Abstract
Background:
The era of multidrug-resistant (MDR) Gram-negative bacilli (GNB) has renewed interest in fosfomycin.
Aim:
The present study evaluated the in vitro activity of fosfomycin against MDR urinary and nonurinary GNB isolates.
Materials and Methods:
Fosfomycin susceptibility was carried out by agar dilution for a total of 279 (142 from urine and 137 from other samples) MDR-GNB. Disk diffusion was done for urinary isolates only.
Results:
Urinary tract isolates had a high degree of susceptibility to fosfomycin (overall susceptibility, 90.8%), whereas only 42.9% of nonurinary isolates retained susceptibility to the drug. Percentage susceptibility rates for urinary and nonurinary isolates of Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Acinetobacter spp. were 99%, 91.3%, 66%, 0% and 62%, 44.4%, 32%, 11%, respectively.
Conclusion:
Fosfomycin showed excellent in vitro activity for uropathogens. Large-scale evaluation of fosfomycin against MDR systemic isolates is required to evaluate its therapeutic efficacy.
Keywords: Fosfomycin, multidrug-resistant Gram-negative bacilli, urinary tract infection
INTRODUCTION
In the era of increasing drug resistance to Gram-negative bacilli (GNB) and lack of new antibiotics, old forgotten antibiotics such as polymyxins and fosfomycin have made an excellent comeback, with polymyxins now enjoying a cult status which it did not in its first innings. The usage of fosfomycin has not been so widespread compared to the polymyxins.
Fosfomycin, originally named phosphonomycin, is a broad-spectrum, bactericidal antibiotic, first identified and reported from various strains of Streptomyces in 1969 in Spain.[1] It is the only member of the epoxide group of antibiotics and is structurally unrelated to any other agent currently approved for clinical use.[1] Fosfomycin interferes cell wall synthesis by inhibiting the initial step of peptidoglycan synthesis involving phosphoenolpyruvate synthetase.[1] It is available as fosfomycin tromethamine and fosfomycin calcium for oral use and as fosfomycin disodium for intravenous (IV) use.[1] Fosfomycin tromethamine (oral form) is recommended as one of the first-line therapies for uncomplicated cystitis and pyelonephritis.[2] The IV form is available only in some selected countries.[1]
The renewed interest in fosfomycin in recent years is mainly to address the treatment of urinary tract infections (UTIs) as an oral agent as well as systemic therapy of severe infections due to multidrug-resistant (MDR)-GNB in hospitalized patients.[1] There are a few technical limitations in the in vitro susceptibility testing as well as in the interpretative criteria of fosfomycin.[3] The Clinical and Laboratory Standards Institute (CLSI) guidelines are available only for the urinary isolates of Escherichia coli and Enterococcus faecalis.[4] Susceptibility breakpoints for these organisms, performed by disk diffusion (DD) and agar dilution (AD) methods by the CLSI, are representative of only the oral formulations.[4] The CLSI has not published IV fosfomycin breakpoints till date.[4] On the other hand, the European Committee of Antimicrobial Susceptibility Testing (EUCAST) recommends AD or broth microdilution methods for minimum inhibitory concentration (MIC) determination of fosfomycin for both oral and IV formulations.[5] In addition, it has recently published zone diameter breakpoints for IV fosfomycin since January 2017.[5] Both the EUCAST and CLSI MIC breakpoints differ [Table 1].[4,5] There is no consensus regarding the breakpoints for non-Enterobacteriaceae isolates such as Pseudomonas aeruginosa and Acinetobacter spp., which are important nosocomial pathogens. Considering the potential utility of fosfomycin against MDR-GNB and the relative paucity of data from India, we undertook the present study to determine the in vitro activity of fosfomycin against MDR-GNB isolates from urinary and nonurinary samples.
Table 1.
Fosfomycin minimum inhibitory concentrations and zone diameter breakpoints for Gram-negative bacilli according to the European Committee of Antimicrobial Susceptibility Testing and Clinical and Laboratory Standards Institute criteria 2017

MATERIALS AND METHODS
The study was performed over a period of 8 months (August 2016–March 2017) at the microbiology laboratory of a tertiary care teaching and referral hospital in the eastern part of India. Consecutive, nonduplicate MDR-GNB isolated from urine and various other samples (pus, blood, and endotracheal secretion/sputum) of the admitted patients were included. MDR was defined as nonsusceptibility to at least one agent in three or more antimicrobial categories.[6]
Fosfomycin susceptibility of all the isolates was determined by both DD and AD. For MIC determination by AD, fosfomycin disodium salt (Sigma Aldrich Corporation, St. Louis, MO. USA, Catalog no: P5396) in solution was added to Mueller–Hinton agar (HiMedia, lab Pvt Ltd, Mumbai, India) supplemented with 25 mg/L glucose-6-phosphate (HiMedia) to provide serial two-fold dilutions ranging in concentrations from 0.25 to 512 mg/l.[7] For DD, commercially available fosfomycin disks (HiMedia) containing 200 μg of fosfomycin and 50 μg of G6P were used.
CLSI MIC and zone diameter breakpoints for E. coli were used to interpret fosfomycin MIC and DD results for urinary isolates of GNB. For nonurinary isolates, EUCAST IV breakpoints were used.[7] The zone diameters of urinary isolates were also interpreted using the current EUCAST DD breakpoints in addition to the CLSI breakpoints. Isolated colonies within the inhibition zone were ignored. The interpretive criteria used are tabulated in Table 1. Categorical agreement (CA) between AD and DD was defined as results within the same susceptibility category.
RESULTS
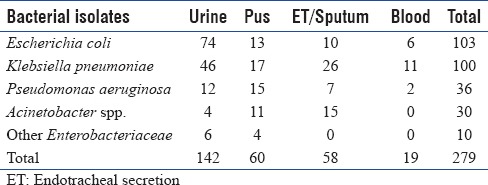
A total of 279 consecutive nonduplicate MDR-GNB were included in the present study. The sample-wise distribution of isolates is summarized in Table 2.
Table 2.
Sample-wise distribution of various isolates
Using the CLSI MIC breakpoints, 129 out of 142 (90.8%) urinary isolates of GNB were susceptible to fosfomycin, whereas 7 (4.9%) and 6 (4.2%) were classified as intermediate and resistant, respectively [Table 3]. There was complete CA between AD and DD results among the susceptible and resistant isolates using CLSI breakpoints. However, all the seven intermediate isolates by AD were observed to be sensitive by DD. Applying EUCAST MIC breakpoints to the urinary isolates, the total number of resistant isolates was 21 (21/142, 14.7%), which included all the 13 isolates classified as intermediate and resistant using the CLSI criteria plus 6 isolates of Klebsiella pneumoniae and 2 isolates of P. aeruginosa with MICs of 64 μg/ml by CLSI.
Table 3.
Minimum inhibitory concentration (μg/ml) distribution of various urinary tract isolates to fosfomycin (Clinical and Laboratory Standards Institute, 2017) (n=142)

A total of 137 nonurinary MDR-GNB isolates were tested for fosfomycin susceptibility applying the EUCAST IV MIC breakpoints for Enterobacteriaceae to nonurinary isolates. Maximum susceptibility was observed for E. coli (18/29, 62%), followed by K. pneumoniae (24/54, 44.4%), P. aeruginosa (8/25, 32%), and Acinetobacter spp. (3/26, 11%) [Table 4].
Table 4.
Minimum inhibitory concentration distribution of various nonurinary tract isolates (n=137) (interpreted by European Committee of Antimicrobial Susceptibility Testing minimum inhibitory concentration, 2017)

When the fosfomycin activity was analyzed as per the site of infection, the highest in vitro susceptibility was observed for urine (121/142, 85.3%), followed by pus (25/58; 43.1%), sputum/tracheal aspirate (17/60, 28.3%), and blood (5/19; 26.3%) [Figure 1]. Thus, a higher resistance rate was detected among isolates recovered from samples other than urine compared to the urinary isolates (57% vs. 9.2%; P < 0.0001).
Figure 1.

Analysis of fosfomycin activity as per the site of infection
DISCUSSION
The present study aimed to assess the in vitro activity of fosfomycin as a suitable oral agent for the treatment of UTI as well as a last resort therapeutic option in severe infections due to MDR-GNB in hospitalized patients.
Isolates recovered from the urinary tract (n = 142) had a high degree of susceptibility to fosfomycin (overall susceptibility, 90.8%) including a high percentage of E. coli and K. pneumoniae, the predominant urinary pathogens, which demonstrated a susceptibility of 99% and 91.3%, respectively. For urinary P. aeruginosa isolates, fosfomycin may be useful as 66.6% isolates were sensitive. We analyzed P. aeruginosa according to the CLSI E. coli breakpoints, which may not be appropriate. Some studies recommend that the ecological cutoff value of 128 mg/L can be used as a reference in interpreting the results in the absence of clinical breakpoints for Pseudomonas.[7] If the cutoff value of 128 mg/L is used, the percentage of susceptible isolates will be 75%. However, the total number of P. aeruginosa urinary isolates is very less (n = 12) to draw any conclusion. Similarly, in the absence of interpretative criteria and very less number of Acinetobacter isolates (n = 5), it is difficult to discuss the findings.
Our findings of fosfomycin susceptibility against uropathogens are similar to that of other Indian studies which have reported susceptibilities in the ranges of 90%–100%.[8] In our study, 99% of urinary E. coli retained susceptibility to fosfomycin. In case of urinary isolates of Klebsiella, in our study, 91.3% retained susceptibility to fosfomycin, whereas other studies from India have reported 95.5%, 90%, and 88.2%.[8] The overall CA between AD and DD was 98.6%, 95.6%, 91.6%, and 25% for E. coli, Klebsiella, P. aeruginosa, and Acinetobacter spp., respectively. The CA between the study by Perdigão-Neto et al. and our study is comparable for Klebsiella spp. (96% vs. 95.6%), whereas wide discrepancies were observed for P. aeruginosa (7% vs. 91.6%) and Acinetobacter spp. (86% vs. 25%).[9]
The second objective of our study was to study the in vitro susceptibility of fosfomycin against systemic infections. Among the 137 isolates tested, 81 (59.12%) were resistant to fosfomycin using the EUCAST breakpoints. Among nonurinary isolates, the rates of resistance for E. coli, Klebsiella, P. aeruginosa, and Acinetobacter spp. were 38%, 55.6%, 68%, and 89%, respectively. Our study found a little high rate of resistance in nonurinary isolates (59.12%) compared to a recent Indian study by Chitra et al. which found 48.8% resistance to fosfomycin among nonurinary isolates of E. coli and Klebsiella spp.[3] In the study by Chitra et al., Klebsiella isolates from blood and sterile body fluid showed increased resistance (24.5%) compared to urinary isolates (5.8%), while E. coli isolates were uniformly susceptible to both blood/body fluids (97%) and urinary isolates (100%).[3]
A notable finding of our study was 99% of MDR E. coli isolates from UTI retained susceptibility to fosfomycin, whereas among nonurinary tract isolates, the fosfomycin sensitivity was 62%. Similarly, 91.3% of MDR K. pneumoniae isolates from UTI retained susceptibility to fosfomycin, whereas among nonurinary tract isolates, the sensitivity was 44.5%. The discrepancy in the rates of resistance between urinary and nonurinary isolates is difficult to explain and more work is needed to understand the differences in susceptibility profile between isolates responsible for UTI and other systemic infections.
CONCLUSION
Although fosfomycin appears promising as a suitable agent for UTI based on in vitro data, high rates of resistance in nonurinary isolates of Enterobacteriaceae is a cause of concern. There is a need for harmonization of CLSI and EUCAST breakpoints and optimization of DD, particularly for nonurinary isolates. Furthermore, breakpoints for Pseudomonas spp. and Acinetobacter spp. need to be defined.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Raz R. Fosfomycin: An old – New antibiotic. Clin Microbiol Infect. 2012;18:4–7. doi: 10.1111/j.1469-0691.2011.03636.x. [DOI] [PubMed] [Google Scholar]
- 2.Gupta K, Hooton TM, Naber KG, Wullt B, Colgan R, Miller LG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: A 2010 update by the infectious diseases society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52:e103–20. doi: 10.1093/cid/ciq257. [DOI] [PubMed] [Google Scholar]
- 3.Chitra C, Kumar D, Shakti L, Diana SR, Balaji V. Technical and interpretative issues of fosfomycin susceptibility testing. Indian J Med Microbiol. 2015;33:611–2. doi: 10.4103/0255-0857.167338. [DOI] [PubMed] [Google Scholar]
- 4.Clinical and Laboratory Standards Institute (CLSI) Wayne: Clinical Laboratory Standards Institute (CLSI); 2017. CLSI document M100S. Performance Standards for Antimicrobial Susceptibility testing – Twenty-Seventh Informational Supplement. [Google Scholar]
- 5.The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone diameters. Version 7.0. [Last accessed on 2017 Jun 01]. Available from: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_7.0_Breakpoint_Tables.pdf .
- 6.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268–81. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 7.Saiprasad PV, Krishnaprasad K. Exploring the hidden potential of fosfomycin for the fight against severe gram-negative infections. Indian J Med Microbiol. 2016;34:416–20. doi: 10.4103/0255-0857.195379. [DOI] [PubMed] [Google Scholar]
- 8.Banerjee S, Sengupta M, Sarker TK. Fosfomycin susceptibility among multidrug-resistant, extended-spectrum beta-lactamase-producing, carbapenem-resistant uropathogens. Indian J Urol. 2017;33:149–54. doi: 10.4103/iju.IJU_285_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perdigão-Neto LV, Oliveira MS, Rizek CF, Carrilho CM, Costa SF, Levin AS, et al. Susceptibility of multiresistant gram-negative bacteria to fosfomycin and performance of different susceptibility testing methods. Antimicrob Agents Chemother. 2014;58:1763–7. doi: 10.1128/AAC.02048-13. [DOI] [PMC free article] [PubMed] [Google Scholar]


