) The indicated untreated cells were mock infected or infected with the indicated virus at an MOI of 3. After 24 h, RNA was prepared and analyzed by real-time quantitative reverse transcriptase PCR (qRT-PCR) for B2.7. (B) qRT-PCR analysis for IE1 (UL123) with RNA generated as described above for panel A. Results from three biological replicates were analyzed with Student's t test. *, P < 0.05; **, P < 0.01; n.s., not significant. (C) The indicated differentiated cells were mock infected or infected with the indicated virus at an MOI of 3. After 24 h, RNA was prepared and analyzed by qRT-PCR for B2.7. (D) qRT-PCR analysis for IE1 (UL123) with RNA generated as described above for panel C. Results from three biological replicates were analyzed with Student's t test. **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.
FIG 4.
The IE1 protein accumulates in differentiated NOKs or hGETs infected with pentamer-competent HCMV. The indicated cells grown on coverslips were infected at an MOI of 3 with the indicated virus for 24 h. IE1 expression was visualized by indirect immunofluorescence microscopy. Results are representative of data from three independent experiments.
FIG 5.

Representative members of each productive-phase kinetic class of viral protein analyzed in differentiated NOKs or hGETs infected with pentamer-competent HCMV. (A) The indicated cells were infected with ADrUL131 at an MOI of 3, and protein lysates harvested at the indicated times were analyzed by Western blotting with the indicated antibodies. Lysates from uninfected, differentiated NOKs and hGETs were analyzed as controls. Results are representative of data from three independent experiments. (B) The indicated cells were infected with ADrUL131 at an MOI of 3, and protein lysates harvested at the indicated times were analyzed by Western blotting with the indicated antibodies. Lysates from uninfected, undifferentiated NOKs and hGETs were analyzed as controls. Results are representative of data from three independent experiments.
FIG 6.
Analysis of pentamer-competent HCMV productive replication in oral epithelial cells. (A) Monolayer RPE cells were infected at an MOI of 1. Infected cells and media were collected at the indicated times, from which virus preparations were generated and titers were determined. Results from three independent biological replicates are shown. Error bars represent the standard errors of the means. (B) Monolayer differentiated cells were analyzed as described above for panel A. Results from three independent biological replicates are shown. Error bars represent the standard errors of the means. (C) Monolayer undifferentiated cells were analyzed as described above for panel A. Results from three independent biological replicates are shown. Error bars represent the standard errors of the means.
HCMV initiates but fails to complete productive infection in differentiated NOKs and hGETs.
We first compared RPE infections to infections of differentiated NOKs (Diff-NOKs) and differentiated hGETs (Diff-hGETs). Diff-NOKs and Diff-hGETs initiate the hallmarks of a pentamer-dependent productive HCMV infection but fail to complete the process. ADrUL131 infection delivered pp71 to the nucleus (Fig. 2), initiated the transcription of both B2.7 (Fig. 3C) and IE1 (UL123) (Fig. 3D), and resulted in IE1 protein accumulation in the nucleus (Fig. 4). AD169 was unable to achieve any of these hallmarks of productive infection initiation and failed to deliver pp71 to the cytoplasm, indicating that a latent infection was not established. Immediate early (IE1) and early (UL44) proteins accumulated in ADrUL131-infected Diff-NOKs and Diff-hGETs (Fig. 5B), as they did in NHDFs and RPE cells (Fig. 5A). The level of the late protein pp28 remained below our limit of detection. Involucrin expression in Diff-NOKs and Diff-hGETs (Fig. 5B) confirmed that these oral epithelial cells were differentiated. Both Diff-NOKs and Diff-hGETs generated few if any infectious viral progeny after infection with ADrUL131 (Fig. 6B). We conclude that AD169 fails to enter Diff-NOKs and Diff-hGETs because it lacks a functional pentamer complex. Furthermore, we conclude that a pentamer-expressing HCMV initiates a productive infection upon entering Diff-NOKs or Diff-hGETs but fails to successfully complete the process.
HCMV replicates productively in undifferentiated NOKs and hGETs.
Interestingly, undifferentiated NOKs and hGETs also illustrated the hallmarks of a pentamer-dependent productive infection, and in these cells, productive replication was successfully completed. ADrUL131 infection delivered pp71 to the nucleus (Fig. 7), initiated the transcription of both B2.7 (Fig. 3A) and IE1 (UL123) (Fig. 3B), and resulted in IE1 protein accumulation in the nucleus (Fig. 8). For all these assays (Fig. 3, 7, and 8), HFs served as a positive control for productive infection. AD169 was unable to achieve any of these hallmarks of productive infection and failed to deliver pp71 to the cytoplasm, indicating that a latent infection was not established. However, AD169 entered NHDFs and expressed both B2.7 (Fig. 3A) and IE1 (UL123) (Fig. 3B) RNAs, indicating the initiation of a productive infection in fibroblasts, as expected. Immediate early (IE1), early (UL44), and late (pp28) proteins accumulated in ADrUL131-infected NOKs and hGETs (Fig. 9A), as they did in NHDFs and RPE cells (Fig. 9B). Involucrin expression in Diff-NOKs and Diff-hGETs, but not in undifferentiated NOKs or hGETs (Fig. 9A), discriminated differentiated from undifferentiated epithelial cells. Both NOKs and hGETs generated infectious viral progeny after infection with ADrUL131 (Fig. 6C). We conclude that AD169 fails to enter NOKs and hGETs because it lacks a functional pentamer complex. Furthermore, we conclude that a pentamer-expressing HCMV initiates a productive infection upon entering NOKs or hGETs, examples of undifferentiated oral epithelial cells.
FIG 7.
Pentamer-competent HCMV delivers tegument-incorporated pp71 to the nucleus of undifferentiated NOKs or hGETs. (A) The indicated cells grown on coverslips were infected at an MOI of 3 with UV-inactivated pentamer-competent (AdrUL131) or UV-inactivated pentamer-deficient (AD169) HCMV for 24 h. The intracellular localization of tegument-delivered pp71 was determined by indirect immunofluorescence microscopy. (B) Undifferentiated NOKs or hGETs grown on coverslips were infected with either live virus or the UV-inactivated virus preparations used for panel A. IE1 expression was visualized by indirect immunofluorescence. Results are representative of data from three independent experiments.
FIG 8.
The IE1 protein accumulates in undifferentiated NOKs or hGETs infected with pentamer-competent HCMV. The indicated cells grown on coverslips were infected at an MOI of 3 with the indicated virus for 24 h. IE1 expression was visualized by indirect immunofluorescence microscopy. Results are representative of data from three independent experiments.
FIG 9.

Representative members of each productive-phase kinetic class of viral protein accumulate in undifferentiated NOKs or hGETs infected with pentamer-competent HCMV. (A) The indicated cells were infected with ADrUL131 at an MOI of 3, and protein lysates harvested at the indicated times were analyzed by Western blotting with the indicated antibodies. Lysates from uninfected, differentiated NOKs and hGETs were analyzed as controls. Results are representative of data from three independent experiments. (B) The indicated control cells were analyzed as described above. Results are representative of data from three independent experiments.
HCMV replication in NOKs and hGETs is cytopathic.
The numbers of cells in which the IE1 protein accumulated during the first 24 h after ADrUL131 infection were similar between undifferentiated and differentiated hGETs but slightly higher for differentiated than for undifferentiated NOK cells (Fig. 10A). Interestingly, tegument-delivered pp71 migrated to the nucleus much faster in NHDFs, differentiated NOKs, and differentiated hGETs than it did in RPE cells, undifferentiated NOKs, or undifferentiated hGETs (Fig. 10B). The rapid delivery of pp71 to the nucleus does not dramatically affect viral gene expression in epithelial cells, nor does it predict infection outcome, and therefore, its significance, if any, is unknown.
FIG 10.

Differentiated NOKs, but not hGETs, display more IE1-positive cells early after infection than do their undifferentiated counterparts. (A) The indicated cells grown on coverslips were infected at an MOI of 3 with ADrUL131 for 24 h. IE1 expression was visualized by indirect immunofluorescence microscopy, and the fraction of positive cells was determined visually. The averages of data from three biological replicates are plotted. Error bars represent the standard errors of the means. The data were analyzed with Student's t test. **, P < 0.01; ns, not significant. (B) The indicated cells grown on coverslips were infected at an MOI of 3 with pentamer-competent (AdrUL131) HCMV for 6 h. The intracellular localization of tegument-delivered pp71 was determined by indirect immunofluorescence microscopy. Results are representative of data from three independent experiments.
Increased cytopathic effect (CPE) was observed upon infection of NOKs or hGETs compared to RPE cells at 5 days postinfection (Fig. 11). Time course studies (Fig. 12) showed that the CPE in undifferentiated NOKs and hGETs required viral replication, increased with infection time, and was more evident than in similar infections of differentiated NOKs or differentiated hGETs. Viral replication and the subsequent CPE may in part explain why we were unable to raft undifferentiated and infected NOKs or hGETs. While uninfected cultures generated typical stratified squamous epithelia, essentially only the collagen matrix remained with infected cultures after 14 days (Fig. 13). Similar results were obtained after 6 days (Fig. 14), a time frame more similar to a single round of productive HCMV replication. UV-inactivated virus had no effect on the rafting of NOKs. hGETs treated with UV-inactivated HCMV still formed rafts (Fig. 14), although their thickness was reduced compared to that in mock-infected cultures. Thus, HCMV replication-induced cell death of undifferentiated NOKs and hGETs occurs with faster kinetics than does raft generation. In total, we conclude that HCMV productively infects undifferentiated oral epithelial cells in vitro in a pentamer-dependent manner.
FIG 11.

HCMV infection of undifferentiated NOKs or hGETs produces a cytopathic effect. Monolayer cells were mock infected or infected with green fluorescent protein (GFP)-expressing ADrUL131 at an MOI of 1 for 5 days, after which bright-field and fluorescence images were captured. Images are representative of results from at least three independent experiments.
FIG 12.
HCMV-induced cytopathic effect in monolayer NOKs and hGETs requires viral replication. Monolayer cells were mock infected or infected with GFP-expressing ADrUL131 at an MOI of 1 for the indicated times, after which bright-field and fluorescence images were captured. Images are representative of results from three independent experiments. (A) NOKs; (B) differentiated NOKs; (C) hGETs; (D) differentiated hGETs. dpi, days postinfection.
FIG 13.

HCMV infection of NOKs or hGETs prevents stratification upon raft culturing. Monolayer cells were mock infected or infected with pentamer-competent (AdrUL131) or pentamer-deficient (AD169) HCMV at an MOI of 3 for 1 h and then rafted. Rafts were cultured for 14 days prior to sectioning, staining, and imaging. Images are representative of results from two independent experiments.
FIG 14.

HCMV-induced cytopathic effect in rafted NOKs or hGETs requires viral replication. (A) Monolayer cells were mock infected or infected with live or UV-inactivated ADrUL131 at an MOI of 3 for 1 h and then rafted. Rafts were cultured for 6 days prior to sectioning, staining, and imaging. Images are representative of results from three independent experiments. (B) NHDFs were infected with the indicated viral stocks used for panel A at an MOI of 3, and protein lysates harvested at 24 h postinfection were analyzed by Western blotting with the indicated antibodies. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
DISCUSSION
Epithelial cells from different anatomical sites are not identical. For example, in vivo, RPEs are postmitotic, monolayer cells (38), whereas oral keratinocytes form multilayer structures composed of differentially located dividing or postmitotic (differentiated) cells (30). Therefore, it might not be surprising if specific characteristics of in vitro infection with HCMV were to differ depending upon the type of epithelial cell studied. Furthermore, the relevance of the results to pathogenesis or dissemination would also seem to rely on the origin of the epithelial cell used for in vitro study. The classic epithelial cell type used to study HCMV infection in vitro has been RPE cells, mainly because of the incidence of HCMV retinitis in pre-HAART-era AIDS patients. As the prevalence of retinitis continues to diminish, epithelial cells from other anatomical sites that model other aspects of HCMV disease or transmission should supplant RPE cells as models of choice for studying HCMV infection in vitro.
We chose to examine in vitro HCMV infection of oral epithelial cells because we were intrigued at the possibility that, in vivo, such infections might provide a latent reservoir important for viral transmission from person to person through saliva. HCMV is known to reside latently in bone marrow hematopoietic progenitor cells and circulating monocytes and may form an additional latent reservoir in microvascular endothelial cells. Reactivation from these sites of carriage seems to reasonably explain intrahost dissemination. However, without a subsequent amplification step (for example, in differentiated epithelial cells), it is unclear if reactivation from the known sites of latency could be directly responsible for most interhost dissemination events. Conversely, reactivations in the oral cavity could easily explain high-level host-to-host transmission through saliva.
There were two major reasons why we hypothesized that oral epithelial cells could represent an undiscovered site of HCMV latency. First, the oral cavity sustains protracted infections, undergoes a high incidence of reactivation events, and appears to be an immune-privileged site for viral persistence, all contributing to viral dissemination via saliva (22). Second, both EBV and KSHV establish latent infections in undifferentiated, basal-layer oral keratinocytes but reactivate upon cellular differentiation (23–25). The known dependence upon differentiation of HCMV reactivation from the myeloid lineage (39) as well as the known characteristics of oral cavity infection mentioned above prompted us to ask if undifferentiated oral epithelial cells supported a productive or a latent infection.
Our results unequivocally demonstrate that undifferentiated oral epithelial cells support productive HCMV infection in vitro. While previous reports of HCMV in vitro infection of epithelial cells sourced from other anatomical sites have all reported productive infection, the cells examined do not generate stratified epithelial layers and thus did not afford the possibility to examine HCMV replication within an undifferentiated subset of cells. The CPE elicited by in vitro infection may contribute to the low titers of infectious progeny generated by these oral epithelial cells. If such CPE were also characteristic of in vivo infections of the oral epithelium, loosely attached infected cells may spread the virus from person to person through saliva. CPE might also provide a recruiting signal for monocytes, which could then carry latent virus to distant anatomical sites within the original host, thereby promoting intrahost viral dissemination. Therefore, these cells represent an opportunity to study and understand the mechanisms and consequences of CPE during an HCMV infection. Immortalization with telomerase does not increase CPE or decrease viral titers during productive infection of fibroblasts (40) and therefore is unlikely to be the cause of these phenotypes in NOKs and hGETs. In addition, the apparent abortive infection in fully differentiated oral epithelial cells provides a chance to identify host factor-dependent late steps critical for productive viral replication that become immediate targets for antiviral drug development.
It is unclear why infection of undifferentiated oral epithelial cells with HCMV, a betaherpesvirus, is substantially different from infection with the gammaherpesviruses EBV and KSHV, or even human papillomavirus, where productive replication is limited to suprabasal cells. In vitro infection of oral epithelial cells with alphaherpesviruses is also understudied; however, two studies with herpes simplex virus 1 (HSV-1) generated data consistent with productive replication in undifferentiated cells (41, 42). Furthermore, HSV-1 productive replication in epithelial cells from other anatomical locations has been reported (43, 44).
Here we introduce NOKs and hGETs as novel models to study in vitro epithelial cell infection. Our initial results with these two new models confirm and extend the absolute dependence for epithelial infection of the viral glycoprotein pentameric complex. In the future, NOKs and hGETs will be useful to us and others for the continued search for the elusive receptor(s) for the HCMV pentamer and in screens for neutralizing antibodies, vaccines, or antiviral drugs that may inhibit epithelial cell infection by alpha-, beta-, and gammaherpesviruses and thereby curtail or eliminate both oral disease and viral dissemination through saliva.
MATERIALS AND METHODS
Cells and viruses.
NOKs are human telomerase reverse transcriptase (hTERT)-expressing cells from human gingival tissue (45), generously provided by Karl Münger. hGETs are hTERT-expressing normal human gingival epithelial progenitor cells (46). NOKs and hGETs were cultured as monolayers in keratinocyte serum-free medium (KSFM; Life Technologies) supplemented with 0.14 μg/ml epidermal growth factor and 0.33 mg/ml bovine pituitary extract at 5% CO2 and 37°C. To maintain their undifferentiated state, the culture medium is changed every other day, and cells are never allowed to approach confluence. NOKs and hGETs were differentiated by treatment with either calcium chloride (1.2 mM CaCl2 and 10% [vol/vol] fetal bovine serum [FBS] in KSFM) or methylcellulose (1.6 g/ml in KSFM without supplements) (catalog number M-0512; Sigma) for 48 h at 37°C. Rafting of NOKs and hGETs was performed as previously described for NIKs (near-diploid immortalized keratinocytes) (47). RPE cells are human retinal pigmented epithelial cells (ARPE-19; ATCC CRL-2302) and were provided by Tom Shenk. RPE cells were cultured in Dulbecco's modified Eagle medium 12 (DMEM-12; Life Technologies) supplemented with 10% (vol/vol) FBS, 15 mM HEPES (pH 7.3), 100 U/ml penicillin, and 100 μg/ml streptomycin at 5% CO2 and 37°C. NHDFs are normal human dermal fibroblasts (Clonetics) and were cultured in DMEM supplemented with 10% (vol/vol) FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin at 5% CO2 and 37°C. The HCMV strains used were AD169 and an AD169 derivative (ADrUL131, generously provided by Tom Shenk) in which the frameshift mutation in the UL131 gene was repaired (13). Viruses were grown and titers were determined on NHDFs. Infections were performed in a minimal volume of culture medium at 37°C for 1 h in a tissue culture incubator, after which additional medium was added.
Assays and antibodies.
Western blot analyses (48), indirect immunofluorescence microscopy (49), and quantitative real-time PCR (50) were performed with primer pairs (51) as previously described. For plaque assays, infected cells and media were harvested, subjected to 1 freeze (−80°C)-thaw (37°C) cycle, and sonicated three times (20 s each, 50% duty cycle, and output control 5 on a Branson 450 sonifier). Debris was removed by low-speed centrifugation. Serial dilutions were used to infect fibroblasts for 1 h, after which they were overlaid with 1% low-melting-point agarose in minimum essential medium Eagle (EMEM) without phenol red and l-glutamine (catalog number 12-668E; BioWhittaker) supplemented with 10% FBS and 0.34% NaHCO3. After 17 days, plates were stained with 0.03% methylene blue in phosphate-buffered saline (PBS), and plaques were counted. Antibodies for tubulin (catalog number DM 1A; Sigma), UL44 (catalog number CA006-100; Virusys), and involucrin (catalog number 19018; Sigma) were obtained from commercial sources. Antibodies against pp71 (IE-233), IE1 (1B12), pp150 (CMV127), and pp28 (CMV157) were described previously (52). Secondary antibodies for immunofluorescence conjugated with Alexa Fluor 594 were obtained from Molecular Probes. Secondary antibodies for Western blots conjugated with IRDye-680 were obtained from LiCor.
ACKNOWLEDGMENTS
We thank Karl Münger for the NOKs, Tom Shenk for the viruses and RPE cells, and Paul Lambert for his generosity.
This work was supported by grants from the NIH to R.F.K. (R01-AI074984) and Paul Lambert (P01-CA022443).
C.W. and R.F.K. designed experiments, analyzed the data, and wrote the paper. C.W. performed all experiments except those presented in Fig. 12 and 14. D.M.N., D.L., and S.C.K. generated and provided the hGETs. N.V.S., C.B.G., and C.W. performed the CPE studies. D.L., C.B.G., and C.W. performed the rafting studies.
REFERENCES
- 1.Boeckh M, Geballe AP. 2011. Cytomegalovirus: pathogen, paradigm, and puzzle. J Clin Invest 121:1673–1680. doi: 10.1172/JCI45449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Britt WJ. 2017. Congenital human cytomegalovirus infection and the enigma of maternal immunity. J Virol 91:e02392-. doi: 10.1128/JVI.02392-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cobbs CS, Harkins L, Samanta M, Gillespie GY, Bharara S, King PH, Nabors LB, Cobbs CG, Britt WJ. 2002. Human cytomegalovirus infection and expression in human malignant glioma. Cancer Res 62:3347–3350. [PubMed] [Google Scholar]
- 4.Ranganathan P, Clark PA, Kuo JS, Salamat MS, Kalejta RF. 2012. Significant association of multiple human cytomegalovirus genomic loci with glioblastoma multiforme samples. J Virol 86:854–864. doi: 10.1128/JVI.06097-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu C, Clark PA, Kuo JS, Kalejta RF. 2017. Human cytomegalovirus-infected glioblastoma cells display stem cell-like phenotypes. mSphere 2:e00137-. doi: 10.1128/mSphere.00137-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campos AB, Ribeiro J, Boutolleau D, Sousa H. 2016. Human cytomegalovirus antiviral drug resistance in hematopoietic stem cell transplantation: current state of the art. Rev Med Virol 26:161–182. doi: 10.1002/rmv.1873. [DOI] [PubMed] [Google Scholar]
- 7.Lischka P, Zhang D, Holder D, Zimmermann H. 2016. Impact of glycoprotein B genotype and naturally occurring ORF UL56 polymorphisms upon susceptibility of clinical human cytomegalovirus isolates to letermovir. Antiviral Res 132:204–209. doi: 10.1016/j.antiviral.2016.06.008. [DOI] [PubMed] [Google Scholar]
- 8.Sinzger C. 2008. Entry route of HCMV into endothelial cells. J Clin Virol 41:174–179. doi: 10.1016/j.jcv.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 9.Britt W. 2007. Chapter 41. Virus entry into host, establishment of infection, spread in host, mechanisms of tissue damage, 737–764. In Arvin A, Campadelli-Fiume G, Mocarski E, Moore PS, Roizman B, Whitley R, Yamanishi K (ed), Human herpesviruses: biology, therapy, and immunoprophylaxis. Cambridge University Press, Cambridge, United Kingdom. [PubMed] [Google Scholar]
- 10.Stewart MW. 2010. Optimal management of cytomegalovirus retinitis in patients with AIDS. Clin Ophthalmol 4:285–299. doi: 10.2147/OPTH.S6700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scholz M, Doerr HW, Cinatl J. 2003. Human cytomegalovirus retinitis: pathogenicity, immune evasion and persistence. Trends Microbiol 11:171–178. doi: 10.1016/S0966-842X(03)00066-0. [DOI] [PubMed] [Google Scholar]
- 12.Detrick B, Rhame J, Wang Y, Nagineni CN, Hooks JJ. 1996. Cytomegalovirus replication in human retinal pigment epithelial cells. Altered expression of viral early proteins. Invest Ophthalmol Vis Sci 37:814–825. [PubMed] [Google Scholar]
- 13.Wang D, Shenk T. 2005. Human cytomegalovirus UL131 open reading frame is required for epithelial cell tropism. J Virol 79:10330–10338. doi: 10.1128/JVI.79.16.10330-10338.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Towler JC, Ebrahimi B, Lane B, Davison AJ, Dargan DJ. 2012. Human cytomegalovirus transcriptome activity differs during replication in human fibroblast, epithelial and astrocyte cell lines. J Gen Virol 93(Part 5):1046–1058. doi: 10.1099/vir.0.038083-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ryckman BJ, Rainish BL, Chase MC, Borton JA, Nelson JA, Jarvis MA, Johnson DC. 2008. Characterization of the human cytomegalovirus gH/gL/UL128-131 complex that mediates entry into epithelial and endothelial cells. J Virol 82:60–70. doi: 10.1128/JVI.01910-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gerna G, Revello MG, Baldanti F, Percivalle E, Lilleri D. 2017. The pentameric complex of human cytomegalovirus: cell tropism, virus dissemination, immune response and vaccine development. J Gen Virol 98:2215–2234. doi: 10.1099/jgv.0.000882. [DOI] [PubMed] [Google Scholar]
- 17.Wang D, Shenk T. 2005. Human cytomegalovirus virion protein complex required for epithelial and endothelial cell tropism. Proc Natl Acad Sci U S A 102:18153–18158. doi: 10.1073/pnas.0509201102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zheng Q, Tao R, Gao H, Xu J, Sun Y, Zhao N, Gu W, Shang S. 2017. Immune modulatory function of human cytomegalovirus-encoded UL128 in the pathogenesis of infection related hearing loss. J Infect Dis Ther 5:322. doi: 10.4172/2332-0877.1000322. [DOI] [Google Scholar]
- 19.Shimamura M, Murphy-Ullrich JE, Britt WJ. 2010. Human cytomegalovirus induces TGF-β1 activation in renal tubular epithelial cells after epithelial-to-mesenchymal transition. PLoS Pathog 6:e1001170. doi: 10.1371/journal.ppat.1001170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Twite N, Andrei G, Kummert C, Donner C, Perez-Morga D, De Vos R, Snoeck R, Marchant A. 2014. Sequestration of human cytomegalovirus by human renal and mammary epithelial cells. Virology 460–461:55–65. doi: 10.1016/j.virol.2014.04.032. [DOI] [PubMed] [Google Scholar]
- 21.Knowles WA. 1976. In-vitro cultivation of human cytomegalovirus in thyroid epithelial cells. Arch Virol 50(1–2):119–124. [DOI] [PubMed] [Google Scholar]
- 22.Campbell AE, Cavanaugh VJ, Slater JS. 2008. The salivary glands as a privileged site of cytomegalovirus immune evasion and persistence. Med Microbiol Immunol 197:205–213. doi: 10.1007/s00430-008-0077-2. [DOI] [PubMed] [Google Scholar]
- 23.Langford A, Kunze R, Timm H, Ruf B, Reichart P. 1990. Cytomegalovirus associated oral ulcerations in HIV-infected patients. J Oral Pathol Med 19:71–76. doi: 10.1111/j.1600-0714.1990.tb00799.x. [DOI] [PubMed] [Google Scholar]
- 24.Jones AC, Freedman PD, Phelan JA, Baughman RA, Kerpel SM. 1993. Cytomegalovirus infections of the oral cavity. A report of six cases and review of the literature. Oral Surg Oral Med Oral Pathol 75:76–85. doi: 10.1016/0030-4220(93)90410-6. [DOI] [PubMed] [Google Scholar]
- 25.Flaitz CM, Nichols CM, Hicks MJ. 1996. Herpesviridae-associated persistent mucocutaneous ulcers in acquired immunodeficiency syndrome. A clinicopathologic study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 81:433–441. doi: 10.1016/S1079-2104(96)80019-9. [DOI] [PubMed] [Google Scholar]
- 26.Dioguardi M, Troiano G, Russo L, Giannatempo G, Rubini C, Bertossi D, Cocchi R, Muzio L. 17 September 2015. Occult co-infection in the oral cavity with cytomegalovirus during immuno-suppression. J Transl Sci http://www.oatext.com/Occult-co-infection-in-the-oral-cavity-with-cytomegalovirus-during-immuno-suppression.php. [Google Scholar]
- 27.Johnson AS, Maronian N, Vieira J. 2005. Activation of Kaposi's sarcoma-associated herpesvirus lytic gene expression during epithelial differentiation. J Virol 79:13769–13777. doi: 10.1128/JVI.79.21.13769-13777.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Temple RM, Zhu J, Budgeon L, Christensen ND, Meyers C, Sample CE. 2014. Efficient replication of Epstein-Barr virus in stratified epithelium in vitro. Proc Natl Acad Sci U S A 111:16544–16549. doi: 10.1073/pnas.1400818111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nawandar DM, Wang A, Makieelski K, Lee D, Ma S, Barlow E, Reusch J, Jiang R, Wille CK, Greenspan D, Greenspan JS, Mertz JE, Hutt-Fletcher L, Johannsen EC, Lambert PF, Kenney SC. 2015. Differentiation-dependent KLF4 expression promotes lytic Epstein-Barr virus infection in epithelial cells. PLoS Pathog 11:e1005195. doi: 10.1371/journal.ppat.1005195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chou SW, Scott KM. 1988. Rapid quantitation of cytomegalovirus and assay of neutralizing antibody by using monoclonal antibody to the major immediate-early viral protein. J Clin Microbiol 26:504–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Penkert RR, Kalejta RF. 2012. Tale of a tegument transactivator: the past, present and future of human CMV pp71. Future Virol 7:855–869. doi: 10.2217/fvl.12.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Penkert RR, Kalejta RF. 2011. Tegument protein control of latent herpesvirus establishment and animation. Herpesviridae 2:3. doi: 10.1186/2042-4280-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rossetto CC, Tarrant-Elorza M, Pari GS. 2013. cis and trans acting factors involved in human cytomegalovirus experimental and natural latent infection of CD14(+) monocytes and CD34(+) cells. PLoS Pathog 9:e1003366. doi: 10.1371/journal.ppat.1003366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andrei G, Duraffour S, Van den Oord J, Snoeck R. 2010. Epithelial raft cultures for investigations of virus growth, pathogenesis and efficacy of antiviral agents. Antiviral Res 85:431–449. doi: 10.1016/j.antiviral.2009.10.019. [DOI] [PubMed] [Google Scholar]
- 35.Murphy GF, Flynn TC, Rice RH, Pinkus GS. 1984. Involucrin expression in normal and neoplastic human skin: a marker for keratinocyte differentiation. J Investig Dermatol 82:453–457. doi: 10.1111/1523-1747.ep12260945. [DOI] [PubMed] [Google Scholar]
- 36.Birdwell CE, Queen KJ, Kilgore PC, Rollyson P, Trutschl M, Cvek U, Scott RS. 2014. Genome-wide DNA methylation as an epigenetic consequence of Epstein-Barr virus infection of immortalized keratinocytes. J Virol 88:11442–11458. doi: 10.1128/JVI.00972-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nawandar DM, Ohashi M, Djavadian R, Barlow E, Makielski K, Ali A, Lee D, Lambert PF, Johannsen E, Kenney SC. 2017. Differentiation-dependent LMP1 expression is required for efficient lytic Epstein-Barr virus reactivation in epithelial cells. J Virol 91:e02438-. doi: 10.1128/JVI.02438-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ao J, Wood JP, Chidlow G, Gillies MC, Casson RJ. 5 December 2017. Retinal pigment epithelium in the pathogenesis of age-related macular degeneration and photobiomodulation as a potential therapy? Clin Exp Ophthalmol doi: 10.1111/ceo.13121. [DOI] [PubMed] [Google Scholar]
- 39.Dupont L, Reeves MB. 2016. Cytomegalovirus latency and reactivation: recent insights into an age old problem. Rev Med Virol 26:75–89. doi: 10.1002/rmv.1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bresnahan WA, Hultman GE, Shenk T. 2000. Replication of wild-type and mutant human cytomegalovirus in life-extended human diploid fibroblasts. J Virol 74:10816–10818. doi: 10.1128/JVI.74.22.10816-10818.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Naqvi AR, Seal A, Shango J, Brambila MF, Martinez G, Chapa G, Hasan S, Yadavalli T, Jaishankar D, Shukla D, Nares S. 15 March 2018. Herpesvirus-encoded microRNAs detected in human gingiva alter host cell transcriptome and regulate viral infection. Biochim Biophys Acta doi: 10.1016/j.bbagrm.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yura Y, Iga H, Terashima K, Yoshida H, Yanagawa T, Hayashi Y, Sato M. 1987. The role of epithelial cell differentiation in the expression of herpes simplex virus type 1 in normal human oral mucosa in culture. Arch Virol 92(1–2):41–53. [DOI] [PubMed] [Google Scholar]
- 43.Visalli RJ, Courtney RJ, Meyers C. 1997. Infection and replication of herpes simplex virus type 1 in an organotypic epithelial culture system. Virology 230:236–243. doi: 10.1006/viro.1997.8484. [DOI] [PubMed] [Google Scholar]
- 44.Andrei G, Van den Oord J, Fiten P, Opdenakker G, De Wolf-Peeters C, De Clercq E, Snoeck R. 2005. Organotypic epithelial raft cultures as a model for evaluating compounds against alphaherpesviruses. Antimicrob Agents Chemother 49:4671–4680. doi: 10.1128/AAC.49.11.4671-4680.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Piboonniyom SO, Duensing S, Swilling NW, Hasskarl J, Hinds PW, Münger K. 2003. Abrogation of the retinoblastoma tumor suppressor checkpoint during keratinocyte immortalization is not sufficient for induction of centrosome-mediated genomic instability. Cancer Res 63:476–483. [PubMed] [Google Scholar]
- 46.Kraus RJ, Yu X, Cordes BA, Sathiamoorthi S, Lempridee T, Nawandar DM, Ma S, Romero-Masters JC, McChesney KG, Lin Z, Makielski KR, Lee DL, Lambert PF, Johannsen EC, Kenney SC, Mertz JE. 2017. Hypoxia-inducible factor-1α plays roles in Epstein-Barr virus's natural life cycle and tumorigenesis by inducing lytic infection through direct binding to the immediate-early BZLF1 gene promoter. PLoS Pathog 13:e1006404. doi: 10.1371/journal.ppat.1006404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee D, Norby K, Hayes M, Chiu YF, Sugden B, Lambert PF. 2016. Using organotypic epithelial tissue culture to study the human papillomavirus life cycle. Curr Protoc Microbiol 41:14B.8.1–14B.8.19. doi: 10.1002/cpmc.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saffert RT, Penkert RR, Kalejta RF. 2010. Cellular and viral control over the initial events of human cytomegalovirus experimental latency in CD34+ cells. J Virol 84:5594–5604. doi: 10.1128/JVI.00348-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saffert RT, Kalejta RF. 2007. Human cytomegalovirus gene expression is silenced by Daxx-mediated intrinsic immune defense in model latent infections established in vitro. J Virol 81:9109–9120. doi: 10.1128/JVI.00827-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Albright E, Kalejta FR. 2013. Myeloblastic cell lines mimic some but not all aspects of human cytomegalovirus experimental latency defined in primary CD34+ cell populations. J Virol 87:9802–9812. doi: 10.1128/JVI.01436-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee SH, Albright ER, Lee JH, Jacobs D, Kalejta RF. 2015. Cellular defense against latent colonization foiled by human cytomegalovirus UL138 protein. Sci Adv 1:e1501164. doi: 10.1126/sciadv.1501164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saffert RT, Kalejta RF. 2006. Inactivating a cellular intrinsic immune defense mediated by Daxx is the mechanism through which the human cytomegalovirus pp71 protein stimulates viral immediate-early gene expression. J Virol 80:3863–3871. doi: 10.1128/JVI.80.8.3863-3871.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]