Abstract
Introduction:
Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of non-Hodgkin's lymphoma. We conducted a retrospective study to analyze the clinicopathological characteristics, cell of origin, response to therapy, and the outcome of patients with DLBCL.
Materials and Methods:
This was a retrospective study which included all patients with DLBCL registered at our center, between May 1, 2013, and July 31, 2015. The data regarding demography, clinical presentation, histopathology, stage, prognostic index, treatment, and treatment-related outcome were collected from prospectively maintained clinical case records of the patients.
Results:
In the study, we included 267 patients. The median age is 49 (20–81) years with male: female ratio of 2:1. B symptoms were seen in 124 (45%) of patients. Early Stages (I and II) were seen in 130 (52%) patients, while advanced Stages (III and 1V) were seen in 119 (48%) patients. Bulky disease (>7.5 cm) was seen in 30% of cases, and bone marrow was involved in 12%. Extranodal involvement is present in 35% of cases. Cell of origin data was available in 160 (60%) of cases, of which 88 (55%) were germinal center and 72 (45%) were activated B cell in origin. The distribution according to the international prognostic index (IPI) was as follows: low risk 40%, intermediate risk 45%, and high risk in 15%. Rituximab was used in 45% of cases. The overall response rate was 84% with a complete response (CR) rate of 70.5%. The CR rates were better with RCHOP compared with CHOP (77% vs. 61.5%, P = 0.001) and good-risk IPI (83.3% vs. 65.2%, P < 0.001) compared with intermediate- and high-risk IPI. Median follow-up period was 24 months, and 2-year event-free survival (EFS) was 70%. The presence of B symptoms, high IPI, failure to attain CR, poor PS, and nonrituximab-based chemotherapy were significantly associated with lower EFS.
Conclusions:
This is the first study from India, which investigated the impact of chemotherapy with or without rituximab in context of cell of origin. Adding rituximab to CHOP showed better response rate and EFS irrespective of cell of origin.
Keywords: Diffuse large B-cell lymphoma, India, outcome, rituximab
Introduction
Diffuse large B-cell lymphoma (DLBCL) constitutes about 40% of newly diagnosed cases of non-Hodgkin's lymphoma (NHL).[1] It is a heterogeneous disease with variable clinical course and outcome. There is a paucity of data regarding the clinicopathologic characteristics, cell of origin, and response and outcome of treatment with chemoimmunotherapy in patients from North India diagnosed with DLBCL.
Materials and Methods
In this retrospective study, all patients of age ≥ 18 years, diagnosed with DLBCL and registered at the lymphoma/leukemia clinic at Dr. B.R.A-IRCH, AIIMS, New Delhi, from May 1, 2013, to July 31, 2015, were included in the study. We excluded patients with primary central nervous system lymphoma, primary mediastinal B-cell lymphoma, and HIV-associated DLBCL for assessment response and outcome because of different treatment and biology. The patients with incomplete record and those who received < 2 cycles of chemotherapy/chemoimmunotherapy were also excluded from the analysis. The patients were classified as germinal center B-cell like (GCB) or activated B-cell (ABC) type using the Hans classification.[2] The clinical stage was evaluated in accordance with Ann Arbor classification (Cotswolds modification) and Lugano classification. Any tumor mass measuring >7.5 cm was labeled as “bulky disease.” Event-free survival (EFS) was defined as time from date of registration to disease relapse, progression, or death, for any cause. Complete response (CR), partial response, progression, refractory disease, and relapse were defined according to International Working Group response criteria for malignant lymphoma.[3] Statistical analysis was done using STAT14.0. (College Station, TX: StataCorp LP). Chi-square test was used to analyze the significance of clinical factors. P <0.05 was kept as statistically significant.
Results
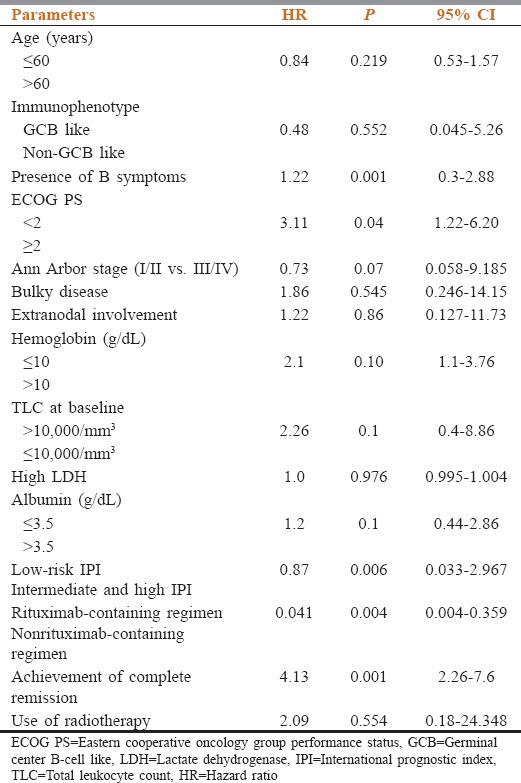
Three hundred and ninety cases of NHL were registered in the lymphoma/leukemia clinic at Dr. B.R.A-IRCH, AIIMS, New Delhi, from May 1, 2013, to July 31, 2015. Of these 267 patients were diagnosed of having DLBCL. A total of 249 cases were available for response assessment and outcome. The median age of patients in this study population was 49 (20–78) years, and 23.5% (60) of the patients had age > 60 years. Base line clinico-pathological features are described in Table 1. In our cohort, 178 (66.6%) patients were male and 89 (33.3%) were female. The male-to-female ratio was 2:1. The Eastern Cooperative Oncology Group (ECOG) performance status > 2 was seen in 57 (22.8%) cases. Extranodal involvement was seen in 93 (34.8%) patients. Fifty patients had primary extranodal lymphoma (PEL). The most frequently affected sites for PEL were the stomach and intestine followed by central nervous system. The other sites of PEL were testis, bone, breast, ovary, parotid, prostate, renal, conjunctival, thyroid, skin and soft tissue, and liver. Bone marrow involvement was noted in 12% of the patients. The information regarding cell of origin (based of Hans algorithm) was available for 160 patients, 88 (55%) were GCB, and 72 (45%) were ABC. Most of the patients were managed with CHOP (cyclophosphamide, vincristine, doxorubicin, and steroid) ± rituximab and rarely with modified CHOP/CVP/CEOP base chemotherapy. Rituximab was used in 45% of cases. Twenty percent needed treatment modification upfront or subsequently because of treatment-related toxicity and upfront poor performance status. Radiotherapy was used in 45% cases of DLBCL for early stage and advanced stage with bulky disease or residual disease. Median chemotherapy cycle used was 6 (range 3–8). Positron emission tomography was used in 33% of patients for baseline imaging and response evaluation. Eight patients were lost to follow-up before response assessment, and three patients died of toxicity. The overall response rate (ORR) was 84% and CR rate was 70.5%. The CR rate was better with RCHOP compared to CHOP (77% vs. 61.5%; P = 0.001) and good-risk international prognostic index (IPI) versus intermediate- and high-risk IPI (83.5% vs. 65.2%, P < 0.001). With a median follow-up of 24 months (range of 3–48 months), a total of 88 events occurred, including 30 deaths, 25 progressive disease, and 33 relapse. Relapses most commonly involved the lymph nodes. The most common salvage therapy includes ifosfamide, carboplatin, etoposide or DHAP (dexamethasone, Ara C, cisplatin). PEPCY (procarbazine, etoposide, prednisolone, cyclophosphamide) and bendamustine plus rituximab (BR) regimens were used in the elderly and/or poor PS patients. The median EFS was not reached, and the 2-year EFS was 70%. The presence of B symptoms, use of rituximab-based therapy, high total leukocyte count (>10,000/mm3) at baseline, advanced stage (III/IV) and high IPI, presence of anemia (Hb < 10 gm/dl), and albumin (<3.5 g/dL) significantly affected EFS in univariate analysis. The presence of B symptoms, high IPI, failure to attain CR, and nonrituximab-based chemotherapy were significantly associated with lower EFS (Table 2). EFS among patients who received RCHOP was superior to CHOP 77% vs. 61%, P = 0.01.
Table 1.
Clinicopathological characteristics of diffuse large B-cell patients
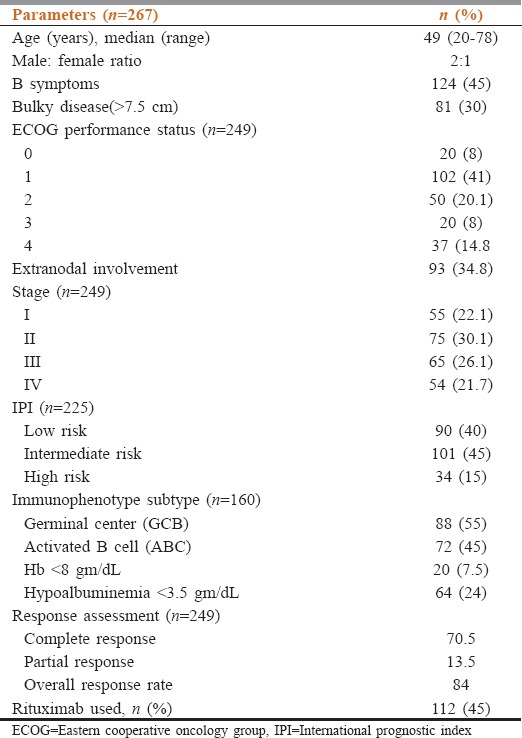
Table 2.
Factors affecting the event-free survival on multivariate analysis
Discussion
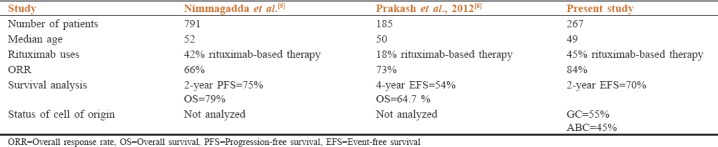
DLBCL is the most common subtype of NHL in our cohort accounting for 68.5% of cases. This is significantly higher as compared to data from earlier studies from both India and the west.[4,5] The median age of patients with DLBCL was 49 years, which is almost a decade and a half younger to those reported in the developed world and similar to those reported from other developing countries.[5,6] The younger average age of Indian patients is consistent with the pattern seen in most other malignancies in India, due to the effect of a younger population in our country or due to the referral bias toward younger patients for treatment at higher center.[5] It is of note that around 30% of cases presented with bulky disease, one-quarter of patients had hypoalbuminemia, and performance status was > 2; this can be in part ascribed to diagnostic delays, lack of access to cancer care, and malnutrition. Majority of our patients (124 [45%]) had B symptoms and 60% of all our patients belonged to high and intermediate IPI groups. This is similar to data reported in previous studies from India, which signified more advanced disease in this part of the world.[6] Comparison with the previous studies from India has shown in Table 3. Extranodal involvement was common in this study and was seen in 93 (35%) patients. This is similar to that seen in a previous study from India.[7] Good response to rituximab-based therapy improved the ORR and CR in our patients and also significantly affects EFS. Patients with high and intermediate IPI had low CR as compared to patients with low IPI. Hans et al. published their first study to differentiate DLBCL by tissue IHC instead of gene expression profiling and reported a 5-year OS rate of 76% for GC and 34% for non-GC types. These results were confirmed by several other studies.[8] In this study, we did not find any correlation with response rate and EFS on the basis of a cell of origin. This might be due to relatively small sample size and short follow-up.
Table 3.
Previous studies from Indian subcontinent related to diffuse large B-cell lymphoma
Conclusion
The present study in northern Indian population shows key differences in the presentation as compared to the west, which include median age of 49 years (almost a decade and half less), higher male-to-female ratio, higher proportion of patients with poor ECOG performance status at diagnosis, higher proportion of patients with high and intermediate IPI risk group, more B symptoms, and extranodal disease. Lack of access to specialized cancer care centers, diagnostic delay, and suboptimal or inappropriate management compounded by socioeconomic factors are probably the factors attributing to the inferior outcome in this part of the world. The outcomes of DLBCL in India can be improved with the creation of regional cancer centers, structured data collection, centralized pathology review of histopathology, uniform chemotherapy protocols, appropriate training, and financial support.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375–90. doi: 10.1182/blood-2016-01-643569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hans CP, Weisenburger DD, Greiner TC, Gascoyne RD, Delabie J, Ott G, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103:275–82. doi: 10.1182/blood-2003-05-1545. [DOI] [PubMed] [Google Scholar]
- 3.Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–86. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 4.Perry AM, Diebold J, Nathwani BN, MacLennan KA, Müller-Hermelink HK, Bast M, et al. Relative frequency of non-Hodgkin lymphoma subtypes in selected centres in North Africa, the middle East and India: A review of 971 cases. Br J Haematol. 2016;172:699–708. doi: 10.1111/bjh.13876. [DOI] [PubMed] [Google Scholar]
- 5.Nimmagadda RB, Digumarti R, Nair R, Bhurani D, Raina V, Aggarwal S, et al. Histopathological pattern of lymphomas and clinical presentation and outcomes of diffuse large B cell lymphoma: A multicenter registry based study from India. Indian J Med Paediatr Oncol. 2013;34:299–304. doi: 10.4103/0971-5851.125250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prakash G, Sharma A, Raina V, Kumar L, Sharma MC, Mohanti BK, et al. Bcell non-Hodgkin's lymphoma: Experience from a tertiary care cancer center. Ann Hematol. 2012;91:1603–11. doi: 10.1007/s00277-012-1491-5. [DOI] [PubMed] [Google Scholar]
- 7.Pai A, Kannan T, Balambika RG, Vasini V. A study of clinical profile of primary extranodal lymphomas in a tertiary care institute in South India. Indian J Med Paediatr Oncol. 2017;38:251–5. doi: 10.4103/ijmpo.ijmpo_82_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ngo L, Hee SW, Lim LC, Tao M, Quek R, Yap SP, et al. Prognostic factors in patients with diffuse large B cell lymphoma: Before and after the introduction of rituximab. Leuk Lymphoma. 2008;49:462–9. doi: 10.1080/10428190701809156. [DOI] [PubMed] [Google Scholar]


