Abstract
Background:
Currently, there are limited data of prognostic clues for neurological recovery in comatose survivors undergoing therapeutic hypothermia (TH). We aimed to evaluate clinical signs and findings that could predict neurological outcomes, and determine the optimal time for the prognostication.
Materials and Methods:
We retrospectively reviewed database of postarrest survivors treated with TH in our hospital from 2006 to 2014. Cerebral performance category (CPC), neurological signs and findings in electroencephalography (EEG) and brain computed tomography (CT) were evaluated. In addition, the optimal time to evaluate neurological status was analyzed.
Results:
TH was performed in 51 postarrest patients. Approximately 53% of TH patients survived at discharge and 33% of the hospital survivors had favorable outcome (CPC1-2). The prognostic clues for unfavorable outcome (CPC3-5) at discharge were lack of pupillary light response (PLR) and/or gag reflex after rewarming, and the absence of at least one of the brainstem reflexes, no eye-opening, or abnormal motor response on the 7th day. Myoclonus and seizure could not be used to indicate poor prognosis. In addition, prognostic values of EEG and CT findings were inconclusive.
Conclusions:
Our study showed the simple neurological signs helped predict short-term neurological prognosis. The most reliable sign determining unfavorable outcome was the lack of PLR. The optimal time to assess prognosis was either at 48–72 h or 7 days after return of spontaneous circulation.
Keywords: Comatose survivor, neurological prognostication, therapeutic hypothermia
INTRODUCTION
It is difficult to manage postcardiac arrest survivors because when treated with therapeutic hypothermia (TH), they commonly remain comatose after rewarming. In addition, previous studies conducted in patients who have undergone TH showed an increase in false-positive prediction for poor neurological outcome.[1] On the other hand, survivors without hypothermia have to be continuously sedated so the neurological prognostications for these survivors cannot be made accurately.[2] As a result of this, it is difficult to make a decision whether to withhold or withdraw life-sustaining treatment to unawake patients which the former choice can incur costly investigations and result in futile treatment, especially for those patients with irreversible conditions. In addition, there are limited data on the optimal time for neurological prognostication. Some recommendations, including those from the American Heart Association, suggest that physicians should delay the assessment beyond 72 h after the return of spontaneous circulation (ROSC).[3,4] Other guidelines recommend the prognostic assessment at 72 h after completing rewarming.[5]
Thus, this study was conducted to evaluate the neurological predictive factors and the appropriate time for prognostication. The hypothesis was that the neurological symptoms, (viz., myoclonus and seizure), simple neurological signs at two different time points, and neurological investigations including electroencephalography (EEG), computed tomography of the brain (brain CT), may be able to predict the neurological outcomes in the survivors treated with hypothermia.
MATERIALS AND METHODS
Patients
We retrospectively reviewed our database for postcardiac arrest survivors who had TH from 2006 to 2014 at the 2 medical Intensive Care Units (ICUs) and 1 CCU at the King Chulalongkorn Memorial Hospital. The patients were identified from the hospital database using “ICD 10 code I 460-cardiac arrest with successful resuscitation” and “ICD9 code 9961-TH” for the diagnosis of postcardiac arrest survivor and intervention of TH, respectively.
Hypothermia protocol
All comatose survivors from cardiac arrest with Glasgow Coma Score (GCS) ≤8 after ROSC were evaluated for TH. In the condition of CCU or ICU-bed availability, all patients who were eligible for TH were cooled to 32°C–34°C for 24 h by external cooling methods with or without internal cooling methods, followed by rewarming. All patients were sedated. Shiverings were treated with extra sedation and neuromuscular blockades. The sedative drugs and neuromuscular blockades were interrupted after completing the rewarming process. In addition, during the 1st week of a postrewarming period, a controlled normothermia protocol was implemented. The patients with postrewarming pyrexia (core temperature >38°C) were decreased their temperature by conventional cooling blankets (for keeping the temperature of 37°C) and treated with acetaminophen if no contraindication. Hemodynamic and respiratory parameters were continuously monitored, and vasopressors or inotropic drugs were administered to maintain hemodynamic stability. Antiepileptic drugs were prescribed if the patients developed clinical or electrical signs of seizure.
Data collection
Baseline characteristics, cooling practice, symptoms, signs, and clinical outcomes were collected from the patients’ medical charts and flow sheets. All data were recorded by ICU staff, including primary physicians, intensivists, neurologists, and critical care nurses. The simple neurological signs at two different time points, immediately after rewarming (48–72 h) and on the 7th day, were evaluated from the recorded data. These signs consisted of (1) GCS: eye-opening, motor response and verbal response, and (2) brainstem reflexes: pupillary light response (PLR), gag reflex, corneal reflex, and Doll's eye reflex. The neurological symptoms, namely seizure and myoclonus, were obtained from the clinical data, EEG, and brain CT images.
Outcome measurements
Glasgow–Pittsburgh cerebral performance category (CPC) at discharge was used to evaluate the neurological outcomes. The outcomes were dichotomized as follows: (1a) favorable outcome: CPC1 = good cerebral performance and CPC2 = moderate disability, and (2a) unfavorable outcome: CPC3 = severe disability, CPC4 = vegetative state and CPC5 = brain death or (1b) regained consciousness: CPC1–3 and (2b) unconsciousness: CPC 4–5.
Data analysis
The patients’ baseline characteristics were described according to the types of variables and the normality of their distributions. Continuous variables were reported as mean (standard deviation) or median (interquartile range). Categorical variables were reported as numbers or percentages. The factors associated with clinical outcomes were analyzed. Unpaired-t-test or Mann–Whitney U-test was used to compare between two groups with continuous variables. Chi-square or Fisher's exact test was used to analyze the association between the two groups with categorical variables. We used a two-sided test, and P < 0.05 was considered statistically significant. In addition, sensitivity, specificity, false positive rate, positive predictive value, negative predictive value, likelihood ratio, and accuracy were analyzed.
The study was approved by the Institutional Review Board, Faculty of Medicine, Chulalongkorn University (IRB No. 219/58).
RESULTS
Patients
All 51 postcardiac arrest survivors treated with TH in the two medical ICUs and CCU during the 8-year period were included in the analysis to assess the neurological prognostic factors. The baseline characteristics and clinical data of the patients are shown in Table 1.
Table 1.
Baseline characteristics of the postcardiac arrest survivors

There were 40 (78%) survivors from out-of-hospital cardiac arrest and 11 (21.6%) survivors from in-hospital cardiac arrest. The majority of primary cardiac rhythms was nonshockable rhythm (78.4%). Cardiovascular events including myocardial infarction and arrhythmia were the main causes of cardiac arrest [Table 1]. Approximately, 57% of the survivors were males and the median age was 59 years. The median time from collapse to ROSC, the median time from ROSC to hypothermia induction and the median time from hypothermia initiation to targeted temperature were 26 min, 5 h, and 4 h, respectively. About 65% of the patients achieved targeted temperature within 6 h after inducing the cooling process, but 15.7% of all patients did not attain the targeted temperature. Seventeen patients developed myoclonus whereas eight patients developed seizures. Overall, 52.9% (27/51) survived and discharged from the hospital with a median LOS of 13 days. Nine of 27 hospital survivors had good neurological recovery (six patients with CPC1 and three patients with CPC2). Eight of the hospital survivors had a severe disability (CPC3) and the others (10) were in vegetative state (CPC4).
A short duration from the time of collapse to ROSC was associated with favorable outcome (P = 0.044). The absence of vasopressor use was clinically independent predictor for good neurological recovery (CPC1–2, P = 0.024), ability to regain consciousness (CPC1–3, P = 0.01), and survival (P < 0.001). Survival improved when the temperature was achieved in < 6 h from ROSC (P = 0.038) and protocol was completed (P = 0.027). Cardiac rhythm, location of arrest, target temperature attainment, rebound hyperthermia, and age were not associated with any clinical outcomes.
Neurological prognostications
Neurological symptoms and signs
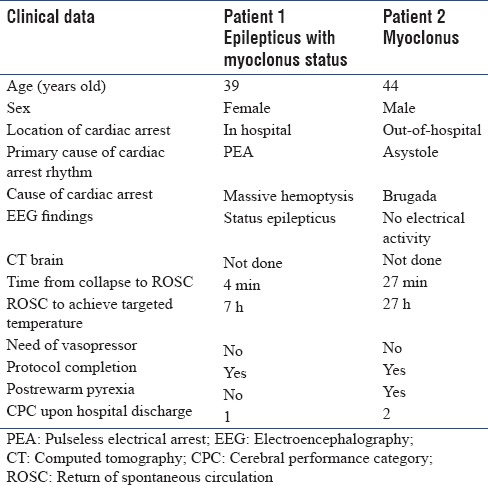
According to the data in Table 1, myoclonus and seizure were not exclusively poor prognostic symptoms. One patient with myoclonus and one patient with both seizure and myoclonus had favorable neurological outcomes post-TH. The clinical data of these patients are shown in Table 2.
Table 2.
Clinical data of the two patients who had myoclonus with or without the epilepticus status with favorable outcomes
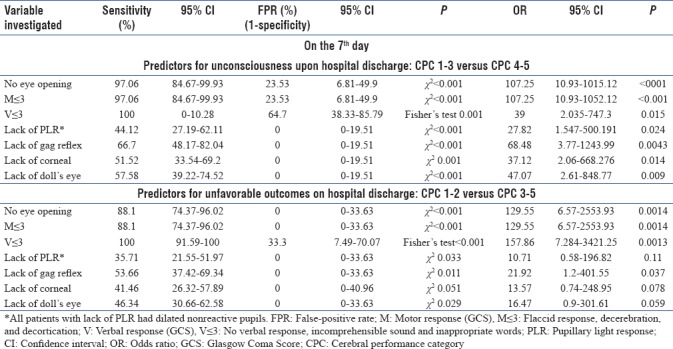
Tables 3 and 4 present the results of the simple neurological signs used as the prognostic tools to evaluate the neurological outcomes post-TH. In the early period of treatment (48–72 h), the absence of PLR and gag reflex were the only two reliable signs predicting the unfavorable neurological outcomes False-positive rate (FPR 0%), but the sensitivities of these signs were low. Noticeably, most of the patients with absent pupillary response (8/9 patients) had dilated nonreactive pupils. There was only one patient with pinpoint pupils who became vegetative at hospital discharge. In addition, any lack of the following responses on the 7th day can be used to precisely (FPR 0%) prognoses unfavorable outcomes: brainstem reflexes, motor response worse than withdraws from pain (M ≤3), and no spontaneous opening of the eyes. Similarly, the accurate predictive signs for unawakening were the absence of PLR and gag responses after rewarming (FPR 0% and 5.9%, respectively), and the absence of any brainstem reflexes on the 7th day (all FPR 0%). However, all of these predictors had a large 95% confidence interval (CI) due to the small sample size.
Table 3.
Sensitivity, false positive rate, positive predictive value, negative predictive value, likelihood ratio, and accuracy of the simple neurological signs immediately after rewarming to predict the outcomes of the patients

Table 4.
Sensitivity, false-positive rate, positive predictive value, negative predictive value, likelihood ratio, and accuracy of the simple neurological signs on the seventh day for predicting the outcome of the patients after therapeutic hypothermia postcardiac arrest
Neurological investigations
Electroencephalography
EEG data were not available for all patients who had TH because of limited resources. EEG monitorings were temporarily performed on patients suspected with or had status epilepticus or patients who had abnormal movements with uncertain diagnoses. Only 15 patients had EEG data. The results of the EEG yielded six patterns: (1) no electrical activity, (2) no epileptiform, (3) status epilepticus, (4) burst suppression, (5) slow-wave pattern, and (6) localized abnormal pattern. The patients from each EEG finding are shown in Table 1. Due to the small number of patients with EEG investigations, we could not evaluate the prognostic value of EEG. However, interestingly enough, we noticed that one of three patients with status epilepticus, confirmed by EEG, subsequently recovered completely as shown in Table 2.
Brain computed tomography images
Twenty-two patients were investigated for the primary causes of cardiac arrest, seizure, or new localizing neurological signs by brain CT. The findings of the brain CT images were as follows: (1) normal, (2) old cerebral infarction, (3) brain atrophy, (4) brain swelling, (5) subarachnoid hemorrhage, and (6) posterior reversible encephalopathy syndrome (PRES). The findings of the brain swelling and PRES may indicate prognostic signs of poor outcomes because all of the three patients with brain swelling had unfavorable outcomes on hospital discharge and one patient with PRES died. However, the predictive value of the brain CT findings was inconclusive because for each brain CT finding, there were few patients. In addition, the brain CT investigations were performed at different time points for purposes not related to TH.
DISCUSSION
TH improves neurological recovery and survival among postcardiac arrest patients. The mechanisms of the neuroprotective effect are avoidance of hyperthermia, reduction in metabolic demand, and reduction in ischemic-reperfusion injury.[6] The prevention of fever reduces cerebral metabolic rate and oxygen consumption, which preserves energy stores and improves brain glucose utilization.[6,7] Besides, TH also prevents fever-related tissue injury. In addition, it is known that ischemia-reperfusion triggers further neuroinflammation, resulting from effects of excitotoxicity, free radical production, blood-brain barrier disruption, blood vessel leakage, and cerebral thermopooling. TH improves cerebral perfusion and decreases hyperemia following reperfusion, which prevents the secondary brain injury and reduces intracranial pressure.[7,8,9,10,11]
The patients undergoing TH have low-metabolic rate and usually receive sedatives and muscle relaxant agents during the first 48 h after ROSC, which interferes the neurological prognostication.[2] The majority of evidence concerning prognostication after cardiac arrest was based on studies conducted in patients not treated with TH. Our study demonstrated the prognostic factors for the unfavorable neurological outcome at hospital discharge in the postcardiac arrest patients undergoing TH. The absence of PLR and/or Gag reflex were the reliable predictors after rewarming, while the absence of at least one of the brainstem reflexes, no eye-opening, or abnormal motor response were the reliable predictors on the 7th day. Nevertheless, myoclonus and seizure were the weak indicators for the unfavorable outcome. In addition, there were inconclusive prognostic values of EEG and CT findings.
According to the good predictive values of the simple signs in the patients treated with TH, physicians could use these signs for early neurological prognostication to avoid unnecessary life-supporting treatment. Our study demonstrated the absence of PLR was the most reliable predictor for unconsciousness and unfavorable outcome which is consistent with other studies shown in Tables 5 and 6. Gag reflex was the second-most reliable sign because it has a little higher FPR. The delayed time of neurological recovery from hypothermia induced low central nervous system metabolism and unpredictable clearance of the sedative drugs can result in high FPR of Doll's eye, corneal reflex, eye-opening, and motor response after rewarming.[19,20,21,22] For example, 25% of the patients with M2-extensor posturing after rewarming regained consciousness later and one of them fully recovered. In addition, 1 of 12 patients with M1-absent motor response after rewarming was awake with minimal disability. Aside from the PLR, other signs such as the eye opening, motor response, and brainstem signs can be used to assess the outcome of TH 7 days after ROSC which is the most appropriate time to conduct the prognostications. Moreover, we observed that CPC scores upon hospital discharge were better than the CPC scores at day 7 by 1 scale in some patients. Therefore, time to reach maximal neurological recovery may take a little longer than 1 week. Furthermore, the patients who regained consciousness after TH protocol completion had good outcomes. None of the patients with CPC1 and 2 after rewarming deteriorated to unfavorable outcome.
Table 5.
Neurological findings to predict the unfavorable outcomes (cerebral performance category 3-5) after therapeutic hypothermia following cardiac arrest
Table 6.
Neurological findings for predicting the patients’ unconsciousness or death (cerebral performance category 4-5) after therapeutic hypothermia following cardiac arrest

As for other symptoms and signs such as myoclonus with or without status, epilepticus cannot be used to indicate poor prognosis. Our study showed a few patients with myoclonus with or without status epilepticus were able to achieve complete or nearly complete recovery. Thus, both myoclonus and status epilepticus were less accurate signs to predict unfavorable prognosis. This finding contradicts some of the data from previous studies, which showed myoclonus was associated with neocortical damage and poor outcome.[23,24] However, recent small studies showed variable FPR of myoclonus in predicting poor outcomes.[25,26,27] Hence, we conclude myoclonus is not an absolute predictor of unfavorable outcome.
Another poor predictor that can be used is the findings from the EEG. However, in this study, approximately 30% of all patients had EEG. It is unfortunate that the EEG was not utilized to its full potential to predict poor prognosis. We observed two patients with burst-suppression pattern that later died which is consistent with the suggestions from experts that burst suppression EEG pattern was associated with poor outcome.[28,29] However, some experts disagreed with this because one study showed that burst-suppression can temporarily appear after inducing hypothermia.[13,30] For this reason, burst-suppression pattern cannot be used as a single predictor for poor outcome. Moreover, continuous EEG monitoring is recommended to detect subtle seizure which is common among comatose survivors.[31,32] One study showed nonconvulsive status epilepticus (NCSE) in 12% of the survivors undergoing TH and the presence of seizure was associated with poor outcome.[33] In our study, more than half of the persistent comatose patients were not monitored with EEG, so we did not know whether NCSE occurred or not. This information may affect the presence or absence of certain neurological signs and clinical outcome.
Other tools that can be used are the brain CT images. For example, loss of gray-white matter differentiation was an early sign of brain edema. Some authors proposed that the attenuations in the grey matter to those in gray-white matter ratio at the basal ganglia and cerebrum in the brain CT within an hour after ROSC of <1.14 can predict unfavorable neurological outcome with FPR of 0% (95% CI 0%–16.1%).[34,35,36,37] In our study, all patients with obvious brain swelling had unfavorable outcome.
Notably, there were quite a high percentage of patients with unfavorable outcomes in our study. The reason for this is because TH was administered to patients with a high probability to develop poor outcomes such as those patients with relative contraindication for TH (e.g., sepsis), patients with prolonged time from collapse to ROSC (more than 30 min) and in-hospital patients who had poor cardiopulmonary reserve.[38] Another explanation for high unfavorable outcomes is that the time from ROSC to cooling induction took a long time to accomplish because there were insufficient number of ICU beds and the primary physicians lacked knowledge of TH. The other explanation is that we included patients with nonshockable rhythm, especially those patients who may have had prolonged time from collapse to CPR initiation or had irreversible diseases.[39] It is also possible that lack of absolute criteria for treatment withdrawal may have resulted in unintentional termination of treatment among patients with reversible conditions. However, due to the high proportion of the patients with the poor outcome, we could demonstrate the reliable prognostic factors for the unfavorable neurological outcome.
Our study had several limitations. It is possible that the retrospective design of the study could have affected the results of the study. Certain data were not available to us because the EEG was selectively performed on patients with clinically suspected seizure. Therefore, it is possible that we may have underdiagnosed patients with nonconvulsive seizure which may affected the prognostic results. Second, there were limited data on serial neurological signs between 3 and 7 days after ROSC. Furthermore, the neurological data obtained from the medical records were collected and interpreted by various staff with different skills, which may be less precise. Third, the study had a small sample size so the power was not enough to detect any significant differences or occurrences. Furhermore, the findings from the brain CT finding is not enough to ascertain the significance of the outcome, especially for the loss of the gray-white matter differentiation. In addition, we did not know to what extent the sedative drugs or neuromuscular blockades would affect the presence of the neurological signs, especially during the early assessment. Last, this study demonstrated only the predictive signs for short-term outcomes in terms of physical disability. Hence, these results cannot predict long-term neurological outcome and functional ability, including cognitive function and quality of life.
CONCLUSIONS
Our analysis showed that the simple neurological signs can help predict the short-term neurological prognosis of comatose survivors undergoing TH. The most reliable sign which determined unconsciousness and unfavorable outcome was the lack of PLR. The optimal time to assess prognosis was either 48–72 h or 7 days after ROSC. Physicians can use these neurological signs to predict the outcomes post-TH. Myoclonus and status epilepticus cannot be used to predict absolutely poor outcomes. However, there were limited data of EEG and brain CT findings in predicting the outcome post-TH.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Kamps MJ, Horn J, Oddo M, Fugate JE, Storm C, Cronberg T, et al. Prognostication of neurologic outcome in cardiac arrest patients after mild therapeutic hypothermia: A meta-analysis of the current literature. Intensive Care Med. 2013;39:1671–82. doi: 10.1007/s00134-013-3004-y. [DOI] [PubMed] [Google Scholar]
- 2.Samaniego EA, Mlynash M, Caulfield AF, Eyngorn I, Wijman CA. Sedation confounds outcome prediction in cardiac arrest survivors treated with hypothermia. Neurocrit Care. 2011;15:113–9. doi: 10.1007/s12028-010-9412-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peberdy MA, Callaway CW, Neumar RW, Geocadin RG, Zimmerman JL, Donnino M, et al. Part 9: Post-cardiac arrest care: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122:S768–86. doi: 10.1161/CIRCULATIONAHA.110.971002. [DOI] [PubMed] [Google Scholar]
- 4.Sayre MR, O’Connor RE, Atkins DL, Billi JE, Callaway CW, Shuster M, et al. Part 2: Evidence evaluation and management of potential or perceived conflicts of interest: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122:S657–64. doi: 10.1161/CIRCULATIONAHA.110.966861. [DOI] [PubMed] [Google Scholar]
- 5.Cronberg T, Brizzi M, Liedholm LJ, Rosén I, Rubertsson S, Rylander C, et al. Neurological prognostication after cardiac arrest – Recommendations from the swedish resuscitation council. Resuscitation. 2013;84:867–72. doi: 10.1016/j.resuscitation.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 6.Karnatovskaia LV, Wartenberg KE, Freeman WD. Therapeutic hypothermia for neuroprotection: History, mechanisms, risks, and clinical applications. Neurohospitalist. 2014;4:153–63. doi: 10.1177/1941874413519802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Polderman KH. Mechanisms of action, physiological effects, and complications of hypothermia. Crit Care Med. 2009;37:S186–202. doi: 10.1097/CCM.0b013e3181aa5241. [DOI] [PubMed] [Google Scholar]
- 8.Erecinska M, Thoresen M, Silver IA. Effects of hypothermia on energy metabolism in mammalian central nervous system. J Cereb Blood Flow Metab. 2003;23:513–30. doi: 10.1097/01.WCB.0000066287.21705.21. [DOI] [PubMed] [Google Scholar]
- 9.Zhao QJ, Zhang XG, Wang LX. Mild hypothermia therapy reduces blood glucose and lactate and improves neurologic outcomes in patients with severe traumatic brain injury. J Crit Care. 2011;26:311–5. doi: 10.1016/j.jcrc.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 10.Wang Q, Li AL, Zhi DS, Huang HL. Effect of mild hypothermia on glucose metabolism and glycerol of brain tissue in patients with severe traumatic brain injury. Chin J Traumatol. 2007;10:246–9. [PubMed] [Google Scholar]
- 11.Yenari MA, Han HS. Neuroprotective mechanisms of hypothermia in brain ischaemia. Nat Rev Neurosci. 2012;13:267–78. doi: 10.1038/nrn3174. [DOI] [PubMed] [Google Scholar]
- 12.Rossetti AO, Urbano LA, Delodder F, Kaplan PW, Oddo M. Prognostic value of continuous EEG monitoring during therapeutic hypothermia after cardiac arrest. Crit Care. 2010;14:R173. doi: 10.1186/cc9276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bisschops LL, van Alfen N, Bons S, van der Hoeven JG, Hoedemaekers CW. Predictors of poor neurologic outcome in patients after cardiac arrest treated with hypothermia: A retrospective study. Resuscitation. 2011;82:696–701. doi: 10.1016/j.resuscitation.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 14.Okada K, Ohde S, Otani N, Sera T, Mochizuki T, Aoki M, et al. Prediction protocol for neurological outcome for survivors of out-of-hospital cardiac arrest treated with targeted temperature management. Resuscitation. 2012;83:734–9. doi: 10.1016/j.resuscitation.2011.12.036. [DOI] [PubMed] [Google Scholar]
- 15.Bouwes A, Binnekade JM, Kuiper MA, Bosch FH, Zandstra DF, Toornvliet AC, et al. Prognosis of coma after therapeutic hypothermia: A prospective cohort study. Ann Neurol. 2012;71:206–12. doi: 10.1002/ana.22632. [DOI] [PubMed] [Google Scholar]
- 16.Legriel S, Hilly-Ginoux J, Resche-Rigon M, Merceron S, Pinoteau J, Henry-Lagarrigue M, et al. Prognostic value of electrographic postanoxic status epilepticus in comatose cardiac-arrest survivors in the therapeutic hypothermia era. Resuscitation. 2013;84:343–50. doi: 10.1016/j.resuscitation.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 17.Dragancea I, Horn J, Kuiper M, Friberg H, Ullén S, Wetterslev J, et al. Neurological prognostication after cardiac arrest and targeted temperature management 33°C versus 36°C: Results from a randomised controlled clinical trial. Resuscitation. 2015;93:164–70. doi: 10.1016/j.resuscitation.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 18.Al Thenayan E, Savard M, Sharpe M, Norton L, Young B. Predictors of poor neurologic outcome after induced mild hypothermia following cardiac arrest. Neurology. 2008;71:1535–7. doi: 10.1212/01.wnl.0000334205.81148.31. [DOI] [PubMed] [Google Scholar]
- 19.Hart RG, Sherman DG, Tegeler CH. Predicting outcome from hypoxic-ischemic coma. JAMA. 1985;254:1171. [PubMed] [Google Scholar]
- 20.Dell’Anna AM, Taccone FS, Halenarova K, Citerio G. Sedation after cardiac arrest and during therapeutic hypothermia. Minerva Anestesiol. 2014;80:954–62. [PubMed] [Google Scholar]
- 21.Empey PE, Miller TM, Philbrick AH, Melick JA, Kochanek PM, Poloyac SM. Mild hypothermia decreases fentanyl and midazolam steady-state clearance in a rat model of cardiac arrest. Crit Care Med. 2012;40:1221–8. doi: 10.1097/CCM.0b013e31823779f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fukuoka N, Aibiki M, Tsukamoto T, Seki K, Morita S. Biphasic concentration change during continuous midazolam administration in brain-injured patients undergoing therapeutic moderate hypothermia. Resuscitation. 2004;60:225–30. doi: 10.1016/j.resuscitation.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 23.Krumholz A, Stern BJ, Weiss HD. Outcome from coma after cardiopulmonary resuscitation: Relation to seizures and myoclonus. Neurology. 1988;38:401–5. doi: 10.1212/wnl.38.3.401. [DOI] [PubMed] [Google Scholar]
- 24.Wijdicks EF, Parisi JE, Sharbrough FW. Prognostic value of myoclonus status in comatose survivors of cardiac arrest. Ann Neurol. 1994;35:239–43. doi: 10.1002/ana.410350219. [DOI] [PubMed] [Google Scholar]
- 25.Bouwes A, van Poppelen D, Koelman JH, Kuiper MA, Zandstra DF, Weinstein HC, et al. Acute posthypoxic myoclonus after cardiopulmonary resuscitation. BMC Neurol. 2012;12:63. doi: 10.1186/1471-2377-12-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seder DB, Sunde K, Rubertsson S, Mooney M, Stammet P, Riker RR, et al. Neurologic outcomes and postresuscitation care of patients with myoclonus following cardiac arrest. Crit Care Med. 2015;43:965–72. doi: 10.1097/CCM.0000000000000880. [DOI] [PubMed] [Google Scholar]
- 27.Lucas JM, Cocchi MN, Salciccioli J, Stanbridge JA, Geocadin RG, Herman ST, et al. Neurologic recovery after therapeutic hypothermia in patients with post-cardiac arrest myoclonus. Resuscitation. 2012;83:265–9. doi: 10.1016/j.resuscitation.2011.09.017. [DOI] [PubMed] [Google Scholar]
- 28.Zandbergen EG, Hijdra A, Koelman JH, Hart AA, Vos PE, Verbeek MM, et al. Prediction of poor outcome within the first 3 days of postanoxic coma. Neurology. 2006;66:62–8. doi: 10.1212/01.wnl.0000191308.22233.88. [DOI] [PubMed] [Google Scholar]
- 29.Zandbergen EG, de Haan RJ, Stoutenbeek CP, Koelman JH, Hijdra A. Systematic review of early prediction of poor outcome in anoxic-ischaemic coma. Lancet. 1998;352:1808–12. doi: 10.1016/S0140-6736(98)04076-8. [DOI] [PubMed] [Google Scholar]
- 30.Wennervirta JE, Ermes MJ, Tiainen SM, Salmi TK, Hynninen MS, Särkelä MO, et al. Hypothermia-treated cardiac arrest patients with good neurological outcome differ early in quantitative variables of EEG suppression and epileptiform activity. Crit Care Med. 2009;37:2427–35. doi: 10.1097/CCM.0b013e3181a0ff84. [DOI] [PubMed] [Google Scholar]
- 31.Friberg H, Westhall E, Rosén I, Rundgren M, Nielsen N, Cronberg T. Clinical review: Continuous and simplified electroencephalography to monitor brain recovery after cardiac arrest. Crit Care. 2013;17:233. doi: 10.1186/cc12699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crepeau AZ, Rabinstein AA, Fugate JE, Mandrekar J, Wijdicks EF, White RD, et al. Continuous EEG in therapeutic hypothermia after cardiac arrest: Prognostic and clinical value. Neurology. 2013;80:339–44. doi: 10.1212/WNL.0b013e31827f089d. [DOI] [PubMed] [Google Scholar]
- 33.Rittenberger JC, Popescu A, Brenner RP, Guyette FX, Callaway CW. Frequency and timing of nonconvulsive status epilepticus in comatose post-cardiac arrest subjects treated with hypothermia. Neurocrit Care. 2012;16:114–22. doi: 10.1007/s12028-011-9565-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cristia C, Ho ML, Levy S, Andersen LW, Perman SM, Giberson T, et al. The association between a quantitative computed tomography (CT) measurement of cerebral edema and outcomes in post-cardiac arrest – A validation study. Resuscitation. 2014;85:1348–53. doi: 10.1016/j.resuscitation.2014.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim SH, Choi SP, Park KN, Youn CS, Oh SH, Choi SM. Early brain computed tomography findings are associated with outcome in patients treated with therapeutic hypothermia after out-of-hospital cardiac arrest. Scand J Trauma Resusc Emerg Med. 2013;21:57. doi: 10.1186/1757-7241-21-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Torbey MT, Selim M, Knorr J, Bigelow C, Recht L. Quantitative analysis of the loss of distinction between gray and white matter in comatose patients after cardiac arrest. Stroke. 2000;31:2163–7. doi: 10.1161/01.str.31.9.2163. [DOI] [PubMed] [Google Scholar]
- 37.Choi SP, Park HK, Park KN, Kim YM, Ahn KJ, Choi KH, et al. The density ratio of grey to white matter on computed tomography as an early predictor of vegetative state or death after cardiac arrest. Emerg Med J. 2008;25:666–9. doi: 10.1136/emj.2007.053306. [DOI] [PubMed] [Google Scholar]
- 38.Piscator E, Hedberg P, Göransson K, Djärv T. Survival after in-hospital cardiac arrest is highly associated with the age-combined charlson co-morbidity index in a cohort study from a two-site Swedish university hospital. Resuscitation. 2016;99:79–83. doi: 10.1016/j.resuscitation.2015.11.023. [DOI] [PubMed] [Google Scholar]
- 39.Terman SW, Hume B, Meurer WJ, Silbergleit R. Impact of presenting rhythm on short- and long-term neurologic outcome in comatose survivors of cardiac arrest treated with therapeutic hypothermia. Crit Care Med. 2014;42:2225–34. doi: 10.1097/CCM.0000000000000506. [DOI] [PMC free article] [PubMed] [Google Scholar]



